Abstract
Soil water‐logging and flooding are common environmental stress conditions that can impair plant fitness. Roots are the first organs to be confronted with reduced oxygen tension as a result of flooding. While anatomical and morphological adaptations of roots are extensively studied, the root system architecture is only now becoming a focus of flooding research. Adventitious root (AR) formation shifts the root system higher up the plant, thereby facilitating supply with oxygen, and thus improving root and plant survival.
We used Arabidopsis knockout mutants and overexpressors of ERFVII transcription factors to study their role in AR formation under hypoxic conditions and in response to ethylene.
Results show that ethylene inhibits AR formation. Hypoxia mainly promotes AR elongation rather than formation mediated by ERFVII transcription factors, as indicated by reduced AR elongation in erfVII seedlings. Overexpression of HRE2 induces AR elongation to the same degree as hypoxia, while ethylene overrides HRE2‐induced AR elongation.
The ERFVII transcription factors promote establishment of an AR system that is under negative control by ethylene. Inhibition of growth of the main root system and promotion of AR elongation under hypoxia strengthens the root system in upper soil layers where oxygen shortage may last for shorter time periods.
Keywords: Adventitious root, Arabidopsis thaliana, ethylene, ethylene response factors of group VII, flooding, HRE2, hypoxia
Introduction
Flooding as a consequence of weather extremes is a major cause of crop loss (Voesenek & Bailey‐Serres 2015). During flooding, plants are exposed to reduced O2 as a result of oxygen consumption and limited gas exchange. In addition to O2 shortage, the gaseous hormone ethylene accumulates due to reduced diffusion rates in water (Grable 1966). Both, reduced O2 and elevated ethylene levels serve as signals that induce flooding stress responses. In flooding‐tolerant species several traits have evolved that improve O2 supply and metabolic adjustments to compensate for limited oxidative ATP synthesis through substrate‐level ATP synthesis and NAD+ recovery in fermentation (Mustroph et al. 2014). To improve gas exchange, flooding‐tolerant plants form aerenchyma (Steffens et al. 2011; Yamauchi et al. 2013), adventitious roots (Lorbiecke & Sauter 1999) and gas films (Herzog et al. 2018), or induce hyponastic growth (Millenaar et al. 2005; Pierik et al. 2005) and stem or petiole elongation (Kende et al. 1998; Millenaar et al. 2005) to raise leaves above the water surface.
Adventitious root (AR) growth is a common adaptive response to flooding, e.g. in deepwater rice (Oryza sativa L.), tamarack (Larix laricina), Rumex palustris and tomato (Solanum lycopersicum; Visser et al. 1996; Lorbiecke & Sauter 1999; Vidoz et al. 2010; Calvo‐Polanco et al. 2012). ARs facilitate gas exchange, uptake of minerals, water and O2 and anchor the plant during the post‐submergence phase. In rice, nodal AR initiation is part of the regular developmental programme. However, the emergence of ARs depends on an environmental stimulus such as flooding, which traps ethylene and triggers the outgrowth of AR primordia.
Arabidopsis thaliana is an intermediate flooding‐tolerant species in which ethylene and low O2 trigger metabolic acclimation (Voesenek & Sasidharan 2013). Petiole elongation and hyponastic growth improve the O2 supply of submerged tissues (Millenaar et al. 2005). The APETALA2/ETHYLENE‐RESPONSE FACTOR (AP2/ERF) transcription factors of group VII (ERFVIIs) are key mediators of low‐oxygen stress responses in rice and Arabidopsis. ERFVIIs regulate metabolic changes and developmental reprogramming under low‐oxygen conditions (Licausi et al. 2013; Abbas et al. 2015; Paul et al. 2016). Arabidopsis has five ERFVIIs, RAP2.2, RAP2.3, RAP2.12, HRE1 and HRE2. Expression of ERFVIIs is differentially controlled by ethylene and low O2 and differs spatially, suggesting that ERFVIIs play specific roles in hypoxia adaptation and act in a tissue‐specific manner. While ethylene induces the expression of HRE1 and RAP2.2, low O2 induces the expression of HRE1 and HRE2 (Hinz et al. 2010; Hess et al. 2011; Bailey‐Serres et al. 2012). ERFVII activity is further controlled at the level of protein stability. Under normoxic conditions ERFVII proteins are degraded via the N‐end rule pathway, whereas in hypoxic conditions they escape degradation (Bailey‐Serres et al. 2012).
Since roots are the first to encounter hypoxic stress during flooding, it is not surprising that the root system adjusts to these conditions, as recently shown for the primary root growth direction in Arabidopsis (Eysholdt‐Derzsó & Sauter 2017). The root system includes a primary root, lateral roots and possibly ARs (Smith & De Smet 2012). In this study, we analysed AR formation in Arabidopsis in response to hypoxia and ethylene, two main signals of flooding stress, with a particular focus on the involvement of ERFVIIs in AR development.
Material and Methods
Plant material and growth conditions
All experiments were carried out with A. thaliana Col‐0. The ein3 eil1 knockout line (An et al. 2010) was kindly provided by Hongwei Guo (Peking‐Tsinghua Center of Life Sciences, Beijing, China). The hre1‐1 and hre2‐1 single knockout, the hre1‐1, hre2‐1 double knockout, the HRE1ox1, HRE2ox1 and HRE2ox5 overexpression lines (Hess et al. 2011; Eysholdt‐Derzsó & Sauter 2017) as well as rap2.3‐2 (Marín‐de la Rosa et al. 2014), rap.2.12‐1 (Gibbs et al. 2014) and the rap2.12 rap2.2 rap2.3 hre1 hre2 pentuple mutant erfVII (Abbas et al. 2015) were described previously. HRE1ox1 and HRE2ox1 were crossed to generate the double overexpressor line HRE1ox1 x HRE2ox1.
Seeds were surface‐sterilised and plated as described (Eysholdt‐Derzsó & Sauter 2017). To induce synchronous germination, seeds were exposed to light for 6 h before they were transferred to the dark for the times indicated. For all treatments, we used 5‐day‐old dark‐grown seedlings that were kept either at 21% O2 or 2% O2 as previously described (Eysholdt‐Derzsó & Sauter 2017), or exposed to 5 μl·l−1 (5 ppm) ethylene (Air Liquide, Paris, France) or 2 μl·l−1 (2 ppm) 1‐methylcyclopropene (1‐MCP) for 6 days in the dark.
Cloning and plant transformation
Gateway technology based on site‐specific recombination was used to generate overexpressing lines of RAP2.3 and RAP2.12 by introducing the open reading frames into the pB7WG2 destination vector through ligation into pENTR 1A DS (Thermo‐Fisher Scientific, Waltham, MA, USA; Karimi et al. 2002). The primer pairs Forrap2.3‐ox1 and Revrap2.3‐ox1, and Forrap2.12‐ox1 and Revrap2.12‐ox1 (Table S1) were used to amplify the open reading frames of RAP2.3 and RAP2.12 from cDNA generated from leaf RNA. The pB7WG2 expression clones were transformed into Agrobacterium tumefaciens strain GV3101, followed by plant transformation of Col‐0 with the floral dip method (Clough & Bent 1998). Plant selection was carried out based on glufosinate resistance from the pB7WG2 vector. Homozygous plants were selected and expression of RAP2.3 and RAP2.12 was analysed in selected plants with RT‐PCR using leaf material for RNA isolation. The gene‐specific primers Forrap2.3‐ox1 and Revrap2.3‐ox1 for RAP2.3, RAPForrap2.12‐ox1 and Revrap2.12‐ox1 for RAP2.12, were used to verify overexpression (Table S1). Actin2 was used as a reference gene with primers Actin2F1 and Actin2R1 (Table S1).
Statistics
Minitab 14 software (www. minitab.com) was used for one‐way anova with Tukey′s post‐hoc test. For non‐parametric samples, a Kruskal–Wallis test with Dunn's post‐hoc test was used. Comparison of two means was performed with Student′s t‐test.
Results
HRE2 overexpression promotes AR formation and elongation growth of AR primordia
To study development of AR in Arabidopsis in response to hypoxic conditions, 5‐day‐old seedlings were transferred to a controlled gas atmosphere with 2% O2, 5 ppm ethylene at 21% or 2% O2 or kept in air for 6 days in the dark. Darkness induced formation of ARs from the hypocotyl of Arabidopsis seedlings (Fig. 1). For a detailed analysis of AR development three categories were distinguished: (i) young AR primordia of stages I–IV according to the nomenclature described previously for lateral root development (Casimiro et al. 2003), (ii) mature primordia prior to emergence at stages V–VII, and (iii) emerged ARs of stage VII as well as elongated ARs (Fig. 1A).
Figure 1.
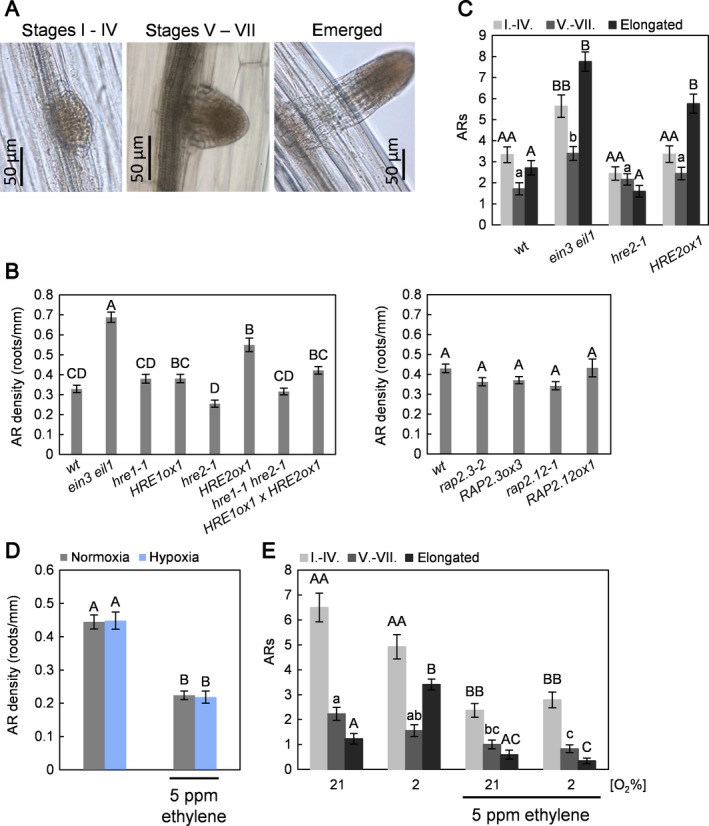
Hypoxia promotes AR elongation while ethylene acts as an inhibitor of AR initiation and growth. A: ARs were grouped into three categories based on the developmental stages described by Casimiro et al. (2003) for lateral root development. Representative ARs are shown from each category. B: AR density of 11‐day‐old wild type and mutant seedlings at normoxia. Error bars represent ± SE from three biological replicates and letters show statistically different values (one‐way anova with Tukey′s test, n = 22–26, P < 0.05). C: AR of wild type, ein3 eil1, hre2‐1 and HRE2ox1 seedlings were counted and categorised as described in (A) in three biological replicates. Values are means (±SE) with single lowercase, capital or double capital letters indicating significant differences within each category (Kruskal–Wallis test, n = 22–26, P < 0.05). D: Average AR densities (±SE) were obtained in three biological replicates. Different letters indicate significantly different values (one‐way anova with Tukey′s test, n = 26–29, P < 0.05). E: 5‐day‐old seedlings were transferred to 21% or 2% O2 with or without 5 μl·l−1 ethylene for 6 days. ARs were categorised as shown in (B). Values are means (±SE) with single lowercase, capital and double capital letters indicating significant changes within the respective developmental category (Kruskal–Wallis test, n = 26–29, P < 0.05).
The gene HRE2, but not its related transcription factor gene HRE1, was shown to be up‐regulated by hypoxia in the shoot (Licausi et al. 2010), and HRE2 overexpression improved anoxia survival (Figure S1). To establish a functional link between hypoxia signalling of AR development and ERFVII transcription factors as hypoxia signal mediators we analysed the roles of the ERFVIIs and of ethylene in AR development using gene knockout and overexpression lines (Fig. 1B and C). HRE2 protein was previously shown to accumulate under non‐stressed conditions when overexpressed (Gibbs et al. 2011). The ein3 eil1 mutant that is deficient in ethylene signalling displayed a significantly higher AR density, indicating that basal ethylene signalling takes place in normoxic seedlings that partially inhibits AR formation (Fig. 1B). Among the knockout mutants and overexpressors of ERFVII transcription factors analysed, HRE2ox1 showed a significantly higher AR density compared to the wild type suggesting that HRE2 and HRE1 have non‐redundant developmental activities. The hypocotyl lengths were not changed in ein3 eil1 or hre1‐1 and hre2‐1 knockout lines compared to the wild type (Figure S2), suggesting that AR organogenesis was a highly specific response to HRE2 overexpression. Knockout and overexpressing mutants of RAP2.3 and RAP2.12 (Fig. 1B; Figure S3) showed a wild‐type phenotype. Based on the finding that HRE2 but not other ERFVIIs, control AR formation, we looked in more detail at AR development in hre2‐1 and HRE2ox1 seedlings in comparison to ein3 eil1 and the wild type (Fig. 1C). Compared to the wild type, ein3 eil1 seedlings had more AR in all three categories, indicating that ethylene inhibits AR initiation but does not alter further AR development. HRE2ox1 seedlings had more ARs than the wild type. Hence, ethylene‐ and hypoxia‐induced HRE2 inversely regulate AR formation. Furthermore, HRE2, unlike ethylene, controlled the late stages of AR development, resulting in more elongated ARs (Fig. 1C).
The AR density did not differ much between seedlings kept in air and seedlings exposed to hypoxia (Figs 1D and 2A). In normoxia, most ARs were at the young primordia stage, whereas hypoxia promoted AR emergence and growth resulting in a significant increase in category three ARs (Fig. 1E). As ethylene is known to control root growth and as it mediates flooding responses (Sasidharan & Voesenek 2015), we next looked into AR development in the presence of 5 μl·l−1 ethylene under normoxic and hypoxic conditions (Fig. 1E). Ethylene inhibited formation of ARs at both O2 levels and inhibited AR development at all developmental stages, overriding hypoxia‐induced AR emergence and growth (Fig. 1E).
Figure 2.
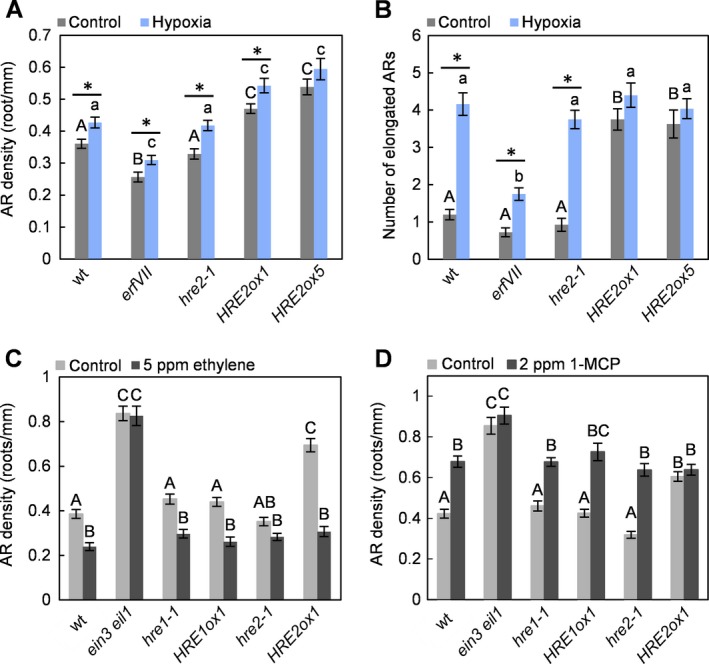
Ethylene inhibits HRE2‐induced AR elongation. A: AR density was analysed in 11‐day‐old wild type, erfVII, hre2‐1,HRE2ox1 and HRE2ox5 seedlings that were exposed to normoxic or hypoxic (2% O2) conditions for 6 days. Capital and lowercase letters indicate significant genotype‐specific differences with a given treatment. Asterisks indicate differences between treatments (one‐way anova with Tukey′s test or two‐sample t‐test, n = 18–31, P < 0.05). B: Number of elongated AR in 11‐day‐old wild type, erfVII, hre2‐1,HRE2ox1 and HRE2ox5 seedlings exposed to normoxic or hypoxic (2% O2) conditions for 6 days. Capital and lowercase letters indicate significant genotype‐specific differences with a given treatment. Asterisks indicate differences between treatments in a given genotype (Kruskal–Wallis test or Mann–Whitney test, n = 18–31, P < 0.05). C: AR density of 11‐day‐old wild‐type and mutant seedlings treated with or without 5 μl·l−1 ethylene. D: AR density of 11‐day‐old wild‐type and mutant seedlings treated with or without 2 μl·l−1 1‐MCP for 6 days. Averages (±SE) in (C) and (D) were obtained in three biological replicates. Letters in (C, D) indicate statistically different values (Kruskal–Wallis test, nC 2H4 = 20–31, nMCP = 20–28, P < 0.05).
Ethylene overrides HRE2 activity
We next analysed the role of HRE2 in AR formation and elongation under hypoxic conditions (Fig. 2A and B). The AR density was not significantly altered in hre2‐1 compared to the wild type, whereas knockout of all ERFVIIs in the erfVII pentuple knockout line resulted in significantly fewer ARs under both normoxic and hypoxic conditions. Overexpression of HRE2 resulted in a higher AR density in two independent HRE2ox lines (Fig. 2A), supporting the idea that HRE2 can induce AR formation when expressed at high enough levels, but knockout of HRE2 is compensated for by one or more other factors. Elongation growth of ARs was several‐fold induced by hypoxia in the wild type, hre2‐1 and erfVII but not in HRE2ox1 and HRE2ox5 seedlings, where the number of elongated AR was already high under normoxic conditions and was not promoted further (Fig. 2B). The results are in accord with the view that HRE2 mediates hypoxia‐induced AR elongation growth.
To test for an interaction of HREs with ethylene signalling, we analysed HRE lines exposed to ethylene or 1‐MCP, an inhibitor of ethylene perception (Fig. 2C and D). AR density was inhibited by 5 μl·l−1 ethylene in the wild type but not in the ethylene‐insensitive ein3 eil1 mutant as would be expected (Fig. 2C). In hre1‐1, HRE1ox1, hre2‐1 and even in HRE2ox1 seedlings that produced more ARs than the wild type, ethylene reduced AR density to wild‐type levels. 1‐MCP treatment resulted in higher AR densities in the wild type, hre1‐1, HRE1ox1 and hre2‐1 (Fig. 2D). AR density in HRE2ox1 did not change with 1‐MCP and was comparable to 1‐MCP‐treated wild‐type seedlings. The observation that HRE2ox1 seedlings are insensitive to 1‐MCP but sensitive to ethylene suggests that HRE2ox1 reduces ethylene responsiveness.
Discussion
Adventitious root growth in rice is a well‐known adaptive trait for survival under prolonged flooding that is primarily regulated by ethylene (Lorbiecke & Sauter 1999; Lin & Sauter 2018). However, apart from favouring ethylene accumulation, flooding causes O2 deprivation that also acts as a submergence signal. In rice and Rumex palustris, AR primordia are formed as part of the developmental programme and emerge upon flooding (Visser et al. 1996; Lorbiecke & Sauter 1999), while in other plants, AR primordia are initiated only when an environmental change occurs. In tomato and sunflower, flooding, and in Arabidopsis, darkness or wounding promote AR formation (Wample & Reid 1978; Vidoz et al. 2010; da Rocha Correa et al. 2012; Dawood et al. 2014).
In this study, we analysed AR formation at the hypocotyl of Arabidopsis seedlings. Our results reveal a role for hypoxia signalling in AR growth and repression of hypoxia‐induced AR growth by ethylene. While in Arabidopsis growth of ARs is enhanced by hypoxia, growth of the main root system is inhibited (Ellis et al. 1999). As a result, the root system architecture changes with a shift of root mass from lower ground toward higher ground, possibly to invest the limited energy reserves in roots that are closer to the soil surface and hence more likely to reach aerated zones. Unlike hypoxia, ethylene inhibits AR initiation (Veloccia et al. 2016). High levels of ethylene inhibited AR initiation to the same degree in normoxic and hypoxic conditions, indicating that ethylene can override the hypoxia signal (Figure S4). In normoxia, AR growth is inhibited by ethylene in a dose‐dependent manner (Veloccia et al. 2016), suggesting that at lower ethylene levels AR are formed that can elongate faster when exposed to hypoxia. Future work may clarify if the sensitivity to ethylene changes under hypoxia. In contrast to Arabidopsis, ethylene has a promotive effect on AR emergence and growth in several flood‐tolerant species including rice (Lorbiecke & Sauter 1999; Lin & Sauter 2018). The contrasting regulation of ARs by ethylene may reflect different adaptive strategies in flooding‐tolerant rice compared to moderately tolerant A. thaliana (Vashisht et al. 2011).
Expression of HRE2 is induced by hypoxia but not ethylene in the shoot and root (Licausi et al. 2010; Hess et al. 2011) and was suggested to induce cell expansion (Lee et al. 2015). Interestingly, loss of HRE2 in hre2‐1 did not reduce or abolish AR elongation under hypoxia, suggesting that a redundant regulatory factor exists. Since the response in erfVII seedlings is altered, redundancy likely comes from the ERFVII group, possibly from RAP2.2 that was not studied here due to the lack of a knockout line. It is further conceivable that knockout of HRE2 results in activation of other ERFVIIs that then compensate for its function. HRE1 expression is induced in roots by hypoxia and ethylene (Licausi et al. 2010; Hess et al. 2011; Eysholdt‐Derzsó & Sauter 2017) and was shown to reduce ethylene inhibition of primary root growth. The molecular mechanism of HRE1 activity is not known, except that it does not alter ethylene production (Yang et al. 2011). While overexpression of HRE1 did not alter AR density, seedlings that overexpressed both HRE1 and HRE2 appeared to have an intermediate phenotype. It is conceivable that HRE1 and HRE2 can heterodimerize, which would contribute to fine‐tuning of flooding responses. Taken together, ERFVIIs promote elongation growth of emerged AR and this growth‐promoting activity can be mimicked by overexpression of HRE2 but not HRE1, RAP2.3 or RAP2.12. Hence, a specific ERFVII contributes to the remodelling of the Arabidopsis root system in response to flooding.
Author contributions
MS conceived the project; EED and MS designed experiments, EED performed and analysed experiments, EED and MS wrote the manuscript.
Supporting information
Figure S1. HRE2 improves and EIN3/EIL1 signalling impairs anoxia survival.
Figure S2. Hypocotyl length in ein3 eil1, HRE1 and HRE2 mutants.
Figure S3. Overexpressing lines of RAP2.3 and RAP2.12.
Figure S4. Model summarising the activities of HRE2 and ethylene in adventitious root development.
Table S1. Primers used for cloning and RT‐PCR.
Acknowledgement
This work was supported by the Deutsche Forschungsgemeinschaft through grant SA 495/11‐1.
References
- Abbas M., Berckhan S., Rooney D.J., Gibbs D.J., Vicente C.J., Sousa C.C., Bassel G.W., Marín‐de la Rosa N., León J., Alabadí D., Blázquez M.A., Holdsworth M.J. (2015) Oxygen sensing coordinates photomorphogenesis to facilitate seedling survival. Current Biology, 25, 1483–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An F., Zhao Q., Ji Y., Li W., Jiang Z., Yu X., Zhang C., Han Y., He W., Liu Y., Zhang S., Ecker J.R., Guo H. (2010) Ethylene‐induced stabilization of ETHYLENE INSENSITIVE3 and EIN3‐LIKE1 is mediated by proteasomal degradation of EIN3 binding F‐Box 1 and 2 that requires EIN2 in Arabidopsis . The Plant Cell, 22, 2384–2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey‐Serres J., Fukao T., Gibbs D.J., Holdsworth M.J., Lee S.C., Licausi F., Perata P., Voesenek L.A.C.J., van Dongen J.T. (2012) Making sense of low oxygen sensing. Trends in Plant Science, 17, 129–138. [DOI] [PubMed] [Google Scholar]
- Calvo‐Polanco M., Señorans J., Zwiazek J.J. (2012) Role of adventitious roots in water relations of tamarack (Larix laricina) seedlings exposed to flooding. BMC Plant Biology, 12, 99–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casimiro I., Beeckman T., Graham N., Bhalerao R., Zhang H., Casero P., Sandberg G., Bennett M.J. (2003) Dissecting Arabidopsis lateral root development. Trends in Plant Science, 8, 165–171. [DOI] [PubMed] [Google Scholar]
- Clough S.J., Bent A.F. (1998) Floral dip: a simplified method for Agrobacterium‐mediated transformation of Arabidopsis thaliana . The Plant Journal, 16, 735–743. [DOI] [PubMed] [Google Scholar]
- Dawood T., Rieu I., Wolters‐Arts M., Derksen E.B., Mariani C., Visser E.J.W. (2014) Rapid flooding‐induced adventitious root development from preformed primordia in Solanum dulcamara . AoB Plants, 6, plt058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis M.H., Dennis E.S., Peacock W.J. (1999) Arabidopsis roots and shoots have different mechanisms for hypoxic stress tolerance. Plant Physiology, 119, 57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eysholdt‐Derzsó E., Sauter M. (2017) Root bending is antagonistically affected by hypoxia and ERF‐mediated transcription via auxin signaling. Plant Physiology, 175, 412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs D.J., Lee S.C., Isa N.M., Gramuglia S., Fukao T., Bassel G.W., Correia C.S., Corbineau F., Theodoulou F.L., Bailey‐Serres J., Holdsworth M.J. (2011) Homeostatic response to hypoxia is regulated by the N‐end rule pathway in plants. Nature, 479, 415–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs D.J., Isa N.M., Movahedi M., Lozano‐Juste J., Mendiondo G.M., Berckhan S., Marín‐de la Rosa N., Vicente Conde J., Sousa Correia C., Pearce S.P., Bassel G.W., Hamali B., Talloji P., Tomé D.F., Coego A., Beynon J., Alabadí D., Bachmair A., León J., Gray J.E., Theodoulou F.L., Holdsworth M.J. (2014) Nitric oxide sensing in plants is mediated by proteolytic control of Group VII ERF transcription factors. Molecular Cell, 53, 369–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grable A.R. (1966) Soil aeration and plant growth In: Norman A.G. (Ed), Advances in agronomy. Academic Press, Cambridge, MA, USA: pp. 57–106. [Google Scholar]
- Herzog M., Konnerup D., Pedersen O., Winkel A., Colmer T.D. (2018) Leaf gas films contribute to rice (Oryza sativa) submergence tolerance during saline floods. Plant, Cell & Environment, 41, 885–897. [DOI] [PubMed] [Google Scholar]
- Hess N., Klode M., Anders M., Sauter M. (2011) The hypoxia responsive transcription factor genes ERF71/HRE2 and ERF73/HRE1 of Arabidopsis are differentially regulated by ethylene. Physiologia Plantarum, 143, 41–49. [DOI] [PubMed] [Google Scholar]
- Hinz M., Wilson I.W., Yang J., Buerstenbinder K., Llewellyn D., Dennis E.S., Sauter M., Dolferus R. (2010) Arabidopsis RAP2.2: an ethylene response transcription factor that is important for hypoxia survival. Plant Physiology, 153, 757–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi M., Inzé D., Depicker A. (2002) GATEWAY™ vectors for Agrobacterium‐mediated plant transformation. Trends in Plant Science, 7, 193–195. [DOI] [PubMed] [Google Scholar]
- Kende H., van der Knaap E., Cho H.‐T. (1998) Deepwater rice: a model plant to study stem elongation. Plant Physiology, 118, 1105–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.‐Y., Hwang E.Y., Seok H.‐Y., Tarte V.N., Jeong M.S., Jang S.B., Moon Y.‐H. (2015) Arabidopsis AtERF71/HRE2 functions as transcriptional activator via cis‐acting GCC box or DRE/CRT element and is involved in root development through regulation of root cell expansion. Plant Cell Reports, 34, 223–231. [DOI] [PubMed] [Google Scholar]
- Licausi F., Van Dongen J.T., Giuntoli B., Novi G., Santaniello A., Geigenberger P., Perata P. (2010) HRE1 and HRE2, two hypoxia‐inducible ethylene response factors, affect anaerobic responses in Arabidopsis thaliana . The Plant Journal, 62, 302–315. [DOI] [PubMed] [Google Scholar]
- Licausi F., Ohme‐Takagi M., Perata P. (2013) APETALA2/ethylene responsive factor (AP2/ERF) transcription factors: mediators of stress responses and developmental programs. New Phytologist, 199, 639–649. [DOI] [PubMed] [Google Scholar]
- Lin C., Sauter M. (2018) Control of adventitious root architecture in rice by darkness, light, and gravity. Plant Physiology, 176, 1352–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorbiecke R., Sauter M. (1999) Adventitious root growth and cell‐cycle induction in deepwater rice. Plant Physiology, 119, 21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marín‐de la Rosa N., Sotillo B., Miskolczi P., Gibbs D.J., Vicente J., Carbonero P., Oñate‐Sánchez L., Holdsworth M.J., Bhalerao R., Alabadí D., Blázquez M.A. (2014) Large‐scale identification of gibberellin‐related transcription factors defines Group VII ETHYLENE RESPONSE FACTORS as functional DELLA partners. Plant Physiology, 166, 1022–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millenaar F.F., Cox M.C.H., van Berkel Y.E.M.d.J., Welschen R.A.M., Pierik R., Voesenek L.A.J.C., Peeters A.J.M. (2005) Ethylene‐induced differential growth of petioles in Arabidopsis. Analyzing natural variation, response kinetics, and regulation. Plant Physiology, 137, 998–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustroph A., Barding G.A. Jr, Kaiser K.A., Larive C.K., Bailey‐Serres J. (2014) Characterization of distinct root and shoot responses to low‐oxygen stress in Arabidopsis with a focus on primary C‐ and N‐metabolism. Plant, Cell and Environment, 37, 2366–2380. [DOI] [PubMed] [Google Scholar]
- Paul M.V., Iyer S., Amerhauser C., Lehmann M., van Dongen J.T., Geigenberger P. (2016) Oxygen sensing via the ethylene response transcription factor RAP2.12 affects plant metabolism and performance under both normoxia and hypoxia. Plant Physiology, 172, 141–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierik R., Millenaar F.F., Peeters A.J.M., Voesenek L.A.C.J. (2005) New perspectives in flooding research: the use of shade avoidance and Arabidopsis thaliana . Annals of Botany, 96, 533–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Rocha Correa L., Troleis J., Mastroberti A.A., Mariath J.E.A., Fett‐Neto A.G. (2012) Distinct modes of adventitious rooting in Arabidopsis thaliana . Plant Biology, 14, 100–109. [DOI] [PubMed] [Google Scholar]
- Sasidharan R., Voesenek L.A.C.J. (2015) Ethylene‐mediated acclimations to flooding stress. Plant Physiology, 169, 3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S., De Smet I. (2012) Root system architecture: insights from Arabidopsis and cereal crops. Philosophical Transactions of the Royal Society, B: Biological Sciences, 367, 1441–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffens B., Geske T., Sauter M. (2011) Aerenchyma formation in the rice stem and its promotion by H2O2 . New Phytologist, 190, 369–378. [DOI] [PubMed] [Google Scholar]
- Vashisht D., Hesselink A., Pierik R., Ammerlaan J.M.H., Bailey‐Serres J., Visser E.J.W., Pedersen O., van Zanten M., Vreugdenhil D., Jamar D.C.L., Voesenek L.A.C.J., Sasidharan R. (2011) Natural variation of submergence tolerance among Arabidopsis thaliana accessions. New Phytologist, 190, 299–310. [DOI] [PubMed] [Google Scholar]
- Veloccia A., Fattorini L., Della Rovere F., Sofo A., D'Angeli S., Betti C., Falasca G., Altamura M.M. (2016) Ethylene and auxin interaction in the control of adventitious rooting in Arabidopsis thaliana . Journal of Experimental Botany, 67, 6445–6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidoz M.L., Loreti E., Mensuali A., Alpi A., Perata P. (2010) Hormonal interplay during adventitious root formation in flooded tomato plants. The Plant Journal, 63, 551–562. [DOI] [PubMed] [Google Scholar]
- Visser E., Cohen J.D., Barendse G., Blom C., Voesenek L. (1996) An ethylene‐mediated increase in sensitivity to auxin induces adventitious root formation in flooded Rumex palustris Sm. Plant Physiology, 112, 1687–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voesenek L.A.C.J., Bailey‐Serres J. (2015) Flood adaptive traits and processes: an overview. New Phytologist, 206, 57–73. [DOI] [PubMed] [Google Scholar]
- Voesenek L.A.C.J., Sasidharan R. (2013) Ethylene – and oxygen signalling – drive plant survival during flooding. Plant Biology, 15, 426–435. [DOI] [PubMed] [Google Scholar]
- Wample R.L., Reid D.M. (1978) Control of adventitious root production and hypocotyl hypertrophy of sunflower (Helianthus annuus) in response to flooding. Physiologia Plantarum, 44, 351–358. [Google Scholar]
- Yamauchi T., Shimamura S., Nakazono M., Mochizuki T. (2013) Aerenchyma formation in crop species: a review. Field Crops Research, 152, 8–16. [Google Scholar]
- Yang C.‐Y., Hsu F.‐C., Li J.‐P., Wang N.‐N., Shih M.‐C. (2011) The AP2/ERF transcription factor AtERF73/HRE1 modulates ethylene responses during hypoxia in Arabidopsis. Plant Physiology, 156, 202–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. HRE2 improves and EIN3/EIL1 signalling impairs anoxia survival.
Figure S2. Hypocotyl length in ein3 eil1, HRE1 and HRE2 mutants.
Figure S3. Overexpressing lines of RAP2.3 and RAP2.12.
Figure S4. Model summarising the activities of HRE2 and ethylene in adventitious root development.
Table S1. Primers used for cloning and RT‐PCR.


