ABSTRACT
Aim
The aim of the present study was to screen and verify downstream genes involved in the epithelial mesenchymal transition (EMT) induced by paired box 2 (PAX2) in NRK‐52E cells.
Methods
NRK‐52E cells were transfected with lentivirus carrying PAX2 gene or no‐load virus respectively. Total RNA was isolated 72 h after transfection from PAX2‐overexpressing cells and control cells. Isolated RNA was then hybridized with the Rat OneArray Plus expression profile chip. The chips were examined by Agilent 0.1 XDR to screen for differentially expressed genes, which were further analyzed to investigate complement‐related genes as genes of interest.
Results
In NRK‐52E cells, PAX2 overexpression promoted EMT followed by upregulation of 298 genes and downregulation of 293 genes. KEGG analysis indicated the differential expression of genes related to cytokines and their receptors, extracellular matrix (ECM), MAPKs, local adhesion, cancer, the complement cascade, and coagulation. Gene oncology analysis screened out genes related to molecular functions (e.g., hydrolase activity, phospholipase activity, components of the ECM) and biological processes (e.g., cell development, signal transduction, phylogeny), and cell components (e.g., cytoplasm, cell membrane, and ECM). Analysis of the complement system revealed upregulation of C3 and downregulation of CD55 and complement regulator factor H (CFH).
Conclusion
PAX2 overexpression upregulates EMT in vitro and may regulate C3, CD55, and CFH.
Keywords: complement genes, epithelial‐mesenchymal cell transition, gene ontology, Kyoto Encyclopedia of Genes and Genomes, NRK‐52E cells, Paired box 2
Summary at a Glance
This molecular analysis examines the effect of overexpressing paired box 2 (PAX2) in a tubule epithelial cell line. Results establish a link between pax2 and both epithelial‐mesenchymal transition (EMT) and the complement pathway.
Paired box 2 (PAX2) encodes a nuclear protein that binds with DNA, serving as a transcription factor. Several studies have shown that PAX2 is an important regulatory factor in embryonic kidney development. PAX2 is continuously expressed during the early, middle and late phases of kidney development, including nephrogenic cord induction and duct formation, ureter germination, nephrogenic branch configuration, and nephron establishment.1, 2 Abnormal expression of PAX2 results in hereditary and acquired kidney diseases.3 Loss of PAX2 during embryonic kidney development can lead to renal‐coloboma, maldevelopment of the kidney, or the absence of a kidney.4, 5 Overexpression of PAX2 potentially leads to dominant polycystic kidney disease (PKD), ureter obstruction, renal cell carcinoma, or lesions on the glomerulus and/or renal tubules.6, 7
Tubulointerstitial fibrosis is a defining characteristic of chronic renal disease, and is associated with upregulation of PAX2 expression.8 In renal tubular epithelial cells, epithelial‐mesenchymal transition (EMT) is a key fibrogenic mechanism of chronic renal disease.9 Our previous in‐vitro research indicated that PAX2 promoted EMT, which facilitated the process of renal interstitial fibrosis.10 However, the underlying mechanism remains unclear.
With the development and advancement of biotechnologies, the gene chip has been widely used to identify differences in gene expression. KEGG (Kyoto Encyclopedia of Genes and Genomes) is a database used to systematically analyze gene function that facilitates research on the gene expression profile as an integrating network.11 Gene ontology (GO) enrichment analysis is likewise another approach used to analyze differentially expressed genes as related to molecular function, biological processes, and cellular components.12 This study sought to investigate the mechanism of EMT induction by PAX2. NRK‐52E rat renal tubular epithelial cells were used to build an EMT cell model by overexpressing PAX2. Downstream genes influenced by PAX2 were identified by specific gene‐chip screening. KEGG and GO enrichment analyses were performed to analyze differential gene expression, which was further verified by real‐time polymerase chain reaction (PCR) and western blotting.
MATERIALS AND METHODS
Materials
A rat renal tubular epithelial cell line (NRK‐52E) was obtained from the cell bank of the Chinese Academy of Sciences. LV‐PAX2 carrying the rat PAX2 gene (NM 001106361) and its lentivirus vector control were obtained from the Genechem Company (Shanghai, China). Rat OneArray Plus® was obtained from the Phalanx Biotech Group (Hsinchu, Taiwan). ROA version 1.1 contains 24 358 probes from the Refseq release 42 and Ensebl release 59 databases. Another 980 control probes were purchased from the Genechem Company.
Methods
Cell culture and lentivirus transfection
Rat renal tubular epithelial NRK‐52E cells were cultured in Dulbecco's modified Eagle's medium (DMEM) containing 10% foetal bovine serum (FBS) at 37°C, in a humidified atmosphere of 5% CO2. One day before transfection, 1 × 104 cells per well were seeded into 24 well plates. Before transfection, cells were washed with sterile PBS. An amount of virus determined by the cell multiplicity of infection (MOI) was added to each well. For a 24 well plate, 5 μL of LV‐PAX2 solution were added to each well in the experimental group, and 1 μL LV‐con solution was added to each well in the control group. Then 500 μL Enis containing 0.5% polybrene was added to each well. The green fluorescent protein (GFP) expression was observed 48 h after transfection. Cell morphology was recorded when fluorescence intensity was >80%. Protein and RNA were isolated from each group at 48, 72, and 96 h after transfection.
Western blot
To obtain total cell lysate, cells were collected by trypsinization, washed twice with ice‐cold 1× PBS and lysed for 1 h on ice by adding cell lysis buffer. Protein concentration was measured using the BCA Protein Assay kit according to the manufacturer's instructions. Total lysates were loaded on SDS‐PAGE, and proteins were separated using SDS running buffer. A PageRuler prestained protein ladder was loaded on the same gel as a standard for protein separation and determination of molecular weight. Proteins were transferred onto polyvinylidene difluoride (PVDF) membranes. After blocking, membranes were incubated overnight with primary antibodies against PAX2 (1 : 400, Santa Cruz, USA), CD55 (1 : 100, Santa Cruz, USA), C3 (1 : 100, Santa Cruz, USA), CFH (1 : 200, Bioss antibodies, China). Subsequently, membranes were washed three times in 1× TBST for 10 min, then incubated with corresponding horseradish peroxidase (HRP)‐conjugated isotype‐specific secondary antibody diluted in 5% non‐fat milk, for 1 h at room temperature. After four washes with 1× TBST, blots were visualized with an ECL + plus™ western blotting system (Amersham, USA) and films. GAPDH (1 : 5000, Bioss antibodies, China) was used as an internal control. The image was analyzed with the quantity ONE system.
Real‐time PCR
Total RNA was extracted by Trizol lysis buffer (Invitrogen, USA) at 72 h after transduction, according to the manufacturer's instructions. RNA was reverse transcribed into cDNA with the PrimeScript RT reagent Kit with a gDNA Eraser (Takara, Japan), according to the manufacturer's instructions. After reverse transcription, mRNA levels of specific genes were quantified by real‐time PCR. The primers used are listed in Table S1.
Gene chip assay and differentially expressed genes analysis
Total RNA was respectively extracted from the samples transfected with empty vector control or PAX2 overexpressing group according to the manufacturer's instructions. RNA quantity and purity were assessed using NanoDrop ND‐1000 (Thermo Scientific, USA). According to pass criteria for absorbance ratios, A260/A280 ≥ 1.8 and A260/A230 ≥ 1.5 indicate acceptable RNA purity. RIN values were ascertained using the Agilent RNA 6000 Nano assay to determine RNA integrity. Pass criteria for RIN values for RNA integrity are established at ≥6. gDNA contamination was evaluated by gel electrophoresis. Target preparation was performed using an Eberwine‐based amplification method with the Amino Allyl MessageAmp II aRNA Amplification Kit (Ambion, AM1753) to generate amino‐allyl antisense RNA (aa‐aRNA). Labelled aRNA coupled with NHS‐CyDye was prepared and purified prior to hybridization. Purified coupled aRNA was quantified using the NanoDrop ND‐1000; the pass criterion for CyDye incorporation efficiency was >15 dye molecules/1000 nt. Two probes per gene were selected from seven consistently expressed housekeeping genes. For each gene, probes were designed from regions of 300–600 bp (S1) or 900–1200 bp (S2), from each 3′ end of the transcripts. The gene expression profiles of each sample were determined using the Rat OneArray Plus® Microarray (Hsinchu, Taiwan). The data obtained were analyzed using Agilent 0.1 XDR. The signal‐segmented file was loaded into Rosetta Resolver® System (Rosetta Biosoftware, USA) for data analysis. Standard selection criteria used to identify differentially expressed genes were as follows: (i) log2 |fold change| ≥ 1 and P < 0.05; and (ii) log2 ratios = ‘NA’ and differences in intensity between samples ≥1000. Gene clustering was performed on selected differentially expressed gene lists after data transformation and mean centring with a linkage algorithm. Gene set enrichment analysis was performed based on the Canonical Pathway database and ‘Gene Symbol’ from the Gene Ontology database (http://geneontology.org).
Statistical analysis
The results were analyzed with spss 17.0 software. Data from three independent experiments are presented as mean ± SD. Statistical comparisons between groups were analyzed using the two‐tailed Student's t‐test. Correlations between variables were assessed with Pearson's correlation test. Differences were considered significant if P < 0.05.
RESULTS
Overexpression of PAX2 upregulates EMT programmes in NRK‐52E cells
NRK‐52E cells were transfected with lentivirus carrying the PAX2 gene (LV‐PAX2) or vector control (LV‐con). Seventy‐two hours after transfection, cells overexpressing PAX2 transformed from round or oval epithelial cells to spindle or needle‐shaped mesenchymal cells (Fig. 1A,C), but control cells did not. Transfection efficiency was visualized as the intensity of GFP staining (Fig. 1B,D). Our previous study confirmed that PAX2 overexpression was sufficient to promote EMT programs in NRK‐52E cells.10
Figure 1.
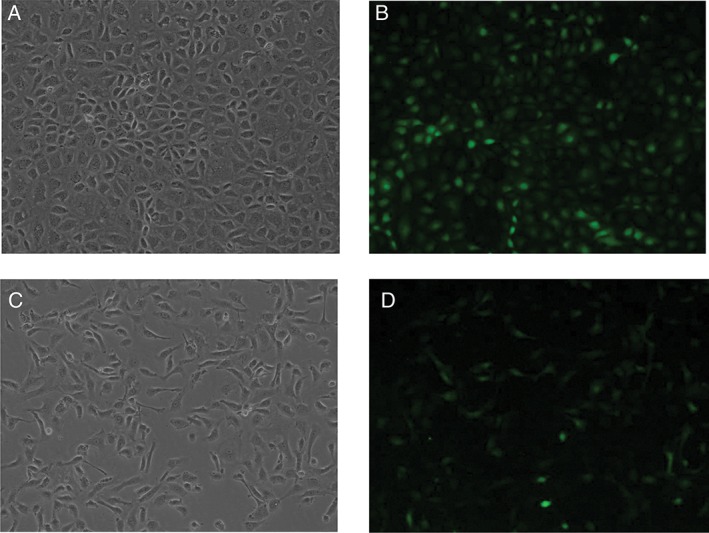
Overexpression of PAX2 in NRK52E cells. (A, B) NRK‐52E cells were transduced with LV‐con for 72 h. Cell morphology was visualized by light or fluorescence microscopy. (C, D) NRK‐52E cells were transduced with LV‐PAX2 for 72 h. Morphology was visualized with light or fluorescence microscopy.
Gene expression profiling and gene set enrichment analysis
RNA samples from the vector control group and PAX2‐overexpressing group were hybridized with the OneArray Plus® Microarray. The hybridization signal was screened by high‐resolution gene‐chip scanner. The results of cluster analysis revealed a difference between groups (P < 0.05) (Fig. 2A‐D). Based on the eligibility criteria that log2 ratio ≥1.0 or ≤−1.0 and P < 0.05, 298 genes were upregulated, and 293 genes were downregulated (Table S2).
Figure 2.
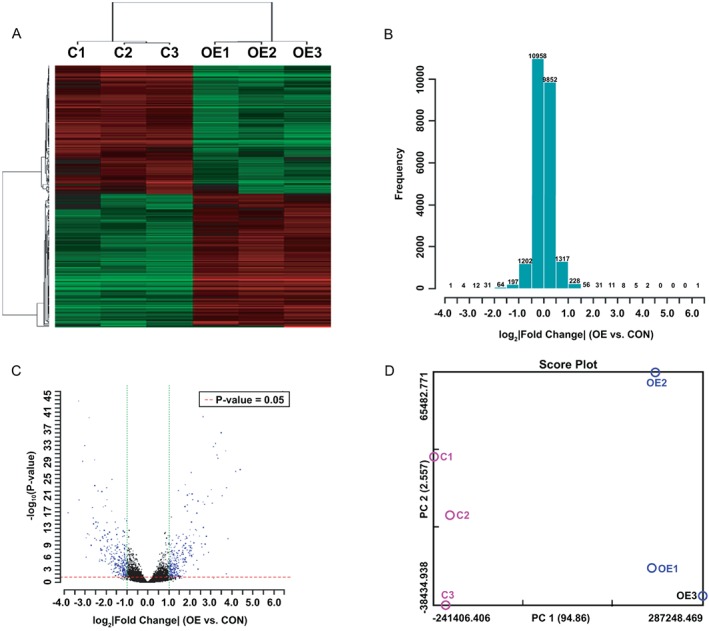
Differential gene expression induced by PAX2 was analyzed using the Rat OneArray® Plus Gene Chip. (A) Clustering was performed to visualize correlations among the replicates and varying sample conditions. Upregulated and downregulated genes are represented in red and green, respectively. A subset of differential genes was selected for clustering analysis. An intensity filter was used to select genes where the difference between the maximum and minimum intensity values >2000 among all microarrays. For this microarray project, the number of genes clustered was 222. (B) The histogram plot shows the distribution of fold‐change for all probes excluding control and flagged probes. Fold change was calculated by Rosetta Resolver 7.2, with the error model adjusted by Amersham Pairwise Ration Builder for signal comparison of samples. (C) Volcano plot of sample OE versus CON. Standard selection criteria to identify differentially expressed genes are established at log2 |fold change| ≧ 1 and P‐value < 0.05. (Blue dots in figure.) (D) PCA plot. The variables for the first three principal components (PC1, PC2, PC3) for this study are 94.86%, 2.56%, and 1.33%, respectively. A subset of differential genes was selected for PCA analysis. Notes: OE represents cells overexpressing PAX2; CON represents control cells. 1, 2 and 3 represent triplicate repetitions.
Based on information from KEGG and Biocarta databases, genes with differential expression upon exposure to PAX2 with log2 ratio ≥1.0 or ≤ − 1.0 (P < 0.05) were selected for enrichment analysis of related signalling pathways. Pathways involving cytokines and their receptors ranked the first in KEGG analysis: six genes (e.g., TNFRSF17) were found to be upregulated; 10 genes (e.g., IL6) were found to be downregulated. KEGG analysis ranked pathways related to extracellular matrix (ECM) second, demonstrating upregulation of COL4A2 and COL6A1 and downregulation of six genes (e.g., ITGA10). Third were genes related to hypertrophic cardiomyopathy: CACNB2 and TPM1 were upregulated after PAX2 overexpression, while six genes (e.g., SGCD) were downregulated. The MAPK signalling pathway was the fourth pathway found to be affected by PAX2 expression, with nine genes (e.g., FGF1) upregulated and seven genes (e.g., PDGFRB) downregulated. Fifth were genes related to focal adhesion: after transduction, COL4A2 was upregulated, and PDGFRB was downregulated (Table 1). Genes related to dilated cardiomyopathy were also influenced by PAX2 expression. CACNB2 and TPM1 were upregulated; four genes, including SGCG, were downregulated. The complement factors and adhesion molecules identified were C3, CD55, CFH, C1S, and SERPING1. C3 was upregulated, and CD55, CFH, C1S, and SERPING1 were downregulated (P < 0.05) (Table 2).
Table 1.
Top 10 pathway terms from gene set enrichment analysis (Based on KEGG and Biocarta databases)
| Geneset name | Genes in overlap (k) | k/K | FDR q‐value |
|---|---|---|---|
| Cytokine and cytokine receptor interaction | 18 | 0.0674 | 3.91E‐08 |
| Extracellular and receptor interaction | 8 | 0.0952 | 1.65E‐04 |
| Hypertrophic cardiomyopathy | 8 | 0.0941 | 1.65E‐04 |
| MAPK signalling pathway | 13 | 0.0487 | 1.65E‐04 |
| Focal adhesion | 10 | 0.0498 | 1.72E‐03 |
| Dilated cardiomyopathy | 7 | 0.0761 | 1.72E‐03 |
| Complement and coagulation cascades | 6 | 0.087 | 2.65E‐03 |
| Pathways in cancer | 12 | 0.0366 | 3.48E‐03 |
| Intestinal immune network for IgA production | 5 | 0.1042 | 3.77E‐03 |
| IL1R pathway | 4 | 0.1212 | 9.88E‐03 |
Table 2.
Differential expression of genes related to complement pathways
| KEGG complement cascades | ID | Entrez_gene | log2 Ratio | P‐value (differentially expressed) | |
|---|---|---|---|---|---|
| OE/CON | OE/CON | ||||
| C3 | Complement component 3 | PH_rn_0004103 | 24 232 | 2.001905869 | 4.29139E‐07 |
| SERPING1 | Serpin peptidase inhibitor, clade G (C1 inhibitor), member 1 | PH_rn_0003815 | 295 703 | −2.701503789 | 1.31049E‐09 |
| CD55 | CD55 molecule, decay accelerating factor for complement | PH_rn_0012112 | 64 036 | −1.073009749 | 0.000107956 |
| C1S | Complement component 1, s subcomponent | PH_rn_0004735 | 192 262 | −1.880664479 | 4.03039E‐05 |
| CFH | complement factor H | PH_rn_0014497 | 155 012 | −1.170529565 | 4.54651E‐13 |
Gene ontology (GO) enrichment analysis was applied to differentially expressed genes, which were classified as related to molecular function, biological processes, or cellular components, and submitted for enrichment ranking. The genes found to have differential gene expression in PAX2‐overexpressing cells performed a broad range of molecular functions, including hydrolase activity (16 genes), high phospholipid hydrolase activity (12 genes), receptor activity (22 genes), phosphoprotein and phosphatase activity (nine genes), and phosphorylated monoester hydrolase activity (10 genes) (Table 3). Moreover, enrichment analysis demonstrated that PAX2 affected genes involved in biological processes include multicellular organ development (51 genes), anatomical structure development (50 genes), system development (45 genes), organ development (40 genes), signal transduction (55 genes), and systemic processes (32 genes) (Table 4). Table 5 presents genes with PAX2‐induced differences in gene expression related to cellular components including the ECM (32 genes [partial characterization, 23 genes]), membrane (60 genes [partial characterization, 51 genes]), plasma membrane (49 genes). In summary, PAX2 overexpression by lentivirus transfection in NRK‐52E cells was sufficient to induce differential gene expression that affected various functional and biological processes.
Table 3.
Top 10 enrichment gene ontology (GO) terms from GO analysis (molecular function)
| Geneset name | Genes in overlap (k) | k/K | FDR q‐value |
|---|---|---|---|
| Hydrolase activity acting on ester bonds | 16 | 0.0595 | 2.56E‐06 |
| Phosphoric ester hydrolase activity | 12 | 0.0784 | 4.90E‐06 |
| Receptor activity | 22 | 0.0377 | 5.68E‐06 |
| Phosphoproten phosphatase activity | 9 | 0.1111 | 6.93E‐06 |
| Phosphoric monoester hydrolase activity | 10 | 0.0893 | 8.51E‐06 |
| Structural molecule activity | 13 | 0.0533 | 3.95E‐05 |
| Substrate specific channel activity | 10 | 0.0641 | 1.31E‐04 |
| Extracellular matrix structural constituent | 5 | 0.1852 | 2.31E‐04 |
| Protein tyrosine phosphatase activity | 6 | 0.1132 | 4.32E‐04 |
| Transmenbrane receptor activity | 15 | 0.0359 | 4.32E‐04 |
Table 4.
Top 10 enrichment gene ontology (GO) terms from GO analysis (biological process)
| Geneset name | Genes in overlap (k) | k/K | FDR q‐value |
|---|---|---|---|
| Multicellular organismal development | 51 | 0.0486 | 0.00E + 00 |
| Anatomical structure development | 50 | 0.0494 | 0.00E + 00 |
| System development | 45 | 0.0523 | 0.00E + 00 |
| Organ development | 33 | 0.0578 | 2.29E‐14 |
| Signal transduction | 55 | 0.0337 | 3.66E‐14 |
| System process | 32 | 0.0568 | 7.63E‐14 |
| Negative regulation of biological process | 30 | 0.0443 | 3.41E‐10 |
| Anatomical structure morphogenesis | 21 | 0.0559 | 9.20E‐09 |
| Cell proliferation | 24 | 0.0468 | 1.40E‐08 |
| Cell development | 25 | 0.0433 | 2.48E‐08 |
Table 5.
Top 10 enrichment gene ontology (GO) terms from GO analysis (cellular component)
| Geneset name | Genes in overlap (k) | k/K | FDR q‐value |
|---|---|---|---|
| Extracellular region | 32 | 0.0716 | 0.00E + 00 |
| Membrane | 60 | 0.0301 | 1.81E‐13 |
| Plasma membrane | 49 | 0.0344 | 4.31E‐13 |
| Membrane part | 51 | 0.0305 | 8.26E‐12 |
| Extracellular region part | 23 | 0.068 | 9.06E‐12 |
| Intrinsic to membrane | 42 | 0.0312 | 4.89E‐10 |
| Integral to membrane | 41 | 0.0308 | 1.03E‐09 |
| Plasma membrane part | 36 | 0.0311 | 1.23E‐08 |
| Proteinaceous extracellular matrix | 11 | 0.1122 | 5.65E‐08 |
| Extracellular matrix | 11 | 0.11 | 6.32E‐08 |
PAX2 overexpression affected expression of complement‐related genes
To further verify the effect of PAX2 overexpression on the expression of complement‐related genes, real‐time PCR was performed. The results showed that RNA levels of C3 were upregulated, while CD55 and CFH levels were downregulated (Fig. 3B). These findings were consistent with the results of gene chip analysis. At the protein level, upregulation of C3 and downregulation of CD55 and CFH in cells overexpressing PAX2 were confirmed by western blot (Fig. 3A,C). In summary, PAX2 regulates the gene expression of C3, CD55 and CFH.
Figure 3.
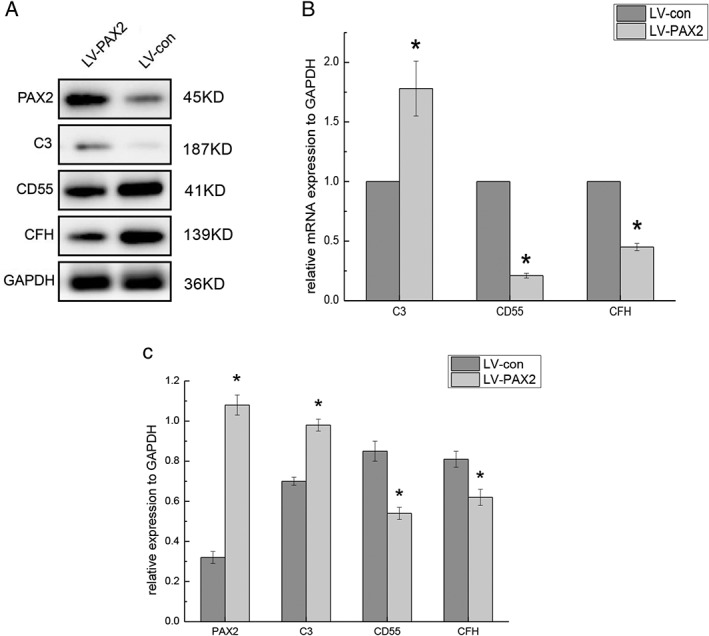
PAX2 overexpression affects the expression of C3, CD55, CFH (A, C) NRK‐52E cells were transduced with lentivirus containing vector controls or rat PAX2 for 72 h. The expression of PAX2, C3, CD55, and CFH was analyzed by western blot after transduction. GAPDH served as loading control, n = 3, *P < 0.05. (B) mRNA expression was measured by qPCR. mRNA expression levels of PAX2, C3, CD55, and CFH in LV‐PAX2 cells were compared with those in LV‐con cells, n = 3, *P < 0.05.
DISCUSSION
Renal interstitial fibrosis is a nonspecific pathological process that is the common final process of chronic kidney disease. Direct injury or other stimulation releases cytokines from renal tubule cells, which results in the accumulation of lymphocytes, macrophages, dendritic cells and mast cells in the renal interstitium. Activated immune cells then produce profibrotic factors to enable renal interstitial fibroblasts, peripheral cells, transdifferentiated renal tubular epithelial cells and endothelial cells transform into matrix‐producing cells.13 Matrix‐producing cells overproduce ECM components such as fibronectin and collagen, ultimately resulting in renal interstitial fibrosis.14 During the process of renal interstitial fibrosis, the conversion of renal tubular cells through EMT is an important source of ECM‐producing cells.15
The expression of PAX2 increases in a variety of kidney diseases, but the role of PAX2 remains to be elucidated. Huang et al. observed the development of EMT in renal tubular epithelial cells under the stimulation of inflammatory factors and reported the re‐expression of Wilm's tumour gene (WT1) and PAX2 in renal tubular epithelial cells. EMT was blocked when WT1 and PAX2 expression levels were suppressed, suggesting that WT1 and PAX2 participate in EMT and that silencing PAX2 could block the EMT induced by IL‐1.16 Doberstein et al. confirmed that PAX2 promoted EMT in renal tumour cells through regulation of L1‐CAM and ADAM10.17 The upper results were consist with our previous results that PAX2 promoted EMT process in vitro.10 In our previous study, the expression of PAX2 was positively correlated with the degree of renal interstitial fibrosis in a rat model of unilateral ureteral obstruction (UUO). Interfering with the expression of PAX2 could relieve renal interstitial fibrosis, which suggested that PAX2 may promote renal interstitial fibrosis by facilitating EMT.18
To further investigate the role of PAX2 in EMT, the NRK‐52E cells transfected with LV‐PAX2 or LV‐con were subjected to high‐throughput differential mRNA screening, which was performed using a Rat OneArray Plus® expression profile chip. Clustering analysis revealed significant differences in gene expression between PAX2‐overexpressing and controls cells, including the upregulation of 298 genes and the downregulation of 293 genes. KEGG pathway analysis verified that these differentially expressed genes were related to cytokines and their receptors, ECM and associated receptors, MAPK signalling, local adhesion, cancer, the complement cascade, and coagulation. GO analysis revealed that these differentially expressed genes were related to: molecular functions including hydrolase activity, phospholipase activity, and ECM functions; processes including cell development, signal transduction, and phylogeny; cell components such as cytoplasm, the cell membrane, and ECM. ECM and related pathways, which are closely related to fibrosis, were ranked within the top 10 in both KEGG and GO enrichment analyses. The effect of PAX2 on upregulation of C3 and downregulation of CD55 and CFH in NRK‐52E cells was further verified by real‐time polymerase chain reaction (PCR) and western blotting.
The complement system includes the classical pathway, the lectin pathway, and the alternative pathway. Most components of the complement system derive from the liver and reach the kidney after circulating through the blood; some of these participate in the pathological processes of various kidney diseases. Early studies have shown that the immune and complement systems play important roles in the development of renal interstitial fibrosis. The main pathological features of autosomal dominant polycystic kidney disease (ADPKD) are renal tubule cystic dilation and renal interstitial fibrosis. Su et al. studied changes in the complement components of urine and kidney tissue in renal cystic patients. As the disease progresses, levels of CFB, SERPING1, and C9 in the urine increase, suggesting the involvement of these complement components in the development of ADPKD.19 Researchers previously used animal models to evaluate the function of C3, CD55, and CFH in renal injury and renal interstitial fibrosis. C3 knockout in UUO mice reduced levels of renin and transforming growth factor‐ß1 (TGF‐ß1), which is a classic profibrotic cytokine.20 Likewise, C3a receptor knockout in an adriamycin‐induced nephropathy mouse model preserved renal function and alleviated renal interstitial inflammation and fibrosis.21 Early studies in a model of ischemia reperfusion injury verified that CD55, as a complement regulator, performs a protective role during the pathological process of renal injury.22
The above results are consistent with a role for C3 in promoting renal interstitial inflammation and fibrosis, while CD55 and CFH may be protective. Our study revealed that PAX2 could upregulate C3 and downregulate CD55 and CFH to promote EMT programmes in NRK‐52E cells. Together with our previous results, these findings suggest that PAX2 upregulates C3 and downregulates CD55 and CFH to promote the EMT program during renal interstitial fibrosis.
Both EMT and endothelial mesenchymal transition (EndMT) have been implicated in the pathogenesis of renal injury and renal interstitial fibrosis. Whether complement components are involved in these process were still not clear. Zhou et al. concluded that C3 induced EMT in mouse epithelial cells through activation of the renin‐angiotensin system in a UUO mouse model.20 Likewise, Tang et al. observed that C3a could induce EMT in human proximal tubular epithelial cells in vitro, while C3a inhibitor could prevent cells from undergoing EMT.21 In addition, Curci et al. examined EndMT using a porcine model of ischaemia reperfusion injury and showed that complement mediates renal interstitial fibrosis via the AKT pathway.23 These results are similar to our finding that complement is involved in the EMT process. In recent years, more and more studies have confirmed that renal interstitial cells can produce complement components that are involved in acute renal injury and the pathological process of chronic kidney disease. Brooimans et al. verified in vitro that IL‐2 induced human proximal tubular epithelial cells to secrete C3 in a dose‐dependent manner.24 Gerritsma et al. suggested that TGF‐ß1 stimulated complement secretion by human proximal tubular epithelial cells.25 In the mouse model of ischaemia–reperfusion injury, C3 generated by the kidney plays a more important role than does C3 from the circulation.26 Recently, Xavier et al. found PDGFRβ‐positive pericytes and CD45‐positive cells could secrete complement components to initiate renal fibrosis. 27 These findings are consistent with our observation that PAX2 may regulate the expression of C3, CD55, and CFH in renal tubular epithelial cells. in vivo experiments will be necessary to further confirm the role of PAX2 in renal tubular epithelial cells.
As a promoter, PAX2 can bind to the promoter zone of C3, CD55, and CFH; the corresponding binding sites are predicted by the Alggen‐Promo database (http://alggen.lsi.upc.es). The 11 sites of where PAX2 may bind to C3 are: GTCAGAG, TGGTGAC, GTCACAC, GTCATGA, GTCATGT, GGTTGAC, CCATGAC, GTCATTC, GAGTGAC, GTCACTC, and CCTTGAC. The eight sites where PAX2 may bind to CD55 are: GTCACAG, GTCACTG, CCCTGAC, GTCAAGT, CAGTGAC, GTCACTG, AGGTGAC, and GGGTGAC. The 12 sites where PAX2 may bind to CFH are: CTTTGAC, GTCAGGA, CCATGAC, GTCACCA, ACGTGAC, TCCTGAC, GAATGAC, CACTGAC, CAATGAC, GCATGAC, GTCACTG, and CACTGAC. The detailed mechanism by which PAX2 regulates the expression of complement components should be investigated further.
In summary, PAX2 overexpression up‐regulates EMT programmes in vitro and affects cytokines and their receptors, ECM and related receptors, MAPKs, local adhesion molecules, cancer, complement, and coagulation. PAX2 may induce EMT through upregulation of C3 and downregulation of CFH and CD55. The potential mechanisms by which PAX2 activates complement to induce EMT programmes require further study.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
Supporting information
Table S1 Real‐time PCR primers.
Table S2 Part of differential gene list.
ACKNOWLEDGEMENTS
This work was supported by grants from the Science Plan Program of Liaoning Province, China (No. 2013021099).
REFERENCES
- 1. Jiang H, Li L, Yang H, Bai Y, Jiang H, Li Y. Pax2 may play a role in kidney development by regulating the expression of TBX1. Mol. Biol. Rep. 2014; 41: 7491–8. [DOI] [PubMed] [Google Scholar]
- 2. Ranghini EJ, Dressler GR. Evidence for intermediate mesoderm and kidney progenitor cell specification by Pax2 and PTIP dependent mechanisms. Dev. Biol. 2015; 399: 296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Harshman LA, Brophy PD. PAX2 in human kidney malformations and disease. Pediatr. Nephrol. 2012; 27: 1265–75. [DOI] [PubMed] [Google Scholar]
- 4. Negrisolo S, Benetti E, Centi S et al PAX2 gene mutations in pediatric and young adult transplant recipients: Kidney and urinary tract malformations without ocular anomalies. Clin. Genet. 2011; 80: 581–5. [DOI] [PubMed] [Google Scholar]
- 5. Fletcher J, Hu M, Berman Y et al Multicystic dysplastic kidney and variable phenotype in a family with a novel deletion mutation of PAX2. J. Am. Soc. Nephrol. 2005; 16: 2754–61. [DOI] [PubMed] [Google Scholar]
- 6. Okumura T, Furuichi K, Higashide T et al Association of PAX2 and other gene mutations with the clinical manifestations of renal Coloboma syndrome. PLoS ONE 2015; 10: e0142843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kuroda N, Agatsuma Y, Tamura M, Martinek P, Hes O, Michal M. Sporadic renal hemangioblastoma with CA9, PAX2 and PAX8 expression: Diagnostic pitfall in the differential diagnosis from clear cell renal cell carcinoma. Int. J. Clin. Exp. Pathol. 2015; 8: 2131–8. [PMC free article] [PubMed] [Google Scholar]
- 8. Xu HL, Ou C, Rong L, Zhou TB. The potential signal pathway between PAX2 and CD2AP in the renal interstitial fibrosis disease. J. Recept. Signal Transduct. Res. 2014; 34: 290–8. [DOI] [PubMed] [Google Scholar]
- 9. Allison SJ. Fibrosis: Targeting EMT to reverse renal fibrosis. Nat. Rev. Nephrol. 2015; 11: 565. [DOI] [PubMed] [Google Scholar]
- 10. Li L, Wu Y, Yang Y. Paired box 2 induces epithelial‐mesenchymal transition in normal renal tubular epithelial cells of rats. Mol. Med. Rep. 2013; 7: 1549–54. [DOI] [PubMed] [Google Scholar]
- 11. Aoki‐Kinoshita KF, Kanehisa M. Glycomic analysis using KEGG GLYCAN. Methods Mol. Biol. 2015; 1273: 97–107. [DOI] [PubMed] [Google Scholar]
- 12. Mazandu GK, Chimusa ER, Mbiyavanga M, Mulder NJ. A‐DaGO‐fun: An adaptable gene ontology semantic similarity‐based functional analysis tool. Bioinformatics 2016; 32: 477–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bersani‐Amado LE, Dantas JA, Damiao MJ et al Involvement of cytokines in the modulation and progression of renal fibrosis induced by unilateral ureteral obstruction in C57BL/6 mice: Effects of thalidomide and dexamethasone. Fundam. Clin. Pharmacol. 2016; 30: 35–46. [DOI] [PubMed] [Google Scholar]
- 14. Perry HM, Okusa MD. Endothelial dysfunction in renal interstitial fibrosis. Nephron 2016; 134: 167–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Menn‐Josephy H, Lee CS, Nolin A et al Renal interstitial fibrosis: An imperfect predictor of kidney disease progression in some patient cohorts. Am. J. Nephrol. 2016; 44: 289–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Discenza MT, He S, Lee TH et al WT1 is a modifier of the Pax2 mutant phenotype: Cooperation and interaction between WT1 and Pax2. Oncogene 2003; 22: 8145–55. [DOI] [PubMed] [Google Scholar]
- 17. Doberstein K, Pfeilschifter J, Gutwein P. The transcription factor PAX2 regulates ADAM10 expression in renal cell carcinoma. Carcinogenesis 2011; 32: 1713–23. [DOI] [PubMed] [Google Scholar]
- 18. Li L, Wu Y, Zhang W. PAX2 re‐expression in renal tubular epithelial cells and correlation with renal interstitial fibrosis of rats with obstructive nephropathy. Ren. Fail. 2010; 32: 603–11. [DOI] [PubMed] [Google Scholar]
- 19. Su Z, Wuthrich RP, Mei C. Response to letter from the editor ‘Complement C3 activation in cyst fluid and urine from autosomal dominant polycystic kidney disease patients’. J. Intern. Med. 2014; 276: 541–2. [DOI] [PubMed] [Google Scholar]
- 20. Zhou X, Fukuda N, Matsuda H et al Complement 3 activates the renal renin‐angiotensin system by induction of epithelial‐to‐mesenchymal transition of the nephrotubulus in mice. Am. J. Physiol. Renal Physiol. 2013; 305: F957–67. [DOI] [PubMed] [Google Scholar]
- 21. Tang Z, Lu B, Hatch E, Sacks SH, Sheerin NS. C3a mediates epithelial‐to‐mesenchymal transition in proteinuric nephropathy. J. Am. Soc. Nephrol. 2009; 20: 593–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yamada K, Miwa T, Liu J, Nangaku M, Song WC. Critical protection from renal ischemia reperfusion injury by CD55 and CD59. J. Immunol. 2004; 172: 3869–75. [DOI] [PubMed] [Google Scholar]
- 23. Curci C, Castellano G, Stasi A et al Endothelial‐to‐mesenchymal transition and renal fibrosis in ischaemia/reperfusion injury are mediated by complement anaphylatoxins and Akt pathway. Nephrol. Dial. Transplant. 2014; 29: 799–808. [DOI] [PubMed] [Google Scholar]
- 24. Brooimans RA, Stegmann AP, van Dorp WT et al Interleukin 2 mediates stimulation of complement C3 biosynthesis in human proximal tubular epithelial cells. J. Clin. Invest. 1991; 88: 379–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gerritsma JS, van Kooten C, Gerritsen AF, van Es LA, Daha MR. Transforming growth factor‐beta 1 regulates chemokine and complement production by human proximal tubular epithelial cells. Kidney Int. 1998; 53: 609–16. [DOI] [PubMed] [Google Scholar]
- 26. Farrar CA, Zhou W, Lin T, Sacks SH. Local extravascular pool of C3 is a determinant of postischemic acute renal failure. FASEB J. 2006; 20: 217–26. [DOI] [PubMed] [Google Scholar]
- 27. Xavier S, Sahu RK, Landes SG et al Pericytes and immune cells contribute to complement activation in tubulointerstitial fibrosis. Am. J. Physiol. Renal Physiol. 2017; 312: F516–F32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Real‐time PCR primers.
Table S2 Part of differential gene list.


