Abstract
Sjögren's syndrome is a lymphoproliferative disease with autoimmune features characterized by mononuclear cell infiltration of exocrine glands, notably the lacrimal and salivary glands. These lymphoid infiltrations lead to dryness of the eyes (keratoconjunctivitis sicca), dryness of the mouth (xerostomia), and, frequently, dryness of other surfaces connected to exocrine glands. Sjögren's syndrome is associated with the production of autoantibodies because B‐cell activation is a consistent immunoregulatory abnormality. The spectrum of the disease extends from an organ‐specific autoimmune disorder to a systemic process and is also associated with an increased risk of B‐cell lymphoma. Current treatments are mainly symptomatic. As a result of the diverse presentation of the syndrome, a major challenge remains to improve diagnosis and therapy. For this purpose an international set of classification criteria for primary Sjögren's syndrome has recently been developed and validated and seems well suited for enrolment in clinical trials. Salivary gland biopsies have been examined and histopathology standards have been developed, to be used in clinical trials and patient stratification. Finally, ultrasonography and saliva meet the need of non‐invasive imaging and sampling methods for discovery and validation of disease biomarkers in Sjögren's syndrome.
Keywords: biomarkers, classification, histopathology, Sjögren's syndrome, ultrasonography
Sjögren's syndrome is named after the Swedish ophthalmologist henrik sjögren (1899–1986). In 1929 he met a patient who complained of dry eyes, oral dryness, and pain in several joints. He noticed that the combination of these symptoms could potentially be a separate disease entity and he started describing patients under the umbrella‐diagnosis of keratoconjunctivitis sicca. Careful examinations were performed both clinically and microscopically. In 1933, henrik sjögren had seen 19 such cases and he then wrote up his thesis ‘Zur Kentniss der Keratoconjunctivitis Sicca’ 1 which was defended the same year. The thesis was received with great enthusiasm but was also criticized by opponents, and were given mediocre marks. Due to the critical evaluation the academic career of henrik sjögren was over. However, he continued his clinical activity and ended up in Jönköping, a town in the southern part of Sweden, in 1935.
Despite all critique of his thesis, henrik sjögren eventually gained an international career, which started with the translation, in 1943, of his thesis into English by an Australian ophthalmologist, bruce hamilton, and the subsequent invitation to become a guest lecturer at the Royal Australian College of Opthalmologists. This tour to Australia was part of a route that took him around the globe.
henrik sjögren was a great clinician, with a broad clinical activity. As an acknowledgement of his work and international reputation, he was bestowed with the title ‘docent’ (≈ associate professor) by the University of Gothenburg in 1957. In 1961 the Swedish government granted him the well‐deserved title ‘professor’.
The purpose of this review was to present recent and internationally accepted classification criteria, including the use of labial salivary gland biopsy, for primary Sjögren's syndrome for its enrolment in clinical trials. Furthermore, we present recent developments regarding the use of serum biomarkers, glandular ultrasonography, and saliva proteomics.
Diagnostic and classification criteria
Sjögren's syndrome is a multisystem disorder that is heterogeneous in its presentation, course, and outcome. There is still no single clinical, laboratory, pathological, or radiological feature that could serve as a ‘gold standard’ for the diagnosis and/or classification of this syndrome. The closest we have got in identifying such a feature is labial salivary gland biopsy with a subsequent histopathological evaluation 2, 3, 4. Consequently, the development of criteria for use in both clinical care as well as research studies has been an important challenge in Sjögren's syndrome.
Classification criteria are often well‐standardized tools that are aimed at selecting properly defined and homogenous groups of patients for research but also guaranteeing comparability across studies. Such criteria are not designed to be used for clinical diagnosis in individual patients and may be unable to identify some cases of disease with a less‐common clinical presentation or course. Diagnostic criteria, on the other hand, are generally less stringent and usually include a wider variety of disease features. Their aim is to identify as many individuals as possible with a similar condition. Therefore, a diagnostic decision made by a clinician has to be based on a combination of symptoms, signs, and diagnostic tests, but also to rule out other diseases. However, in daily practice, classification criteria are normally regarded as useful guides for the diagnosis and they may also have a role in the education and training of medical personnel.
The most recent and new American College of Rheumatology/European League Against Rheumatism (ACR‐EULAR) classification criteria for primary Sjögren's syndrome are the end result of an international collaboration and have been derived using a well‐established and validated methodology 5 (Table 1). These criteria describe the key shared features defining the disorder and they may represent the common language to be used in the future to make the scientific communication easier and more correct, favour the exchange of information, and stimulate the development of collaborative studies 6. However, these new classification criteria do not discriminate between primary and secondary Sjögrens's syndrome.
Table 1.
American College of Rheumatology/European League Against Rheumatism (ACR‐EULAR) classification criteria for primary Sjögren's syndrome
| Item | Weight/score |
|---|---|
| Labial salivary gland with focal lymphocytic sialadenitis and focus score of ≥1 foci/4 mm2 | 3 |
| Anti‐SSA/anti‐Ro positive | 3 |
| Ocular staining score ≥5 (or van Bijsterveld score ≥ 4) in at least one eye | 1 |
| Schirmer's test ≤5 mm/5 min in at least one eye | 1 |
| Unstimulated whole saliva flow rate ≤0.1 ml min−1 | 1 |
The classification of primary Sjögren's syndrome applies to any individual who meets the inclusion criteria*; does not have any of the conditions listed as exclusion criteria**; and has a score of ≥4 when the weights from the five criteria in the table are summed. This is a simplified version of table 3 in shiboski et al. 5. *The inclusion criteria are applicable either to any patient with at least one symptom of ocular or oral dryness, defined as a positive response to at least one of the following: (i) Have you had daily, persistent, troublesome dry eyes for more than 3 months? (ii) Do you have a recurrent sensation of sand or gravel in the eyes? (iii) Do you use tear substitutes more than three times a day? (iv) Have you had a daily feeling of dry mouth for more than 3 months? (iv) Do you frequently drink liquids to aid in swallowing dry food? or in whom there is suspicion of Sjögren's syndrome (SS) from the European League Against Rheumatism SS Disease Activity Index questionnaire (at least one domain with a positive item), **Exclusion criteria include prior diagnosis of any of the following conditions, which would exclude diagnosis of Sjögren's syndrome and participation in Sjögren's syndrome studies or therapeutic trials because of overlapping clinical features or interference with criteria tests: (i) history of head and neck radiation treatment, (ii) active hepatitis C infection (with confirmation by PCR), (iii) AIDS, (iv) sarcoidosis, (v) amyloidosis, (vi) graft‐vs.‐host disease, (vii) IgG4‐related disease].
Biomarkers in Sjögren's syndrome
In order to improve the diagnosis, the search for biomarkers has become very important. However, biomarkers are not only quantitative measures to allow a more precise diagnosis but can also be used for assessing a disease process as well as monitoring response(s) to treatment. Indeed, biomarkers can be considered as the foundation for therapy. By definition, a biomarker can be considered ‘a characteristic that is objectively measured and evaluated as an indicator of normal biologic processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention’ 7.
In the pharmaceutical industry, biomarkers are crucial for efficient development of medical products. However, as a consequence of scientific, economic, and regulatory factors, biomarker development has lagged significantly behind therapeutic development. Many potential biomarkers have been discovered, but relatively few studies have fulfilled the more laborious validation process.
There are a number of biomarker examples in Sjögren's syndrome. The most obvious are those in serum (e.g. autoantibodies and cytokines) and in DNA (identified using gene profiling and/or genome‐wide association studies), as well as in cells or at the cellular level (identified according to the different phenotypes or properties/functions of cells). Even tissue reactions are good examples of biomarkers (e.g. focal inflammation and germinal center reactions), and very tempting sources of biomarkers in Sjögren's syndrome are saliva or tears, products released directly from the target organs.
Diagnostic markers in serum/plasma for primary Sjögren's syndrome
Validated biomarkers are important for providing a rapid and accurate diagnosis, as well as for the classification, treatment, and follow‐up of patients. Peripheral blood samples (serum/plasma) are easily accessible, making them the most obvious biomaterials when searching for biomarkers. We published a list of potential biomarkers in 2001 8 and, together with recent reviews by tong et al . 9 and fayyaz et al . 10, this should give a good overview of biomarkers that are associated with Sjögren's syndrome.
Autoantibodies to the autoantigens Ro/SSA and La/SSB are the most important biomarkers identified to date and have been incorporated into the classification criteria for primary Sjögren's syndrome 5, 11, 12, 13, 14. After all these years of scientific scrutiny, anti‐Ro/SSA and anti‐La/SSB are still today important for the disease classification just as they serve as diagnostic markers for Sjögren's syndrome. Ro/SSA and La/SSB actually comprise three different cellular proteins Ro52, Ro60, and La48 – based on their molecular weight, which was discovered in the late 1960s and early 1970s 14, 15, 16, 17. Depending on the testing method used, the selection criteria for primary Sjögren's syndrome, and the patient cohorts, the prevalences of seropositivity are approximately 70% for Ro52, 40% for Ro60, and 50% for La48 8, 18. The Ro and La proteins were first isolated and characterized for their partition in ribonucleoprotein (RNP) complexes with human Y RNAs, which indicated involvement in cellular post‐transcriptional regulation. More specific functions were subsequently attributed, with Ro52 (also referred to as TRIM21) shown to be a ubiquitin E3 ligase, and Ro60 and La48 have both been shown to be RNA‐binding proteins 19, 20 A data‐driven study of international patient cohorts 5, 14 has shown that anti‐Ro/SSA is the second‐best predictor of Sjögren's syndrome after focus score, when the most recent criteria for Sjögren's syndrome are employed.
Anti‐nuclear antibodies (ANA) 21 and rheumatoid factor (RF) 22, 23, 24 are also parameters commonly measured in Sjögren's syndrome as clinical and diagnostic tools but have only partially made a position in the classification criteria.
In recent years, a few biomarkers have stood up as promising diagnostic markers. Muscarinic type 3 receptor (M3R) is one of the new promising biomarkers with direct biological and functional links to exocrine secretion. It is suspected that antibodies towards M3R may potentially inhibit saliva secretion 25, 26, 27, 28, 29, 30, 31. Some have reported a 60–80% concordance of anti‐M3R with Sjögren's syndrome 32 but the usage has been hampered by reproducibility issues and anti‐M3R is therefore not widely used as a biomarker 33 in clinical settings. Improvement in analytical techniques may change this.
Calprotectin is a complex of the S100A8 and S100A9 proteins found abundantly in neutrophils. In the presence of calcium (Ca2+), calprotectin has inflammatory and antimicrobial activities. Salivary, but not blood, calprotectin has shown strong correlation with clinical signs of Sjögren's syndrome 34, 35, 36. Serum calprotectin has more recently been shown to be a marker for carotid atherosclerosis in primary Sjögren's syndrome 37.
Carbamylation is the process of modification of lysine in proteins to homocitrulline. Elevated levels of carbamylated proteins have been associated with increased focal lymphocytic infiltration, formation of ectopic GC‐like structures in minor salivary glands, and diminished salivary gland function 38.
During the past 40 years many potential candidate molecules have been studied in relation to Sjögren's syndrome and disease development (Table 2). However, these have not become validated diagnostic markers as they have not provided improved descriptive power over the biomarkers already established in Sjögren's syndrome diagnosis. It is remarkable that no new biomarker has been established after all these years and the major efforts made. This indicates that we are not much closer to understanding the underlying disease mechanisms than we were years ago. Although we have made progress in describing the clinical features and potential treatment measures in Sjögren's syndrome, the causes are still obscure. This may be because of the complexity of the disease, just as it is a question if there is indeed more than one disease. New biomarkers may be valuable in describing subphenotypes of primary Sjögren's syndrome and hence optimizing treatment for subgroups of patients 10.
Table 2.
Diagnostic biomarkers in use and potential candidates for further validation
| Biomarkers | References |
|---|---|
| Regularly used | |
| Ro/SSA – Ro52 (TRIM21) and Ro60 | 14, 15, 16, 17, 18 |
| La/SSB – La48 | 14, 15, 16, 17, 18 |
| Occasionally used | |
| Rheumatoid factor (RF) | 22, 23, 24 |
| Anti‐nuclear antibodies (ANA) | 21 |
| New potential biomarkers | |
| Muscarinic type 3 receptor (M3R) | 25, 26, 27, 28, 29, 30, 31, 32, 33 |
| Calprotectin | 34, 35, 36, 37 |
| Carbamylated proteins (homocitrulline) | 38 |
Another very important aspect is that biomarkers/diagnostic markers may be present many years before the onset of clinical symptoms. We have shown that it is possible to detect anti‐Ro and anti‐La immunoglobulins up to 18–20 years before the appearance of symptoms and diagnosis of Sjögren's syndrome (Fig. 1) 39, 40. The potential benefit of early diagnostic testing may be that it initiates early treatment and thus prevents significant tissue damage.
Figure 1.
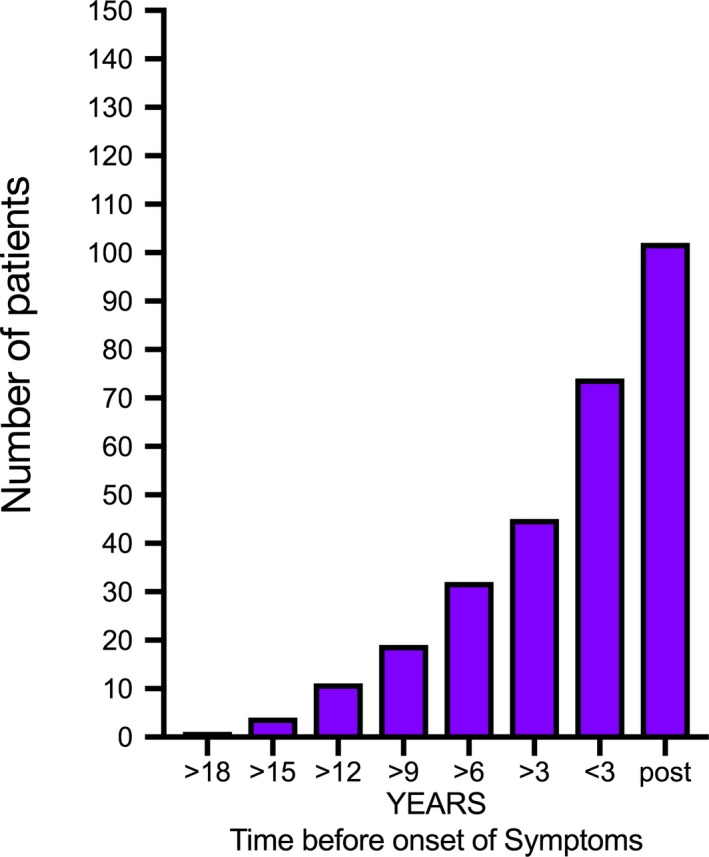
Number of patients, from a cohort of 171 patients, with positive anti‐nuclear antibody (ANA) score before (>18 yr) and after (post) onset of disease. Adapted from jonsson et al. 39 and theander et al. 40.
Histopathology of minor salivary glands in Sjögren's syndrome
Labial salivary gland (LSG) biopsy has played an important role in the diagnosis of Sjögren's syndrome since it was first described over 40 years ago 2, 3. Biopsy currently remains the best method for diagnosing the salivary gland component of Sjögren's syndrome, mainly because of its high disease specificity and limited invasiveness 41. The diagnostic role of salivary gland biopsy is widely accepted in both the established 2002 American‐European Consensus Criteria (AECG) for Sjögren's syndrome 12 and in the newly proposed ACR classification 13, 42. Recent evidence suggests that LSG biopsies may also provide useful information for prognostication and stratification of patients with Sjögren's syndrome 43, 44, 45.
Biopsy of LSGs is usually performed for diagnosis of Sjögren's syndrome, although some centres prefer biopsy of parotid glands performed by an experienced clinician because this is associated with a low complication rate 46. The typical histopathological changes in the minor salivary glands are well‐defined foci of mostly lymphocytes surrounding ducts or small vessels. A positive LSG biopsy has been defined as a focal mononuclear infiltrate with a focus score of ≥1 per 4 mm2 of glandular tissue 41. The focal infiltrate should contain 50 or more cells, mostly lymphocytes in a periductal location, typically adjacent to acini with a normal appearance.
The dominating cell populations in the focal infiltrates are T and B lymphocytes. Certain lymphocytic subsets are currently under investigation to delineate their possible role in different phases of disease development. Elevated numbers of cells of the T‐helper subset, Th17, mainly defined by secretion of the cytokine interleukin (IL)‐17, have been detected in the periphery and also in the salivary gland tissue of patients with primary Sjögren's syndrome 47, 48. The follicular T‐helper‐cell subset is another focus of interest as these cells are involved in the crosstalk between T and B cells under the stimulus of IL‐12 secreted by dendritic cells. It is noteworthy that macrophages, natural killer (NK) cells, and dendritic cells are also present in varying numbers during progression of Sjögren's syndrome disease.
B‐cell hyperactivity represents a key feature in the pathogenesis of Sjögren's syndrome, and alterations of B‐cell subtypes have been detected in patients with primary Sjögren's syndrome 49, 50. Notably, higher focus scores are associated with an increased B/T‐cell ratio. As the focal infiltrates increase in size, lymphoid organization in the form of germinal center (GC)‐like structures may start in approximately 20–25% of the patients 51, 52. These GC‐like structures have been suggested to be a possible predictor of lymphoma development because the majority of patients who developed lymphoma later, presented with GC‐like structures in the diagnostic minor salivary gland biopsy 44. In haematoxylin and eosin‐stained sections, detection of GC‐like structures in the minor salivary glands can be more challenging than detection of such structures in the secondary lymphoid organs. Accordingly, additional staining with CD21 (a marker of follicular dendritic cells), as well as CD20 (a marker of B cells) and CD3 (a marker of T cells), has recently been advised by an expert consensus group, to improve the reliability and consistency of GC identification 4.
The focal inflammation observed in the minor salivary gland tissue of patients with Sjögren's syndrome is commonly accompanied by acinar atrophy, duct dilation, and fibrosis. Another prominent feature is the presence of adipose tissue, which can occupy a large fraction of the glandular tissue. To date, little is known about the diagnostic and pathological significance of fatty replacement in Sjögren's syndrome lesions. Our own studies indicated a higher incidence of adipose tissue in the salivary glands of patients with Sjögren's syndrome compared with non‐Sjögren's syndrome controls 53. However, others suggest that fatty infiltration can be a selective feature of ageing rather than disease 54. Regardless, adipocytes may be active players in immune reactions because adipocytes have been localized in certain cytokine‐ and chemokine‐rich niches of salivary glands from patients with Sjögren's syndrome 53, 55, 56. Future studies are necessary to verify this concept.
While some patients are willing to have two or even three biopsies removed for the purposes of follow up and monitoring of the effect of treatment in Sjögren's syndrome, there is an ethical limit on the number of consecutive lip biopsies that can be made.
pijpe et al. 46 proposed that histopathology of the parotid gland should be included in the classification criteria for Sjögren's syndrome as an alternative to histopathology of labial glands. Histopathological conditions of the minor and major glands were reported to be comparable. The approach presents some limitations, in particular related to the fact that biopsy of the parotid gland requires specific surgical skills and that biopsy of the labial gland is more easily performed. Therefore, labial gland biopsy is still preferred by most groups. However, recent studies highlight the diagnostic potential of parotid gland tissue, including the possibility of identifying parotid mucosa‐associated lymphoid tissue (MALT) lymphoma at an earlier stage. Furthermore, the possibility of repeated biopsies in the same parotid gland can facilitate evaluation of treatment efficacy in clinical trials and may also serve as a guide to personalized treatment 57, 58. The diagnostic potential of the parotid gland should therefore be reconsidered. Nevertheless, the concordance of parotid gland biopsy compared with labial gland biopsy is currently a matter of debate and there is a need for larger and comparative studies 59.
Major salivary gland ultrasonography
There have been a number of attempts to find an alternative to labial salivary gland biopsies in Sjögren's syndrome. Salivary gland ultrasonography (SGUS) is a non‐invasive, non‐irradiating imaging modality for assessment of the parotid and submandibular glands as part of the diagnostic work‐up, and possibly follow‐up, of natural history or treatment outcome in primary and secondary Sjögren's syndrome 60. The method may be used to evaluate echogenicity in general, as well as structural changes (such as fibrosis and calcification) and degenerative changes, visualized as inhomogeneity and hyper‐ and hypoechogenic areas (Fig. 2).
Figure 2.
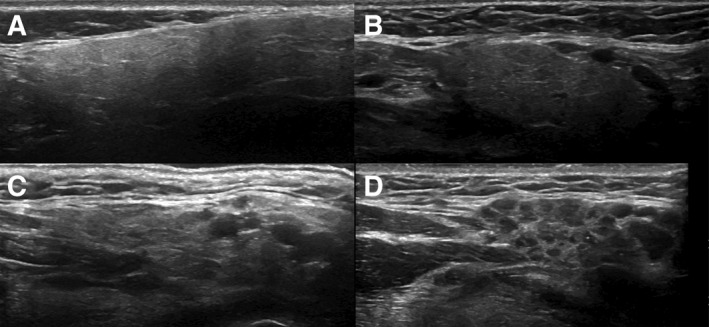
Ultrasonographic images of four left submandibular glands illustrating varying grades of normal/non‐specific to pathological changes. Grades 0–3 were used when evaluating ultrasonographic images of submandibular and parotid glands from each patient. (A) Grade 0. (B) Grade 1. (C) Grade 2. (D) Grade 3. Grades 0–1 were considered to correspond to normal morphology/non‐specific changes, whereas grades 2–3 were considered to correspond to pathological changes, possibly related to primary Sjögren's syndrome. Reproduced from hammenfors et al. 61, with permission.
Several studies have shown correlations between SGUS findings and focus score in the minor salivary glands 61, 62, as well as in parotid salivary gland biopsies 57. The definition and emphasis on various SGUS abnormalities vary in previously published studies. theander et al. 63 suggested grading parenchymal homogeneity in salivary glands from 0 to 3, with grades 0 (normal) and 1 (mild inhomogeneity) being interpreted as normal or unspecific, and grades 2 (several rounded) and 3 (numerous or confluent hypoechoic lesions) as Sjögren's syndrome typical. Specificity and positive predictive values of abnormal SGUS for Sjögren's syndrome were both 98%, with sensitivity and negative predictive values being 52% and 53%, respectively. Patients with pathological SGUS had significantly more signs and symptoms of systemic complications, higher disease activity, and, more frequently, markers of lymphoma development, such as salivary gland swelling, skin vasculitis, GC‐like structures in minor salivary gland biopsy, and CD4+ T‐lymphocytopenia 63.
In a study by shimizu et al. 64, Doppler was added to SGUS in order to use parotid gland vascularity as a means to improve the SGUS diagnosis of Sjögren's syndrome 64. Patients with Sjögren's syndrome showed significantly higher degree of vascularity than patients without Sjögren's syndrome. Abnormal vascularity correlated with histopathological grades, but not with sialographic grades; the highest mean vascular score determined by Doppler was observed for the sialographic initial stage and cavitary‐destructive stages, suggesting that hypervascularity may represent ongoing processes in the gland parenchyma. With vascular information, the sensitivity, specificity, and accuracy of SGUS changed from 44%, 97%, and 65%, to 63%, 90%, and 74%, respectively.
jousse‐joulin et al. 65 used colour Doppler SGUS to evaluate the major salivary gland treatment response following rituximab treatment. Doppler waveform analysis of the transverse facial artery of parotid glands showed significant differences between untreated patients and controls, before, but not after, lemon juice stimulation. Following rituximab treatment, both the parotid and submandibular glands showed significant reduction in size compared with baseline. Response to rituximab treatment was also investigated in a multicentre, randomized, double‐blind, placebo‐controlled trial termed ‘Tolerance and Efficacy of Rituximab in Primary Sjögren's Syndrome’ (TEARS) 66. Patients with primary Sjögren's syndrome were examined with SGUS before the first placebo/rituximab infusion and 6 months after. At each examination, SGUS of the parotid and submandibular glands was performed and the parameters echostructure (score 0–4), size of each gland, and vascularization based on the resistive index of the transverse facial artery of the parotid gland, were recorded before and after lemon juice stimulation. Parotid parenchyma echostructure improved in 50% of the rituximab‐treated patients compared with 7% of the placebo‐treated patients (P = 0.03). Submandibular gland echostructure also improved in a larger proportion of rituximab‐treated patients, although not statistically significantly. The size of the glands and the resistive index remained unchanged.
For diagnostic purposes, recent studies suggest that use of a simplified approach for SGUS evaluation of the major salivary glands may be sufficient. The reliability of SGUS was investigated in a two‐step process using both static and acquisition SGUS images 67. Selected SGUS echostructural abnormalities in Sjögren's syndrome (echogenicity, homogeneity, hyperechoic bands, number and location of hypo/anechoic areas, presence of abnormal lymph nodes in the glands, calcifications, visibility of posterior border, and diagnosis advice of primary Sjögren's syndrome based on the seven items) were defined and set up in a preliminary atlas. Although it is not yet established what the hypoechogenic areas reflect, echogenicity and homogeneity were identified as two reliable items, whereas the reliability of other core items was slight or poor. Parotid gland SGUS echogenicity provided substantial interobserver reliability with regard to suggested diagnosis of Sjögren's syndrome 67.
Investigations by mossel et al. 68 suggested the evaluation of parenchymal echogenicity, homogeneity, hypoechogenic areas, hyperechogenic reflections, and salivary gland posterior border [as previously described by hocevar et al. 69], in either the right or left parotid and submandibular glands, to be sufficient to predict the ACR‐EULAR classification. Even using only parenchymal echogenicity and hypoechogenic areas contributed independently to the ACR‐EULAR classification, as did using only hypoechogenic areas on one side, further increasing feasibility of SGUS in outpatient clinics worldwide 68.
Early detection of primary Sjögren's syndrome was investigated by baldini et al. 70, who also found that SGUS score correlated with minor salivary gland focus score and unstimulated whole saliva flow. A SGUS cut‐off of ≥1 was associated with a sensitivity of 66%, a specificity of 98%, a positive predictive value of 97%, and a negative predictive value of 73% for the diagnosis of primary Sjögren's syndrome.
Salivary gland ultrasonography may play a role in the diagnosis of patients lacking extractable nuclear antibodies who need to have a labial salivary gland biopsy to fulfil the diagnostic criteria. Accordingly, SGUS imaging of the major salivary glands was investigated as a predictor of the histology, to explore whether SGUS can help in stratifying patients with Sjögren's syndrome and reduce the need for biopsy. In a blinded, retrospective study of minor salivary gland histopathology and major salivary gland ultrasonography, SGUS had a positive predictive value of 85% and a negative predicative value of 96% for the histology results. Overall concordance between SGUS and histopathology was 91% 71.
Salivary gland ultrasonography has also been assessed with regard to the ability to distinguish patients with Sjögren's syndrome from individuals with xerostomia and/or keratoconjunctivitis and a diagnosis of stable undifferentiated connective tissue disease (UCTD). Patients diagnosed with Sjögren's syndrome according to the AECG criteria 12 presented with higher SGUS score compared with patients with UCTD. Setting the SGUS cut‐off score at a value of >2 provided a sensitivity of 65%, a specificity of 96%, a positive predictive value of 95%, and a negative predictive value of 73% for the diagnosis of Sjögren's syndrome 72.
The diagnostic performance of SGUS in the AECG criteria was investigated in a cohort with suspected Sjögren's syndrome 73, and applying the AECG criteria 12 alone, the sensitivity of Sjögren's syndrome diagnosis was 77.9% and specificity 98.7% compared with expert opinion. Compared with the AECG criteria alone, adding pathological SGUS findings to the AECG criteria increased sensitivity to 87.0% but did not markedly alter specificity (96.1% vs. 98.7%). In another series of patients with suspected Sjögren's syndrome, SGUS increased the diagnostic performance of the ACR criteria from 64.4% to 84.4% and only slightly decreased specificity, from 91.1% to 89.3% 74.
Similar findings were presented 75 for SGUS as an additional item in the ACR classification of Sjögren's syndrome, in individuals classified as having primary Sjögren's syndrome or non‐Sjögren's syndrome according to the AECG criteria 12. Salivary gland ultrasonography was performed in selected patients who had scored at least two positive or at least two negative results according to the ACR criteria 13. Incorporation of the SGUS criteria as an alternative to one of the three ACR classification items achieved 89–91% sensitivity, 87–96% specificity, and 89% or 92% accuracy, comparable with the original ACR classification.
A recent study by le goff et al. assessed how SGUS might improve the classification of patients with suspected primary Sjögren's syndrome 76. Concordance between AECG and ACR/EULAR criteria was excellent, and 58% of these patients had pathological SGUS findings. Patients fulfilling only the ACR/EULAR criteria had similar age and symptom duration, but lower frequencies of keratoconjunctivitis, xerostomia, and salivary gland dysfunction, than patients fulfilling both sets of criteria. In patients not fulfilling either set of criteria (n = 165), SGUS was abnormal in 12%. Using the physicians’ diagnosis as reference standard, sensitivity was increased from 87.4% to 91.1% when including SGUS among the ACR/EULAR criteria.
Combining positive SGUS findings and anti‐Ro/SSA gives a highly predictive score of Sjögren's syndrome according to the AECG, ACR, and ACR‐EULAR classification criteria 57 in a prospective inception cohort study derived from daily clinical practice. Interestingly, the agreement between SGUS and salivary gland biopsies was slightly higher for parotid gland biopsies than for labial gland biopsies. When parotid gland biopsy was used as a classification factor, the lack of anti‐Ro/SSA with or without negative SGUS indicates no presence of Sjögren's syndrome. However, when the outcome of labial gland biopsy was considered as a criterion, the combination of negative SGUS with absence of anti‐Ro/anti‐SSA could not exclude diagnosis of Sjögren's syndrome.
Work regarding incorporation of SGUS into the ACR‐EULAR criteria has also been carried out using patient vignettes, and preliminary findings indicate that the addition of SGUS as an item to both AECG and ACR/EULAR 2017 classification criteria increased sensitivity from 90% to 96%, but did not change specificity (84%) 77.
Juvenile Sjögren's syndrome
Juvenile Sjögren's syndrome is a rare, poorly defined, and possibly underdiagnosed condition 78, 79 affecting children and adolescents, with a mean age at diagnosis of 10.7 yr 80. A variety of organ systems may be affected, resulting in neurological, dermatological, musculoskeletal, vascular, gastrointestinal, respiratory, renal, and haematological manifestations 81, 82. Extraglandular manifestations have been reported with a prevalence of 51.3% in juvenile Sjögren's syndrome 78.
Diagnosis of juvenile Sjögren's syndrome is based on clinical symptoms and presence of autoantibodies, after exclusion of infectious or lymphoproliferative diseases. Diagnosis, treatment, and follow‐up of juvenile Sjögren's syndrome is generally based on clinical experience from primary Sjögren's syndrome in adults; however, compared with primary Sjögren's syndrome in adults, patients with juvenile Sjögren's syndrome often display swelling of the major salivary glands as an initial symptom 80, 83. Recurrent parotitis in childhood is most commonly of infectious origin or because of retention of saliva. In juvenile Sjögren's syndrome, parotid swelling usually precedes regular oral and ocular symptoms, while typical serological findings may be absent 84. Salivary gland ultrasonography shows features typical of primary Sjögren's syndrome/juvenile Sjögren's syndrome that can add useful information, and SGUS has been suggested as a routine imaging tool in patients with recurrent parotitis and autoantibodies 85.
Exploration of saliva as a diagnostic fluid in Sjögren's syndrome
Although salivary flow rates are heterogeneous among patients with Sjögren's syndrome, salivary constituents have been studied in this context since the early 1970s and are reviewed elsewhere 86, 87. More recently, by navigating the methodological complexities associated with emerging ‘omics’ technologies, researchers with an interest in Sjögren's syndrome have taken different approaches with respect to salivary biomarkers and their potential application. The main focus has been the discovery of biomarkers for diagnosis and patient cohort stratification (Figs 3 and 4). Regrettably, the demand for an analytical test allowing detection of Sjögren's syndrome at an early stage using saliva has not yet been addressed sufficiently.
Figure 3.
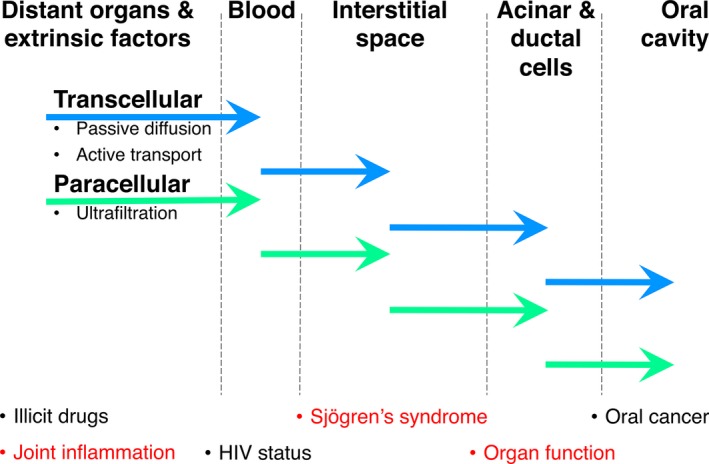
Barriers and modes of transport for components originating from distant organs, the bloodstream, the interstitial space, acinar and ductal cells, or the oral cavity, to become detectable in saliva. The ability to monitor tissue‐related changes in saliva relies on the paradigm that a specific tissue state is reflected in the spectrum and quantity of specific components liberated into specific biofluids.
Figure 4.
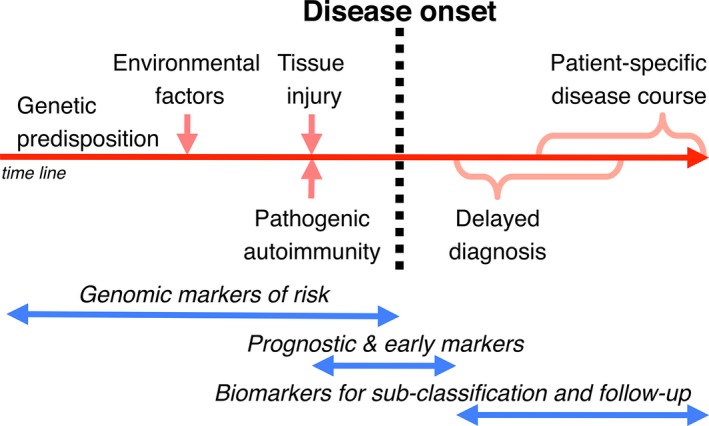
Schematic representation of the long covert phase of Sjögren's syndrome before disease onset followed by a commonly late diagnosis and currently limited options for biomarker‐based patient follow‐up. Ease of collection, repeatability, and close vicinity to the target organ make saliva a prime biofluid for biomarker discovery and clinical application in Sjögren's syndrome.
Mass spectrometry‐based analyses of saliva pooled according to disease‐group membership have highlighted comprehensive and distinct protein patterns characteristic for Sjögren's syndrome 88, 89, 90. These profiles mostly comprise secretory proteins, enzymes, and highly abundant immune system‐related molecules. Interestingly, the profile associated with secondary Sjögren's syndrome has, in some patients, been found to resemble that of primary Sjögren's syndrome, while in others it was found to be more similar to that of healthy subjects 89. More recently, efforts have also been made to characterize the metabolome of patients with Sjögren's syndrome and this has also suggested the existence of different subpopulations of Sjögren's syndrome 91.
While the true diagnostic value of the classical Sjögren's syndrome autoantibodies (anti‐Ro/SSA and anti‐La/SSB) measured in saliva remains to be fully determined 92, protein arrays assessing a wider spectrum of autoantibodies in saliva highlight the value of such analyses to discriminate between Sjögren's syndrome, systemic lupus erythematosus, and asymptomatic controls 93.
Using a similar concept but focussing on relating 187 key salivary proteins to the biological state of the salivary glands, we were, on the basis of a 6‐plex salivary biomarker signature, able to discriminate patients with Sjögren's syndrome from patients with rheumatoid arthritis and asymptomatic controls with an accuracy of >94% 94 (Fig. 5A). Functional annotation of the great number of coordinated alterations suggests very high fidelity of saliva when conveying the histopathological and immunological hallmarks of Sjögren's syndrome‐associated sialadenitis. Following the same strategy for patient stratification we also identified compact biomarker signatures capable of categorizing patients with Sjögren's syndrome with respect to their individual presentation of hyposalivation and ectopic GC‐like structure formation 95 (Fig. 5B). As a result of their increased predisposition to develop B‐cell lymphoma 44, the latter individuals may be viewed as important beneficiaries of novel tools to monitor the state of their salivary gland in a non‐invasive manner.
Figure 5.
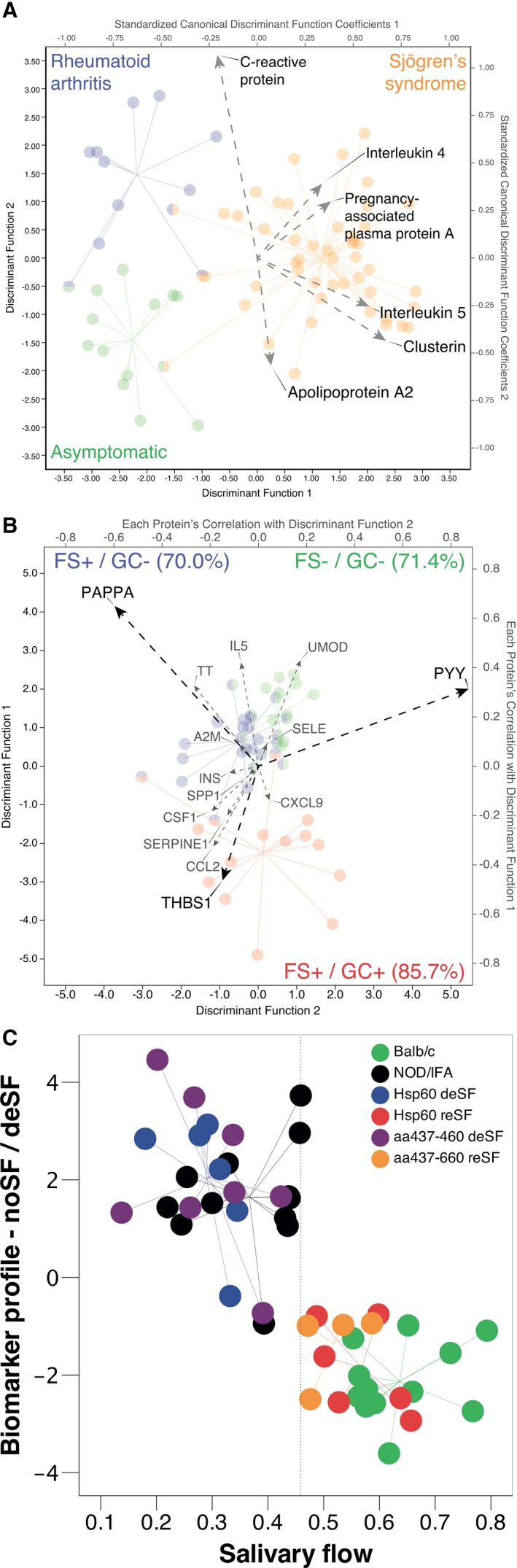
Salivary biomarker signatures serving in the diagnosis and stratification of patients as well as in predicting treatment responses in experimental models of Sjögrens syndrome. (A) 6‐plex [C‐reactive protein, interleukin (IL)‐4, pregnancy‐associated plasma protein A (PAPPA), IL‐5, clusterin, and apolipoprotein A2] salivary biomarker signature‐based recapitulation of the American–European Consensus Group criteria‐based classification. Discrimination between patients with Sjögren's syndrome, patients with rheumatoid arthritis, and asymptomatic controls was, at 94%, found to be highly accurate. Borrowed with permission from delaleu et al. 94. (B) 3‐plex [PAPPA, thrombospondin 1 (THBS‐1), and peptide YY (PYY)] biomarker signature‐based prediction of the nature of salivary gland inflammation for individual patients with Sjögren's syndrome. Borrowed with permission from delaleu et al. 95. A2M, alpha‐2‐macroglobulin; CCL2, chemokine (C‐C motif) ligand 2; CSF1, colony stimulating factor 1; CXCL9, chemokine (C‐X‐C motif) ligand 9; FS, focus score; GC, ectopic germinal centre; IL5, interleukin 5; INS, insulin; TT, transferrin; SELE, selectin E; SERPINE1, serpin family E member 1; SPP1, secreted phosphoprotein 1; UMOD, uromodulin. (C) Potential of a biomarker signature, combining granulocyte chemotactic protein 2 and IL‐1alpha measured in serum, and myeloperoxidase, myoglobin, and macrophage inflammatory protein 3b measured in saliva, to predict reinstatement of a physiological salivary secretion rate in a murine model of spontaneous Sjögren's syndrome after experimental treatment. Red and orange, responders to treatment; green, asymptomatic controls; purple and blue, non‐responders to treatment; black, disease control (accuracy = 93.8%). The x‐axis shows the salivary flow rate in microliters per minute per gram; the y‐axis represents the discriminant score of the biomarker signature. Borrowed with permission from delaleu et al. 96. aa, amino acids; deSF, decreased salivary flow; Hsp60, 60‐kd heat‐shock protein; IFA, Freund's incomplete adjuvant; NOD, nonobese diabetic mice; reSF, retained salivary flow. Circles represent individual patients (A, B) and mice (C).
A field that has been little explored thus far is assessment of the usefulness of complementing clinical studies with analyses using exploratory salivary biomarkers. The ability to infer understanding regarding how experimental drugs shape the molecular landscape of spontaneous Sjögren's syndrome‐like disease in mice depending on treatment response 96 is, in our opinion, sufficiently promising to justify adaptation of this concept for clinical trials (Fig. 5C).
Finally, the ability to prescreen individuals at risk for Sjögren's syndrome on the basis of a drop of saliva may significantly reduce the number of undiagnosed cases and shorten the delay from disease onset to diagnosis. Improvements in both respects would open new possibilities for disease management and the technology for implementing such approaches is maturing at a fast pace.
Conclusion
An international set of classification criteria for primary Sjögren's syndrome has recently been developed and validated using guidelines from ACR and EULAR. These criteria performed well in validation analyses and seem well suited for use in enrollment in clinical trials. In the continued work of patient stratification, standardized evaluation of labial salivary gland biopsies has been used systematically in disease classification and in clinical trials. Finally, ultrasonographic examination of salivary glands and collecting saliva are examples of non‐invasive sampling methods. These methods can be carried out repetitively, are not associated with adverse effects and have a high compliance with the patients. To further explore and develop validated diagnostic tests based on these methods should be of high priority.
Conflicts of interests
The authors declare that they have no conflict of interest.
Acknowledgements
Work by the authors referred to in the text has more recently been supported by the Broegelmann Foundation, EU H2020 contract HarmonicSS (H2020‐SC1‐2016‐RTD/731944), the Western Norway Regional Health Authorities (grant nr. 912065) and the University of Bergen.
Jonsson R, Brokstad KA, Jonsson MV, Delaleu N, Skarstein K. Current concepts on Sjögren's syndrome – classification criteria and biomarkers. Eur J Oral Sci 2018; 126(Suppl. 1): 37–48. © 2018 Eur J Oral Sci
[The copyright line for this article was changed on 10 May 2019 after original online publication.]
References
- 1. Sjögren H. Zur Kenntnis der Keratoconjunctivitis sicca. Acta Ophthalmol 1933; 11(Suppl): 1–151. [DOI] [PubMed] [Google Scholar]
- 2. Chisholm DM, Mason DK. Labial salivary gland biopy in Sjögren's disease. J Clin Pathol 1968; 21: 656–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Greenspan JS, Daniels TE, Talal N, Sylvester RA. The histopathology of Sjögrens's syndrome in labial salivary gland biopsies. Oral Surg Oral Med Oral Pathol 1974; 37: 221–229. [DOI] [PubMed] [Google Scholar]
- 4. Fisher BA, Jonsson R, Daniels T, Bombardieri M, Brown RM, Morgan P, Bombardieri S, Ng W‐F, Tzioufas AG, Vitali C, Shirlaw P, Haacke E, Costa S, Bootsma H, Devauchelle‐Pensec V, Radstake TR, Mariette X, Richards A, Stack R, Bowman SJ, Barone F. Standardisation of labial salivary gland histopathology in clinical trials in primary Sjögren's syndrome. Ann Rheum Dis 2017; 76: 1161–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shiboski CH, Shiboski SC, Seror R, Criswell LA, Labetoulle M, Lietman TM, Rasmussen A, Scofield H, Vitali C, Bowman SJ, Mariette X; International Sjögren's Syndrome Criteria Working Group . 2016 American College of Rheumatology/European League Against Rheumatism classification criteria for primary Sjögren's syndrome: A consensus and data‐driven methodology involving three international patient cohorts. Ann Rheum Dis 2017; 76: 9–16. [DOI] [PubMed] [Google Scholar]
- 6. Vitali C, Del Papa N. Classification and diagnostic criteria in Sjögrens's syndrome: a long‐standing and still open controversy. Ann Rheum Dis 2017; 76: 1953–1954. [DOI] [PubMed] [Google Scholar]
- 7. Biomarkers Definitions Working Group . Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther 2001; 69(3): 89–95. [DOI] [PubMed] [Google Scholar]
- 8. Jonsson R, Brokstad K. Sjögren′s syndrome In: Austen KF, Frank MM, Atkinson JP, Cantor H, eds. Samter′s Immunologic Diseases, 6th edn Philadelphia, PA: Lippincott Williams & Wilkins, 2001; chapter 40: 495–504. [Google Scholar]
- 9. Tong L, Koh V, Thong BY. Review of autoantigens in Sjögren's syndrome: an update. J Inflamm Res 2017; 10: 97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fayyaz A, Kurien BT, Scofield RH. Autoantibodies in Sjögren's Syndrome. Rheum Dis Clin North Am 2016; 42: 419–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vitali C, Bombardieri S, Moutsopoulos HM, Balestrieri G, Bencivelli W, Bernstein RM, Bjerrum KB, Braga S, Coll J, de Vita S. Preliminary criteria for the classification of Sjögren's syndrome. Results of a prospective concerted action supported by the European Community. Arthritis Rheum 1993; 36: 340–347. [DOI] [PubMed] [Google Scholar]
- 12. Vitali C, Bombardieri S, Jonsson R, Moutsopoulos HM, Alexander EL, Carsons SE, Daniels TE, Fox PC, Fox RI, Kassan SS, Pillemer SR, Talal N, Weisman N; European Study Group on Classification Criteria for Sjögren's Syndrome . Classification criteria for Sjögren's syndrome: a revised version of the European criteria proposed by the American‐European Consensus Group. Ann Rheum Dis 2002; 61: 554–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shiboski SC, Shiboski CH, Criswell L, Baer A, Challacombe S, Lanfranchi H, Schiødt M, Umehara H, Vivino F, Zhao Y, Dong Y, Greenspan D, Heidenreich AM, Helin P, Kirkham B, Kitagawa K, Larkin G, Li M, Lietman T, Lindegaard J, McNamara N, Sack K, Shirlaw P, Sugai S, Vollenweider C, Whitcher J, Wu A, Zhang S, Zhang W, Greenspan J, Daniels T; Sjögren's International Collaborative Clinical Alliance (SICCA) Research Groups . American College of Rheumatology classification criteria for Sjögren's syndrome: a data‐driven, expert consensus approach in the Sjögren's International Collaborative Clinical Alliance cohort. Arthritis Care Res (Hoboken) 2012; 64: 475–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Clark G, Reichlin M, Tomasi TB Jr. Characterization of a soluble cytoplasmic antigen reactive with sera from patients with systemic lupus erythematosus. J Immunol 1969; 102: 117–122. [PubMed] [Google Scholar]
- 15. Mattioli M, Reichlin M. Heterogeneity of RNA protein antigens reactive with sera of patients with systemic lupus erythematosus. Description of a cytoplasmic nonribosomal antigen. Arthritis Rheum 1974; 17: 421–429. [DOI] [PubMed] [Google Scholar]
- 16. Alspaugh MA, Talal N, Tan EM. Differentiation and characterization of autoantibodies and their antigens in Sjögren's syndrome. Arthritis Rheum 1976; 19: 216–222. [DOI] [PubMed] [Google Scholar]
- 17. Venables PJ, Smith PR, Maini RN. Purification and characterization of the Sjögren's syndrome A and B antigens. Clin Exp Immunol 1983; 54: 731–738. [PMC free article] [PubMed] [Google Scholar]
- 18. Garberg H, Jonsson R, Brokstad KA. The serological pattern of autoantibodies to the Ro52, Ro60, and La48 autoantigens in primary Sjögren's syndrome patients and healthy controls. Scand J Rheumatol 2005; 34: 49–55. [DOI] [PubMed] [Google Scholar]
- 19. Wada K, Kamitani T. Autoantigen Ro52 is an E3 ubiquitin ligase. Biochem Biophys Res Commun 2006; 339: 415–421. [DOI] [PubMed] [Google Scholar]
- 20. Campos‐Almaraz MD, Barbosa‐Cisneros O, Herrera‐Esparza R. Ro60 ribonucleoprotein inhibits transcription by T3 RNA polymerase in vitro. Rev Rheum Engl Ed 1999; 66: 310–314. [PubMed] [Google Scholar]
- 21. Tan EM. Antinuclear antibodies: diagnostic markers for autoimmune diseases and probes for cell biology. Adv Immunol 1989; 44: 93–151. [DOI] [PubMed] [Google Scholar]
- 22. Markusse HM, Otten HG, Vroom TM, Smeets TJ, Fokkens N, Breedveld FC. Rheumatoid factor isotypes in serum and salivary fluid of patients with primary Sjögren's syndrome. Clin Immunol Immunopathol 1993; 66: 26–32. [DOI] [PubMed] [Google Scholar]
- 23. Atkinson JC, Fox PC, Travis WD, Popek E, Katz RW, Balow JE, Pillemer SR. IgA rheumatoid factor and IgA containing immune complexes in primary Sjögren's syndrome. J Rheumatol 1989; 16: 1205–1210. [PubMed] [Google Scholar]
- 24. Elagib KE, Børretzen M, Jonsson R, Haga HJ, Thoen J, Thompson KM, Natvig JB. Rheumatoid factors in primary Sjögren's syndrome (pSS) use diverse VH region genes, the majority of which show no evidence of somatic hypermutation. Clin Exp Immunol 1999; 117: 388–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bacman S, Sterin‐Borda L, Camusso JJ, Arana R, Hubscher O, Borda E. Circulating antibodies against rat parotid gland M3 muscarinic receptors in primary Sjögren's syndrome. Clin Exp Immunol 1996; 104: 454–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bacman S, Perez Leiros C, Sterin‐Borda L, Hubscher O, Arana R, Borda E. Autoantibodies against lacrimal gland M3 muscarinic acetylcholine receptors in patients with primary Sjögren's syndrome. Invest Ophthalmol Vis Sci 1998; 39: 151–156. [PubMed] [Google Scholar]
- 27. Robinson CP, Brayer J, Yamachika S, Esch TR, Peck AB, Stewart CA, Peen E, Jonsson R, Humphreys‐Beher MG. Transfer of human serum IgG to NOD. Igμnull mice reveals a role for autoantibodies in the loss of secretory function of exocrine tissues in Sjögren's syndrome. Proc Natl Acad Sci USA 1998; 95: 7538–7543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Waterman SA, Gordon TP, Rischmueller M. Inhibitory effects of muscarinic receptor autoantibodies on parasympathetic neurotransmission in Sjögren's syndrome. Arthritis Rheum 2000; 43: 1647–1654. [DOI] [PubMed] [Google Scholar]
- 29. Humphreys‐Beher MG, Brayer J, Yamachika S, Peck AB, Jonsson R. An alternative perspective to the immune response in autoimmune exocrinopathy: induction of functional quiescence rather than destructive autoaggression. Scand J Immunol 1999; 49: 7–10. [DOI] [PubMed] [Google Scholar]
- 30. Sumida T, Tsuboi H, Iizuka M, Asashima H, Matsumoto I. Anti‐M3 muscarinic acetylcholine receptor antibodies in patients with Sjögren's syndrome. Mod Rheumatol 2013; 23: 841–845. [DOI] [PubMed] [Google Scholar]
- 31. Sumida T, Tsuboi H, Iizuka M, Hirota T, Asashima H, Matsumoto I. The role of M3 muscarinic acetylcholine receptor reactive T cells in Sjögren's syndrome: acritical review. J Autoimmun 2014; 51: 44–50. [DOI] [PubMed] [Google Scholar]
- 32. Deng C, Hu C, Chen S, Li J, Wen X, Wu Z, Li Y, Zhang F, Li Y. Meta‐analysis of anti‐muscarinic receptor type 3 antibodies for the diagnosis of Sjögren syndrome. PLoS ONE 2015; 10: e0116744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Roescher N, Kingman A, Shirota Y, Chiorini JA, Illei GG. Peptide‐based ELISAs are not sensitive and specific enough to detect muscarinic receptor type 3 autoantibodies in serum from patients with Sjogren's syndrome. Ann Rheum Dis 2011; 70: 235–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Brun JG, Cuida M, Jacobsen H, Kloster R, Johannesen AC, Høyeraal HM, Jonsson R. Sjögren's syndrome in inflammatory rheumatic diseases: analysis of the leukocyte protein calprotectin in plasma and saliva. Scand J Rheumatol 1994; 23: 114–118. [DOI] [PubMed] [Google Scholar]
- 35. Cuida M, Brun JG, Johannessen AC, Jonsson R. Immunohistochemical characterization of the cellular infiltrates in Sjögren's syndrome, rheumatoid arthritis and osteoarthritis with special reference to calprotectin‐producing cells. APMIS 1996; 104: 881–890. [DOI] [PubMed] [Google Scholar]
- 36. Nordal HH, Brun JG, Halse AK, Madland TM, Fagerhol MK, Jonsson R. Calprotectin (S100A8/A9), S100A12, and EDTA‐resistant S100A12 complexes (ERAC) in primary Sjögren's syndrome. Scand J Rheumatol 2014; 43: 76–78. [DOI] [PubMed] [Google Scholar]
- 37. Balarini GM, Zandonade E, Tanure L, Ferreira GA, Sardenberg WM, Serrano ÉV, Dias CC, Navarro TP, Nordal HH, Mydel PM, Brun JG, Brokstad KA, Gerdts E, Jonsson R, Valim V. Serum calprotectin is a biomarker of carotid atherosclerosis in patients with primary Sjögren's syndrome. Clin Exp Rheumatol 2016; 34: 1006–1012. [PubMed] [Google Scholar]
- 38. Bergum B, Koro C, Delaleu N, Solheim M, Hellvard A, Binder V, Jonsson R, Valim V, Hammenfors DS, Jonsson MV, Mydel P. Antibodies against carbamylated proteins are present in primary Sjögren's syndrome and are associated with disease severity. Ann Rheum Dis 2016; 75: 1494–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jonsson R, Theander E, Sjöström B, Brokstad K, Henriksson G. Autoantibodies present before symptom onset in primary Sjögren syndrome. JAMA 2013; 310: 1854–1855. [DOI] [PubMed] [Google Scholar]
- 40. Theander E, Jonsson R, Sjöström B, Brokstad K, Olsson P, Henriksson G. Prediction of Sjögren's syndrome years before diagnosis and identification of patients with early onset and severe disease course by autoantibody profiling. Arthritis Rheumatol 2015; 67: 2427–2436. [DOI] [PubMed] [Google Scholar]
- 41. Vitali C, Moutsopoulos HM, Bombardieri S. The European Community study group on dianostic criteria for Sjögrens's syndrome. Sensitivity and specificity of tests for ocular and oral involvement for Sjögren's syndrome. Ann Rheum Dis 1994; 53: 637–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bowman SJ, Fox RI. Classification criteria for Sjögren’ syndrome: nothing ever stands still. Ann Rheum Dis 2014; 73: 1–2. [DOI] [PubMed] [Google Scholar]
- 43. Risselada AP, Kruize AA, Goldschmeding R, Lafeber FP, Bijlsma JW, van Roon JA. The prognostic value of routinely performed minor salivary gland assessments in primary Sjogren's syndrome. Ann Rheum Dis 2014; 73: 1537–1540. [DOI] [PubMed] [Google Scholar]
- 44. Theander E, Vasaitis L, Baecklund E, Nordmark G, Warfvinge G, Liedholm R, Brokstad K, Jonsson R, Jonsson MV. Lymphoid organisation in labial salivary gland biopsies is a possible predictor for the development of malignant lymphoma in primary Sjogren's syndrome. Ann Rheum Dis 2011; 70: 1363–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cornec D, Costa S, Devauchelle‐Pensec V, Chiche L, Saraux A, Pers JO. Do high numbers of salivary gland‐infiltrating B cells predict better or worse outcomes after rituximab in patients with primary Sjogren's syndrome? Ann Rheum Dis 2016; 75: e33–e209300. [DOI] [PubMed] [Google Scholar]
- 46. Pijpe J, Kalk WW, van der Wal JE, Vissink A, Kluin PM, Roodenburg JL, Bootsma H, Kallenberg CG, Spijkervet FK. Parotid gland biopsy compared with labial biopsy in the diagnosis of patients with primary Sjögrens syndrome. Rheumatology (Oxford) 2007; 46: 335–341. [DOI] [PubMed] [Google Scholar]
- 47. Sallusto F, Lanzavecchia A. Human Th17 cells in infection and autoimmunity. Microbes Infect 2009; 11: 620–624. [DOI] [PubMed] [Google Scholar]
- 48. Alunno A, Petrillo MG, Nocentini G, Bistoni O, Bartoloni E, Caterbi S, Bianchini R, Baldini C, Nicoletti I, Riccardi C, Gerli R. Characterization of a new regulatory CD4 + T cell subset in primary Sjögren's syndrome. Rheumatology (Oxford) 2013; 52: 1387–1396. [DOI] [PubMed] [Google Scholar]
- 49. Bohnhorst JØ, Bjørgan MB, Thoen JE, Jonsson R, Natvig JB, Thompson KM. Abnormal B cell differentiation in primary Sjögren's syndrome results in a depressed percentage of circulating memory B cells and elevated levels of soluble CD27 that correlate with Serum IgG concentration. Clin Immunol 2002; 103: 79–88. [DOI] [PubMed] [Google Scholar]
- 50. Aqrawi LA, Brokstad KA, Jakobsen K, Jonsson R, Skarstein K. Low number of memory B cells in the salivary glands of patients with primary Sjögren's syndrome. Autoimmunity 2012; 45: 547–555. [DOI] [PubMed] [Google Scholar]
- 51. Salomonsson S, Jonsson M, Skarstein K, Brokstad KA, Hjelmström P, Wahren‐Herlenius M, Wahren‐Herlenius M, Jonsson R. Cellular basis of ectopic germinal center formation and autoantibody production in the target organ of patients with Sjögren's syndrome. Arthritis Rheum 2003; 48: 3187–3201. [DOI] [PubMed] [Google Scholar]
- 52. Jonsson MV, Skarstein K. Follicular dendritic cells confirm lymphoid organization in the minor salivary glands of primary Sjögren's syndrome. J Oral Pathol Med 2008; 37: 515–521. [DOI] [PubMed] [Google Scholar]
- 53. Skarstein K, Aqrawi LA, Øijordsbakken G, Jonsson R, Liaaen Jensen JC. Adipose tissue is prominent in salivary glands of Sjögren's syndrome patients and appears to influence the microenvironment in these organs. Autoimmunity 2016; 49: 338–346. [DOI] [PubMed] [Google Scholar]
- 54. Leehan KM, Pezant NP, Rasmussen A, Grundahl K, Moore JS, Radfar L, Lewis DM, Stone DU, Lessard CJ, Rhodus NL, Segal BM, Kaufman CE, Scofield RH, Sivils KL, Montgomery C, Farris AD. Fatty infiltration of the minor salivary glands is a selective feature of aging but not Sjögren's syndrome. Autoimmunity 2017; 50: 451–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Szyszko EA, Brokstad KA, Oijordsbakken G, Jonsson MV, Jonsson R, Skarstein K. Salivary glands of primary Sjögrens syndrome patients express factors vital for plasma cell survival. Arthritis Res Ther 2011; 13: R2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Aqrawi LA, Liaaen Jensen JC, Øijordsbakken G, Ruus A‐K, Nygård S, Holden M, Jonsson R, Galtung HK, Skarstein K. Signalling pathways identified in salivary glands from primary Sjögren's syndrome patients reveal enhanced adipose tissue development. Autoimmunity 2018; 51: 135–146. [DOI] [PubMed] [Google Scholar]
- 57. Mossel E, Delli K, van Nimwegen JF, Stel AJ, Kroese FGM, Spijkervet FKL, Vissink A, Bootsma H. Ultrasonography of major salivary glands compared with parotid and labial gland biopsy and classification criteria in patients with clinically suspected primary Sjögren's syndrome. Ann Rheum Dis 2017; 76: 1883–1889. [DOI] [PubMed] [Google Scholar]
- 58. Haacke EA, van der Vegt B, Vissink A, Spijkervet FKL, Bootsma H, Kroese FGM. Germinal centres in diagnostic labial gland biopsies of patients with primary Sjögren's syndrome are not predictive for parotid MALT lymphoma development. Ann Rheum Dis 2017; 76: 1781–1784. [DOI] [PubMed] [Google Scholar]
- 59. Alegria GC, Costa S, Jousse‐Joulin S, Marcorelles P, Pers JO, Saraux A, Devauchelle‐Pensec V, Cornec D. What is the agreement between pathological features of parotid gland and labial salivary gland biopsies? Ann Rheum Dis 2017; 77: e37 10.1136/annrheumdis-2017-212289. [DOI] [PubMed] [Google Scholar]
- 60. Jonsson MV, Baldini C. Major salivary gland ultrasonography in the diagnosis of Sjögren's syndrome: A place in the diagnostic criteria? Rheum Dis Clin North Am 2016; 42: 501–517. [DOI] [PubMed] [Google Scholar]
- 61. Hammenfors DS, Brun JG, Jonsson R, Jonsson MV. Diagnostic utility of major salivary gland ultrasonography in primary Sjögren's syndrome. Clin Exp Rheumatol 2015; 33: 56–62. [PubMed] [Google Scholar]
- 62. Kim JW, Lee H, Park SH, Kim SK, Choe JY, Kim JK. Salivary gland ultrasonography findings are associated with clinical, histological, and serologic features of Sjögren's syndrome. Scand J Rheumatol 2018; Feb 7: 1–8. 10.1080/03009742.2017.1374451. [Epub ahead of print] PMID: 29411664. [DOI] [PubMed] [Google Scholar]
- 63. Theander E, Mandl T. Primary Sjogren's syndrome: The diagnostic and prognostic value of salivary gland ultrasonography using a simplified scoring system. Arthritis Care Res 2014; 66: 1102–1107. [DOI] [PubMed] [Google Scholar]
- 64. Shimizu M, Okamura K, Yoshiura K, Ohyama Y, Nakamura S. Sonographic diagnosis of Sjögren syndrome: evaluation of parotid gland vascularity as a diagnostic tool. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2008; 106: 587–594. [DOI] [PubMed] [Google Scholar]
- 65. Jousse‐Joulin S, Devauchelle‐Pensec V, Morvan J, Guias B, Pennec Y, Pers JO, Daridon C, Jamin C, Renaudineau Y, Roué IQ, Cochener B, Bressollette L, Youinou P, Saraux A. Ultrasound assessment of salivary glands in patients with primary Sjögren's syndrome treated with rituximab: Quantitative and Doppler waveform analysis. Biologics 2007; 1: 311–319. [PMC free article] [PubMed] [Google Scholar]
- 66. Jousse‐Joulin S, Devauchelle‐Pensec V, Cornec D, Marhadour T, Bressollette L, Gestin S, Pers JO, Nowak E, Saraux A. Brief report: ultrasonographic assessment of salivary gland response to rituximab in primary Sjögren's syndrome. Arthritis Rheumatol 2015; 67: 1623–1628. [DOI] [PubMed] [Google Scholar]
- 67. Jousse‐Joulin S, Nowak E, Cornec D, Brown J, Carr A, Carotti M, Fisher B, Fradin J, Hocevar A, Jonsson MV, Luciano N, Milic V, Rout J, Theander E, Stel A, Bootsma H, Vissink A, Baldini C, Baer A, Ng WF, Bowman S, Alavi Z, Saraux A, Devauchelle‐Pensec V. Salivary gland ultrasound abnormalities in primary Sjögren's syndrome: consensual US‐SG core items definition and reliability. RMD Open 2017; 3: e000364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Mossel E, Arends S, van Nimwegen JF, Delli K, Stel AJ, Kroese FGM, Spijkervet FKL, Vissink A, Bootsma H, EULAR US‐pSS study group . Scoring hypoechogenic areas in one parotid and one submandibular gland increases feasibility of ultrasound in primary Sjögren's syndrome. Ann Rheum Dis 2018; 77: 556–562. [DOI] [PubMed] [Google Scholar]
- 69. Hocevar A, Ambrozic A, Rozman B, Kveder T, Tomsic M. Ultrasonographic changes of major salivary glands in primary Sjögren's syndrome. Diagnostic value of a novel scoring system. Rheumatology (Oxford) 2005; 44: 768–772. [DOI] [PubMed] [Google Scholar]
- 70. Baldini C, Luciano N, Tarantini G, Pascale R, Sernissi F, Mosca M, Caramella D, Bombardieri S. Salivary gland ultrasonography: a highly specific tool for the early diagnosis of primary Sjogren's syndrome. Arthritis Res Ther 2015; 17: 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Astorri E, Sutcliffe N, Richards PS, Suchak K, Pitzalis C, Bombardieri M, Tappuni AR. Ultrasound of the salivary glands is a strong predictor of labial gland biopsy histopathology in patients with sicca symptoms. J Oral Pathol Med 2016; 45: 450–454. [DOI] [PubMed] [Google Scholar]
- 72. Luciano N, Baldini C, Tarantini G, Ferro F, Sernissi F, Varanini V, Donati V, Martini D, Mosca M, Caramella D, Bombardieri S. Ultrasonography of major salivary glands: a highly specific tool for distinguishing primary Sjögren's syndrome from undifferentiated connective tissue diseases. Rheumatology (Oxford) 2015; 52: 2198–2204. [DOI] [PubMed] [Google Scholar]
- 73. Cornec D, Jousse‐Joulin S, Pers JO, Marhadour T, Cochener B, Boisrame‐Gastrin S, Nowak E, Youinou P, Saraux A, Devauchelle‐Pensec V. Contribution of salivary gland ultrasonography to the diagnosis of Sjögren's syndrome: toward new diagnostic criteria? Arthritis Rheum 2013; 65: 216–225. [DOI] [PubMed] [Google Scholar]
- 74. Cornec D, Jousse‐Joulin S, Marhadour T, Pers JO, Boisrame‐Gastrin S, Renaudineau Y, Saraux A, Devauchelle‐Pensec V. Salivary gland ultrasonography improves the diagnostic performance of the 2012 American College of Rheumatology classification criteria for Sjögren's syndrome. Rheumatology (Oxford) 2014; 53: 1604–1607. [DOI] [PubMed] [Google Scholar]
- 75. Takagi Y, Sumi M, Nakamura H, Sato S, Kawakami A, Nakamura T. Salivary gland ultrasonography as a primary imaging tool for predicting efficacy of xerostomia treatment in patients with Sjögren's syndrome. Rheumatology (Oxford) 2016; 55: 237–245. [DOI] [PubMed] [Google Scholar]
- 76. Le Goff M, Cornec D, Jousse‐Joulin S, Guellec D, Costa S, Marhadour T, Le Berre R, Genestet S, Cochener B, Boisrame‐Gastrin S, Renaudineau Y, Pers JO, Saraux A, Devauchelle‐Pensec V. Comparison of 2002 AECG and 2016 ACR/EULAR classification criteria and added value of salivary gland ultrasonography in a patient cohort with suspected primary Sjögren's syndrome. Arthritis Res Ther 2017; 19: 269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Jousse‐Joulin S, Gatineau F, Baldini C, Baer AN, Barone F, Bootsma H, Bowman S, Brito‐Zeron P, Cornec D, Doerner T, DeVita S, Fisher B, Hammenfors DS, Jonsson MV, Mariette X, Milic V, Nakamura H, Ng W‐F, Nowak E, Ramos‐Casals M, Rasmussen A, Seror R, Shiboski C, Nakamura T, Vissink A, Saraux A, Devauchelle‐Pensec V. Modification of the classification criteria for primary Sjögren syndrome: An International vignette survey. Arthritis Rheumatol 2017; 69: 1286–1288. Abstract Number: 877. [Google Scholar]
- 78. Anaya JM, Ogawa N, Talal N. Sjogren's syndrome in childhood. J Rheumatol 1995; 22: 1152–1158. [PubMed] [Google Scholar]
- 79. Lieberman SM. Childhood Sjogren syndrome: insights from adults and animal models. Curr Opin Rheumatol 2013; 25: 651–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Cimaz R, Casadei A, Rose C, Bartunkova J, Sediva A, Falcini F, Picco P, Taglietti M, Zulian F, Ten Cate R, Sztajnbok FR, Voulgari PV, Drosos AA. Primary Sjögren syndrome in the paediatric age: a multicentre survey. Eur J Pediatr 2003; 162: 661–665. [DOI] [PubMed] [Google Scholar]
- 81. Nikitakis NG, Rivera H, Lariccia C, Papadimitriou JC, Sauk JJ. Primary Sjögren syndrome in childhood: report of a case and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2003; 96: 42–47. [DOI] [PubMed] [Google Scholar]
- 82. Bogdanovic R, Basta‐Jovanovic G, Putnik J, Stajic N, Paripovic A. Renal involvement in primary Sjogren syndrome of childhood: case report and literature review. Mod Rheumatol 2013; 23: 182–189. [DOI] [PubMed] [Google Scholar]
- 83. Fang QG, Liu FY, Sun CF. Recurrent submandibular gland swelling as a first manifestation in a child with primary Sjogren syndrome. J Craniofac Surg 2013; 24: e413–e415. [DOI] [PubMed] [Google Scholar]
- 84. de Souza TR, Silva IH, Carvalho AT, Gomes VB, Duarte AP, Leão JC, Gueiros LA. Juvenile Sjögren syndrome: distinctive age, unique findings. Pediatr Dent 2012; 34: 427–430. [PubMed] [Google Scholar]
- 85. Nieto‐Gonzalez JC, Monteagudo I, Bello N, Martinez‐Estupinan L, Naredo E, Carreno L. Salivary gland ultrasound in children: a useful tool in the diagnosis of juvenile Sjögren's syndrome. Clin Exp Rheumatol 2014; 32: 578–580. [PubMed] [Google Scholar]
- 86. Jonsson MV, Delaleu N, Marthinussen MC, Jonsson R. Oral and dental manifestations of Sjögren's syndrome: current Approaches to diagnostics and therapy In: Fox RI, Fox C, eds. Sjögren's Syndrome: Current Topics of Pathogenesis and Therapy, 1st edn New York: Springer, 2011; 221–242. [Google Scholar]
- 87. Katsiougiannis S, Wong DT. The Proteomics of Saliva in Sjögren's Syndrome. Rheum Dis Clin North Am 2016; 42: 449–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Hu S, Wang J, Meijer J, Ieong S, Xie Y, Yu T, Zhou H, Henry S, Vissink A, Pijpe J, Kallenberg C, Elashoff D, Loo JA, Wong DT. Salivary proteomic and genomic biomarkers for primary Sjögren's syndrome. Arthritis Rheum 2007; 56: 3588–3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Baldini C, Giusti L, Ciregia F, Da Valle Y, Giacomelli C, Donadio E, Sernissi F, Bazzichi L, Giannaccini G, Bombardieri S, Lucacchini A. Proteomic analysis of saliva: a unique tool to distinguish primary Sjögren's syndrome from secondary Sjögren's syndrome and other sicca syndromes. Arthritis Res Ther 2011; 13: R194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Ambatipudi KS, Swatkoski S, Moresco JJ, Tu PG, Coca A, Anolik JH, Gucek M, Sanz I, Yates JR, Melvin JE. Quantitative proteomics of parotid saliva in primary Sjögren's syndrome. Proteomics 2012; 12: 3113–3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Kageyama G, Saegusa J, Irino Y, Tanaka S, Tsuda K, Takahashi S, Sendo S, Morinobu A. Metabolomics analysis of saliva from patients with primary Sjögren's syndrome. Clin Exp Immunol 2015; 182: 149–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Sreebny L, Zhu WX. Whole saliva and the diagnosis of Sjögren's syndrome: an evaluation of patients who complain of dry mouth and dry eyes. Part 2: Immunologic findings. Gerodontology 1996; 13: 44–48. [DOI] [PubMed] [Google Scholar]
- 93. Hu S, Vissink A, Arellano M, Roozendaal C, Zhou H, Kallenberg CG, Wong DT. Identification of autoantibody biomarkers for primary Sjögren's syndrome using protein microarrays. Proteomics 2011; 11: 1499–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Delaleu N, Mydel P, Kwee I, Brun JG, Jonsson MV, Jonsson R. High fidelity between saliva proteomics and the biologic state of salivary glands defines biomarker signatures for primary Sjögren's syndrome. Arthritis Rheumatol 2015; 67: 1084–1095. [DOI] [PubMed] [Google Scholar]
- 95. Delaleu N, Mydel P, Brun JG, Jonsson MV, Alimonti A, Jonsson R. Sjögren's syndrome patients with ectopic germinal centers present with a distinct salivary proteome. Rheumatology (Oxford) 2016; 55: 1127–1137. [DOI] [PubMed] [Google Scholar]
- 96. Delaleu N, Madureira AC, Immervoll H, Jonsson R. Inhibition of experimental Sjögren's syndrome through immunization with HSP60 and its peptide amino acids 437‐460. Arthritis Rheum 2008; 58: 2318–2328. [DOI] [PubMed] [Google Scholar]


