Abstract
Alginate‐based hydrogels represent promising microenvironments for cell culture and tissue engineering, as their mechanical and porous characteristics are adjustable toward in vivo conditions. However, alginate scaffolds are bioinert and thus inhibit cellular interactions. To overcome this disadvantage, bioactive alginate surfaces were produced by conjugating tyramine molecules to high‐molecular‐weight alginates using the carbodiimide chemistry. Structural elucidation using nuclear magnetic resonance spectroscopy and contact angle measurements revealed a surface chemistry and wettability of tyramine‐alginate hydrogels similar to standard cell culture treated polystyrene. In contrast to stiff cell culture plastic, tyramine‐alginate scaffolds were found to be soft (60–80 kPa), meeting the elastic moduli of human tissues such as liver and heart. We further demonstrated an enhanced protein adsorption with increasing tyramine conjugation, stable for several weeks. Cell culture studies with human mesenchymal stem cells and human pluripotent stem cell‐derived cardiomyocytes qualified tyramine‐alginate hydrogels as bioactive platforms enabling cell adhesion and contraction on (structured) 2‐D layer and spherical matrices. Due to the alginate functionalization with tyramines, stable cell–matrix interactions were observed beneficial for an implementation in biology, biotechnology, and medicine toward efficient cell culture and tissue substitutes. © 2018 The Authors. Journal of Biomedical Materials Research Part A published by Wiley Periodicals, Inc. J Biomed Mater Res Part A: 107A: 114–121, 2019.
Keywords: alginate, tyramine, biointerface engineering, mesenchymal stem cells, cardiomyocytes
INTRODUCTION
Controlling the interface between biomaterials and biological entities such as proteins and cells represents the key for sophisticated cell cultures, implants, and disease models for drug screening. In the last decades, the design of biomaterials was widely investigated to direct cellular behavior.1, 2 Besides the surface characteristics of the biomaterial such as its wettability,3 charge,4 chemistry,5 and topography,6 the materials mechanical stability7 and porosity8 were defined as crucial parameters for cell–matrix interactions. The mechanical microenvironment of the cells affects cellular processes like adhesion,9 gene expression,10 and differentiation.11 Nevertheless, standard cell culture processes are still mainly carried out on stiff plastic neglecting the significance of the cell–matrix interface.
To meet the requirements of the complex, native cell environment soft and porous materials with adjustable mechanical properties are needed. Here, alginate as a widely applied biomaterial in tissue engineering12, 13, 14 represents a promising cell microenvironment, as this biocompatible polymer is able to form hydrogels with adaptable mechanical characteristics and porosities.15 However, native alginate inhibits cell adhesion due to its repelling anionic surface and lacking cell interaction sites.16 Hence, this study aims to address cell–alginate interactions via the functionalization of the cross‐linked alginate scaffold (ALG).
Standard cell culture surfaces are mainly based on polystyrene (PS) which were modified by sulfuric acid treatment and physical methods (corona discharge, gas plasma, or irradiation) to introduce hydroxylic, phenolic, and carboxylic groups to the carbon backbone.17 In comparison, alginate consists of 1,4‐glycosidically linked mannuronic and guluronic acids bearing hydroxylic and carboxylic functional groups. Consequently, the surface chemistry of standard cell culture plastic can be simulated via the introduction of phenolic groups to the alginate scaffolds. Tyramine, a biogenic amine occurring in plants and animals, owns a phenolic component and a terminal amino group which represents a suitable binding site for the amidation with alginate using the carbodiimide chemistry. In previous studies, tyramine was coupled to a wide range of natural polymers such as hyaluronic acid,18 dextran,19 and alginate20, 21, 22, 23 to induce an enzyme‐mediated gelation using horseradish peroxidase (HRP) and hydrogen peroxide.18, 19, 20, 22, 23 These bioresponsive systems were mainly applied for encapsulations18, 20, 22 and 3D printing21 as in situ‐forming, injectable hydrogels.
In contrast, this study targets the functionalization of ALG with tyramine to enable a stable cell adhesion to soft, bioactive scaffolds applicable for the demands of future tissue engineering approaches in, for example, personalized medicine.
MATERIALS AND METHODS
ALG fabrication
High‐molecular‐weight alginates extracted from the brown algae Lessonia nigrescens (LN) and Lessonia trabeculata (LT) (Alginatec, Riedenheim, Germany) were applied for scaffold fabrication as 0.7% (w/v%) solutions in isotonic, 0.9% sodium chloride solution (NaCl; B. Braun, Melsungen, Germany), and 1:1 mixtures to adjust a defined mannuronate/guluronate ratio.
Planar and structured ALG
Planar alginate layers were used as scaffolds positioned on round standard cell culture treated polystyrene‐based coverslips (Thermanox™, Ø 13 mm, Thermo Fisher Scientific, Dreieich, Germany) and dishes (Ø 35 mm, Corning, New York, NY) treated with poly‐l‐lysine (pLL; Sigma‐Aldrich, Taufkirchen, Germany) in phosphate‐buffered saline (PBS; Gibco, Thermo Fisher Scientific, Darmstadt, Germany) for 30 min at 37°C. The alginate solution (LN/LT 1:1, 0.7% [wt %/vol %]) were placed on the treated, dried plastic surfaces and gelled with 20 mM barium chloride solution (BaCl2) for 20 min at room temperature (RT). The obtained alginate layers were washed three times with NaCl and stored at 4°C until usage.
Structured, planar alginate surfaces were manufactured using a μ‐contact printer (GeSiM mbH, Rossendorf, Germany; see Supporting Information, Fig. 1). The alginate solution was placed on an agarose gel (4% [wt/vol %]; Sigma‐Aldrich, Taufkirchen, Germany) containing 100 mM BaCl2 and structured for 3 min by a stamp based on polydimethylsiloxane (PDMS; Sylgard 184 elastomer kit, Sigma‐Aldrich, Taufkirchen, Germany). The structured hydrogel was transferred to a pLL‐treated plastic dish, post‐cross‐linked with 20 mM BaCl2 for 10 min, washed three times with NaCl, and stored at 4°C until usage.
Spherical ALG
In terms of spherical scaffolds, alginate was processed as described previously.24 Briefly, the alginate solution was dispersed into droplets using a coaxial air stream and polymerized while dropping into the aqueous 20 mM BaCl2. After 20 min of cross‐linking, spherical ALG were washed three times with NaCl and stored at 4°C until usage.
ALG modification
To introduce bioactive entities, alginate's carboxylic groups were activated by aqueous carbodiimide chemistry resulting in the conjugation of N‐hydroxysuccinimide (NHS; Sigma‐Aldrich, Taufkirchen, Germany). The obtained alginate‐NHS esters were subsequently coupled with tyramine hydrochloride (Sigma‐Aldrich, Taufkirchen, Germany) in various quantities (2.5, 7.5, and 12.5 mg/cm2 surface area) for 24 h at RT. Matrigel‐coated surfaces were produced by the incubation of tyramine‐conjugated alginate scaffolds (ALGTYR) with Matrigel (8.7 μg/cm2 surface area, Corning, NY) for 15 h at RT. Final modified ALG were washed three times with NaCl.
Nuclear magnetic resonance (NMR) spectroscopy
For structural elucidation of the (modified) ALG, NMR spectroscopy was applied. Ultrasonically, freeze‐dried alginate samples were dissolved in D2O (7.5 mg/mL) containing sodium‐3‐trimethylsilylpropionate‐2,2,3,3‐d4 (TSP) as an internal reference and analyzed with 128 scans and water suppression (presaturation) at 80°C. 1H NMR spectra were recorded using a Bruker DRX 500 (Bruker, MA) and processed (integrations and deconvolutions) using the software TopSpin.
Wettability analysis using captive bubble technique
Contact angles were measured using an OCA device with a conventional goniometer contact angle apparatus with an additional bracket for flat samples (SHC 20), a glass cuvette (GC 40), an upward curved dispensing needle (SNC 052/026), a digital image capture, and data analysis software (SCA 20) (all from DataPhysics Instruments, Filterstadt, Germany) at RT. Three seconds after placing an air bubble (3 μL) onto the hydrogel surface, which is surrounded with NaCl, a digital image was captured and analyzed using the SCA 20 software. To measure contact angles after protein adsorption, the (modified) ALG were incubated in DMEM/F‐12 (Gibco, Thermo Fisher Scientific, Darmstadt, Germany) supplemented with 10% (v/v%) fetal calf serum (FCS; Sigma‐Aldrich, Taufkirchen, Germany) at 37°C for 24 h and were subsequently washed with NaCl before analyzing.
Zeta potential measurements
Zeta potentials of (modified) spherical ALG (Ø 50 μm, 10 cm2 total surface area) suspended in PBS were measured at 25°C in a capillary cell (Malvern Instruments, Worcestershire, England) using the Zetasizer Nano‐ZS PN3702 (Malvern Instruments, Worcestershire, England). Surface charges after protein adsorption were analyzed after the incubation (24 h) of spherical ALG in DMEM/F‐12 supplemented with 10% FCS at 37°C and three washing steps with NaCl before analyzing.
Compression tests
(Modified) ALG (Ø 13 mm, height: 1.4 mm) surrounded with NaCl were compressed until 35% of strain with a deformation rate of 0.5 mm/s using TA.XTplus (Stable Micro Systems, Godalming, United Kingdom). The elastic moduli were calculated as the slope of the stress–strain curve (10–30% of strain).
Protein adsorption assays
Ellman's reagent
The sulfhydryl groups of adsorbed proteins were determined using 5,5′‐dithio‐bis‐(2‐nitrobenzoic acid) (DTNB, Sigma‐Aldrich, Taufkirchen, Germany). Therefore, the (modified) ALG were incubated in DMEM/F‐12 supplemented with 10% FCS at 37°C for 24 h and subsequently washed with PBS. After the addition of 0.1 mM DTNB solution and 2 min of incubation at RT, the absorbance of the test samples was measured at 412 nm with the Infinite F200 microplate reader (Tecan, Maennedorf, Switzerland).
Immunofluorescence staining
Spherical ALGTYR were treated with laminin (1.0 μg/cm2 surface area; L2020, Sigma Aldrich, Taufkirchen, Germany) dissolved in DMEM/F‐12 for 4 h at RT and subsequently washed with PBS. Immunostaining against laminin were carried out using a primary antibody against laminin (rabbit polyclonal; Abcam, Cambridge, United Kingdom) and the secondary antibody Alexa Fluor 488 (goat anti‐rabbit IgG H + L; Thermo Fisher Scientific, Dreieich, Germany). After washing steps with PBS images were taken immediately as well as after 7 and 14 days of storage at 4°C using a fluorescence microscope (Nikon Eclipse TS100; Nikon Instruments Europe, Amsterdam, The Netherlands).
Cell culture
Human mesenchymal stem cells (MSCs)
Cell culture of MSCs (Wharton's jelly, PromoCell GmbH, Heidelberg, Germany) was performed in standard cell culture treated flasks (75 cm2 growth area; Corning, NY) using DMEM/F‐12 supplemented with 10% FCS, 100 units/mL penicillin/streptomycin and 1 ng/mL basic fibroblast growth factor (all from Gibco, Thermo Fisher Scientific, Darmstadt, Germany) as culture media. MSCs were passaged using 0.05% trypsin/EDTA (Gibco, Thermo Fisher Scientific, Darmstadt, Germany) once a week or at confluency of ~80% with a cell density of at least 1000 cells/cm2 for inoculation.
Human pluripotent stem cell‐derived cardiomyocytes (hiPSC‐CMs)
hiPSC‐CMs (Cor.4U) were cultured using the Cor.4U medium (both from Axiogenesis, Cologne, Germany), which was changed 1–2 times per week before cell experiments. Cell detachment was carried out according to the instructions of the manufacturer.
Cell adhesion and beat frequency studies
MSCs (2.5 × 105 cells/scaffold) were seeded onto PS, ALG, and ALGTYR surfaces and the respective adhesion (number adherent cells) was quantified manually after 6 and 12 h of inoculation via live cell imaging (1 image/15 min) using the Biostation IM (Nikon Instruments Europe, Amsterdam, The Netherlands).
hiPSC‐CMs (2.5 × 105 cells/scaffold) were seeded onto planar and structured, Matrigel‐coated ALGTYR surfaces and cultivated for 21 days. Their adhesion behavior was documented after 1, 3, 7, 14, and 21 days using the microscope Eclipse TE 2000 (Nikon Instruments Europe, Amsterdam, The Netherlands). To study the beat frequency of adherent cells, hiPSC‐CMs (4000 cells/cm2) were cultured for 8 days on spherical ALGTYR coated with different Matrigel quantities (0, 2.2, 4.3, 6.5, and 8.7 μg/cm2 surface area). The respective beat frequencies were determined manually analyzing the image sequences.
Statistical evaluation
Graphical illustration of data and statistical analyses were performed using OriginPro and IBM SPSS Statistics. Differences between groups were considered significant by p < 0.05 and were evaluated with univariate analyses of variance (ANOVA) with simple contrasts.
RESULTS
Scaffold preparation and characteristics
Tyramine was conjugated reproducibly to ALG via aqueous carbodiimide chemistry. The modification process was confirmed by the structural elucidation using 1H NMR spectroscopy [Figs. 1 and 2(A)]. The spectra of ALG illustrate the typical homo‐ and heteropolymeric block fractions of alginates consisting of mannuronate and guluronate. After the amidation between ALG and tyramine using EDC and NHS, the aromatic protons of tyramines phenolic group were present within the ALGTYR spectra (6.88–7.23 ppm). Without using EDC/NHS, tyramine did not bind to ALG excluding an unspecific reaction.
Figure 1.
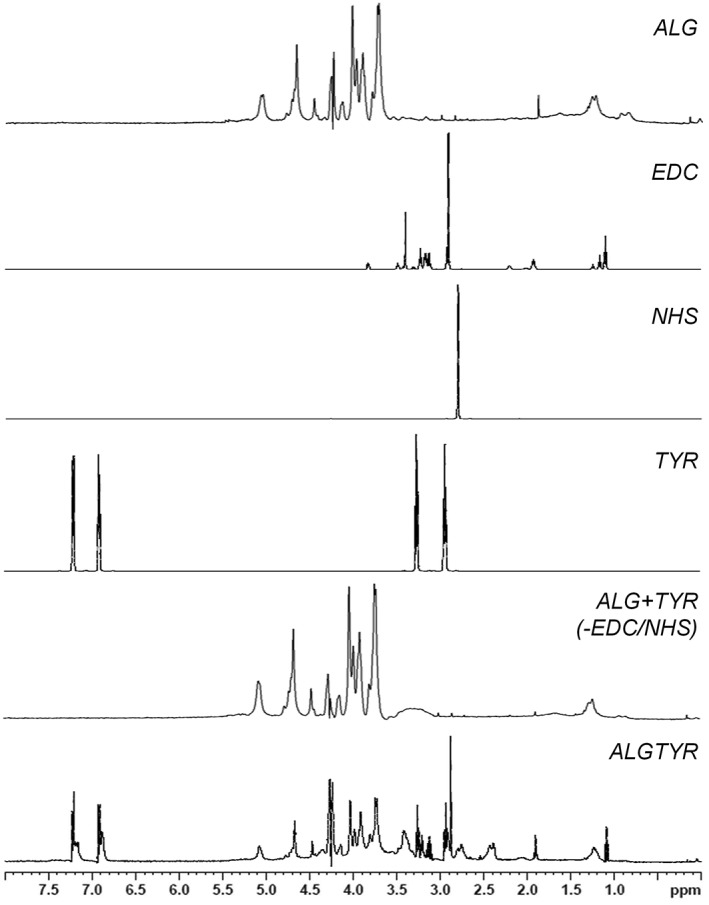
The functionalization of alginate hydrogels (ALG) with tyramine (TYR) using the carbodiimide chemistry (EDC, NHS) was confirmed by the structural elucidation via 1H NMR spectroscopy. Besides the typical homo‐ and heteropolymeric block fractions of alginates consisting of mannuronate and guluronate, the aromatic protons of the introduced tyramines phenolic group (6.88–7.23 ppm) were present within the tyramine‐alginate (ALGTYR) spectra. Without using EDC/NHS, tyramine did not bind to ALG excluding an unspecific reaction (ALG + TYR (−EDC/NHS)).
Figure 2.
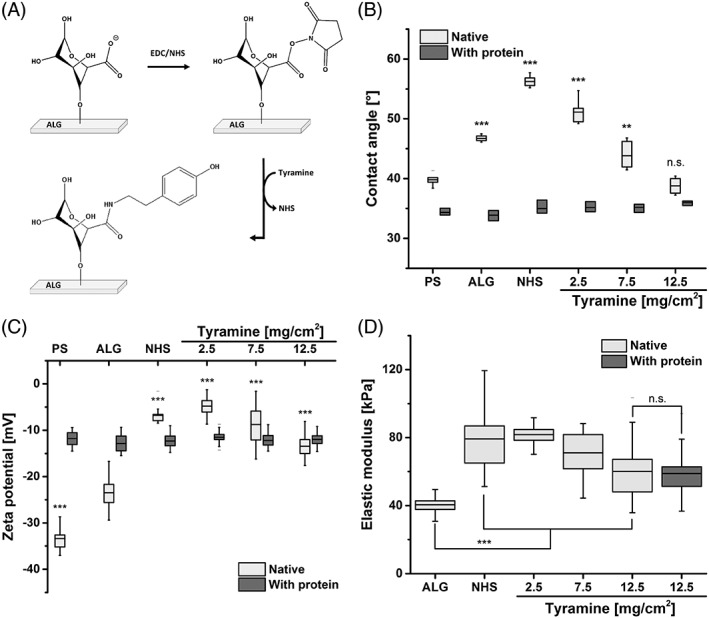
A: Reaction scheme of the functionalization of alginate scaffolds (ALG) with tyramines using the carbodiimide chemistry (EDC/NHS). B: Wettability studies of modified scaffolds compared to standard cell culture‐treated polystyrene (PS) and native ALG. The coupling of 12.5 mg tyramine to 1 cm2 ALG creates a surface wettability comparable to PS. C: Using zeta potential measurements, the surface charges of the scaffolds were analyzed. D: The elastic moduli describing the mechanical stabilities of the scaffolds were measured via compression tests. Alginate‐based hydrogels were found to be soft meeting the mechanical characteristics of human tissues. Differences of groups compared to ALG were considered significant by p < 0.01 (**) and p < 0.001 (***) (n.s.: not significant).
The captive bubble method was used to characterize the wettability of the investigated surfaces [Fig. 2(B)]. Cell culture‐treated PS were found to be hydrophilic (39.8 ± 0.9°). ALG exhibited contact angles of 46.7 ± 0.5°. The ALG functionalization with carbodiimide and different tyramine quantities resulted in contact angles of 56.2 ± 0.9° (EDC/NHS), 51.1 ± 0.5° (2.5 mg TYR/cm2), 43.9 ± 2.2° (7.5 mg TYR/cm2), and 38.8 ± 1.3 ° (12.5 mg TYR/cm2). The surface charge of the scaffolds was analyzed using zeta potential measurements [Fig. 2(C)]. PS and unmodified ALG surfaces exhibited highly negative charges (−33.4 ± 2.5 and −23.5 ± 2.9 mV, respectively). The conjugation of tyramines to ALG resulted in increased zeta potentials. However, zeta potentials were reduced with increasing tyramine content.
Besides the surface characteristics, the mechanical stability of the fabricated scaffolds was tested using compression studies [Fig. 2(D)]. ALG possessed an elastic modulus of 40.5 ± 4.7 kPa. The modification with EDC/NHS resulted in stiffer gels (78.8 ± 29.2 kPa). However, with increasing tyramine conjugation (2.5, 7.5, and 12.5 mg TYR/cm2), the hydrogel scaffolds became softer (81.7 ± 5.8, 71.0 ± 14.4, and 60.1 ± 14.9 kPa, respectively) compared to carbodiimide‐treated ALG.
Protein adsorption to tyramine‐conjugated alginate surfaces
To study the interaction between the prepared scaffolds and proteins, the protein adsorption was evaluated in terms of protein content using Ellman's reagent and stability via immunofluorescence staining against laminin. In addition, the impact of proteins on the scaffolds characteristics was determined. As shown in Figure 3(A), protein adsorption increased with tyramine conjugation to ALG. In comparison to PS, proteins adsorbed up to five times more on ALGTYR (12.5 mg TYR/cm2). Immunofluorescence staining against laminin showed that the adsorption of laminins to ALGTYR was stable for at least 14 days of observation [Fig. 3(B)]. Due to the attached proteins, the surface characteristics altered. After protein adsorption, the wettability of all surface were equalized at 34.9 ± 0.9° [Fig. 2(B)]. Moreover, similar surface charges were found (−12.1 ± 1.5 mV) [Fig. 2(C)]. However, protein coating did not affect the mechanical stability of ALGTYR scaffolds [Fig. 2(D)].
Figure 3.
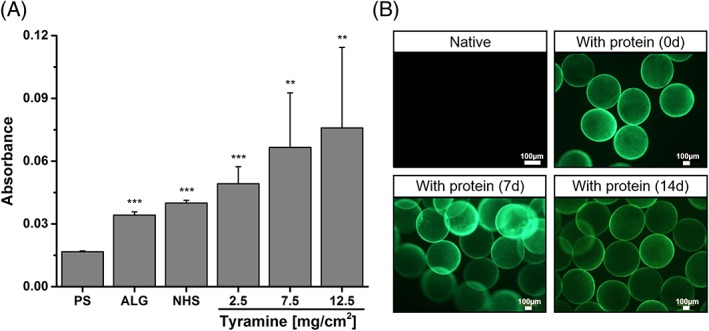
A: Using Ellman's reagent, the content of proteins adsorbed to the different surfaces was determined (PS: standard cell culture treated polystyrene; ALG: alginate scaffold; NHS: N‐hydroxysuccinimide‐modified ALG). Protein adsorption was found to be enhanced with increasing tyramine conjugation. Differences of groups compared to PS were considered significant by p < 0.01 (**) and p < 0.001 (***). B: Immunofluorescence staining against laminin revealed a stable protein adsorption for at least 14 days.
Cell behavior of MSCs and hiPSC‐CMs
ALGTYR surfaces were examined in cell culture experiments with MSCs and hiPSC‐CMs in comparison to PS and ALG. No cell adhesion was observed on unmodified ALG surfaces [Fig. 4(A/D)]. The cells remained round in suspension. In contrast, MSCs adhered and spread with high adhesion rates on PS and ALGTYR surfaces [Fig. 4(B–D)]. To exclude that intermediates of the tyramine coupling process to ALG trigger the cell adhesion, MSCs were cultivated using the hanging droplet method for 8 days on spherical ALG with different modifications (Supporting Information, Fig. 2). Only ALGTYR microcarriers showed MSC adhesion.
Figure 4.
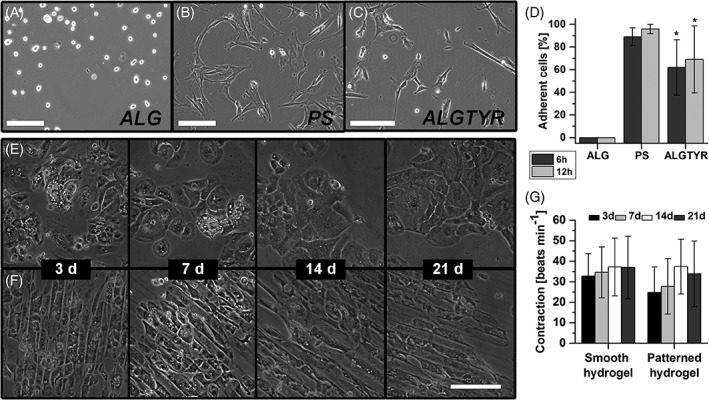
In comparison to native alginate scaffolds (ALG, A) and standard cell culture treated polystyrene surfaces (PS, B) tyramine‐alginate scaffolds (ALGTYR, C) were examined in cell culture experiments with MSCs (A–D) and hiPSC‐CMs (E–G). ALGTYR surfaces coated with Matrigel (E,F) were found to be a bioactive surface supporting cell adhesion and contraction of hiPSC‐CMs. Providing structured surfaces using μ‐contact printing resulted in oriented contraction of hiPSC‐CMs according to the pattern of parallel lines. Differences of groups were considered significant by p < 0.05 (*).
To further validate the potential of the modified scaffolds, hiPSC‐CMs were applied exemplarily as cells that require a more complex microenvironment. Therefore, ALGTYR surfaces were coated with the protein mixture Matrigel. hiPSC‐CMs were seeded onto planar and structured modified scaffolds and attached to both soft scaffolds. During the 21 days of cultivation, the cells adhered without a certain pattern on planar surfaces [Fig. 4(E)]. However, providing structured surfaces using μ‐contact printing resulted in the orientation of the hiPSC‐CMs according to the pattern of parallel lines within 3 days [Fig. 4(F)]. The beating frequencies of the hiPSC‐CMs appeared to be independent from the surface structure [Fig. 4(G)]. Nevertheless, the provided structures resulted in a more uniform contraction of the hiPSC‐CMs in comparison to those on planar surfaces. Moreover, the cultivation of hiPSC‐CMs was successfully translated from 2D layer to spherical matrices (Supporting Information, Fig. 3). Coating the spherical ALGTYR microcarriers with 2.2 μg Matrigel/cm2 surface area was sufficient for stable cell adhesion and contraction similar to hiPSC‐CMs spheroids.
DISCUSSION
Alginate‐based hydrogels represent promising biomaterials due to their biocompatibility and adjustable mechanical and porous characteristics.12, 13, 14, 15 As native alginate inhibits cell adhesion,16 functionalization strategies are needed to address cell–matrix contacts. Hence, this study aimed to enable stable cell adhesion via the conjugation of tyramine to ALG mimicking the surface chemistry of standard cell culture‐treated PS.
Therefore, carbodiimide chemistry was used to initialize the amidation between tyramine and ALG. The reproducible introduction of phenolic groups to ALG was confirmed using 1H NMR spectroscopy (Fig. 1). In consequence, ALGTYR bearing hydroxyl, carboxyl, and phenolic groups possess functional units comparable with the chemical composition of PS.17 Without the presence of EDC/NHS, no covalent binding of tyramine to ALG takes place. In contrast, the esterification of ALG with NHS to active intermediates results in scaffolds with increased hydrophobicity and surface charge due to the substitution of the carboxyl OH group with succinimidyl units (Fig. 2). Moreover, compression studies revealed an increased stiffness of the NHS‐conjugated alginate gels indicating a functionalization process not only on the scaffold surface but also of the porous bulk toward denser networks. After that, the succinimidyl units of the active NHS‐alginate scaffolds are substituted by tyramine resulting in increasing hydrophilicity and negative surface charge due to the introduced, phenolic OH groups. Here, the coupling of 12.5 mg tyramine to 1 cm2 ALG creates a surface wettability comparable to cell culture treated PS surfaces (PS: 39.8 ± 0.9° and 12.5ALGTYR: 38.8 ± 1.3°). In contrast to stiff PS (Young's modulus = 3 × 109 Pa25), ALGTYR scaffolds are porous and soft hydrogels (60–80 × 103 Pa) meeting the mechanical stabilities of native tissues (e.g., human liver: 20–60 × 103 Pa26 and human heart: 10–150 × 103 Pa27).
Moreover, protein adsorption was found to be enhanced with increasing tyramine conjugation. As tyrosine interactions are known to stabilize protein assemblies,28 the proteins tyrosine residues interact with ALGTYR resulting in an increasing content of adsorbed proteins which attached firmly for several weeks. As a consequence of the protein coating, various surfaces exhibit the wettability and surface charges of the protein layer. Hence, different protein compositions are applied for cell cultivation instead of diverse material characteristics. These protein‐based binding sites are crucial to promote cellular interactions. In contrast to existing strategies to bioactivate alginates via the conjugation of RGD29, 30 or YIGSR motifs,31 ALGTYR scaffolds allow noncovalent and universal surface coatings with adhesion molecules of different sources (e.g., serum proteins, Matrigel, laminins, etc.) adjustable in terms of protein type and quantity. As a consequence, an appropriate presence of proteins on the alginate hydrogel surface is created to induce cell adhesion compared to the previous noncovalent mixing of proteins with the alginate sol before gelation.32
Subsequently, MSCs and hiPSC‐CMs were used to examine the bioactivity of native and modified scaffolds in comparison to PS. Cell culture experiments revealed the bioinertness of unmodified alginates. Although proteins adsorb to the material, no cell adhesion occurred on ALG. The bioinert characteristic of ALG is attributed to tremendous conformational changes of adsorbed domains to denaturated proteins.33 Active NHS intermediates also prevent cell adhesion, as carbodiimide are known to be cytotoxic.34 The functionalization to ALGTYR results in bioactive scaffolds on which cells like MSCs and hiPSC‐CMs adhere. In contrast to the work of Zhu et al., which revealed no adhesion of NIH 3T3 mouse fibroblasts to the gel surface of their prepared HRP‐mediated alginate‐tyramine hydrogels,22 adsorbed proteins on ALGTYR surfaces in this study enable stable cell adhesion and proliferation comparable to PS‐based cell culture. Moreover, the soft, bioactive ALGTYR matrices support the functional cell performance like the beating of hiPSC‐CMs (see Supporting Information, Movie JBMRA_suppmov1_PS for contraction of hiPSC‐CMs on PS coated with Matrigel and Movie JBMRA_suppmov2_ALGTYR for contraction on ALGTYR surfaces with Matrigel coating at day 7). Uniform hiPSC‐CMs contractions become feasible due to the alignment of the cells using μ‐contact printed structures. In contrast to stiff cell culture plastic, ALGTYR hydrogels can be compressed by the cellular tension while mimicking the in vivo mechanical properties and cell alignment.35 The stable protein adsorption and cell–matrix interactions on soft alginates surfaces qualify ALGTYR hydrogels as a promising platform for bioactive scaffolds applicable in stem cell culture and tissue engineering. For an enhanced understanding of the established cell–matrix interactions, future studies involve gene expression analyses and in vivo evaluations. Here, the differentiation potential and metabolic profiles of the seeded (stem) cells and the biointegration in vivo are of particular interest.
CONCLUSION
Tyramines were conjugated reproducibly to alginate hydrogels using carbodiimide to overcome the bioinertness of alginate scaffolds and establish stable cell–matrix interactions. Due to the introduction of the tyramines phenolic units to the alginate backbone, a surface chemistry comparable to standard cell‐culture plastic PS was created. As a consequence, ALGTYR hydrogels exhibit similar wettability to PS and enable robust protein adsorption to soft and porous scaffolds possessing mechanical characteristics of human tissues. Cell culture studies with MSCs and hiPSC‐CMs revealed stable cell adhesion and contraction on (structured) 2‐D layer and spherical matrices. Hence, the functionalization of bioinert alginate‐based hydrogels with tyramines represents an efficient technique to produce bioactive scaffolds beneficial for robust cell culture and tissue engineering. According to the generated data, ALGTYR hydrogels bear the potential to provide a versatile surface for universal coatings with adhesion molecules. Thus, using ALGTYR hydrogels soft and porous cell microenvironments become accessible for a wide range of applications in biology, biotechnology, and medicine such as cell expansion and differentiation, cell‐based models, and tissue substitutes.
Disclosure
The authors declare that they have no conflict of interest.
Supporting information
Supporting Information, Figure 1 Fabrication technique of micropatterned alginates. A: Setup to produce structured alginate scaffolds. B: Detailed view of the print head while structuring with the individual components. C: Processing steps for the patterning of alginate hydrogels: (1) Positioning of the print head, stamp, and agarose block. (2) Addition of an alginate solution onto the agarose block. (3) Bonding of the stamp and the alginate solution through the print head resulting in the Ba2+‐mediated alginate gelation. (4) Lifting the printhead ends the structuring process. (5) Isolation of the patterned alginate scaffold from the structuring platform.
Supporting Information, Figure 2. Representative images of human mesenchymal stem cells cultivated in hanging droplets on spherical alginate microcarriers with different modifications. Using carbodiimide chemistry (EDC/NHS), tyramine (TYR) was conjugated to the alginate hydrogels. Only tyramine‐alginate beads (+EDC/NHS + TYR) showed mesenchymal stem cell adhesion.
Supporting Information, Figure 3. Adhesion (left) and contraction (right) of cardiomyocytes cultivated on tyramine‐alginate microcarrier (A) without Matrigel coating, (B) with 25%, (C) 50%, (D) 75%, (E) 100% Matrigel, and (F) without microcarrier. Coating the spherical tyramine‐alginate microcarriers with 2.2 μg Matrigel/cm2 surface area (B, 25%) was sufficient for stable cell adhesion and contraction similar to spheroids consisting of cardiomyocytes (F).
Appendix S1: Supporting Information, movie JBMRA_suppmov1_PS
Appendix S2: Supporting Information, movie JBMRA_suppmov2_ALGTYR
ACKNOWLEDGMENT
The authors kindly acknowledge Wencke Lubojanski for her statistical support and careful proof‐reading.
How to cite this article: Schulz A, Gepp MM, Stracke F, von Briesen H, Neubauer JC, Zimmermann H. 2019. Tyramine‐conjugated alginate hydrogels as a platform for bioactive scaffolds. J Biomed Mater Res Part A 2019:107A:114–121.
References
- 1. Dhowre HS, Rajput S, Russell NA, Zelzer M. Responsive cell–material interfaces. Nanomedicine 2015;10(5):849–871. [DOI] [PubMed] [Google Scholar]
- 2. Schaap‐Oziemlak AM, Kühn PT, van Kooten TG, van Rijn P. Biomaterial–stem cell interactions and their impact on stem cell response. RSC Adv 2014;4(95):53307–53320. [Google Scholar]
- 3. Hao L, Yang H, Du C, Fu X, Zhao N, Xu S, Cui F, Mao C, Wang Y. Directing the fate of human and mouse mesenchymal stem cells by hydroxyl–methyl mixed self‐assembled monolayers with varying wettability. J Mater Chem B 2014;2(30):4794–4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. de Luca I, Di Salle A, Alessio N, Margarucci S, Simeone M, Galderisi U, Calarco A, Peluso G. Positively charged polymers modulate the fate of human mesenchymal stromal cells via ephrinB2/EphB4 signaling. Stem Cell Res 2016;17(2):248–255. [DOI] [PubMed] [Google Scholar]
- 5. Cao B, Peng Y, Liu X, Ding J. Effects of functional groups of materials on nonspecific adhesion and chondrogenic induction of mesenchymal stem cells on free and micropatterned surfaces. ACS Appl Mater Interfaces 2017;9(28):23574–23585. [DOI] [PubMed] [Google Scholar]
- 6. Yim EKF, Darling EM, Kulangara K, Guilak F, Leong KW. Nanotopography‐induced changes in focal adhesions, cytoskeletal organization, and mechanical properties of human mesenchymal stem cells. Biomaterials 2010;31(6):1299–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Discher DE, Janmey P, Wang Y‐l. Tissue cells feel and respond to the stiffness of their substrate. Science 2005;310(5751):1139–1143. [DOI] [PubMed] [Google Scholar]
- 8. Teng W, Long TJ, Zhang Q, Yao K, Shen TT, Ratner BD. A tough, precision‐porous hydrogel scaffold: Ophthalmologic applications. Biomaterials 2014;35(32):8916–8926. [DOI] [PubMed] [Google Scholar]
- 9. Pelham RJ, Wang Y‐l. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc Natl Acad Sci 1997;94(25):13661–13665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Forte G, Pagliari S, Ebara M, Uto K, van Tam JK, Romanazzo S, Escobedo‐Lucea C, Romano E, Di Nardo P, Traversa E. Substrate stiffness modulates gene expression and phenotype in neonatal cardiomyocytes in vitro. Tissue Eng Part A 2012;18(17–18):1837–1848. [DOI] [PubMed] [Google Scholar]
- 11. Engler AJ, Griffin MA, Sen S, Bönnemann CG, Sweeney HL, Discher DE. Myotubes differentiate optimally on substrates with tissue‐like stiffness: Pathological implications for soft or stiff microenvironments. J Cell Biol 2004;166(6):877–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li Z, Ramay HR, Hauch KD, Xiao D, Zhang M. Chitosan–alginate hybrid scaffolds for bone tissue engineering. Biomaterials 2005;26(18):3919–3928. [DOI] [PubMed] [Google Scholar]
- 13. Gepp MM, Fischer B, Schulz A, Dobringer J, Gentile L, Vásquez JA, Neubauer JC, Zimmermann H. Bioactive surfaces from seaweed‐derived alginates for the cultivation of human stem cells. J Appl Phycol 2017;29(5):2451–2461. [Google Scholar]
- 14. Zimmermann H, Zimmermann D, Reuss R, Feilen PJ, Manz B, Katsen A, Weber M, Ihmig FR, Ehrhart F, Geßner P, Behringer M, Steinbach A, Wegner LH, Sukhorukov VL, Vásquez JA, Schneider S, Weber MM, Volke F, Wolf R, Zimmermann U. Towards a medically approved technology for alginate‐based microcapsules allowing long‐term immunoisolated transplantation. J Mater Sci Mater Med 2005;16(6):491–501. [DOI] [PubMed] [Google Scholar]
- 15. LeRoux MA, Guilak F, Setton LA. Compressive and shear properties of alginate gel: Effects of sodium ions and alginate concentration. J Biomed Mater Res A 1999;47(1):46–53. [DOI] [PubMed] [Google Scholar]
- 16. Schneider S, Feilen PJ, Brunnenmeier F, Minnemann T, Zimmermann H, Zimmermann U, Weber MM. Long‐term graft function of adult rat and human islets encapsulated in novel alginate‐based microcapsules after transplantation in immunocompetent diabetic mice. Diabetes 2005;54(3):687–693. [DOI] [PubMed] [Google Scholar]
- 17. Ryan JA. Evolution of cell culture surfaces. BioFiles 2008;3(8):21. [Google Scholar]
- 18. Darr A, Calabro A. Synthesis and characterization of tyramine‐based hyaluronan hydrogels. J Mater Sci Mater Med 2009;20(1):33–44. [DOI] [PubMed] [Google Scholar]
- 19. Teixeira LSM, Bijl S, Pully VV, Otto C, Jin R, Feijen J, van Blitterswijk CA, Dijkstra PJ, Karperien M. Self‐attaching and cell‐attracting in‐situ forming dextran‐tyramine conjugates hydrogels for arthroscopic cartilage repair. Biomaterials 2012;33(11):3164–3174. [DOI] [PubMed] [Google Scholar]
- 20. Sakai S, Kawakami K. Both ionically and enzymatically crosslinkable alginate–tyramine conjugate as materials for cell encapsulation. J Biomed Mater Res A 2008;85(2):345–351. [DOI] [PubMed] [Google Scholar]
- 21. Nguyen D, Hägg DA, Forsman A, Ekholm J, Nimkingratana P, Brantsing C, Kalogeropoulos T, Zaunz S, Concaro S, Brittberg M. Cartilage tissue engineering by the 3D bioprinting of iPS cells in a nanocellulose/alginate bioink. Sci Rep 2017;7(1):658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hou J, Li C, Guan Y, Zhang Y, Zhu XX. Enzymatically crosslinked alginate hydrogels with improved adhesion properties. Polym Chem 2015;6(12):2204–2213. [Google Scholar]
- 23. Prodanovic O, Spasojevic D, Prokopijevic M, Radotic K, Markovic N, Blazic M, Prodanovic R. Tyramine modified alginates via periodate oxidation for peroxidase induced hydrogel formation and immobilization. React Funct Polym 2015;93:77–83. [Google Scholar]
- 24. Zimmermann H, Ehrhart F, Zimmermann D, Müller K, Katsen‐Globa A, Behringer M, Feilen PJ, Gessner P, Zimmermann G, Shirley SG. Hydrogel‐based encapsulation of biological, functional tissue: Fundamentals, technologies and applications. Appl Phys A Mater Sci Process 2007;89(4):909–922. [Google Scholar]
- 25. Oral I, Guzel H, Ahmetli G. Measuring the Young's modulus of polystyrene‐based composites by tensile test and pulse‐echo method. Polym Bull 2011;67(9):1893–1906. [Google Scholar]
- 26. Nava A, Mazza E, Furrer M, Villiger P, Reinhart WH. In vivo mechanical characterization of human liver. Med Image Anal 2008;12(2):203–216. [DOI] [PubMed] [Google Scholar]
- 27. Grover CN, Cameron RE, Best SM. Investigating the morphological, mechanical and degradation properties of scaffolds comprising collagen, gelatin and elastin for use in soft tissue engineering. J Mech Behav Biomed Mater 2012;10:62–74. [DOI] [PubMed] [Google Scholar]
- 28. Vashi AV, Ramshaw JA, Glattauer V, Elvin CM, Lyons RE, Werkmeister JA. Controlled surface modification of tissue culture polystyrene for selective cell binding using resilin‐inspired polypeptides. Biofabrication 2013;5(3):35005. [DOI] [PubMed] [Google Scholar]
- 29. Alsberg E, Anderson KW, Albeiruti A, Franceschi RT, Mooney DJ. Cell‐interactive alginate hydrogels for bone tissue engineering. J Dent Res 2001;80(11):2025–2029. [DOI] [PubMed] [Google Scholar]
- 30. Grigore A, Sarker B, Fabry B, Boccaccini AR, Detsch R. Behavior of encapsulated MG‐63 cells in RGD and gelatine‐modified alginate hydrogels. Tissue Eng Part A 2014;20(15–16):2140–2150. [DOI] [PubMed] [Google Scholar]
- 31. Dhoot NO, Tobias CA, Fischer I, Wheatley MA. Peptide‐modified alginate surfaces as a growth permissive substrate for neurite outgrowth. J Biomed Mater Res A 2004;71(2):191–200. [DOI] [PubMed] [Google Scholar]
- 32. Sang L, Luo D, Xu S, Wang X, Li X. Fabrication and evaluation of biomimetic scaffolds by using collagen–alginate fibrillar gels for potential tissue engineering applications. Mater Sci Eng C 2011;31(2):262–271. [Google Scholar]
- 33. Schulz A, Katsen‐Globa A, Huber EJ, Mueller SC, Kreiner A, Pütz N, Gepp MM, Fischer B, Stracke F, von Briesen H, Neubauer JC, Zimmermann H. Poly (amidoamine)‐alginate hydrogels: Directing the behavior of mesenchymal stem cells with charged hydrogel surfaces. J Mater Sci Mater Med 2018;29(7):105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Moshnikova AB, Afanasyev VN, Proussakova OV, Chernyshov S, Gogvadze V, Beletsky IP. Cytotoxic activity of 1‐ethyl‐3‐(3‐dimethylaminopropyl)‐carbodiimide is underlain by DNA interchain cross‐linking. Cell Mol Life Sci 2006;63(2):229–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zimmermann W‐H, Schneiderbanger K, Schubert P, Didie M, Münzel F, Heubach JF, Kostin S, Neuhuber WL, Eschenhagen T. Tissue engineering of a differentiated cardiac muscle construct. Circ Res 2002;90(2):223–230. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information, Figure 1 Fabrication technique of micropatterned alginates. A: Setup to produce structured alginate scaffolds. B: Detailed view of the print head while structuring with the individual components. C: Processing steps for the patterning of alginate hydrogels: (1) Positioning of the print head, stamp, and agarose block. (2) Addition of an alginate solution onto the agarose block. (3) Bonding of the stamp and the alginate solution through the print head resulting in the Ba2+‐mediated alginate gelation. (4) Lifting the printhead ends the structuring process. (5) Isolation of the patterned alginate scaffold from the structuring platform.
Supporting Information, Figure 2. Representative images of human mesenchymal stem cells cultivated in hanging droplets on spherical alginate microcarriers with different modifications. Using carbodiimide chemistry (EDC/NHS), tyramine (TYR) was conjugated to the alginate hydrogels. Only tyramine‐alginate beads (+EDC/NHS + TYR) showed mesenchymal stem cell adhesion.
Supporting Information, Figure 3. Adhesion (left) and contraction (right) of cardiomyocytes cultivated on tyramine‐alginate microcarrier (A) without Matrigel coating, (B) with 25%, (C) 50%, (D) 75%, (E) 100% Matrigel, and (F) without microcarrier. Coating the spherical tyramine‐alginate microcarriers with 2.2 μg Matrigel/cm2 surface area (B, 25%) was sufficient for stable cell adhesion and contraction similar to spheroids consisting of cardiomyocytes (F).
Appendix S1: Supporting Information, movie JBMRA_suppmov1_PS
Appendix S2: Supporting Information, movie JBMRA_suppmov2_ALGTYR


