ABSTRACT
In vitro chondrogenesis of mesenchymal stem cells (MSCs) mimics in vivo chondrogenesis of MSCs. However, the size of the cartilage pellets that can be attained in vitro is limited by current methods; therefore, some modifications are required to obtain larger pellets. Petaloid pieces of recombinant peptide (petaloid RCP) have the advantage of creating spaces between cells in culture. The RCP used here is based on the alpha‐1 sequence of human collagen type I and contains 12 Arg‐Gly‐Asp motifs. We examined the effect and mechanisms of adding petaloid RCP on the in vitro chondrogenesis of human synovial MSCs by culturing 125k cells with or without 0.125 mg petaloid RCP in chondrogenic medium for 21 days. The cartilage pellets were sequentially analyzed by weight, sulfated glycosaminoglycan content, DNA retention, and histology. Petaloid RCP significantly increased the weight of the cartilage pellets: The petaloid RCP group weighed 7.7 ± 1.2 mg (n = 108), whereas the control group weighed 5.3 ± 1.6 mg. Sulfated glycosaminoglycan and DNA contents were significantly higher in the petaloid RCP group than in the control group. Light and transmission electron microscopy images showed that the petaloid RCP formed the framework of the pellet at day 1, the framework was broken by production of cartilage matrix by the synovial MSCs at day 7, and the cartilage pellet grew larger, with diffuse petaloid RCP remaining, at day 21. Therefore, petaloid RCP formed a framework for the pellet, maintained a higher cell number, and promoted in vitro cartilage formation of synovial MSCs. © 2018 The Authors. Journal of Orthopaedic Research® Published by Wiley Periodicals, Inc. J Orthop Res 37:1350–1357, 2019.
Keywords: petaloid recombinant peptide, synovial mesenchymal stem cell (MSC), chondrogenesis, regenerative medicine, scaffold
The in vitro chondrogenesis of mesenchymal stem cells (MSCs) by a pellet culture system was first described by Johnstone et al.1 The increase in size of the pellet was due to the production of extracellular matrix, rather than the proliferation of cells, so the pellet size is a convincing indicator of in vitro chondrogenesis in a population of MSCs.2 The in vitro chondrogenesis by MSCs also imitated the in vivo chondrogenesis by MSCs, and a population of MSCs that could differentiate into a larger cartilage pellet in vitro also produced a larger amount of cartilage matrix when transplanted into a cartilage defect.3 We previously compared the chondrogenic potential of MSCs derived from several kinds of mesenchymal tissues, and found that synovial MSCs produced the largest cartilage pellets.4 However, the size of pellets that can be produced is still limited, indicating that some modifications are required to obtain sufficiently large cartilage pellets in vitro for clinical purposes.
MSCs can be induced to differentiate to form larger cartilage pellets by growth on biomaterials.5, 6, 7 One candidate biomaterial is petaloid pieces of recombinant peptide (petaloid RCP) (Fig. 1A). This RCP is based on the alpha‐1 sequence of human collagen type I and contains 12 Arg‐Gly‐Asp (RGD) motifs in a single molecule, which increases its cell‐adhesion ability.8, 9 The collagen type 1 antigenic site has been removed from the RCP to reduce immunogenicity after transplantation. This RCP is produced by yeast, so it poses no risk of infection from diseases such as bovine spongiform encephalopathy. The shape of the RCP pieces influences its effects, and the petaloid shape was chosen in this study for its ability to create spaces between cells when mixed with MSCs.9
Figure 1.
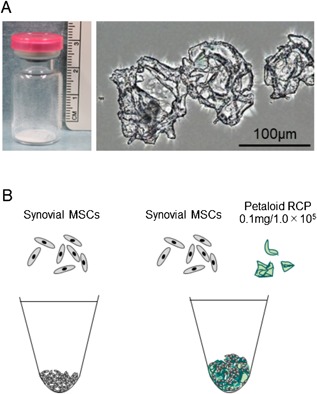
Appearance of petaloid RCP and scheme of the study. (A) Macroscopic and microscopic appearance of petaloid RCP. (B) Scheme of the study. For chondrogenesis, synovial MSCs with or without petaloid RCP in chondrogenic medium were pelleted and cultured for 21 days.
In this study, we examined the effect of addition of petaloid RCP on in vitro chondrogenesis by synovial MSCs. The effect was evaluated by determining the cartilage pellet weight and the amount of glycosaminoglycan and DNA per pellet. We further analyzed the process of cartilage formation by light and electron microscopy examination of the morphology of the complex formed between the synovial MSCs and petaloid RCP. The findings from this in vitro study will contribute to future in vivo studies aimed at advancing cartilage regeneration therapy.
MATERIALS AND METHODS
Preparation of Synovial MSCs
The present study was approved by the Medical Research Ethics Committee of Tokyo Medical and Dental University. All study subjects provided informed consent. Human synovium was harvested from nine female donors (76 ± 6 years old; mean ± SD) during total knee arthroplasty and digested with 3 mg/ml collagenase (Sigma–Aldrich by Merck, NJ) at 37°C for 3 h. The separated cells were filtered through a 70 µm cell strainer (Greiner Bio‐One GmbH, Frichenhausen, Germany). The nucleated cells were cultured in 150 cm2 culture dishes (Nalge Nunc International, Rochester, NY) in 20 ml α‐minimum essential medium (α‐MEM; Thermo Fisher Scientific, MA) containing 10% fetal bovine serum (FBS; Thermo Fisher Scientific) for 14 days as passage 0. The cells were replated at 50 cells/cm2, cultured for 14 days, and used as passage 1 for further analyses. An exception was made for the passage 0 cells from donor 6, which were cryopreserved. These frozen cells were slowly thawed, plated, and incubated for 4 days as passage 1. These passage 1 cells were replated at 50 cells/cm2, cultured for 14 days, and passage 2 cells were used for further analyses.
In Vitro Chondrogenesis
Synovial MSCs were transferred to a 15 ml tube (Corning Falcon, NY) and cultured in chondrogenic induction medium containing 10 ng/ml transforming growth factor‐β3 (Miltenyi Biotec, Tokyo, Japan), 500 ng/ml bone morphogenetic protein 2 (Medtronic, TN), 10−7 M dexamethasone (Sigma–Aldrich), 50 µg/ml ascorbate‐2‐phosphate, 40 µg/ml proline, 100 µg/ml pyruvate, and 50 mg/ml ITS Premix (Corning); this induction medium was changed every 3–4 days. After 21 days, images of cartilage pellets were obtained with a Leica M165 FC stereoscopic microscope (Leica, Wetzlar, Germany), and the pellets were weighed. The optimal cell number was determined by culturing an initial 250k, 125k, 63k, or 31k synovial MSCs; 125k cells were used for further analyses.
Preparation of Petaloid RCP
Petaloid RCP (Fig. 1A) (FUJIFILM Corporation, Tokyo, Japan) was prepared by crushing and fractionating a freeze‐dried 4% RCP solution (cellnest lyophilized; FUJIFILM Corporation, Tokyo, Japan).9 The collected pieces were thermally cross‐linked under reduced pressure at 160°C for 24 h. Two milliliter of chondrogenic induction medium was placed into a vial containing 2 mg of the prepared petaloid RCP (Fig. 1A), and the suspended petaloid RCP was adjusted to a concentration of 0.1 mg per 1.0 × 105 synovial MSCs for in vitro chondrogenesis (Fig. 1B).
Measurement of Sulfated Glycosaminoglycan (sGAG)
After 0, 7, 14, and 21 days of culture, each pellet was digested for 18 h at 65°C in 200 mg/ml papain buffer (Sigma–Aldrich) and dissolved in 200 mM phosphate buffer containing 8 mg/ml sodium acetate, 0.8 mg/ml cysteine‐HCl, and 4 mg/ml EDTA. The sulfated glycosaminoglycan (sGAG) concentration of the supernatant was determined by the Blyscan assay (Biocolor Ltd, Newtonabbay, Ireland), according to the manufacturer's instructions.
Measurement of DNA
After 21 days of culture, each pellet was digested in papain buffer, and the amount of DNA per pellet was evaluated using a PicoGreen kit (Molecular Probes, Eugene, OR).
Histology (Safranin‐o)
Cartilage pellets were fixed in formalin for 1 day and then embedded in paraffin wax. The specimens were cut into sections 5 µm thick and stained with safranin‐o/fast green. Micrographs were taken with an Olympus BX53 digital microscope (Olympus, Tokyo, Japan). The histology of the cartilage pellets was evaluated by two independent observers using Bern's scoring system10 in a blinded manner, and the mean score for each donor was recorded.
Immunostaining
Paraffin‐embedded sections were deparaffinized and activated by proteinase K (Dako, Glostrup, Denmark), and endogenous peroxidases were quenched using 0.3% hydrogen peroxidase in methanol for 10 min. After blocking with Protein Block Serum‐Free (Dako), 5 µm thick sections were incubated with 2.5 μg/ml COL2 antibody (Kyowa Pharma Chemical, Toyama, Japan) overnight at 4°C. After washing three times, secondary antibodies (Chemmate Envision HRP‐polymer, Dako) were added, followed by incubation for 30 min at room temperature. The sections were stained with DAB+ solution (Dako), and counterstained with hematoxylin.
Histology (Toluidine Blue Staining)
Cartilage pellets were fixed with 2.5% glutaraldehyde in 0.1 M PBS for 2 h, washed overnight at 4°C in the same buffer, and post‐fixed with 1% OsO4 buffered with 0.1 M PBS for 2 h. The pellet was dehydrated in a graded ethanol series and embedded in Epon 812. Semi‐thin (1 µm) sections were collected on glass slides, stained for 30 s with toluidine blue, and observed by light microscopy.11
Transmission Electron Microscopy
Cartilage pellets were fixed in 2.5% glutaraldehyde in 0.1 M phosphate buffer. The samples were washed with 0.1 M phosphate buffer, post‐fixed in 1% OsO4 buffered with 0.1 M phosphate buffer for 2 h, dehydrated in a graded ethanol series, and embedded in Epon 812. Ultrathin 90 nm sections were collected on copper grids, double‐stained with uranyl acetate and lead citrate, and then observed by transmission electron microscopy.11
Statistical Analyses
All data were statistically evaluated with GraphPad Prism 6 (GraphPad Software, La Jolla, CA). The results are presented as means ± standard deviation. Statistical analyses included the Wilcoxon matched‐pairs signed‐rank test and Mann‐Whitney's U‐test. p‐Values of <0.05 were considered statistically significant.
RESULTS
Petaloid RCP Increased the Cartilage Weight
The cartilage pellets appeared larger in the petaloid RCP group than in the control group for all starting numbers of MSCs (31–250k synovial MSCs/pellet) (Fig. 2A). Quantitative analysis demonstrated that the cartilage pellets were significantly heavier in the petaloid RCP group than in the control group, irrespective of the initial cell number per pellet (Fig. 2B). In the control group, the weight of the cartilage pellet depended on the initial cell number per pellet, whereas in the petaloid RCP group, the resulting cartilage pellet from an initial 125k cells was as heavy as that resulting from an initial 250k cells. The largest difference in cartilage pellet weight was obtained with pellets arising from 125k cells, so this cell number was used in all further analyses.
Figure 2.
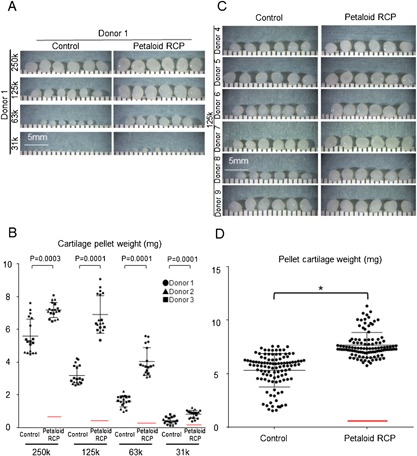
Macroscopic appearance and weight of cartilage pellets derived from synovial MSCs with or without petaloid RCP. (A) Macroscopic appearance of cartilage pellets from initial cell numbers of 250k, 125k, 63k, or 31k synovial MSCs (n = 6). (B) Wet weight of the cartilage pellets from different initial cell numbers of synovial MSCs. Synovial MSCs derived from donors 1 to 3 were cultured with or without petaloid RCP in chondrogenic medium for 21 days. Error bars denote means ± SD (n = 18). Red bars show theoretical weight of petaloid RCP. p < 0.05 by Wilcoxon matched‐pairs signed‐rank test. (C) Macroscopic appearance of cartilage pellets from an initial cell number of 125k synovial MSCs (n = 6). (D) Wet weight of cartilage pellets from an initial cell number of 125k synovial MSCs. Synovial MSCs derived from donors 4 to 9 were cultured with or without petaloid RCP in chondrogenic medium for 21 days. Bars denote means ± SD (n = 108). Red bars show theoretical weight of petaloid RCP. *p < 0.05 by Mann–Whitney's U‐test.
Cartilage pellets appeared larger in the petaloid RCP group than in the control group for six donors (Fig. 2C). The cartilage pellet weight was 7.7 ± 1.2 mg in the petaloid RCP group and 5.3 ± 1.6 mg in the control group, indicating that the use of petaloid RCP significantly increased the weight of the cartilage pellet produced by synovial MSCs (Fig. 2D).
Petaloid RCP Increased the Amounts of sGAG and DNA Retention in the Cartilage Pellets
We evaluated in vitro chondrogenesis by measuring the amounts of sulfated glycosaminoglycan (sGAG) per pellet. The in vitro chondrogenesis by the synovial MSCs resulted in a time‐dependent increase in the mass of sulfated glycosaminoglycan per pellet for six donors (Fig. 3A). The amount of sulfated glycosaminoglycan amount per pellet was significantly higher in the petaloid RCP group than in the control group in most cases at days 7, 14, and 21 for each donor.
Figure 3.
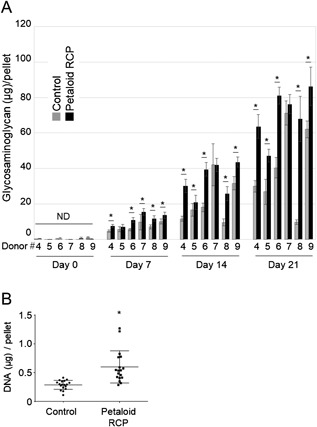
The amount of sulfated glycosaminoglycan per pellet and DNA retention per pellet in synovial MSCs cultured with or without petaloid RCP. (A) The amount of sulfated glycosaminoglycan/pellet derived from synovial MSCs with or without petaloid RCP. An initial 125k synovial MSCs derived from donors 4 to 9 were cultured with or without petaloid RCP in chondrogenic medium for 0, 7, 14, and 21 days. Bars denote means ± SD (n = 6). *p < 0.05 by Wilcoxon matched‐pairs signed‐rank test. ND indicates “not detectable,” as these values were below the detection limit. (B) The amount of DNA/pellet derived from synovial MSCs with/without petaloid RCP. An initial 125k synovial MSCs derived from donors 6, 8, and 9 were cultured with or without petaloid RCP in chondrogenic medium for 21 days. Bars denote means ± SD (n = 18). *p < 0.05 by Mann–Whitney's U‐test.
We also measured the DNA content per pellet to determine the numbers of viable cells. At day 21, the amount of DNA per pellet was 600 ± 279 ng in the petaloid RCP group and 289 ± 78 ng in the control group, indicating that the addition of petaloid RCP resulted in a significantly higher retention of DNA in the cartilage pellets of the synovial MSCs when compared to the control group (Fig. 3B).
Petaloid RCP Improved the Size of the Cartilage Pellets
After 21 days of in vitro chondrogenesis by the synovial MSCs, the extracellular matrix of the pellets from eight donors in the control group showed positive staining with safranin‐o and positive immunostaining for type II collagen (Fig. 4A). The inclusion of petaloid RCP increased the safranin‐o staining and type II collagen immunostaining in the extracellular matrix of the pellet for most donor samples. The Bern score was significantly higher in the petaloid RCP group than in the control group (Fig. 4B). Sequential observations at days 7, 14, and 21 showed a time‐dependent increase in the stainability with safranin‐o in the control group (Fig. 5). In the petaloid RCP group, the pellet was mainly constructed of petaloid RCP at day 7, but it was positively stained with safranin‐o at day 14 and more intensely stained at day 21.
Figure 4.
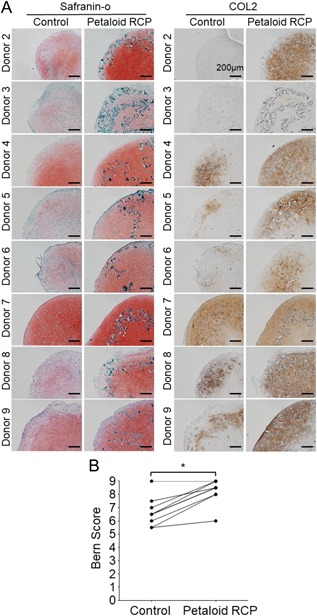
Histological appearance of the cartilage pellet derived from synovial MSCs cultured with or without petaloid RCP. An initial 1.25 × 105 synovial MSCs derived from donors 2 to 9 were cultured with or without petaloid RCP in chondrogenic medium for 21 days. (A) Histological appearance of the cartilage pellets stained with safranin‐o and immunostained for type II collagen. Bars = 200µm. (B) Bern score (0–9) for evaluation of the safranin‐o stained cartilage pellet. *p < 0.05 by Wilcoxon matched‐pairs signed‐rank test.
Figure 5.
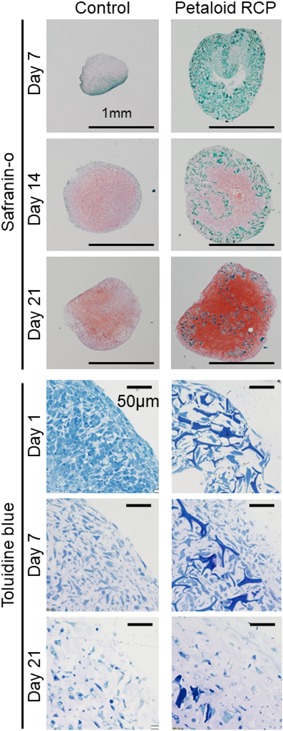
Time course of the changes in histological appearance during in vitro chondrogenesis of synovial MSCs cultured with or without petaloid RCP. An initial 125k synovial MSCs were cultured with or without petaloid RCP in chondrogenic medium for 7, 14, and 21 days for safranin‐o staining and for 1, 7, and 21 days for toluidine blue staining.
Observations of toluidine blue stained sections from the early phase showed that control synovial MSCs were condensed at day 1, slightly spaced apart from each other at day 7, and farther spread out at day 21. In the petaloid RCP group, the pellet was constructed of petaloid RCP, and the cells were located in the spaces created by petaloid RCP at day 1. By day 7, the framework of petaloid RCP had been disrupted due to the production of cartilage matrix by the synovial MSCs. By day 21, most of the pellet volume was occupied by cartilage matrix rather than petaloid RCP. Observation by transmission electron microscopy revealed that the control synovial MSCs were condensed firmly at day 1, and the intercellular spaces increased due to production of collagen fibrils at day 7, with a further increase by day 21 (Fig. 6). In the petaloid RCP group, empty spaces were created by the petaloid RCP, and synovial MSCs were in contact with the petaloid RCP at day 1. The empty spaces were lost and collagen fibrils were observed along the petaloid RCP at day 7. The extracellular space was filled with a greater amount of collagen fibrils by day 21.
Figure 6.
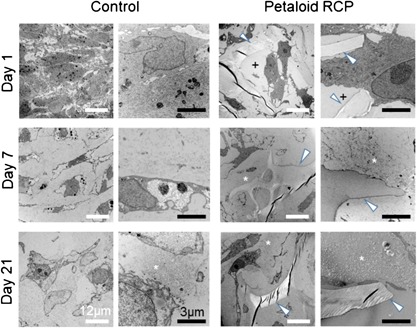
Time course of changes in transmission electron microscopy images during in vitro chondrogenesis of synovial MSCs cultured with or without petaloid RCP. An initial 125k synovial MSCs were cultured with or without petaloid RCP in chondrogenic medium for 1, 7, and 21 days. White arrowheads indicate petaloid RCP. “+” indicates an empty space. Asterisks indicate collagen fibrils.
DISCUSSION
We investigated the effect of the use of petaloid RCP on in vitro chondrogenesis in synovial MSCs. The inclusion of petaloid RCP significantly increased the weight of the cartilage pellets and the mass of sulfated glycosaminoglycans within the cartilage pellets formed by synovial MSCs. These results demonstrated that inclusion of petaloid RCP enhanced in vitro cartilage formation by synovial MSCs.
In the petaloid RCP group, the cartilage pellet formed by an initial 125k cells per pellet was as heavy as that formed by 250k cells per pellet, whereas the weight of the cartilage pellet formed by the control cells was proportional to the initial cell number per pellet (Fig. 2A and B). We previously examined the pellets formed by 200–1,600k human synovial MSCs per pellet without petaloid RCP and found that the cartilage pellet size increased with an initial cell number up to 800k cells per pellet. The diameter of the cartilage pellet formed from an initial 800k cells per pellet was approximately 3 mm and was similar to that formed by an initial 1,600 cells per pellet.12 Successive experiments on in vitro chondrogenesis of human synovial MSCs indicated that the maximum cartilage pellet size was approximately 3 mm. This possibly reflects a failure of nutrient penetration into the center of the cartilage pellets when the pellet exceeds 3 mm in diameter. In the current study, the size of cartilage pellet formed from an initial 125k MSCs per pellet was approximately 3 mm, and this was also the maximum size of the pellet in the petaloid RCP group. Therefore, the weight of the cartilage pellet formed in the petaloid RCP group from an initial 125k cells per pellet was similar to that formed by an initial 250k cells per pellet.
The inclusion of petaloid RCP increased the safranin‐o staining and type II collagen immunostaining in the extracellular matrix of the cartilage pellet at day 21 (Fig. 4A). The Bern score for safranin‐o staining was significantly higher in the petaloid RCP group than in the control group (Fig. 4B). The Bern score accounts for “uniformity and intensity of matrix staining,” “cell density/extent of matrix produced,” and “cellular morphologies.” The Bern score has three criteria, with each scored from 0 to 3, and the sum of the score is a minimum of 0 and a maximum of 9.10 In this study, the Bern score was higher for matrix staining and cellular morphology in the petaloid RCP group than in the control group.
We also analyzed the process of cartilage formation in the complex formed by synovial MSCs and petaloid RCP from a morphological point of view by light and transmission electron microscopy. These observations indicated that the framework of the pellet was constructed from petaloid RCP, and the cells were located in the spaces formed by the petaloid RCP. The spaces were then filled with cartilage matrix, and the framework established by petaloid RCP was disrupted by the production of the cartilage matrix by the synovial MSCs. The cartilage pellet increased in size and diffuse petaloid RCP remained (Fig. 7). The amount of DNA per pellet decreased during in vitro chondrogenesis of MSCs, but the DNA content per pellet was maintained when petaloid RCP was used. This possibly reflected the intercellular spaces created by petaloid RCP. The shape of RCP is particularly well designed to create spaces between the cells, and these spaces may have enabled nutrients to diffuse farther into the center of the MSCs‐petaloid RCP complex, thereby reducing cell necrosis.
Figure 7.
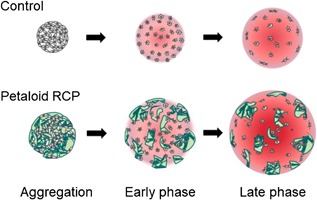
Scheme for morphological events occurring during in vitro chondrogenesis of synovial MSCs with or without petaloid RCP. In the absence of petaloid RCP, synovial MSCs were condensed at the early phase, produced cartilage matrix at the middle phase, and then showed an increase in the size of the cartilage pellet at the late phase. With the inclusion of petaloid RCP, the framework of the pellet was constructed from petaloid RCP, and the cells were located in the spaces created by the petaloid RCP at the early phase. These spaces were filled with cartilage matrix, and the framework petaloid RCP formed was disrupted by production of cartilage matrix by the synovial MSCs during the middle phase. The cartilage pellet increased further in size at the late phase and showed diffuse petaloid RCP remaining.
Petaloid RCP is based on the alpha‐1 sequence of human collagen type I and contains 12 RGD motifs in each molecule that increase its cell‐adhesion ability.9 The RGD peptide was identified in extracellular matrix protein as the minimal sequence required for recognition by cell membrane integrins, such as α5β1 and α6β3.13 These were found on chondrocytes, bone marrow MSCs, and synovial MSCs.14, 15, 16 Re'em et al. reported that RGD peptide immobilized in alginate scaffolds facilitated chondrogenesis of bone marrow MSCs by promoting cell adhesion, proliferation, and activation of Smad2 and extracellular signal‐regulated kinases.17 The RGD peptides in petaloid RCP in our study may also have promoted chondrogenesis by the synovial MSCs. One limitation of the present study was that we did not compare our petaloid RCP with RGD motifs to other petaloid RCPs without RGD motifs.
In the present study, scaffolds could have aided in the in vitro differentiation of MSCs into larger cartilage pellets.5, 6, 7 The significance of scaffolds is that they generate an environment that allows for appropriate exchange of nutrients, oxygen, and metabolic waste, and they allow establishment of intimate cell‐matrix contacts to ensure optimal cell viability.18, 19, 20 Scaffolds such as PLGA and alginate are known to promote cartilage formation,21, 22 but these scaffolds have the disadvantages of releasing substances that are harmful to the living body and that induce degeneration of the degeneration.19, 20, 23 These issues regarding scaffold materials are difficult to overcome,24 but the use of petaloid RCP may provide a solution.8, 9
The first report of petaloid RCP use was by Nakamura et al., who demonstrated that petaloid RCP increased ATP activity of bone marrow MSCs at 7 days in a spheroid culture. They also examined the effect of petaloid RCP in a streptozotocin‐induced diabetic model of mice. In these mice, co‐transplantation of pancreatic islets with bone marrow MSCs improved the function of the islets,9 so a mixture of islets and an MSC suspension was injected subcutaneously with or without petaloid RCP. The inclusion of petaloid RCP improved the diabetic condition in the mice, as indicated by a decreased blood glucose level and improved glucose tolerance.9
In the present study, the inclusion of petaloid RCP promoted the in vitro cartilage formation by synovial MSCs. This suggests that transplantation of synovial MSCs with petaloid RCP has the potential to improve cartilage regeneration. We plan two future in vivo studies: One is to prepare a micromass consisting of 125k synovial MSCs and 0.125 mg petaloid RCP and to implant a number of these micromasses into a cartilage defect. We have previously shown cartilage regeneration following transplantation of 5–100 micromasses of synovial MSCs in a rabbit model.25 The other plan is to prepare suspensions of synovial MSCs and petaloid RCP at a concentration of 0.1 mg RCP per 105 cells and to place this suspension onto cartilage defects for transplantation. We have previously transplanted 50 million synovial MSCs onto cartilage defects in the clinic.26
CONCLUSIONS
Petaloid RCP enhanced in vitro cartilage formation by synovial MSCs. Petaloid RCP also maintained the DNA content per pellet. A pellet framework was initially constructed from the petaloid RCP, and that framework was subsequently disrupted as synovial MSCs produced a cartilage matrix. Larger cartilage pellets were obtained with RCP than in control cultures without RCP.
AUTHORS’ CONTRIBUTIONS
MN: data collection and manuscript writing; MM: conception and design, data analysis, and manuscript writing; HK: conception and design, manuscript writing; KO: material collection; KK, SF: data collection; SI: TEM analysis; NO: conception and design, data analysis; KT, HK, TM: data interpretation; IS: conception and design, financial support, manuscript writing, final approval of manuscript.
ACKNOWLEDGMENTS
We would like to thank Ms. Mika Watanabe and Ms. Kimiko Takanashi for the management of our laboratory and Ms. Ellen Roider for English editing. Petaloid RCP was provided by FUJIFILM Corporation, Tokyo, Japan.
REFERENCES
- 1. Johnstone B, Hering TM, Caplan AI, et al. 1998. In vitro chondrogenesis of bone marrow‐derived mesenchymal progenitor cells. Exp Cell Res 238:265–272. [DOI] [PubMed] [Google Scholar]
- 2. Sekiya I, Vuoristo JT, Larson BL, et al. 2002. In vitro cartilage formation by human adult stem cells from bone marrow stroma defines the sequence of cellular and molecular events during chondrogenesis. Proc Natl Acad Sci USA 99:4397–4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Koga H, Muneta T, Nagase T, et al. 2008. Comparison of mesenchymal tissues‐derived stem cells for in vivo chondrogenesis: suitable conditions for cell therapy of cartilage defects in rabbit. Cell Tissue Res 333:207–215. [DOI] [PubMed] [Google Scholar]
- 4. Sakaguchi Y, Sekiya I, Yagishita K, et al. 2005. Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis Rheum 52:2521–2529. [DOI] [PubMed] [Google Scholar]
- 5. Kon E, Roffi A, Filardo G, et al. 2015. Scaffold‐based cartilage treatments: with or without cells? A systematic review of preclinical and clinical evidence. Arthroscopy 31:767–775. [DOI] [PubMed] [Google Scholar]
- 6. Bernhard JC, Vunjak‐Novakovic G. 2016. Should we use cells, biomaterials, or tissue engineering for cartilage regeneration? Stem Cell Res Ther 7:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang W, Ouyang H, Dass CR, et al. 2016. Current research on pharmacologic and regenerative therapies for osteoarthritis. Bone Res 4:15040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nakamura K, Tabata Y. 2010. A new fluorescent imaging of renal inflammation with RCP. J Control Release 148:351–358. [DOI] [PubMed] [Google Scholar]
- 9. Nakamura K, Iwazawa R, Yoshioka Y. 2016. Introduction to a new cell transplantation platform via recombinant peptide petaloid pieces and its application to islet transplantation with mesenchymal stem cells. Transpl Int 29:1039–1050. [DOI] [PubMed] [Google Scholar]
- 10. Grogan SP, Barbero A, Winkelmann V, et al. 2006. Visual histological grading system for the evaluation of in vitro‐generated neocartilage. Tissue Eng 12:2141–2149. [DOI] [PubMed] [Google Scholar]
- 11. Ichinose S, Muneta T, Koga H, et al. 2010. Morphological differences during in vitro chondrogenesis of bone marrow‐, synovium‐MSCs, and chondrocytes. Lab Invest 90:210–221. [DOI] [PubMed] [Google Scholar]
- 12. Shirasawa S, Sekiya I, Sakaguchi Y, et al. 2006. In vitro chondrogenesis of human synovium‐derived mesenchymal stem cells: optimal condition and comparison with bone marrow‐derived cells. J Cell Biochem 97:84–97. [DOI] [PubMed] [Google Scholar]
- 13. Ruoslahti E. 1996. RGD and other recognition sequences for integrins. Annu Rev Cell Dev Biol 12:697–715. [DOI] [PubMed] [Google Scholar]
- 14. Shimaya M, Muneta T, Ichinose S, et al. 2010. Magnesium enhances adherence and cartilage formation of synovial mesenchymal stem cells through integrins. Osteoarthritis Cartilage 18:1300–1309. [DOI] [PubMed] [Google Scholar]
- 15. Chastain SR, Kundu AK, Dhar S, et al. 2006. Adhesion of mesenchymal stem cells to polymer scaffolds occurs via distinct ECM ligands and controls their osteogenic differentiation. J Biomed Mater Res A 78:73–85. [DOI] [PubMed] [Google Scholar]
- 16. Enomoto‐Iwamoto M, Iwamoto M, Nakashima K, et al. 1997. Involvement of α5β1 integrin in matrix interactions and proliferation of chondrocytes. J Bone Miner Res 12:1124–1132. [DOI] [PubMed] [Google Scholar]
- 17. Re'em T, Tsur‐Gang O, Cohen S. 2010. The effect of immobilized RGD peptide in macroporous alginate scaffolds on TGFβ1‐induced chondrogenesis of human mesenchymal stem cells. Biomaterials 31:6746–6755. [DOI] [PubMed] [Google Scholar]
- 18. Correa D, Lietman SA. 2017. Articular cartilage repair: current needs, methods and research directions. Semin Cell Dev Biol 62:67–77. [DOI] [PubMed] [Google Scholar]
- 19. Huey DJ, Hu JC, Athanasiou KA. 2012. Unlike bone, cartilage regeneration remains elusive. Science 338:917–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. O'Brien FJ. 2011. Biomaterials & scaffolds for tissue engineering. Mater Today 14:88–95. [Google Scholar]
- 21. Zhang Y, Yang F, Liu K, et al. 2012. The impact of PLGA scaffold orientation on in vitro cartilage regeneration. Biomaterials 33:2926–2935. [DOI] [PubMed] [Google Scholar]
- 22. Wang CC, Yang KC, Lin KH, et al. 2012. Cartilage regeneration in SCID mice using a highly organized three‐dimensional alginate scaffold. Biomaterials 33:120–127. [DOI] [PubMed] [Google Scholar]
- 23. Puppi D, Chiellini F, Piras AM, et al. 2010. Polymeric materials for bone and cartilage repair. Prog Polym Sci 35:403–440. [Google Scholar]
- 24. Makris EA, Gomoll AH, Malizos KN, et al. 2015. Repair and tissue engineering techniques for articular cartilage. Nat Rev Rheumatol 11:21–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Suzuki S, Muneta T, Tsuji K, et al. 2012. Properties and usefulness of aggregates of synovial mesenchymal stem cells as a source for cartilage regeneration. Arthritis Res Ther 14:R136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sekiya I, Muneta T, Horie M, et al. 2015. Arthroscopic transplantation of synovial stem cells improves clinical outcomes in knees with cartilage defects. Clin Orthop Relat Res 473:2316–2326. [DOI] [PMC free article] [PubMed] [Google Scholar]


