Abstract
This retrospective study presents our 4‐year experience of preemptive treatment of early anti‐HLA donor specific antibodies with IgA‐ and IgM‐enriched immunoglobulins. We compared outcomes between patients with antibodies and treatment (case patients) and patients without antibodies (control patients). Records of patients transplanted at our institution between March 2013 and November 2017 were reviewed. The treatment protocol included one single 2 g/kg immunoglobulin infusion followed by successive 0.5 g/kg infusions for a maximum of 6 months, usually combined with a single dose of anti‐CD20 antibody and, in case of clinical rejection or positive crossmatch, with plasmapheresis or immunoabsorption. Among the 598 transplanted patients, 128 (21%) patients formed the case group and 452 (76%) the control group. In 116 (91%) patients who completed treatment, 106 (91%) showed no antibodies at treatment end. Fourteen (13%) patients showed antibody recurrence thereafter. In case versus control patients and at 4‐year follow‐up, respectively, graft survival (%) was 79 versus 81 (P = .59), freedom (%) from biopsy‐confirmed rejection 57 versus 53 (P = .34), and from chronic lung allograft dysfunction 82 versus 78 (P = .83). After lung transplantation, patients with early donor‐specific antibodies and treated with IgA‐ and IgM‐enriched immunoglobulins had 4‐year graft survival similar to patients without antibodies and showed high antibody clearance.
Keywords: clinical research/practice, graft survival, immunosuppression/immune modulation, intravenous immunoglobulin/IVIG, lung (allograft) function/dysfunction, lung transplantation/pulmonology, major histocompatibility complex (MHC), rejection: antibody‐mediated (ABMR)
Short abstract
Lung‐transplanted patients who develop early anti‐HLA donor‐specific antibodies and are treated with a protocol based on successive infusion of IgA‐ and IgM‐enriched intravenous immunoglobulins show good antibody clearance and graft survival, similar to the survival of patients without early donor‐specific antibodies.
Abbreviations
- AMR
antibody‐mediated rejection
- CI
confidence interval
- CLAD
chronic lung allograft dysfunction
- CMV
Cytomegalovirus
- ECMO
extracorporeal membrane oxygenation
- eDSA
early anti‐HLA donor specific antibodies
- EVLP
ex‐vivo lung perfusion
- FEV1
forced expiratory volume in 1 second
- FFP
fresh frozen plasma
- HLA
human leucocyte antigen
- ICU
intensive care unit
- IgGAM
IgA‐ and IgM‐enriched intravenous human immunoglobulins
- IQR
interquartile range
- ISHLT
International Society for Heart and Lung Transplantation
- MFI
mean fluorescence index
- PC
platelets concentrate
- PGD
primary graft dysfunction
- PRBCs
peripheral red blood cells
- SD
standard deviation
- tPE
therapeutic plasmapheresis
1. INTRODUCTION
The development of antibodies against donor human leukocyte antigens (donor specific antibodies, DSA) after lung transplantation has been associated with antibody‐mediated rejection (AMR), chronic lung allograft dysfunction (CLAD) and patient mortality.1, 2, 3, 4, 5, 6, 7, 8, 9
However, there are many open questions concerning DSA and AMR treatment.10 Different protocols have been used, making any conclusion about treatment efficacy difficult.11, 12, 13, 14, 15, 16, 17 Treatment of clinical AMR has shown suboptimal efficacy, since the graft dysfunction may not be reversible anymore.12, 17
Since March 2013, at our institution, patients who developed DSA early after transplantation (eDSA) have been treated with a protocol based on successive infusion of IgA‐ and IgM‐enriched intravenous human immunoglobulins (IgGAM, Pentaglobin, Biotest AG, Dreieich, Germany). In our experience, treated patients showed good eDSA clearance and short‐term graft survival that was comparable to survival of patients without eDSA.14
This retrospective study presented our 4‐year experience of early DSA treatment with IgGAM in lung transplantation. We compared outcomes between patients with eDSA treated with IgGAM and patients without eDSA.
2. METHODS
2.1. Patients
The in‐hospital and follow‐up records of patients who underwent lung transplantation at our institution between March 2013 and November 2017 were retrospectively reviewed.
Patients who showed eDSA after transplantation and were treated with IgGAM formed the eDSA+/IgGAM+ group (case group). The outcomes of eDSA+/IgGAM+ patients were compared to the outcomes of patients who did not show eDSA after transplantation (eDSA− patients, control group).
Patients, who showed eDSA and were treated without IgGAM (eDSA+/IgGAM− patients), and the few patients who showed eDSA but were not treated at all (eDSA+/no‐treatment patients), were excluded from the study. However, their results were reported in the supporting information section.
Follow‐up ended on November 1, 2017 and was 100% completed.
The hospital ethical review board waived the need of patient consent to the study, since all patients had given their consent to handle their personal data for research purposes at the time of listing to lung transplantation. In addition, in eDSA+/IgGAM+ patients, a patient consent was obtained to perform the additional DSA controls at follow‐up.
2.2. Variable definition
The present study focused on the treatment of early DSA, which were defined as DSA, which were detected during initial hospitalization after lung transplantation, before hospital discharge.
eDSA clearance was defined as absence of DSA in two consecutive Luminex‐based SPA (LIFECODES, Immucor Transplant Diagnostics, Inc., Stamfort, CT) controls. DSA recurrence was defined as a renewed positivity of previously cleared DSA at Luminex‐based SPA control.
The definitions of other variables and outcomes are reported elsewhere.3, 13, 14, 18, 19, 20 Details on patient management after transplantation at our institution are reported in the supporting information section of this manuscript.3, 13, 14
2.3. eDSA detection protocol
All patients were screened for anti‐HLA antibodies at the time of listing to lung transplantation, and for eDSA, immediately before lung transplantation, on day 14 and before hospital discharge or upon indication. In the Luminex analysis, a low threshold of 1000 mean fluorescence index (MFI) was used to detect eDSA.
At follow‐up, in eDSA+/IgGAM+ patients, Luminex‐based DSA controls were performed at the beginning of each IgGAM treatment session and, after treatment end, every 6 months. In eDSA− as well as excluded eDSA+/IgGAM− and eDSA+/no‐treatment patients, DSA were not regularly assessed, but only upon indication.
2.4. eDSA treatment protocols
In March 2013, an IgGAM‐based treatment protocol replaced the previous rather ineffective eDSA treatment protocol which had been based only on therapeutic plasmapheresis (tPE) and a single dose of anti‐CD 20 antibody (Rituximab).13, 14 Pentaglobin was used, since it has been demonstrated that its IgA and IgM components conferred additional immunomodulatory and antimicrobial effects.21 eDSA treatment with IgGAM represents an off‐label use of IVIG.
Treatment was usually performed preemptively, since most of the patients showed only serologic evidence of eDSA (possible subclinical AMR9). In those patients with graft dysfunction, dysfunction was defined as worsening of blood oxygenation and/or lung function tests, unexplained by concomitant infection. In this case, diagnosis of definite clinical AMR was not made, since transbronchial biopsies were usually not performed early after transplantation for safety reasons (possible clinical AMR9).
IgGAM therapy consisted of a first infusion of 2 g/kg of IgGAM followed by additional infusions of 0.5 g/kg of IgGAM every 4 weeks until eDSA clearance or for a maximum of 6 months. Other procedures and drugs, comprising 3 distinct successive treatment protocols, were added to the first IgGAM infusion (Figure 1).
Figure 1.
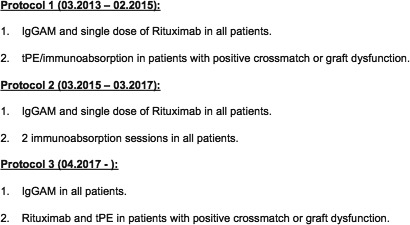
During the study period, three IgGAM‐based treatment protocol were employed at our institution. In the first protocol, 3 or 5 sessions of tPE preceded the first IgGAM dose in those patients with graft dysfunction or positive crossmatch. In the second protocol, 2 sessions of immunoabsorption using tryptophan columns preceded the first IgGAM dose in all patients, in an effort to shorten treatment time. In both protocols, a single dose of Rituximab (375 mg/m2) was administered following the first IgGAM dose. Since April 2017, immunoabsorption has been eliminated, and tPE and Rituximab were given only in case of presence of positive crossmatch or graft dysfunction. IgGAM, IgA‐ and IgM‐enriched intravenous human immunoglobulins; tPE, therapeutic plasmapheresis
More treatment details are reported in the supporting information section.
2.5. Statistics
IBM SPSS 24.0 (IBM, NY) was used for the data analysis. Primary endpoints were graft survival and eDSA clearance at treatment end. Secondary endpoints were patient survival, freedom from pulsed‐steroid therapy, biopsy‐confirmed acute rejection, CLAD, retransplant and infection requiring hospitalization.
Categorical and continuous variables were summarized as percentages and median with interquartile range (IQR), respectively. The non‐parametric Mann‐Whitney test and the Chi‐squared test or the Fisher's exact test were used for group comparisons of continuous and categorical variables, respectively.
Survival estimates along with freedom from endpoints were calculated by the product‐limit method of Kaplan‐Meier. Differences between groups were quantified using the log‐rank test.
In order to account for the influence on outcomes of the variables which showed a statistical significant difference (P ≤ .05) among included eDSA+/IgGAM+ and eDSA− patients, propensity scores were developed based on 4 covariates in a logistic regression model with IgGAM treatment for eDSA as the dependent variable. The variables were age at transplantation under 18 years old, pulmonary artery hypertension as indication to transplantation, lung retrieval with portable ex‐vivo lung perfusion (EVLP), and evidence of antibodies against HLA class II before transplantation (Tables 1, 2, 3, 4).
Table 1.
Preoperative recipient data
| Variable | eDSA+/IgGAM+ (n = 128) | eDSA− (n = 452) | P value |
|---|---|---|---|
| Female sex | 61 (48) | 213 (47) | .84 |
| Age (y) | 49 (31‐58) | 52 (38‐59) | .25 |
| Age < 18 y | 18 (14) | 26 (6) | .002 |
| Age > 60 y | 19 (15) | 66 (15) | .95 |
| BSA (m2) | 1.70 (1.54‐1.90) | 1.74 (1.56‐1.94) | .76 |
| Transplant indication | |||
| COPD | 38 (30) | 116 (26) | .33 |
| Pulmonary fibrosis | 36 (28) | 160 (35) | .12 |
| Cystic fibrosis | 24 (19) | 99 (22) | .44 |
| Pulmonary hypertension | 15 (12) | 17 (4) | <.001 |
| Re‐transplant | 11 (9) | 32 (7) | .56 |
| Other | 5 (4) | 28 (6) | .32 |
| Associated pulmonary artery hypertension | 47 (37) | 182 (40) | .47 |
| LAS score | 36.1 (32.6‐42.4) | 36.1 (33.2‐41.6) | .99 |
| Preoperative mechanical ventilation | 3 (2) | 15 (3) | .57 |
| Preoperative intensive care unit | 14 (11) | 40 (9) | .47 |
| Preoperative ECMO/iLA | 13 (10) | 25 (6) | .062 |
Values are expressed as median (IQR, interquartile range) or N of patients (%). BSA, body surface area; COPD, chronic obstructive pulmonary disease; ECMO, extracorporeal membrane oxygenation; eDSA, early donor‐specific antibodies; IgGAM, IgA‐ and IgM‐enriched intravenous human immunoglobulins; iLA, interventional Lung Assist Novalung; LAS, lung allocating score.
Table 2.
Donor and intraoperative recipient characteristics
| Variable | eDSA+/IgGAM+ (n = 128) | eDSA− (n = 452) | P value |
|---|---|---|---|
| Donor characteristics | |||
| Female sex | 71 (56) | 212 (47) | .091 |
| Age (y) | 51 (38‐59) | 50 (37‐59) | .49 |
| Age > 70 y | 7 (6) | 30 (7) | .63 |
| BSA (m2) | 1.90 (1.77‐2.05) | 1.91 (1.77‐2.08) | .83 |
| Ventilation time (d) | 4 (2‐8) | 4 (2‐7) | .87 |
| pO2 (100%, mmHg) | 397 (329‐453) | 377 (312‐441) | .48 |
| Smoking history | 55 (43) | 183 (41) | .63 |
| Contusion | 13 (10) | 37 (8) | .49 |
| Aspiration | 7 (6) | 26 (6) | .90 |
| Lung preservation | |||
| Celsior | 113 (88) | 367 (83) | .15 |
| Portable EVLP | 3 (2) | 32 (7) | .047 |
| Intraoperative recipient characteristics | |||
| Single lung | 3 (2) | 12 (3) | .86 |
| Double lung | 125 (98) | 440 (97) | .84 |
| Cardiopulmonary bypass | 2 (2) | 9 (2) | 1.00 |
| Intraoperative ECMO | 34 (27) | 118 (26) | .95 |
| Postoperative extended ECMO | 16 (13) | 39 (9) | .19 |
| Ischemic time (min) | |||
| First lung | 400 (315‐477) | 401 (319‐495) | .96 |
| Second lung | 507 (429‐590) | 507 (414‐604) | .97 |
Values are expressed as median (IQR, interquartile range) or N of patients (%). BSA, body surface area; ECMO, extracorporeal membrane oxygenation; eDSA, early donor‐specific antibodies; EVLP, ex‐vivo lung perfusion; IgGAM, IgA‐ and IgM‐enriched intravenous human immunoglobulins.
Table 3.
Anti‐HLA antibodies
| Variable | eDSA+/IgGAM+ (n = 128) | eDSA− (n = 452) | P value |
|---|---|---|---|
| Preoperative anti‐HLA antibodies | |||
| Anti‐HLA I | 26 (20) | 83 (18) | .62 |
| Anti‐HLA II | 40 (31) | 86 (19) | .003 |
| Anti‐HLA I + anti‐HLA II | 9 (7) | 24 (5) | .46 |
| Cumulative mismatches | |||
| HLA A + B | 3 (2‐4) | 3 (3‐4) | .04 |
| HLA A + B + DR | 5 (4‐6) | 5 (4‐5) | <.001 |
| Postoperative anti‐HLA antibodiesa | |||
| Anti‐HLA I | 56 (44) | 98 (22) | <.001 |
| Anti‐HLA II | 111 (87) | 116 (26) | <.001 |
| Anti‐HLA I + anti‐HLA II | 43 (34) | 45 (10) | <.001 |
| Postoperative anti‐HLA eDSA | |||
| HLA A | 15 (12) | ||
| HLA B | 21 (16) | ||
| HLA C | 2 (2) | ||
| HLA DR | 12 (9) | ||
| HLADQ | 103 (81) | ||
| Positive crossmatch | 10 (8) | ||
Values are expressed as median (IQR) or N of patients (%). eDSA, early donor specific antibodies; HLA, human leukocyte antigen; IgGAM, IgA‐ and IgM‐enriched intravenous human immunoglobulins.All patients who developed anti‐HLA antibodies after lung transplantation were considered, independently of DSA positivity.
Table 4.
Postoperative data
| Variable | eDSA+/IgGAM+ (n = 128) | eDSA− (n = 452) | P value |
|---|---|---|---|
| PGD score grade 2 or 3 | |||
| 24 h | 20 (16) | 51 (11) | .18 |
| 48 h | 21 (17) | 57 (13) | .27 |
| 72 h | 17 (13) | 44 (10) | .24 |
| Rethoracotomy for bleeding | 8 (6) | 36 (8) | .51 |
| New dialysis | 6 (5) | 38 (8) | .16 |
| Postoperative pulsed steroid therapy | 49 (38) | 133 (30) | .061 |
| Secondary ECMO | 2 (2) | 9 (2) | 1.00 |
| Tracheostomy | 12 (9) | 35 (8) | .55 |
| Ventilation time, h | 11 (8‐14) | 11 (8‐17) | .84 |
| ICU stay, d | 2 (1‐5) | 2 (1‐4) | .23 |
| Hospital stay, d | 25 (22‐34) | 22 (21‐27) | <.001 |
| In‐hospital mortality | 4 (3) | 21 (5) | .45 |
| Immunosuppressive therapy at discharge after transplantationa | |||
| Cyclosporine | 0 | 3 (1) | 1.00 |
| Tacrolimus | 124 (100) | 428 (99) | 1.00 |
| Mycofenolate mofetil | 123 (99) | 431 (100) | .22 |
| Immunosuppressive therapy at last outpatient controla | |||
| Cyclosporine | 4 (3) | 54 (13) | .003 |
| Tacrolimus | 117 (95) | 375 (87) | .012 |
| Mycofenolate mofetil | 112 (92) | 401 (93) | .76 |
| Everolimus | 11 (9) | 21 (5) | .088 |
Values are expressed as median (IQR, interquartile range) or N of patients (%). ECMO, extracorporeal membrane oxygenation; eDSA, early donor‐specific antibodies; ICU, intensive care unit; IgGAM, IgA‐ and IgM‐enriched intravenous human immunoglobulins' PGD, primary graft dysfunction.
In‐hospital deaths (n = 25) are excluded.
Study endpoints were thus evaluated using propensity scores as balancing scores in two ways22: first, 123 eDSA+/IgGAM+ patients were 1:1 matched to 123 eDSA− patients. Second, all included patients were stratified into quintiles on the basis of having similar propensity scores. Each endpoint was then evaluated within each quintile.
P‐values ≤ .05 were considered significant.
3. RESULTS
3.1. Patient groups
Between March 2013 and November 2017, among the 598 patients who underwent lung transplantation at our institution, 146 (24%) patients showed a positive crossmatch or eDSA, and the remaining 452 (76%) patients did not (control group). Percentage of eDSA+/crossmatch+ patients for each study year is reported in Figure S1. Among the 146 patients, 128 (88%) patients underwent treatment with IgGAM (eDSA+/IgGAM+ group, case group). Among the remaining 18 (12%) patients, 8 (5%) patients were treated only with tPE and a single dose of Rituximab (eDSA+/IgGAM− group), and 10 (7%) patients were not treated at all (eDSA+/no‐treatment group). Patient groups are reported in Figure 2. Pretransplant, intraoperative, and posttransplant recipient and donor characteristics in eDSA+/IgGAM+ vs. eDSA− patients are reported in Tables 1 to 4 and in Tables S1 and S2.
Figure 2.
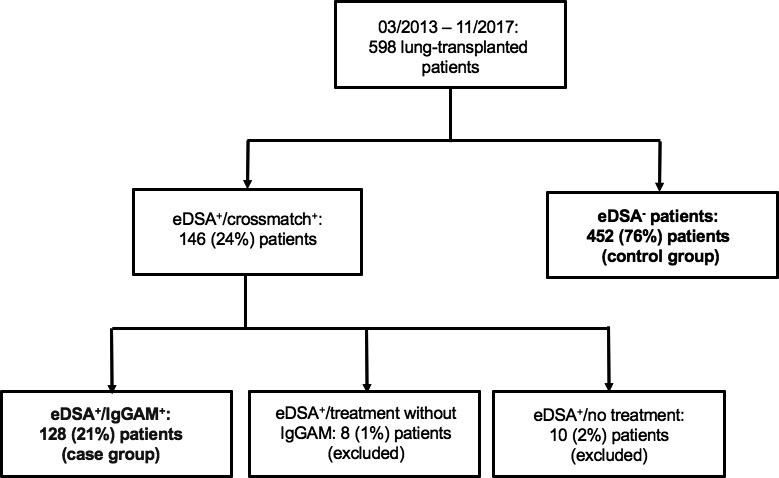
Figure 2 shows patient groups. Patients who developed eDSA and were treated with IgGAM (n = 128) formed the case group. Patients without eDSA (n = 452) formed the control group. Both groups are marked in bold. eDSA, early donor‐specific antibodies; IgGAM, IgA‐ and IgM‐enriched intravenous human immunoglobulins
3.2. eDSA
In case group, 21 (16%) patients showed pre‐formed eDSA. The remaining 107 (84%) patients developed de‐novo eDSA. eDSA were more often against donor HLA class II than I antigens (81% vs. 25%, Table 3). Twelve (9%) patients showed eDSA against both HLA class antigens. Median time to eDSA positivity was 14 (11‐20) days. Before treatment start, median MFI value was 4279 (2264‐9983). Median cumulative MFI value was 4961 (2290‐11 197).
3.3. eDSA treatment and IgGAM side effects
Treatment was performed preemptively in 110 (86%) patients. The remaining 18 (14%) patients had evidence of graft dysfunction.
Before the first IgGAM infusion, 18 (14%) patients underwent tPE (3 sessions in 13 patients and 5 sessions in 5 patients), and 37 (29%) patients 2 sessions of immunoabsorption. A single dose of Rituximab was given in 112 (88%) patients after the first IgGAM infusion. A hundred and eight (84%) patients underwent at least one consecutive 0.5 g/kg IgGAM infusion (median 3, [2‐5] infusions) at follow‐up (median treatment time 3 [2‐5] months). Figure S2 shows eDSA treatment. There was no difference between protocols 1 and 2 regarding the number of additional 0.5 g/kg IgGAM infusions (median 4 vs. 3, P = .35) or treatment time (median 4. vs. 3 months, P = .16).
Overall, 493 IgGAM infusions (2 g/kg, n = 128, and 0.5 g/kg, n = 365) were performed. During IgGAM infusions, anemia, defined as a drop of the haemoglobin value below 8 g/dl or of at least 2 g/dl after IgGAM infusion, was detected 26 (5%) times; allergic reaction, 6 (1.2%) times; nausea and abdominal pain, 22 (4.5%) times. In one (0.7%) patient, IgGAM treatment was withdrawn earlier as intended per protocol due to recurrent abdominal pain at each IgGAM infusion.
3.4. eDSA clearance
eDSA clearance is reported in Figure 3 and Table 5. Among the 128 eDSA+/IgGAM+ patients, 116 (91%) patients completed treatment as intended per protocol at follow‐up end. Among the remaining 12 (9%) patients, 4 (3%) patients had died in‐hospital, 4 (3%) patients were still on treatment, and 4 (3%) patients terminated treatment earlier as intended per protocol (due to evidence of carcinoma, n = 1; IgGAM side effects, n = 1; early retransplant, n = 1; recurrent hospital stays due to infection, n = 1). At treatment end, eDSA were cleared in 106 (91%) out of 116 patients. Among these 106 patients, the same eDSA recurred in 14 (13%) patients at a median of 9 (6‐18) months after treatment end. No new DSA was detected. At the last DSA control, performed at a median of 23 (7‐36) months after transplantation, 98 (92%) out of 106 patients did not show any DSA. eDSA clearance was worse in patients with preformed than de novo eDSA and in patients with graft dysfunction (Table 5).
Figure 3.
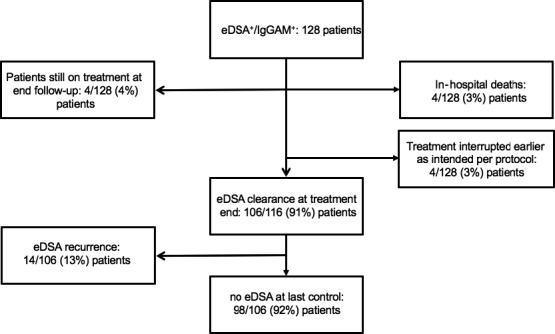
Figure 3 shows eDSA clearance, at treatment end and at last DSA control performed at a median of 23 (7‐36) months after transplantation. eDSA, early donor‐specific antibodies
Table 5.
eDSA clearance at treatment end
| Stratification | Clearance at treatment end (n = 106/116a, 91%) |
|---|---|
| HLA class | |
| I (n = 27) | 24 (83%) |
| II (n = 96) | 88 (92%) |
| P value | .60 |
| Pre‐formed vs. de novo DSA | |
| De novo (n = 101) | 98 (97%) |
| Preformed (n = 15) | 8 (53%) |
| P value | <.001 |
| MFI values before treatment | |
| Cleared (n = 106) | 3654 (2084‐9164) |
| Not cleared (n = 10) | 8360 (4428‐12 089) |
| P value | .082 |
| Cumulativeb MFI values before treatment | |
| Cleared (n = 106) | 4729 (2186‐9898) |
| Not cleared (n = 10) | 7716 (3940‐15 351) |
| P value | .13 |
| Crossmatch | |
| Positive (n = 8) | 7 (88) |
| Negative (n = 108) | 99 (92) |
| P value | .52 |
| tPE/immunoabsorption | |
| Yes (n = 48) | 42 (88) |
| No (n = 68) | 64 (94) |
| P value | .31 |
| Rituximab | |
| Yes (n = 106) | 97 (92) |
| No (n = 10) | 9 (90) |
| P value | .61 |
| Treatment protocol | |
| Protocol 1 (n = 81) | 74 (91) |
| Protocol 2 (n = 32) | 29 (91) |
| P value | .90 |
Values are expressed as median (IQR) or N of patients (%). DSA, donor specific antibodies; MFI, mean fluorescence index, tPE, therapeutic plasmapheresis.
12 patients were not considered in this analysis (4 patients still on IgGAM treatment; 4 patients died in‐hospital; in the remaining 4 patients, treatment was interrupted earlier as per protocol).
Sum of the single MFI, in case a patient showed eDSA against more than one antigen.
Among the 10 eDSA+/no‐treatment patients, 9 (90%) did not show DSA at last control, performed at a median of 17 (6‐28) months after transplantation. eDSA+/no‐treatment patients showed no pre‐formed eDSA and had a lower prevalence of eDSA against donor HLA class II antigens (60% vs. 81%, P = .094). The median MFI value at first positive DSA control was lower in eDSA+/no‐treatment than eDSA+/IgGAM+ patients (2037, IQR 1506‐3191, P = .012).
3.5. Outcomes
Median follow‐up was 24 (11‐40) months and did not differ between eDSA+/IgGAM+ and eDSA− patients (P = .76). Outcomes of eDSA+/IgGAM+ versus eDSA− did not show significant statistical differences between groups (Table 6 and Figure 4A‐D). However, freedoms from biopsy confirmed rejection (Figure 4B) and from pulsed steroid therapy (Figure 4C) at 6 months after transplantation were higher in eDSA+/IgGAM+ than eDSA− patients. These results were confirmed after propensity score matching and stratification according to quintiles of propensity scores (Tables S3‐S6).
Table 6.
Outcomes at follow‐up
| Variable | eDSA+/IgGAM+ (n = 128) | eDSA− (n = 452) | P value |
|---|---|---|---|
| Patient survival (%) | |||
| 1 y | 94 ± 2 | 92 ± 1 | |
| 4 y | 82 ± 4 | 83 ± 3 | .59 |
| Graft survival (%) | |||
| 1 y | 93 ± 2 | 91 ± 1 | |
| 4 y | 79 ± 5 | 81 ± 3 | .58 |
| Causes of death after hospital dischargea | |||
| CLAD | 4 (3) | 8 (2) | .35 |
| Infection | 4 (3) | 5 (1) | .11 |
| Malignancy | 4 (3) | 6 (1) | .18 |
| Cardiac | 0 | 1 (0.2) | 1.00 |
| Other | 1 (1) | 8 (2) | .69 |
| Freedom from biopsy‐confirmed rejection (%) | |||
| 6 mo | 74 ± 4 | 63 ± 3 | |
| 1 y | 67 ± 5 | 61 ± 3 | |
| 4 y | 57 ± 5 | 53 ± 3 | .34 |
| ISHLT biopsy grade | |||
| A1 | 34 (32) | 128 (34) | .62 |
| A2 | 10 (9) | 41 (11) | .63 |
| A3 | 0 | 3 (1) | 1.00 |
| Freedom from pulsed steroid therapy (%) | |||
| 6 mo | 73 ± 4 | 64 ± 2 | |
| 1 y | 58 ± 5 | 60 ± 3 | |
| 4 y | 43 ± 5 | 47 ± 3 | .82 |
| Freedom from CLAD (%) | |||
| 1 y | 99 ± 1 | 99 ± 1 | |
| 4 y | 82 ± 5 | 78 ± 4 | .83 |
| Freedom from re‐transplant (%) | |||
| 1 y | 98 ± 1 | 99 ± 1 | |
| 4 y | 95 ± 3 | 97 ± 1 | .28 |
| Freedom from infection (%) | |||
| 1 y | 74 ± 4 | 78 ± 2 | |
| 4 y | 48 ± 8 | 63 ± 3 | .15 |
Values are expressed as mean ± SD (%) or N of patients (%). CLAD, chronic lung allograft dysfunction; ISHLT, International Society of Heart and Lung Transplantation.
Patients who died before hospital discharge (n = 25) were not considered.
Figure 4.
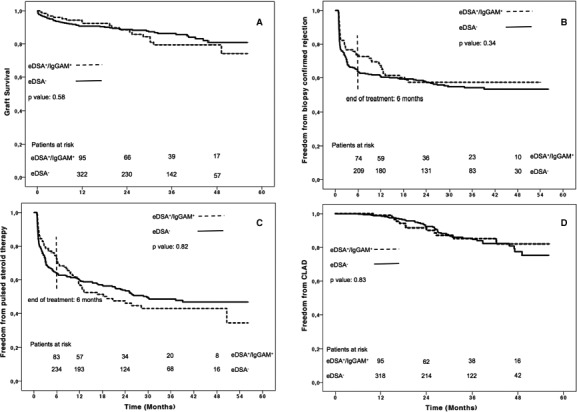
Figure 4 shows graft survival (A), freedom from biopsy confirmed rejection (B), freedom from pulsed steroid therapy (C), and freedom from CLAD (D), between eDSA +/IgGAM + vs. eDSA − patients. Patients at risk are reported above the X axis. In B and C a dotted line at 6‐month follow‐up marks the treatment end. CLAD, chronic lung allograft dysfunction; eDSA, early donor‐specific antibodies; IgGAM, IgA‐ and IgM‐enriched intravenous human immunoglobulins
eDSA+/IgGAM+ patients showed better graft survival (P = .005) and freedom from retransplant (P = .02) than excluded eDSA+ patients (Table S7), and particularly better freedom from retransplant (P = .003) than eDSA+/no‐treatment patients (Table S8). However, this could have been confounded by the small number of excluded patients.
In eDSA+/IgGAM+ patients, outcomes did not differ after stratification according to presence of preformed versus de novo eDSA, use of tPE or immunoabsorption, use of treatment protocol 1 versus 2, and eDSA clearance at treatment end (Tables S9, S10, S11, S12, respectively). However, eDSA+/IgGAM+ patients who had a negative crossmatch, did not have graft dysfunction at treatment time, and received Rituximab, had better graft survival (Tables S13, S14, S15, respectively).
Finally, outcomes were similar between a small number of eDSA+/no‐treatment patients and eDSA− patients, except for a higher incidence of retransplant in eDSA+/no‐treatment patients (Table S16).
Median forced respiratory volume in 1 second (FEV1) values (% predicted) did not differ between eDSA+/IgGAM+ versus eDSA− patients at discharge (68 vs. 64, P = .88), at 1‐year follow‐up (87 vs. 88, P = .23), and at last outpatient assessment (80 vs. 84, P = .29), performed at 24 (12‐37) months after transplantation.
4. DISCUSSION
This study represents the largest single‐centre case series on treatment of early DSA in lung transplantation published so far.11, 12, 13, 15, 16, 17
IVIG are a consolidated component of AMR treatment protocols in renal transplantation.23, 24 In lung transplantation, conversely, there is no consensus on when and how AMR must be treated.11, 12, 13, 14, 15, 16, 17
In the first published case series on preemptive DSA treatment with IVIG after transplantation, Hachem et al showed a DSA clearance of 65% at treatment end. Outcomes were worse in patients who did not clear DSA than in patients who did.11 Witt et al reported that treatment with IVIG and Rituximab cleared DSA in 9 out of 21 (43%) patients with acute AMR. Six (29%) patients died in‐hospital of refractory AMR. Among survivors, 14 (93%) patients developed CLAD.12 Vacha et al treated 16 patients with acute AMR using a combination of Bortezomib, Rituximab, tPE, and successive 0.5 g/kg IVIG infusions. DSA cleared in only 3 out of 11 patients (27.7%) at 6 months after treatment. Survival was 56.2% following treatment.17 Finally, in the case series of Islam et al, 72 (22.2%) patients developed de novo DSA after lung transplantation and, in 25 (34.7%) patients, DSA cleared spontaneously. They treated only patients with graft dysfunction using tPE, Rituximab and IVIG, showing a DSA clearance of 53%.16
All these studies reconfirm that current treatment protocols are ineffective in cases of AMR with established graft dysfunction. Therefore, at our institution, we treat patients as soon as eDSA are detected, mainly preemptively (possible subclinical AMR). In our opinion, eDSA represent just the early measurable part of general allosensitization of host versus graft.25 We observed that survival and outcomes were similar in treated patients versus patients without eDSA. In accordance with the previously reported literature, those patients with graft dysfunction (possible clinical AMR) showed worse survival and eDSA clearance than patients with only eDSA (possible subclinical AMR).
Freedom from biopsy confirmed rejection and from pulsed steroid therapy were higher during treatment time (Figure 4B,C) and decreased after treatment end, reconfirming that IgGAM may have a protective role against rejection. IgGAM are not per se immunosuppressive and have pleiotropic immunomodulatory effects, since they act on different points of the immunologic cascade.21, 24 IgGAM contain IgG (76%), IgM (12%), and IgA (12%), and can neutralize DSA in the periphery and scavenge activated complement through the IgM, IgG, and IgA components; inhibit the activation of antibody dependent cell mediated cytotoxicity through the IgG component; inhibit tissue migration of activated neutrophilic granulocytes and monocytes through the IgA component; and activate T regulatory cells through the IgG component.21, 26, 27, 28, 29 Moreover, the IgM component also confers a protection against infections through pathogen opsonisation.21 In our study, freedom from infection was similar among groups during treatment, but worsened thereafter in previously treated patients. This trend may be due to a late effect of Rituximab.
During the study period, we developed three different IgGAM‐based protocols to treat eDSA, looking for the most appropriate therapy. In fact, therapies of AMR may also provoke side effects, and the benefit of treatment must be carefully evaluated against the risk of side effects, particularly in asymptomatic patients with eDSA. We usually combined IgGAM with a single dose of Rituximab and, in some patients, with tPE or immunoabsorption. No difference was found in clearance and outcomes between protocol 1 and 2. The addition of 2 immunoabsorptions in all patients with eDSA did not add any benefit and did not reduce treatment time. Thus, since April 2017, we use a combination protocol with IgGAM, Rituximab and tPE for patients with a positive crossmatch or presence of graft dysfunction (possible clinical AMR), and only IgGAM in asymptomatic patients with eDSA (possible subclinical AMR, Figure 1).
Finally, 90% of untreated patients (n = 10) showed spontaneous eDSA clearance. Outcomes were mostly similar to treated patients, yet freedom from CLAD and re‐transplant were worse in untreated patients. Moreover, in a recent publication, spontaneous DSA clearance was observed in 34.7% of patients and was associated with a lower risk of acute rejection.16 Therefore, a randomized trial is required to demonstrate the real treatment efficacy by comparing outcomes between patients with DSA and treated versus patients with DSA without treatment.
5. STUDY LIMITATIONS
A control group made of eDSA+/no‐treatment patients would have been more robust than a control group made of patients without eDSA, to demonstrate treatment effect. The choice of eDSA− patients instead of eDSA+/no‐treatment patients was motivated by the fact that only few eDSA+ patients were not treated, and that, according to the recent evidence in literature,1, 2, 3, 4, 5, 6, 7, 8 DSA− patients have better graft function and survival than DSA+ patients.
Moreover, in the present study, we investigated the efficacy of IgGAM therapy only in patients with early DSA. Therefore, the results of this study might not be necessarily extended to patients who develop late DSA. This aspect was not investigated, because, at follow‐up, DSA were only controlled upon indication in patients without eDSA.
6. CONCLUSIONS
After lung transplantation, outcomes of treated patients with eDSA were similar to the outcomes of patients without eDSA. These results were confirmed after matching and stratification into quintiles of propensity scores. Treated patients showed high antibody clearance, that persisted at follow‐up end. However, further studies are required to demonstrate that IgAM therapy really improves outcomes and directly leads to eDSA clearance, since most of the eDSA+/no‐treatment patients cleared eDSA spontaneously.
DISCLOSURE
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation. Dr. Fabio Ius and Dr. Gregor Warnecke report personal and congress fees paid from Biotest, outside the submitted work. Dr. Tobias Welte reports personal fees from Boehringer and from Roche outside the submitted work. The other authors have no conflicts of interest to disclose.
Supporting information
ACKNOWLEDGMENTS
The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agreed to the manuscript as written.
Ius F, Verboom M, Sommer W, et al. Preemptive treatment of early donor‐specific antibodies with IgA‐ and IgM‐enriched intravenous human immunoglobulins in lung transplantation. Am J Transplant. 2018;18:2295–2304. 10.1111/ajt.14912
Igor Tudorache and Gregor Warnecke share senior authorship.
[The copyright line for this article was changed on October 2, 2018, after original online publication]
REFERENCES
- 1. Lobo LJ, Aris RM, Schmitz J, et al. Donor‐specific antibodies are associated with antibody‐mediated rejection, acute cellular rejection, bronchiolitis obliterans syndrome, and cystic fibrosis after lung transplantation. J Heart Lung Transplant. 2013;32:70‐77. [DOI] [PubMed] [Google Scholar]
- 2. Smith JD, Ibrahim MW, Newell H, et al. Pre‐transplant donor HLA‐specific antibodies: characteristics causing detrimental effects on survival after lung transplantation. J Heart Lung Transplant. 2014;33:1074‐1082. [DOI] [PubMed] [Google Scholar]
- 3. Ius F, Sommer W, Tudorache I, et al. Early‐donor‐specific antibodies in lung transplantation: risk factors and impact on survival. J Heart Lung Transplant. 2014;33:1255‐1263. [DOI] [PubMed] [Google Scholar]
- 4. Safavi S, Robinson DR, Soresi S, et al. De novo donor HLA‐specific antibodies predict development of bronchiolitis obliterans syndrome after lung transplantation. J Heart Lung Transplant. 2014;33:1273‐1281. [DOI] [PubMed] [Google Scholar]
- 5. Morrell MR, Pilewski JM, Gries CJ, et al. De novo donor‐specific HLA antibodies are associated with early and high‐grade bronchiolitis obliterans syndrome and death after lung transplantation. J Heart Lung Transplant. 2014;33:1288‐1294. [DOI] [PubMed] [Google Scholar]
- 6. Roux A, Bendib Le Lan I, Holifanjaniaina S, et al. Antibody‐mediated rejection in lung transplantation: clinical outcomes and donor‐specific antibody characteristics. Am J Transplant. 2016;16:1216‐1228. [DOI] [PubMed] [Google Scholar]
- 7. Le Pavec J, Suberbielle C, Lamrani L, et al. De‐novo donor‐specific anti‐HLA antibodies 30 days after lung transplantation are associated with worse outcomes. J Heart Lung Transplant. 2016;35:1067‐1077. [DOI] [PubMed] [Google Scholar]
- 8. Reinsmoen NL, Mirocha J, Ensor CR, et al. A 3‐center study reveals new insights into the impact of non‐HLA antibodies on lung transplantation outcome. Transplantation. 2017;101:1215‐1221. [DOI] [PubMed] [Google Scholar]
- 9. Levine DJ, Glanville AR, Aboyoun C, et al. Antibody‐mediated rejection of the lung: a consensus report of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2016;35:397‐406. [DOI] [PubMed] [Google Scholar]
- 10. Westall GP, Snell GI. Antibody‐mediated rejection in lung transplantation: fable, spin, or fact? Transplantation. 2014;98:927‐930. [DOI] [PubMed] [Google Scholar]
- 11. Hachem RR, Yusen RD, Meyers BF, et al. Anti‐HLA antibodies and preemptive antibody‐directed therapy after lung transplantation. J Heart Lung Transplant. 2010;29:973‐980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Witt CA, Gaut JP, Yusen RD, et al. Acute antibody‐mediated rejection after lung transplantation: a retrospective, single center, case series. J Heart Lung Transplant. 2013;32:1034‐1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ius F, Sommer W, Tudorache I, et al. Preemptive treatment with therapeutic plasma exchange and rituximab for early donor‐specific antibodies after lung transplantation. J Heart Lung Transplant. 2015;34:50‐58. [DOI] [PubMed] [Google Scholar]
- 14. Ius F, Sommer W, Kieneke D, et al. IgM‐enriched human intravenous immunoglobulin‐based treatment of patients with early donor specific anti‐HLA antibodies after lung transplantation. Transplantation. 2016;100:2682‐2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tinckam KJ, Keshavjee S, Chaparro C, et al. Survival in sensitized lung transplant recipients with perioperative desensitization. Am J Transplant. 2015;15:417‐426. [DOI] [PubMed] [Google Scholar]
- 16. Islam AK, Sinha N, DeVos JM, et al. Early clearance vs persistence of de novo donor‐specific antibodies following lung transplantation. Clin Transplant. 2017;31(8):e13028. [DOI] [PubMed] [Google Scholar]
- 17. Vacha M, Chery G, Hulbert A, et al. Antibody depletion strategy for the treatment of suspected antibody‐mediated rejection in lung transplant recipients: does it work? Clin Transplant. 2017;31(3):e12886. [DOI] [PubMed] [Google Scholar]
- 18. Lee JC, Christie JD, Keshavjee S. Primary graft dysfunction: definition, risk factors, short‐ and long‐term outcomes. Semin Respir Crit Care Med. 2010;31:161‐171. [DOI] [PubMed] [Google Scholar]
- 19. Verleden SE, Todd JL, Sato D, et al. Impact of CLAD phenotype on survival after lung re‐transplantation: a multicenter study. Am J Transplant. 2015;15:2223‐2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stewart S, Fishbein MC, Snell GI, et al. Revision of the 1996 working formulation for the standardization of nomenclature in the diagnosis of lung rejection. J Heart Lung Transplant. 2007;26:1229‐1242. [DOI] [PubMed] [Google Scholar]
- 21. Walpen AJ, Laumonier T, Aebi C, et al. Immunoglobulin M‐enriched intravenous immunoglobulin inhibits classical pathway complement activation, but not bactericidal activity of human serum. Xenotransplantation. 2004;11:141‐148. [DOI] [PubMed] [Google Scholar]
- 22. Blackstone EH. Comparing apples and oranges. J Thorac Cardiovasc Surg. 2002;123:8‐15. [DOI] [PubMed] [Google Scholar]
- 23. Jordan SC, Toyoda M, Kahwaji J, et al. Clinical aspects of intravenous immunoglobulin use in solid organ transplant recipients. Am J Transplant. 2011;11:196‐202. [DOI] [PubMed] [Google Scholar]
- 24. Tedla FM, Roche‐Recinos A, Brar A. Intravenous immunoglobulin in kidney transplantation. Curr Opin Organ Transplant. 2015;20:630‐637. [DOI] [PubMed] [Google Scholar]
- 25. Koenig A, Mariat C, Mousson C, et al. B cells and antibodies in transplantation. Transplantation. 2016;100:1460‐1464. [DOI] [PubMed] [Google Scholar]
- 26. Lobo PI. Role of natural autoantibodies and natural IgM anti‐leucocyte autoantibodies in health and disease. Front Immunol. 2016;7:198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Djoumerska IK, Tchorbanov AI, Donkova‐Petrini VD, et al. Serum IgM, IgG and IgA block by F(ab′)‐dependent mechanism the binding of natural IgG autoantibodies from therapeutic immunoglobulin preparations to self‐antigen. Eur J Haematol. 2005;74:101‐110. [DOI] [PubMed] [Google Scholar]
- 28. Jordan SC, Toyoda M, Vo AA. Regulation of immunity and inflammation by intravenous immunoglobulin: relevance to solid organ transplantation. Expert Rev Clin Immunol. 2011;7:341‐348. [DOI] [PubMed] [Google Scholar]
- 29. Shevach EM. Mechanisms of foxp3 + T regulatory cell‐mediated suppression. Immunity. 2009;30:636‐645. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


