Abstract
Purpose
To investigate in vitro central and peripheral corneal endothelial cell (EC) migration from Quarter–Descemet membrane endothelial keratoplasty (Quarter‐DMEK) grafts.
Methods
Quarter‐DMEK grafts were obtained from 10 corneas ineligible for transplantation but with intact and viable ECs. Ten Quarter‐DMEK grafts were ‘sandwiched’ between two glass slides and cultured over 1 week in a humidified atmosphere at 37 °C and 5% CO2. Cell migration was evaluated by light microscopy at standardized time intervals. In addition, immunohistochemistry analyses were performed to assess the detailed structural organization of ECs in the corneal centre and far periphery.
Results
Endothelial cell (EC) migration occurred from the radial cut graft edges, but not from the far peripheral area. Cell migration followed three different migration patterns: (1) individual cell migration, (2) uncoordinated cell migration of cell clusters and (3) collective migration in which ECs moved as a sheet. Immunostaining showed the presence of ECs up to the far periphery but with different expression patterns of phenotypical markers ZO‐1, Na+/K+ ‐ATPase and vimentin compared to central ECs.
Conclusion
In vitro EC migration from Quarter‐DMEK grafts occurs along the radial cut edges with a decrease in migration activity towards the corneal far periphery. No migration occurred along the outer peripheral corneal edge possibly due to a different anatomical matrix in the far periphery. Hence, ECs from the far periphery may not contribute to corneal clearance of the adjacent bare area after Quarter‐DMEK surgery, but these cells may constitute a valuable cellular reserve on the graft.
Keywords: corneal clearance, Descemet membrane endothelial keratoplasty, endothelial cell migration, immunohistochemistry, peripheral endothelial cells, Quarter‐DMEK
Introduction
Recently, we have introduced several modifications of Descemet membrane endothelial keratoplasty (DMEK) including Quarter‐DMEK, a technique that potentially allows to retrieve four quadrants from a full‐size Descemet membrane (DM) and therefore to utilize four endothelial grafts from a single donor cornea (Gerber‐Hollbach et al. 2016; Lie et al. 2016; Müller et al. 2017a,2017b). In a first series, Quarter‐DMEK eyes showed visual outcomes similar to conventional (circular) DMEK (Müller et al. 2017b). At the slit lamp, however, Quarter‐DMEK eyes typically showed a different corneal clearance pattern with clearing primarily occurring adjacent to the radial cut graft edges but not along the ‘limbal’ round edge of the Quarter‐DMEK grafts. This finding would suggest that donor endothelial cell (EC) migration varies over these grafts, with an almost complete absence of migration in the far peripheral anatomical area of DM.
The aim of this study was to further evaluate how EC migration may vary over different anatomical corneal areas, by studying in vitro EC migration from organ‐cultured Quarter‐DMEK grafts, and to determine how Quarter‐DMEK grafts may be positioned best onto the posterior recipient corneal surface during surgery in order to obtain a homogenous redistribution of donor ECs postoperatively.
Materials and Methods
Corneas
Ten human corneas ineligible for transplantation but with an intact and viable EC layer were obtained from seven donors (mean age 72 (±13) years; range 51–84 years; Table 1). All donors had stated to have no objection against transplanted‐related research.
Table 1.
Donor demographics
| Donor information | Indicators |
|---|---|
| Donor data | |
| Gender | |
| Female | 4 |
| Male | 3 |
| Mean age (±SD), yrs (range) | 72 (±13), (51–84) |
| Mean storage time (±SD), days (range) | 11 (±5), (3–16) |
| Cause of death | |
| Cardio/stroke | 2 |
| Respiratory | 5 |
Mean storage time = time between death and culture of first isolated Descemet membrane‐endothelial cell tissue; SD = standard deviation; yrs = years.
Quarter–Descemet membrane endothelial keratoplasty graft preparation
Quarter‐DMEK grafts for these experiments were prepared at Amnitrans EyeBank Rotterdam as previously described (Müller et al. 2017a). Briefly, after decontamination of the globes, corneo‐scleral rims were excised within 36 hours post‐mortem. After EC morphology and viability were evaluated and digital photographs were made with inverted light microscopy (Zeiss Axiovert 40C; Carl Zeiss International, Zaventem, Belgium), the excised corneo‐scleral rims were stored in organ culture medium (CorneaMax; Eurobio, Courtaboeuf, France) at 30 °C until further processing. To peel the Quarter‐DMEK grafts, corneo‐scleral rims were placed endothelial‐side‐up on a custom made holder with a suction cup. The endothelium was then stained for visualization with 0.04% hypotonic trypan blue solution (Hippocratech, Rotterdam, the Netherlands) for 10 second. Next, the DM‐EC sheet including trabecular meshwork (TM) was loosened over 360° from the scleral spur towards the corneal centre and the corneo‐scleral button was divided into four equally sized parts with a surgical blade (no. 24 knife; Swann‐Morton, Sheffield, UK). The DM was then centripetally stripped from the posterior stroma by grasping the TM with McPherson forceps (Moria, Medical Workshop, Groningen, the Netherlands), thereby obtaining four Quarter‐DMEK grafts. After stripping, four rolls formed spontaneously with the endothelium on the outer side and the TM still attached to facilitate later graft handling. Endothelial cell morphology and viability were again assessed, and images of each Quarter‐DMEK graft were evaluated using the fixed frame method. For all ten corneas, the endothelial cell density (ECD) determined in the eye bank after DMEK graft preparation was on average 2743 (±185) cells/mm2, with no significant ECD difference between the four quarters (p > .05) deriving from the same cornea. Each Quarter‐DMEK graft was then stored separately in organ culture medium (CorneaMax; Eurobio) before being evaluated for chemotactic cell ability or immunohistochemistry analysis.
Cell migration study
To analyse cell migration patterns of the corneal endothelium on a Quarter‐DMEK graft, 10 individual Quarter‐DMEK rolls with the TM attached, obtained from 10 different corneas, were unfolded endothelial‐side‐up on a FNC‐coated (fibronectin, collagen and albumin coating mix; Athena ES™ Baltimore, MD, USA) glass coverslip and evaluated in vitro. Unfolding for all grafts was performed in a ‘no‐touch’ manner by grabbing the graft only at the TM site with a McPherson forceps, and dropping organ culture medium onto the graft while the glass coverslip was kept tilted under a small angle.
Next, the TM was carefully removed from the Quarter‐DMEK grafts and the endothelium was submerged in serum‐containing culture medium to ensure cell viability during the experiments. Serum‐containing culture medium consisted of 15% fetal bovine serum in Dulbecco's modified Eagle's medium supplemented with 2 mm l‐Glutamine, 2 ng/ml fibroblast growth factor (bFGF), 0.3 mm l‐ascorbic acid 2‐phosphate (all from Sigma‐Aldrich, Zwijndrecht, the Netherlands) and 10 000 U‐ml Pen/Strep (Carl Roth GmbH + Co. KG, Karlsruhe, Germany). A second FNC‐coated glass coverslip that was spatially separated from the flattened Quarter‐DMEK graft by a suture wire (Supramid TS194‐0, non‐absorbable, Hueber Medica) was then carefully placed on top. The Quarter‐DMEK graft, now ‘sandwiched’ between the two glass slides, was then transferred to a 24‐well plate and kept over 9 days in a humidified atmosphere at 37 °C and 5% CO2. For routine maintenance, medium was replaced with fresh culture medium every 2–3 days. To assess cell morphology and the degree of cell migration, the Quarter‐DMEK grafts were photographed daily with an AxioVert.A1 microscope with AxioCam ERc 5s stand‐alone functionality camera (Zeiss, Oberkochen, Germany).
Immunohistochemistry
To evaluate the expression of continuous zonula occludens‐1 (ZO‐1) at the cell–cell borders, thin cortical vimentin cytoskeleton and pump function through Na+/K+‐ATPase, immunohistochemistry analysis was performed at room temperature on Quarter‐DMEK grafts obtained from the same corneas as the grafts used for the cell migration experiments. Quarter‐DMEK grafts were unfolded and flattened on silane‐precoated glass slides (Sigma‐Aldrich) before fixation in 4% paraformaldehyde (Sigma‐Aldrich) for 30 min. Following fixation, the grafts were first washed with phosphate‐buffered saline (PBS), then permeabilized using permeabilization buffer (0.1% Triton X‐100 in PBS; Sigma‐Aldrich) and finally incubated with blocking buffer (5% bovine serum albumin in PBS; Sigma‐Aldrich) for 1 hr to prevent non‐specific staining. Blocking buffer was also used for primary and secondary antibody (Life Technology, Bleiswijk, the Netherlands) dilutions. Incubation with primary antibodies anti‐ZO‐1 tight junction protein (anti‐ZO‐1/TJP1; dilution 1:100), anti‐vimentin filamentous protein (anti‐vimentin, dilution 1:100) and anti‐sodium/potassium‐ATPase (anti‐Na+/K+‐ATPase, dilution 1:100) was performed for 1 hr and was followed by several PBS washing steps. Samples were then incubated with secondary antibodies (dilution 1:200) for 1 hr. As control, an antibody to smooth muscle actin (anti‐α SMA, dilution 1:100) was included as a marker for smooth muscle cells and myofibroblasts. After washing with PBS, the samples were stained with 4′,6‐diamidino‐2‐phenylindole (DAPI; Sigma‐Aldrich) to visualize the nuclear DNA and then imaged using an inverted fluorescence microscope connected to a camera (Axiovert; Zeiss).
Results
Structural analysis of endothelial cell distribution on Quarter‐DMEK grafts
With light microscopy, all Quarter‐DMEK grafts showed an intact endothelium up to the radial cut edges (Fig. 1A). From the central to far peripheral corneal areas, the ECs showed a different morphology, with a relatively homogenous cell distribution in the corneal centre and less densely packed cells with a more heterogeneous morphology towards the periphery (Fig. 1A–C). In the area directly adjacent to the TM, collagen fibres intermingled with the ECs in a spiral‐like pattern (Fig. 1C).
Figure 1.
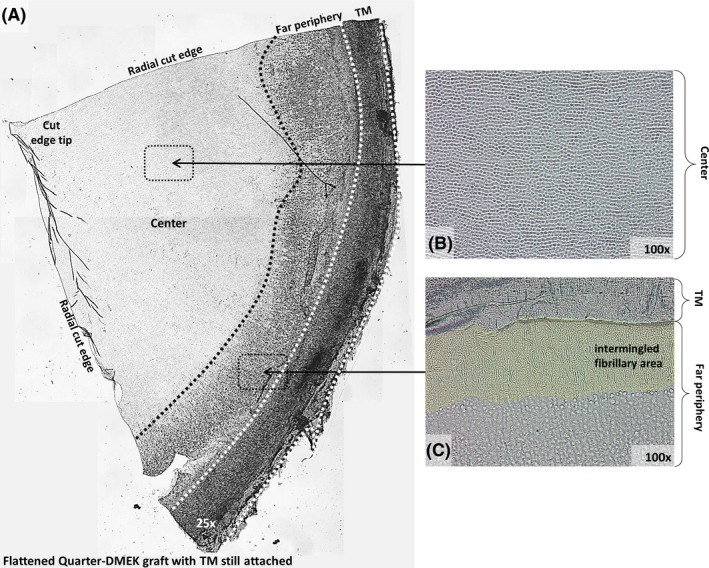
General view of a Quarter‐Descemet membrane endothelial keratoplasty (DMEK) graft flattened on a glass support. (A) Overview of a flattened Quarter‐DMEK graft with the trabecular meshwork (TM) still attached. Corneal centre (B) and far periphery (C) show structural differences, which become more distinct when displayed at higher magnifications (×100). (B) Corneal centre with closely packed hexagonal endothelial cells. (C) Far peripheral area is dominated by a fibrillary area adjacent to the TM.
Cell migration study
All grafts showed substantial cell migration from day 4 up to day 6 with EC migration along the radial cut edges up to the central tip of the graft (Figs 2 and 3). The degree of migration decreased towards the peripheral graft area. Over the far periphery, that is the intermingled fibrillary area, no cell migration was observed, that is no cells crossed the rounded graft edge at any time‐point (Figs 2B and 3A–C).
Figure 2.
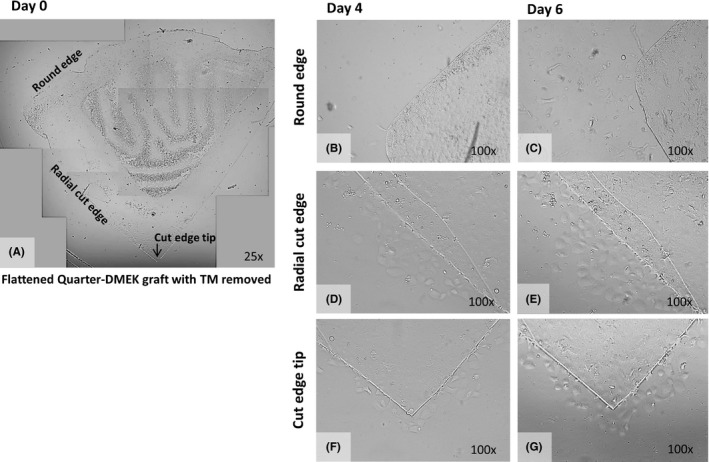
Example of individual and uncoordinated in vitro endothelial cell (EC) migration. (A) Collage of light microscopy images (×25 magnification) to create an overview of a flattened Quarter‐Descemet membrane endothelial keratoplasty (DMEK) graft without trabecular meshwork attached, at the start of the cell migration experiment (Day 0). (B–G) Light microscopy images of the round edge (B,C), the radial cut edge (D,E) and the cut edge tip (F,G) of the Quarter‐DMEK graft taken at Day 4 (left) and Day 6 (right) with ×100 magnification. (B) In the area of the round edge of the Quarter‐DMEK graft, no apparent cell migration is observed across the round graft edge with (C) only individual cells migrating across the far peripheral cut edge of the graft. (D,E) Along the radial cut edge of the Quarter‐DMEK graft, single bleb‐like ECs migrating onto the glass coverslip are observed. (F,G) At the cut edge tip of the Quarter‐DMEK graft, individual cells migrate onto the glass coverslip.
Figure 3.
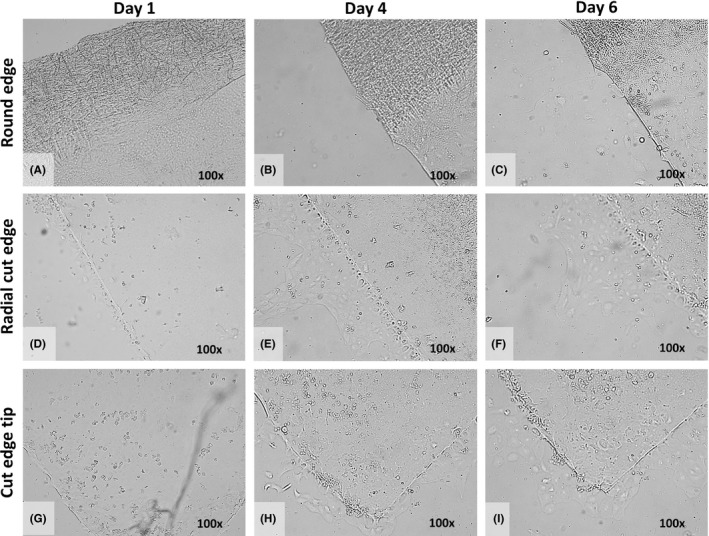
Example of collective in vitro endothelial cell (EC) migration. (A–I) Light microscopy images of the round edge (A–C), the radial cut edge (D–F) and the cut edge tip (G–I) of the Quarter‐Descemet membrane endothelial keratoplasty (DMEK) graft taken at Day 1 (left), Day 4 (middle) and Day 6 (right) with ×100 magnification. (A–C) In the area of the round edge and far periphery of the Quarter‐DMEK graft, no EC migration onto the glass slide was observed up to Day 6. (D–F) Along the radial cut edges of the Quarter‐DMEK graft, collective EC migration in a form of a monolayer was observed; leader cells at the front edge of the advancing cell sheet are identifiable. (G–I) Around the cut edge tip of the Quarter‐DMEK graft, the collective migration pattern was most evident at Day 6.
At the radial cut edges, three types of cell migration patterns could be observed: (1) individual cell migration in an exploratory manner lacking a directional stimulation (i.e. random cell migration) was present in five of 10 grafts (Fig. 2C), (2) migration of cell clusters, with cells coexisting at the leading migratory edge, not forming a continuous monolayer (i.e. uncoordinated cell migration) was observed in four of 10 grafts (Fig. 2D–G) and (3) migration of interconnected cells that collectively departed the DM with a leading ‘cell group’ at the front edge (i.e. collective cell migration) was observed in one of 10 graft (Fig. 3D–I).
Immunohistochemistry
Immunohistochemistry analysis confirmed the presence of ECs up to the far peripheral area of the Quarter‐DMEK grafts, that is up to the round edge of the graft (Fig. 4). However, ECs in the centre and far periphery revealed different expression patterns of the typical endothelial markers ZO‐1, vimentin and Na+/K+‐ATPase (Fig. 4). While ZO‐1 expression at the apical junctions in the central graft area showed the typical hexagonal cell borders, the distribution of ZO‐1 towards the intermingled fibrillary area in the far periphery was more discontinuous and also revealed larger cells than in the central area (Fig. 4A,B). Differences in cell size and shape between ECs in the centre and far periphery were also shown by the expression of vimentin that showed a mat of filaments within the EC cytoplasm in the far periphery (Fig. 4C,D). Towards the far periphery, also Na+/K+‐ATPase pumps were expressed more irregularly (Fig. 4E,F). The absence of α‐SMA‐positive cells in both central and peripheral regions of the Quarter‐DMEK graft verified the absence of any transformed ECs (Fig. 4G,H).
Figure 4.
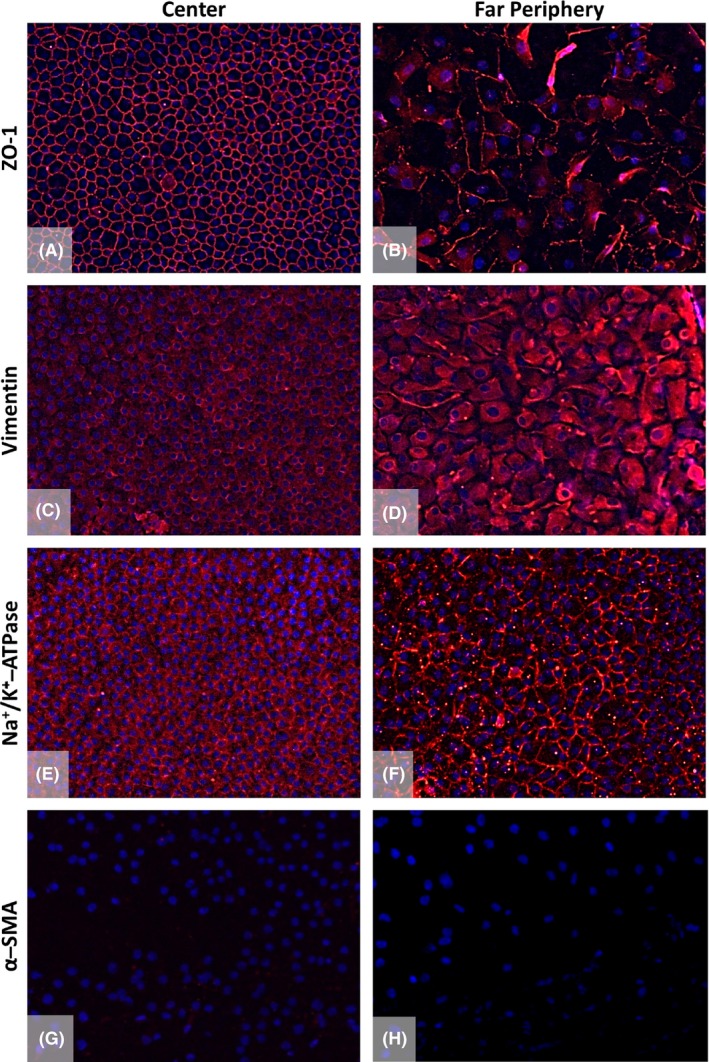
Immunofluorescence staining of the Quarter‐Descemet membrane endothelial keratoplasty (DMEK) graft in the centre compared to the far periphery. Expression of ZO‐1 (A,B), vimentin (C,D), Na+/K+‐ATPase (E,F) and α‐smooth muscle actin (α‐SMA; G,H) was analysed. The central endothelium showed characteristic expressions for the tight junction protein ZO‐1 (A, red), structural protein vimentin (C, red) and functional protein Na+/K+‐ATPase (E, red) counterstained with 4′,6‐diamidino‐2‐phenylindole (blue). The presence of markers in the far peripheral area (B,D,F) verified the presence of endothelial cells (ECs) up to the round edge of the Quarter‐DMEK graft. However, the cells in the far periphery showed a different expression pattern for these endothelial markers (B,D,F red) as compared to the central area. α‐SMA, used as a negative control for the ECs, was absent in the centre and in the far periphery of the endothelium (G,H). ×200 magnification.
Discussion
In this study, we evaluated how EC migration may vary over different anatomical corneal areas, by studying in vitro EC migration from organ‐cultured Quarter‐DMEK grafts.
Our results showed that corneal ECs migrate from the radial cut edges but not from the round edge of a Quarter‐DMEK graft, that is the far, ‘limbal’ periphery of DM. The lack of EC migration from the peripheral round edge may be explained by the structural organization of the peripheral DM. Immunolocalization showed expression of the structural (ZO‐1 and vimentin) and functional (Na+/K+‐ATPase) markers up to the far periphery, however, although ECs in the far periphery formed a cellular monolayer, these cells did not show the typical hexagonal cell structure.
He et al. (2012) showed that in the corneal far periphery, ECs were organized in small radial rows induced by the furrow‐like distribution of the underlying collagen fibres. It was suggested that this anatomical organization is to direct the migration of ECs from specific niches in the far periphery towards the centre of the cornea throughout life, limiting the migration in the other direction. Hence, if migration would not occur or is limited from the limbal edge of a Quarter‐DMEK graft, it may be important to position the graft eccentrically, with its radial cut edges near the pupillary area while the peripheral round edge is positioned peripherally, to avoid slowly resolving corneal oedema in the visual axis (Zygoura et al. 2018). Further, it may be beneficial for the smaller Quarter‐DMEK graft – compared to a circular conventional graft – that not all cells are able to ‘leave’ the graft. This could possibly enable early stabilization of the EC density over time.
Although detailed knowledge about the movement of corneal ECs is lacking, migration of other cell sheets has been studied more extensively, especially in vitro (Vitorina & Meyer 2008; Mayor & Carmona‐Fontaine 2010; Gov 2011; Tambe et al. 2011; Trepat & Fredberg 2011). Under normal conditions, cells maintain strong adhesions with neighbouring cells. When a wound is created, the released transient chemical signals enhance cellular motility near the edge of the wound and cells at the wound edge extend large polarized lamellipodia towards the free surface producing an overall traction force that is directed towards the wound. (Gov 2011; Tambe et al. 2011; Trepat & Fredberg 2011). Another important denominator of cell migration is growth factor signalling, which is necessary for directional migration (Vitorina & Meyer 2008). For instance, in vitro, fibroblast growth factor 2 (FGF‐2, bFGF), has been shown to induce human umbilical vein ECs near the boundary of a sheet to move into open space, whereas in the absence of bFGF, cells migrated with normal speed but failed to sense open space or to respond with directed movement (Vitorina & Meyer 2008).
While collective migration patterns were observed for other cell types, in our study, uncoordinated or individual migration patterns were more prevalent, which might be partly explained by the experimental set‐up that might induce a limited number of viable neighbouring cells, for example due to cell damage during tissue handling. However, differences in cell migration from the radial cut edges and from the peripheral round edge were clearly distinguishable.
Recent studies on the effect of Rho‐associated kinase (ROCK) inhibitors showed that topical administration of ROCK inhibitors after induced surgical injury of rabbit corneal endothelium triggered cell adhesive changes which contributed to enhanced proliferation and migration (Meekins et al. 2016). A similar observation was made after cases of surgical ‘descemetorhexis only’ procedures, that is stripping of a diseased DM–endothelium layer without subsequent corneal graft transplantation, followed by topical administration of ROCK inhibitors (Moloney et al. 2017). Although, corneas after Quarter‐DMEK may show sufficient clearance (Zygoura et al. 2018), ROCK inhibitors may potentially enhance EC migration and corneal clearance. However, as ROCK inhibitors were not administered after in vivo Quarter‐DMEK surgery, they were also not added as an agent to the serum‐containing growth media in our experiments to ensure better comparability of in vitro and in vivo EC migration patterns.
In conclusion, asymmetrical EC migration of Quarter‐DMEK grafts may explain the corneal clearance pattern after Quarter‐DMEK surgery, with cell migration predominantly from the radial cut edges, but not the rounded, limbal edge. While the ECs from the graft's far periphery may not contribute to corneal clearance after Quarter‐DMEK surgery, these cells may constitute a valuable cellular reserve on the graft.
Dr Melles is a consultant for DORC International/Dutch Ophthalmic USA and SurgiCube International. Dr Baydoun is a consultant for DORC International/Dutch Ophthalmic USA. The other authors do not have any potential conflict of interests to disclose.
Part of the project leading to this study has received funding from the European Union's Horizon 2020 Research and Innovation Programme under grant agreement number 667400 (ARREST BLINDNESS Consortium).
References
- Gerber‐Hollbach N, Parker J, Baydoun L, Liarakos VS, Ham L, Dapena I & Melles GR (2016): Preliminary outcome of hemi‐Descemet membrane endothelial keratoplasty for Fuchs endothelial dystrophy. Br J Ophthalmol 100: 1564–1568. [DOI] [PubMed] [Google Scholar]
- Gov N (2011): Cell mechanics: moving under peer pressure. Nat Mater 10: 412–414. [DOI] [PubMed] [Google Scholar]
- He Z, Campolmi N, Gain P et al. (2012): Revisited microanatomy of the corneal endothelial periphery: new evidence for continuous centripetal migration of endothelial cells in humans. Stem Cells 30: 2523–2534. [DOI] [PubMed] [Google Scholar]
- Lie JT, Lam FC, Groeneveld‐van Beek EA, van der Wees J & Melles GR (2016): Graft preparation for hemi‐Descemet membrane endothelial keratoplasty (hemi‐DMEK). Br J Ophthalmol 100: 420–424. [DOI] [PubMed] [Google Scholar]
- Mayor R & Carmona‐Fontaine C (2010): Keeping in touch with contact inhibition of locomotion. Trends Cell Biol 20: 319–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meekins LC, Rosado‐Adames N, Maddala R, Zhao JJ, Rao PV & Afshari NA (2016): Corneal endothelial cell migration and proliferation enhanced by Rho Kinase (ROCK) inhibitors in in vitro and in vivo models. Invest Ophthalmol Vis Sci 57: 6731–6738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moloney G, Petsoglou C, Ball M et al. (2017): Descemetorhexis without grafting for Fuchs endothelial dystrophy — supplementation with topical ripasudil. Cornea 36: 642–648. [DOI] [PubMed] [Google Scholar]
- Müller TM, Baydoun L & Melles GR (2017a): 3‐Year update on the first case series of hemi‐Descemet membrane endothelial keratoplasty. Graefes Arch Clin Exp Ophthalmol 255: 213–215. [DOI] [PubMed] [Google Scholar]
- Müller TM, Lavy I, Baydoun L, Lie JT, Dapena I & Melles GR (2017b): Case report of Quarter‐Descemet membrane endothelial keratoplasty for Fuchs endothelial dystrophy. Cornea 36: 104–107. [DOI] [PubMed] [Google Scholar]
- Tambe DT, Hardin CC, Angelini TE et al. (2011): Collective cell guidance by cooperative intercellular forces. Nat Mater 10: 469–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trepat X & Fredberg JJ (2011): Plithotaxis and emergent dynamics in collective cellular migration. Trends Cell Biol 21: 638–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitorina P & Meyer T (2008): Modular control of endothelial sheet migration. Genes Dev 22: 3268–3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zygoura V, Baydoun L, Ham L et al. (2018): Quarter‐Descemet membrane endothelial keratoplasty (Quarter‐DMEK) for Fuchs endothelial corneal dystrophy: 6 months clinical outcome. Br J Ophthalmol [Epub ahead of print 2018 Jan 17]. [DOI] [PubMed] [Google Scholar]


