Abstract
Objectives
There is evidence that the gut microbiota plays a major role in the pathogenesis of diseases of the central nervous system through the gut–brain axis. The aim of the present study was to analyze gut microbiota composition in bipolar disorder (BD) and its relation to inflammation, serum lipids, oxidative stress, tryptophan (TRP)/kynurenine (KYN) levels, anthropometric measurements and parameters of metabolic syndrome. Further, microbial community differences of individuals with BD compared with healthy controls (HC) were explored.
Methods
In this cross‐sectional study, we performed 16S rRNA gene sequencing of stool samples from 32 BD individuals and 10 HC. Laboratory parameters included inflammatory markers, serum lipids, KYN, oxidative stress and anthropometric measures. Microbial community analysis and correlation to clinical parameters was performed with QIIME, differential abundance analysis of taxa encompassed linear discriminant analysis effect size (LEfSe).
Results
We found a negative correlation between microbial alpha‐diversity and illness duration in BD (R = −0.408, P = 0.021). Furthermore, we identified bacterial clades associated with inflammatory status, serum lipids, TRP, depressive symptoms, oxidative stress, anthropometrics and metabolic syndrome in individuals with BD. LEfSe identified the phylum Actinobacteria (LDA= 4.82, P = 0.007) and the class Coriobacteria (LDA= 4.75, P = 0.010) as significantly more abundant in BD when compared with HC, and Ruminococcaceae (LDA= 4.59, P = 0.018) and Faecalibacterium (LDA= 4.09, P = 0.039) as more abundant in HC when compared with BD.
Conclusions
The present findings suggest that causes and/or consequences of BD may also lie outside the brain. Exploratory research of the gut microbiota in affective disorders like BD may identify previously unknown underlying causes, and offer new research and therapeutic approaches to mood disorders.
Keywords: 16S rRNA gene, bipolar disorder, diversity, gut–brain axis, gut microbiota, illness duration, inflammation, metabolic syndrome, oxidative stress, tryptophan
1. INTRODUCTION
Bipolar affective disorder (BD) is one of the top 10 causes of global disability and premature mortality with enormous socioeconomic impact. Nevertheless, the neurobiological basis of BD is not sufficiently characterized. Relevant factors include structural brain changes, disturbances in neuroplasticity as well as chronobiology. Pathophysiological causes are genetic and environmental factors, including defects in apoptotic, immune‐inflammatory, neurotransmitter, neurotrophin and calcium signaling pathways. Additionally, alterations in oxidative and nitrosative stress, cellular bioenergetics, and membrane or vesicular transport have been found in BD.1 To date, one of the most innovative und significant areas that still need to be investigated in terms of potential environmental factors contributing to BD is the intestinal community of microbes. As recurring affective episodes in BD are associated with a progressing decline in cognitive function, it is especially interesting that an expanding volume of evidence supports the view that cognitive and emotional processes can be altered by microbes acting through the gut–brain axis.2, 3 Key findings show that on the one hand stress influences the composition of the gut microbiota, and bidirectional communication between the gut microbiota and the central nervous system (CNS) influences stress reactivity.4 Several studies have indicated that gut microbiota influences behavior as well as immune provocation influences anxiety‐ and depressive‐like behavior associated with alterations in microbiota.4, 5 Alterations in microbiota might modulate plasticity and related serotonergic and γ‐aminobutyric acid (GABA)ergic signaling pathways in the CNS.5
Currently, most gut microbiota findings result from animal studies, and research in humans is only at its early stages. To our knowledge, up to now, only two studies have involved individuals with BD.6, 7 Evans et al7 compared the gut microbiota of individuals with BD and healthy controls (HC). They found a decreased fractional representation of Faecalibacterium in individuals with BD compared with HC, and found that the abundance of Faecalibacterium was negatively associated with self‐reported burden of disease measures in BD individuals. The second study investigating microbiota in BD6 focused on differences between individuals with BD taking or not taking atypical antipsychotics. A decreased species diversity was only found in females treated with atypical antipsychotics compared with females not taking the respective medication. More specifically, they found a reduction of Lachnospiraceae, Akkermansia and Sutterella. However, besides incomplete mood state information, both studies lack a proper characterization of BD individuals.
Bipolar disorder is known to be associated with a high risk of obesity and metabolic disturbances, which consequently lead to further medical complications, including a more complex illness presentation and a poorer prognosis.8 Several mechanisms underlying the high prevalence of metabolic disturbances and obesity in BD have been discussed so far. Psychopharmacological medication,9 genetic factors,10 serotonergic dysfunction,11 inflammatory mediators12 and increased oxidative stress parameters13 may (beside others) all be involved in the onset and maintenance of obesity in BD. In addition, there is strong evidence that behavioral factors including poor eating behavior and physical inactivity contribute to the progression of the disease.14 As gut microbes are responsible for an enormous array of metabolic activities, for example they harvest energy from food,15 the possibility of a bidirectional relationship between obesity in BD and microbiota changes seems likely. In this context, Hamdani et al16 found that a manic episode could be treated by the application of charcoal, and supposed a role of the gut–brain axis influencing the clinical course of BD. Additionally, fermentation products of bacteria such as short chain fatty acids were found to control various immune responses and regulate the T‐cell network.17 As chronic low‐grade inflammatory processes are known to play an important pathophysiological role in BD,18 interactions of the gut microbiota with inflammatory processes are likely and of interest. In this context, also the production of neurotransmitters in the gut and the presence of different neurotransmitter receptors come into mind. Serotonin (5‐HT), a metabolite of tryptophan (TRP), is mainly produced in the intestine and known to play an important role in the regulation of mood. Another metabolite of TRP is kynurenine (KYN), which is synthesized by indoleamine 2,3‐dioxygenase (IDO), an enzyme that is upregulated in neuropsychiatric disorders related to immune system dysfunction.19 It has been shown that the plasma 5‐HT levels of conventional mice are significantly higher than in germ‐free mice, who have no intestinal microbiota,20 which is demonstrating the capacity of the microbiota to influence 5‐HT levels. Studies relating microbiota composition with blood parameters (TRP, inflammatory and metabolic factors) in humans are strongly needed to confirm the described associations of the gut–brain axis found in animal studies.
Against this background, we hypothesized that the composition of the gut microbiota correlates with distinct changes in inflammatory and metabolic factors in individuals with BD and differs from that of HC. The objectives of the present study were: (i) to delineate the gut microbiota composition in relation to inflammatory and metabolic parameters as well as TRP/KYN levels in individuals with BD; and (ii) to characterize possible differences of the gut microbiota in terms of diversity and/or abundance of bacterial taxa between BD individuals and HCs.
2. MATERIALS AND METHODS
2.1. Participants
Stool samples of 32 inpatients (18 males; 20–65 years old; mean = 41.3 years, SD = 14.7 years) with the Diagnostic and Statistical Manual of Mental Disorders (DSM‐IV)21 diagnosis of bipolar I disorder were collected at the Department of Psychiatry and Psychotherapeutic Medicine, Medical University of Graz. The diagnosis had been established using a structured interview for DSM‐IV (SCID‐I)22 conducted by an experienced psychiatrist or psychologist. All individuals with BD had been hospitalized because of a depressive episode. A detailed medical and psychiatric history was taken. For a clinical rating of the depressive symptoms, participants completed the Beck's Depression Inventory (BDI‐II)23 and were assessed with the Hamilton depression rating scale (HAMD).24 At the time of collecting the stool samples, 13 patients still scored in a depressive range in the BDI‐score (>18) seen as clinically relevant.
All BD individuals were medicated: 24/32 individuals were treated with atypical neuroleptics; 8/32 individuals with lithium; 11/32 individuals with anticonvulsants; and 23/32 individuals with antidepressants. Furthermore, we enrolled 10 HCs (four male) free of any psychiatric or somatic disease. Exclusion criteria for both groups were antibiotic or antifungal treatment within the previous month, regular intake of prebiotics or probiotics, acute or chronic diseases or infections (including rheumatoid arthritis, systemic lupus erythematosus, neurodegenerative and neuroinflammatory disorders), severe alcohol or drug abuse, dementia, history of digestive disease such as inflammatory bowel disease and irritable bowel syndrome, history of gastrointestinal surgery (other than appendectomy), current pregnancy or breast‐feeding.
The investigation was carried out in accordance with the Helsinki Declaration. The study was approved by the institutional review board of the Medical University of Graz (protocol no. 24–123 ex 11/12), and written informed consent was obtained from all participants.
2.2. Measurements
2.2.1. Laboratory testing and biological assays
Fasting blood samples were collected between 8:00 am and 9:30 am, and different parameters including inflammatory markers (C‐reactive protein [CRP] and interleukin‐6 [IL‐6]) and lipids (total cholesterol, high‐density lipoprotein [HDL], and low‐density lipoprotein [LDL]) were analyzed. Markers of TRP availability and metabolism (TRP and KYN serum concentrations) were assessed by high‐performance liquid chromatography.25 The chromatographic system was composed of a Waters Acquity UPLC separations module connected to a Xevo TQ MS triple‐quadrupole mass spectrometer, equipped with a Z‐spray ESI ion source (Waters, Milford, MA, USA). Separation was carried out using a Kinetex XB‐C18, 2.6 μm, 2.1 × 150 mm column (Phenomenex, Torrance, CA, USA). Laboratory testing was performed at the Institute of Laboratory Medicine, Medical Center of Ludwig Maximilian University (LMU), Munich, Germany.
Oxidative stress parameters (levels of thiobarbituric acid reactive substances [TBARS] and malondialdehyde [MDA]) were measured with a method based on TBA‐reaction and high‐performance liquid chromatography (HPLC), which is broadly used in other scientific reports.26 The TBA‐reaction followed by HPLC and fluorometric detection was used to detect TBARS (Merck‐Hitachi, Stuttgart, Germany).
2.2.2. Anthropometric measures and metabolic rating
To assess overweight and obesity, anthropometric parameters were collected (body mass index [BMI], waist‐to‐hip ratio, waist‐to‐height‐ratio). The BMI was calculated as the weight in kilograms divided by the square of height in meters. We defined normal‐weight, overweight and obesity using the BMI cut‐offs according to the World Health Organization (WHO) as follows: normal weight: BMI = 18.50–24.99; overweight: BMI = 25.0 29.9; obesity: BMI ≥ 30.00 (WHO, 2008).
In our sample, we defined “metabolic syndrome” using the criteria of the International Diabetes Federation, including central obesity (waist circumference ≥94 cm in males and ≥80 cm in females) plus any two of the following factors: raised triglycerides (>150 mg/dL or specific treatment for this lipid abnormality), reduced HDL cholesterol (<40 mg/dL in males, <50 mg/dL in females or specific treatment for this lipid abnormality), raised blood pressure (BP; systolic BP ≥130 or diastolic BP ≥85 mm Hg or treatment of previously diagnosed hypertension), raised fasting plasma glucose (≥100 mg/dL) or previously diagnosed type 2 diabetes.27
2.2.3. Stool sample – protocol for patients
All stool samples used for microbiota analysis were collected at the same day or 1 day after collection of the blood sample, and immediately frozen and stored at −20°C before DNA isolation. Interim storage and long‐term sample storage (up to 14 years) at −20°C of faecal specimens guarantees high sample quality for 16S sequencing without altering results.28, 29
2.3. Microbiota analysis
2.3.1. DNA Isolation and PCR amplification
DNA was extracted from stool samples with the PowerLyzer PowerSoil DNA Isolation Kit (MO BIO Laboratories, CA, USA). DNA concentration was measured with Picogreen fluorescence. The V1–V2 region of the 16S rRNA gene was amplified by polymerase chain reaction (PCR) from 50 ng fecal DNA with primers GATTGCCAGCAGCCGCGGTAA and GGACTACCAGGGTATCTAAT. PCR amplification was performed with the Mastermix 16S Complete PCR Kit (Molzym, Bremen, Germany). The first PCR reaction product was subjected to a second round of PCR with primers fusing the 16S primer sequence to the A and P adapters (for Ion Torrent sequencing). Additionally, a barcode sequence was included to multiplex up to 96 samples at the same time. PCR products were subjected to agarose gel electrophoresis. QIAquick (Qiagen, Hilden, Germany) gel extraction system was used to purify the band of the expected length (330 nt). DNA concentration of the final PCR product was raised with Picogreen fluorescence.
2.3.2. Sequencing
Amplicons were pooled equimolarly. Subsequently, emulsion PCR was performed using the Ion Torrent One Touch 2.0 Kit (CatNr: 4480974; Thermo Fisher Scientific, Waltham, MA, USA) according to manufacturer's protocols (Ion PGM Template OT2 200 Kit User Guide, CatNr: 4480974; Thermo Fisher Scientific). After emulsion PCR, the beads were purified on Ion ES station and loaded onto Ion Torrent 318 chips. Sequencing reactions were performed on Ion Torrent PGM using the Ion 400BP Sequencing Kit (Thermo Fisher Scientific). Sequences were split by barcode and transferred to the Torrent suite server. Unmapped bam files were used as input for bioinformatics analysis.
2.3.3. Phylogenic analysis and bioinformatics
All sequences were initially trimmed by a sliding window quality filter with a width of 20 nt and a cutoff of Q20. DeconSeq30 was used to eliminate reads shorter than 100 nucleotides or reads mapping the human genome. The reads were then subjected to error correction using the Acacia tool,31 which led to an error correction of 10%–20% of reads. Subsequently, PCR chimeras were removed by Usearch algorithm32 in de‐novo and reference‐based settings.32 The final sequence files were then analyzed by QIIME 1.8 workflow scripts.33 OTU search was performed using the parallel_pick_open_reference_otus workflow script and the greengenes 13_8 reference database. OTUs were clustered using a 97% similarity threshold.
2.3.4. Statistical analysis and visualization
Groupings supplied in the mapping file were tested for statistical significance using the QIIME implementation of the Adonis test (compare_categories.py script of QIIME version 1.8.0), and significance of individual bacterial strains was determined using the Kruskal–Wallis test (group_significance.py script of QIIME version 1.8.0). LEfSe34 analysis was performed to detect statistically relevant strains in the study groupings. Species richness was calculated with the number of observed species and Chao‐1‐estimator. Data were rarified to 8000 sequences per sample. All remaining statistical calculations were performed in IBM SPSS Statistics Version 22.
3. RESULTS
3.1. Sample characteristics
Sample characteristics of BD patients are displayed in Table 1 in which the means and standard deviations are given.
Table 1.
Demographic data of BD patients
| Individuals with BD | |
|---|---|
| Total number | 32 |
| Number female | 14 |
| Mean age (SD) (years) | 41.31 (14.73) |
| Mean BMI (SD) (kg/m2) | 28.44 (6.08) |
| Number of patients with severe obesity (BMI > 35) | 4 |
| Number of patients with diabetes | 1 |
| Mean illness duration (SD) (years) | 17.53 (13.33) |
| BDI (SD) | 16.45 (11.41) |
| HAMD (SD) | 6.94 (4.37) |
BD, bipolar disorder; BDI, Beck Depression Inventory; BMI, body mass index; HAMD, Hamilton depression rating scale; SD, standard deviation.
3.2. Microbiota analysis in BD patients
Microbiota analysis was performed on a total of 42 stool samples (32 BD patients and 10 HC). After preprocessing, we obtained a total of 1.696.331 sequences with an average of 40.388 sequences per sample (range 12 353.0–69107.0). In a first step, we investigated disease parameters of BD patients in relation to microbiota composition and, in a second step, we examined differences between the BD and HC groups.
3.2.1. Alpha‐diversity and disease parameters
To determine associations between alpha‐diversity (Chao‐1‐estimator and number of observed species) and disease parameters within the BD group, we used Spearman's correlation analysis. Illness duration was inversely correlated with the number of observed species (R = −0.408, P = 0.021), indicating that the longer patients suffered from BD, the less bacterial species were present in their fecal microbiota (Figure 1).
Figure 1.
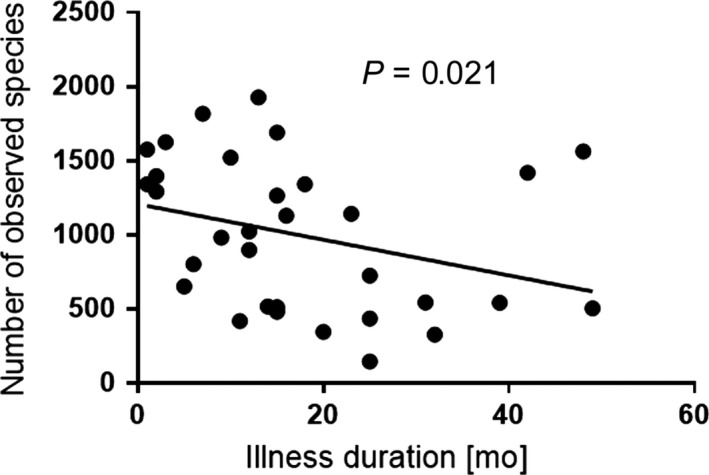
Correlation of illness duration (months) with the number of observed bacterial species
We did not find significant correlations of microbial diversity with serum concentrations of CRP, IL‐6, lipid, TRP, KYN, oxidative stress parameters and depression levels. Furthermore, there were no significant correlations between alpha‐diversity and anthropometric measurements.
3.2.2. Bacterial taxa and disease parameters
We used LEfSe analysis to test for differential abundant microbial taxa in BD patients.34 The median was used to divide the BD group into individuals showing high or low expressions of disease parameters.
3.2.3. Inflammatory markers
First, we used the median to divide the group of BD patients according to inflammatory marker levels. LEfSe analysis revealed that BD patients with high IL‐6 levels showed significantly higher abundances of the order Lactobacillales (LDA = 4.47, P = 0.005), the family of Lactobacillaceae (LDA = 4.42, P = 0.006), the genus Lactobacillus (LDA = 4.43, P = 0.006), the family of Streptococcaceae (LDA = 3.76, P = 0.013) and the genus Streptococcus (LDA = 3.75, P = 0.012) compared with BD individuals with lower IL‐6. There was no difference between the high and low CRP groups in bacterial clades to any significant extent.
3.2.4. Lipids
The BD group with high total cholesterol levels harbored significantly more Clostridiaceae (LDA = 3.48, P = 0.004) compared with BD patients with low cholesterol. Individuals with low LDL cholesterol showed significantly higher abundances of Prevotellaceae (LDA = 4.28, P = 0.025) and the genus Prevotella (LDA = 4.28, P = 0.025). There was no significant difference in bacterial taxa between high and low HDL groups.
3.2.5. Tryptophan‐metabolites
Individuals with high TRP differed significantly in the genus Lactobacillus (LDA = 4.73, P < 0.001), the family of Lactobacillaceae (LDA = 4.73, P < 0.001), the family of Coriobacteriaceae (LDA = 4.41, P = 0.019) and Clostridiaceae (LDA = 3.68, P = 0.044) from individuals with low TRP, while KYN showed no significant differences.
3.2.6. Markers of oxidative stress
Individuals with BD showing high levels of TBARS also had significantly higher abundances of the genus Eubacterium (LDA = 2.30, P = 0.029). Low MDA levels occurred simultaneously with high relative abundances of the genus Faecalibacterium (LDA = 4.22, P = 0.031).
3.2.7. Anthropometric measures and metabolic syndrome parameters
We divided individuals with BD into two groups depending on their median‐split BMI. The genus Lactobacillus (LDA = 4.77, P = 0.015), the family of Lactobacillaceae (LDA = 4.77, P = 0.015) and the class of Bacilli (LDA = 4.81, P = 0.0319) were significantly more abundant in patients with higher BMI. Furthermore, LEfSe identified the family of Lactobacillaceae (LDA = 4.64, P = 0.009), the genus Lactobacillus (LDA = 4.64, P = 0.009) and the family of Coriobacteriaceae (LDA = 3.74, P < 0.001) to be more abundant in BD patients with metabolic syndrome according to the criteria mentioned above.
3.2.8. Clinically relevant depressive symptoms
We conducted a LEfSe analysis to show probable differences of bacterial clades between patients with and without clinically relevant depressive symptoms (BDI cut‐off score 18), whereas three discriminative features could be identified: the family of Enterobacteriaceae (LDA = 3.12, P = 0.044) was more abundant in depressive BD patients, while the family of Clostridiaceae (LDA = 3.41, P = 0.048) and the genus Roseburia (LDA = 3.13, P = 0.016) were more abundant in already healthier BD patients.
3.3. Microbiota of BD patients vs HC
In a second step, we included the HC group in the analysis. The characteristics of BD patients and HC are listed in Table 2.
Table 2.
Demographic data of BD individuals and HC
| BD individuals | HC | P‐value | |
|---|---|---|---|
| Total number | 32 | 10 | |
| Number female | 14 | 6 | 0.381 |
| Mean age (SD) (years) | 41.31 (14.73) | 31.4 (7.61) | 0.009 |
| Mean BMI (SD) (kg/m2) | 28.44 (6.08) | 24.26 (3.76) | 0.047 |
BD, bipolar disorder; BMI, body mass index; HC, healthy controls; SD, standard deviation.
P‐values < 0.05 were regarded as statistically significant.
3.3.1. Alpha‐diversity between groups
Alpha‐diversity was compared between BD patients and HC using the number of observed species, Chao‐1‐estimator, Shannon index and Simpson index. Figure 2 shows rarefaction plots for the number of observed species and Chao‐1. Using the highest available sequencing depth (8000 sequences), no significant differences in the number of observed species (P = 0.179), Chao‐1‐diversity index (P = 0.767), Shannon index (P = 0.217) and Simpson index (P = 0.379) could be observed between the groups. As diabetes and obesity could affect gut microbiota diversity, we excluded one BD patient suffering from diabetes mellitus and four patients with severe obesity (BMI > 35) to enhance the accuracy of our results. Again, there were no significant differences in the number of observed species (P = 0.133), Chao‐1‐diversity index (P = 0.696), Shannon index (P = 0.169) and Simpson index (P = 0.339).
Figure 2.
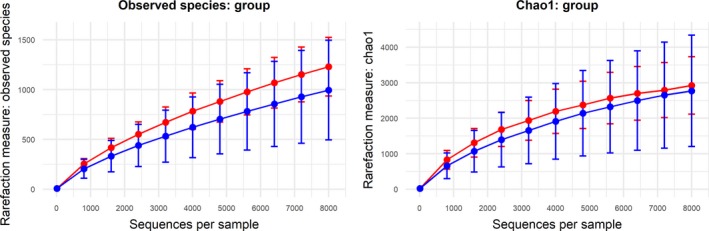
Alpha‐diversity measures observed species (left) and the Chao‐1‐index (right). Bipolar disorder (BD) individuals are shown in blue, healthy controls (HC) in red [Colour figure can be viewed at wileyonlinelibrary.com]
3.3.2. Beta‐diversity between groups
Using beta‐diversity analysis, both weighted (A) and unweighted (B) principal component analysis (PCoA) UniFrac plots were generated in QIIME using a total of 42 participants. PCoA plots are a multivariate statistical method to depict phylogenetic distances between samples. The weighted PCoA UniFrac plot includes the relative abundances of OTUs among groups, while the unweighted PCoA UniFrac plot represents the phylogenetic distances based on the presence or absence of OTUs. Each dot represents an individual sample. No significant differences could be detected (Figure S1A and B). After exclusion of the patient with diabetes and the patients with severe obesity (n = 4), still no significant differences could be detected (Figure S2A and B).
3.3.3. Differentially abundant bacterial clades between BD patients and HC
LEfSe was used to identify differential microbial abundances between BD patients and HC. This analysis revealed significant differences in bacterial clades from phylum to species level between BD patients and HC. Importantly, six features showed a markedly high LDA score (LDA ≥ 4.5) and total abundances in percent (Table 3). The results after exclusion of the patient with diabetes and the patients with severe obesity are depicted in Table S1.
Table 3.
Differentially abundant features of BD individuals in comparison to HC with abundances in the percentage range
| Domain | LDA score | P‐value |
|---|---|---|
| More abundant in BD individuals | ||
| Phylum Actinobacteria | 4.82 | 0.007 |
| Class Coriobacteria | 4.75 | 0.010 |
| Order Coriobacteriales | 4.75 | 0.011 |
| Family Coriobacteriaceae | 4.75 | 0.010 |
| More abundant in HC | ||
| Family Ruminococcaceae | 4.59 | 0.018 |
| Genus Faecalibacterium | 4.09 | 0.039 |
BD, bipolar disorder; HC, healthy controls; LDA, linear discriminative analysis.
4. DISCUSSION
In the current study, we describe associations between the gut microbiota and clinical parameters in a well‐characterized cohort of individuals with BD, and identify differentially abundant bacterial taxa in comparison to a HC group. As hypothesized, we were able to find significant relationships between representations of several bacterial taxa and disease parameters in individuals with BD, and distinct differences between patients and HCs. Our data represent the first investigation of BD disease parameters, such as markers of inflammation, immune activation, metabolism and oxidative stress along with analysis of the gut microbiota in BD.
Alpha‐diversity, which describes the number of different bacterial taxa prevalent in the gut, has been proved to be of potential relevance to the pathogenesis of various diseases. For example, a lower bacterial diversity is related to inflammatory bowel disease35 and anorexia nervosa.36 Conversely, in a recent study Jiang et al found increased alpha‐diversity in patients with acute major depression compared with a HC group.37 In our study we showed that alpha‐diversity correlated negatively with illness duration in BD individuals. This might be due to inflammatory processes in BD, which cause neurobiological and functional impairment increasing with illness duration.38 Recently, Tatay‐Manteiga et al described an association between biomarkers of inflammation and poorer functional outcome of BD patients by depicting differences in inflammation markers in BD patients in different stages of the disease.39 On the other hand, our results could also be attributed to the continuous or prolonged use of psychopharmacological medication.40 This would be in line with Jiang et al, where the increase of bacterial alpha‐diversity was only found in active depression but not in responded major depressive patients after psychopharmacological treatment.37 Another potential influencing factor for lower alpha‐diversity in BD individuals with long illness duration might be poor nutrition. Poor diet quality was associated with twice the odds for BD.41 Therefore, dietary patterns may be a factor influencing alpha‐diversity in the long term. However, longitudinal studies are needed to show whether the number of observed species decreases during the course of BD. Findings of longitudinal studies may also be helpful to support the literature on the clinical use of biomarkers of neuroprogression and clinical staging models in BD.38, 42
Looking more detailed into our analyses, we could identify Faecalibacterium as a feature discriminating between BD individuals and HC, given that HC showed higher abundances of Faecalibacterium. This goes in line with a recent study by Evans et al7 who found a group difference in Faecalibacterium abundance in their analysis of stool samples between BD patients and HC, suggesting a depletion of the beneficial bacterium in BD associated with worse self‐reported health outcomes. Of note, reduced levels of Faecalibacterium have also been observed in patients suffering from major depression when compared with HC.37 We further found significant differences in the phylum of Actinobacteria and the family of Coriobacteriaceae between individuals with BD and HCs. Actinobacteria and Coriobacteriaceae are common inhabitants of the GI‐tract involved in lipid metabolism43 and shown to correlate with cholesterol levels,44 which could be the reason for finding them more abundant in the BD group.
As several studies have recognized an increased risk of individuals with BD to suffer from somatic comorbidities such as obesity, autoimmune disorders, diabetes and cardiovascular disease,45 we hypothesized that distinct alterations of clinical parameters in BD are related to particular changes in the composition of the gut microbiota. BD has been related to alterations of the immune system; however, the exact mechanisms of immune dysfunction in BD remain unclear. As the gut microbiota has been shown to severely affect immune function, gut dysbiosis might be a factor contributing to immune alterations in BD.46 For example, meta‐analysis data depict increased activation of innate and cell‐mediated immune processes in BD.12 In our study, BD individuals with higher IL‐6 levels showed significantly higher abundances of Lactobacillales, Streptococcaceae and Bacilli. An analogous correlation was found in the study of Yamashiro et al47 in patients with ischemic stroke, where a high prevalence of Lactobacillus ruminis was positively correlated with IL‐6 levels.In the study of Million et al, the genus Lactobacillus was found to be more abundant in obese study participants compared with lean controls.48 Similarly, in our study, individuals with BD and higher BMI harbored significantly more Lactobacilli when compared with the lower BMI group. Moreover, the family of Lactobacillaceae and the genus Lactobacillus were more abundant in BD individuals with metabolic syndrome. Therefore, Lactobacilli could be a factor contributing to obesity in BD; however, we cannot deduce a causal relationship from the results of our cross‐sectional study.
Importantly, oxidative stress has been found to trigger rapid shifts in intestinal microbial groups,49 and obesity is associated with a highly prevalent state of chronic oxidative stress in BD. In our study, individuals with higher oxidative stress, measured by MDA levels, showed high abundances of Faecalibacterium. Noteworthy, the BD individuals investigated in the current study had significantly higher BMI values than HC. In obese individuals, antioxidant defenses are attenuated because of a decreased intake of antioxidants or an increased utilization of these molecules,50 which could potentially have influenced microbial community composition.
From a psychiatric perspective, it is interesting that the association between neurotransmitters and gut microbiota has already been investigated. The results showed that most of our neurotransmitters can be produced by gut bacteria. For example, Lactobacillus species produce acetylcholine and GABA; Bifidobacterium species also synthesize GABA; Escherichia produces noradrenaline, 5‐HT and dopamine; Streptococcus and Enterococcus synthesize 5‐HT; and Bacillus species produce dopamine and noradrenaline.3 TRP, which is a 5‐HT precursor, has been shown to be altered in BD along with changes in KYN pathway metabolites.51
In our study, high TRP levels were associated with the family of Lactobacillaceae and the order of Lactobacillales. This is especially interesting as in animal studies, certain Lactobacillus species have been shown to decrease IDO activity, which goes along with higher available TRP levels.52 However, as the TRP level could be altered by psychopharmacological agents and our BD patients received psychopharmacological treatment, results should be interpreted with caution. Nevertheless, certain gut bacteria may be considered as psychobiotics that act on the gut–brain axis by producing neurotransmitters.53
In light of this, future research should examine microbiota‐targeted therapies, such as prebiotics, probiotics and nutritional interventions, for psychiatric patients.54, 55 Nutritional psychiatry is an emerging field highlighting the need for dietary quality for the prevention and treatment of psychiatric disorders.56 Therefore, nutritional interventions on the one hand and bacterial‐based interventions with health benefit (psychobiotics53) on the other hand might represent a significant advance in future BD therapies. Accordingly, the longitudinal scientific investigation of personalized interventions addressed to target the gut–brain axis in BD patients is urgently warranted.
It is important to note that our study has some limitations. Gut microbiota research in BD is still in its infancy, and the communication pathways between the microbiota and the brain have not yet been identified in detail. Because of the dynamic and changing microbial community, which is highly modifiable by external lifestyle parameters such as diet and physical activity, any mechanistic relationships are difficult to study. Currently, the potential direct effects of acute states of BD on gut microbiota are largely unknown, and especially precise consequences remain unclear. All our BD inpatients were in an acute episode of bipolar depression. Acute episodes may influence microbial diversity by various mechanisms like stress associated with the recent relapse, lifestyle changes due to hospital admission (changes in diet, physical activity, smoking habits, sleep quality), and the need of higher doses of medication and polypharmacy. We can underline this (for example) by our results of differences of bacterial clades between BD patients with still relevant clinical depressive symptoms and already healthier patients. Depressed BD individuals showed significantly higher abundances of Enterobacteriaceae, while already healthier BD patients showed higher abundances of Clostridiaceae and Roseburia. However, the results must be interpreted with caution due to another limitation of the study, the small sample size of groups. Nevertheless, our results support the current literature on the complex interaction between the gut–brain axis and BD. Further studies on state‐ and trait‐related effects and influences of BD on gut microbiota are highly interesting and needed.
A further limitation is that neither individuals with BD nor controls received a standardized diet. The gut microbiota composition is influenced by dietary changes,57 which must be taken into account when interpreting our results. This can also be said for lifestyle parameters,58, 59, 60 which were not explicitly assessed in our study participants. Additionally, BD individuals remained on their usual pharmacotherapy, which could have had an impact on the gut microbiota.6 Therefore, the correlation between low species richness and long disease duration could in part be due to medication effects. Psychopharmacological agents such as antidepressants and lithium have been shown to have antibiotic properties,61 which could affect the gut microbiota in the short and long term. In general, studies with larger sample size need to confirm this finding.
Therefore, it remains difficult to establish, in the clinical setting, causality between gut dysbiosis and an episode of BD, and to deduce whether gut dysbiosis causes BD or vice versa. It remains uncertain whether we see differences in microbial features because of the presence of metabolic parameters, medication or because of BD itself. As BD is highly comorbid with inflammatory diseases and obesity, it is conceivable that complex interactions between the investigated parameters are responsible for the detected changes of the gut microbiota.
Nevertheless, our observations are relevant to the microbiota–psychiatric illness link because of the need of studies in humans to confirm postulated alterations of cognitive and emotional processes caused by microbes in animal studies, and contribute valuable knowledge about additional causes and consequences of the increased incidence of obesity and metabolic syndrome associated with mood disorders, especially BD.
We can conclude that metabolic syndrome and low‐grade inflammation are important trademarks for BD that could be possibly affected by the gut microbiota. Our results point out that BD may not uniquely be an affective disorder secondary to cerebral monoamine dysregulations, but could also be understood in the wider context of gut–brain axis dysfunction. Further research involving interventional studies is needed to discover novel treatment strategies influencing the gut microbiota and the gut–brain axis in BD patients, and to elucidate potentially beneficial effects of prebiotics, probiotics and antibiotics. These treatment strategies might as well have effects and provide alternative therapeutic options for BD and its common comorbidities such as obesity and inflammation.
DISCLOSURES
Financial support from the “Stadt Graz” (City of Graz, Austria) has been received for this study (Grant Number: A16 – 28180/2009). All authors declare that they have no conflicts of interest. There have been no commercial or other relationships that could constitute a conflict of interest.
Supporting information
ACKNOWLEDGEMENTS
The authors thank all participants in the study as well as staff of the Department of Psychiatry Graz, Medical University of Graz, Austria, with special thanks to Renate Unterweger for her additional support.
Painold A, Mörkl S, Kashofer K, et al. A step ahead: Exploring the gut microbiota in inpatients with bipolar disorder during a depressive episode. Bipolar Disord. 2019;21:40–49. 10.1111/bdi.12682
Annamaria Painold and Sabrina Mörkl contributed equally to the contents of this manuscript and therefore share first authorship.
REFERENCES
- 1. Sigitova E, Fisar Z, Hroudova J, Cikankova T, Raboch J. Biological hypotheses and biomarkers of bipolar disorder. Psychiatry Clin Neurosci. 2016;71:77‐103. [DOI] [PubMed] [Google Scholar]
- 2. Forsythe P, Sudo N, Dinan T, Taylor VH, Bienenstock J. Mood and gut feelings. Brain Behav Immun. 2010;24:9‐16. [DOI] [PubMed] [Google Scholar]
- 3. Cryan JF, Dinan TG. Mind‐altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13:701‐712. [DOI] [PubMed] [Google Scholar]
- 4. Bailey MT, Dowd SE, Galley JD, Hufnagle AR, Allen RG, Lyte M. Exposure to a social stressor alters the structure of the intestinal microbiota: implications for stressor‐induced immunomodulation. Brain Behav Immun. 2011;25:397‐407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Foster JA, McVey Neufeld KA. Gut‐brain axis: how the microbiome influences anxiety and depression. Trends Neurosci. 2013;36:305‐312. [DOI] [PubMed] [Google Scholar]
- 6. Flowers SA, Evans SJ, Ward KM, McInnis MG, Ellingrod VL. Interaction between atypical antipsychotics and the gut microbiome in a bipolar disease cohort. Pharmacotherapy. 2017;37:261‐267. [DOI] [PubMed] [Google Scholar]
- 7. Evans SJ, Bassis CM, Hein R, et al. The gut microbiome composition associates with bipolar disorder and illness severity. J Psychiatr Res. 2017;87:23‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Thompson WK, Kupfer DJ, Fagiolini A, Scott JA, Frank E. Prevalence and clinical correlates of medical comorbidities in patients with bipolar I disorder: analysis of acute‐phase data from a randomized controlled trial. J Clin Psychiatry. 2006;67:783‐788. [DOI] [PubMed] [Google Scholar]
- 9. Virk S, Schwartz T, Jindal S, Nihalani N, Jones N. Psychiatric medication induced obesity: an aetiologic review. Obes Rev. 2004;5:167‐170. [DOI] [PubMed] [Google Scholar]
- 10. Winham S, Cuellar‐Barboza A, Oliveros A, et al. Genome‐wide association study of bipolar disorder accounting for effect of body mass index identifies a new risk allele in TCF7L2. Mol Psychiatry. 2014;19:1010‐1016. [DOI] [PubMed] [Google Scholar]
- 11. Reininghaus EZ, McIntyre RS, Reininghaus B, et al. Tryptophan breakdown is increased in euthymic overweight individuals with bipolar disorder: a preliminary report. Bipolar Disord. 2014;16:432‐440. [DOI] [PubMed] [Google Scholar]
- 12. Munkholm K, Vinberg M, Kessing LV. Cytokines in bipolar disorder: a systematic review and meta‐analysis. J Affect Disord. 2013;144:16‐27. [DOI] [PubMed] [Google Scholar]
- 13. Bengesser S, Lackner N, Birner A, et al. Peripheral markers of oxidative stress and antioxidative defense in euthymia of bipolar disorder – gender and obesity effects. J Affect Disord. 2015;172:367‐374. [DOI] [PubMed] [Google Scholar]
- 14. Wildes JE, Marcus MD, Fagiolini A. Obesity in patients with bipolar disorder: a biopsychosocial‐behavioral model. J Clin Psychiatry. 2006;67:904‐915. [DOI] [PubMed] [Google Scholar]
- 15. Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity‐associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027‐1131. [DOI] [PubMed] [Google Scholar]
- 16. Hamdani N, Boukouaci W, Hallouche MR, et al. Resolution of a manic episode treated with activated charcoal: evidence for a brain–gut axis in bipolar disorder. Aust N Z J Psychiatry. 2015;49:1221‐1223. [DOI] [PubMed] [Google Scholar]
- 17. Furusawa Y, Obata Y, Fukuda S, et al. Commensal microbe‐derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504:446‐450. [DOI] [PubMed] [Google Scholar]
- 18. Kunz M, Ceresér KM, Goi PD, et al. Serum levels of IL‐6, IL‐10 and TNF‐α in patients with bipolar disorder and schizophrenia: differences in pro‐ and anti‐inflammatory balance. Revista brasileira de psiquiatria. 2011;33:268‐274. [DOI] [PubMed] [Google Scholar]
- 19. Capuron L, Miller AH. Immune system to brain signaling: neuropsychopharmacological implications. Pharmacol Ther. 2011;130:226‐238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Collins SM, Bercik P. The relationship between intestinal microbiota and the central nervous system in normal gastrointestinal function and disease. Gastroenterology. 2009;136:2003‐2014. [DOI] [PubMed] [Google Scholar]
- 21. Association AP. Diagnostic and Statistical Manual of Mental Disorders. 4th edn Washington, DC: American Psychiatric Press; 1994. [Google Scholar]
- 22. First MB, Spitzer RL, Gibbon M, Williams JW. Structured Clinical Interview for DSM‐IV Axis I Disorders, Clinician Version (SCID‐CV). Washington, DC: American Psychiatric Press; 1996. [Google Scholar]
- 23. Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561‐571. [DOI] [PubMed] [Google Scholar]
- 24. Hamilton M. Hamilton Depression Scale. ECDEU Assessment Manual for Psychopharmacology, Revised Edition. Rockville, MD: National Institute of Mental Health; 1976:179‐192. [Google Scholar]
- 25. Widner B, Werner ER, Schennach H, Wachter H, Fuchs D. Simultaneous measurement of serum tryptophan and kynurenine by HPLC. Clin Chem. 1997;43:2424‐2426. [PubMed] [Google Scholar]
- 26. Bengesser SA, Lackner N, Birner A, et al. Mood stabilizers, oxidative stress and antioxidative defense in euthymia of bipolar disorder. CNS Neurol Disord Drug Targets. 2016;15:381‐389. [DOI] [PubMed] [Google Scholar]
- 27. Zimmet P, Alberti KG, Serrano Rios M. A new International Diabetes Federation (IDF) worldwide definition of the metabolic syndrome: the rationale and the results. Rev Esp Cardiol. 2005;58:1371‐1375. [PubMed] [Google Scholar]
- 28. Kia E, Mackenzie BW, Middleton D, et al. Integrity of the human faecal microbiota following long‐term sample storage. PLoS ONE. 2016;11:e0163666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bassis CM, Moore NM, Lolans K, et al. Comparison of stool versus rectal swab samples and storage conditions on bacterial community profiles. BMC Microbiol. 2017;17:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schmieder R, Edwards R. Fast identification and removal of sequence contamination from genomic and metagenomic datasets. PLoS ONE. 2011;6:e17288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bragg L, Stone G, Imelfort M, Hugenholtz P, Tyson GW. Fast, accurate error‐correction of amplicon pyrosequences using Acacia. Nat Methods. 2012;9:425‐426. [DOI] [PubMed] [Google Scholar]
- 32. Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26:2460‐2461. [DOI] [PubMed] [Google Scholar]
- 33. Caporaso JG, Kuczynski J, Stombaugh J, et al. QIIME allows analysis of high‐throughput community sequencing data. Nat Methods. 2010;7:335‐336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Segata N, Izard J, Waldron L, et al. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12:R60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sartor RB. Microbial influences in inflammatory bowel diseases. Gastroenterology. 2008;134:577‐594. [DOI] [PubMed] [Google Scholar]
- 36. Morkl S, Lackner S, Muller W, et al. Gut microbiota and body composition in anorexia nervosa inpatients in comparison to athletes, overweight, obese, and normal weight controls. Int J Eat Dis. 2017;50:1421‐1431. [DOI] [PubMed] [Google Scholar]
- 37. Jiang H, Ling Z, Zhang Y, et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav Immun. 2015;48:186‐194. [DOI] [PubMed] [Google Scholar]
- 38. Gama CS, Kunz M, Magalhães PV, Kapczinski F. Staging and neuroprogression in bipolar disorder: a systematic review of the literature. Revista Brasileira de Psiquiatria. 2013;35:70‐74. [DOI] [PubMed] [Google Scholar]
- 39. Tatay‐Manteiga A, Balanzá‐Martínez V, Bristot G, Tabarés‐Seisdedos R, Kapczinski F, Cauli O. Clinical staging and serum cytokines in bipolar patients during euthymia. Prog Neuropsychopharmacol Biol Psychiatry. 2017;77:194‐201. [DOI] [PubMed] [Google Scholar]
- 40. Ticinesi A, Milani C, Lauretani F, et al. Gut microbiota composition is associated with polypharmacy in elderly hospitalized patients. Sci Rep. 2017;7:11 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jacka FN, Pasco JA, Mykletun A, et al. Diet quality in bipolar disorder in a population‐based sample of women. J Affect Disord. 2011;129:332‐337. [DOI] [PubMed] [Google Scholar]
- 42. Kapczinski F, Magalhães P, Balanzá‐Martinez V, et al. Staging systems in bipolar disorder: an international society for bipolar disorders task force report. Acta Psychiatr Scand. 2014;130:354‐363. [DOI] [PubMed] [Google Scholar]
- 43. Clavel T, Lepage P, Charrier C. The Family Coriobacteriaceae. The Prokaryotes: Springer; 2014:201‐238. [Google Scholar]
- 44. Lahti L, Salonen A, Kekkonen RA, et al. Associations between the human intestinal microbiota, Lactobacillus rhamnosus GG and serum lipids indicated by integrated analysis of high‐throughput profiling data. PeerJ. 2013;1:e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. SayuriYamagata A, Brietzke E, Rosenblat J, Kakar R, McIntyre RS. Medical comorbidity in bipolar disorder: the link with metabolic‐inflammatory systems. J Affect Disord. 2017;211:99‐106. [DOI] [PubMed] [Google Scholar]
- 46. Borre YE, O'Keeffe GW, Clarke G, Stanton C, Dinan TG, Cryan JF. Microbiota and neurodevelopmental windows: implications for brain disorders. Trends Mol Med. 2014;20:509‐518. [DOI] [PubMed] [Google Scholar]
- 47. Yamashiro K, Tanaka R, Urabe T, et al. Gut dysbiosis is associated with metabolism and systemic inflammation in patients with ischemic stroke. PLoS ONE. 2017;12:e0171521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Million M, Angelakis E, Maraninchi M, et al. Correlation between body mass index and gut concentrations of Lactobacillus reuteri, Bifidobacterium animalis, Methanobrevibacter smithii and Escherichia coli . Int J Obes. 2013;37:1460‐1466. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 49. Weiss GA, Hennet T. Mechanisms and consequences of intestinal dysbiosis. Cell Mol Life Sci. 2017;74:2959‐2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Savini I, Catani MV, Evangelista D, Gasperi V, Avigliano L. Obesity‐associated oxidative stress: strategies finalized to improve redox state. Int J Mol Sci. 2013;14:10 497‐10 538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Birner A, Platzer M, Bengesser SA, et al. Increased breakdown of kynurenine towards its neurotoxic branch in bipolar disorder. PLoS ONE. 2017;12:e0172699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Valladares R, Bojilova L, Potts AH, et al. Lactobacillus johnsonii inhibits indoleamine 2, 3‐dioxygenase and alters tryptophan metabolite levels in BioBreeding rats. FASEB J. 2013;27:1711‐1720. [DOI] [PubMed] [Google Scholar]
- 53. Dinan TG, Stanton C, Cryan JF. Psychobiotics: a novel class of psychotropic. Biol Psychiat. 2013;74:720‐726. [DOI] [PubMed] [Google Scholar]
- 54. R Caso J, Balanza‐Martinez V, Palomo T, Garcia‐Bueno B. The microbiota and gut‐brain axis: contributions to the immunopathogenesis of schizophrenia. Curr Pharm Des. 2016;22:6122‐6133. [DOI] [PubMed] [Google Scholar]
- 55. Dinan TG, Cryan JF. Melancholic microbes: a link between gut microbiota and depression? Neurogastroenterol Motil. 2013;25:713‐719. [DOI] [PubMed] [Google Scholar]
- 56. Sarris J, Logan AC, Akbaraly TN, et al. Nutritional medicine as mainstream in psychiatry. Lancet Psychiatry. 2015;2:271‐274. [DOI] [PubMed] [Google Scholar]
- 57. David LA, Maurice CF, Carmody RN, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559‐563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bressa C, Bailén‐Andrino M, Pérez‐Santiago J, et al. Differences in gut microbiota profile between women with active lifestyle and sedentary women. PLoS ONE. 2017;12:e0171352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. David LA, Materna AC, Friedman J, et al. Host lifestyle affects human microbiota on daily timescales. Genome Biol. 2014;15:R89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Capurso G, Lahner E. The interaction between smoking, alcohol and the gut microbiome. Best Pract Res Clin Gastroenterol. 2017;31:579‐588. [DOI] [PubMed] [Google Scholar]
- 61. Lieb J. The immunostimulating and antimicrobial properties of lithium and antidepressants. J Infect. 2004;49:88‐93. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


