Abstract
High‐dose ivermectin, co‐administered for 3 days with dihydroartemisinin‐piperaquine (DP), killed mosquitoes feeding on individuals for at least 28 days posttreatment in a recent trial (IVERMAL), whereas 7 days was predicted pretrial. The current study assessed the relationship between ivermectin blood concentrations and the observed mosquitocidal effects against Anopheles gambiae s.s. Three days of ivermectin 0, 300, or 600 mcg/kg/day plus DP was randomly assigned to 141 adults with uncomplicated malaria in Kenya. During 28 days of follow‐up, 1,393 venous and 335 paired capillary plasma samples, 850 mosquito‐cluster mortality rates, and 524 QTcF‐intervals were collected. Using pharmacokinetic/pharmacodynamic (PK/PD) modeling, we show a consistent correlation between predicted ivermectin concentrations and observed mosquitocidal‐effects throughout the 28‐day study duration, without invoking an unidentified mosquitocidal metabolite or drug‐drug interaction. Ivermectin had no effect on piperaquine's PKs or QTcF‐prolongation. The PK/PD model can be used to design new treatment regimens with predicted mosquitocidal effect. This methodology could be used to evaluate effectiveness of other endectocides.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
☑Ivermectin has been shown, in vitro and in vivo, to kill malaria mosquitoes after feeding on human blood. Previous clinical studies showed an effect for 7 days posttreatment. A recent clinical trial showed a prolonged effect for at least 28 days posttreatment.
WHAT QUESTION DID THIS STUDY ADDRESS?
☑Using data from the recent trial, this study explored the PK/PD relationship between ivermectin (when co‐administered with the antimalarial DP) and the observed mosquitocidal effects, in order to understand whether an unidentified metabolite or an ivermectin‐piperaquine drug interaction could be contributing to the prolonged effect.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
☑The study shows a time‐independent PK/PD relationship between ivermectin exposure in individuals and its mosquitocidal activity, without the need to invoke unidentified variables, such as an active metabolite or a drug‐drug interaction.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
☑A comprehensive PK/PD model able to predict the mosquitocidal effect of varying ivermectin regimens in this population can be utilized in guiding future studies and mass drug administration for malaria elimination programs.
Mass drug administration (MDA) with the long‐acting antimalarial dihydroartemisinin‐piperaquine (DP) is being evaluated in several malaria endemic countries for malaria transmission reduction and elimination.1, 2, 3 Ivermectin is an antiparasitic drug, which also kills mosquitoes feeding on recently treated individuals. Adding ivermectin to DP has been proposed as an innovative tool to increase the impact of MDA for malaria.4 However, the single dose of 150–200 mcg/kg ivermectin used for onchocerciasis and lymphatic filariasis control has only a small and short‐lived effect (< 7 days) on mosquito mortality.5 Ivermectin is documented to be remarkably well tolerated, even up to doses of 2,000 mcg/kg.6, 7
In a recent randomized, double‐blind, placebo‐controlled trial in western Kenya, ivermectin 300 and 600 mcg/kg/day, co‐administered for 3 days with DP, were shown to kill mosquitoes feeding on individuals for at least 28 days posttreatment.8 This was significantly longer than the 7‐day effectiveness expected based on the predicted time ivermectin would remain above the in vitro median half‐maximal lethal concentration (LC50) for Anopheles gambiae in pretrial simulations.5 Two possible explanations are an unidentified mosquitocidal metabolite with a longer active half‐life than the parent compound ivermectin or a drug‐drug interaction that results in a slower clearance of ivermectin from the circulation. As ivermectin and piperaquine are both metabolized by cytochrome P450 3A4 (CYP3A4), it has been hypothesized that an interaction could occur.5 Using the trial data, the current pharmacokinetic (PK) and pharmacodynamic (PD) analysis aimed to determine whether a drug interaction or an unidentified ivermectin metabolite could be contributing to the prolonged mosquitocidal effect of ivermectin.
Results
Ivermectin PK
Ivermectin PKs were best described by a two‐compartment oral absorption model (Figure S1, Table S1). The initial sequential model, utilizing only plasma concentration data to fit the PK data, resulted in the parameters described in Table 1. Goodness‐of‐fit plots are shown in Figure 1 (observed capillary‐venous plots are shown in Figure S2). In the ivermectin arms posttreatment, concentrations were below the limit of quantification (BLOQ) for 277 of 805 (34.4%) venous and 44 of 224 (19.6%) capillary samples, predominantly at later time points (Table S2).
Table 1.
Primary and secondary PK parameters of ivermectin and piperaquine
| Parameter | Ivermectin sequential PK/PD model | Ivermectin simultaneous PK/PD model | Piperaquine |
|---|---|---|---|
| Median (p5–p95) ± interindividual variability (%) | Median (p5–p95) ± interindividual variability (%) | Median (p5–p95) ± interindividual variability (%) | |
| V/F (L) | 147.0 (36.2–582.0) × (WT/60) ± 103.0 | 161.7 (70.9–760.4) × (WT/60) ± 112.7 | 803.7 (149.1–1,999) × (WT/60) ± 69.0 |
| CL/F (L/hour) | 9.6 (6.5–14.6) × (WT/60)0.75 ± 39.1 | 10.9 (6.6–26.1) × (WT/60)0.75 ± 57.4 | 97.1 (20.0–177.3) × (WT/60)0.75 ± 49.1 |
| k a (hour−1) | 0.22 (0.082–1.79) ± 157.1 | 0.474 (0.15–6.93) ± 163.5 | 1 (Fixed) |
| Q1/F (L/hour) | 19.0 (7.7–113.1) ± 104.7 | 21.1 (9.7–116.4) ± 100.5 | 1,017.0 (197.5–4,079) ± 87.5 |
| V P1/F (L) | 1,148.1 (413.1–3,845) ± 86.6 | 612.4 (253.0–1,879) ± 82.1 | 3,796 (415.1–14,918) ± 165.8 |
| Q2/F (L/hour) | NA | NA | 156.4 (32.6–545.7) ± 82.8 |
| V P2/F (L) | NA | NA | 35,993 (7,586–146,968) ± 96.2 |
| Capillary/venous ratio | 1.32 (1.1–1.6) ± 18.7 | 1.33 (0.98–1.63) ± 29.1 | 1.55 (1.1–2.8) ± 36.1 |
| Secondary parameters | |||
| Cmax (ng/mL) | |||
| All subjects | NA | NA | 252.4 (95.5–1,072.5) |
| 0 mcg/kg/day IVM arm | NA | NA | 250.1 (99.4–727.7) |
| 300 mcg/kg/day IVM arm | 64.1 (29.7–129.9) | 69.4 (34.1 –196.3) | 263.6 (84.6–1,268.2) |
| 600 mcg/kg/day IVM arm | 105.2 (44.5–482.5) | 118.9 (45.2–455.1) | 246.2 (98.8–990.1) |
| Tmax (hour) after last dose | |||
| All subjectsa | 4.8 (0.58–8.7) | 2.9 (0.46–7.8) | 1.4 (1.1–3.6) |
| 0 mcg/kg/day IVM arm | NA | NA | 1.4 (1.1–3.5) |
| 300 mcg/kg/day IVM arm | 5.0 (1.4–8.8) | 3.9 (0.75–7.6) | 1.4 (1.1–3.0) |
| 600 mcg/kg/day IVM arm | 3.5 (0.40–7.5) | 2.3 (0.43–8.2) | 1.5 (1.1–3.8) |
| t1/2 (hour) | |||
| All subjectsa | 4.9 (1.9–12.9) | 3.1 (0.93–11.4) | 17.7 (2.6–81.8) |
| 0 mcg/kg/day IVM arm | NA | NA | 18.2 (5.7–89.8) |
| 300 mcg/kg/day IVM arm | 5.1 (2.2–12.6) | 2.9 (1.1–7.8) | 17.2 (2.8–94.6) |
| 600 mcg/kg/day IVM arm | 4.7 (1.7–12.5) | 3.2 (0.90–8.5) | 17.9 (1.5–42.3) |
| AUC0–28 days (hour/mcg/mL) | |||
| All subjects | NA | NA | 21.4 (6.3–47.9) |
| 0 mcg/kg/day IVM arm | NA | NA | 21.3 (10.4–44.9) |
| 300 mcg/kg/day IVM arm | 5.5 (2.5–8.3) | 5.0 (1.6–8.3) | 23.7 (6.9–49.7) |
| 600 mcg/kg/day IVM arm | 10.0 (1.7–22.3) | 9.3 (2.1–25.0) | 21.2 (6.5–46.5) |
Ivermectin parameters are reported using either the sequential PK/PD model (where PK analysis was done on venous and capillary PK data only) or the simultaneous PK/PD model (where PK/PD analysis was performed simultaneously on venous and capillary exposure data as well as PD outputs defined as corresponding mosquito mortality rates for each venous sample).
AUC, area under the curve; IVM, ivermectin; PD, pharmacodynamic; PK, pharmacokinetic; NA, not available; Tmax, time of maximum plasma concentration; t1/2, terminal half‐life; WT, bodyweight.
For ivermectin models, only subjects in the ivermectin arms.
Figure 1.
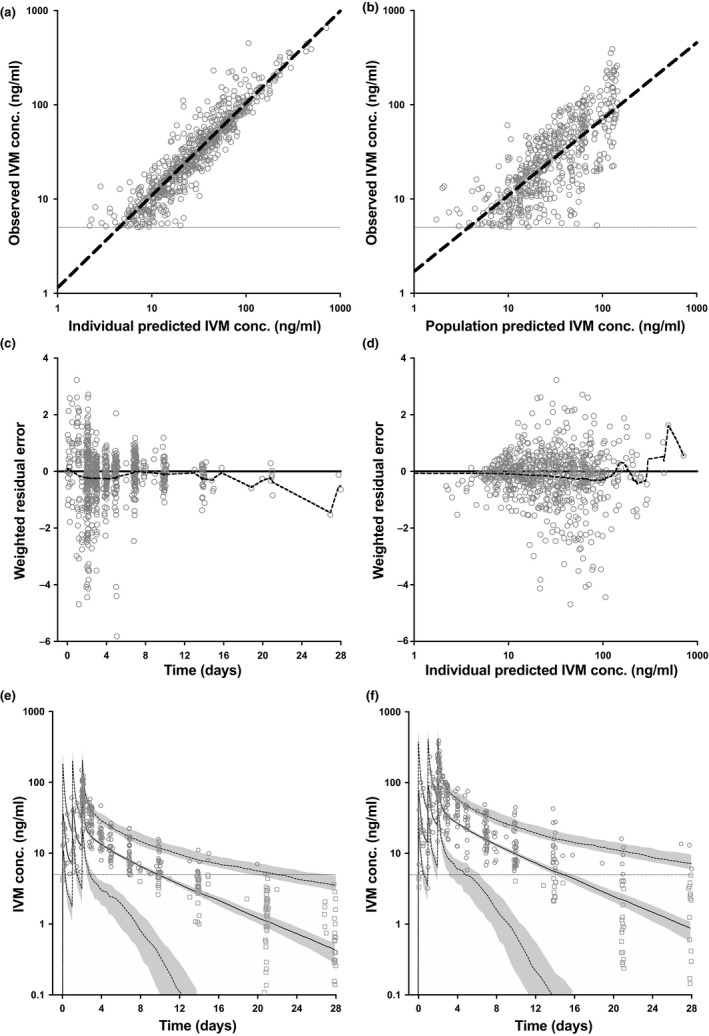
Ivermectin pharmacokinetic model (sequential approach) using venous and capillary concentrations: goodness‐of‐fit and simulation. (a) Ivermectin individual predicted concentrations (n = 1,029) vs. observed concentrations (n = 708) (slope = 0.98, R 2 = 0.8652). (b) Ivermectin population predicted concentrations vs. observed concentrations (slope = 0.81, R 2 = 0.5793). (c) Weighted residual error distribution of predicted vs. observed ivermectin concentrations over time (mean = −0.23 over 28 days) (dashed black line = locally weighted scatterplot smoothing (LOESS) curve fit through residuals). (d) Weighted residual error distribution of predicted vs. observed ivermectin concentrations over predicted ivermectin concentration (mean = −0.23 over a range of 1–353 ng/mL; dashed black line = LOESS curve fit through residuals). (e) Observed ivermectin venous concentrations (gray circles) with predicted concentrations for those unobserved (gray squares), overlaid with simulation of ivermectin 300 mcg/kg/day for 3 days (solid black line = median; dashed gray lines = 5% and 95% percentiles; shaded gray area = 95% confidence interval for the percentiles). (f) Similar to e with ivermectin 600 mcg/kg/day for 3 days. IVM, ivermectin; conc., concentration; lower limit of quantification, 5 ng/mL (horizontal gray line). Simulations included 1,000 individuals of 60 kg bodyweight.
The subsequent simultaneous PK/PD model resulted in remarkably similar PK parameters to the initial sequential approach (Table 1), meaning the PD element did not disturb the PK prediction, which supports the notion that it is unnecessary to invoke an active metabolite to explain the PD output of ivermectin.
Piperaquine PK
Piperaquine PKs were best described using a three‐compartment oral absorption model (Figure S1, Table S1), resulting in the parameters displayed in Table 1. Concentrations were BLOQ for 3 of 1,248 (0.2%) venous and 0 of 333 (0%) capillary posttreatment samples (Table S2). Goodness‐of‐fit graphs, including both capillary and venous concentrations, are shown in Figure 2 (observed capillary‐venous plots are shown in Figure S2). A visual predictive check (VPC), incorporating variation in patient weights, resulted in a profile that accurately fits the observed population PK data (Figure 2 e). Post hoc analysis showed that piperaquine PK was not influenced by ivermectin with area under the curve (AUC), peak plasma concentration (Cmax), terminal half‐life (t1/2; Table 1) and the overall PK profile (Figure 2 f) showing no differences across all three study arms.
Figure 2.
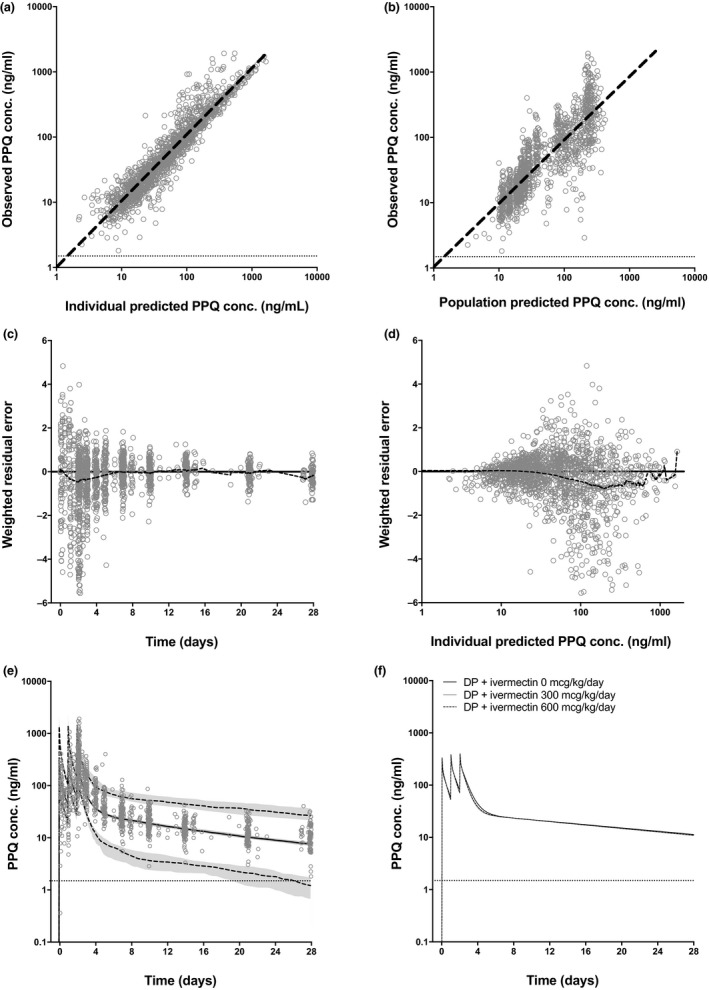
Piperaquine pharmacokinetic model using venous and capillary concentrations: goodness‐of‐fit and simulation. (a) Piperaquine individual predicted concentrations (n = 1,581) vs. observed concentrations (n = 1,578; slope = 1.04, R 2 = 0.9273). (b) Piperaquine population predicted concentrations vs. observed concentrations (slope = 0.93, R 2 = 0.8332). (c) Weighted residual error distribution of predicted vs. observed piperaquine concentrations over time (mean = −0.23 over 28 days; dashed black line = locally weighted scatterplot smoothing (LOESS) curve fit through residuals). (d) Weighted residual error distribution of predicted vs. observed piperaquine concentrations over predicted ivermectin concentration (mean = −0.20 over a range of 2–1,421 ng/mL; dashed black line = LOESS curve fit through residuals) (e) Observed piperaquine venous concentrations (gray circles) overlaid with simulation of piperaquine 960 mg/day for 3 days (solid black line = median; dashed gray lines = 5% and 95% percentiles; shaded gray area = 95% confidence interval for the percentiles). (f) Simulation of piperaquine 960 mg/day for 3 days based on parameters derived from patients concomitantly receiving ivermectin 0 mcg/kg/day (solid black line), ivermectin 300 mcg/kg/day (solid gray line) or ivermectin 600 mcg/kg/day (black dashed line). Conc., concentration; DP, dihydroartemisinin‐piperaquine; lower limit of quantification, 1.5 ng/mL (horizontal gray line); PPQ, piperaquine. Simulations included 1,000 individuals of 60 kg bodyweight.
Ivermectin PD
PD analysis of ivermectin activity was performed initially by building an exposure‐effect relationship between pooled observed venous ivermectin concentrations in patients and the corresponding mosquitocidal activity of each blood sample (Figure 3 a). This generated ivermectin's predicted half‐maximal effective concentration (EC50) of 11.3 ng/mL and maximal mosquito mortality rate (maximum effect (Emax) + minimum effect (Emin)) of 48.8 deaths per 100 mosquito days observed (an incidence density rate). Baseline mortality rate (Emin) was fixed to 3.7 deaths/100 days based on mortality rates observed in 392 mosquito clusters feeding on ivermectin‐free blood from baseline and control samples.
Figure 3.
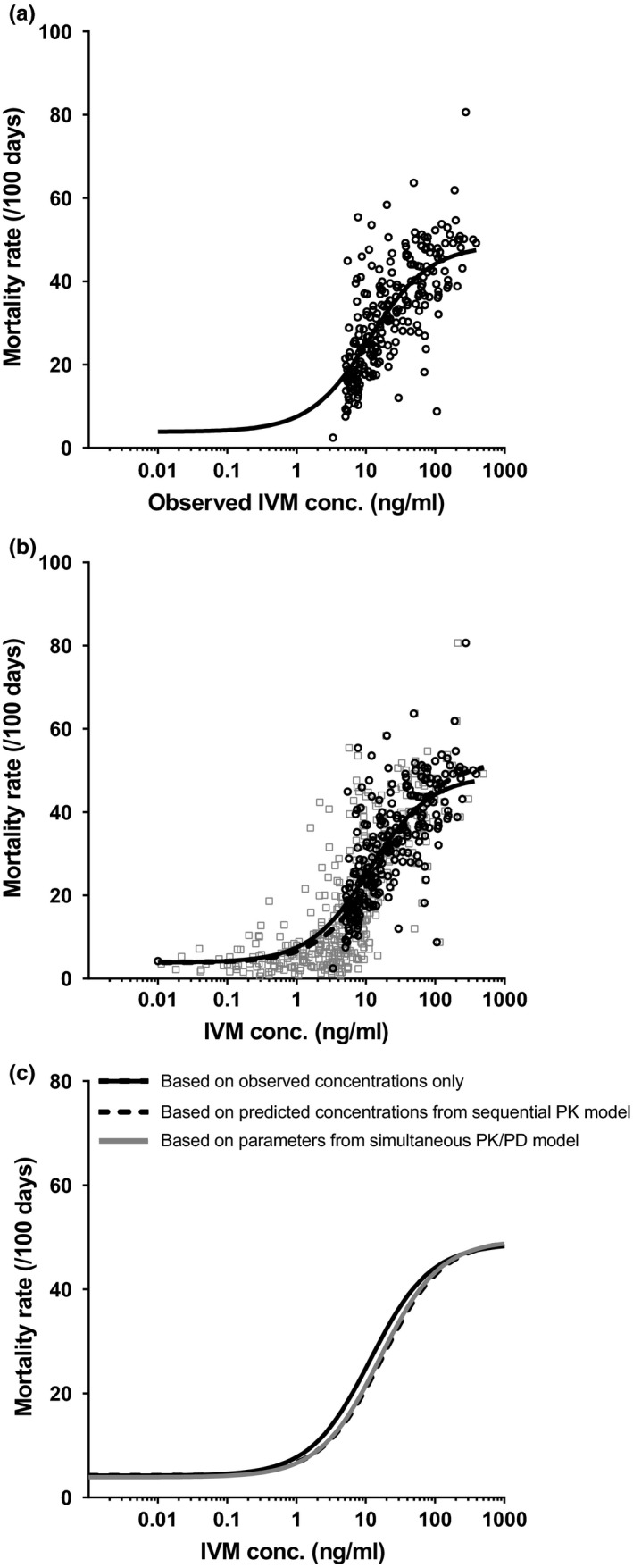
(a) Relationship between observed ivermectin venous concentration and mosquito mortality rate (/100 days). Open circles (n = 246 concentrations above lower limit of quantification (LLOQ) with paired mortality rate) represent observed data. The solid line represents sigmoidal three‐parameter maximum effect (Emax) fit. (b) Similar to a, however, now overlaid with predicted ivermectin venous concentrations for all samples (including those that were below LLOQ) with observed mosquito mortality rates in patients that received ivermectin (gray squares, n = 567). The dashed line represents the sigmoidal three‐parameter Emax fits for the predicted concentrations. (c) A comparison between the exposure relationship of a, b, and the exposure‐effect relationship generated using the simultaneous pharmacokinetic/pharmacodynamic (PK/PD) model, which incorporated PD data in the process of PK modeling and vice versa. IVM, ivermectin; conc., concentration.
The relationship between mosquitocidal activity and pooled predicted ivermectin concentrations from the sequential PK/PD model (including 321 BLOQ venous and capillary concentrations that were predicted but not observed) resulted in a similar predicted PD response, compared to analyzing observed data only (Table 2 and Figure 3 b). A sequential population method, incorporating individual PK parameters (assessed previously) and individuals’ corresponding PD output, gave similar results: predicted median maximal mosquito mortality rate (Emax + Emin) of 57.3 (p5–p95: 42.1–68.8) deaths per 100 mosquito days, median EC50 of 20.5 ng/mL (p5–p95: 6.1–49.7), and median Emin of 3.84 (p5–p95: 3.6–4.4) deaths per 100 mosquito days. Finally, the simultaneous PK/PD model resulted in PD parameters that are very similar to those obtained from utilizing the sequential models or observed data only (Table 2 and Figure 3 c).
Table 2.
PD parameters of ivermectin
| Models based on: | Observed data: obs. venous conc. vs. obs. mosq. effect (pooled analysis) | Sequential PK/PD model: pred. venous conc. vs. obs. mosq. effect (pooled analysis) | Simultaneous PK/PD model: pred. venous conc. vs. pred. mosq. effect (population modeling) |
|---|---|---|---|
| Parameter | Median (p5–p95) | Median (p5–p95) | Median (p5–p95) |
| Mosquitocidal effect | |||
| Emin (deaths/100 days) | 3.7 (fixed) | 3.7 (fixed) | 3.9 (2.9–5.8) |
| E 1 (deaths/100 days) | 4.18 (4.16–4.21) | 4.22 (4.19–4.25) | 4.40 (4.23–4.53) |
| E 5 (deaths/100 days) | 6.14 (6.02–6.25) | 6.31 (6.18–6.47) | 6.38 (5.54–7.08) |
| E 50 (deaths/100 days) | 28.1 (23.2–29.3) | 29.9 (28.5–31.4) | 28.7 (20.1–35.5) |
| E 95 (deaths/100 days) | 50.1 (47.7–52.3) | 53.4 (50.8–56.2) | 50.7 (34.9–64.1) |
| Emax + Emin (deaths/100 days) | 52.5 (50.0–54.9) | 56.0 (53.3–59.0) | 53.4 (36.7–67.5) |
| Effective concentration | |||
| EC1 (ng/mL) | 0.11 (0.095–0.14) | 0.17 (0.14–0.19) | 0.16 (0.053–0.37) |
| EC5 (ng/mL) | 0.90 (0.51–1.14) | 0.84 (0.74–1.0) | 0.83 (0.28–1.9) |
| EC50 (ng/mL) | 11.3 (9.5–13.6) | 16.5 (14.1–19.2) | 15.9 (5.3–36.4) |
| EC95 (ng/mL) | 214.3 (178.2–259.0) | 314 (269.3–374.3) | 302.1 (100.7–691.6) |
| Time above EC (ivermectin 300 mcg/kg/day for 3 days) | |||
| T 1 (days) | 33.3 (19.0–130.5) | 30.1 (17.9– 115.4) | 24.4 (10.5–63.0) |
| T 5 (days) | 22.6 (13.4–56.4) | 20.3 (12.2–50.9) | 17.0 (8.0–42.6) |
| T 50 (days) | 5.6 (2.4–12.7) | 3.9 (2.2–8.9) | 4.1 (NA–8.1) |
| T 95 (days) | NA | NA | NA |
| Time above EC (ivermectin 600 mcg/kg/day for 3 days) | |||
| T 1 (days) | 38.0 (21.6–150.1) | 34.5 (20.0–142.2) | 28.1 (11.5–72.8) |
| T 5 (days) | 27.4 (15.4–66.0) | 24.8 (14.3–60.4) | 20.1 (9.3–52.9) |
| T 50 (days) | 9.0 (3.0–20.7) | 7.1 (2.6–16.4) | 6.8 (2.5–14.7) |
| T 95 (days) | NA | NA | NA |
Emin was fixed to 3.7 for (i) and (ii) based on baseline and control mortality. Exx is the percentile of effect between Emin and Emin + Emax. ECxx is the concentration at which Exx is achieved. Txx is the time posttreatment at which ECxx and Exx are achieved.
Conc., concentration; EC, effective concentration; Emin, minimum effect; Emax, maximum effect relative to Emin; mosq. mosquitocidal; Obs., observed; PD, pharmacodynamic; PK, pharmacokinetic; pred., predicted.
The relationship between ivermectin exposure and its mosquitocidal effect was investigated separately for each day of follow‐up (days: 2 + 4 hours, 7, 10, 14, 21, and 28). As most samples beyond day 14 resulted in unquantifiable concentrations (BLOQ), which would have restricted the ability of PD analysis, predicted concentrations from the sequential PK model (using PK data from all days) were used for PD modeling; this was justified as predicted concentrations matched observed values accurately (Figures 1 a and 3 b). Figure 4 shows the relationship between predicted ivermectin exposure and mosquito mortality rate at each study visit. The results indicate that the mosquito mortality rate has the same relationship with predicted ivermectin concentration at all posttreatment feeding days of the study, including days 21 and 28 when most ivermectin exposures were BLOQ. There is no effect of time posttreatment on the shape of the sigmoidal relationship between predicted ivermectin concentration and observed mortality rate. Similar to this sequential PK/PD approach, the simultaneous PK/PD model showed no bias over time in residual analysis of PD predictions; meaning ivermectin's concentration‐effect relationship (i.e., EC50 and Emax) was consistent throughout the study duration (Figure S4).
Figure 4.
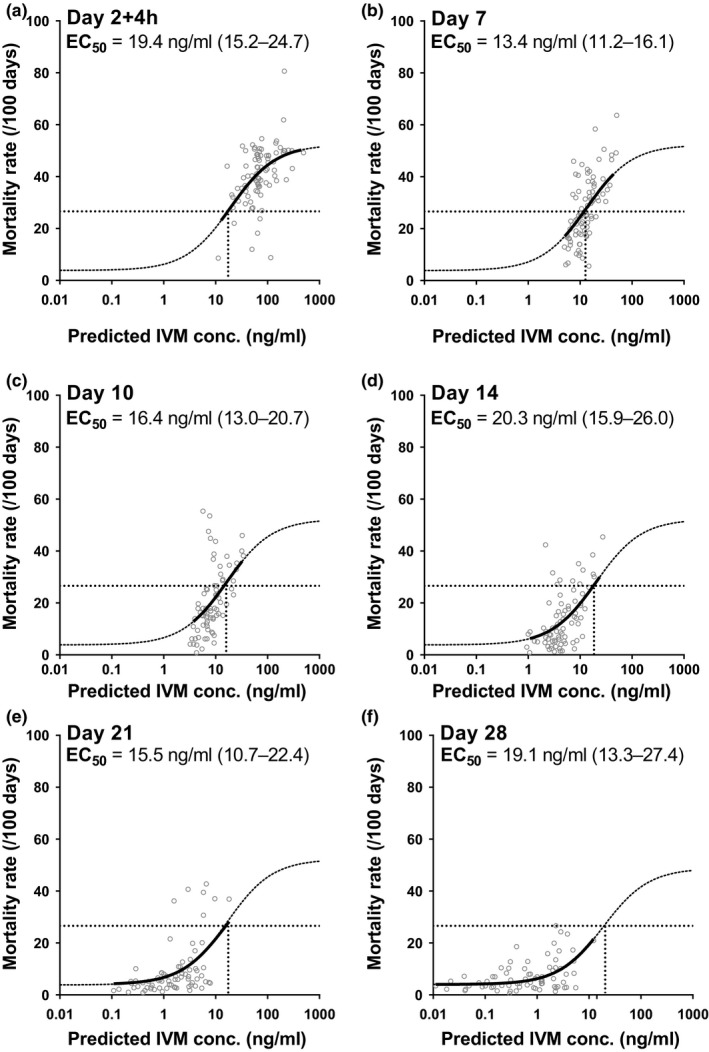
The exposure‐effect relationship between predicted ivermectin (IVM) concentrations (from the sequential pharmacokinetic (PK) model, using PK data from all days) and corresponding observed mosquito mortality rates separated by day of analysis after initiation of treatment. Minimum effect (Emin) and maximum effect (Emax) were fixed to the values determined by analyzing the entire dataset and half‐maximal effective concentration (EC50) concentrations (95% confidence intervals) were estimated as shown in the figure.
Mosquito mortality was also analyzed as a proportion (%) to calculate the ivermectin concentration able to kill 50% of mosquitoes by a specific time point (LC50), unadjusted and adjusted for baseline mortality (Figure S3 and Table S3).
Ivermectin PK/PD simulation
A VPC of the PK/PD simulation did not show any bias in comparison with observed PD data in either arm of the study (Figure 5).
Figure 5.
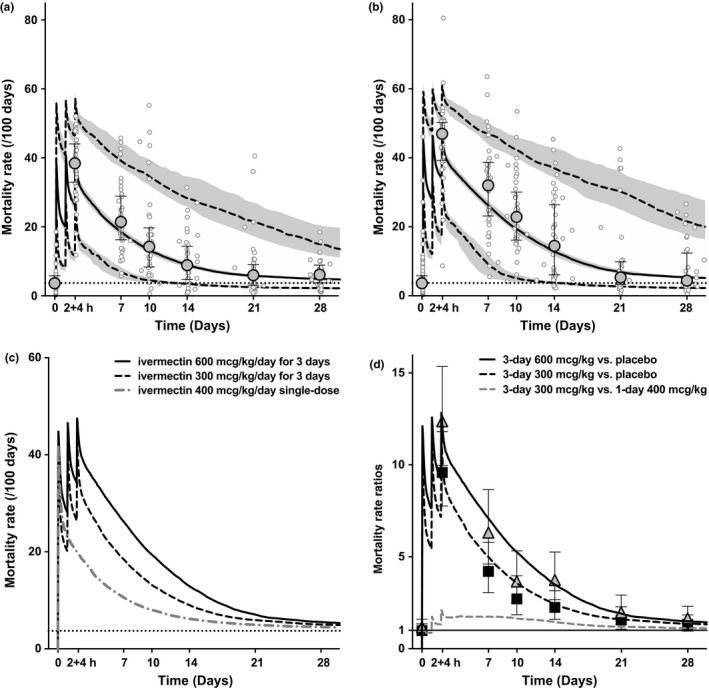
Ivermectin pharmacokinetic (PK) and pharmacodynamic (PD) simulation of mosquito mortality rate (using simultaneous approach) with (a) 300 mcg/kg/day for 3 days, and (b) 600 mcg/kg/day for 3 days. Mosquito mortality rate simulated median (solid black line), 5th and 95th percentiles (dashed lines), and 95% confidence intervals (CIs; shaded gray areas), with observed mosquito mortality rates per sample (open circles), observed median ± interquartile range mortality rate per study visit (ball‐whiskers), and minimum effect (Emin; horizontal dashed line). (c) Comparison of both regimens with a simulation of a 400 mcg/kg single‐dose. Simulations included 1,000 individuals of 60 kg bodyweight. (d) Mortality rate ratios calculated as incidence rate ratios using the PK/PD model (incidence rate ratio; lines) and as hazard ratios (HRs) with 95% CIs as per main efficacy results8 (HR; triangles: 600 mcg/kg/day for 3 days vs. placebo, squares: 300 mcg/kg/day for 3 days vs. placebo, and whiskers: 95%CIs). Conc., concentration; PPQ, piperaquine.
Piperaquine PD
Piperaquine's effect upon QTcF was analyzed using a conventional linear model as well as a dynamic Emax model. Using the linear model, piperaquine resulted in a mean QTcF‐prolongation of 3.71 ms (95% confidence interval (CI): 3.12–4.29) per 100 ng/mL with a baseline intercept of 6.76 ms (95% CI: 4.99–8.53) (Figure S5). Using the dynamic Emax population model, that was simultaneously fitted with piperaquine PK using Pmetrics, we show that from a median baseline QTcF‐interval of 399.3 ms (p5–p95: 377.5–416.3) the maximal ΔQTcF‐interval was estimated at 53.5 ms (31.1–122.9), resulting in a maximal QTcF‐interval of 449.8 ms (415.1–520.0). The median concentration required to achieve an effect half‐way between baseline and maximal possible prolongation (EC50) was 181.7 ng/mL (16.0–1200.0). Parasitemia, present at low levels and only at baseline, was not associated with QTcF at baseline vs. day 28, at day 2 + 4 hours vs. day 0, or at day 2 + 4 hours vs. day 28.
Piperaquine concentration correlated with QTcF (Figure S5), however, post hoc analysis showed only a weak (ρ < 0.3), albeit significant, correlation between piperaquine Cmax and D2 + 4 hours QTcF (Table S6). Piperaquine AUC was not correlated with day 2 + 4 hours QTcF and ΔQTcF (Table S6). Ivermectin dose did not modify piperaquine's QTcF‐prolonging effect (Tables S4 and S5).
Discussion
Ivermectin's PD effect on A. gambiae mortality was much stronger and persisted much longer posttreatment in the current trial than reported in previous studies. The PK/PD analysis showed that the entire 28 days of mosquitocidal effect posttreatment observed in the main trial8 could be explained by ivermectin concentrations alone, without invoking unidentified variables, such as an active metabolite or a drug‐drug interaction. Furthermore, ivermectin exerted no modification of piperaquine's PKs or QTcF‐prolongation. This ivermectin PK/PD model can be used to design new treatment regimens and predict their mosquitocidal effect. The methodology could also be used to assess new endectocides.
Ivermectin PK
Ivermectin's PK properties in this study were similar to those reported in earlier studies, which used venous concentrations from adults receiving single doses ranging between 150 and 2,000 mcg/kg,7, 9, 10, 11, 12, 13, 14 and repeated doses up to 1,000 mcg/kg given three times in 1 week.7 Ivermectin clearance has been reported as 19.2 L/hour (SD ± 14.8),10 17.3 L/hour (SD ± 9.2),14 and, in a study with co‐administration of azithromycin and albendazole, as 11.8 L/hour (RSE 32.8%) overall, and 12.3 L/hour (RSE 42.6%) when allowing for different subpopulations.11 Clearance in the current study using a simultaneous model was 10.9 L/hour for an average 60 kg patient (Table 1), which is similar to previous studies and makes a drug‐drug interaction in the current study unlikely.
Ivermectin PD
Ivermectin's mosquitocidal effect has been assessed in several in vitro studies with spiked blood and three previous clinical trials, one of which measured plasma concentrations. Outcomes are often expressed as the LC50 by a specified time postfeeding (e.g., by 7 days), although assay durations vary widely, as does adjustment for background mortality rate, hampering comparability. In vivo adjusted LC50 values in the current trial were 0.6, 2.6, 4.3, 4.7, and 7.1 times lower than previous studies (Figure S3 and Table S3).15, 16, 17, 18 Noticeably, there was no major difference between the current study and the previous in vivo trial (adjusted LC50: 2.6 vs. 6.5 ng/mL), especially considering the later value could be an overestimation due to a scarcity of data in the upper ranges of concentrations (maximum value achieved: 15.6 ng/mL) and mosquito mortality rates (2 of 233 values were >90%).15 Any major difference between LC50 estimates obtained from in vivo and in vitro studies could suggest the presence of an active metabolite, however, comparisons between the current in vivo trial and the in vitro studies were inconclusive, some showing either lower (0.6‐fold) or higher (7.1‐fold) values. Nevertheless, our PK/PD model showed that the entire mosquitocidal effect, including its prolonged effect beyond day 7 up to day 28 posttreatment, could be explained by ivermectin concentrations alone, without the need to invoke an unidentified metabolite (Figure 5, Table 2 , and Figure S4). This indicates that ivermectin in its parent form is likely the sole contributor to the observed mosquitocidal effect for the duration of the study, or if an active metabolite is present, then it must have a remarkably similar PK profile to ivermectin itself.
Ivermectin effect‐duration
The 28‐day exposure‐effect relationship exceeded the prediction from the pretrial PD simulation, which estimated an effect‐duration of only 7 days.5 The simulation was based on previously reported 7‐day LC50 value of ivermectin of 15.9 ng/mL,17 whereas, in the current trial, the 7‐day LC50 was 3.4 ng/mL. Importantly, this study also illustrated the value of using thresholds below the LC50 to help explain the effect‐duration, such as 5% and 1% of maximal activity (EC5 0.83 ng/mL and EC1 0.16 ng/mL), which were reached at 20.1 and 28.1 days posttreatment with the 3‐day 600 mcg/kg/day regimen (Table 2). The latter is consistent with the observed 28‐day mosquitocidal effects of the main trial.8 Future PD simulations should consider using these EC1–EC5 concentrations as thresholds to predict effect‐durations.
With this PK/PD model we also show that the high‐dose regimens can extend the time that 5% of maximal activity against mosquitoes is achieved from 13 days with a single‐dose of 400 mcg/kg to 23 days with the 3‐day 600 mcg/kg/day regimen; additionally, the overall kill rate using the 3‐day 600 or 300 mcg/kg/day regimens is predicted to be several fold faster in the first 2 weeks of treatment when compared to the 400 mcg/kg single‐dose (Figure 5).
Assessing endectocides
Other aspects of the methodology followed in this analysis may also be useful for further assessment and comparisons of new endectocides and regimen optimization.19 We propose to collect the following data for each drug: Emax, Emin, EC50, Hill coefficient (in this study equaling 1), and PK parameters (e.g., CL, V, Q 1/F, V P1/F, and k a) to allow for PK/PD simulation. The E is specific to the mosquito species studied. We also propose to express the PD effect of each endectocide on mosquito mortality as an incidence density rate (IDR) by day 14 (i.e., deaths per 100 mosquito days observed, during a 14‐day mosquito survival assay). The 14‐day follow‐up is required to capture the cumulative effect during a prolonged extrinsic cycle. The use of IDRs instead of hazard ratios (HRs) is recommended, as rates are easier to interpret and incorporate in PK/PD models. For example, in the PK/PD simulation it was possible to simulate an IDR for any desired drug regimen at any time posttreatment. By dividing the drug IDR (E) by the baseline mortality rate (Emin) it was then possible to calculate the incidence rate ratio (IRR = E/Emin) at each timepoint (see IVERMAL dose‐response calculator (Text S3) and Figure 5 d). Both IRRs and HRs assess the relative differences in incidence rates, can be interpreted in roughly the same way,20 and equally incorporated into population models. As MDA rounds are likely to be spaced at least 1 month apart, determining an endectocide's IRR at 28 days posttreatment (either directly or by simulation) may be a useful measure to assess prolonged effectiveness.
Ivermectin capillary concentrations
No previous studies assessed capillary concentrations of ivermectin; the current study identified a capillary‐venous ratio of 1.33 (0.98–1.63) ± 29.1%, which was consistent over time from days 2–7 posttreatment (Table 1 and Figure S2 ). In the current assays, mosquitoes were fed venous blood via artificial membranes. As capillary concentrations of ivermectin were found to be higher (Table 1), and when mosquitoes bite humans directly they are more likely to feed from capillary blood, the mosquitocidal effects of ivermectin might be higher than presented in the current study. To assess this, an analysis is ongoing comparing mosquito mortality following direct skin feeding (from capillary blood) vs. membrane feeding (from venous blood).
Piperaquine PK
Piperaquine PKs have been described using meta‐analysis, across age groups, and using capillary sampling, with clearance reported as 55.4 L/hour (RSE 4.2%; for an average 54 kg patient),21 which was somewhat higher in the current study at 97.1 L/hour (interindividual variability ± 49.1%; for an average 60 kg patient). The majority of previous studies were performed in Asian patients, whereas this study was performed in Kenyan patients. Although the current clearance value had a relatively wide percentile range (p5–p95: 20.0–177.3), more studies in African patients might be needed to investigate whether genetics plays a role in piperaquine clearance. The plasma capillary‐venous ratio of 1.55 ± 36.1% was similar to those reported previously for blood (1.66)22 and plasma (1.9,23 1.63,24 and 1.4125). Using a linear model, piperaquine's PD effect on QT‐interval was also similar to previous studies,26, 27, 28, 29 with the current trial predicting a ΔQTcF‐prolongation of 3.71 ms (95% CI: 3.12–4.29) per 100 ng/mL of piperaquine. Additionally, using a dynamic Emax model that simultaneously incorporated piperaquine PK and PD data, we estimated maximal QTcF (449.8 ms) and maximal ΔQTcF (53.5 ms) using population methods (Table S5 and Figure S5). Importantly, neither of the two ivermectin regimens used in the study altered piperaquine's PKs or PDs (Table 1 , Figure 2 f, Tables S4 and S5).
Drug interaction
It is not possible to completely rule out an effect of DP on ivermectin's PKs or PDs, as all groups received DP. Nonetheless, a substantial drug‐drug interaction is unlikely as ivermectin PK parameters were very similar to previously reported values (Table 1) and within our prediction, which was based on published data where ivermectin was given alone.5 Future studies comparing ivermectin alone vs. ivermectin with DP could help to further assess any potential effect of DP on ivermectin PK/PD.
Limitations and future studies
A limitation of this PK/PD analysis was that the lower limit of quantification (LLOQ) for ivermectin was relatively high (venous: 5 ng/mL, capillary: 10 ng/mL), resulting in undetectable concentrations for 34% of venous and 20% of capillary samples in the ivermectin arms posttreatment, predominantly at later timepoints. Previous studies used venous LLOQs of: 0.2,30 1.0,31 0.5,7 2.5,11 0.2,15 and 0.8,32 ng/mL. Based on the number of samples available, it was still possible to generate an accurate PK/PD model, however, future studies should attempt to use assays with lower LLOQs to make full use of the available samples and detect ivermectin at the very low concentrations that it still has a mosquitocidal effect. Another possible limitation is that this study used a homogeneous laboratory‐reared mosquito colony, which may not be reflective of wild populations. Although previous work has demonstrated that ivermectin affects survival of all tested anophelines (≥ 17 species tested),33 future studies would be beneficial to examine possible (heterogeneity of) effects of ivermectin against wild populations.8 Finally, this study included only (nonpregnant) adults, whereas the targeted population for MDA also includes children and pregnant women. Children are hypothesized to have faster ivermectin metabolism based on increased CYP3A4 expression.34 Ivermectin is currently contra‐indicated in pregnancy, however, inadvertent exposures during MDA for lymphatic filariasis and onchocerciasis have not led to an increase in adverse events.35 Further studies assessing the safety, efficacy, and PKs of (high‐dose) ivermectin are needed in children and pregnant women.
Prolonging mosquitocidal effects
Additional effort is also required to further increase ivermectin's effect‐duration beyond 28 days, as a longer effect‐duration would allow MDA rounds to be more widely spaced and reduced in number. Consideration could be given to developing slow‐release tablet formulations, to developing gastric‐resident devices,36 or to other similar drugs with a long half‐life, such as moxidectin, albeit its LC50 of 2,789 ng/mL against A. gambiae does not seem to make it very suited for malaria control.12, 37
CONCLUSION
In conclusion, the current PK/PD model predicted that the mosquitocidal effect of high‐dose ivermectin lasted for at least 28 days posttreatment, which is consistent with the in vivo observations from the main trial.8 This effect could be explained by ivermectin concentrations alone, without invoking unidentified variables, such as an active metabolite or a drug‐drug interaction. Furthermore, ivermectin exerted no modification of piperaquine's PKs or QTcF‐prolongation. The presented ivermectin PK/PD model can be used to design new treatment regimens and predict their mosquitocidal effect. The presented methodology may be useful for the assessment of new endectocides.
Methods
Trial design
Details of the trial design, procedures, and main results were published elsewhere.5, 8 Briefly, the study was a randomized, double‐blind, placebo‐controlled, parallel three‐arm, superiority trial (ClinicalTrials.gov: NCT02511353). One hundred forty‐one adults with uncomplicated malaria in western Kenya were randomly assigned (1:1:1), stratified by sex and body mass index, to receive 3 days of ivermectin 600 mcg/kg/day (n = 47), 300 mcg/kg/day (n = 48), or placebo (n = 46), all co‐administered with 3 days of DP. The primary outcome was the effect of ivermectin dose on mosquito mortality. For safety, the effect of ivermectin dose on piperaquine concentration and piperaquine‐induced QTcF‐prolongation were also assessed. Venous plasma was collected from day 0 to day 28 using both rich and population sampling strategies. Additional capillary plasma was collected using finger‐pricks from day 2 + 4 hours (4 hours after the third dose) up to day 7, concomitantly with the venous population sampling. Capillary sampling might be useful for subsequent pediatric or field trials and might be more representative of the blood that mosquitoes feed on.
Observed plasma concentrations and outcome data
During 28 days of follow‐up, 1,393 venous and 335 paired capillary plasma samples, 850 mosquito‐cluster mortality rates (from 91,109 mosquitoes), and 524 QTcF‐intervals were collected (Table S2). Drug analytical quantification was performed by liquid chromatography tandem mass spectrometry (Text S1); only quantifiability and not detectability was considered. Ivermectin venous concentrations were above the LLOQ (5 ng/mL) for 534 samples, of which 246 had paired mortality rates, and capillary concentrations were above the LLOQ (10 ng/mL) for 181 samples. Piperaquine venous concentrations were above the LLOQ (1.5 ng/mL) for 1,246 samples, of which 251 had paired QTcF‐intervals, and capillary concentrations were above the LLOQ (1.5 ng/mL) for 333 samples.
PK and PD analysis
Pooled exposure‐effect analyses were performed using Prism v7.00 (GraphPad Software, La Jolla, CA). Population PK/PD modeling and fitting of data were independently performed for ivermectin and piperaquine concentrations using Pmetrics v1.5.0 (LAPKB, University of Southern California, Los Angeles, CA).38 All PK and PK/PD modeling done with Pmetrics utilized the nonparametric adaptive grid for finding the nonparametric maximum likelihood estimate of the population distribution. Samples that were BLOQ were indicated in the Pmetrics data file as “‐99” and the LLOQ was incorporated into the overall standard population error model. A proportional scaling factor was used to fit venous and capillary concentrations simultaneously for each drug model (Eqs. S1–S13 in Text S2>).
Residual analysis was performed for all predicted PK and PD data in Pmetrics. This included goodness‐of‐fit comparisons of individual and population predictions against observed data, as well as weighted residuals over time and concentration, to investigate any biases toward higher concentrations or later timepoints. The VPCs were performed using the Pmetrics simulator tool, in which 1,000 subjects were simulated based on the generated median PK parameters, their variances and correlation matrices as generated in Pmetrics for each drug. Additionally, weight variation among patients and associated dosing brackets were included in the simulation. The weight variation was set to match that of the study population and a median of 60 kg patient weight were used for the simulation. The Pmetrics simulator predicted patients’ weights for 1,000 patients in the range of 45–101 kg, which is the range of this study. The median, 5%, and 95% percentiles with their 95% CIs were plotted against observed data for the VPC graphs. A similar simulation was done using a simultaneous PK/PD model with 1,000 subjects to predict the overall mosquitocidal activity in the study population. To avoid the complexity of adding different dosing brackets to different patient weights, simulations were performed with a standard patient weight only, which is 600 mcg/kg/day or 300 mcg/kg/day administered to 60 kg patients. The wide margins used for CL/F and V/F would account for more variability than that introduced by variability in weight.
Ivermectin PK/PD analysis
Modeling ivermectin was performed using multiple approaches to address several questions about its PK/PD relationship against mosquitoes. As it had been hypothesized that ivermectin's mosquitocidal effects at later timepoints could be due a metabolite or drug‐drug interaction, an initial sequential analysis (using three approaches, see below) was performed to justify a final simultaneous analysis. The sequential analysis, in which the PK model is blinded to PD data, assessed the consistency of ivermectin's exposure‐effect relationship over time. The simultaneous analysis presumes ivermectin concentrations alone can explain the mosquitocidal activity; this allows for the model to predict best PK and PD parameter estimates using both PK and PD data simultaneously to achieve well‐informed parameters that are derived from two sets of data (PK and PD data) rather than parameters being informed by a single set of data (i.e., PK information only, as in the first step of the sequential model).
Sequential modeling approach
For both the sequential and simultaneous approaches, PK analysis was performed using a two‐compartment model (Figure S1), which displayed a better fit than a one‐compartment model (Table S1). A three‐compartment model fit was not attempted for ivermectin as VPCs showed that ivermectin follows a two‐compartment model trend and does not display a tri‐exponential trend. The two‐compartment PK model was performed using Eqs. S1–S13 in Text S2.
PD analysis of the relationship between ivermectin concentrations and mosquitocidal effect was performed according to the following Emax sigmoidal equation39:
| (1) |
Where E represents the mosquitocidal effect of ivermectin expressed as the mortality rate of mosquitoes per 100 days, Emin is the baseline natural mortality rate, Emax is the maximal possible effect relative to Emin, EC50 is the concentration necessary to achieve a rate halfway between baseline and maximal effect (Emax + Emin), and C is ivermectin concentration in grams/liter.
The sequential PD analysis was performed using three approaches. The first was fitting an Emax function through observed mosquito mortality rates and pooled observed concentrations in Prism v7. The second followed the same methodology using predicted concentrations in order to assess whether (BLOQ) concentrations predicted by the Pmetrics PK model (blinded to PD data) showed a similar exposure‐effect relationship to observed concentrations. The third approach used Pmetrics for the population modeling of both PK and PD separately, in which each patient in the PD analysis had fixed PK parameters that matched their individual predicted parameters in the PK run. This allowed predicting population variability on PD parameters using the sequential PK/PD model (which cannot be assessed using pooled data in the previous two approaches).
The relationship between ivermectin exposure and its mosquitocidal effect was investigated separately for each day of the study (days: 2 + 4 hours, 7, 10, 14, 21, and 28), using predicted PK parameters (second sequential model), as any time‐dependent variation could potentially suggest the presence of an unidentified mosquitocidal metabolite.
Simultaneous modeling approach
The simultaneous approach used the same equations above, however, all available PD data (ivermectin mosquito mortality rates) were entered into the initial modeling process and both the PK and the PD data for all subjects were modeled simultaneously, allowing for PD data to enhance the PK fit, and vice versa, based on the assumption that ivermectin in its parent form is solely responsible for any increase in the mosquito mortality rate throughout the study duration.
Piperaquine PK
Piperaquine PK was analyzed using a three‐compartment model (Figure S1) according to Eqs. S1–S13 in Text S2. This was compared to one‐compartment and two‐compartment models, which resulted in poorer fits as evidenced from −2 log‐likelihood and Akaike information criterion and Bayesian information criteria (Table S1).
Piperaquine PD
Piperaquine effect upon QTcF‐interval was analyzed using two methods: (i) a linear model, according to Eq. (2) below, which is commonly used in similar published analyses, and (ii) an Emax sigmoidal model, utilizing previously discussed Eq. (1), with the effect (E) being QTcF‐interval in milliseconds at any given concentration, and C being piperaquine concentration. The data was either analyzed using pooled observed piperaquine concentration vs. QTcF‐interval (linear model) or simultaneously fitted using a population method with Pmetrics, as described for the simultaneous ivermectin PK/PD model (Emax model).
| (2) |
The slope represents the relationship between piperaquine concentration in ng/mL and the change in QTcF (∆QTcF) in milliseconds.
Secondary PK parameters post hoc statistical analysis
AUC0–28 days is defined as the predicted area under the curve for 3 doses of ivermectin or piperaquine over a period of 28 days and was determined using the AUC trapezoidal approximation algorithm in Pmetrics for each subject individually. The Cmax represents the maximal predicted concentration for each subject in the study. The Tmax is the time at which Cmax is achieved for each individual and t1/2 is calculated from the predicted PK slope for each individual between 27 and 29 days after treatment.
The primary PK parameters for ivermectin (k a, Q 1/F, V P1/F, CL/F, and V/F) and primary PK parameters for piperaquine (k a, Q 1/F, V P1/F, Q 2/F, V P2/F, CL/F, and V/F) and secondary PK parameters for both drugs (Cmax and AUC0–28 days) for each subject in the study were correlated to each other as well as with age, sex, weight, height, QTcF interval (measured at days 0, 2, 2 + 4 hours, and 28 after dosing initiation) or mosquito mortality rate (at each study timepoint). Correlation analysis was done using SPSS v.24 (IBM, Armonk, NY) using Spearman bivariate correlation to identify any possible effects that require further analysis. This included any effect of ivermectin exposure upon piperaquine, exploring any possible interaction between study arm (i.e., ivermectin dose 0, 300, or 600 mcg/kg/day), interaction between all structural parameters (e.g., CL, V, Q 1/F, V P1/F, and k a), and interaction between observed and predicted Cmax, and AUC for both drugs. Correlations were considered significant if ρ > 0.3 and P < 0.05.
Funding
This study is funded by the Malaria Eradication Scientific Alliance (MESA), through a sub‐grant from the Bill and Melinda Gates Foundation (BMGF). Neither MESA nor the BMGF had any role in the design of the study, the collection, analysis, and interpretation of data, or in the writing the manuscript. Co‐funding was provided by the US Centers for Disease Control and Prevention, through a Cooperative Agreement between the CDC and LSTM.
Conflicts of Interest
The authors declared no competing interests for this work.
Author Contributions
M.R.S., E.O.O., Da.W., T.K.K., B.O.A., T.B., N.M.B., J.E.G., A.M.S., M.R.D., P.A.P.H., S.K.K., Du.W., F.O.t.K., S.A.W., and G.A. wrote the manuscript. M.R.S., G.A., E.O.O., S.A.W., and F.O.t.K. designed the research. M.R.S., E.O.O., T.K.K., B.O.A., and Da.W. performed the research. M.R.S. and G.A. analyzed the data. Da.W. and S.A.W. contributed new reagents/analytical tools.
Supporting information
Figure S1. Flowchart of pharmacokinetic model applied separately to both ivermectin and piperaquine.
Figure S2. Capillary vs. venous concentration ratios for ivermectin and piperaquine.
Figure S3. Ivermectin vs. mosquito mortality (%) dose‐response curves (LC50 method).
Figure S4. Residual analysis of mosquito mortality rates (i.e., ivermectin’s pharmacodynamic effect) over time, as predicted by the simultaneous PK/PD model vs. observed data.
Figure S5. Piperaquine vs. QTcF dose‐response curves.
Table S1. Compartmental model fits for ivermectin and piperaquine.
Table S2. Number of observed concentrations and outcomes (ivermectin and piperaquine).
Table S3. Ivermectin LC50 by assay duration, and vs. previous studies.
Table S4. Piperaquine concentration and QTcF interval (observed data).
Table S5. Piperaquine concentration and QTcF interval (population fitted data).
Table S6. Correlations.
Text S1. Drug analytical quantification of ivermectin and piperaquine.
Text S2. Equations.
Text S3. IVERMAL dose‐response calculator.
Acknowledgments
Many thanks to all those who made this study possible, including the study participants and staff, the members of the Trial Steering Committee (Teun Bousema, Kevin Kobylinski, and Brian Foy), and of the Data Monitoring & Ethics Committee (Alejandro Krolewiecki, James Oloo, Timothy Collier, and Carlos Chaccour). Further thanks to the Mundo Sano Foundation and Elea Laboratories for donating the ivermectin, and NeurOptics for donating pupillometers. Finally, we would also like to thank the Jaramogi Oginga Odinga Teaching and Referral Hospital (JOOTRH) and the Kisumu County Ministry of Health (MoH), for hosting the study, Donald Tjia, Ophthalmologist, for his support, and Jacco Veldhuyzen, Emergency Medicine Physician, for assisting in interpreting electrocardiograms. This article is published with the permission of KEMRI Director. The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and Prevention.
References
- 1. Eisele, T.P. et al Short‐term impact of mass drug administration with dihydroartemisinin plus piperaquine on malaria in Southern province Zambia: a cluster‐randomized controlled trial. J. Infect. Dis. 214, 1831–1839 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mwesigwa, J. et al Mass drug administration and reactive case detection for malaria elimination. ASTMH 2017 session. Am. J. Trop. Med. Hyg. 97(suppl. 5), 411–413 (2017). [Google Scholar]
- 3. von Seidlein, L. et al Targeted malaria elimination in the greater Mekong subregion using mass drug administration. ECTMIH 2017 Session. Trop. Med. Int. Health 22, 394–396 (2017). [Google Scholar]
- 4. Chaccour, C.J. et al Ivermectin to reduce malaria transmission: a research agenda for a promising new tool for elimination. Malar. J. 12, 153 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Smit, M.R. et al Efficacy and safety of high‐dose ivermectin for reducing malaria transmission (IVERMAL): protocol for a double‐blind, randomized, placebo‐controlled, dose‐finding trial in Western Kenya. JMIR Res. Protoc. 5, e213 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gardon, J. , Boussinesq, M. , Kamgno, J. , Gardon‐Wendel, N. , Demanga, N. & Duke, B.O. Effects of standard and high doses of ivermectin on adult worms of Onchocerca volvulus: a randomised controlled trial. Lancet 360, 203–210 (2002). [DOI] [PubMed] [Google Scholar]
- 7. Guzzo, C.A. et al Safety, tolerability, and pharmacokinetics of escalating high doses of ivermectin in healthy adult subjects. J. Clin. Pharmacol. 42, 1122–1133 (2002). [DOI] [PubMed] [Google Scholar]
- 8. Smit, M.R. et al Safety and mosquitocidal efficacy of high‐dose ivermectin when co‐administered with dihydroartemisinin‐piperaquine in Kenyan adults with uncomplicated malaria (IVERMAL): a randomised, double‐blind, placebo‐controlled trial. Lancet Infect. Dis. 18, 615–626 (2018). [DOI] [PubMed] [Google Scholar]
- 9. Gonzalez Canga, A. , Sahagun Prieto, A.M. , Diez Liebana, M.J. , Fernandez Martinez, N. , Sierra Vega, M. & Garcia Vieitez, J.J. The pharmacokinetics and interactions of ivermectin in humans—a mini‐review. AAPS J. 10, 42–46 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Amsden, G.W. , Gregory, T.B. , Michalak, C.A. , Glue, P. & Knirsch, C.A. Pharmacokinetics of azithromycin and the combination of ivermectin and albendazole when administered alone and concurrently in healthy volunteers. Am. J. Trop. Med. Hyg. 76, 1153–1157 (2007). [PubMed] [Google Scholar]
- 11. El‐Tahtawy, A. , Glue, P. , Andrews, E.N. , Mardekian, J. , Amsden, G.W. & Knirsch, C.A. The effect of azithromycin on ivermectin pharmacokinetics—a population pharmacokinetic model analysis. PLoS Negl. Trop. Dis. 2, e236 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Prichard, R. , Menez, C. & Lespine, A. Moxidectin and the avermectins: consanguinity but not identity. Int. J. Parasitol. Drugs Drug Resist. 2, 134–153 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Miyajima, A. et al Effect of high‐fat meal intake on the pharmacokinetic profile of ivermectin in Japanese patients with scabies. J. Dermatol. 43, 1030–1036 (2016). [DOI] [PubMed] [Google Scholar]
- 14. Munoz, J. et al Safety and pharmacokinetic profile of fixed‐dose ivermectin with an innovative 18 mg tablet in healthy adult volunteers. PLoS Negl. Trop. Dis. 12, e0006020 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ouedraogo, A.L. et al Efficacy and safety of the mosquitocidal drug ivermectin to prevent malaria transmission after treatment: a double‐blind, randomized, clinical trial. Clin. Infect. Dis. 60, 357–365 (2015). [DOI] [PubMed] [Google Scholar]
- 16. Kobylinski, K.C. et al The effect of oral anthelmintics on the survivorship and re‐feeding frequency of anthropophilic mosquito disease vectors. Acta Trop. 116, 119–126 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kobylinski, K.C. , Foy, B.D. & Richardson, J.H. Ivermectin inhibits the sporogony of Plasmodium falciparum in Anopheles gambiae . Malar. J. 11, 381 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fritz, M.L. , Siegert, P.Y. , Walker, E.D. , Bayoh, M.N. , Vulule, J.R. & Miller, J.R. Toxicity of bloodmeals from ivermectin‐treated cattle to Anopheles gambiae s.l. Ann. Trop. Med. Parasitol. 103, 539–547 (2009). [DOI] [PubMed] [Google Scholar]
- 19. Burrows, J.N. et al New developments in anti‐malarial target candidate and product profiles. Malar. J. 16, 1–29 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hernan, M.A. . The hazards of hazard ratios. Epidemiology (Cambridge, MA). 21, 13–15 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hoglund, R.M. et al Population pharmacokinetic properties of piperaquine in falciparum malaria: an individual participant data meta‐analysis. PLoS Med. 14, e1002212 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ashley, E.A. et al Comparison of plasma, venous and capillary blood levels of piperaquine in patients with uncomplicated falciparum malaria. Eur. J. Clin. Pharmacol. 66, 705–712 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tarning, J. et al Population pharmacokinetics and pharmacodynamics of piperaquine in children with uncomplicated falciparum malaria. Clin. Pharmacol. Ther. 91, 497–505 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zongo, I. et al Efficacy and day 7 plasma piperaquine concentrations in African children treated for uncomplicated malaria with dihydroartemisinin‐piperaquine. PLoS One 9, e103200 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tarning, J. et al Population pharmacokinetics and antimalarial pharmacodynamics of piperaquine in patients with plasmodium vivax malaria in Thailand. CPT Pharmacometrics Syst. Pharmacol. 3, e132 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vanachayangkul, P. et al Piperaquine population pharmacokinetics and cardiac safety in Cambodia. Antimicrob. Agents Chemother. 61, pii: e02000‐16 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chotsiri, P. et al Population pharmacokinetics and electrocardiographic effects of dihydroartemisinin‐piperaquine in healthy volunteers. Br. J. Clin. Pharmacol. 83, 2752–2766 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Darpo, B. et al Evaluation of the QT effect of a combination of piperaquine and a novel anti‐malarial drug candidate OZ439, for the treatment of uncomplicated malaria. Br. J. Clin. Pharmacol. 80, 706–715 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wattanakul, T. et al Population pharmacokinetics and cardiovascular safety of piperaquine in African patients with uncomplicated malaria. Annual Meeting of the Population Approach Group in Europe 2017. <http://www.page-meeting.org/?abstract=7242>.
- 30. Edwards, G. , Dingsdale, A. , Helsby, N. , Orme, M.L. & Breckenridge, A.M. The relative systemic availability of ivermectin after administration as capsule, tablet, and oral solution. Eur. J. Clin. Pharmacol. 35, 681–684 (1988). [DOI] [PubMed] [Google Scholar]
- 31. Krishna, D.R. & Klotz, U. Determination of ivermectin in human plasma by high‐performance liquid chromatography. Arzneimittelforschung 43, 609–611 (1993). [PubMed] [Google Scholar]
- 32. Kobylinski, K. et al Pharmacokinetic and pharmacodynamic properties of ivermectin: ivermectin for malaria in Southeast Asia (IMSEA), Thailand. American Society of Tropical Medicine and Hygiene Annual Meeting; Baltimore, MD (2017).
- 33. Chaccour, C.J. et al Establishment of the Ivermectin Research for Malaria Elimination Network: updating the research agenda. Malar. J. 14, 243 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Upreti, V.V. & Wahlstrom, J.L. Meta‐analysis of hepatic cytochrome P450 ontogeny to underwrite the prediction of pediatric pharmacokinetics using physiologically based pharmacokinetic modeling. J. Clin. Pharmacol. 56, 266–283 (2016). [DOI] [PubMed] [Google Scholar]
- 35. Brown, K.R. Changes in the use profile of Mectizan: 1987‐1997. Ann. Trop. Med. Parasitol. 92(Suppl 1), S61–S64 (1998). [DOI] [PubMed] [Google Scholar]
- 36. Bellinger, A.M. et al Oral, ultra‐long‐lasting drug delivery: application toward malaria elimination goals. Sci. Transl. Med. 8, 365ra157 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Butters, M.P. et al Comparative evaluation of systemic drugs for their effects against Anopheles gambiae . Acta Trop. 121, 34–43 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Neely, M.N. , van Guilder, M.G. , Yamada, W.M. , Schumitzky, A. & Jelliffe, R.W. Accurate detection of outliers and subpopulations with Pmetrics, a nonparametric and parametric pharmacometric modeling and simulation package for R. Ther. Drug Monit. 34, 467–476 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Holford, N.H. & Sheiner, L.B. Understanding the dose‐effect relationship: clinical application of pharmacokinetic‐pharmacodynamic models. Clin. Pharmacokinet. 6, 429–453 (1981). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Flowchart of pharmacokinetic model applied separately to both ivermectin and piperaquine.
Figure S2. Capillary vs. venous concentration ratios for ivermectin and piperaquine.
Figure S3. Ivermectin vs. mosquito mortality (%) dose‐response curves (LC50 method).
Figure S4. Residual analysis of mosquito mortality rates (i.e., ivermectin’s pharmacodynamic effect) over time, as predicted by the simultaneous PK/PD model vs. observed data.
Figure S5. Piperaquine vs. QTcF dose‐response curves.
Table S1. Compartmental model fits for ivermectin and piperaquine.
Table S2. Number of observed concentrations and outcomes (ivermectin and piperaquine).
Table S3. Ivermectin LC50 by assay duration, and vs. previous studies.
Table S4. Piperaquine concentration and QTcF interval (observed data).
Table S5. Piperaquine concentration and QTcF interval (population fitted data).
Table S6. Correlations.
Text S1. Drug analytical quantification of ivermectin and piperaquine.
Text S2. Equations.
Text S3. IVERMAL dose‐response calculator.


