Abstract
Background
In the first 2 years of grass tablet sublingual immunotherapy treatment, we have previously demonstrated a progressive development of a regulatory T‐cell response, which was preceded by an early decrease in the frequency of both IL‐4+ cells and sIgE levels. A progressive increase in sIgG 4 levels and FAB blockage were also found.
Methods
By monitoring immunological kinetics during 3 years of active treatment + 2 years of follow‐up, we aimed to identify key immunological parameters that could explain sustained clinical benefit of grass tablet sublingual immunotherapy.
Results
Thirty patients completed the 5‐year clinical trial protocol. Although individual responses were heterogeneous, reduction in both sIgE and circulating IL‐4+ cells compared to the initial 1‐ to 4‐month peak was maintained throughout the 3‐year treatment period and for 2 years after discontinuation. Meanwhile, after a 2‐year increase in sIgG4, the levels were stabilized during the third year and decreased post‐therapy. FAB inhibition remained significantly inhibited throughout the study compared to preimmunotherapy in 83% of patients. A sustained regulatory T‐cell response, after IT cessation, occurs in two‐thirds of the patients. There was a statistical association between this regulatory response, the maintenance of lower eosinophil counts during grass pollen seasons, and sIgE titers lower than before immunotherapy treatment, and the latter were significantly associated with clinical response.
Conclusion
Our results suggest that the immunological mechanisms underlying the sustained response after 2 years of cessation of immunotherapy (3‐year treatment period) are linked to the acquisition and maintenance of a regulatory T‐cell response.
Keywords: allergic rhinitis, IL‐4, regulatory T cells, sIgE, sIgG4, sublingual immunotherapy
Highlights.
Early effect is governed by effector cell desensitization.
SIgG4 interference is generated a few months after SIT initiation and is maximum in the first two treatment years.
amT regulatory response is consolidated after 3 continuous treatment years and is key for sustained benefit two years after SIT cessation.
Abbreviations
- AIT
allergy immunotherapy
- amTreg
activated memory regulatory T cell
- FAB
facilitated allergen binding
- GPS
grass pollen seasons
- PBMC
peripheral blood mononuclear cells
- SLIT
sublingual allergen immunotherapy
1. INTRODUCTION
When used as a tolerance‐inducing treatment for allergic diseases, allergy immunotherapy (AIT) acts through several mechanisms, including the generation of B and T regulatory responses.1, 2 AIT often induces a transient increase in specific IgE and promotes synthesis of allergen‐specific IgG antibodies, which can block the allergen‐IgE interaction. This blockade consequently interferes with the IgE‐mediated allergic inflammation cascade, the IgE‐facilitated allergen presentation to T cells, and with the increased IgE production during high allergen exposure.3, 4, 5 Induction of allergen‐specific IgG accompanies the clinical benefits of AIT, with allergen specificity determining antibody ability to block allergen‐dependent IgE activity.6, 7 Regulatory T cells are also decisive for the suppression of allergic inflammation and long‐term tolerance.8
Sublingual allergen immunotherapy (SLIT) has a well‐established profile for safety and effectiveness in the treatment of allergic rhinitis and asthma.9, 10 In a two‐year longitudinal study on the systemic effects of SLIT, we have previously described the early and late phases of immune modulation in peripheral blood induced by a registered AIT with Phleum pratense tablets in peripheral blood samples.11 Briefly, SLIT induced an initial exacerbation of Th2 responses, measured as an increase in the levels of both allergen‐specific IgE and IgG, as well as in the percentage of IL‐4+ cells at months 1 to 4 of therapy; this was followed by a progressive downregulation orchestrated by continued production of allergen‐specific antibodies other than IgE (such as sIgG4) and by generation of a CD4+ T cell subset with an activated memory/induced regulatory phenotype (amTreg).11 This clinical trial was extended by 9 months of additional active therapy and 2 years of follow‐up period to evaluate sustained immunological effects. To determine which treatment‐induced tolerance mechanisms persist after cessation of therapy, we evaluated several immune parameters, including phenotypic characterization of T regulatory cell (amTreg) subsets; allergen‐specific IgE, IgG4, and IgG1 levels; and frequency of IL‐4‐producing cells.
2. PATIENTS AND METHODS
2.1. Study population
Fifty‐eight adult patients were enrolled in the prospective protocol in September 2009 by the Allergy Dept. of Hospital de La Princesa (Madrid, Spain), forty remained in the study 2 years later, and thirty completed the 5 years (three active treatment years and two‐year follow‐up). Inclusion criteria were a clinical history suggesting grass pollen allergic rhinitis, together with positive specific IgE (CAP class ≥2) and skin prick test (Soluprick SQ, ALK) to Phleum pratense. Patient characteristics are described in Table S1. Exclusion criteria included previous AIT, a clinical history of symptomatic perennial allergic rhinitis or asthma, patients with contraindications for AIT as established by the immunotherapy subcommittee of the EAACI and by the Summary of Product Characteristics of GRAZAX®, and pregnancy.12 The institutional review board approved the study protocol; all subjects were informed of the aim of the trial and gave written consent.
All patients received daily sublingual administration of GRAZAX® Hørsholm, Denmark (Phleum pratense, 75,000 SQ‐T tablets ALK, Denmark) during the initial 3‐year treatment period. Immunological evaluations were performed out of pollen season and during peak pollination (May‐June). The baseline was established in September‐October 2009 before beginning AIT, as previously described.11 During the 2‐year trial extension, samples were evaluated twice yearly, in the grass pollen season (GPS) and 3 months later. Only data from the patients completing the study are included in the current report, and values from first‐year therapy are reproduced when needed for comparison.
2.2. Clinical improvement in patients under and post‐grass tablet SLIT
To analyze long‐term clinical efficiency as an indicator of therapy success, we used patient self‐assessment. Subjective clinical evaluation during a post seasonal visit was based on comparison between the most recent grass season and baseline pollen season. A numerical value was assigned to all questions, ranging from −2 and −1 (degree in worsening in symptoms), 0 (no change), to 1 and 2 (degree of improvement). Cumulative values of the assessments from years 1 + 2 + 3 yield the end‐of‐therapy clinical index. Cumulative values of the assessments from years 4 + 5 yield post‐therapy clinical index, ranging from −4 to −1 (degree in worsening in symptoms both or only 1 year), 0 (no changes), to 1 to 4 (degree in improvement in symptoms both or only 1 year).
2.3. PBMC separation and immunophenotypic and flow cytometric analysis
Peripheral blood mononuclear cells (PBMC) were separated on Ficoll‐Hypaque (GE Healthcare Bio‐Sciences AB, Uppsala, Sweden) and incubated (20 hours) with Phleum extract protein (20 μg/mL). For cytokine analyses, protein secretion was blocked with 2 μg/mL brefeldin A (Calbiochem, Billerica, MA) in cultures for the last 5 hours. No significant cell death was observed under the experimental conditions.
For multidimensional flow cytometric analyses of phenotype and cytokine‐secreting lymphocytes, we used a Gallios flow cytometer (Beckman Coulter). Flow‐Check Fluorospheres (Beckman Coulter, Indianapolis IN, United States) were used daily to assess flow cytometer optical alignment and fluidics system performance. To ensure maintenance of instrument settings and consistency over time, standardization was performed every round of visits using Flow‐Set Fluorospheres (Beckman Coulter) to re‐establish settings of all fluorescence parameters. Compensation was performed using an aliquot of frozen human PBMC cultured overnight and stained with anti‐human CD4 labeled with each marker for each dye combination. ADC software (Beckman Coulter) was used for automatic compensation and verified using the same PBMC sample labeled with the antibodies in our protocols to ensure satisfactory automatic coefficient calculation.
Kaluza software (Beckman Coulter) was used to analyze listmode data. One million events were acquired and lymphocytes gated using side‐scatter properties.
Fractionated cell subsets were stained with Live/Dead dye (Invitrogen, Carlsbad, CA) and identified using the following anti‐human mAb:
AmCyan‐anti‐CD4 (mIgG1; Becton Dickinson, San Diego, CA), Pacific Blue‐anti‐CD127 (mIgG1; eBiosciences, San Diego, CA), ECD‐anti‐CD45RA (mIgG1; all Beckman Coulter), and APC‐PC7‐anti‐CD25 (rat IgG1; eBiosciences).
Cells were surface‐stained according to the manufacturer's protocols, and subset phenotype analysis was determined as previously described.11
2.4. Allergen‐specific IgE, IgG4, and IgG1
Allergen‐specific IgE (to the major allergens Phleum p 1 and p 5) was measured using the ADVIA Centaur platform (Siemens Healthcare Diagnostics, Inc., Tarrytown, NY. United States) according to standard methods. Phleum p 1‐ and p 5‐specific IgG4 and IgG1 were determined by ELISA.11
2.5. FAB assay
FAB analysis was performed using standard methods. A defined indicator serum containing high sIgE concentrations was preincubated with allergen alone or with patient serum and further incubated with EBV‐transformed B cells. Surface binding of allergen‐IgE complexes was detected by flow cytometry.11
2.6. Data presentation and statistical analysis
Data are represented as boxes showing sample mean ± 95% confidence intervals. Statistical analysis was performed with Prism v5.0 software (GraphPad Software, La Jolla, CA). Unless otherwise specified, the one‐way ANOVA test (one‐way analysis of variance, repeated measures, and the pair‐based Dunnett test for longitudinal assays) was used in individual parameters and Pearson's and Fisher's exact test for pairwise correlations. P values <0.05 were considered significant.
3. RESULTS
3.1. Humoral response and FAB in grass pollen SLIT
After an initial 1‐month peak, a reduction in sIgE production was observed, which was maintained throughout the 3‐year treatment period (Figure 1A, black boxes) and for 2 years after discontinuation (Figure 1A, red boxes); but the individual responses were heterogeneous. By the end of therapy (GPSIII), 46% of patients (n=14) showed sIgE levels similar to or lower than baseline values, which reached 76% by GPSIV, when sIgE values were lowest. From this time on, 33% of patients (n=10) showed an increase in sIgE levels, and, at the end of trial, a two‐ to fivefold increase in sIgE values compared to baseline. In the remaining 67% of patients (n=20), sIgE remained lower than both during therapy and preimmunotherapy levels (Figure 1A, dotted line). Specific IgG4 levels increased in 85% of treated patients (n=35) during the first 2 years of treatment; in all cases, sIgG4 decreased during the third year and post‐therapy (Figure 1B), but remained higher than preimmunotherapy in 60% of patients (n=19) (Figure 1B, dotted line). Patients with detectable sIgG1 levels (56%) showed the same pattern; in 46% (n=14) of them, end‐of‐trial values remained higher than baseline (Figure 1C). sIgE/sIgG4 ratios at the end of trial were lower than preimmunotherapy ratios in 70% (n=21) of patients (Figure 1D). FAB remained lower than preimmunotherapy in 83% of patients (n=25) (Figure 2A). Inverse correlation was found between the sIgG4 increase and the change from baseline in IgE‐FAB at the end of therapy and afterward (Figure 2B).
Figure 1.
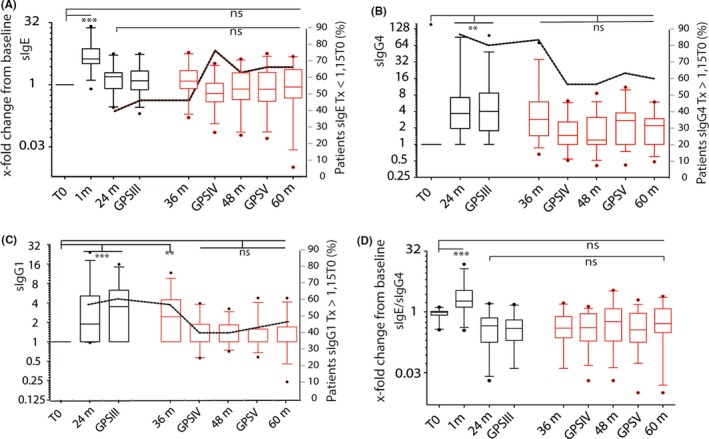
Modulation of allergen‐specific Ig by grass tablet SLIT. sIgE (A), sIgG 4 (B), sIgG 1 (C), and sIgE/sIgG 4 (D) levels are shown as the x‐fold change from baseline for the two main Phleum allergens, Phl p 1+p 5. ***P ≤ 0.001, **P ≤ 0.01, ns: not significant. Dotted line indicates the percentage of the population (n = 30) showing lower sIgE levels (A) and higher sIgG 4 (B) or sIgG 1 (C) levels than preimmunotherapy
Figure 2.
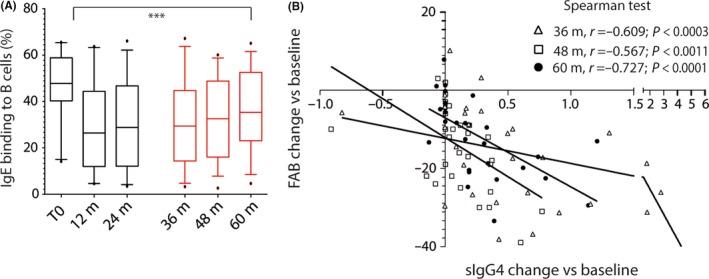
A, Blocking effect of patient serum in allergen‐IgE binding to B cells over time. ANOVA tests for longitudinal assays were applied; ***P ≤ 0.001, ns: not significant. B, Decrease in IgE‐FAB correlates with increased sIgG 4 levels from baseline at 36 (△), 48 (□), and 60 months (●)
3.2. Acquisition of regulatory response by the end of the trial is associated with sustained reduced sIgE and GPS‐eosinophil counts
The increase in circulating IL‐4+ cells during the initial 1‐ to 4‐month therapy period was followed by a significant decrease in these cells during treatment (Figure 3A). At the end of active therapy, circulating IL‐4+ cell levels were lower than at time 0 in 89% of patients. This reduction remained at year 4 and 5 in 80% (n 24) of patients. We detected a decrease in circulating IL‐4+ cells during the pollen seasons after therapy cessation, which was more pronounced in GPSIV, but not statistically significant when compared to the end of treatment (Figure 3A).
Figure 3.
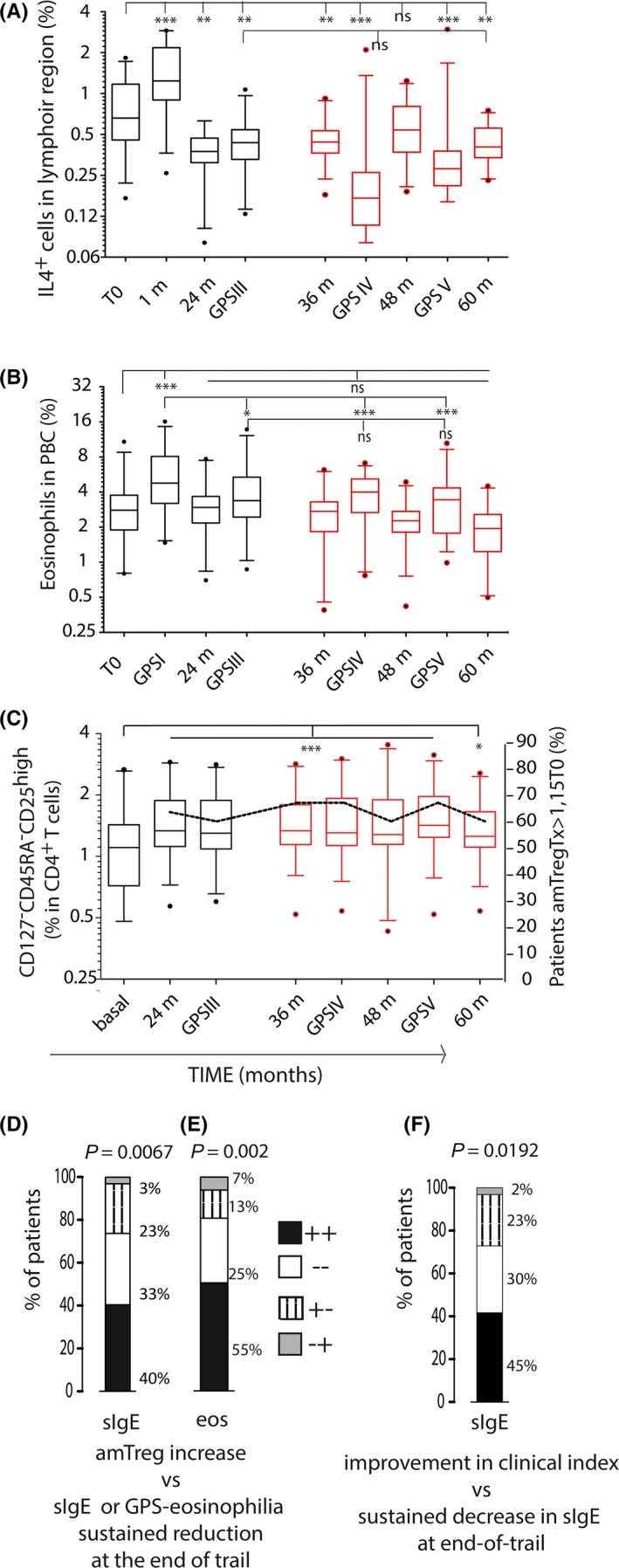
Modulation of cellular and cytokine profiles by grass tablet SLIT. A‐B, Percentages of circulating IL4+ (A) cells and eosinophils (B) throughout the trial (n = 30). C, Sustained generation of a amTreg‐like cell‐mediated response over time. ***P ≤ 0.001, **P ≤ 0.01, *P < 0.05, ns: not significant. D‐F, Contingency tables of variables distribution in the population; Fisher's test was applied. Percentage of patients showing at the end of trial both increase in amTreg‐like cell and low sIgE levels compared to baseline (black bars), neither (white bars), or only one condition (amTreg cell generation, dashed bars; sIgE values lower than T0, gray bars) (D); and data as above, comparing amTreg cell generation and sustained reduction in GPS‐associated eosinophil counts (E). Percentage of patients showing both end‐of‐trial sustained reduction in sIgE and improvement in clinical index (black bars), neither (white bars), or only one condition (sustained improvement in clinical index, dashed bars; end‐of‐trial sustained reduction in sIgE levels, gray bars) (F)
Peripheral eosinophil counts during GPS were used as an indicator of patient allergic response to environmental pollen. A significant reduction in number of eosinophils was detected at GPSIII vs GPSI; this reduction was sustained in GPSIV and V post‐therapy (Figure 3B). In addition, a significant increase in the rate of the CD4+ T‐cell subset with the amTreg phenotype was detected after 2 years of treatment and thereafter; this amTreg subset was generated in 63% of patients by the end of the second year of therapy11 and persisted above 60% both throughout therapy and for the 2 years of follow‐up (Figure 3C).
By comparing variables we found, in 40% of patients, a significant coincidence of sustained increase in the amTreg‐like cell pool with the presence of end‐of‐trial sIgE levels lower than those at preimmunotherapy; meanwhile, 33% of the population showed neither increase in the amTreg pool nor end‐of‐trial reduced sIgE levels, and 23% of patients with induction in the amTreg‐like cell pool showed post‐therapy increase in sIgE values (Figure 3D). Eosinophil counts lower during GPS than in the first year of therapy were also significantly associated to the generation of the amTreg‐like cell subset (Figure 3E). Positive contingency was also found between the clinical score and the changes in sIgE levels post‐therapy: 41% of patients showed sustained decrease in sIgE post‐therapy and improvement in clinical index (Table 1); 30% of the population showed an increase in sIgE levels post‐therapy and worsening in clinical index; while 26% of those with improved clinical index showed post‐therapy increase in sIgE values (Figure 3F and Table 1).
Table 1.
Clinical index
| No. | 1st | 2nd | 3rd | 4th | 5th | End‐of‐therapy CI | Post‐therapy CI | sIgE ratio Year 5/4 | sIgE ratio Year 5/3 | sIgE ratio Year 5/0 |
|---|---|---|---|---|---|---|---|---|---|---|
| 04 | 1 | 2 | 2 | 0 | 1 | 5 | 1 | 1.13 | 3.94 | 2.26 |
| 05 | 1 | 2 | 2 | 0 | −1 | 5 | −1 | 1.29 | 0.75 | 0.95 |
| 07 | 1 | 1 | 1 | 2 | 0 | 3 | 2 | 0.75 | 0.36 | 0.39 |
| 08 | 2 | 2 | −1 | 1 | 1 | 3 | 2 | 0.18 | 0.05 | 0.01 |
| 11 | 2 | 2 | 1 | 1 | 5 | 0.87 | 0.37 | 0.88 | ||
| 13 | 2 | 2 | −1 | 2 | −1 | 3 | 1 | 0.79 | 0.95 | 0.69 |
| 14 | 2 | 2 | 2 | −1 | 1 | 6 | 0 | 1.09 | 0.56 | 0.48 |
| 15 | 2 | 0 | 1 | 1 | 1 | 3 | 2 | 1.66 | 0.80 | 4.83 |
| 16 | 2 | 1 | 0 | −1 | 1 | 3 | 0 | 0.76 | 0.56 | 0.91 |
| 19 | 1 | −1 | 1 | 0 | 1 | 1 | 1 | 3.99 | 1.48 | 0.58 |
| 20 | 2 | 2 | 1 | 2 | −1 | 5 | 1 | 1.14 | 0.32 | 0.34 |
| 21 | 1 | 1 | 1 | −1 | 1 | 3 | 0 | 1.02 | 1.71 | 3.89 |
| 22 | 1 | −1 | 0 | 1 | 0 | 0 | 1 | 1.51 | 1.73 | 2.43 |
| 23 | 0 | 1 | 2 | 0 | 0 | 3 | 0 | 0.83 | 0.60 | 0.88 |
| 25 | 2 | 1 | 1 | 1 | 1 | 4 | 2 | 1.02 | 0.34 | 0.22 |
| 28 | 1 | 1 | 2 | −1 | −1 | 4 | −2 | 1.29 | 0.52 | 0.79 |
| 31 | 1 | 1 | 1 | 0 | −1 | 3 | −1 | 1.03 | 1.03 | 0.86 |
| 32 | 2 | 2 | −1 | 2 | −1 | 3 | 1 | 1.84 | 0.67 | 1.11 |
| 33 | 1 | 2 | 1 | 1 | 1 | 4 | 2 | 1.80 | 0.78 | 2.13 |
| 35 | 2 | 1 | 0 | 2 | 1 | 3 | 3 | 1.49 | 1.11 | 1.01 |
| 39 | 2 | 1 | 2 | 1 | −1 | 5 | 0 | 0.96 | 0.61 | 0.65 |
| 41 | 1 | 1 | 2 | 0 | −1 | 4 | −1 | 0.83 | 1.57 | 3.46 |
| 42 | 1 | 2 | −2 | −1 | −3 | 1.56 | 2.94 | 4.69 | ||
| 48 | 1 | 1 | 0 | 0 | −1 | 2 | −1 | 1.34 | 0.73 | 2.63 |
| 51 | 1 | 1 | 0 | −1 | −1 | 2 | −2 | 1.47 | 0.78 | 0.75 |
| 52 | 2 | 1 | 0 | 2 | 0 | 3 | 2 | 1.09 | 0.39 | 0.47 |
| 54 | 1 | 1 | 0 | 1 | 1 | 2 | 2 | 2.24 | 0.83 | 4.36 |
| 55 | 1 | 1 | 1 | 1 | 1 | 3 | 2 | 1.00 | 0.99 | 0.16 |
| 57 | 1 | 2 | 2 | −1 | 0 | 5 | −1 | 1.16 | 0.79 | 2.30 |
| 59 | 1 | 1 | 0 | 2 | 2 | 0.64 | 0.18 | 0.04 |
Subjective clinical evaluation during a postseasonal visit was based on comparison between the most recent grass season and the previous one. A numerical value was assigned to all questions, ranging from −2 and −1 (degree in worsening in symptoms), 0 (no change), to 1 and 2 (degree of improvement). Cumulative values from year 1 + 2 + 3 assessment yield the end‐of‐therapy clinical index. Cumulative values from year 4 + 5 assessment yield post‐therapy clinical index ranging from −4 to −1 (degree in worsening in symptoms both or only 1 year), 0 (no changes), to 1 to 4 (degree in improvement in symptoms both or only 1 year).
3.3. Differential patterns of response in individual patients
Patients displayed a great diversity of immunological responses, both in strength and in time of onset. As an example, sIgE, sIgG4, amTreg, and FAB responses of representative individual patients along 3 years of treatment and 2 years of follow‐up are shown in Figure 4.
Figure 4.
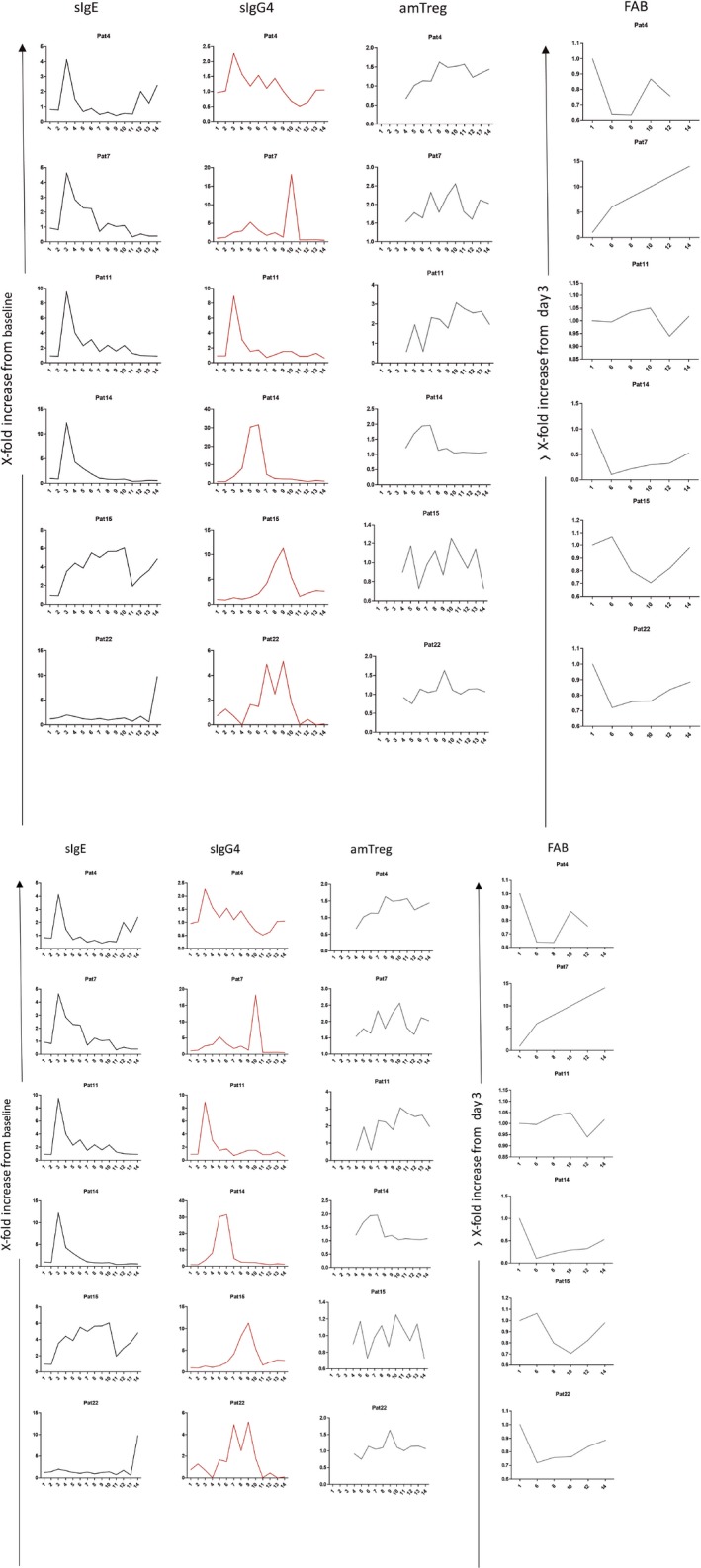
Kinetics of representative patients. IgE (black line), IgG4 (red line), amTreg (gray line), and FAB (green line). Y‐axis represents fold increase vs T0 (beginning of the trial), and x‐axis represents time point along the trials 1 (3 days), 2 (8 d), 3 (1 mo), 4 (4 mo), 5 (GPSI), 6 (12 mo), 7 (GPSII), 8 (24 mo), 9 (GPSIII), 10 (36 mo), 11 (GPSIV), 12 (48 mo), 13 (GPSV), and 14 (60 mo)
One of the few consistent results is the peak of sIgE at the first month of treatment (around 10‐fold increase) (Figure 4; patients 4, 11, 14, 28, 32, 33, 39, 52). In fact, a lack of an early increase in sIgE, or subsequent early decline, is suggestive of treatment failure (Figure 4; patients 15, 22). This early sIgE peak is consistent with the activation of memory B cells10 and is a sign of immunological interaction. Other responses, such as the level of sIgG4 or its kinetic along time, as well as the percentage of amTreg and peak of regulation along time, varied substantially among patients as well. As can be seen in Figure 4, FAB maximum improvement was observed around the second treatment year. In spite of a clear trend to associate with sIgG4, some patients, as Patient 32, show FAB improvement in the absence of IgG4‐ or IgG1‐specific responses.
Interestingly, individual kinetics revealed that even without detectable levels of sIgG4 (Figure 4; Patient 32) or with differential kinetics along time, with a sIgG4 peak at either early (Figure 4; patients 4, 11, 14, 28) or late time points along the treatment (Figure 4; patients 7, 15, 22, 33, 39, 52), the acquisition of a regulatory phenotype during the first two treatment years (in around 70% of the subjects) and its maintenance by the end of the trial (in a 63% of the patients) was not affected. Moreover, we could not find any correlation between the time of onset or strength of sIgG4 and the development of an amTreg response, suggesting that sIgG4/FAB‐associated mechanisms act independently of the Treg response.
4. DISCUSSION
To our knowledge, this is the first immunological study performed during specific allergen immunotherapy treatment over a complete five‐year period (3 + 2 years) investigating both humoral and cellular changes. The specific IT product used in the study is the first and only documented product in prospective 5‐year trials both in adult and in child populations.9, 10, 13 This makes this approach of special interest to understand the immunological mechanisms, to generate new hypothesis, and to link them to the already known clinical profile.
The main finding of this 5‐year clinical trial is the existence of an association between the maintenance of an amTreg response and the downregulation of eosinophil counts and sIgE. Beyond this finding, the current study provides a rational framework to understand the effect of the SLIT product during and after the end of treatment.
We can thus analyze short‐term therapy effect (first year), effects of long‐term treatment (3 years) and sustained effect (5 years). The understanding of these mechanisms will be critical for the design of better specific AIT intervention strategies and for a rational use of existing therapy products.
4.1. Short‐term effect (first year)
There is a clear increase in both sIgE and circulating IL‐4+ cells during the first 4 months of therapy, which is followed by a progressive decrease later on (Figure 1A). Levels of sIgE will be lower than baseline values only after 3 years of therapy. sIgG4 responses will be developed after 4 months of therapy. FAB inhibition shows, in parallel, a slight worsening during the first month,14 with significant improvement only after 1 year of therapy. All these data support that no clear peripheral improvement of allergy is observed during the first month of therapy.
On the other hand, previous reports4, 5, 15 documented a clear clinical benefit after only a few months of therapy, which seems to be a contradiction. Effector cell desensitization could be the leading immunological mechanism during early AIT phase. Recently, Rosace et al16 have shown that oral mucosa from allergic patients undergo structural changes which facilitate immunological interaction. Co‐localization of CD11+ and CD4+ cells supports this interaction and could constitute the missing link to understand how sublingual immunotherapy products interact with immune cells.
Moreover, it has been documented that relatively high antigen doses are needed to induce clinical effect in both subcutaneous and sublingual therapy approaches.17 These high doses may be needed for effector cell modulation. In the same way, accelerated schedules in hymenoptera venom AIT induce immunological tolerance in a few days.18 Making effector cells unresponsive might also have a critical effect for subsequent immunological changes by shutting down key pro‐inflammatory cascades. Increases in blocking antibodies were detected after 3 months of treatment in a previous study,19 so this element may also contribute to the effect of AIT during the first treatment year. Interestingly, in a recent publication on the GRAZAX® asthma prevention study13, grass pollen allergic children treated with the same registered product GRAZAX® were assessed for asthma symptoms both during summer and out of season (winter), where the latter could be related to general airway inflammation. An effect on these out‐of‐season asthma symptoms was observed in the third winter season and especially in follow‐up seasons, indicating that peripheral improvement of general airway inflammation coincides with the onset of a regulatory response.
4.2. Effect of long‐term treatment (3 years)
In our previous publication,11 we found that it took 2 years of continuous therapy to develop a statistically significant amTreg response. This regulatory response was significantly associated with an early control of IL‐4+ cells. A third year of therapy is not linked to an increase in patients entering regulation. Interestingly, Swamy et al20 described a progressive reduction in the methylation of CpG sites within the Foxp3 locus in a clinical trial of sublingual therapy performed for 2 years. Moreover, in a recent clinical trial,21 2 years of AIT with SLIT tablets (GRAZAX®) were not enough to detect clinical benefit 1 year after discontinuation. These data suggest that a third year of treatment might be pivotal for the epigenetic fixation of specific regulatory phenotype, especially for patients entering late into regulation. In the same study, the similar clinical outcomes were observed with a high‐dose intact allergen subcutaneous regime (Alutard SQ), suggesting similar mechanisms or both administration routes. Recently, Arroyo‐Hornero et al22 pointed out that CD4+ CD25 + CD127‐/lo population contained a subpopulation of Treg with differential maintenance of TSDR (regulatory T cell‐specific demethylation region) demethylation status, CD27 expression, cytokine production, and ability to suppress when exposed to immunosuppressants during in vitro expansion. A better understanding of T reg dynamics will be critical for the design of new specific immunotherapy intervention strategies.
Regarding the sIgE/sIgG4 axis, a huge variability of individual responses can be observed. In many patients, the production of sIgG4 toward the major allergens Phl p 1 and p 5 presents a clear peak that can be developed at any time during the treatment period. In general, sIgG4/sIgE ratios and FAB inhibition support the generation of an immunological interference along the treatment, with different strength for each individual patient. The lack of association with the amTreg response points in the direction of independent mechanisms, and could explain why changes in sIgG4 alone are not directly predicting clinical effect. In fact, as can be seen in the Figure 4 (Patient 32), even in the absence of a sIgG4 response, an amTreg response might be generated, and vice versa (Patient 15).
The late onset of the amTreg response might be essential to understand why numerous clinical trials with a T‐cell focus have failed in the last years. These approaches have included T‐cell peptides (https://clinicaltrials.gov NCT02040844), allergen fragments (https://clinicaltrials.gov NCT02943720), modified allergens (https://clinicaltrials.gov NCT02382718), or low‐dose intact allergens (https://clinicaltrials.gov NCT02166268). All of them share a relatively short active treatment phase and primarily aim to modulate T‐cell responses, whereas they generally lack targeting of desensitization routes. Interestingly, some patients in the current study seem to develop an amTreg response after a shorter treatment time. This fact could explain why some pivotal trials primarily targeting T cells with a very limited number of patients were suggestive of clinical benefit, and bigger phase II/III trials have failed so far. It will be interesting to study immunological kinetics with perennial allergen vaccines, such as mites, to investigate whether the onset of an amTreg response is generated in an earlier phase due to perennial allergen exposure. In fact, recent publications on sublingual immunotherapy in LTP‐mediated food allergy,23, 24 suggest that a T regulatory response (PD‐L1+/IL‐10+Treg) might be generated earlier.
4.3. Sustained effect (2 years after cessation)
The results provided by this study demonstrate end‐of‐trial sIgE levels lower than those at preimmunotherapy. In fact, by comparing readouts in those patients, we have found an association of this result with both a significant increase in amTreg and a decrease in eosinophil counts at the end of the trial. Moreover, this sustained reduction in sIgE levels is also associated with clinical improvement. In general, the amTreg cell population is maintained during the follow‐up period even though some variation is observed for individual patients, suggesting that both reduction in sIgE and increased amTreg numbers should be further investigated as markers of sustained AIT effect.
5. CONCLUDING REMARKS
Our data provide evidence that simple at‐hand biomarkers, such as sustained reduction in sIgE or in GPS‐associated eosinophil counts, are connected to the generation of an amTreg‐like population during SLIT therapy, which in turn is related to clinical improvement. To our knowledge, we have conducted the first five‐year prospective study exploring various immunological mechanisms associated with sustained effect of specific SLIT allergy intervention. We decided to maximize the chance of understanding immunological changes by allocating all patients to the active group. This was possible because the product used is documented as safe and clinically effective in multiple previous prospective and well‐controlled studies for up to 5 years.9, 10, 13 We formulated the hypothesis that the maintenance of the amTreg response after discontinuation should play a pivotal clinical role. Considering the data from the five‐year study period, we can conclude that about 30% of the patients do not develop an amTreg response to the therapy. Up to 2 years of therapy are needed to induce such a response in the rest of the patients. A third year of therapy does not add extra patients to the amTreg+ group. After 2 years of discontinuation, the percentage of amTreg+ patients is maintained, even though a reduction is seen for some patients. This way of understanding immunotherapy effects on amTreg induction may open new biomarker strategies to identify early, late, and sustained responders, and thus to design better intervention strategies that, for example, might have clear consequences for clinical trial design.
In Figure 5, a summary of the sequential mechanisms involved for patients attending our study is shown. It should be stressed that the mechanism suggested is based on detailed investigations of various immunological parameters in the current study. The tested product presents a good balance between safety and efficacy during continuous treatment for up to 3 years.9, 10, 13 Thus, these findings are only applicable to this product (grass extract freeze‐dried fast‐dissolving tablet formulation) and grass allergic patients and are not applicable to other products or different allergic disease conditions. The diversity of immunological mechanisms involved, with different dose/effect, unique pharmacologic profile, and a different bioavailability,25 makes extrapolation impossible and supports the need for performing independent investigations of the immunological mechanism for different immunotherapy products.
Figure 5.
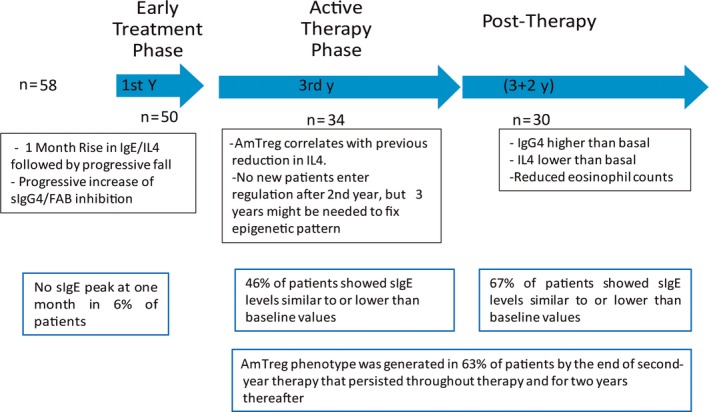
Schematic representation of the sequential mechanisms involved in SLIT
CONFLICTS OF INTEREST
Ana C. Carrera has received Research support from ALK‐Abello A/S. Lucia Jimeno, Agustin Galán, Peter A. Würtzen, Alicia Marín and Santiago Martin are employed by ALK‐Abello. Carlos Blanco has received lecture fees from ALK‐Abello and Merck. Domingo Barber was employed at ALK‐Abello until March 2013 and has received advisory fees from ALK‐Abello.
AUTHOR CONTRIBUTIONS
DB conceived the study, analyzed the data, and contributed to the article writing. CB designed and coordinated clinical trial execution and contributed to the writing. RV performed the experimental immunological part and contributed to the article writing. ACC designed the experimental immunological setup; TR, FV, and AM included patients and supervised all ethical and legal clinical research procedures. LJ and AG performed all serological determinations. SM and MME analyzed the data and contributed to the writing. PAW performed IgE‐FAB studies and contributed to the writing.
Supporting information
ACKNOWLEDGMENTS
This work was supported by Genoma España and ALK‐Abello A/S.
Varona R, Ramos T, Escribese MM, et al. Persistent regulatory T‐cell response 2 years after 3 years of grass tablet SLIT: Links to reduced eosinophil counts, sIgE levels, and clinical benefit. Allergy. 2019;74:349–360. 10.1111/all.13553
Blanco and Barber contributed equally to this work.
REFERENCES
- 1. Palomares O, Akdis M, Martin‐Fontecha M, Akdis CA. Mechanisms of immune regulation in allergic diseases: the role of regulatory T and B cells. Immunol Rev. 2017;278:219‐236. [DOI] [PubMed] [Google Scholar]
- 2. Akdis M, Akdis CA. Mechanisms of allergen‐specific immunotherapy: multiple suppressor factors at work in immune tolerance to allergens. J Allergy Clin Immunol. 2014;133:621‐631. [DOI] [PubMed] [Google Scholar]
- 3. Flicker S, Valenta R. Renaissance of the blocking antibody concept in type I allergy. Int Arch Allergy Immunol. 2003;132:13‐24. [DOI] [PubMed] [Google Scholar]
- 4. Novak N, Mete N, Bussmann C, et al. Early suppression of basophil activation during allergen‐specific immunotherapy by histamine receptor 2. J Allergy Clin Immunol. 2012;130:1153‐1158. [DOI] [PubMed] [Google Scholar]
- 5. Santos AF, James LK, Bahnson HT, et al. IgG4 inhibits peanut‐induced basophil and mast cell activation in peanut‐tolerant children sensitized to peanut major allergens. J Allergy Clin Immunol. 2015;135:1249‐1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dodev TS, Bowen H, Shamji MH, et al. Inhibition of allergen‐dependent IgE activity by antibodies of the same specificity but different class. Allergy. 2015;70:720‐724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. James LK, Shamji MH, Walker SM, et al. Long‐term tolerance after allergen immunotherapy is accompanied by selective persistence of blocking antibodies. J Allergy Clin Immunol. 2011;127:509‐516. [DOI] [PubMed] [Google Scholar]
- 8. Jay DC, Nadeau KC. Immune mechanisms of sublingual immunotherapy. Curr Allergy Asthma Rep. 2014;14:473. [DOI] [PubMed] [Google Scholar]
- 9. Durham SR, Emminger W, Kapp A, et al. Long‐term clinical efficacy in grass pollen‐induced rhinoconjunctivitis after treatment with SQ‐standardized grass allergy immunotherapy tablet. J Allergy Clin Immunol. 2010;125:131‐138. [DOI] [PubMed] [Google Scholar]
- 10. Durham SR, Emminger W, Kapp A, et al. SQ‐standardized sublingual grass immunotherapy: confirmation of disease modification 2 years after 3 years of treatment in a randomized trial. J Allergy Clin Immunol. 2012;129:717‐725. [DOI] [PubMed] [Google Scholar]
- 11. Suarez‐Fueyo A, Ramos T, Galan A, et al. Grass tablet sublingual immunotherapy downregulates the TH2 cytokine response followed by regulatory T‐cell generation. J Allergy Clin Immunol. 2014;133:130‐138. [DOI] [PubMed] [Google Scholar]
- 12. Pitsios C, Demoly P, Bilo MB, et al. Clinical contraindications to allergen immunotherapy: an EACCI position paper. Allergy. 2015;70:897‐909. [DOI] [PubMed] [Google Scholar]
- 13. Valovirta E, Petersen TH, Piotrowska T, et al. Results from the 5‐year SQ grass sublingual immunotherapy tablet asthma prevention (GAP) trial in children with grass pollen allergy. J Allergy Clin Immunol. 2018;141:529‐538. [DOI] [PubMed] [Google Scholar]
- 14. Horak F, Zieglmayer P, Zieglmayer R, et al. Early onset of action of a 5‐grass‐pollen 300‐IR sublingual immunotherapy tablet evaluated in an allergen challenge chamber. J Allergy Clin Immunol. 2009;124:471‐477. [DOI] [PubMed] [Google Scholar]
- 15. Calderon MA, Birk AO, Andersen JS, Durham SR. Prolonged preseasonal treatment phase with Grazax sublingual immunotherapy increases clinical efficacy. Allergy. 2007;62:958‐961. [DOI] [PubMed] [Google Scholar]
- 16. Rosace D, Gómez‐Casado C, Fernández P, et al. Profilin‐mediated food‐induced allergic reactions are associated with oral epithelial remodeling. J Allerg Clin Immunol. 2018. 10.1016/j.jaci.2018.03.013 [Epub ahead of print] PMID: 29705246 [DOI] [PubMed] [Google Scholar]
- 17. Shamji MH, Durham SR. Mechanisms of allergen immunotherapy for inhaled allergens and predictive biomarkers. J Allergy Clin Immunol. 2017;140:1485‐1498. [DOI] [PubMed] [Google Scholar]
- 18. Cox L. Accelerated immunotherapy schedules: review of efficacy and safety. Ann Allergy Asthma Immunol. 2006;97:126‐137. [DOI] [PubMed] [Google Scholar]
- 19. Aasbjerg K, Backer V, Lund G, et al. Immunological comparison of allergen immunotherapy tablet treatment and subcutaneous immunotherapy against grass allergy. Clin Exp Allergy. 2014;44:417‐428. [DOI] [PubMed] [Google Scholar]
- 20. Swamy RS, Reshamwala N, Hunter T, et al. Epigenetic modifications and improved regulatory T‐cell function in subjects undergoing dual sublingual immunotherapy. J Allergy Clin Immunol. 2012;130:215‐224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Scadding GW, Calderon MA, Shamji MH, et al. Effect of 2 years of treatment with sublingual grass pollen immunotherapy on nasal response to allergen challenge at 3 years among patients with moderate to severe seasonal allergic rhinitis: the GRASS randomized clinical trial. JAMA. 2017;317:615‐625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Arroyo‐Hornero R, Betts GJ, Sawitzki B, Vogt K, Harden PN, Wood KJ. CD45RA distingishes CD+CD25 + CD127‐/low TSDR demethylated regulatory T cell subpopulations with differential stability and susceptibility to tacrolimus‐mediated inhibition suppression. Transplantation. 2017;101:302‐309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Palomares F, Gomez F, Bogas G, et al. Immunological changes induced in peach allergy patients with systemic reactions by Pru p 3 sublingual immunotherapy. Mol Nutr Food Res. 2018;62: 10.1002/mnfr.201700669. Epub 2018 Jan 8. PMID: 29105313. [DOI] [PubMed] [Google Scholar]
- 24. Gomez F, Bogas G, Gonzalez M, et al. The clinical and immunological effects of Pru p 3 sublingual immunotherapy on peach and peanut allergy in patients with systemic reactions. Clin Exp Allergy. 2017;47:339‐350. [DOI] [PubMed] [Google Scholar]
- 25. Ohashi‐Doi K, Kito H, Du W, et al. Bioavailability of house dust mite allergens in sublingual allergy tablets is highly dependent on the formulation. Int Arch Allergy Immunol. 2017;174:26‐34. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


