Abstract
Purpose
Antivascular endothelial growth factor agents are increasingly used in diabetic macular oedema (DME); however, there are few studies exploring their use in DME in real‐world settings.
Methods
POLARIS was a noninterventional, multicentre study to monitor 12‐month outcomes in patients starting ranibizumab treatment in routine practices. The primary outcome was mean change in visual acuity (VA) from baseline to month 12 (last observation carried forward approach). Other outcomes included mean change in central retinal thickness (CRT) and resource utilization. Visual acuity (VA) outcomes were also stratified by country, baseline visual acuity score (VAS), sex, age and injection frequency.
Results
Outcomes were analysed from all treated patients (n = 804) and from first‐year completers (patients who had a visual acuity assessment at 12 months; n = 568). The mean (SD) baseline VAS was 59.4 (15.9) letters, and the mean change in visual acuity was 4.4 letters (95% confidence interval: 3.3–5.4) at month 12 (study eye; first‐year completers). The mean number of injections (study eye) was 4.9, and the mean number of all visits (any eye) was 10 (58% were injection visits) over 12 months (first‐year completers). The mean (SD) baseline CRT was 410.6 (128.8) μm, and the mean change in CRT was −115.2 μm at month 12 (study eye; first‐year completers). Visual acuity (VA) outcomes were generally comparable across most countries and subgroups and were greatest in patients with the lowest baseline VAS (≤60 letters).
Conclusion
POLARIS showed that real‐world outcomes in DME patients starting treatment with ranibizumab were lower than those observed in clinical studies, in spite of extensive monitoring.
Keywords: anti‐VEGF, diabetic macular oedema, ranibizumab
Introduction
It is estimated that 285 million adults worldwide currently have diabetes mellitus, with 31 million having vision‐threatening diabetic retinopathy or diabetic macular oedema (DME) (Lee et al. 2015). Diabetic macular oedema can occur at any stage of retinopathy and is typically characterized by retinal thickening and leakage of extracellular fluid, which are linked with hypoxia and upregulation of vascular endothelial growth factor (VEGF) (Fong et al. 2003). Current treatment options include laser photocoagulation and dexamethasone implants. Laser is effective in stabilizing disease, but its ability to reverse vision loss is low. Laser is also associated with thermal complications, which can still manifest many years after treatment (Early Treatment Diabetic Retinopathy Study Research Group 1985; Cheung et al. 2010). Since their introduction, anti‐VEGF agents have been used increasingly in the treatment of DME (Jiang et al. 2015; Dugel et al. 2016). These agents not only target VEGF, notably VEGF‐A, but are clinically effective and well tolerated in randomized studies in patients with DME (Mitchell et al. 2011; Nguyen et al. 2012; Papadopoulos et al. 2012; Simo et al. 2014; Wells et al. 2015, 2016; Prunte et al. 2016).
A direct comparison of three anti‐VEGF agents [ranibizumab 0.3 mg, intravitreal aflibercept (IVT‐AFL) 2 mg and bevacizumab 1.25 mg] in the Protocol T study showed that all agents were effective in improving VA at 1 year (the primary outcome) and over 2 years in patients with DME, but IVT‐AFL was significantly more effective at improving 1‐year outcomes in patients with worse baseline VA (<69 letters) compared with ranibizumab (p = 0.003) and bevacizumab (p < 0.001) (Wells et al. 2015, 2016). However, the Diabetic Retinopathy Clinical Research Network added that caution must be exercised when extrapolating these results into routine clinical practices (Heier et al. 2016). This is particularly important for considering any clinical differences between the US Food and Drug Administration–approved dose of ranibizumab (0.3 mg), which was used in Protocol T, and the European Union–approved dose (0.5 mg). In addition, these studies did not consider the impact of the recent European label change for ranibizumab; the recommended approach was switched from as‐needed with monthly monitoring to a more flexible regimen. Observational studies in other indications, such as neovascular age‐related macular degeneration (nAMD), have shown that ranibizumab dosing is low and VA outcomes are not maintained over time (Writing Committee for the UK Age‐Related Macular Degeneration EMR Users Group. 2014; Holz et al. 2015, 2016a,2016b). Such studies were also performed when ranibizumab had a less flexible regimen per label. To date, there are a few small studies exploring these issues in patients with DME (Brynskov et al. 2013; Hrarat et al. 2016; Patrao et al. 2016).
POLARIS was an international, large‐scale, noninterventional, multicentre study to monitor 12‐month outcomes in patients with DME who started treatment with ranibizumab (this was the only anti‐VEGF agent approved at the start of this study). This study aimed at reflecting a real‐world setting, and any subsequent treatment decisions were made by the treating physician in accordance with their normal routine practice. Patients were enrolled from clinical practices across eight European countries.
Patients and Methods
Study design
POLARIS (NCT01771081) was a noninterventional, real‐world study to monitor 12‐month outcomes and resource use in patients with DME who started treatment with ranibizumab. The study was conducted from 27 September 2012 (first patient visit) to 30 January 2015 (last patient visit) in 75 centres across eight European countries: France, Germany, Greece, Portugal, Russia, Slovakia, Spain and the UK. The protocol and its amendments were approved by the independent ethics committees and institutional review boards for all participating centres as required by country laws and regulations.
Participants
Patients diagnosed with type 1 or 2 diabetes mellitus and DME with central involvement, which was defined as the centre subfield area on optical coherence tomography (OCT), were enrolled. Patients were enrolled after the diagnosis and decision to treat DME with ranibizumab had been made by the physician, and patients had to receive the first treatment on or after 01 October 2012. Patients were excluded if they had received any anti‐VEGF prior to study enrolment or if they had participated in any investigational study. To reduce selection bias, each consecutive patient examined during the observation period had to be screened and documented in an anonymous patient log file (independent of prescribed treatment), including information on the treatment given. All patients provided written informed consent to participate.
Treatment and data collection
Patients started treatment with ranibizumab (this was the only anti‐VEGF agent approved at the start of this study). Any retreatment decisions (including interval and dose) were made by the treating physician in accordance with their normal routine practice. As this study aimed at reflecting a real‐world setting, patients could also receive ocular surgeries, laser or switch to other available anti‐VEGF agents such as pegaptanib sodium or bevacizumab based on the physician's decisions and usual practice (IVT‐AFL was not approved or available for this indication at the time of study start). These patients were included in the analyses. This decision was preplanned to monitor actual treatment use and associated outcomes in a real‐world setting.
Patients were followed up for 12 months during routine (treatment and monitoring) visits. Information on study eye(s), drug, dose and date administered, together with the reasons for discontinuation or retreatment, was recorded. All data were collected by study investigators who were blinded to patient details, and patients were identified by a central identification code only.
Patients could be enrolled prospectively (i.e. first treatment on/after 01 October 2012 with prospective follow‐up) or retrospectively (i.e. data collected retrospectively from the date of first treatment with prospective follow‐up) from enrolment; this allowed sufficient numbers to be consecutively included without prolonging recruitment. Data were collected from medical records, patient interviews or during initial and follow‐up visits, and included socio‐demographic characteristics, medical history, previous and current DME therapy and routine tests. All data were provided by the treating ophthalmologist/physician and recorded in electronic case report forms, which were audited for accuracy using source data verification overseen by the sponsor (Bayer AG, Berlin, Germany).
Assessments
Data were recorded using both eyes (study eye/fellow eye), but only outcomes in the study eye were included in analyses. The study eye was defined as the eye with the worse visual acuity (VA) at the start of therapy. However, if both eyes had the same VA at therapy start, then the right eye was defined as the study eye. In the case of bilateral disease, the second eye treated (treated fellow eye) was analysed separately. The primary outcome was the mean change in VA (letters) from baseline up to month 12. As the study was noninterventional, VA was measured according to physician's own practice [e.g. Early Treatment Diabetic Retinopathy Study (ETDRS) letters, Snellen fractions or logarithm of the minimum angle of resolution (logMAR)] and was converted to a standardized visual acuity score (VAS) (letters) using published conversion charts (Ferris et al. 1982; Holladay 1997; Gregori et al. 2010). Additional data were collected on the socio‐demographic and clinical profile of the patient; resource utilization (visits, injections and diagnostics) including the proportion of patients requiring laser therapy and mean change in central retinal thickness (CRT) on OCT were recorded. The use of OCT (any type) as an assessment depended on the clinic's access to it, and need for it, and would therefore vary during the study. All spectral domain measurements were subtracted with 43.1 μm to fit the measurements of the time domain machines.
Statistical analyses
A sample size of 330 patients was required to estimate the change from baseline in VA (letters) within a 95% confidence interval (CI) of ±1.5 letters. This assumed a 95% probability of obtaining a CI with a width of ≤3 letters using a standard deviation (SD) of 13 for the change in VA. To ensure that POLARIS was reasonably well matched against the additional criteria used in the VIVID/VISTA‐DME and RESTORE studies (Mitchell et al. 2011; Korobelnik et al. 2014), it was assumed that a higher patient number would be needed mainly to estimate VA changes with the precision used in these studies, and a sample size of 807 patients (assuming a 10% dropout) was used.
Statistical analyses were of an exploratory and descriptive nature. Analyses were performed using data from all patients who received ≥1 anti‐VEGF treatment and had baseline and ≥1 postbaseline assessment of VA for the study eye and from patients who also had a VA assessment at month 12 (360 ± 60 days after baseline; first‐year completers).
For the primary outcome, the mean change in VA from baseline (initial visit prior to first ranibizumab therapy injection) up to month 12 (360 ± 60 days after baseline) and 95% CI were calculated; if the patient had two visits during 360 ± 60 days, then the visit closest to 360 days was used. All other follow‐up visits were scheduled within a 30‐day window [e.g. visit 1 (30 ± 15 days) and visit 12 (360 ± 15 days)]. Visits were defined as injection or monitoring (noninjection) visits and could relate to either study or fellow eye. If injection and monitoring visits occurred within a 7‐day period, then they were counted as one injection visit. An additional post hoc analysis was also undertaken to recount visits on separate dates as separate visits. To account for missing data, a last observation carried forward (LOCF) approach was used for each window from study start. VA outcomes were stratified by country and subgroups [sex, age (65, ≥65 years) and baseline VAS (<60, 61–73, >73 letters)]. Post hoc analyses were undertaken to explore the association between VA outcomes, baseline VA and injection frequency. The statistical evaluation was performed using the software package sas release 9.2 SP4 (SAS Institute Inc., Cary, NC, USA).
Results
Participants
Overall, 1674 patients were screened and 983 (58.7%) were enrolled. Of the 691 patients who were not enrolled, 672 were due to screen failures and 19 were due to study closure (Fig. S1). Outcomes were analysed from 804 patients (all patients) and from 568 patients (first‐year completers). The main reasons for exclusion were no baseline (n = 85) or postbaseline (n = 49) visual acuity assessments (all patients) and no visual acuity assessment within the month 12 window (360 ± 60 days) (n = 236) (first‐year completers). Overall, 98% of patients had some prospective data, 45% of patients had full prospective data (all patients), 99% of patients had some prospective data, and 45% of patients had full prospective data (first‐year completers). The mean age at baseline was 64.1 years, and most patients were male (58.3%), white (65.7%) and had DME in both eyes (66.4%) (first‐year completers) (Table 1). All patients received at least one ranibizumab injection in the study eye. A total of 31 patients (all patients) and 25 patients (first‐year completers) also received ≥1 injection of another anti‐VEGF agent.
Table 1.
Patients' socio‐demographic and clinical characteristics at baseline
| Mean (SD) unless stated | All patients (n = 804) | First‐year completers (n = 568) |
|---|---|---|
| Male, n (%) | 473 (58.8) | 331 (58.3) |
| Age at baseline, years | 64.1 (10.7) | 64.1 (10.6) |
| BMI at baseline, kg/m2 | 30.04 (5.45) | 30.13 (5.71) |
| Race, n (%) | ||
| White | 501 (62.3) | 373 (65.7) |
| Asian | 23 (2.9) | 17 (3.0) |
| Black | 5 (0.6) | 5 (0.9) |
| Other | 2 (0.2) | 2 (0.4) |
| Missing or not reporteda | 273 (34.0) | 171 (30.1) |
| Haemoglobin A1C (%) [n = 216/166] | 7.33 (1.18) | 7.26 (1.09) |
| Type of diabetes mellitus, n (%) | ||
| Type I | 79 (9.8) | 54 (9.5) |
| Type II | 724 (90.0) | 513 (90.3) |
| Missing | 1 (0.1) | 1 (0.2) |
| Eye affected by DME, n (%) | ||
| Right | 143 (17.8) | 94 (16.5) |
| Left | 139 (17.3) | 97 (17.1) |
| Both | 522 (64.9) | 377 (66.4) |
| Study eye | ||
| Left | 399 (49.6) | 289 (50.9) |
| Right | 405 (50.4) | 279 (49.1) |
| Baseline visual acuity score, letters [n = 795/562] | 59.7 (15.8) | 59.4 (15.9) |
| Baseline visual acuity score categories, n (%) | ||
| <69 letters | 526 (65.4) | 369 (65.0) |
| ≥69 letters | 278 (34.6) | 199 (35.0) |
| Baseline visual acuity score categories, n (%) | ||
| ≤60 letters | 384 (47.8) | 272 (47.9) |
| 61–73 letters | 261 (32.5) | 182 (32.0) |
| >73 letters | 159 (19.8) | 114 (20.1) |
| CRT, μm [n = 664/484] | 403.5 (128.9) | 410.6 (128.8) |
| Prior laser treatment (study eye), n (%) | 468 (58.2) | 341 (60.0) |
| Focal/grid | 277 (34.5) | 211 (37.1) |
| Panretinal | 268 (33.3) | 194 (34.2) |
| Laser | 35 (4.4) | 27 (4.8) |
| Employment status, n (%) | ||
| Employed | 133 (16.5) | 100 (17.6) |
| Seeking work | 7 (0.9) | 5 (0.9) |
| Retired | 327 (40.7) | 237 (41.7) |
| Student | 2 (0.2) | 1 (0.2) |
| Keeping house | 19 (2.4) | 12 (2.1) |
| Self‐employed | 11 (1.4) | 10 (1.8) |
| Other | 12 (1.5) | 6 (1.1) |
| Not reported/missing | 293 (36.4) | 197 (34.7) |
BMI = body mass index, CRT = central retinal thickness, DME = diabetic macular oedema, SD = standard deviation.
Not reported for France and Germany.
Functional and anatomical outcomes
The outcomes for both populations were similar. The mean (SD) baseline VAS was 59.4 (15.9) letters, and the proportions of patients with baseline VAS <69 letters or ≥69 letters were 65% and 35%, respectively (study eye; first‐year completers). There was a gradual improvement to 4.2 letters by day 90, which was maintained throughout the study; the final mean change in visual acuity at month 12 was 4.4 letters (95% CI: 3.3–5.4) (study eye; first‐year completers; LOCF) (Fig. 1A and Table 2) and 4.3 letters (95% CI: 3.2–5.3) (study eye; observational cohort). Overall, 29.4% of patients achieved best‐corrected visual acuity gains of ≥10 letters, 17.1% achieved best‐corrected visual acuity gains of ≥15 letters, and 6.7% lost ≥15 letters at month 12 (study eye; first‐year completers) (Fig. 1B). The proportion of patients achieving ≥69 letters at month 12 was 47.4% (study eye; first‐year completers). For OCT recordings, most measurements [91.8% (all patients) and 90.9% (first‐year completers)] were with spectral domain machines at the last visit. There was a mean reduction in CRT of −115.2 μm (study eye; first‐year completers).
Figure 1.
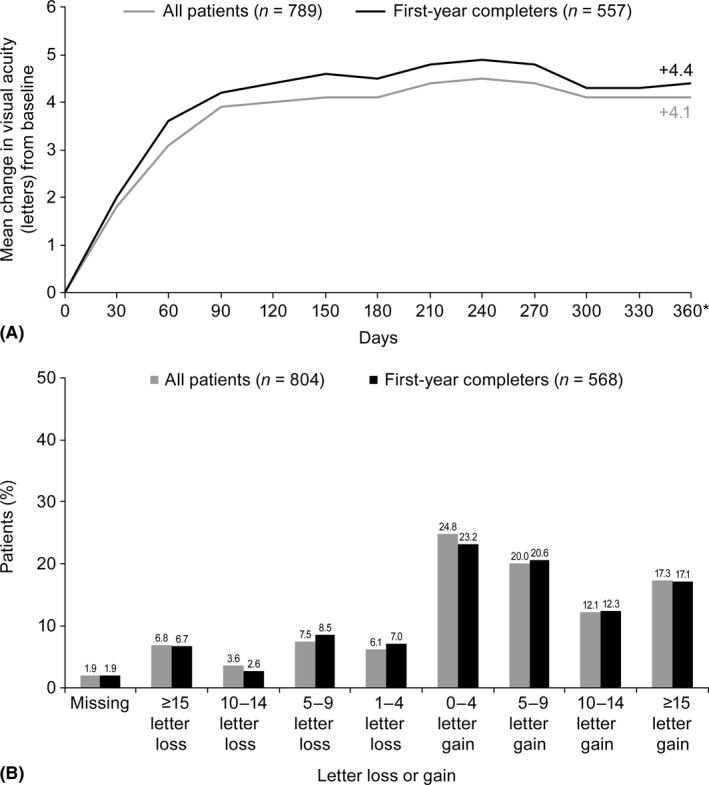
Visual acuity outcomes for all patients and first‐year completers including (A) mean change in visual acuity (LOCF) from baseline to month 12 and (B) loss or gain of letters (LOCF) at month 12. LOCF = last observation carried forward. *Definition of first‐year visit: the visit closest to 360 days from baseline within 360 ± 60 day window. Data not available for all patients at all time points.
Table 2.
Visual acuity outcomes (LOCF) for all patients stratified by country, subgroups and injections
| n | Mean baseline visual acuity score (letters) | Mean change in visual acuity at month 12 (letters) | 95% CI | |
|---|---|---|---|---|
| First‐year completers | 562 | 59.4 | 4.4 | 3.3–5.4 |
| Countrya | ||||
| France | 56 | 60.7 | 4.5 | 0.9–8.1 |
| Germany | 95 | 61.4 | 3.9 | 1.2–6.6 |
| Greece | 32 | 61.6 | 3.4 | −0.3 to 7.1 |
| Portugal | 5 | 57.6 | 0.0 | −10.5 to 10.5 |
| Russia | 39 | 44.1 | 8.4 | 3.5–13.2 |
| Slovakia | 137 | 62.0 | 3.1 | 1.5–4.6 |
| Spain | 73 | 56.9 | 4.8 | 2.2–7.5 |
| UK | 125 | 60.3 | 5.1 | 2.5–7.7 |
| Sexa | ||||
| Male | 327 | 62.0 | 4.5 | 3.1–5.8 |
| Female | 235 | 55.9 | 4.3 | 2.6–6.0 |
| Agea | ||||
| <65 years | 268 | 59.6 | 4.5 | 3.0–6.1 |
| ≥65 years | 294 | 59.3 | 4.2 | 2.8–5.6 |
| Baseline visual acuity score categoriesa | ||||
| ≤60 letters | 266 | 46.3 | 8.8 | 7.2–10.4 |
| 61–73 letters | 182 | 67.4 | 1.5 | –0.0 to 2.9 |
| >73 letters | 114 | 77.3 | –1.1 | –3.1 to 0.8 |
| Injection categories (4‐level)a | ||||
| <4 | 184 | 58.6 | 4.6 | 2.9–6.3 |
| 4–5 | 156 | 60.4 | 4.3 | 2.2–6.5 |
| 6–7 | 116 | 59.6 | 4.4 | 2.1–6.7 |
| >7 | 106 | 59.2 | 4.0 | 1.6–6.4 |
CI = confidence interval, SD = standard deviation.
First‐year completers.
Resource utilization
Resource use was similar in both populations (Table 3). The mean number of injections (study eye) was 4.9 over the 12‐month period (first‐year completers). The mean number of all visits (for monitoring or injections relating to either eye) was 10.0 (first‐year completers) over the 12‐month period according to the predefined study set‐up. Only 58% (n = 5.8/10.0) were injection visits. The predefined method allowed multiple visits made within a window of 7 days to be documented as a single visit. A post hoc analysis was undertaken to count visits made on different days as separate visits to more closely reflect the patient and clinic burden. On this basis, the mean (SD) number of all visits increased only slightly to 11.3 (4.3) (first‐year completers). It must also be noted that 64.9% (all patients)/66.4% (first‐year completers) of patients had both eyes affected by DME; a breakdown of visits shows that the mean (SD) injections/year in the fellow eye was 3.8 (2.2)/year (all patients). The percentage of patients receiving concomitant laser in the study eye was 27.4% (all patients) and 28.2% (first‐year completers), and the percentage receiving ocular surgery in the study eye was 9.5% (all patients) and 10.6% (first‐year completers).
Table 3.
Resource use for all patients and by countrya (LOCF)
| Mean (SD in brackets) unless stated | All patients | First‐year completers | France | Germany | Greece | Portugal | Russia | Slovakia | Spain | UK |
|---|---|---|---|---|---|---|---|---|---|---|
| n | 804 | 568 | 60 | 95 | 32 | 5 | 40 | 137 | 74 | 125 |
| Injectionsb | 4.5 (2.3) | 4.9 (2.4) | 4.5 (2.0) | 5.1 (2.1) | 4.2 (1.9) | 5.6 (0.9) | 2.0 (1.2) | 4.6 (1.8) | 4.1 (2.3) | 6.7 (2.3) |
| All visits | 9.1 (4.0) | 10.0 (3.9) | 9.3 (2.2) | 12.5 (6.7) | 9.3 (3.1) | 7.6 (0.5) | 5.8 (1.4) | 10.9 (2.9) | 8.7 (2.1) | 9.8 (2.4) |
| Injection visits | 5.4 (2.9) | 5.8 (2.9) | 5.0 (2.2) | 6.3 (3.4) | 5.3 (2.5) | 5.8 (0.8) | 2.3 (1.4) | 6.2 (2.8) | 4.7 (2.5) | 7.4 (2.4) |
| Monitoring visits | 3.7 (3.2) | 4.1 (3.4) | 4.3 (2.1) | 6.2 (6.0) | 3.9 (2.3) | 1.8 (0.8) | 3.5 (1.2) | 4.6 (2.1) | 4.0 (2.8) | 2.4 (2.0) |
| Visual acuity tests | 9.1 (4.0) | 10.0 (3.9) | 9.3 (2.2) | 12.5 (6.7) | 9.3 (3.1) | 7.6 (0.5) | 5.8 (1.4) | 10.9 (2.9) | 8.7 (2.1) | 9.8 (2.4) |
| OCT | 5.9 (3.4) | 6.8 (3.3) | 6.4 (2.2) | 4.2 (3.5) | 7.2 (2.4) | 5.0 (1.4) | 4.7 (1.6) | 9.4 (3.2) | 6.9 (2.2) | 6.7 (2.8) |
| Ophthalmoscopy | 7.2 (5.9) | 8.1 (6.3) | 3.9 (3.8) | 9.3 (4.3) | 7.2 (5.8) | 3.2 (1.1) | 8.2 (2.6) | 14.8 (6.8) | 5.8 (2.3) | 3.5 (3.7) |
| FA | 0.7 (1.0) | 0.7 (1.0) | 0.4 (0.6) | 1.7 (1.3) | 0.7 (0.5) | 0.4 (0.5) | 0.1 (0.3) | 0.8 (0.9) | 0.6 (0.7) | 0.2 (0.4) |
| Concomitant laserb, n (%) | 220 (27.4) | 160 (28.2) | 11 (18.3) | 26 (27.4) | 10 (31.3) | 1 (20.0) | 16 (40.0) | 61 (44.5) | 18 (24.3) | 17 (13.6) |
FA = fluorescein angiography, LOCF = last observation carried forward, OCT = optical coherence tomography, SD = standard deviation.
First‐year completers.
Study eye.
Outcomes in subgroups
Visual acuity (VA) outcomes for the study eye stratified by country and subgroups are shown in Table 2. In general, the majority of countries were clustered around a mean four‐letter gain, with the exception of Portugal and Russia, where the letter gains were 0 letters and 8.4 letters, respectively. Outcomes based on sex and age subgroups were comparable and similar to the overall mean. There was a gain of 8.8 letters in patients with the lowest baseline VAS (≤60 letters) and a loss of −1.1 letters in patients with the highest baseline VAS (>73 letters). However, there was no clear association between injection numbers and VA outcomes (Table 2); the mean change in VA at month 12 was 4.6 letters (<4 injections) and 4.0 letters (>7 injections). After adjusting for baseline VA, there was still no apparent association between injection numbers and VA outcomes (Table 4).
Table 4.
Visual acuity outcomesa by baseline visual acuity score and number of injections
| Letters | Patients (n) | Mean baseline visual acuity score (letters) | Mean change in visual acuity at month 12 (letters) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ≤60 | 61–73 | >73 | ≤60 | 61–73 | >73 | ≤60 | 61–73 | >73 | |
| <4 injections | 83 | 62 | 39 | 43.3 | 67.7 | 76.9 | 8.6 | 2.4 | 1.0 |
| 4–5 injections | 72 | 52 | 32 | 46.8 | 67.7 | 79.0 | 9.5 | 0.8 | −3.8 |
| 6–7 injections | 56 | 38 | 22 | 48.3 | 66.9 | 75.8 | 8.8 | 0.3 | −0.5 |
| >7 injections | 55 | 30 | 21 | 48.3 | 66.6 | 77.0 | 8.3 | −0.7 | 0.0 |
First‐year completers.
Reasons for ending the study
The majority of patients [84.1% (all patients) and 95.2% (first‐year completers)] completed the study; the main reasons for ending the study early included loss to follow‐up (2.8%), change of physician (0.5%) and effectiveness achieved (0.4%) (first‐year completers) (Fig. S1).
Discussion
POLARIS provides an important overview of the 12‐month outcomes for patients with DME treated with ranibizumab under real‐world settings. The study showed that the mean change in visual acuity (VA) was a gain of 4.4 letters at 12 months. This was observed in the majority of countries, with the exception of Portugal and Russia; however, these differences may be partly due to the small sample size in Portugal (five patients) and the lower mean baseline VA in Russia (44 letters). Diabetic macular oedema was also associated with a high treatment burden for patients and physicians. Although patients attended approximately 10 visits over the year, only 58% of these were treatment visits.
These findings were similar to those of other observational studies (Brynskov et al. 2013; Mitchell 2016). An interim analysis of the LUMINOUS study showed that the mean change in VA at 12 months was 4.4 letters in treatment‐naïve DME patients and the mean number of ranibizumab injections was 3.7 (i.e. a gain of one letter per injection over 12 months) (Mitchell 2016). However, they do not mirror those achieved in the active phases of randomized studies. The mean change in VA at 12 months was approximately six letters with ranibizumab as‐needed or treat‐and‐extend (RESTORE, RETAIN) and approximately 12 letters with monthly dosing (RISE/RIDE) (Mitchell et al. 2011; Nguyen et al. 2012; Prunte et al. 2016). When a subgroup of patients in the RISE/RIDE studies were switched to as‐needed ranibizumab treatment from months 36 to 54 (Boyer et al. 2015), the mean number of injections was 4.5, which was sufficient to maintain VA gains (around 12 letters). Based on these outcomes and those observed in Protocol I and Protocol T, which both had intensive monthly initiation phases, these authors advocate intensive therapy to maximize letter gains followed by a reduction in treatment burden. The regimen used in POLARIS may be insufficient to maximize initial letter gains in patients who were treatment‐naïve; this may be a limitation with as‐needed dosing in routine practice.
Other potential reasons for marked differences in VA outcomes achieved in real‐world studies compared with those achieved in clinical studies may relate to the fact that measurements in randomized studies are usually best‐corrected visual acuity, whereas observational studies rely on whatever method the clinic uses. Before analysis, VA values were converted to a standard scale using a published standardization chart. Although this is the normal approach used in observational studies, it may introduce inconsistencies among clinics and bias in the conversion chart used. Other issues include any differences/similarities in baseline characteristics (including definition of worse‐ or better‐seeing eye), data collection (notably prospective versus retrospective collection), retreatment criteria and ophthalmologist adherence to these criteria. For example, the mean baseline VAS in POLARIS was 59.4 letters (first‐year completers), which was lower than that observed in RESTORE (63.5 letters) (Mitchell et al. 2011). The outcomes in RESTORE could be limited by a ceiling effect, but based on this, we would have expected the VA change outcomes to be greater in POLARIS.
Although all patients started treatment with ranibizumab, some also received another anti‐VEGF agent (4%) during the observational period and were included in preplanned analyses. This is consistent with practice in a real‐world setting, and the protocol in the pivotal Protocol T study also permitted laser or other DME treatment based on specific criteria. In the ranibizumab group of Protocol T, 46% received laser (from week 24 to year 1) and <1% received other treatment. This is somewhat of an ethical imperative, and if many patients need additional therapy, then a data set could be diminished by excluding such patients. However, it does mean that some patients receiving additional treatment are included in analyses resulting in some data mixing. To remove these patients in POLARIS would be a violation of the noninterventional protocol, and given the small numbers, it is unlikely that the outcome would be impacted. We acknowledge that it is a limitation with respect to the outcomes.
Furthermore, IVT‐AFL was not approved at study start, and it was not possible to derive information on how many patients would use it. In Protocol T, IVT‐AFL was associated with a mean gain of 13.3 letters with 9.2 injections, and ranibizumab was associated with a mean gain of 11.2 letters with 9.4 injections in year 1; this study followed a protocol in which injections were initially given monthly depending on criteria (Wells et al. 2015). The impact of adjuvant laser was difficult to monitor due to small patient numbers. Macular laser was applied to ~ 30% of eyes receiving ranibizumab 0.5 mg in RISE/RIDE over 3 years (Brown et al. 2013). In Protocol T, deferred laser was used in 41% of IVT‐AFL patients, 64% of bevacizumab patients and 52% of ranibizumab patients over 2 years; a subanalysis revealed that laser treatment could have positively impacted the central subfield thickness reductions in patients with worse vision in the bevacizumab group (Jampol et al. 2016; Wells et al. 2016). In RESTORE, VA gains at 12 months were similar in patients receiving ranibizumab with or without concomitant laser (Mitchell et al. 2011).
It must be noted that there was a large difference in patient numbers between the ‘all patients’ and ‘first‐year completers’; this does not indicate selection bias, but highlights the lack of strict inclusion criteria in an observational design study and reflects clinical practice with respect to treatment use at 12 months. It was also difficult to determine associations between number of injections and VA outcomes in subgroups due to small patient numbers, and the study was not designed to assess these in detail. As opposed to randomized studies, which follow strict protocols, dosing in real‐world studies may be reactive (i.e. in response to outcomes), and achievement of short‐term gains may result in reduction of future injections despite the possibility of further improvement; this was evidenced in POLARIS (Table 4), which indicates that outcomes seem to drive injections as much as injections drive outcomes when using as‐needed regimens in DME. This may partly explain the lack of association between injection frequency and VA outcomes. It could also be driven by disease characteristics at baseline.
We did not determine how patients’ access to treatment and arrangement for reimbursement of provider costs in each healthcare system affected resource use during the study and, therefore, outcomes. It is notable that like AURA (Holz et al. 2015), injection rates and VA gains were generally greater in the UK than in other countries (Table 2); this is similar to findings observed in another real‐world study in the UK setting in which 164 patients with DME received an average of 7.2 ranibizumab injections and an increase of 6.6 letters over 12 months (Patrao et al. 2016). Patients of the UK National Health Service are not required to seek approval for physician‐prescribed treatment with ranibizumab or IVT‐AFL or to make copayments for treatments or visits. Restrictions in healthcare systems may, in part, account for some of the variation in outcomes observed between countries. Access to ophthalmology facilities may also be an issue in some countries (Marques et al. 2015). This also highlights that any differences in the number of patients enrolled between different clinics and countries could introduce bias in the current study.
In conclusion, POLARIS showed that real‐world outcomes with ranibizumab were suboptimal in comparison with clinical studies, in spite of extensive monitoring. It is potentially linked to an insufficient number of intravitreal injections in real‐life studies, but it must be noted that a real‐world setting is very different from a randomized study. Since data collection for the current analysis, the labelled ranibizumab regimen changed from as‐needed with monthly monitoring to a more flexible approach guided by the physician; the impact of this change on treatment practice needs to be fully determined.
Supporting information
Figure S1. Patient disposition during the study.
Appendix 1.
The POLARIS study investigators are: Souied E, Lalloum F, Querques G, Ayello‐Scheer S, Coriat C, Girens J F, Sahel J‐A, Creuzot‐Garcher C, Bremond‐Gignac D, Chiambaretta F, Farguette F, Delhay C, Baillif‐Gostoli S, Maschi C, Kodjikian L, Fajnkuchen F, Milazzo S, Benzerroug M, Théron J P, Schmickler S, Zywien A, Bopp S, Höh H, Câmpean P, Schattmann K, Fromberg I, Fromberg C, Fromberg D, Spital G, Heimes B, Emmerich K‐H, Lang M, Krieb A, Xafis G, Stock L, Klotz N, Ungerechts R, Matuschek A, Radermacher M, Thelen U, Tetz M, Denisiuk M, Berens U, Schumacher A, Neuhann T, Lange O, Richard G, Wieland M, Filev F, Bittersohl D, Wiedemann P, Lorenz K, Wasielica‐Poslednik J, Rosbach J, Dave H, Wirtz N, Weber B, Gelisken F, Wilhelm B, Peters T, König T, Kampik A, Abbasova S, Wolf A, Kurz S, Herold T, Ulbig M, Arend N, Dabov S, Prause K, Fazekas C, Märtz J, Bayerl K, Heuer U, Bischoff G, Künne C, Lorenz B, Jäger M, Schiel H, Datseris I, Diamanti‐Ramza A, Charonis A, Straga I, Babouli N, Brevetti C, Tranos P, Perganta G, Panayiotis T, Angeliki A, Dinioti T, Tsironi E, Kotoula M, Brazitikos P, Nanas D, Figueira J, Ribeiro L, Izmailov A, Molodkina N, Abdulaeva E, Pashtaev N, Ovchinnikova V, Yurieva T, Vaycheslav B, Liya R, Ahlers J, Zmatlova I, Popovcova M, Bajacek J, Panisova J, Struharova K, Sturova L, Jamrichova Z, Krasnik V, Stefanickova J, Krajcova P, Hasa J, Piovarciova E, Gajdosova M, Vida R, Janco L, Leskova V, Demsky P, Alexik M, Falatova A, Lipkova B, Stubna M, Tomaskova D, Herle D, Martinez Alday N, Sanchez Aparicio J A, Martinez Anton M, Lopez Galvez M I, Manzanas Leal L, Juberias Sanchez R, Perez Belmonte L, Fernandez Vega A, Fernandez‐Vega Sanz B, Villota Deleu E, Gloria D L T C, Canga S, De Santiago Rodiguez M A, Ramos Gonzalez D, Prieto Maratin J F, Franco Suarez‐Barcena I, Casado Prieto A, Hernandez Galilea E, Gomez Ledesma I, de Juan Marco L, Mendivil Soto M P, Bearan I, Nuñez M, Lopez Garrido J L, Rodriguez Raton A, Cincunegui J, Vazquez Cruchaga E, Quiroga de la Hera P, Fernandez Rodriguez M, Rodriguez Cid M J, Méndez Martínez S, Gonzalez Martinez A, Gomez‐Ulla F, Garcia Garcés I, Martinez Perez L, Mansilla Cuñarro R, Abraldes Lopez‐Veiga M, Rodriguez Nuñez M, Piñeiro Figuera M C, Rodriguez Ferro F, Menon G, North L, Chandran M, Retnamma R, Sivaprasad S, Taylor S, Scanlon P, Johnston R, Pearce I, Chong V, Mall S, Bailey C, Varma D, Talks J, Lotery A, Thulasidharan S, Eckstein M, Fahd Q, Koshy Z, Hanumanthu S, Kelly S, Evangelos S, Ghanchi F, Asaria R, Harris M, Derdeb T, Dipa G and Mahuma I.
Ranibizumab was the only anti‐vascular endothelial growth factor agent approved for diabetic macular oedema at the time of the study.
Medical writing assistance was provided by PAREXEL and was funded by Bayer AG.
Jana Stefanickova is a consultant for Bayer, Novartis and Regeneron. Michael Ulbig is a consultant for Alimera, Allergan, Bayer, Novartis and Regeneron. Ian Pearce is a consultant for Alcon, Allergan, Bayer, Novartis and Regeneron. Laurent Kodjikian is a consultant for AbbVie, Alcon, Allergan, Bayer, Krys, Novartis, Regeneron and Thea. Jose Cunha‐Vaz, Alvaro Fernández‐Vega Sanz, Panagiotis Theodossiadis and Alexander Izmailov are consultants for Bayer and Regeneron. Dominic Muston, Zdravko Vassilev, Benedicte Lamotte, Claudia Tückmantel, Sabine Friedl, Andreas Altemark and Todd Katz are employees of Bayer. Hans‐Jörg Schwarz is an employee of Kantar, who was contracted by Bayer to provide data analysis.
Contributor Information
Jose Cunha‐Vaz, Email: cunhavaz@aibili.pt.
the POLARIS study investigators:
E Souied, F Lalloum, G Querques, S Ayello‐Scheer, C Coriat, J F Girens, J‐A Sahel, C Creuzot‐Garcher, D Bremond‐Gignac, F Chiambaretta, F Farguette, C Delhay, S Baillif‐Gostoli, C Maschi, F Fajnkuchen, S Milazzo, M Benzerroug, J P Théron, S Schmickler, A Zywien, S Bopp, H Höh, P Câmpean, K Schattmann, I Fromberg, C Fromberg, D Fromberg, G Spital, B Heimes, K‐H Emmerich, M Lang, A Krieb, G Xafis, L Stock, N Klotz, R Ungerechts, A Matuschek, M Radermacher, U Thelen, M Tetz, M Denisiuk, U Berens, A Schumacher, T Neuhann, O Lange, G Richard, M Wieland, F Filev, D Bittersohl, P Wiedemann, K Lorenz, J Wasielica‐Poslednik, J Rosbach, H Dave, N Wirtz, B Weber, F Gelisken, B Wilhelm, T Peters, T König, A Kampik, S Abbasova, A Wolf, S Kurz, T Herold, N Arend, S Dabov, K Prause, C Fazekas, J Märtz, K Bayerl, U Heuer, G Bischoff, C Künne, B Lorenz, M Jäger, H Schiel, I Datseris, A Diamanti‐Ramza, A Charonis, I Straga, N Babouli, C Brevetti, P Tranos, G Perganta, T Panayiotis, A Angeliki, T Dinioti, E Tsironi, M Kotoula, P Brazitikos, D Nanas, J Figueira, L Ribeiro, N Molodkina, E Abdulaeva, N Pashtaev, V Ovchinnikova, T Yurieva, B Vaycheslav, R Liya, J Ahlers, I Zmatlova, M Popovcova, J Bajacek, J Panisova, K Struharova, L Sturova, Z Jamrichova, V Krasnik, P Krajcova, J Hasa, E Piovarciova, M Gajdosova, R Vida, L Janco, V Leskova, P Demsky, M Alexik, A Falatova, B Lipkova, M Stubna, D Tomaskova, D Herle, N Martinez Alday, J A Sanchez Aparicio, M Martinez Anton, M I Lopez Galvez, L Manzanas Leal, R Juberias Sanchez, L Perez Belmonte, B Fernandez‐Vega Sanz, E Villota Deleu, D L T C Gloria, S Canga, M A De Santiago Rodiguez, D Ramos Gonzalez, J F Prieto Maratin, I Franco Suarez‐Barcena, A Casado Prieto, E Hernandez Galilea, I Gomez Ledesma, L de Juan Marco, M P Mendivil Soto, I Bearan, M Nuñez, J L Lopez Garrido, A Rodriguez Raton, J Cincunegui, E Vazquez Cruchaga, P Quiroga de la Hera, M Fernandez Rodriguez, M J Rodriguez Cid, S Méndez Martínez, A Gonzalez Martinez, F Gomez‐Ulla, I Garcia Garcés, L Martinez Perez, R Mansilla Cuñarro, M Abraldes Lopez‐Veiga, M Rodriguez Nuñez, MC Piñeiro Figuera, F Rodriguez Ferro, G Menon, L North, M Chandran, R Retnamma, S Sivaprasad, S Taylor, P Scanlon, R Johnston, V Chong, S Mall, C Bailey, D Varma, J Talks, A Lotery, S Thulasidharan, M Eckstein, Q Fahd, Z Koshy, S Hanumanthu, S Kelly, S Evangelos, F Ghanchi, R Asaria, M Harris, T Derdeb, G Dipa, and I Mahuma
References
- Boyer DS, Nguyen QD, Brown DM, Basu K & Ehrlich JS (2015): Outcomes with as‐needed ranibizumab after initial monthly therapy: long‐term outcomes of the phase III RIDE and RISE trials. Ophthalmology 122: 2504–2513. [DOI] [PubMed] [Google Scholar]
- Brown DM, Nguyen QD, Marcus DM et al. (2013): Long‐term outcomes of ranibizumab therapy for diabetic macular edema: the 36‐month results from two phase III trials: RISE and RIDE. Ophthalmology 120: 2013–2022. [DOI] [PubMed] [Google Scholar]
- Brynskov T, Laugesen CS & Sorensen TL (2013): Intravitreal ranibizumab for diabetic macular oedema: 1‐year experiences in a clinical setting. Acta Ophthalmol 91: e243–e244. [DOI] [PubMed] [Google Scholar]
- Cheung N, Mitchell P & Wong TY (2010): Diabetic retinopathy. Lancet 376: 124–136. [DOI] [PubMed] [Google Scholar]
- Dugel PU, Layton A & Varma RB (2016): Diabetic macular edema diagnosis and treatment in the real world: an analysis of Medicare claims data (2008 to 2010). Ophthalmic Surg Lasers Imaging Retina 47: 258–267. [DOI] [PubMed] [Google Scholar]
- Early Treatment Diabetic Retinopathy Study Research Group (1985): Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Arch Ophthalmol 103: 1796–1806. [PubMed] [Google Scholar]
- Ferris FL III, Kassoff A, Bresnick GH & Bailey I (1982): New visual acuity charts for clinical research. Am J Ophthalmol 94: 91–96. [PubMed] [Google Scholar]
- Fong DS, Aiello L, Gardner TW, King GL, Blankenship G, Cavallerano JD, Ferris FL III & Klein R (2003): Diabetic retinopathy. Diabetes Care 26: 226–229. [DOI] [PubMed] [Google Scholar]
- Gregori NZ, Feuer W & Rosenfeld PJ (2010): Novel method for analyzing Snellen visual acuity measurements. Retina 30: 1046–1050. [DOI] [PubMed] [Google Scholar]
- Heier JS, Bressler NM, Avery RL et al. (2016): Comparison of aflibercept, bevacizumab, and ranibizumab for treatment of diabetic macular edema: extrapolation of data to clinical practice. JAMA Ophthalmol 134: 95–99. [DOI] [PubMed] [Google Scholar]
- Holladay JT (1997): Proper method for calculating average visual acuity. J Refract Surg 13: 388–391. [DOI] [PubMed] [Google Scholar]
- Holz FG, Tadayoni R, Beatty S et al. (2015): Multi‐country real‐life experience of anti‐vascular endothelial growth factor therapy for wet age‐related macular degeneration. Br J Ophthalmol 99: 220–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz FG, Tadayoni R, Beatty S et al. (2016a): Key drivers of visual acuity gains in neovascular age‐related macular degeneration in real life: findings from the AURA study. Br J Ophthalmol 100: 1623–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz FG, Tadayoni R, Beatty S et al. (2016b): Determinants of visual acuity outcomes in eyes with neovascular AMD treated with anti‐VEGF agents: an instrumental variable analysis of the AURA study. Eye (Lond) 30: 1063–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrarat L, Fajnkuchen F, Boubaya M et al. (2016): Outcomes after a 1‐year treatment with ranibizumab for diabetic macular edema in a clinical setting. Ophthalmologica 236: 207–214. [DOI] [PubMed] [Google Scholar]
- Jampol LM, Glassman AR, Bressler NM, Wells JA & Ayala AR (2016): Anti‐Vascular Endothelial Growth Factor Comparative Effectiveness Trial for Diabetic Macular Edema: additional efficacy post hoc analyses of a randomized clinical trial. JAMA Ophthalmol 134 10.1001/jamaophthalmol.2016.3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang S, Barner JC, Park C & Ling YL (2015): Treatment patterns of anti‐vascular endothelial growth factor and laser therapy among patients with diabetic macular edema. J Manag Care Spec Pharm 21: 735–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korobelnik JF, Do DV, Schmidt‐Erfurth U et al. (2014): Intravitreal aflibercept for diabetic macular edema. Ophthalmology 121: 2247–2254. [DOI] [PubMed] [Google Scholar]
- Lee R, Wong TY & Sabanayagam C (2015): Epidemiology of diabetic retinopathy, diabetic macular edema and related vision loss. Eye Vis (Lond) 2: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques AP, Macedo AF, Perelman J, Aguiar P, Rocha‐Sousa A & Santana R (2015): Diffusion of anti‐VEGF injections in the Portuguese National Health System. BMJ Open 5: e009006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P (2016): Good real‐world outcomes in DME patients with poor baseline visual acuity at 1 year: results from LUMINOUS™ [abstract]. Association for Research in Vision and Ophthalmology (ARVO) 2016 Meeting.01‐05 May 2016, Seattle, WA, USA. Abstract 2100‐B0332. [Google Scholar]
- Mitchell P, Bandello F, Schmidt‐Erfurth U et al. (2011): The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology 118: 615–625. [DOI] [PubMed] [Google Scholar]
- Nguyen QD, Brown DM, Marcus DM et al. (2012): Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology 119: 789–801. [DOI] [PubMed] [Google Scholar]
- Papadopoulos N, Martin J, Ruan Q et al. (2012): Binding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF Trap, ranibizumab and bevacizumab. Angiogenesis 15: 171–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrao NV, Antao S, Egan C, Omar A, Hamilton R, Hykin PG, Sivaprasad S & Rajendram R (2016): Real‐world outcomes of ranibizumab treatment for diabetic macular edema in a United Kingdom National Health Service setting. Am J Ophthalmol 172: 51–57. [DOI] [PubMed] [Google Scholar]
- Prunte C, Fajnkuchen F, Mahmood S et al. (2016): Ranibizumab 0.5 mg treat‐and‐extend regimen for diabetic macular oedema: the RETAIN study. Br J Ophthalmol 100: 787–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simo R, Sundstrom JM & Antonetti DA (2014): Ocular Anti‐VEGF therapy for diabetic retinopathy: the role of VEGF in the pathogenesis of diabetic retinopathy. Diabetes Care 37: 893–899. [DOI] [PubMed] [Google Scholar]
- Wells JA, Glassman AR, Ayala AR et al. (2015): Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med 372: 1193–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells JA, Glassman AR, Ayala AR et al. (2016): Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema: two‐year results from a comparative effectiveness randomized clinical trial. Ophthalmology 123: 1351–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Writing Committee for the UK Age‐Related Macular Degeneration EMR Users Group. (2014): The neovascular age‐related macular degeneration database: multicenter study of 92 976 ranibizumab injections: report 1: visual acuity. Ophthalmology 121: 1092–1101. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Patient disposition during the study.


