Abstract
Objectives
The Dolutegravir Monotherapy for HIV (DOMONO; NCT02401828) study showed that maintenance monotherapy with dolutegravir (DTG) is associated with virological failure (VF) and leads to DTG resistance and as a result should not be used. However, data on clinical and virological factors associated with VF during DTG monotherapy are lacking. We identified factors associated with VF during DTG monotherapy.
Methods
A randomized trial was carried out in which patients on combination antiretroviral therapy (cART) with an HIV‐1 RNA zenith < 100 000 copies/mL and a CD4 T‐cell nadir ≥ 200 cells/μL, who had never experienced VF, switched to DTG monotherapy. Clinical and virological factors were compared between patients with and without VF, using univariate analyses.
Results
Eight of the 95 patients developed VF during DTG monotherapy. A total of 78 participants had reached week 48 when the study was discontinued. The median CD4 T‐cell nadir was lower in patients with VF than in patients without VF [260 (interquartile range (IQR) 223–320) versus 380 (IQR 290–520) cells/μL, respectively; P = 0.011]. Patients with VF had a longer time between HIV diagnosis and cART initiation than those without VF [median 49 (IQR 27–64) versus 15 (IQR 1–38) months, respectively; P = 0.015]. The median total peripheral blood mononuclear cell (PBMC) HIV DNA copy number was higher in patients with VF than in those without VF [417 (range 85–4151) versus 147 (range 16–4132) copies/106 PBMCs, respectively; P = 0.022].
Conclusions
A lower CD4 nadir, a longer time between HIV diagnosis and cART initiation, and a higher HIV DNA copy number at the time of DTG monotherapy initiation were associated with VF. While there clearly is no future role for DTG monotherapy, ongoing and future studies on the efficacy of maintenance dual therapy (e.g. DTG lamivudine) may have to take these variables into account in their study design and analysis.
Keywords: combination antiretroviral therapy, dolutegravir monotherapy, HIV integrase inhibitors, HIV‐1, virological failure
Introduction
Dolutegravir (DTG)‐based combination antiretroviral therapy (cART) is one of the preferred treatment options in current guidelines for HIV‐1 treatment. Given the high genetic barrier of DTG maintenance monotherapy to resistance based on previously determined in vitro data 1, 2, 3, 4, 5, we studied its efficacy in the Dolutegravir Monotherapy for HIV (DOMONO; NCT02401828) study 6. In DOMONO, 95 virologically suppressed patients on cART, selected on strict criteria regarding CD4 T‐cell nadir and HIV RNA zenith, started DTG monotherapy. The study was discontinued prematurely, because virological failure (VF) was observed in eight patients, of whom three had integrase inhibitor resistance‐associated mutations 6, 7, 8. Previous studies on protease inhibitor (PI) maintenance monotherapy identified time on cART, drug adherence during monotherapy, the presence of very low level viraemia (< 50 HIV‐1 RNA copies/mL) at baseline and CD4 T‐cell nadir as predictors of failure 9, 10. Additionally, the peripheral blood mononuclear cell (PBMC) HIV DNA copy number was associated with the risk of VF in the MONOI study and PROTEA study 9, 11. Predictors of VF during integrase inhibitor monotherapy have not been described. Here, we determined which clinical and virological factors were associated with VF during DTG monotherapy.
Methods
The DOMONO study (NCT02401828) was a randomized clinical noninferiority trial. Participants provided written informed consent, and the study was approved by the ethics committee (METC Erasmus MC; MEC2015‐043) and performed in accordance with the Helsinki Declaration. Details can be found elsewhere, but, in brief, 95 patients who were virologically suppressed on cART, had never failed any antiretroviral regimen, and had a CD4 T‐cell nadir ≥ 200 cells/μL and an HIV RNA zenith < 100 000 copies/mL consented to switch from cART to DTG monotherapy 6. The primary outcome of this study was virological suppression at week 24 during DTG monotherapy, and we defined VF as a confirmed plasma HIV RNA > 200 copies/mL. Clinical and virological factors were compared between patients with and without VF using unpaired t‐tests, Mann–Whitney U‐tests, and Fisher's exact tests, when applicable. As a consequence of the relatively low number of patients with VF at the time when the study was discontinued, a multivariable analysis could not be performed. Factors included were as follows: age, sex, the pre‐cART HIV RNA zenith and CD4 T‐cell nadir, the CD4 T‐cell count at the start of DTG monotherapy, and the time between HIV diagnosis and cART initiation. Other evaluated factors were the type of cART regimen before the switch to DTG monotherapy (nonnucleoside reverse transcriptase inhibitor‐ versus PI‐ versus integrase strand transfer inhibitor‐containing cART), the time on cART, whether the patient had a detectable viral load at the start of DTG monotherapy (defined as an HIV plasma viral load of > 20 copies/mL or an HIV plasma viral load that was detectable but < 20 copies/mL), DTG plasma concentration, and the total HIV DNA copy number in PBMCs at the start of DTG monotherapy. Total HIV DNA quantification was performed by droplet digital polymerase chain reaction (ddPCR), as described elsewhere, and could be carried out in 77 patients (eight patients with VF and 69 without VF) from whom PBMCs had been successfully harvested 12, 13, 14.
Results
A total of Seventy‐eight of the 95 participants had reached the week 48 endpoint when the study was discontinued prematurely in accordance with one of the predefined stopping rules. At the time of study discontinuation, VF had been observed in eight patients. The median follow‐up duration was 59 [interquartile range (IQR) 48–71] weeks and for 17 patients, including five with VF, the follow‐up was < 48 weeks. The characteristics of the patients with and without VF are described in Table 1. According to the inclusion criteria the median HIV RNA zenith was low and the median CD4 T‐cell nadir was relatively high with a minimum of 200 cells/μL. The median CD4 T‐cell nadir was significantly lower in patients with VF [260 (IQR 223–320) cells/μL] than in those without VF [380 (IQR 290–520) cells/μL] (P = 0.011). Also, the median time between HIV diagnosis and cART initiation was longer in patients with VF: 49 (IQR 27–64) months versus 15 (1–38) months for patients without VF on DTG monotherapy (P = 0.015). At the start of DTG monotherapy, no significant differences were observed between patients with and without VF regarding the number of patients with detectable plasma HIV RNA, the CD4 T‐cell count, the CD4:CD8 ratio, or the C‐reactive protein (CRP) concentration. In contrast, the median total HIV DNA copy number in PBMCs at the time of DTG monotherapy initiation differed significantly between the two groups: 147 (range 16–4132) versus 417 (range 85–4151) copies per 106 PBMCs in those without and with VF, respectively (P = 0.022). DTG plasma levels were adequate (i.e. > 0.1 mg/L) in all patients with VF, and no difference in median DTG plasma concentration was observed between the patients with VF and 20 randomly selected patients without VF: 1.70 (range 0.70–2.90) mg/L versus 1.65 (range 0.70–4.50) mg/L, respectively. See Figure 1 for boxplots of CD4 T‐cell nadir, time between HIV diagnosis and start of cART, total HIV DNA copy number in PBMCs and DTG plasma concentration in patients with and without VF.
Table 1.
Baseline characteristics of patients with and without virological failure (VF) during dolutegravir (DTG) maintenance monotherapy, including P‐values for the univariate analysis
| No VF during DTG monotherapy (n = 87) | VF during DTG monotherapy (n = 8) | P‐value (test) | |
|---|---|---|---|
| Age (years) [mean (SD)] | 47 (11.0) | 47 (11.2) | 0.891 (UTT) |
| Male sex [n (%)] | 80 (92) | 8 (100) | 1.00 (FET) |
| HIV RNA zenith (copies/mL) | 37000 (12 950, 65 625) | 27350 (17 750, 64 325) | 0.973 (MWU) |
| Residual Viraemia at start of DTGa [n (%)] | 10 (11.5) | 2 (25.0) | 0.266 (FET) |
| HIV DNA (copies/106 PBMCs) | 147 (69, 338) | 417 (181, 837) | 0.022 (MWU) |
| Log10 HIV DNA (log10 copies/106 PBMCs) [mean (SD)] | 2.16 (0.53) | 2.57 (0.40) | 0.037 (UTT) |
| CD4 T‐cell nadir (cells/μL) | 380 (290, 520) | 260 (223, 320) | 0.011 (MWU) |
| CD4 T‐cell count at start of DTG (cells/μL) | 650 (540, 825) | 830 (573, 1030) | 0.153 (MWU) |
| CD4:CD8 ratio at start of DTG | 1.05 (0.74, 1.50) | 1.41 (0.74, 2.00) | 0.507 (MWU) |
| C‐reactive protein at start of DTG (mg/L) | 1.20 (0.40, 2.70) | 1.45 (0.73, 3.08) | 0.673 (MWU) |
| DTG plasma concentration (mg/L) | 1.65 (1.23, 3.75) | 1.70 (1.05, 2.40) | 0.308 (MWU) |
| % deviation of DTG plasma concentration from population average | 12.9 (−43.2, 55.2) | 10.9 (−27.6, 45.5) | 0.879 (MWU) |
| cART before DTG [n (%)] | |||
| NNRTI | 69 (79.3) | 7 (87.5) | 0.783 (CST) |
| PI | 4 (4.6) | 0 (0) | |
| INI | 14 (16.1) | 1 (12.5) | |
| Time between HIV diagnosis and start of cART (months) | 15 (1, 38) | 49 (27, 64) | 0.015 (MWU) |
| Time suppressed on cART (months) | 31 (20, 54) | 57 (28, 94) | 0.104 (MWU) |
Data shown are median (Q1, Q3), unless otherwise stated.
SD, standard deviation; UTT, unpaired t‐test; FET, Fisher's exact test; MWU, Mann–Whitney U‐test; PBMCs, peripheral blood mononuclear cells; cART, combination antiretroviral therapy; NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor; INI, integrase inhibitor; CST, χ2 test.
Residual viraemia is defined as HIV RNA detectable but < 20 copies/mL or HIV RNA > 20 copies/mL.
Figure 1.
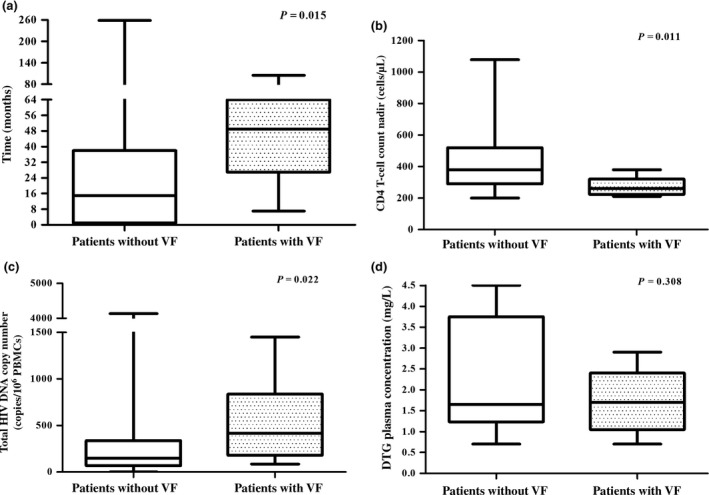
Distributions of (a) time between HIV diagnosis and start of combination antiretroviral therapy (cART), (b) CD4 T‐cell nadir, (c) total HIV DNA copy number in peripheral blood mononuclear cells (PBMCs) and (d) dolutegravir (DTG) plasma concentration in patients without and with virological failure (VF) during DTG maintenance monotherapy. Mann–Whitney U‐tests were used.
Discussion
In the DOMONO study, we clearly showed that DTG maintenance monotherapy is associated with VF and the development of DTG resistance and should not be used as maintenance monotherapy. In the current study, we evaluated potential predictors of VF during integrase inhibitor monotherapy. We showed that a higher cell‐associated total HIV DNA copy number at the start of monotherapy, a lower CD4 T‐cell count nadir, and a longer time between HIV diagnosis and start of cART were significantly associated with VF. A lower CD4 T‐cell count nadir and a higher cell‐associated HIV DNA copy number have previously also been described as risk factors for virological failure during PI monotherapy 9, 10, 11. In contrast, none of the following ten factors were associated with VF during DTG monotherapy: gender, age, CD4 T‐cell count, CRP concentration, CD4:CD8 ratio, type of cART regimen, whether the patient had detectable plasma HIV RNA (all at the time of DTG monotherapy initiation), DTG plasma concentrations during monotherapy, the duration of viral suppression on cART, and the HIV RNA zenith before DTG monotherapy initiation. Also, no differences were observed in plasma viral load detectability in the 12 months preceding DTG monotherapy initiation: three of 87 patients without VF versus zero of eight patients with VF had HIV RNA > 20 copies/mL in the 12 months preceding the switch to DTG monotherapy.
Various PI monotherapy studies identified suboptimal adherence as a risk factor for VF 9, 15, 16. We were unable to analyse adherence as a predictor of VF because the inclusion and exclusion criteria of the study led to the selection of a very therapy‐adherent study population: no history of VF on any previous cART regimen, self‐reported adherence during DTG monotherapy > 95%, and therapeutic DTG plasma concentrations in all patients with and without VF. DTG plasma concentrations were adequate in both groups, and there was no significant difference in DTG plasma concentration between patients with and without VF, which is consistent with previous studies which did not identify lower PI plasma concentrations as a risk factor for VF in patients receiving PI monotherapy 16, 17. It must be noted that drug level measurement was only performed at single time‐points, so the possibility of temporary nonadherence between study visits cannot be ruled out.
A limiting factor of this study is the relatively small number of patients who experienced VF in comparison to the above‐mentioned PI studies. Even if we had considered an isolated and unconfirmed viral load > 50 copies/mL as VF (as observed in 14 patients), the number of patients with VF would have been too small to enable a multivariate analysis to be performed. Therefore, we were not able to assess whether CD4 T‐cell count nadir, time between HIV diagnosis and start of cART, and cell‐associated HIV DNA level are independent risk factors for VF during DTG maintenance monotherapy. Actually, CD4 T‐cell count nadir, time between HIV diagnosis and start of cART, and cell‐associated HIV DNA level could very well be correlated. Indeed, Boulassel et al. 18 previously showed that there was an inverse relationship between CD4 T‐cell nadir and cell‐associated HIV DNA level, and that a longer time between HIV diagnosis and start of cART was associated with higher cell‐associated HIV DNA level 19. This implies that the size of the viral reservoir is probably the most important determinant of VF, as the cell‐associated total HIV DNA level is a measure of the size of the viral reservoir in virologically suppressed patients. The reactivation of HIV from latently infected cells is a stochastic process, which occurs on average every 5–8 days, and depends on the size of the replication‐competent viral reservoir 20. Our observation that a higher HIV DNA level was associated with VF is in agreement with stochastic reactivation of pre‐existing provirus harbouring a single mutation associated with integrase inhibitor resistance. It would have been useful to provide data on the size of the reservoir at the time of VF. Unfortunately, we did not collect PBMCs at the time of VF, and therefore we are not able to provide these data.
In conclusion, a longer time between HIV diagnosis and cART initiation, a lower CD4 count nadir, and a higher total HIV DNA copy number increased the risk of VF during DTG monotherapy. While there clearly is no future role for DTG monotherapy, ongoing and future studies on the efficacy of maintenance dual therapy (e.g. DTG with lamivudine) should take these variables into account in their study design and analysis.
Author contributions
BR and CR designed the study and wrote the protocol. IW, JK, CR and BR contributed to the literature search. IW and BR contributed to the conduct of the study; IW contributed to the preparation of figures and tables; IW, BR, JK, CB, AV, DB, SR and LV contributed to the collection, analysis and interpretation of the data; IW, SR, CR, DB, AV, JK, CB, BR and LV contributed to the writing and review of the manuscript.
Acknowledgements
This work was supported by the Erasmus University Rotterdam Trustfund (grant number 97030.34/15). IW reports a personal fee from Gilead Sciences, outside the submitted work. SR received a strategic basic research fund from Research Foundation – Flanders (FWO, 1S32916N). CR reports a research grant from MERCK outside the context of this work, and personal fees from Gilead and ViiV outside the context of the submitted work. DB, AV and JK have nothing to disclose. CB reports support from Viiv Healthcare outside the submitted work. BR reports grants from the EUR Trust Fund during the conduct of the study, and personal fees and nonfinancial support from ViiV outside the submitted work. LV reports grants from MSD België (Merck) and ViiV Healthcare outside the submitted work.
References
- 1. Cahn P, Pozniak AL, Mingrone H et al Dolutegravir versus raltegravir in antiretroviral‐experienced, integrase‐inhibitor‐naive adults with HIV: week 48 results from the randomised, double‐blind, non‐inferiority SAILING study. Lancet 2013; 382: 700–708. [DOI] [PubMed] [Google Scholar]
- 2. Walmsley S, Baumgarten A, Berenguer J et al Brief report: dolutegravir plus abacavir/lamivudine for the treatment of HIV‐1 infection in antiretroviral therapy‐naive patients: week 96 and week 144 results from the SINGLE randomized clinical trial. J Acquir Immune Defic Syndr 2015; 70: 515–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Molina JM, Clotet B, van Lunzen J et al Once‐daily dolutegravir is superior to once‐daily darunavir/ritonavir in treatment‐naïve HIV‐1‐positive individuals: 96 week results from FLAMINGO. J Int AIDS Soc 2014; 17: 19490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Raffi F, Jaeger H, Quiros‐Roldan E et al Once‐daily dolutegravir versus twice‐daily raltegravir in antiretroviral‐naive adults with HIV‐1 infection (SPRING‐2 study): 96 week results from a randomised, double‐blind, non‐inferiority study. Lancet Infect Dis 2013; 13: 927–935. [DOI] [PubMed] [Google Scholar]
- 5. Marcelin AG, Grude M, Charpentier C et al French national survey of resistance to integrase inhibitors shows high differences of resistance selection rate in case of virological failure in a context of routine hospital care (ANRS‐AC11 virology network). International Congress of Drug Therapy in HIV Infection. Glasgow, UK, 23‐26 October 2016 [Abstract O332].
- 6. Wijting IEA, Rokx C, Boucher CAB et al Dolutegravir as maintenance monotherapy for HIV (DOMONO): a phase 2, randomised non‐inferiority trial. Lancet HIV 2017; 4: e547–e554. [DOI] [PubMed] [Google Scholar]
- 7. Wijting IEA, Lungu C, Rijnders BJA et al HIV‐1 resistance dynamics in patients failing dolutegravir maintenance monotherapy. J Infect Dis 2018; 218: 688–679. 10.1093/infdis/jiy176 [DOI] [PubMed] [Google Scholar]
- 8. Pham HT, Labrie L, Wijting IEA et al The S230R integrase substitution associated with viral rebound during DTG monotherapy confers low levels INSTI drug resistance. J Infect Dis 2018; 218: 698–706. 10.1093/infdis/jiy175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lambert‐Niclot S, Flandre P, Valantin MA et al Factors associated with virological failure in HIV‐1‐infected patients receiving darunavir/ritonavir monotherapy. J Infect Dis 2011; 204: 1211–1216. [DOI] [PubMed] [Google Scholar]
- 10. Gianotti N, Cozzi‐Lepri A, Antinori A et al Refining criteria for selecting candidates for a safe lopinavir/ritonavir or darunavir/ritonavir monotherapy in HIV‐infected virologically suppressed patients. PLoS ONE 2017; 12: e0171611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rutsaert S, De Spiegelaere W, De Clercq L et al HIV DNA as a predictive marker for virologic failure of darunavir/r monotherapy: a substudy of the PROTEA trial to define a cut‐off for success. European aids Clinical Society Conference. Milan, Italy, October 2017 [Abstract PS6/2]. Available at http://resourcelibrary.eacs.cyim.com/mediatheque/media.aspx?mediaId=34850&channel=28172 (accessed 28 May 2018).
- 12. Malatinkova E, Spiegelaere WD, Bonczkowski P et al Impact of a decade of successful antiretroviral therapy initiated at HIV‐1 seroconversion on blood and rectal reservoirs. Elife 2015; 4: e09115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Trypsteen W, Vynck M, De Neve J et al ddpcRquant: threshold determination for single channel droplet digital PCR experiments. Anal Bioanal Chem 2015; 407: 5827–5834. [DOI] [PubMed] [Google Scholar]
- 14. Schvachsa N, Turk G, Burgard M et al Examination of real‐time PCR for HIV‐1 RNA and DNA quantitation in patients infected with HIV‐1 BF intersubtype recombinant variants. J Virol Methods 2007; 140: 222–227. [DOI] [PubMed] [Google Scholar]
- 15. Torres‐Cornejo A, Benmarzouk‐Hidalgo OJ, Gutiérrez‐Valencia A et al Cellular HIV reservoir replenishment is not affected by blip or intermittent viremia episodes during darunavir/ritonavir monotherapy. AIDS 2014; 28: 201–208. [DOI] [PubMed] [Google Scholar]
- 16. Lopez‐Cortes LF, Ruiz‐Valderas R, Sánchez‐Rivas E et al Lopinavir plasma concentration and virological outcome with lopinavir‐ritonavir monotherapy in HIV‐1‐infected patients. Antimicrob Agents Chemother 2013; 57: 3746–3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Campo RE, Da Silva BA, Cotte L et al Predictors of loss of virologic response in subjects who simplified to lopinavir/ritonavir monotherapy from lopinavir/ritonavir plus zidovudine/lamivudine. AIDS Res Hum Retroviruses 2009; 25: 269–275. [DOI] [PubMed] [Google Scholar]
- 18. Boulassel MR, Chomont N, Pai NP, Gilmore N, Sékaly RP, Routy JP. CD4 T cell nadir independently predicts the magnitude of the HIV reservoir after prolonged suppressive antiretroviral therapy. J Clin Virol 2012; 53: 29–32. [DOI] [PubMed] [Google Scholar]
- 19. Avettand‐Fènoel V, Hocqueloux L, Ghosn J et al Total HIV‐1 DNA, a marker of viral reservoir dynamics with clinical implications. Clin Microbiol Rev 2016; 29: 859–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pinkevych M, Cromer D, Tolstrup M et al HIV reactivation from latency after treatment interruption occurs on average every 5‐8 days – implications for HIV remission. PLoS Pathog 2015; 11: e1005000. [DOI] [PMC free article] [PubMed] [Google Scholar]


