Abstract
Purpose
To determine the cost (loss of visual function associated with the procedure) and benefit (long‐term preservation of the visual field) of glaucoma surgery.
Methods
We included 100 patients who underwent glaucoma surgery (Baerveldt glaucoma implant [BGI], n = 61; trabeculectomy [TE], n = 39). Preoperatively, the median (interquartile range [IQR]) standard automated perimetry mean deviation (MD) was −12 (−16 to −6) dB. We analysed the change in visual acuity (BCVA) and MD due to the procedure and, in a subset with at least 5 years of perimetric follow‐up both pre‐ and postoperatively (n = 20), the change in rate of progression (ROP; time rate of change in MD). For the surgery‐induced change in ROP, we also performed a meta‐analysis including the current and previously published studies. From the surgery‐induced decrease in MD and change in ROP, we calculated the average postoperative duration needed for the benefit to surpass the cost.
Results
Mean (standard deviation) MD decline was 1.3 (2.7) and 1.0 (2.3) dB for BGI (p < 0.001) and TE (p = 0.009), respectively; no significant surgery‐induced changes in BCVA were found (p = 0.08 and p = 0.12, respectively). In our study, surgery was associated with a non‐significant deceleration of ROP (from −0.37 [0.52] to −0.15 [0.48] dB/year; p = 0.23). The meta‐analysis, based on eight studies, showed an overall surgery‐induced change in ROP of 0.44 (95% confidence interval 0.25 to 0.64; p < 0.0001) dB/year.
Conclusion
Glaucoma surgery significantly reduces the progression velocity in glaucoma. On average, the benefit of glaucoma surgery surpasses the cost after approximately 1.5 years.
Keywords: Baerveldt glaucoma implant, glaucoma drainage device, perimetry, progression, rate of progression, trabeculectomy
Introduction
Glaucoma is one of the leading causes of avoidable blindness, characterized by a degeneration of the optic nerve and corresponding visual field loss. To slow down this chronic, progressive eye disease, treatment is focused on the lowering of the intraocular pressure (IOP) by restricting the production of aqueous or by increasing its outflow. The initial approach to achieve a lower IOP is the daily use of IOP‐lowering drugs and/or one or more laser treatments. Surgery is generally considered after impermissible progression of visual field loss despite maximal tolerable medical and laser treatment. Glaucoma surgery is not without risk and may in itself compromise visual function. It is a clinical challenge to weigh the risk and benefit in individual patients.
Within glaucoma surgery, the creation of a fistula (filtering surgery) or implanting a drainage system has earned their spurs. Although invented in the same time period, filtering surgery (trabeculectomy; TE) was the first surgical choice for decades. Nowadays, the popularity of glaucoma drainage devices is growing (Gedde et al. 2012; Arora et al. 2015; Islamaj et al. 2018). The success of surgery might come with a cost; however, as patients often complain of a decrease in visual function after surgery. This observation gives the clinician the sense of a drawback of surgery. However, the literature showed all three possible outcomes: an improvement in visual function, a decline and no effect (Hagiwara et al. 2000; Sehi et al. 2010; Balekudaru et al. 2014; Caprioli et al. 2016). The crucial question is, if there is a surgery‐induced loss, if it counterbalances the loss avoided due to the IOP lowering. The IOP lowering aims to yield a lower rate of progression (ROP), that is, a less negative time rate of change in the standard automated perimetry (SAP) mean deviation (MD) – thus delaying blindness.
The aim of this study was to determine the cost and benefit of glaucoma surgery regarding visual function. For this purpose, we analysed the change in visual acuity and SAP MD due to the procedure (the cost) and the change in rate of progression (ROP), that is the time rate of change in the MD (the benefit). These analyses were performed primarily in the cohort of the Groningen Longitudinal Glaucoma Study (GLGS; Heeg et al. 2005). For the surgery‐induced change in ROP, we also performed a meta‐analysis including the current and previously published studies. From the surgery‐induced decrease in MD and change in ROP, we calculated the average postoperative duration needed for the benefit to surpass the cost.
Materials and Methods
Study population and data acquisition
The present study is part of the GLGS, a prospective observational cohort study performed in a clinical setting. The GLGS started in 2000 and is still ongoing. The ethics board of the University Medical Center Groningen (UMCG) approved that for the current study no informed consent had to be obtained because the study comprised a retrospective anonymous analysis of ophthalmic examination and visual field data collected during regular glaucoma care. The study followed the tenets of the Declaration of Helsinki.
The subpopulation selected for the present study consisted of patients who were treated with a Baerveldt glaucoma implant (BGI) or TE and who had at least (1) two (not including the first visual field, which was discarded because of learning effects) reliable visual fields (measured with SAP [Humphrey Field Analyzer 30‐2 SITA fast; Carl Zeiss, Jena, Germany]) both pre‐ and post‐intervention and (2) a follow‐up of at least one year after the intervention, in the operated eye. If both eyes met the inclusion criteria, a random eye was chosen (based on even or uneven case number in the database). For the analysis concerning the ROP, a perimetric follow‐up of at least five years was required both pre‐ and post‐intervention (Jansonius 2010; Junoy Montolio et al. 2012). Visual fields had to be reliable. A test result was considered unreliable if false positives exceeded 10% or if both false negatives and fixation losses exceeded 10% and 20%, respectively. We pooled false negatives and fixation losses because they were reported to have a much smaller influence on the MD (Junoy Montolio et al. 2012) and especially the false negative are not informative in glaucoma (Bengtsson & Heijl 2000). Both primary and secondary glaucoma was allowed. We included both phakic and pseudophakic patients; patients who underwent a cataract extraction simultaneously with the glaucoma surgery were excluded. We also excluded patients who underwent a second glaucoma operation during follow‐up.
Surgical procedure
Indication for surgery and method (BGI or TE) was made by either of the two authors who also performed the operations (RM and NJ). In general, in our hospital, surgery is delayed until we observe progression too rapid for the age of the patient (Wesselink et al. 2011), with maximum tolerable medical treatment and after considering or performing laser surgery. Early surgery is considered in cases with high baseline IOP and limited IOP lowering on non‐surgical treatment, especially in young patients with already moderate or severe glaucoma at the time of diagnosis. Trabeculectomy (TE) is the first choice in primary glaucoma in phakic eyes with a clear lens; during the last decade we moved from TE to BGI in pseudophakic eyes. Baerveldt glaucoma implant (BGI) is the first choice in secondary glaucoma.
Baerveldt glaucoma implant
A BGI with 350 mm2 plate was implanted in the superior‐temporal quadrant. The tube was closed with a vicryl 7‐0 suture to restrict short‐term excessive drainage before encapsulation. The tube was placed in the anterior chamber through a peripheral iridotomy made with the 23 G needle used to create a scleral entry for the tube, aiming at a position of the tube as close to the iris as possible to prevent endothelial cell loss (Tan et al. 2017). For this, the anterior chamber was temporary deepened with an air bubble and the needle entered the eye 3 mm posterior to the limbus, in parallel with the iris plane. The tube entrance was covered with a patch of donor sclera. Intraocular pressure (IOP)‐lowering drugs were continued unchanged until tube opening (typically six weeks after the intervention) and then tapered depending on the IOP. As of 2009, some patients got a drainage suture for early IOP reduction (Rietveld et al. 2009; van Hoefen Wijsard et al. 2018). Antibiotic drops were given for two weeks (chloramphenicol 0.4% three times a day); steroids for 10 weeks (dexamethasone 0.1% three times a day for eight weeks followed by two times a day for two weeks).
Trabeculectomy
A TE with fornix‐based conjunctival flap and limbus‐based scleral flap was performed superiorly with the application of mitomycin C (0.2 mg/ml during 2–4 min; applied before making the scleral flap). The scleral flap was sutured with two or three nylon 10‐0 sutures, with the possibility for laser suture lysis afterwards. Conjunctiva was closed with two or three vicryl 7‐0 sutures. Antibiotic drops were given for two weeks (chloramphenicol 0.4% three times a day); steroids for 12 weeks (dexamethasone 0.1% six times a day for six weeks followed by four times a day for four weeks and finally two times a day for two weeks; oral prednisone 30 mg for 10 days was added if the inflammation was above average in the early postoperative phase).
Statistical analysis
As most of the characteristics of the study population were not normally distributed (according to the Shapiro–Wilk test), we used nonparametric descriptive statistics (median with interquartile range [IQR]) to describe the study population. Groups were compared using a Mann–Whitney test; for paired data, we used a Wilcoxon test. Proportions were compared with a chi‐squared test. For pre‐intervention and post‐intervention visual acuity, IOP and the number of IOP‐lowering medications, we took the last value before the intervention and a value measured as close as possible to 1 year after the operation; for MD, we took the average value of the last two tests before the intervention and the first two tests after the intervention. Inevitably, a perimetric gap exists because of a waiting list before the intervention and delayed testing after the intervention (until full recovery or a situation deemed stable was reached). Hence, the observed MD decline is a composite of a surgery‐induced decline and a decline due to intrinsic disease progression during this perimetric gap (see Discussion section). Rate of progression (ROP) was determined using linear regression. Changes (post‐intervention minus pre‐intervention) were evaluated with a paired t‐test or Wilcoxon test, depending on the distribution of the paired changes. Similarly, we used Pearson or Spearman correlation coefficients depending on the distribution of the concerning variables. A p value of 0.05 or less was considered statistically significant.
Meta‐analysis
For the meta‐analysis, we included glaucoma surgery studies regarding either BGI or TE that reported on preoperative and postoperative ROP of MD based on a mean follow‐up of at least 3 years with SAP. Studies also had to report a paired comparison of the change in ROP with standard error, confidence interval, standard deviation or p value. We searched for literature in PubMed with search string ‘(glaucom*) AND ((shunt OR tube) OR (trabeculect*) OR surgery) AND ((Visual field OR HFA OR perimetr*)) AND (progression OR (Rate of progression))’ and in Embase. Additionally, we searched through the reference list of the identified articles. The search was performed in June 2017.
We calculated the mean of the change in ROP using a random‐effects model (DerSimonian & Laird 1986). If the standard error of the change (or confidence interval or standard deviation) was not provided, it was calculated from the reported p value; if no exact p value was given, we used the upper limit (for example, 0.05 in case of p < 0.05), yielding a conservative estimate of the standard error (Fu et al. 2013). We used a random‐effects model because we expected significant heterogeneity amongst the included studies (indication of surgery, type of surgery, cataract extraction during follow‐up, disease stage, postoperative care, ethnicity, etc.). Heterogeneity was evaluated using the I 2 statistic (Higgins et al. 2003). I 2 is the percentage of the total variation across the studies, that is due to heterogeneity. Values up to 25%, 25–49%, 50–74% and 75% and above are considered no, low, moderate and high heterogeneity, respectively (Higgins & Thompson 2002). We performed a sensitivity analysis to evaluate the contribution of each individual study to the heterogeneity by sequentially leaving out one study and re‐analysing the pooled estimate for the remaining studies (Tobias 1999). Publication bias was assessed with Egger's regression asymmetry test (Egger et al. 1997) and Begg's adjusted rank correlation test (Begg & Mazumdar 1994).
Results
Table 1 presents the general characteristics of the study population. As can be seen in this Table, 61 patients received a BGI and 39 a TE. For only 20 (11 BGI and 9 TE) patients, we had a sufficiently long follow‐up duration (at least 5 years both pre‐ and postoperatively) to compare ROP pre‐ versus postoperatively, and for that reason, we pooled BGI and TE for this analysis. Baerveldt glaucoma implant (BGI) and TE patients were comparable in age at surgery and gender. Preoperatively, the BGI patients had a lower BCVA (p = 0.043), a slightly higher IOP (p = 0.027), and a similar MD (p = 0.92), compared to the TE patients. Pre‐ compared to postoperatively, both the BGI and the TE patients had a significantly lower IOP with less medication (p < 0.001).
Table 1.
General characteristics of the study population
| BGI (n = 61) | TE (n = 39) | pb | |
|---|---|---|---|
| Age [year; median (IQR)] | 70 (57–75) | 68 (59–73) | 0.60 |
| Gender (% female) | 53 | 51 | 0.91 |
| Visual acuity pre‐op [logMAR, median (IQR)] | 0.14 (0.02–0.28) | 0.10 (0.00–0.17) | 0.043 |
| IOP pre‐op [mmHg; median (IQR)] | 21 (17–25) | 18 (15–21) | 0.027 |
| IOP post‐op [mmHg; median (IQR)] | 13 (11–16) | 13 (9–15) | 0.40 |
| Pre‐op number of IOP‐lowering medications [median (IQR)] | 3 (2–4) | 3 (2–4) | 0.68 |
| Post‐op number of IOP‐lowering medications [median (IQR)] | 2 (1–3) | 0 (0–0) | <0.001 |
| Pre‐op mean deviation [dB; median (IQR)] | −12 (−16 to −6) | −11 (−16 to −7) | 0.92 |
| Pseudophakic (%)a | 54.1 | 10.3 | <0.001 |
| Secondary glaucoma (%) | 23.0 | 5.1 | 0.035 |
BGI = Baerveldt glaucoma implant; dB = decibels; IOP = intraocular pressure; IQR = interquartile range; TE = trabeculectomy.
Before surgery
Mann–Whitney test for medians, chi‐squared test for proportions.
Figure 1 shows the change in BCVA as a function of preoperative visual field loss. No significant correlations were found (p = 0.92 and p = 0.55 for BGI and TE, respectively). A non‐significant mean (SD) increase in logMAR BCVA (i.e. a decrease in decimal BCVA) of 0.03 (0.14) was found for BGI (p = 0.08) and of 0.04 (0.14) for TE (p = 0.12).
Figure 1.
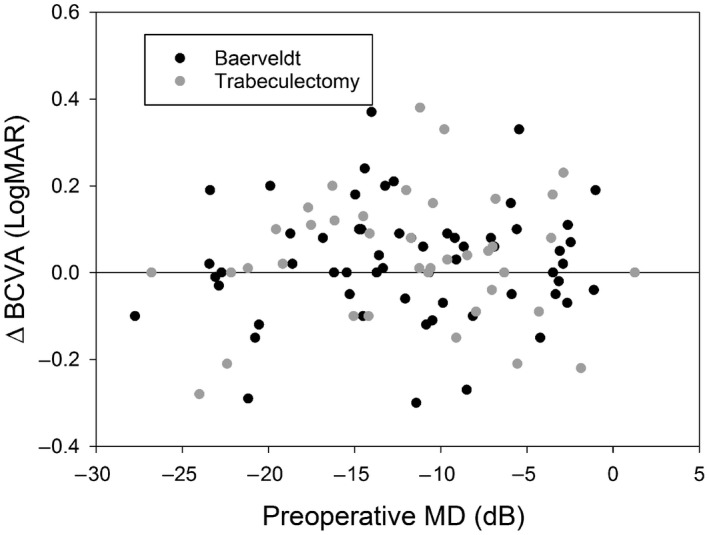
Surgery‐induced change in best‐corrected visual acuity (BCVA) as a function of preoperative visual field loss.
Figure 2 illustrates the surgery‐induced visual field change as a function of preoperative visual field loss. Again, no significant correlations were found (p = 0.19 and p = 0.57 for BGI and TE, respectively). A significant mean (SD) MD decline of 1.3 (2.7) dB was found for BGI (p < 0.001) and of 1.0 (2.3) dB for TE (p = 0.009). Of the 61 patients with a BGI, 18 had received a drainage suture for early IOP reduction. Mean deviation (MD) decline was 1.4 dB (p = 0.004) without drainage suture and 1.1 dB (p = 0.025) with drainage suture. The decline did not differ between the subgroups (p = 0.73). The midpoint of the last two preoperative visual fields was 6 months before surgery; the midpoint of the first two postoperative visual fields was 1.5 years after surgery.
Figure 2.
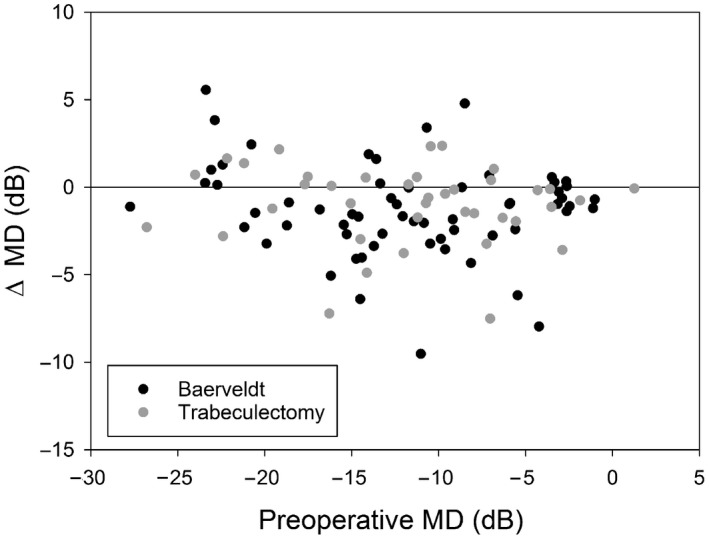
Surgery‐induced change in visual field mean deviation (MD) as a function of preoperative visual field loss.
For the longitudinal analysis, the median (IQR) follow‐up was 7.0 (5.7–8.8) years preoperatively and 7.6 (6.3–9.3) years postoperatively. The mean (SD) preoperative rate of progression was −0.37 (0.52) dB/year. After surgery, this was −0.15 (0.48) dB/year; the paired difference (standard error) was 0.22 (0.18) dB/year (p = 0.23). Of the 20 patients with a longitudinal follow‐up, 4 (3 BGI, 1 TE) underwent a cataract extraction before the glaucoma surgery and 4 (2 BGI, 2 TE) after the surgery. Exclusion of these patients would result in a mean pre‐op ROP of −0.37 dB/year and a mean post‐op ROP of −0.24 dB/year (to be compared to −0.37 and −0.15 dB/year in Table 2). Hence, the bias seems limited.
Table 2.
Characteristics of the studies included in the meta‐analysis
| Study | Number of Patients | Mean follow‐up (pre/post; year) | pre‐op ROP [mean (SD); dB/year] | post‐op ROP [mean (SD); dB/year] | Change in ROP [mean (SE); dB/year]a | p |
|---|---|---|---|---|---|---|
| Folgar et al. (2010) | 28 | 3.6/3.5 | −1.48 (1.4) | −0.43 (0.8) | 1.05 (0.41) | 0.01 |
| Bhardwaj et al. (2013; pre‐op progressors) | 9 | 5.8/4.5 | −1.0 (0.9) | −0.2 (0.38) | 0.80 (0.34) | 0.02 |
| Bhardwaj et al. (2013; pre‐op non‐progressors) | 8 | 5.8/4.5 | 0.1 (0.8) | −0.2 (0.4) | −0.30 (0.35) | 0.40 |
| Bertrand et al. (2014) | 52 | 3.8/3.9 | −0.36 (0.79) | −0.16 (0.58) | 0.20 (0.14) | 0.15 |
| Mataki et al. (2014) | 34 | 4.6/5.7 | −0.70 (0.52) | −0.25 (0.50) | 0.45 (0.11) | <0.001 |
| Caprioli et al. (2016) | 74 | 5.1/5.4 | −0.7 (1.1) | −0.1 (0.8) | 0.60 (0.18) | <0.001 |
| Iverson et al. (2016) | 9 | 7.6/5.4 | −1.05 (0.66) | −0.25 (0.86) | 0.80 (0.41) | 0.05 |
| Oie et al. (2017) | 17 | 5.9/15.6 | −0.86 (0.51) | −0.19 (0.2) | 0.67 (0.20) | <0.001 |
| Junoy Montolio et al. | 20 | 7.0/7.6 | −0.37 (0.52) | −0.15 (0.48) | 0.22 (0.18) | 0.23 |
ROP = rate of progression; SD = standard deviation; SE = standard error.
Estimated SE in case of non‐exact p value.
Complications for patients receiving BGI (n = 61) included ptosis (n = 2), persistent (i.e. orthoptic consultation was warranted) diplopia (n = 1), and additional surgery (cyclodiode laser therapy; n = 1). Trabeculectomy (TE) (n = 39) associated complications were persistent bleb leakage (that is, bleb leakage requiring surgical repair; n = 2), failure (i.e. no visible bleb in combination with a higher than desired IOP; n = 2), hypotony with choroidal detachment closer than two disc diameters from the optic disc (n = 1), and hypotony associated maculopathy (n = 1; Abbas et al. 2018).
Table 2 shows the characteristics of the studies included in the meta‐analysis. Eight studies were included, of which one (Bhardwaj et al. 2013) reported two subgroups. All but two (Folgar et al. 2010; this study) included only TE. Figure 3 shows the preoperative and postoperative ROP with standard error. As can be seen in this figure, there was a wide variety in ROP preoperatively but not postoperatively (see Discussion section). The weighted mean (95% CI) surgery‐induced change in ROP was 0.44 (0.25–0.64; p < 0.0001) dB/year, indicating that glaucoma surgery results in a significant deceleration of the ROP. I 2 was 47%, that is, the studies showed a low heterogeneity. Sensitivity analysis showed that one of the subgroups of the study of Bhardwaj et al. (2013) substantially influenced the pooled estimate. After excluding this study, the overall effect size was 0.45 (95% CI 0.32–0.57; p < 0.0001) dB/year with a remaining I 2 of 34%. There was no evidence of publication bias (Egger's test: p = 0.48; Begg's test: p = 0.30).
Figure 3.
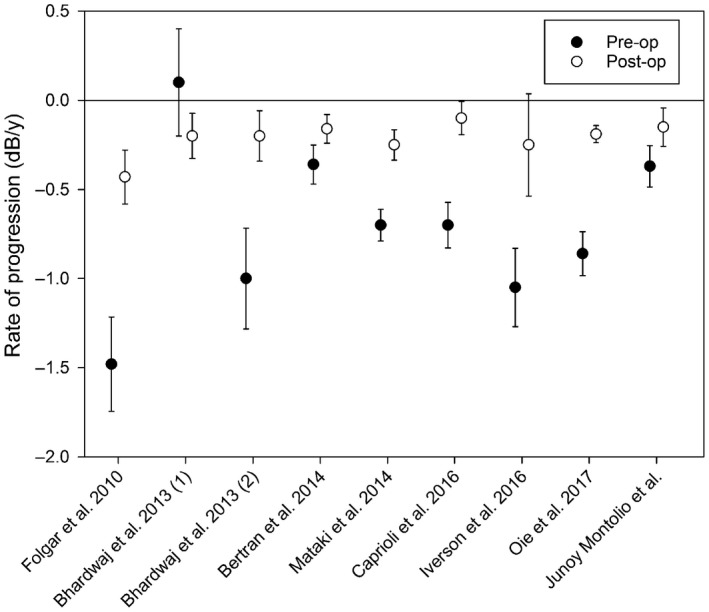
Preoperative and postoperative ROP with standard error for the studies included in the meta‐analysis.
Discussion
In our study, both TE and BGI were associated with a small but significant MD loss; no surgery‐induced changes in BCVA were found. Surgery was associated with a non‐significant deceleration of the ROP. In the meta‐analysis, based on eight studies, the overall effect size indicated that glaucoma surgery significantly reduces the progression velocity in glaucoma.
Several studies reported an unchanged BCVA after glaucoma surgery, for both TE (Bevin et al. 2008; Bhardwaj et al. 2013; Balekudaru et al. 2014; Bertrand et al. 2014; Iverson et al. 2016) and BGI (Namavari et al. 2016), and in a recent randomized clinical trial (RCT) comparing primary BGI with TE (Islamaj et al. 2018). This is in agreement with our results. Only a few studies described BCVA loss after surgery (Gedde et al. 2007; Stead & King 2010; Christakis et al. 2017). Stead & King (2010) found, in patients with very severe glaucoma (mean [SD] MD −25.3 [3.5] dB) who underwent a TE, that nine of 104 patients lost two lines of Snellen or more – deemed to be related to glaucoma – at the end of the follow‐up (i.e. on average three years postoperatively). In the multicentre RCT of Gedde et al. (2007) comparing BGI with TE, the BCVA decreased significantly, on average (SD) from 0.43 (0.54) and 0.37 (0.38) logMAR preoperatively to 0.61 (0.75) and 0.49 (0.56) after one year for the BGI and TE patients, respectively. The authors described that the main reasons for the loss of BCVA were cataract and other, not glaucoma‐related, reasons. Christakis et al. (2017) found in their multicentre RCT comparing Ahmed drainage devices with BGI a mean (SD) decrease in BCVA from 1.1 (1.0) to 1.3 (1.2) logMAR in BGI patients after one year. They described that 34% of the patients lost two or more lines of Snellen, of which 18% was caused by glaucoma, 15% by cataract and 68% by macular disease or other causes. Harju et al. (2018) studied the long‐term results of deep sclerectomy. Four of 37 patients had a VA loss of two lines or more; in two cases this was attributed to cataract formation. The main difference between these four studies and our study is the much higher preoperative BCVA in our study (Table 1). This suggests that a low preoperative visual acuity is an indicator of further surgery‐induced loss, a plausible hypothesis from a clinical point of view. The limited variability in our preoperative BCVA preludes a detailed check of this hypothesis in our data. Development of cataract could be an issue, as progression of cataract is found within 1 year after surgery, particularly in eyes with postoperative complications (Gedde et al. 2007). However, this seemed not to be the case in our data. Subgroup analysis comparing patients who were pseudophakic before surgery with patients who were phakic did not reveal any difference in visual acuity change, neither in the BGI group nor in the TE group.
Only a few earlier studies reported on a surgery‐induced MD decline. Most studies found no influence on the MD (Tavares et al. 2006; Sehi et al. 2010; Yamazaki & Hayamizu 2012; Balekudaru et al. 2014; Wright et al. 2015; Islamaj et al. 2018); one study found a deterioration of the MD after a TE (Hagiwara et al. 2000). In the latter study, the postoperative MD was assessed at the end of the follow‐up (mean follow‐up duration was 4.75 years). In most of the studies that found no effect, the authors compared the first visual field after the operation to the last visual field before the operation; in two studies, they performed two visual fields at the same time‐point. We reported a mean surgery‐induced MD decline of 1.3 and 1.0 dB for BGI and TE, respectively. This decline was based on the last two tests before the intervention and the first two tests after the intervention. This method was chosen to reduce variability and to compensate for a possible regression to the mean effect: a poor VF could trigger the clinician to initiate surgery and considering only this field could thus mask surgery‐induced damage. By using four visual fields, however, a part of the observed decline will actually be caused by progression that would have occurred anyway – the fields were not clustered (as would have been done in a trial) but made as part routine clinical care. This is illustrated in Fig. 4. If we assume an observed surgery‐induced MD decline of 1.2 dB, an interval of two years between the time‐point halfway the last two tests before the intervention and the time‐point halfway the first two tests after the intervention (0.5 years before the intervention and 1.5 years after the intervention; based on our 61 + 39 interventions), and a ROP of −0.63 dB/year before the intervention and −0.19 dB/year after the intervention (inverse‐variance estimates based on the meta‐analysis), then 50% (0.5*0.63 + 1.5 + 0.19 = 0.6 of 1.2 dB) of the observed decline could be attributed to the perioperative perimetric gap and the other 50% to the surgery itself.
Figure 4.
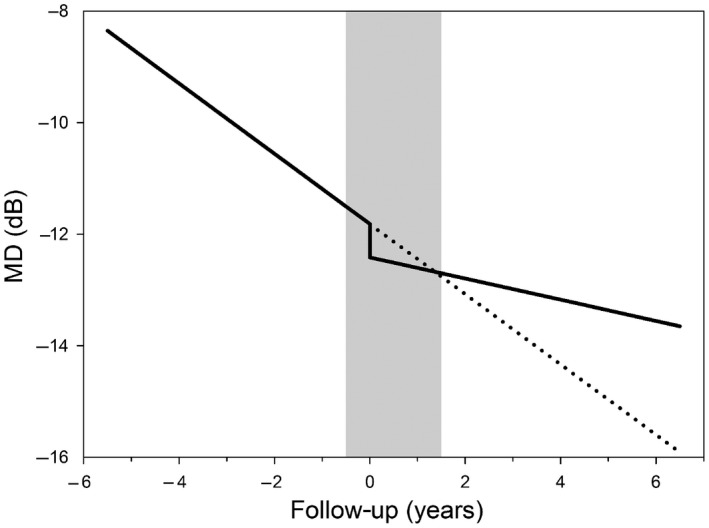
Schematic time–course of the visual field mean deviation (MD) with (continuous line) and without (dashed line) surgery. The surgery itself is performed at time‐point 0 years. The surgery‐induced drop in MD is counterbalanced by the slower rate of progression, on average 1.5 years after surgery (crossing of continuous and dashed line). The grey bar depicts the period in which no perimetry is performed (from 6 months before to 1.5 years after surgery; see Results section). The observed drop in MD, that is the difference in MD between the beginning and the end of the grey bar, is about twice as large as the actual drop in MD, because of autonomous disease progression in the period without perimetry.
In our study, glaucoma surgery yielded a non‐significant deceleration of the ROP. The non‐significance could be related to the small sample size and/or remaining variability in the ROP estimate in individual patients – despite a relatively long follow‐up duration, or may actually denote no effect. The small sample size was related to the exclusion of combined surgery (glaucoma surgery and cataract extraction simultaneously; see next paragraph) and the required long follow‐up duration; in our tertiary referral centre, most glaucoma surgeries are performed on patients with secondary glaucoma (De Vries et al. 2016) and on referred patients. Both groups often lack an uninterrupted perimetric follow‐up. We confined the current study to the regular visitors of our department, for whom we collect observational data prospectively as of 2000 – as part of the GLGS. Interestingly, all identified published studies that reported on ROP before and after glaucoma surgery pointed in the same – beneficial – direction, but the change in ROP was not always statistically significant. The overall effect size of the meta‐analysis, however, was highly significant, indicating the value of meta‐analysis as a statistical tool to provide a solid ground for performing surgery.
We excluded patients who underwent a cataract extraction simultaneously with the glaucoma surgery but we did not exclude those who underwent a cataract extraction during the longitudinal follow‐up. Both inclusion and exclusion of patients who underwent a cataract extraction during the longitudinal follow‐up could induce bias. As shown in the Results section, this bias seems limited in this population.
Before the operation, the mean ROP varied largely between the studies. This might reflect, amongst others, differences in policy regarding indicating surgery. After the operation, the mean ROP was close to −0.2 dB/year in most of the studies (Table 2, Fig. 3). Interestingly, a value of −0.2 dB/year is the typical mean value as reported in several glaucoma cohorts (Heijl et al. 2002; De Moraes et al. 2012; Wesselink et al. 2012). This indicates that glaucoma surgery is indeed able to bring rapid progressors back into the normal ROP range. Despite the preoperative variability in ROP, the studies in the meta‐analysis showed a low heterogeneity. The majority of the heterogeneity could be attributed to the subgroup of the study of Bhardwaj et al. (2013) in which patients were included without preoperative progression.
Limitations of this study were the observational design of the GLGS and the limited sample size in especially the longitudinal part of the current study. Reasons for the limited sample size were given above; we addressed this by performing a meta‐analysis. By combining many studies that showed not always significant results on their own, we were able to determine the highly significant benefit of glaucoma surgery. The observational nature of the GLGS implies that the patients included in the current study may form a biased sample. In case of a real disaster, post‐op visual fields may be lacking, resulting in exclusion and bias towards good outcomes. On the other hand, visual fields may also be taken less frequently in cases with a well‐regulated IOP (as they are less urgent for clinical decision making), yielding bias towards poor outcomes. Clearly, an observational study, and even not a meta‐analysis of observational studies, can beat a large randomized clinical trial (RCT). However, RCTs performed in the field of glaucoma surgery thus far reported only on IOP and visual field change defined as a post‐op progression event (Ederer et al. 1994; Musch et al. 1999; Anderson et al. 2003; Gedde et al. 2012); no RCT data regarding change in ROP are available. We required a follow‐up of at least 5 years pre‐ and postoperatively for our own longitudinal analysis (see Methods section); we had to weaken this to 3 years for the meta‐analysis (Table 2). With 3 years, ROP assessment in individual patients is still very noisy, as was argued by modelling (Jansonius 2010) and shown in patient data (Junoy Montolio et al. 2012; their table 3 shows that a short follow‐up causes a spurious widening of the ROP distribution).
Glaucoma surgery could be an ungrateful intervention to perform as most patients have little complains preoperatively, while experience the perioperative and postoperative hassle and possible visual acuity and visual field loss. Therefore, it is very important to understand what patients relinquish to prevent future blindness. Figure 4 illustrates the cost and benefit of glaucoma surgery, compiled from our cost data (surgery‐induced MD decline) and the ROP results from the studies included in the meta‐analysis. It shows that, on average 1.5 years after the operation (depicted as time‐point 0 years in Fig. 4), patients that were operated are better off than patients who declined surgery (crossing of continuous and dashed line in Fig. 4). Note that the end of the perimetric gap (right side of grey bar) and the crossing of the lines coincide coincidentally; this may give the clinician, when restarting perimetry after surgery, the spurious impression that the surgery did not have any effect. Figure 4 may also be used to inform the patient properly, and it illustrates that the decision to operate is not always an easy one, but should involve the weighing of, amongst others, current damage, ROP, IOP and life expectancy (Wesselink et al. 2011).
In conclusion, the cost of glaucoma surgery is the loss of visual function associated with the procedure; the benefit is the long‐term preservation of the visual field due to the reduced rate of progression. Both are significant. On average, the benefit of glaucoma surgery surpasses the cost after approximately 1.5 years.
This study has been presented at the national meeting of the Dutch Ophthalmological Society (NOG) 2016, Maastricht, The Netherlands.
References
- Abbas A, Agrawal P & King AJ (2018): Exploring literature‐based definitions of hypotony following glaucoma filtration surgery and the impact on clinical outcomes. Acta Ophthalmol 96: e285–e289. [DOI] [PubMed] [Google Scholar]
- Anderson DR, Drance SM, Schulzer M & CNTG study group (2003): Factors that predict the benefit of lowering intraocular pressure in normal tension glaucoma. Am J Ophthalmol 136: 820–829. [DOI] [PubMed] [Google Scholar]
- Arora KS, Robin AL, Corcoran KJ, Corcoran SL & Ramulu PY (2015): Use of various glaucoma surgeries and procedures in Medicare beneficiaries from 1994 to 2012. Ophthalmology 122: 1615–1624. [DOI] [PubMed] [Google Scholar]
- Balekudaru S, George R, Panday M, Singh M, Neog A & Lingam V (2014): Prospective evaluation of early visual loss following glaucoma‐filtering surgery in eyes with split fixation. J Glaucoma 23: 211–218. [DOI] [PubMed] [Google Scholar]
- Begg CB & Mazumdar M (1994): Operating characteristics of a rank correlation test for publication bias. Biometrics 50: 1088. [PubMed] [Google Scholar]
- Bengtsson B & Heijl A (2000): False‐negative responses in glaucoma perimetry: indicators of patient performance or test reliability? Invest Ophthalmol Vis Sci 41: 2201–2204. [PubMed] [Google Scholar]
- Bertrand V, Fieuws S, Stalmans I & Zeyen T (2014): Rates of visual field loss before and after trabeculectomy. Acta Ophthalmol 92: 116–120. [DOI] [PubMed] [Google Scholar]
- Bevin TH, Molteno ACB & Herbison P (2008): Otago glaucoma surgery outcome study: long‐term results of 841 trabeculectomies. Clin Experiment Ophthalmol 36: 731–737. [DOI] [PubMed] [Google Scholar]
- Bhardwaj N, Niles PI, Greenfield DS, Hymowitz M, Sehi M, Feuer WJ & Budenz DL (2013): The impact of surgical intraocular pressure reduction on visual function using various criteria to define visual field progression. J Glaucoma 22: 632–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caprioli J, de Leon JM, Azarbod P, Chen A, Morales E, Nouri‐Mahdavi K, Coleman A, Yu F & Afifi A (2016): Trabeculectomy can improve long‐term visual function in glaucoma. Ophthalmology 123: 117–128. [DOI] [PubMed] [Google Scholar]
- Christakis PG, Zhang D, Budenz DL, Barton K, Tsai JC, Ahmed IIK & ABC‐AVB Study Groups (2017): Five‐year pooled data analysis of the Ahmed Baerveldt comparison study and the Ahmed versus Baerveldt study. Am J Ophthalmol 176: 118–126. [DOI] [PubMed] [Google Scholar]
- De Moraes CG, Demirel S, Gardiner SK, Liebmann JM, Cioffi GA, Ritch R, Gordon MO & Kass MA (2012): Effect of treatment on the rate of visual field change in the ocular hypertension treatment study observation group. Invest Ophthalmol Vis Sci 53: 1704–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries MM, Müskens RPHM, Renardel de Lavalette VW, Hooymans JMM & Jansonius NM (2016): Glaucoma drainage device surgery after vitreoretinal surgery: incidence and risk factors. Acta Ophthalmol 94: 135–139. [DOI] [PubMed] [Google Scholar]
- DerSimonian R & Laird N (1986): Meta‐analysis in clinical trials. Control Clin Trials, 7: 177–188. [DOI] [PubMed] [Google Scholar]
- Ederer F, Gaasterland DE & Sullivan EK, AGIS Investigators (1994) The Advanced Glaucoma Intervention Study (AGIS): 1. Study design and methods and baseline characteristics of study patients. Control Clin Trials 15: 299–325. [DOI] [PubMed] [Google Scholar]
- Egger M, Davey Smith G, Schneider M & Minder C (1997): Bias in meta‐analysis detected by a simple, graphical test. BMJ 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folgar FA, de Moraes CGV, Prata TS, Teng CC, Tello C, Ritch R & Liebmann JM (2010): Glaucoma surgery decreases the rates of localized and global visual field progression. Am J Ophthalmol 149: 258–264. [DOI] [PubMed] [Google Scholar]
- Fu R, Vandermeer BW, Shamliyan TA, O'Neil ME, Yazdi F, Fox SH & Morton SC (2013): Handling continuous outcomes in quantitative synthesis. Methods Guide for Comparative Effectiveness Reviews. AHRQ Publication No. 13‐EHC103‐EF. Rockville, MD: Agency for Healthcare Research and Quality. [PubMed] [Google Scholar]
- Gedde SJ, Schiffman JC, Feuer WJ, Herndon LW, Brandt JD & Budenz DL (2007): Treatment outcomes in the tube versus trabeculectomy study after one year of follow‐up. Am J Ophthalmol 143: 9–22. [DOI] [PubMed] [Google Scholar]
- Gedde SJ, Schiffman JC, Feuer WJ, Herndon LW, Brandt JD & Budenz DL & Tube versus Trabeculectomy Study Group (2012): Treatment outcomes in the Tube Versus Trabeculectomy (TVT) study after five years of follow‐up. Am J Ophthalmol 153: 789–803.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara Y, Yamamoto T & Kitazawa Y (2000): The effect of mitomycin C trabeculectomy on the progression of visual field defect in normal‐tension glaucoma. Graefes Arch Clin Exp Ophthalmol 238: 232–236. [DOI] [PubMed] [Google Scholar]
- Harju M, Suominen S, Allinen P & Vesti E (2018): Long‐term results of deep sclerectomy in normal‐tension glaucoma. Acta Ophthalmol 96: 154–160. [DOI] [PubMed] [Google Scholar]
- Heeg GP, Blanksma LJ, Hardus PLLJ & Jansonius NM (2005): The Groningen longitudinal glaucoma study. I. Baseline sensitivity and specificity of the frequency doubling perimeter and the GDx nerve fibre analyser. Acta Ophthalmol Scand 83: 46–52. [DOI] [PubMed] [Google Scholar]
- Heijl A, Leske MC, Bengtsson B, Hyman L, Bengtsson B, Hussein M & Early Manifest Glaucoma Trial Group (2002): Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol 120: 1268–1279. [DOI] [PubMed] [Google Scholar]
- Higgins JPT & Thompson SG (2002): Quantifying heterogeneity in a meta‐analysis. Stat Med 21: 1539–1558. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Thompson SG, Deeks JJ & Altman DG (2003): Measuring inconsistency in meta‐analyses. BMJ 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hoefen Wijsard M, Haan M, Rietveld E & vanRijn LJ (2018): Donor sclera versus bovine pericardium as patch graft material in glaucoma implant surgery and the impact of a drainage suture. Acta Ophthalmol, 10.1111/aos.13721. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islamaj E, Wubbels RJ & de Waard PTW (2018): Primary Baerveldt versus trabeculectomy study after one year follow‐up. Acta Ophthalmol 10.1111/aos.13658. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Iverson SM, Schultz SK, Shi W, Feuer WJ & Greenfield DS (2016): Effectiveness of single‐digit IOP targets on decreasing global and localized visual field progression after filtration surgery in eyes with progressive normal‐tension glaucoma. J Glaucoma 25: 408–414. [DOI] [PubMed] [Google Scholar]
- Jansonius NM (2010): On the accuracy of measuring rates of visual field change in glaucoma. Br J Ophthalmol 94: 1404–1405. [DOI] [PubMed] [Google Scholar]
- Junoy Montolio FG, Wesselink C, Gordijn M & Jansonius NM (2012): Factors that influence standard automated perimetry test results in glaucoma: test reliability, technician experience, time of day, and season. Invest Ophthalmol Vis Sci 53: 7010–7017. [DOI] [PubMed] [Google Scholar]
- Mataki N, Murata H, Sawada A, Yamamoto T, Shigeeda T & Araie M (2014): Visual field progressive rate in normal tension glaucoma before and after trabeculectomy: a subfield‐based analysis. Asia Pac J Ophthalmol (Phila) 3: 263–266. [DOI] [PubMed] [Google Scholar]
- Musch DC, Lichter PR, Guire KE & Standardi CL (1999): The collaborative initial glaucoma treatment study: study design, methods, and baseline characteristics of enrolled patients. Ophthalmology 106: 653–662. [DOI] [PubMed] [Google Scholar]
- Namavari A, Hyde RA, Wang D, Vajaranant TS & Aref AA (2016): Primary Baerveldt shunt implantation: outcomes and complications. Ophthalmol Therapy 5: 253–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oie S, Ishida K & Yamamoto T (2017): Impact of intraocular pressure reduction on visual field progression in normal‐tension glaucoma followed up over 15 years. Jpn J Ophthalmol 61: 314–323. [DOI] [PubMed] [Google Scholar]
- Rietveld E, Jansonius NM & Muskens RPHM (2009): Immediate pressure reduction with Baerveldt glaucoma implants. ARVO Meeting Abstract; Invest Ophthalmol Visual Sci 50: 445. [Google Scholar]
- Sehi M, Grewal DS, Goodkin ML & Greenfield DS (2010): Reversal of retinal ganglion cell dysfunction after surgical reduction of intraocular pressure. Ophthalmology 117: 2329–2336. [DOI] [PubMed] [Google Scholar]
- Stead RE & King AJ (2010): Outcome of trabeculectomy with mitomycin C in patients with advanced glaucoma. Br J Ophthalmol 95: 960–965. [DOI] [PubMed] [Google Scholar]
- Tan AN, Webers CAB, Berendschot TTJM, de Brabander J, de Witte PM, Nuijts RMMA, Schouten JSAG & Beckers HJM (2017): Corneal endothelial cell loss after Baerveldt glaucoma drainage device implantation in the anterior chamber. Acta Ophthalmol 95: 91–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavares IM, Melo LAS Jr, Prata JA Jr, Galhardo R, Paranhos A Jr & Mello PAA (2006): No changes in anatomical and functional glaucoma evaluation after trabeculectomy. Graefes Arch Clin Exp Ophthalmol 244: 545–550. [DOI] [PubMed] [Google Scholar]
- Tobias A (1999): Assessing the influence of a single study in the meta‐analysis estimate. Stata Tech Bull 47: 15–17. [Google Scholar]
- Wesselink C, Stoutenbeek R & Jansonius NM (2011): Incorporating life expectancy in glaucoma care. Eye 25: 1575–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesselink C, Marcus MW & Jansonius NM (2012): Risk factors for visual field progression in the groningen longitudinal glaucoma study: a comparison of different statistical approaches. J Glaucoma 21: 579–585. [DOI] [PubMed] [Google Scholar]
- Wright TM, Goharian I, Gardiner SK, Sehi M & Greenfield DS (2015): Short‐term enhancement of visual field sensitivity in glaucomatous eyes following surgical intraocular pressure reduction. Am J Ophthalmol 159: 378–85.e1. [DOI] [PubMed] [Google Scholar]
- Yamazaki Y & Hayamizu F (2012): Effect of trabeculectomy on retrobulbar circulation and visual field progression in patients with primary open‐angle glaucoma. Clin Ophthalmol 6: 1539–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]


