Abstract
Background
There are limited options for percutaneous mechanical circulatory support (pMCS) in patients requiring high‐risk percutaneous coronary intervention.
Objectives
This first‐in‐human, single‐center study aimed to evaluate the safety and feasibility of a novel pMCS device in high‐risk percutaneous coronary intervention patients.
Methods
Aortix (Procyrion, Houston, Texas) is a pMCS device deployed in the descending aorta via the femoral artery that uses axial flow to provide cardiac unloading and augment renal and systemic perfusion. We assessed the use and effect of the Aortix device in six patients undergoing high‐risk PCI. All patients had impaired left ventricular function, complex coronary disease, renal dysfunction, and suitable iliofemoral anatomy for Aortix placement via transfemoral approach. We recorded periprocedural events including hemodynamic effects of the device on cardiac output and urine output. We then followed patients up to 30 days following the PCI procedure for adverse events.
Results
Aortix delivery (18 Fr sheath) took 4–9 min, mean support time was 70 (range 47–95) min, and mean flow rate through the device was 3.5 L/min. During support, mean rate of urine output increased 10‐fold (range 2.5–25.0x). Estimated GFR improved at discharge compared with baseline (mean increase 6.95 ± 8.09 mL/min). There were no device failures and PCI was successful in all patients. Aortix was removed and hemostasis was achieved with a vascular closure device and manual pressure. No patients experienced adverse events or hemodynamic compromise. No clinically significant hemolysis occurred (mean LDH 239.2 ± 73.6 mU/mL at baseline and 206.4 ± 82.2 mU/mL at discharge). No vascular access complications were observed.
Conclusions
Aortix, a novel pMCS device, was successfully deployed and retrieved in all initial patients undergoing high‐risk PCI. We noted no significant hemolysis with temporary use of this axial flow device. Improvement in eGFR suggests a potential renal protective effect and is an important area for future investigation in patients with impaired left ventricular function and renal dysfunction.
Keywords: first‐in‐human, high‐risk PCI, left ventricular dysfunction, percutaneous mechanical circulatory support
1. INTRODUCTION
Percutaneous coronary intervention (PCI) is one of the cornerstones of the management of ischemic heart disease and, compared with coronary artery bypass grafting (CABG), provides a minimally invasive method for revascularization to provide flow to ischemic myocardial territories.1 In patients with high‐risk clinical or angiographic characteristics, temporary percutaneous mechanical circulatory support (pMCS) may be helpful in reducing overall morbidity during the PCI procedure.2
Currently available pMCS options during complex, high‐risk indicated PCI (CHIP) include the intraaortic balloon pump (IABP), TandemHeart, and Impella devices.2 Although the IABP is easily inserted and increases coronary perfusion while decreasing myocardial oxygen demand, it provides only minimal circulatory support. The TandemHeart requires transseptal puncture to cannulate the left atrium. The Impella crosses the aortic valve and rests in the ascending aorta and may increase stroke risk. Thus, there is a significant clinical need for additional temporary pMCS options in patients at high risk for complications during PCI.
A novel, axial‐flow pMCS system (Aortix, Procyrion Inc, Houston, TX) was developed to address some of the shortcomings with currently available devices. We report the first‐in‐human experience with this novel pMCS device in the setting of CHIP. The objective of this study was to: (1) demonstrate the feasibility of percutaneous Aortix implantation and retrieval and (2) evaluate the safety and efficacy of the Aortix device in CHIP patients.
2. METHODS
This first‐in‐human study was designed as a single‐arm, open‐label, prospective evaluation of a pMCS device in patients undergoing CHIP. All procedures were performed at the Sanitorio Italiano Hospital (Asuncion, Paraguay). The study protocol was approved by the Paraguay National Board of Health Bioethics Committee.
2.1. Study population
We identified patients undergoing elective CHIP with an indication for pMCS. Inclusion criteria were: angiographic and clinical indication for PCI, left ventricular systolic dysfunction with ejection fraction ≤35%, and at least one other high‐risk feature from one of the following: last patent conduit to the heart, unprotected left main coronary artery, three‐vessel coronary disease, bifurcation left anterior descending artery or left circumflex artery disease felt to represent a large myocardial territory, or ejection fraction less than 20%. Key exclusion criteria included: ongoing myocardial, cardiogenic shock or recent cardiac arrest, contraindicated anatomy for the Aortix device (iliac artery diameter < 6.5 mm, descending thoracic aorta diameter > 27 mm), severe bleeding risk, or severe medical comorbidity or acute illness [systolic blood pressure < 80 mmHg or treatment with an intravenous vasopressor, renal failure (estimated glomerular filtration rate < 15 mL/min) OR current use of dialysis, or evidence of hemolysis at time of screening]. All patients were followed for 30 days following PCI.
2.2. Aortix system and preliminary data
The Aortix device is a novel intraaortic pMCS device that can be deployed percutaneously via a transfemoral approach using an 18Fr delivery system (Figure 1). Aortix is a 6 mm axial flow pump that can support up to 5.0 L/min of pump flow via a 2 mm transarterial electrical lead. Aortix is designed to unload the ventricle and perfuse end organs. Aortix is designed to be deployed in the descending thoracic aorta prior to the start of a PCI and remains in place for the duration of the procedure. PCI may be achieved via contralateral transfemoral access or via transradial access. Following PCI, Aortix is removed in the catheter lab and a percutaneous closure is performed. Initial safety and efficacy data were obtained in a porcine model and demonstrated proof of concept (n = 9).3
Figure 1.
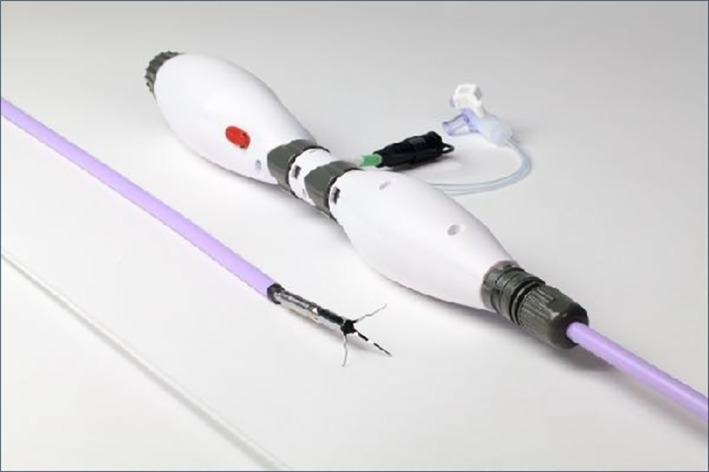
Aortix pump and delivery system. This figure displays the Aortix pump and the 18 French delivery system [Color figure can be viewed at wileyonlinelibrary.com]
2.3. Aortix and PCI procedure
The study flow is shown in Figure 2. Patients with left ventricular systolic dysfunction (ejection fraction <35%) being considered for PCI were screened for basic (noninvasive) study entry criteria and written informed consent was obtained. A diagnostic coronary angiogram was performed and patients received standard therapy as needed. Each case was reviewed by a cardiologist who assessed the acceptability of PCI as a therapeutic option for the participant. During the high‐risk PCI procedure, iliofemoral and descending aortic angiography was performed, and if the anatomy was suitable using the specifications in the study eligibility criteria, the Aortix device was delivered via an 18 Fr delivery sheath using a percutaneous transfemoral approach. After deployment of the Aortix device, PCI was performed via contralateral femoral approach. Heparin was used for anticoagulation in all patients to achieve a goal activated clotting time of greater than 250 sec. Recorded laboratory values of interest included creatinine, estimated GFR, B‐type natriuretic peptide, lactate dehydrogenase, and plasma free hemoglobin. Invasive hemodynamics and urine output were measured at 30 min intervals during the procedure. Urine output was recorded for approximately 1 hr prior to pump placement until pump removal. Vital signs (heart rate and mean arterial pressure), femoral artery insertion site, and distal pulses were monitored continuously until the device was removed.
Figure 2.
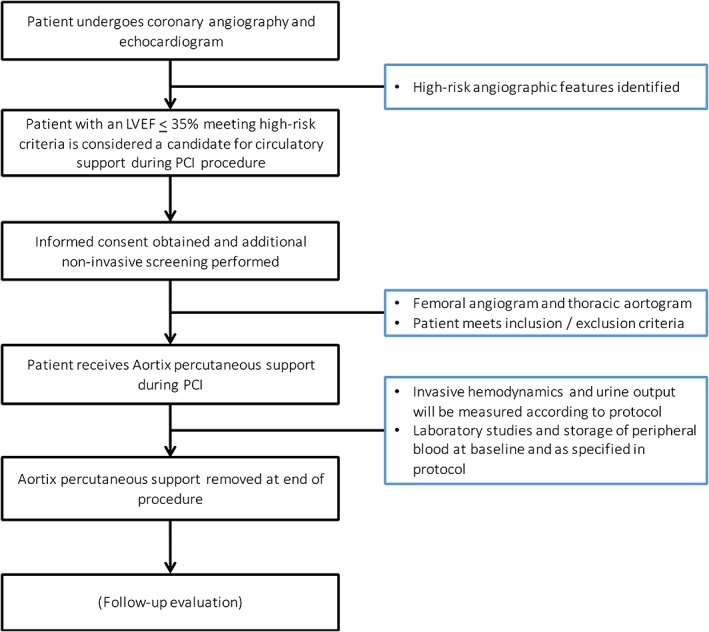
Study flow. This figure displays patient flow in the study. Abbreviation: PCI, percutaneous coronary intervention [Color figure can be viewed at wileyonlinelibrary.com]
After PCI, the Aortix device was retrieved via the 18‐French delivery system (Figure 3). Hemostasis was achieved with the use of one or two Perclose Proglide (Abbott Vascular, Abbott Park, IL) devices and manual pressure. Following the procedure, patients were monitored for significant clinical events, including: signs of worsening myocardial ischemia or infarction, addition of vasopressors or inotropes, initiation of mechanical ventilation, arrhythmias, or signs/symptoms of heart failure. Patients were then followed up at 30 days after discharge.
Figure 3.
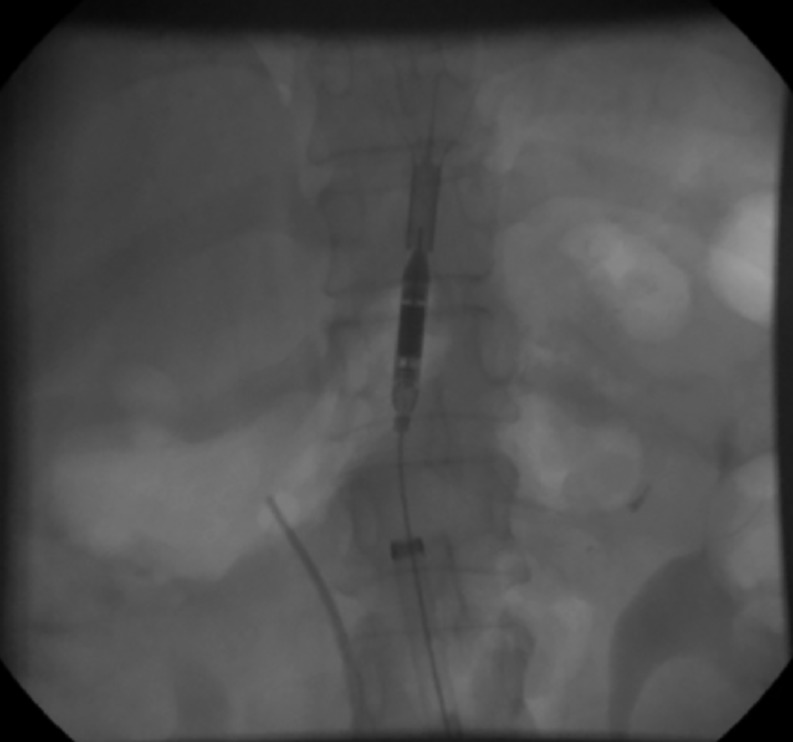
Aortix pump deployed. This figure demonstrates the optimal position of the Aortix device. It is delivered via the transfemoral sheath to the level of the diaphragm, proximal to the renal arteries
2.4. Study endpoints
The primary endpoints of the study were successful delivery/deployment of device and successful removal of the device, with freedom from adverse events. Safety endpoints included hemolysis assessment, angiographic success, major adverse cardiac events (MACE), and arrhythmia requiring cardioversion. We also assessed renal effects (urine output, creatinine) as well as hemodynamics (HR, proximal and distal pressure, cardiac output).
2.5. Statistical analysis
We evaluated the time to delivery of the pump, mean support duration, and mean flow rate in all patients. We evaluated baseline urine output and hemodynamic data in patients prior to delivery of the Aortix device, during pump activity, and after retrieval of the device. All data were manually entered into Microsoft Excel (Redmond, Washington).
3. RESULTS
Thirteen patients were screened. Seven patients failed the inclusion criteria (one had normal ejection fraction, one had dilated aorta, and five had small caliber and highly tortuous femoral arteries). Six patients met inclusion criteria for the study and were enrolled. Their baseline characteristics are presented in Table 1. The mean age of participants was 61.5 years (range 49–71 years), and 83.3% were male. All had left ventricular systolic dysfunction with LVEF ≤35% (range 20–28%). Anatomic characteristics of the treated lesions is described in Table 1.
Table 1.
Patient characteristics
| Pt | Age | Sex | LVEF | sCr | Anatomy | Lesions treated |
|---|---|---|---|---|---|---|
| 1 | 68 | M | 20% | 2.52 | Unprotected LM, 3 vessel disease, LVEF ≤20% | 4 |
| 2 | 56 | F | 34% | 1.13 | Unprotected LM | 1 |
| 3 | 49 | M | 35% | 1.16 | 3 vessel disease | 1 |
| 4 | 58 | M | 30% | 1.58 | Bifurcation disease representing large territory | 1 |
| 5 | 67 | M | 35% | 1.35 | Bifurcation disease representing large territory | 1 |
| 6 | 71 | M | 28% | 1.54 | Unprotected LM | 1 |
The device was successfully delivered in all patients within 4–9 min (mean 5.8 min) after the vessel was accessed percutaneously. The mean support time was 70 min (range 47–95 min), and the mean flow rate during support was estimated to be 3.5 L/min. The device was resheathed and repositioned in two patients to improve the deployment position of the device.
PCI was successful in all six patients. After completion of the PCI procedure, the Aortix device was successfully retrieved using the 18 Fr delivery system in all patients. Vascular hemostasis was achieved using 1–2 Perclose Proglide devices (implanted prior to pump delivery via the Preclose technique) and manual pressure. Primary surgical repair of the femoral artery was not necessary in any patient.
Urine output prior to Aortix delivery and during support are shown in Figure 4. All patients received 500–1,000 cm3 of heparinized saline during the procedure. Urine output data were only available in four patients; the urine collection protocol was not implemented in Patient #1, and in Patient #3 there was misplacement of the indwelling urinary catheter and no urine was collected. The mean baseline urine output was 52.5 mL/hr and increased to 257 mL/hour during support.
Figure 4.
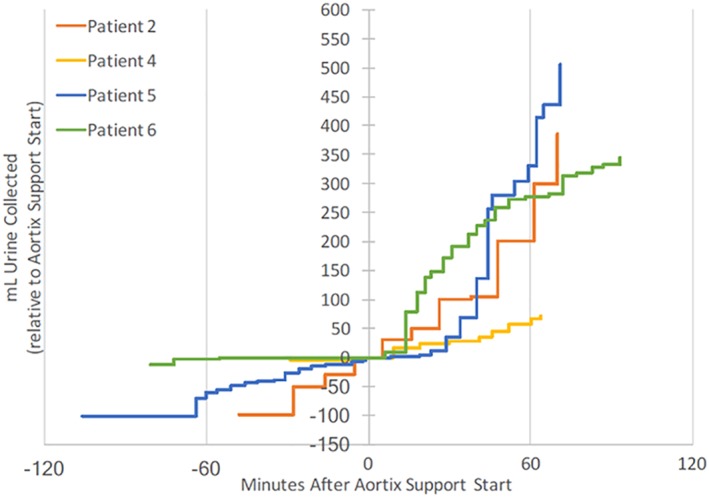
Urine output during Aortix implantation. This figure demonstrates the change in urine output during Aortix device implantation. Time 0 is activation of the Aortix device [Color figure can be viewed at wileyonlinelibrary.com]
Table 2 describes pooled hemodynamic data for all patients at four timepoints: baseline (prior to device insertion), maximum support (30,000 RPM, flow of 5 L/min), mean support (25,000, flow of 3.5 L/min), and after removal of the device. Baseline hemodynamics were supported in all patients at all timepoints. No clinically significant hemolysis was observed (mean LDH was 239.2 mU/mL at baseline and 206.4 mU/mL at discharge among the five patients for whom it was collected). Plasma‐free hemoglobin was less than 30 mg/dL (below the lower reference limit) at both time points post‐PCI (immediately post‐PCI cath lab and at discharge).
Table 2.
Hemodynamics
| Metric | Baseline | Max support(30 000 RPM, 5 L/min flow) | Mean support(25 000 RPM, 3.5 L/min flow) | After support |
|---|---|---|---|---|
| HR (bpm) | 72 ± 9 | 69 ± 8 | 68 ± 11 | 69 ± 16 |
| Prox aorta (mmHg) | 90 ± 16 | 86 ± 20 | 90 ± 17 | 92 ± 16 |
| Dist aorta (mmHg) | 94 ± 9 | 101 ± 13 | 97 ± 9 | N/A |
| Pulmonary artery diastolic pressure (mmHg) | 21.0 ± 7.2 | 15.0 ± 5.0 | 20.8 ± 6.3 | 21.4 ± 7.5 |
| Central venous pressure (mmHg) | 7.8 ± 4.4 | 6.5 ± 4.1 | 8.1 ± 4.9 | 7.5 ± 4.5 |
| Cardiac index (L/min/m2) | 2.9 ± 0.4 | 3.3 ± 0.6 | 3.0 ± 0.6 | 2.8 ± 0.9 |
Patients were discharged on day 0–1. At time of discharge, estimated GFR improved modestly compared with baseline (mean increase 6.95 ± 8.09 mL/min). There were no reported adverse events from procedure completion to discharge.
4. DISCUSSION
In this study, we report the first‐in‐human experience with the Aortix pump, a novel pMCS device implanted in the descending aorta via the transfemoral approach that delivers up to 5. 0 L/min of flow. The Aortix pump was successfully delivered and retrieved in all six attempted patients with LV systolic dysfunction undergoing CHIP. The marked increase in urine output and the modest improvement in eGFR suggests a potential renal protective effect and the functional improvement in kidney function may be beneficial in patients with impaired left ventricular function and renal dysfunction.
This initial safety and feasibility study focused on patients with severe LV systolic dysfunction undergoing CHIP. In these patients, current expert consensus recommends consideration of temporary MCS support during PCI, specifically those requiring multivessel, left main, or last patent conduit interventions, especially in the setting of severely decreased ejection fraction.2 Elective placement of temporary MCS may improve procedural success by maintaining hemodynamic stability and minimizing myocardial ischemia, especially during periods of decreased coronary perfusion caused by temporary coronary occlusion by PCI devices. Counter‐pulsation provided by IABPs has been used to provide temporary percutaneous MCS during high‐risk procedures.4, 5 Although a number of observational studies have supported the use of IABPs in this setting, the only randomized trial evaluating routine IABP use in high‐risk PCI did not show significant reduction in major adverse cardiovascular or cerebrovascular events at 6 months, though patients undergoing routine IABP use had significantly fewer major procedural complications compared to patients with no planned IABP use (1.3% vs 10.7%, P < 0.001).6 More recently, other mechanical circulatory support devices have been evaluated for use during high‐risk PCI procedures, including the TandemHeart and Impella devices. The TandemHeart is a transseptal left‐atrium to femoral artery system that connects to an external centrifugal pump to provide circulatory support and has been used in patients undergoing high‐risk PCI. The Impella device is a percutaneously inserted axial flow pump that is advanced across the aortic valve into the left ventricle and can provide 2.5–5.0 L/min of flow,7 though it did not show benefit compared with IABP in CHIP.8 Each of these systems has distinct advantages and disadvantages: while the IABP is widely used and relatively simple to implant, it provides modest circulatory support. Conversely, the TandemHeart can deliver more robust support but require transseptal puncture to cannulate the left atrium. The Impella rests in the ascending aorta across the aortic valve and theoretically may increase risk of stroke or valvular damage.
The Aortix device is a novel, axial‐flow pMCS device that is implanted in the descending aorta at the level of the diaphragm using an 18Fr delivery system via a transfemoral approach. The device is powered and controlled via a 2 mm electrical lead attached to the device, a lead that is envisioned to be tunneled out of the body for short‐term implantation of the device for support therapy in the future. Prior animal studies with this device demonstrated improvement in ejection fraction, cardiac output, and reduced myocardial oxygen extraction, along with improved urine output and renal perfusion 3, 9, 10. As the device does not traverse the ascending aorta or the great vessels, there is minimal risk of ischemic cerebrovascular accident. In this initial safety and feasibility study, we successfully deployed and retrieved the device in all attempted patients. We employed a percutaneous approach to access the vessel and deliver the device. PCI was performed successfully in all patients, and pump failure or malfunction was not observed. There was no evidence of hemolysis in this initial study as well.
We found that urine output markedly increased with pump activity, as much as 25‐fold in one evaluated participant (10‐fold mean). We speculate that this may be due to significantly increased renal arterial flow with device activation though other factors likely played a role including improved coronary perfusion. We also observed modest improvement in eGFR among patients during their hospitalization, or at least no decrement in renal function in the setting of a contrast load. This suggests that the device may serve as a useful adjunct in patients with compromised renal function. Future studies will evaluate the hemodynamic and renal effects in patients with acute heart failure and impaired renal function.
Almost 6 million adults in the United States have heart failure, and about half of these patients have reduced ejection fraction. Among patients with advanced heart failure that are eligible for advanced therapies such as heart transplant or surgical placement of a left ventricular assist device, there is significant variability in the use of pMCS as a bridge to more definitive therapy. A prior ultrafiltration device‐based strategy to provide renal decongestion among patients with decompensated heart failure and cardiorenal syndrome did not demonstrate benefit over a pharmacologic approach,11 though this system focused on ultrafiltration instead of augmenting renal perfusion. Among patients with advanced heart failure and renal dysfunction, the Aortix device may be an attractive option to facilitate renal perfusion and systemic decongestion, and additional studies are necessary to determine if this is a safe and beneficial strategy. Future iterations of the device will allow for tunneling of the cable, allowing for short‐term ambulatory support up to 30 days.
Our study has a number of important limitations. In this early safety and feasibility study, we did not observe a signal concerning for adverse device‐related events, though the device was implanted in only six patients. Given that this was a safety and feasibility study for short‐term support, there was no control arm to assess the effect of device support on PCI success. We did not measure overall contrast load, which may have affected the diuretic response during and following the procedure. In the setting of these limitations, a larger, multicenter study may be necessary to detect less frequent adverse events and to assess the safety and the efficacy of the Aortix device across CHIP and advanced heart failure indications.
5. CONCLUSIONS
In this first‐in‐human study, the Aortix device was safely delivered and retrieved in all attempted patients with LV systolic dysfunction undergoing CHIP via a transfemoral approach. The increase in urine output and modest improvement in renal function suggest a potential role for this device in patients with impaired left ventricular function and renal dysfunction undergoing PCI.
CONFLICT OF INTEREST
AN Vora: Dr. Vora reports no relevant disclosures.
WS Jones: Dr. Jones reports research grants from the Agency for Healthcare Research and Quality, AstraZeneca, American Heart Association, Bristol‐Myers Squibb, Doris Duke Charitable Foundation, Merck, Patient‐Centered Outcomes Research Institute; honoraria/other from the American College of Physicians, Bayer, Bristol‐Myers Squibb, Daiichi Sankyo, and Janssen Pharmaceuticals.
AD Devore: Dr. Devore reports research support from the American Heart Association, Amgen, NHLBI, and Novartis; consulting for Novartis.
A Ebner: Dr. Ebner reports no relevant disclosures.
W Clifton: Dr. Clifton is an employee and shareholder of Procyrion, Inc.
MR Patel: Dr. Patel reports research grants from AstraZeneca, Bayer, Janssen, Procyrion, NHLBI, consulting for Janssen, Bayer, AstraZeneca.
Supporting information
Appendix S1. Supporting Information
Vora AN, Schuyler Jones W, DeVore AD, Ebner A, Clifton W, Patel MR. First‐in‐human experience with Aortix intraaortic pump. Catheter Cardiovasc Interv. 2019;93:428–433. 10.1002/ccd.27857
Funding information Procyrion, Inc
REFERENCES
- 1. Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention: A report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines and the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol. 2011;58:e44‐e122. [DOI] [PubMed] [Google Scholar]
- 2. Rihal CS, Naidu SS, Givertz MM, et al. 2015 SCAI/ACC/HFSA/STS clinical expert consensus statement on the use of percutaneous mechanical circulatory support devices in cardiovascular care: Endorsed by the American HEART ASSOCIATION, the Cardiological Society of India, and Sociedad Latino Americana de Cardiologia Intervencion; affirmation of value by the Canadian Association of Interventional Cardiology‐Association Canadienne de Cardiologie d'intervention. J Am Coll Cardiol. 2015;65:e7‐e26. [DOI] [PubMed] [Google Scholar]
- 3. Shabari FR, George J, Cuchiara MP, et al. Improved hemodynamics with a novel miniaturized intra‐aortic axial flow pump in a porcine model of acute left ventricular dysfunction. ASAIO J. 2013;59:240‐245. [DOI] [PubMed] [Google Scholar]
- 4. Anwar A, Mooney MR, Stertzer SH, et al. Intra‐aortic balloon counterpulsation support for elective coronary angioplasty in the setting of poor left ventricular function: A two center experience. J Invas Cardiol. 1990;2:175‐180. [PubMed] [Google Scholar]
- 5. Kahn JK, Rutherford BD, McConahay DR, Johnson WL, Giorgi LV, Hartzler GO. Supported "high risk" coronary angioplasty using intraaortic balloon pump counterpulsation. J Am Coll Cardiol. 1990;15:1151‐1155. [DOI] [PubMed] [Google Scholar]
- 6. Perera D, Stables R, Thomas M, et al. Elective intra‐aortic balloon counterpulsation during high‐risk percutaneous coronary intervention: A randomized controlled trial. J Am Med Assoc. 2010;304:867‐874. [DOI] [PubMed] [Google Scholar]
- 7. Sjauw KD, Konorza T, Erbel R, et al. Supported high‐risk percutaneous coronary intervention with the Impella 2.5 device the Europella registry. J Am Coll Cardiol. 2009;54:2430‐2434. [DOI] [PubMed] [Google Scholar]
- 8. O'Neill WW, Kleiman NS, Moses J, et al. A prospective, randomized clinical trial of hemodynamic support with Impella 2.5 versus intra‐aortic balloon pump in patients undergoing high‐risk percutaneous coronary intervention: The PROTECT II study. Circulation. 2012;126:1717‐1727. [DOI] [PubMed] [Google Scholar]
- 9. Clifton WL, Heuring J, Hertzog B. Initial results with a novel intra‐aortic cardiorenal support device. J Am Coll Cardiol. 2014;63:A813. [Google Scholar]
- 10. del Rio C, Clifton W, Heuring J, Hertzog B, Ueyama Y, Youngblood B, and McConnell PI. AORTIX™, a novel catheter‐based intra‐vascular device, provides cardio‐renal support while improving Ventriculo‐arterial coupling and myocardial demand in sheep with induced chronic ischemic heart failure. J Am Coll Cardiol. 2015;65:A800. [Google Scholar]
- 11. Bart BA, Goldsmith SR, Lee KL, et al. Ultrafiltration in decompensated heart failure with cardiorenal syndrome. N Engl J Med. 2012;367:2296‐2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting Information


