Abstract
Objectives
To assess the safety and effectiveness of the Stellarex™ drug‐coated angioplasty balloon (DCB) to inhibit restenosis in the superficial femoral and/or popliteal artery.
Background
Treatment of peripheral arterial disease is challenged by restenosis, requiring revascularization procedures to maintain patency. DCBs are designed to deliver an anti‐proliferative drug to the vessel wall to diminish smooth muscle cell proliferation and maintain patency.
Methods
This prospective, single‐arm, multicenter study enrolled 50 patients with 58 lesions in the first cohort that required pre‐dilatation with an uncoated angioplasty balloon prior to inflation of the DCB. The primary effectiveness endpoint was 6‐month late lumen loss (LLL). The major secondary endpoint was major adverse event (MAE) rate at 6 months, defined as cardiovascular death, amputation, and/or ischemia‐driven target lesion revascularization.
Results
The mean lesion length was 7.2 cm and baseline stenosis was 75.1%. Calcification was present in 62.1% of lesions and 12.1% were occluded. Both endpoints met their prespecified performance goals; at 6 months, the MAE rate was 4% and the mean LLL was 0.54 mm. The primary patency rate was 89.5% at 12 months and 80.3% at 24 months. The freedom from clinically‐driven target lesion revascularization rate, per Kaplan‐Meier estimate, was 90.0% at 12 months and 85.8% at 24 months. Additionally, there were no amputations or cardiovascular deaths reported through 24 months.
Conclusions
The Stellarex DCB provides safe and durable clinical outcomes for treatment of femoropopliteal artery disease through 24 months. © 2015 Wiley Periodicals, Inc.
Keywords: peripheral arterial disease, claudication, paclitaxel‐coated balloon
INTRODUCTION
Peripheral artery disease affects an estimated 27 million adults in Europe and North America and is associated with significant morbidity and mortality 1. Total disease prevalence has been estimated at between 3% and 10%, increasing to 15 to 20% in subjects over 80 years old 2.
The superficial femoral artery (SFA) is the longest artery in the human body and travels a tortuous course that is exposed to compression, extension, bending, and torsion. The SFA has consistently proven to be a challenging environment in which to maintain patency following revascularization. A meta‐analysis of percutaneous transluminal angioplasty (PTA) to treat SFA lesions up to 15 cm in length reported a 12‐month primary patency rate of only 33% 3. Treatment with bare metal self‐expanding stents yields higher but variable one‐year patency rates that range between 52% 4 and 81% 5. Stents coated with paclitaxel, an antiproliferative drug, present a promising advancement 6, 7. However, intrinsic limitations of all stents include implantation of a foreign body, risk of fractures, which are associated with higher restenosis rates 8 and future treatment challenges related to in‐stent restenosis. Coating an angioplasty balloon with paclitaxel is an appealing concept because it facilitates delivery of paclitaxel without leaving a stent behind. Presented here are the first‐in‐human (FIH) data with the next generation Stellarex™ drug‐coated angioplasty balloon (DCB; The Spectranetics Corp., Colorado Springs, CO), with a lower dose density of 2 µg/mm2 surface concentration of paclitaxel.
MATERIALS AND METHODS
The ILLUMENATE FIH study was a prospective, single‐arm, multi‐center study. The purpose of the study was to assess the safety and effectiveness of the Stellarex™ DCB to inhibit restenosis in the SFA and/or popliteal arteries. The study was comprised of sequentially enrolled cohorts. Lesions in the first 50 patients were treated with traditional predilatation with an uncoated angioplasty balloon prior to inflation of the DCB. The subsequent 30 patients were enrolled into the direct DCB (e.g., no predilation) cohort; data from this cohort are still being collected.
The study was conducted in accordance with Good Clinical Practices and the Declaration of Helsinki. Prior to enrollment, each site's Ethics Committee reviewed and approved the study protocol and a signed informed consent was required for all patients. The study included independent oversight of adverse events, as well as, independent evaluation of study outcomes. A Clinical Events Committee (CEC) composed of independent physicians adjudicated adverse events. Core laboratories analyzed angiographic images (SynvaCor, Springfield, IL) and duplex ultrasound images (VasCore, Massachusetts General Hospital, Boston, MA).
Eligible patients had symptomatic peripheral arterial disease [Rutherford Clinical Classification (RCC) 2, 3, or 4] with de novo or restenotic lesions located in the SFA and/or popliteal artery. Lesions were located at least 1 cm distal to the femoral bifurcation and at least 3 cm proximal to the intercondylar fossa. Lesions were 3‐15cm in length and were at least 70% stenosed. Patients with inadequate distal outflow, significant inflow disease (≥ 50% stenosis), and/or a thrombotic target vessel were excluded. Treatment of multiple lesions meeting the eligibility criteria was allowed. Use of adjunctive therapy was not permitted. As such, lesions with calcification that precluded adequate PTA treatment were excluded. All eligibility criteria are listed in Table 1.
Table 1.
Inclusion and Exclusion Criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
| • Subjects with symptomatic leg ischemia, requiring treatment of SFA or popliteal (P1 segment) artery | • Pregnant or lactating females |
| • Male or nonpregnant female > 18 years of age | • Coexisting clinically significant aneurismal disease of the abdominal aorta, iliac, or popliteal arteries |
| • De novo or restenotic lesion(s) >70% stenosed within the SFA and popliteal (P 1 segment) arteries in a single limb | • Significant gastrointestinal bleeding or any coagulopathy that would contraindicate the use of antiplatelet therapy |
| • ≥3 cm and ≤15 cm in cumulative length (by visual estimation) | • Known or intolerance to study medications, paclitaxel, or contrast agents |
| • Subject is willing to provide informed consent and comply with the required follow‐up visits, testing schedule, and medication regimen | • Doubts in the willingness or capability of the subject to allow follow‐up examinations |
| • Successful wire crossing of lesion | • Subject actively participating in another investigational device or drug study |
| • Target vessel reference diameter ≥3 and ≤7 mm (by visual estimation) | • History of hemorrhagic stroke within 3 months |
| • Target lesion(s) can be treated with a maximum of two DCBs | • Previous or planned surgical or interventional procedure within 30 days of the index procedure |
| • At least one patency (<50% stenosis) tibio‐peroneal run‐off vessel confirmed by baseline angiography or prior Magnetic resonance angiography or CT angiography | • Prior vascular surgery of the target lesion |
| • Lesion length <3 cm or >15 cm or there is no normal proximal arterial segment in which duplex ultrasound velocity ratios can be measured | |
| • Life expectancy < 1 year | • Known inadequate distal outflow |
| • Rutherford classification of 2, 3, or 4 | • Significant inflow disease |
| • Acute or subacute thrombus in target vessel | |
| • Use of adjunctive therapies (i.e., laser, atherectomy, cryoplasty, scoring/cutting balloons, and brachytherapy) | |
| • Outflow arteries (distal popliteal, anterior or posterior tibial or peroneal arteries) with significant lesions (≥50% stenosis) may not be treated during the same procedure | |
| • Presence of prohibitive calcification that precludes adequate PTA treatment | |
| • Subjects held in custody in an institution or official or court order |
Study Devices and Procedure
All subjects were treated with the Stellarex™ DCB. This DCB is a 0.035″ compatible over‐the‐wire device with a coating consisting of paclitaxel (2 µg/mm2 balloon surface) and polyethylene glycol, an excipient that facilitates drug transfer into the vessel wall.
Subjects were prescribed aspirin and clopidogrel (ticlopidine was permitted in cases of allergies to clopidogrel) per hospital standard of care (for three days prior to procedure or a loading dose on the day of the intervention). During the procedure, subjects were anticoagulated per standard hospital practice. Pre‐dilatation was performed with an uncoated balloon catheter. After predilatation, the treating physician determined the suitability of the lesion for treatment with PTA alone or the requirement for a self‐expandable stent. The DCBs were sized to ensure the full length of the lesion was treated. Up to two DCBs could be used per lesion. If two balloons were required, they were overlapped by at least 1 cm. Inflations were at least 1 minute. In cases of residual stenosis >50% or significant dissection, postdilatation was performed. Stenting with a nitinol stent was limited to provisional or bail‐out situations where the stenosis or dissection persisted after postdilatation. Geographic miss, or edge to edge misalignment between the predilated and DCB treatment areas > 5 mm, was assessed by the angiographic core laboratory.
Angiograms of the target vessel were captured at baseline, during each step of the procedure, and at 6 months postprocedure. The same projection angles and contrast medium were used for the procedure and the 6‐month angiogram. An adhesive radiopaque ruler was placed in the same position during the procedure and 6‐month visit to provide lesion location and a calibration source. All images were sent to the angiographic core laboratory for assessment.
Follow‐Up and Endpoints
Clinical evaluations at baseline, pre‐discharge, and at 1, 6, 12, and 24 months postprocedure included concomitant medications, Doppler ultrasound of the target lesion(s), ankle‐brachial index (ABI), Rutherford‐Becker classification, walking impairment questionnaire (WIQ), walking distance (either by treadmill or 6‐minute walk test) and adverse events. The 6‐month visit included an angiogram as well.
The primary endpoint was late lumen loss (LLL) at 6 months postprocedure defined as the difference between the minimum lumen diameter (MLD) after the intervention and at follow‐up as determined by the angiographic core lab. The major secondary endpoint was the rate of major adverse events (MAE) at 6 months postprocedure. This was defined as a composite rate of cardiovascular death, index limb amputation, and ischemia‐driven target lesion revascularization (TLR), as determined by the CEC.
“Ischemia‐driven” TLR was defined in the protocol as revascularization associated with ≥50% stenosis and a worsening of RCC by more than 2 categories (or to a RCC 5 or 6). “Clinically‐driven” TLR is a more frequently reported term and was defined as revascularization associated with >50% stenosis via angiogram and worsening of RCC ≥1 or ABI decrease of >0.15 from the maximum early postprocedure level, that is clearly referable to the target lesion. The CEC utilized both definitions to adjudicate each TLR.
Other secondary endpoints included clinically‐driven TLR and primary patency, defined as freedom from clinically‐driven TLR and Doppler ultrasound stenosis >50% (PSVR ≥ 2.5). Lesion success was defined as a final residual diameter stenosis <50% following treatment, including predilatation and postdilatation (if it was required), without a device malfunction. Procedure success was defined as lesion success without the occurrence of an MAE during the procedure. Change in ankle‐brachial index and walking distance were assessed. Additionally, a modified walking impairment questionnaire was administered. Walking impairment scores were determined by dividing the sum of the weighted scores [degree of difficulty on a 0 (did not do) to 3 (no difficulty) scale multiplied by a weight for distance or speed] by the maximum possible weighted score and multiplying by 100. Each score ranges from 0–100 with lower scores indicating lower performance. Items coded as “did not do” were treated as “unable to do.” Missing items were removed from the denominator of the weighted score to calculate a percent score based on the items that remained. For walking distance, the weight is based on the number of meters walked, using the estimate of 6 meters as the weight for walking about the house. For walking speed, weights were assigned based on the approximate speed of walking 90 meters (1.5 for 2.4 km/h, 2 for 3.2 km/h, 3 for 4.8 km/h, and 5 for 8 km/h).
Statistical Analysis
A sample size of 50 subjects was selected to provide statistical power of 81% to detect a 0.65 mm difference in angiographic LLL at 6 months postprocedure between the DCB group (SD = 1.0 mm) and a historical control (SD = 1.4 mm) with 1‐sided alpha of 0.05 and 10% attrition rate. Assumptions for this sample size calculation were based on results from the FemPac 9, THUNDER 10, and LEVANT I 11 trials.
Continuous data are expressed as mean ± standard deviation. Changes of clinical assessments from baseline were evaluated with the t‐test or Wilcoxon signed‐rank test. Categorical variables are expressed as counts and percentage. Kaplan‐Meier estimates are presented for patency. Six month angiographic LLL is presented with the 95% confidence interval and deemed successful if the upper bound was less than the historical control. The 6‐month MAE rate was successful if the upper limit of the exact 95% confidence interval was less than the pre‐specified objective performance criterion of 30%.
Exploratory post hoc subgroup comparisons were performed to evaluate differences by gender, smoking status, diabetes, and calcification. The 6‐month MAE rate was evaluated with Fisher's Exact Test, 6‐month angiographic LLL with the Wilcoxon Rank Sum Test, and patency with the log‐rank test. All statistical analyses were performed using Statistical Analysis System (SAS) for Windows version 9.1 or higher (SAS Institute, Cary, NC). P‐values are two‐sided, with values <0.05 deemed statistically significant.
RESULTS
Enrollment began in December, 2011 and ended in November, 2012. Eighty subjects were enrolled at three centers in Germany. Lesions in the first 50 subjects were treated by traditional means of inflating an uncoated balloon (“predilatation”) prior to inflation of the Stellarex™ DCB. The subsequent 30 subjects were enrolled as a separate group in the direct DCB (e.g., no predilatation) cohort and follow‐up is ongoing.
Fifty subjects with 58 lesions were enrolled in the pre‐dilatation cohort. Subject demographics and baseline lesion characteristics are listed in Table 2. The mean age (±SD) was 69 ± 9.3 years, 62% were male, 52% were currently smoking, 34% had diabetes; the majority had hyperlipidemia (80%) and hypertension (90%). The mean lesion length was 7.2 ± 4.7 cm and the mean baseline percent stenosis was 75.1 ± 17%.
Table 2.
Baseline Demographics
| Subject demographic | |
|---|---|
| Age (Years) | 69.0 ± 9.3 (50) |
| Female | 38.0% (19/50) |
| Medical History | |
| Current smoker | 52.0% (26/50) |
| Hypertension | 90.0% (45/50) |
| Hyperlipidemia | 80.0% (40/50) |
| Diabetes | 34.0% (17/50) |
| Angina | 10.0% (5/50) |
| Previous PCI/CABG | 34.0% (17/50) |
| Rutherford CC | |
| 2 | 12% (6/50) |
| 3 | 86% (43/50) |
| 4 | 2% (1/50) |
| Ankle‐brachial index | 0.78 ± 0.18 (50) |
| Lesion characteristics | |
| Number of lesions treated | 58 |
| Lesion location | |
| Proximal SFA | 22.4% (13/58) |
| Mid SFA | 50.0% (29/58) |
| Distal SFA | 22.4% (13/58) |
| Proximal popliteal | 5.2% (3/58) |
| Baseline lesion length (cm) | 7.2±4.7 (58) |
| Baseline percent stenosis | 75.1±17.0 (58) |
| Baseline RVD (mm) | 5.2±0.9 (58) |
| Baseline MLD (mm) | 1.3±1.0 (58) |
| Calcified | 62.1% (36/58) |
| Severe | 13.8% (8/58) |
| Total occlusion | 12.1% (7/58) |
| Eccentric lesion | 6.9% (4/58) |
| Number of run‐off vessels | |
| 0 | 1.7% (1/58) |
| 1 | 19.0% (11/58) |
| 2 | 39.7% (23/58) |
| 3 | 39.7% (23/58) |
Data Presented as Mean ± SD (N) or % (n/N).
CABG: coronary artery bypass graft; CC: clinical classification; PCI: percutaneous coronary intervention; MLD: minimum luminal diameter; RVD: reference vessel diameter; SFA: superficial femoral artery.
Procedural outcomes are provided in Table 3. All lesions were predilated. One lesion (1.7%) required a stent following predilatation due to dissection. Two (3.4%) additional lesions were stented following DCB treatment due to residual stenosis > 50%. The mean diameter stenosis was 41.9% following predilatation. The mean inflation pressure was 11.6 atm. Geographic miss occurred in three (5.6%) lesions as assessed by the angiographic core laboratory. Lesion and procedural success were achieved in 100% of lesions and subjects.
Table 3.
Procedural Outcomes
| Outcome | |
|---|---|
| Predilatation | 100.0% (58/58) |
| Predilatation inflation pressure (atm) | 7.9 ± 1.2 |
| Postpredilatation stent | 1.7% (1/58) |
| Balloon‐artery ratio | 1.1 ± 0.1 (53) |
| DCB inflation pressure (atm) | 11.6 ± 2.0 |
| Geographic miss | 5.6% (3/54) |
| Additional treatment | 13.8% (8/58) |
| Postdilatation | 12.1% (7/58) |
| Post‐DCB Stent | 3.4% (2/58) |
| Postprocedure percent stenosis | |
| Mean ± SD (N) | 19.1 ± 9.7 (58) |
| Median (IQR) | 19.5 (12 − 26) |
| Postprocedure MLD (mm) | 4.3 ± 0.7 (58) |
| Technical successa (per lesion) | 94.8% (55/58) |
| Lesion successb (per lesion) | 100.0% (58/58) |
| Procedural successc (Per Subject) | 100.0% (50/50) |
Data Presented as Mean ± SD (N), or % (n/N).
DCB: drug‐coated balloon; MLD: minimum luminal diameter.
Defined as <50% diameter stenosis by angiographic core laboratory, following postdilatation if required (stent implantation was considered a failure).
Defined as <50% diameter stenosis by angiographic core laboratory, postprocedure.
Defined as Lesion Success without the occurrence of an MAE during the procedure.
Periprocedural complications as observed by the angiographic core lab and adjudicated by the CEC included two distal embolizations and one arterio‐venous‐fistula. There were two Grade C dissections, one observed following predilatation and the other following the DCB inflation. There were no Grade D‐F dissections.
Angiograms at 6 months were available for 47 (94%) subjects, and 42 subjects were assessed via duplex ultrasound at 24 months. Subject follow‐up compliance and attrition are illustrated in Fig. 1.
Figure 1.
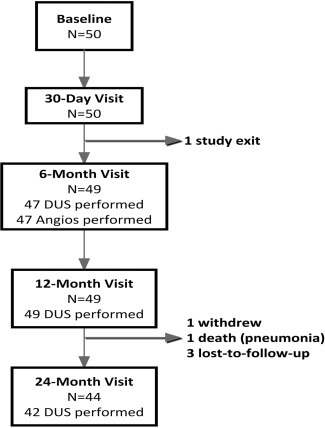
Follow‐up Compliance Chart. All study subjects except one were available for the 12‐month follow‐up visit.
Quantitative angiographic analysis of the primary endpoint (LLL) was 0.54 mm (95%CI: 0.28‐0.81), statistically significantly below the historical control (1.1 mm); therefore, the primary endpoint was met. The major secondary endpoint was the 6‐month MAE rate. Using the protocol‐definition of “ischemia‐driven”, the 6‐month MAE rate was 0% as no subject with a TLR experienced a shift of more than 2 RCC grades. When the more traditional definition of “clinically‐driven” TLR was applied, the observed rate was 4% (95% CI: 0.5–13.7%). The lower bound of the confidence interval (13.7%) was below the pre‐specified objective performance criterion of 30%; therefore, the endpoint was met.
Long‐term outcomes are provided in Table 4. The freedom from clinically‐driven target lesion revascularization rate at 12 and 24 months, as estimated by Kaplan‐Meier survival estimate, was 90.0 and 85.8%, respectively (Fig. 2). The primary patency rate at 12 and 24 months, as estimated by Kaplan‐Meier survival estimates was 89.5 and 80.3%, respectively (Fig. 3). Subgroup analyses are reported in Table 5. When stratified by gender, the 24‐month patency rate in females (n = 19) was 90 vs. 74.5% in males (n = 31, P = 0.273). The patency rates observed in subjects with diabetes (n = 17) was 80.4% at 12 months and 63.3% at 24 months. These rates trended lower (P = 0.056) than the observed rates in subjects without diabetes (n = 33), which were 94.6% at 12 months and 88.9% at 24 months. There were 7 occluded lesions included. The mean length in this subgroup was 11.5 ± 5.8 cm. All occlusions were patent through 24 months.
Table 4.
Long‐Term Outcomes
| Outcome | 12 months | 24 months |
|---|---|---|
| Cumulative MAEs | 12% (6/50) | 14.9% (7/47) |
| Clinically‐driven TLR | 12% (6/50) | 14.9% (7/47) |
| Index limb amputation | 0% (0/49) | 0.0% (0/44) |
| Cardiovascular death | 0% (0/49) | 0.0% (0/44) |
| Freedom from clinically driven TLR | 90.0% | 85.8% |
| Primary patency | 89.5% | 80.3% |
| Change in ABI compared with baselinea | 0.21±0.18b (36) | 0.22±0.13b (32) |
Data presented as % (n/N), survival percentage from KM analysis or Mean ± SD (N). ABI: ankle‐brachial index; TLR: target lesion revascularization.
Includes patients with baseline ABI < 0.9 only.
P‐value < 0.001 compared with baseline, paired t‐test.
Figure 2.
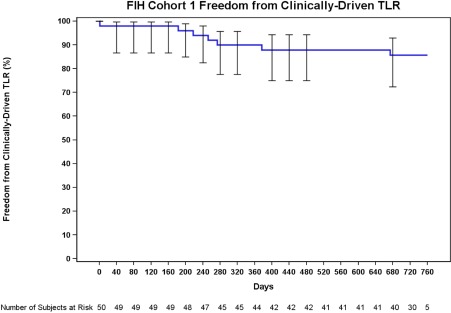
The freedom from clinically‐driven TLR was 90.0% at 12 months (day 365) and 87.9% at day 395, the upper end of the follow‐up window. The rate was 85.8% throughout the 24‐month follow‐up window (at day 730 and day 760). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Figure 3.
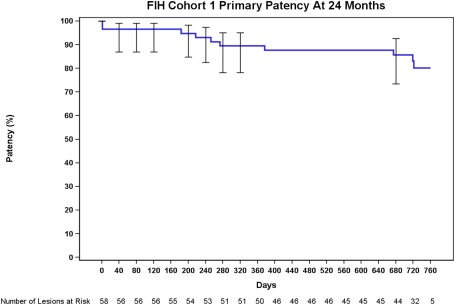
The primary patency rate was 89.5% at 12 months (day 365) and 87.7% at day 395, the upper end of the follow‐up window. The rate was 80.3% throughout the 24‐month follow‐up window (at day 730 and day 760). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Table 5.
Subgroup Analyses
| Subgroup | Diabetic status | Calcification | Gender | Smoking status | ||||
|---|---|---|---|---|---|---|---|---|
| Diabetic | Not diabetic | Calcification | No Calcification | Female | Male | Current smoker | Not a current smoker | |
| Sample Size (subjects/lesions) | 17/21 | 33/37 | 31/36 | 20/22 | 19/20 | 31/38 | 26/28 | 24/30 |
| Mean Lesion Length | 7.0 ± 4.8 | 7.3 ± 4.7 | 8.0 ± 4.7 | 6.0 ± 4.4 | 7.8 ± 5.1 | 6.9 ± 4.5 | 7.0 ± 4.7 | 7.4 ± 4.7 |
| Calcification | 61.9% (13/21) | 62.2% (23/37) | 100% (36/36) | 0% | 50% (10/20) | 68.4% (26/38) | 64.3% (18/28) | 60.0% (18/30) |
| Severe Calcification | 9.5% (2/21) | 16.2% (6/37) | 22.2% (8/36) | 0% | 15% (3/20) | 13.2% (5/38) | 7.1% (2/28) | 20% (6/30) |
| Total occlusions | 4.8% (1/21) | 16.2% (6/37) | 16.7% (6/36) | 4.5% (1/22) | 10% (2/20) | 13.2% (5/38) | 17.9% (5/28) | 6.7% (2/30) |
| 12‐Month patencya | 80.4% | 94.6% | 86.4% | 91.4% | 90% | 89.3% | 92.9% | 86.4% |
| 24‐Month patencya | 63.3% | 88.9% | 74.5% | 83.6% | 90% | 74.5% | 84.8% | 76.5% |
| P‐valueb | 0.056 | 0.393 | 0.273 | 0.588 | ||||
Per Kaplan‐Meier Estimate.
Log‐Rank Test.
Data from a modified version of the walking impairment questionnaire are summarized in Fig. 4. Treadmill tests were conducted in a sub‐set of subjects (n = 34) where treadmills were available. The mean baseline walking distance was 114±125 meters. The mean walking distance was significantly (P ≤ 0.01) increased at 6, 12, and 24 month visits with distances of 185 ± 182 m, 139 ± 95 m, and 190 ± 87 m, respectively.
Figure 4.
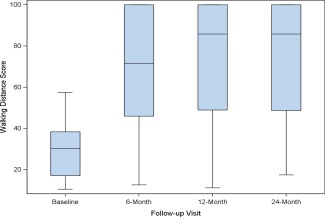
Walking Impairment Questionnaire Outcomes, Box plot diagrams demonstrate WIQ‐derived walking distance (Panel A) and walking speed scores (Panel B) at baseline and follow‐up visits. The bold line denotes the median value, and the box denotes the upper and lower quartiles. There was a significant improvement from baseline at each follow‐up visit for both assessments. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Improvement in ABI was assessed in subjects with a baseline ABI < 0.9 (n = 38). The mean baseline ABI in these subjects was 0.71 ± 0.13. At 6 months, the mean increased significantly (P < 0.001) to 0.91 ± 0.16 and remained increased through 24 months.
DISCUSSION
The results presented here demonstrate, for the first time, the effectiveness of the Stellarex DCB for treatment of atherosclerotic lesions in the SFA and popliteal arteries. With a paclitaxel surface concentration of 2 μg/mm2, this balloon has a lower dose density than the first generation balloons with a surface concentration of 3.5 μg/mm2 that were evaluated in PACIFIER and the Italian Registry 12, 13. These initial results with Stellarex show that the lower dose density of paclitaxel is clinically effective. While the data presented here are based solely on the results of 50 subjects, a high rate of follow‐up compliance was achieved, demonstrating thorough data collection. After 6 months, angiography was performed in 47/50 subjects and a Duplex examination was performed in 47/50 subjects. Sonographic follow‐up was performed after 12 months in 49/50 subjects and after 24 months in 42/50 subjects. Clinical follow‐up was possible after 6 and 12 months in 49/50 subjects and after 24 months in 44/50 subjects.
Lesion length and occlusions are predictors of restenosis and must be considered when evaluating outcomes 14. The mean length of treated lesions in this study was 7.2 cm and was roughly comparable to the lesion lengths in THUNDER 10 (7.4 cm), LEVANT I 11 (8.1 cm) and PACIFIER 9 (7.0 cm), with a somewhat lower rate of complete occlusions (12.1% versus 27%/41%/22.7%, respectively). The primary patency rate observed in this study was 89.5% after 12 months and 80.3% after 24 months, which compares favorably to the 24‐month patency rates currently published which include LEVANT I 11 (57%) and the Italian Registry 13 (72.4%; Fig. 5). Stents were implanted in 3/58 lesions (5.2%), so only a small influence of stent placement on the evaluated data is to be expected, in comparison to the stent rate of 27% in LEVANT I 11. It has been our experience that stenting dissections that are not flow‐limiting is not necessary when treating with DCBs. In many instances, positive remodeling in these cases is noticeable in subsequent angiograms as shown in Fig. 6. This observation is in alignment with the sub‐analysis of the dissected lesions in the THUNDER study, which suggests stents are not necessary in dissections following DCB treatment that do not limit flow 15.
Figure 5.
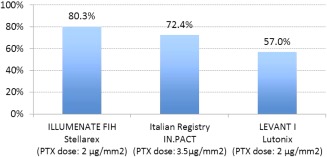
24‐Month Primary Patency Rate, 24‐month primary patency rates reported in FIH studies for various drug‐coated balloons. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Figure 6.
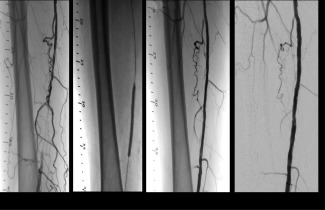
Procedure and Follow‐ up Angiograms, Case example of a subject; Panel A is the initial angiogram showing an occlusion in the SFA. Panel B shows the inflated 5 × 80 mm DCB. Panel C shows the post‐DCB results where a residual stenosis of 30% and a small nonflow limiting dissection was obtained. Panel D shows the patent 6‐month follow‐up angiogram without restenosis and with visible positive remodeling.
The primary endpoint of 6‐month angiographic LLL was documented at 0.54 mm. This is comparable to previously reported data (0.4 mm in the THUNDER Trial 10, 0.46 mm in LEVANT I 11, and −0.05 mm in PACIFIER 9). In 15 lesions, positive remodeling, or a negative LLL, was observed at 6 months. Note there was no revascularization in these lesions during this time period, which would have impacted the LLL assessment. The mean lumen gain observed in these lesions was 0.3 ± 0.2 mm. The mean lesion length in this subgroup was 7.3 ± 3.8 cm; two were severely calcified and two were total occlusions.
Freedom from TLR rates in this study were 90.0% and 85.8% after 12 and 24 months, respectively, which are comparable with the results of the Italian Registry 13 (92%/85%) and the PACIFIER Trial 9 (93/85%) and are meaningfully higher than the results from the LEVANT I Trial 11 (71%/64%). Lower TLR rates have important positive implications on the health economics of DCBs and cost of treatment over time 16.
Due to the excellent long‐term data, the DCB formulation used in this study seems particularly useful in patients with long lesions, and rapidly progressive atherosclerotic changes in the femoral arteries. Those two conditions have shown a high rate of disease recurrence. In addition, the analysis of subgroups did not reveal significantly different results with regard to the gender of the subjects, smoking status, or the presence of calcification. The impact of calcification on DCB effectiveness has been reported elsewhere 17. The lack of a significant difference observed in this study was likely because lesions with calcification that precluded adequate PTA treatment were excluded.
Limitations of the study include the lack of randomization and small proportion of CTOs (12.1%). However, all occluded lesions were patent through 24 months, which suggests adequate effectiveness of this DCB in CTOs. Further assessment in complex disease is warranted. The study also lacked objective criteria for the exclusion criterion “prohibitive” calcification, which may have left room for investigator bias. Larger studies with robust designs are currently enrolling patients, including two randomized controlled trials and one global registry.
CONCLUSION
The Stellarex DCB is a next‐generation balloon with an effective mechanism of action and excellent 2‐year data with improved patency rates compared to historical data and outcomes comparable with the best published DCB studies. Treatment of atherosclerotic lesions with the Stellarex DCB is a safe and effective option that offers an important economic benefit due to the high patency rate and low TLR rate.
ACKNOWLEDGMENTS
The authors would like to thank the following: Michael R. Jaff, DO and Krishna Rocha‐Singh, MD for core laboratory oversight and study design, Philippe Marco, MD from CV Ingenuity for study design; Sarah Verdoliva, MS from NAMSA for statistical analysis and Meghan Schadow, MS from Spectranetics for writing assistance.
Conflict of interest: Henrik Schroeder is a consultant with Spectranetics and Stephan Duda is a former holder of CV Ingenuity stock options and has a consulting agreement with Spectranetics. The other authors have no conflicts of interest to disclose.
REFERENCES
- 1. Belch JJ, Topol EJ, Agnelli G, Bertrand M, Califf RM, Clement DL, Creager MA, Easton JD, Gavin JR III, Greenland P, Hankey G, Hanrath P, Hirsch AT, Meyer J, Smith SC, Sullivan F, Weber MA. Prevention of atherothrombotic disease N. Critical issues in peripheral arterial disease detection and management: A call to action. Arch Intern Med 2003;163:884–892. [DOI] [PubMed] [Google Scholar]
- 2. Fowkes FG, Rudan D, Rudan I, Aboyans V, Denenberg JO, McDermott MM, Norman PE, Sampson UK, Williams LJ, Mensah GA, Criqui MH. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: A systematic review and analysis. Lancet 2013;382:1329–1340. [DOI] [PubMed] [Google Scholar]
- 3. Rocha‐Singh KJ, Jaff MR, Crabtree TR, Bloch DA, Ansel G, Viva PI. Performance goals and endpoint assessments for clinical trials of femoropopliteal bare nitinol stents in patients with symptomatic peripheral arterial disease. Catheter Cardiovasc Interv 2007;69:910–919. [DOI] [PubMed] [Google Scholar]
- 4. Sabeti S, Schillinger M, Amighi J, Sherif C, Mlekusch W, Ahmadi R, Minar E. Primary patency of femoropopliteal arteries treated with nitinol versus stainless steel self‐expanding stents: Propensity score‐adjusted analysis. Radiology 2004;232:516–521. [DOI] [PubMed] [Google Scholar]
- 5. Laird JR, Katzen BT, Scheinert D, Lammer J, Carpenter J, Buchbinder M, Dave R, Ansel G, Lansky A, Cristea E, Collins TJ, Goldstein J, Jaff MR. Nitinol stent implantation versus balloon angioplasty for lesions in the superficial femoral artery and proximal popliteal artery: Twelve‐month results from the resilient randomized trial. Circ Cardiovasc Interv 2010;3:267–276. [DOI] [PubMed] [Google Scholar]
- 6. Dake MD, Ansel GM, Jaff MR, Ohki T, Saxon RR, Smouse HB, Zeller T, Roubin GS, Burket MW, Khatib Y, Snyder SA, Ragheb AO, White JK, Machan LS. Zilver PTXI . Paclitaxel‐eluting stents show superiority to balloon angioplasty and bare metal stents in femoropopliteal disease: Twelve‐month zilver ptx randomized study results. Circ Cardiovasc Interv 2011;4:495–504. [DOI] [PubMed] [Google Scholar]
- 7. Dake MD, Scheinert D, Tepe G, Tessarek J, Fanelli F, Bosiers M, Ruhlmann C, Kavteladze Z, Lottes AE, Ragheb AO, Zeller T, Zilver PTXS‐ ASI . Nitinol stents with polymer‐free paclitaxel coating for lesions in the superficial femoral and popliteal arteries above the knee: Twelve‐month safety and effectiveness results from the zilver ptx single‐arm clinical study. J Endovasc Ther 2011;18:613–623. [DOI] [PubMed] [Google Scholar]
- 8. Scheinert D, Scheinert S, Sax J, Piorkowski C, Braunlich S, Ulrich M, Biamino G, Schmidt A. Prevalence and clinical impact of stent fractures after femoropopliteal stenting. J Am Coll Cardiol 2005;45:312–315. [DOI] [PubMed] [Google Scholar]
- 9. Werk M, Langner S, Reinkensmeier B, Boettcher HF, Tepe G, Dietz U, Hosten N, Hamm B, Speck U, Ricke J. Inhibition of restenosis in femoropopliteal arteries: Paclitaxel‐coated versus uncoated balloon: Femoral paclitaxel randomized pilot trial. Circulation 2008;118:1358–1365. [DOI] [PubMed] [Google Scholar]
- 10. Tepe G, Zeller T, Albrecht T, Heller S, Schwarzwalder U, Beregi JP, Claussen CD, Oldenburg A, Scheller B, Speck U. Local delivery of paclitaxel to inhibit restenosis during angioplasty of the leg. N Engl J Med 2008;358:689–699. [DOI] [PubMed] [Google Scholar]
- 11. Scheinert D, Duda S, Zeller T, Krankenberg H, Ricke J, Bosiers M, Tepe G, Naisbitt S, Rosenfield K. The LEVANT 1 (lutonix paclitaxel‐coated balloon for the prevention of femoropopliteal restenosis) trial for femoropopliteal revascularization: First‐in‐human randomized trial of low‐dose drug‐coated balloon versus uncoated balloon angioplasty. JACC Cardiovasc Interv 2014;7:10–19. [DOI] [PubMed] [Google Scholar]
- 12. Micari A, Cioppa A, Vadala G, Castriota F, Liso A, Marchese A, Grattoni C, Pantaleo P, Cremonesi A, Rubino P, Biamino G. 2‐year results of paclitaxel‐eluting balloons for femoropopliteal artery disease: Evidence from a multicenter registry. JACC Cardiovasc Interv 2013;6:282–289. [DOI] [PubMed] [Google Scholar]
- 13. Micari A, Cioppa A, Vadala G, Castriota F, Liso A, Marchese A, Grattoni C, Pantaleo P, Cremonesi A, Rubino P, Biamino G. Clinical evaluation of a paclitaxel‐eluting balloon for treatment of femoropopliteal arterial disease: 12‐month results from a multicenter italian registry. JACC Cardiovasc Interv 2012;5:331–338. [DOI] [PubMed] [Google Scholar]
- 14. Shammas NW, Shammas G, Bryan D, Rauba J, Dippel E, Jerin M. Predictors of target lesion revascularization in patients undergoing lower extremity percutaneous interventions. J Invasive Cardiol 2009;21:266–269. [PubMed] [Google Scholar]
- 15. Tepe G, Zeller T, Schnorr B, Claussen CD, Beschorner U, Brechtel K, Scheller B, Speck U. High‐grade, non‐flow‐limiting dissections do not negatively impact long‐term outcome after paclitaxel‐coated balloon angioplasty: An additional analysis from the thunder study. J Endovasc Ther 2013;20:792–800. [DOI] [PubMed] [Google Scholar]
- 16. Pietzsch JB, Geisler BP, Garner AM, Zeller T, Jaff MR. Economic analysis of endovascular interventions for femoropopliteal arterial disease: A systematic review and budget impact model for the United states and Germany. Catheter Cardiovasc Interv 2014;84:546–554. [DOI] [PubMed] [Google Scholar]
- 17. Fanelli F, Cannavale A, Gazzetti M, Lucatelli P, Wlderk A, Cirelli C, d'Adamo A, Salvatori FM. Calcium burden assessment and impact on drug‐eluting balloons in peripheral arterial disease. Cardiovasc Intervent Radiol 2014;37:898–907. [DOI] [PubMed] [Google Scholar]


