Abstract
Organs are complex systems composed of different cells, proteins and signalling molecules that are arranged in a highly ordered structure to orchestrate a myriad of functions in our body. Biofabrication strategies can be applied to engineer 3D tissue models in vitro by mimicking the structure and function of native tissue through the precise deposition and assembly of materials and cells. This approach allows the spatiotemporal control over cell-cell and cell-extracellular matrix communication and thus the recreation of tissue-like structures. In this Review, we examine biofabrication strategies for the construction of functional tissue replacements and organ models, focusing on the development of biomaterials, such as supramolecular and photosensitive materials, that can be processed using biofabrication techniques. We highlight bioprinted and bioassembled tissue models and survey biofabrication techniques for their potential to recreate complex tissue properties, such as shape, vasculature and specific functionalities. Finally, we discuss challenges, such as scalability and the foreign body response, and opportunities in the field and provide an outlook to the future of biofabrication in regenerative medicine.
Tissue engineering and regenerative medicine aim to develop replacement tissues for our body1. Various cell types, biomaterials and stimulatory signals (for example, growth factors and mechanical signalling), either alone or in combination, have been explored for their potential to support tissue repair and regeneration and to recreate the structure and/or function of tissues. Progress in cell and material technologies, such as automation of cell culture, techniques for cell selection and new material formulations for photolithography and bioprinting, has led to the development of more efficient therapies for the repair of simple tissues in the laboratory and in preclinical models. However, self-sustaining solutions that facilitate full tissue integration and homeostasis in a timely manner remain elusive2,3.
Biofabrication technologies enable the fabrication of biological constructs with precise control over the positioning of cells and biomaterials (BOX 1). Bioprinting and bioassembly constitute the two major biofabrication pillars, and various techniques have been developed (FIGS. 1, 2). Bioprinting allows the spatial arrangement of cells, materials and biologically active factors, whereas bioassembly facilitates the automated assembly of cell containing building blocks4. These techniques provide a high level of biomimicry by recreating the complexity of tissues and organs, and they can be upscaled for manufacturing and production. Importantly, biofabrication strategies allow the spatiotemporal modulation of cell-cell and cell-extracellular matrix (ECM) interacttions5,6 through the formulation and use of engineered materials, such as hydrogels, that enable cells to migrate and that can be remodelled for ECM deposition. In bioprinting, the synthetic or natural materials used to recreate tissues are processed together with cells and/or biomolecules and are often termed bioinks.
Box 1 |. Biofabrication.
Biofabrication
In regenerative medicine, biofabrication is the automated generation of structurally organized, biologically functional products from living cells, bioactive molecules, biomaterials, cell aggregates such as microtissues or hybrid cell-material constructs through bioprinting or bioassembly, and subsequent tissue maturation processes4,15.
Bioprinting
Bioprinting is the use of computer-aided transfer processes for the patterning and assembly of living and non-living materials with a defined 2D or 3D architecture to produce bioengineered structures for regenerative medicine, pharmacokinetic and basic cell biology studies. This includes the additive manufacturing of scaffolds designed to control cell activity for tissue repair or regeneration (for example, through hierarchical structure or surface engineering).
Bioassembly
Bioassembly is the fabrication of hierarchical constructs with a defined 2D or 3D organization through automated assembly of preformed cell-containing fabrication units generated through cell-driven self-organization or assembly of hybrid cell-material building blocks, which is typically done by applying microfabricated moulds or microfluidics4,15.
Biomaterials
Biomaterials are used as (part of) a medical device or an advanced medical product to replace, restore or regenerate a tissue or organ and its function15. Biomaterials comprise non-toxic synthetic or natural polymers, such as hydrogels (water-swollen polymer networks), extracellular matrices, shape memory materials, ceramics and metals. If biomaterial properties are designed to modulate cell activity in vitro and in vivo, they are referred to as instructive biomaterials.
Bioinks
Bioinks are biomaterials that are processed by bioprinting and that contain biological molecules and/or cells15.
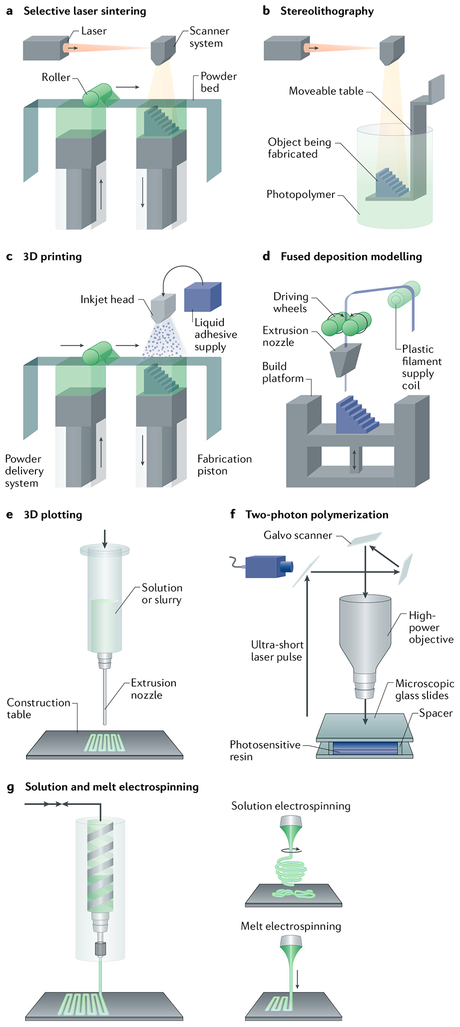
Fig. 1 |. Bioprinting and bioassembly techniques.
a | Selective laser sintering creates scaffolds by scanning a powder bed with a laser beam and by locally sintering the hit grains. b | Stereolithography creates scaffolds by selectively exposing a photopolymer with a light source. c | 3D printing is used to fabricate scaffolds by ejecting a binder onto a powder bed of material. d,e | Fused deposition modelling and 3D plotting fabricate scaffolds by extruding a material (either in filament or pellet form) through a nozzle by pressure. f | Two-photon polymerization is applied to develop scaffolds through focusing a light source on a specific point within a biomaterial. g | Solution and melt electrospinning are used to produce fibrous structures from polymer melts and solutions by applying electric force. Panels a–c and f are adapted from Peltola, S. M. et al. A review of rapid prototyping techniques for tissue engineering purposes, Annals. of Medicine (2008) REF238, by permission of Taylor & Francis Ltd. Panels e and g are adapted with permission from REF15, Elsevier.
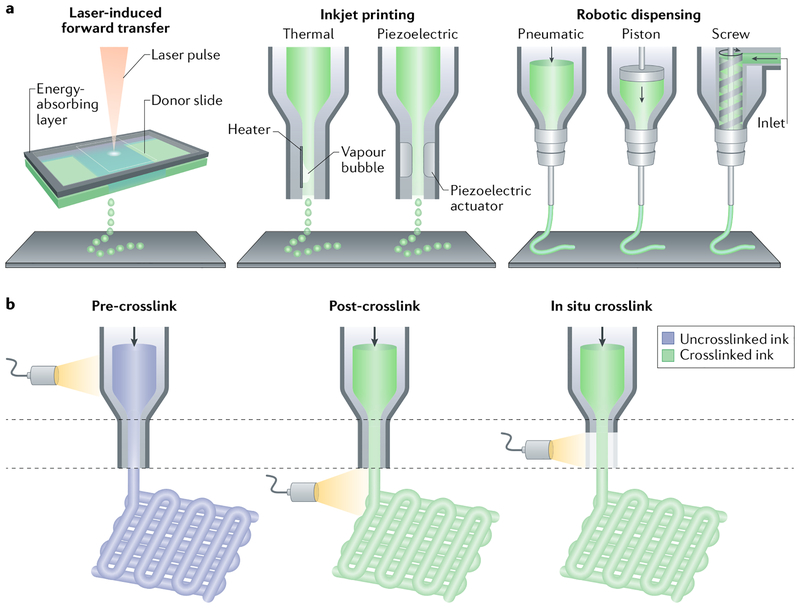
Fig. 2 |. Hydrogel bioprinting.
a | Cell-laden hydrogel scaffolds are created by applying laser light (laser-induced forward transfer) or by extrusion (inkjet printing with or without robotic dispension). b | Light-induced crosslinking strategies for the bioprinting of photocrosslinkable bioinks are shown. Crosslinking can be triggered before (pre-crosslink), after (post-crosslink) or during (in situ crosslink) extrusion. Panel a is adapted with permission from REF239, John Wiley and Sons. Panel b is adapted with permission from REF23, John Wiley and Sons.
High spatial and temporal resolution is important for the fabrication of complex tissues for therapies and for the creation of 3D in vitro models to investigate biological processes7–9. Traditional approaches to fabricating engineered scaffolds, such as porogen leaching or gas foaming, do not allow for the simultaneous incorporation of biologically relevant signals and cells with high spatial control. Biofabrication technologies can be used to pattern cells and materials at such high resolution; however, whole organ regeneration is not yet possible. Better control over the biological processes guiding tissue regeneration needs to be achieved, and materials and technologies need to be developed that can adequately and dynamically replicate these processes at (sub)cellular resolution.
In this Review, we discuss biofabrication technologies in the context of tissue and organ models and implantable constructs. We highlight advances in biomaterials engineering for bioprinting and bioassembly and investigate bioinks for their ability to address challenges regarding compatibility with different printing technologies and functionality.
Bioink development and processing
Biomaterials can be derived from natural or synthetic materials and can be static or dynamic; for example, materials can be biodegradable through hydrolysis or enzymatic degradation. Biomaterials with complex, dynamic functionality can be created using chemistries such as photo-mediated degradation or crosslinking10. High-throughput screening of different material variations11 enables the rapid design of specific material formulations, and various biomaterials are in use as cell-instructive and biomimetic environments for regenerative medicine12–14.
Materials used in biofabrication must meet specific criteria, depending on the respective technique (for example, extrusion or inkjet bioprinting, photopatterning and lithography or formation and assembly of modular components (FiGS. 1, 2, 3)). The spatial resolution of bioprinted structures ranges from the nanometre to the millimetre scale and is dependent on the biofabrication technology15. Biomaterials termed bioinks refer to biomaterials that incorporate cells.
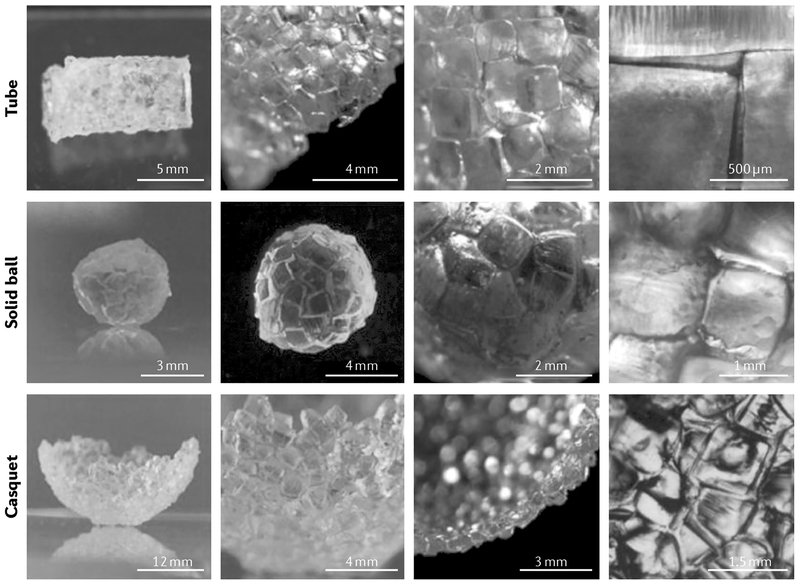
Fig. 3 |. Bioassembly of tissue-like constructs.
Tubular, spherical and casquet-shaped tissue-like structures can be created by automated assembly of cellular spheroids or cell-laden hydrogel building blocks that fuse together because of tissue liquidity principles (cellular spheroids) or secondary interactions (cell-laden microgels). Reproduced with permission from REF207, John Wiley and Sons.
Biomaterials for extrusion bioprinting
Extrusion-based bioprinting is one of the most commonly used biofabrication techniques (FiGS. 1, 2a). In this technique, a material must exhibit steady flow until deposition and must rapidly stabilize upon delivery. Historically, material formulations used in bioprinting have been dominated by materials that rapidly stabilize from a non-viscous state, for example, alginate, which is rapidly crosslinked by calcium ions, and gelatin-methacrylate (GelMA), which crosslinks through cooling or light16–18 (FIG. 2b). Because ofits innate biofunctionality and tunability, GelMA has been used alone or in combination with silk fibroin for the bioprinting of constructs with optimized biophysical properties19,20. Alginate continues to be useful in stabilizing other materials to enable inkjet printing of silk fibroin21,22 or to support heterogeneous, multimaterial extrusion techniques. Modifications to printer hardware, such as photopermeable nozzles and switchable print heads fed from multiple reservoirs, will expand the possibilities for materials23 and complexity22,24 in biofabrication.
Supramolecular materials as bioinks
Materials crosslinked by physical, non-covalent bonds have increased solution viscosity and thus exhibit shear thinning during flow. For example, hydrogen bonds in β-sheets of recombinant spider silk proteins25 or in guest-host interactions of β-cyclodextrin or adamantane in modified hyaluronic acid (HA) polymers26 are broken under shear stress, enabling bioprinting by extrusion, which is followed by rapid stabilization upon deposition. Covalent crosslinking can then be applied to stabilize the structure. Supramolecular DNA hybridization has also been used in combination with inkjet bioprinting to crosslink polypeptides that are functionalized with single-stranded DNA, resulting in a material that can be degraded by either proteases or nucleases27. Alternatively, complementary peptide binding domains can be grafted onto polymers, such as alginate, to maintain a homogeneous cell suspension and to help shield cells from shear stress during the extrusion process. In the case of modified alginate, calcium can provide a stabilizing secondary crosslink upon extrusion28. Finally, bioinks made of short, self-assembling peptides form soft, injectable hydrogels and thus offer possibilities for customized bioprinted constructs owing to their ease of functionalization29.
Hydrogels as support materials
Shear-thinning hydrogels and viscoplastic materials can be used as dynamic support materials for bioprinting. For example, the printed material can be directly deposited into a reservoir of support material30, or alternatively, poloxamers can be extruded to create a pattern of divergent and convergent channels31. Hydrogel inks can also be directly deposited into a gelatin–particle slurry by the use of granular medium that fluidizes upon the movement of the print head. This approach can be applied to support physiological structures with large void spaces, such as heart constructs32 (FIG. 4a). Similarly, jammed carbomer microparticles33 can support the printing of complex structures, such as hierarchical multiscale branching structures, which cannot be bioprinted without support (FIG. 4b). A self-healing support hydrogel can be designed by introducing supramolecular guest–host modifications, which enable rearrangement of the polymeric network and thus adaption to the bioprinted material34. This approach allows the disposition of arbitrary, high-resolution structures in 3D (FIG. 4c). A bisphosphonate-modified HA, crosslinked through coordination with calcium ions, can also serve as support material35.
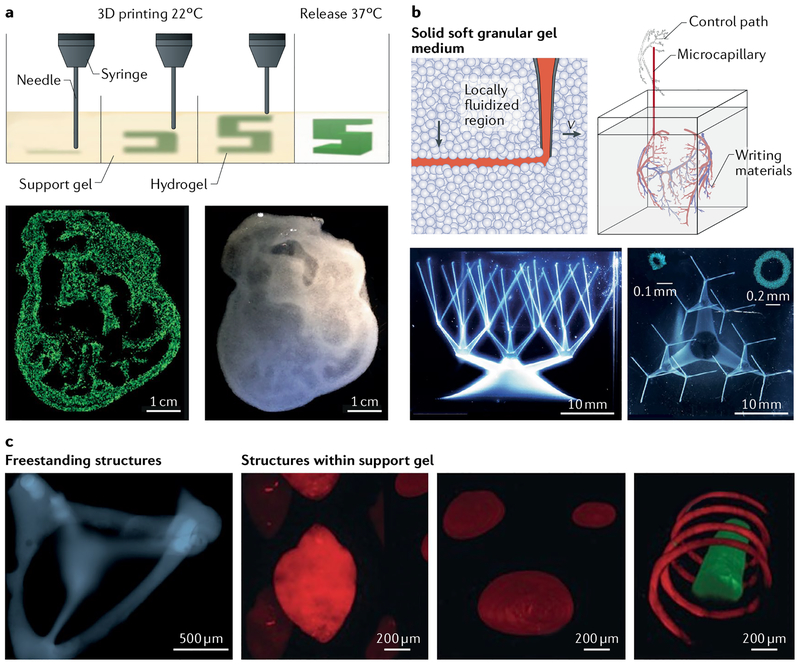
Fig. 4 |. Bioprinting in support materials.
Self-healing hydrogels can be used as support media for bioprinting to enable the 3D fabrication of structures. a | A gelatin slurry can be used for the fabrication of hydrogel structures with large void spaces, for example, heart constructs. The hydrogel (green) is extruded and crosslinked within the gelatin slurry support (yellow). The 3D object is then released through melting of gelatin at 37 °C. Using this method, an embryonic chicken heart can be fabricated on the basis of a 3D computer-aided design model. The bioprinted tissue construct made using fluorescent alginate (green) exhibits the same internal trabecular structure as an embryonic chicken heart. b | A granular medium composed of carbomer microgels enables 3D printing of multiscale hierarchical structures, for example, continuous branched tubular networks of hollow vessels. The network of hollow tubes shown in the microscopy images was printed using polyvinyl alcohol, starting from a 25 mm diameter circular base and tapering to 27 capillaries with a diameter of 100 μm and a wall thickness of 100 μm. The insets show confocal cross sections. c | Hydrogels crosslinked by non-permanent, shear-thinning and self-healing bonds support the printing of high-resolution structures. Support material or printed ink can be removed after processing to produce complex structures that are freestanding or that contain voids and channels. The confocal microscopy images show a freestanding 3D tetrahedron made of photocrosslinked methacrylate-modified hyaluronic acid (blue), a rhodamine-labelled spherical structure (red) in an unlabelled support hydrogel and a fluorescein-labelled filament (green) with a rhodamine-labelled spiral (red) in an unlabelled support hydrogel. Panel a is adapted from REF32, CC-BY-4.0. Panel b is adapted from REF33, CC-BY-4.0. Panel c is adapted with permission from REF34, John Wiley and Sons.
The use of support materials enables the direct patterning of structures and functionalities into hydrogels34,35, the creation of fine channels31–34,36,37 and the deposition of hydrogel structures with large internal voids32–34. Bioprinting into a granular medium allows the fabrication of structures from a range of materials and supports scaffold-free deposition of cells38, facilitating the direct deposition of cell aggregates39. Therefore, the amount of biomaterial required to deposit cells can be reduced to better reflect the architecture of native tissues40. For example, cell-sheet-based bioinks41 enable the bioprinting of cell aggregates to compose dense cellular structures42.
High-viscosity and low-viscosity materials
The deposition of highly viscous, thixotropic43 and non-viscous materials has been facilitated through adaptions in the curing process during bioprinting23. Viscous inks exhibiting kinetics that prevent deposition in the solution phase can be extruded as microparticles, which are cured before deposition. For example, poly(dimethylsiloxane) (PDMS) microspheres have a long cure time. When wetted with a thin layer of uncured liquid precursor, the particles are held together through capillary action, which enables their curing into robust, elastomeric structures43. Low degrees of covalent crosslinking oflow-viscosity solutions before extrusion also facilitate the precise control of rheological properties of bioinks to ensure printability; for example, gelatin and fibrinogen can be crosslinked with an amine-reactive polyethylene glycol (PEG) crosslinker44. The bioprinting process can also be designed to allow photocrosslinking through a transparent nozzle and thus low-viscosity materials can be printed as stable filaments. Light exposure after deposition results in material flow, and light exposure before extrusion leads to material fracture23. This technique enables the bioprinting of bioinks from a wide range of materials commonly used in tissue engineering, such as norbornene-modified HA, GelMA and PEG diacrylate (PEGDA)23.
Photocrosslinkable bioinks
Light-based chemistries allow for the development and spatiotemporal control of materials with dynamic physicochemical properties10,45. Bioinks that react in the presence of light are important for standard additive manufacturing technologies and enable the fabrication of 3D structures through photopatterning. Many photochemical reactions are limited by oxygen attenuation of radicals; however, this issue can be addressed using thiolene photoactivated chemistries46. Allyl-functionalized and thiol-functionalized linear poly(glycidol) combined with a photoinitiator is rapidly crosslinked upon bioprinting in the presence of UV light47, yielding high bioprint fidelity to computer design48. For example, allylated gelatin constitutes a highly tuneable bioink that can be used for a variety of printing methods49. Similarly, the highly specific thiolnorbornene click chemistry reaction can be exploited to crosslink a norbornene-modified poly(glycerol sebacate) as an elastomeric ink50. Thiol–ene crosslinking allows the design of bioprintable hydrogels with dynamic properties, for example, through the incorporation of enzymatically degradable dithiol crosslinks.
Photocrosslinkable bioinks are central to lithography-based biofabrication techniques51 (FiG. 2b). The multiscale capabilities oflithography-based bioprinting enable the printing of materials, such as biocompatible PEGDA and PEG dimethacrylate (PEG-DMA)52, that polymerizethrough a radical chain growth mechanism into single multiscale and multimaterial structures. In this process, oxygen inhibition results in the formation of a thin unreacted layer to which additional material is bound. Similarly, digital light processing can be used to fabricate structures on a cellular scale in PEGDA and GelMA with high fabrication speed53. Digital light processing can also be used for the patterning of high-resolution vasculature using GelMA scaffolds that contain HA54.
Photodegradable materials
In addition to photoactive materials used in additive lithographic approaches, materials can also be designed to locally degrade in response to a light cue, which can be exploited for the spatial control of material dynamics. By directing the focus of a nanosecond pulsed laser to specific volumes of a material, fine channels and micro-fluidic networks can be patterned into hydrogels, which are fabricated from ECM proteins, polysaccharides or synthetic polymeric networks through the physical ablation of covalent bonds55. For example, high-resolution channel structures can be patterned into PEG hydrogels through photolysis of synthetic peptide crosslinks that contain ortho-nitrobenzyl ester functionalities56. Such powerful, high-resolution laser-based patterning methods considerably improve the resolution of biofabrication techniques.
Natural biomaterials
An important area of research is the development of bioinks from natural materials57, such as collagen58,59, decellularized ECM60–62, gelatin23,63–65, alginate66,67, HA23,26,34,35 and silk21,68,69, because of their inherent biocompatibility and the possibility to harvest the biochemical and biophysical cues present in natural cellular microenvironments to control cell behaviour70. Decellularized materials from a variety of tissue types can be formulated as bioinks and deposited using poly^-caprolactone) (PCL) supports61. Biofabrication enables the deposition of natural materials to reproduce the structural and chemical organization of native tissues. However, challenges remain to make natural materials printable and to achieve biologically relevant mechanical properties57.
Cells in bioinks
The term bioink refers to biomaterials that incorporate cells. Bioprinting of materials that contain cells faces several challenges. Stress, such as physical or chemical perturbations that occur during the bioprinting process, may affect cell behaviour and survival. The cell density needs to be sufficiently high to achieve multicellular architectures in the bioprinted material. The incorporation of cells may also alter the properties of the biomaterial, and finally, the material can be toxic for cells.
Cells are typically included in bioinks at concentrations on the order of ten million cells per millilitre; thisconcentration corresponds to approximately ≤5% of the total bioink volume. At these concentrations, the presence of cells has a negligible effect on the rheological properties of the bioink during extrusion71. By contrast, the incorporation of cells affects droplet formation in jetting processes72,73. Bioink properties are also expected to change with increasing cell numbers, which is similar to composite material systems, in which high densities of included cells and particles modify mechanical and rheological properties74,75. Notably, how the nonlinear viscoelastic behaviour of cells76 impacts the properties of bioinks remains elusive.
Bioprinting of cell clusters39,77 and shielding of dense cell populations from shear stress through core-shell flows78 can be applied to increase the viability of cells in a bioprinted construct. Cell viability can also be improved by decoupling the cells from the bioink, for example, through the use of microspheres as cell carriers79 or the encapsulation of cells in protective microgels80. Ultimately, both bioink formulations and biofabrication processes need to be tailored to maintain cell viability to yield high densities of viable cells.
In vitro 3D models
In vitro 3D tissue models offer the opportunity to investigate the safety and efficacy of biochemical agents, for example, for drug development, and to model biological processes, such as tissue development and disease. Numerous normal and diseased 3D tissue models have been developed, such as cellular spheroids, cell-laden hydrogel constructs, miniorgans and microfluidic organs-on-a-chip81,82; for example, in vitro models containing perfused microfluidic chambers and one cell type have been used. More complex systems can include multiple cell types organized along a porous membrane with integrated microchannels. However, most in vitro 3D models created by traditional methods such as biomedical microelectromechanical systems cannot recreate the dynamic, multicellular, spatially and functionally complex microscale architecture of tissues and organs83. To create a physiologically relevant 3D model platform, it is important to build tissue-like or organ-like miniatures that have similar structural and functional characteristics to native tissues.
Bioprinted in vitro tissue models
Skin.
Bioprinting strategies can be employed to re-create multilayered skin tissue84–86. Layers of keratinocytes and fibroblasts can be bioprinted to construct a bioengineered skin tissue composed of epidermis and dermis, which can be used as an in vitro skin model. For example, a collagen hydrogel containing fibroblasts can be bioprinted, and melanocytes and keratinocytes can be sequentially deposited on top of the fibroblast layer to mimic the architecture of native skin84. A 3D human skin wound model can be engineered through the bioprinting of multilayered skin tissues on a non-planar PDMS surface86. Such in vitro skin models can be used to study skin corrosion, irritation, permeability and the safety of chemical compounds.
Liver.
Bioprinted hepatic models can serve as a platform for the investigation of physiological phenomena in the liver and for the accurate prediction of drug and toxic responses9,83,87–89. A liver micro-organ chamber device can be engineered by bioprinting a hepatocyte-laden alginate hydrogel in a microfluidic chamber, thereby creating a physiologically relevant pharmacokinetic model. This device can be operated at continuous perfusion flow while maintaining cell viability and hepatocyte-specific functions such as albumin and fibrinogen production. A human induced pluripotent stem cell (hiPSC)-laden alginate bioink can be bioprinted into a 3D mini-liver by the use of a dual-head jetting bioprinter7. The hiPSCs in the bioprinted construct were differentiated into hepatocyte-like cells, which expressed hepatocyte-specific markers and secreted albumin. The function of 3D liver models can be evaluated through the analysis of protein synthesis (for example, fibrinogen and prothrombin), bile acid synthesis for digestion and the transformation of carbohydrates to fatty acids90–92.
Lung.
In vitro 3D lung models have been considered for high-throughput screening and drug discovery93,94. A human in vitro air–blood barrier model, composed of three layers of endothelial cells, basement membrane and lung epithelial cells, can be fabricated using an extrusion-based bioprinter95. The 3D bioprinting process enables the construction of very thin and uniform layers of cells and Matrigel (as the basement membrane), which resemble the physiology and function of native lung tissue. A more realistic lung model can be achieved through introducing simulated physiological breathing motion by cyclic mechanical strain96.
Heart.
Multimaterial bioprinting of sacrificial dextran, flexible thermoplastic polyurethane (TPU), conductive carbon black nanoparticles (CB) and PDMS allows for the creation of a cardiac microphysiological device5. In this device, TPU microfilaments guide car diomyocyte alignment, and the deposited conductive CB-TPU composite is able to measure tissue contraction. Functional assessment of the in vitro cardiac tissue model includes cardiac cell synchronization, beating behaviour, electrophysiological properties and contractile force measurement97,98.
Kidney.
An in vitro model of the human proximal tubule interstitial interface can be fabricated by bio printing of renal fibroblasts, endothelial cells and primary human renal proximal tubule epithelial cells99. The in vitro proximal tubule tissue can be used to study the mechanisms of nephrotoxicity and to investigate epithelial–interstitial interactions involved in kidney pathogenesis.
Body-on-a-chip.
Body-on-a-chip (or human-on-a-chip) devices aim to integrate multiple human tissue models within microfluidic devices100 to mimic human physiology. Bioprinting strategies101 can contribute to the development of such devices, for example, by providing a miniature 3D heart model that actively pumps fluid through the entire system and/or a 3D lung model to oxygenate, a 3D liver model to metabolize and a 3D kidney model to purify the circulating blood substitute.
Cancer models.
In vitro 3D tumour models, such as cancer cell spheroids, are frequently used for therapeutic screening. Cellular spheroids mimic the cell-cell and cell-matrix interactions in the tumour microenvironment102,103. However, such models do not recapitulate all aspects of the complex tumour microenvironment, such as the associated vasculature and neural network. Therefore, bioprinting technology can be employed to create multicellular, controllable and reproducible tumour models. For example, a 3D cervical tumour model can be fabricated by the extrusion printing of HeLa cells (a human cell line derived from cervical cancer tissue)104. Bioprinted cancer cells form spheroids in the 3D bioprinted microenvironment and exhibit high chemoresistance. A bioprinted ovarian tumour model that features a multicellular acini structure consisting of human ovarian cancer cells and normal fibroblasts can be engineered using inkjet bioprinting. This technique allows for precise control of cell density, droplet size and the spatial distance between the droplets. The bioprinted ovarian tumour model has been applied for high-throughput screening105. A breast tumour model can be created by the direct bio printing of cell spheroids composed of breast cancer cells in the core, mammary fibroblasts and adipose cells into multi-well plates106. Such bio printed tumour models provide an in vitro tool for the development of anticancer therapeutics.
Bioassembled in vitro tissue models
Bioassembly involves the integration of various-shaped cellular building blocks to reconstruct organomimetic macroscopic cellular tissues. Cell-laden hydrogels are commonly used as building blocks because their shapes can be varied using microfluidic and microfabrication techniques. Hydrogel-based building blocks are categorized into point-shaped, line-shaped and plane-shaped cell-laden structures107.
Point-shaped structures.
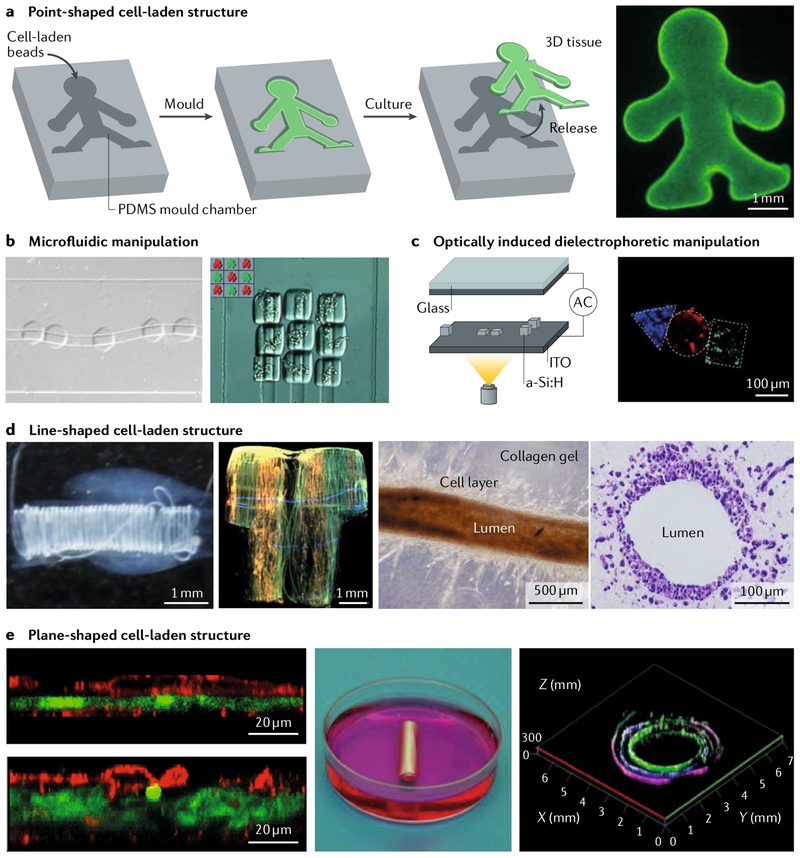
Point-shaped cell-laden structures are easily prepared by culturing cells with hydrogel beads made of alginate108,109, PEG110,111 or collagen112,113. Such structures are fabricated using microfluidic devices with T junction, flow-focusing and nozzleshaped microchannels. Moulding is a popular method for the assembly of cell-laden beads. In this method, the beads are packed into a mould and integrated through cell adhesion, resulting in the construction of millimetre to centimetre-sized tissues with specific shapes defined by the shape of the mould108,111,112,114,115 (FIG. 5a). Moulding of point-shaped cell-laden structures has been applied for the construction of hepatic tissues112, connective tissues112,115 and neural tissues114, for biological analyses in 3D culture conditions (for example, albumin secretion from hepatic tissue) and to investigate cell-cell interactions (for example, 3D neural networks). Organoids are point-shaped cell-laden structures that contain cellular aggregates of patient derived stem cells and possess diameters up to several millimetres. Organoids are promising macroscopic models for the physiologically relevant reconstruction of diseases116, and their assembly will be facilitated by bioassembly using moulds. Point-shaped cell-laden structures that contain different types of cells can be precisely arranged within macroscopic tissues by applying microfluidic and dielectrophoretic forces in microchannels in a high-throughput manner. This approach enables the spatial control of co-culture of different tissues117,118 (FIG. 5b,c).
Fig. 5 |. Bioassembly of macroscopic tissue structures.
a | Cell-laden beads are assembled by moulding in poÌy(dimethyÌsiÌoxane). The microscopy image shows a human doll-shaped tissue made of fluorescently labelled fibroblasts (green) and collagen beads. b | Point-shaped cell-laden structures containing human epithelial cells transfected with green fluorescent protein and human embryonic kidney cells transfected with red fluorescent protein can be delivered and subsequently assembled by microfluidic flow, c | Optically induced dielectrophoretic force-based manipulation for the assembly of point-shaped cell-laden structures can also be used. The device consists of a top glass substrate with transparent and conductive indium oxide (ITO) coating, a working chamber and a bottom ITO glass substrate coated with a thin photoconductive hydrogenated amorphous silicon (a-Si:H) film. The microscopy image shows assembled point-shaped structures containing fibroblasts (green), human embryonic kidney cells (blue) and human metastatic mammary carcinoma cells (red). d | Assembly of line-shaped cell-laden structures is also used. Helical tubes are formed by reeling of a hepatocyte-laden and a fibroblast-laden fibre with a rod. A T-shirt-shaped structure is formed by weaving cell-laden fibres with fibroblasts (green), hepatocytes (red) and small lung carcinoma cells (blue). Blood vessellike structures can be fabricated by dissolving smooth muscle cell-laden and endothelial cell-laden alginate gel fibres in a collagen block. e | Assembly of plane-shaped cell-laden structures is also used. Cell-laden sheets are stacked by sandwiching a hepatocyte-laden sheet (green) between endothelial cell-laden sheets (red). Tubular structures are created by rolling of a cell-laden sheet containing endothelial cells (green), smooth muscle cells (blue) and fibroblasts (magenta). The tubular structure has multiple cell layers. Panel a is reproduced from REF117, Macmillan Publishers Limited. Panel b is adapted with permission from REF118, John Wiley and Sons. Panel c is adapted from REF121, Macmillan Publishers Limited. Panel d is adapted with permission from REF123, American Chemical Society Panel e is adapted with permission from REFS128,129, John Wiley and Sons.
Line-shaped structures.
Line-shaped cell-laden structures, such as cell-laden fibres and tubes, are formed using laminar flow and nozzle-shaped microchannels, alginate119,120 and/or collagen121,122. The assembly of cellladen fibres and tubes is facilitated through rotation of rods or plates at the outlet of the microchannels, allowing the reeling of fibres and tubes120,121. Using the reeling method, the different cell-laden fibres can be arranged on support structures in 3D (FIG. 5d). Alternatively, weaving can be applied without support materials121. Using the weaving method, centimetre-sized 3D cellular tissues can be constructed on the basis of precisely arranged cell-laden fibres. For example, hepatic tissues can be formed through the reeling and weaving of hepatocyte-laden fibres and fibroblast-laden fibres. Such 3D co-culture conditions promote cellular functions (for example, albumin secretion). Furthermore, cell-laden fibres and tubes can be used to create lumen structures by embedding vascular endothelial cells in the fibres and tubes. The vascular channels, formed by the endothelial cells, provide nutrients and oxygen123,124 (FIG. 5d). The mechanical flexibility of cell-laden fibres and tubes allows for the formation of wavy-shaped vascular channels at arbitrary locations in the tissue. Thus, line-shaped cell-laden structures can be used as cellular building blocks for the construction of large-scale, 3D, vascularized tissues.
Plane-shaped structures.
Plane-shaped cell-laden structures, for example, cell-laden sheets, can be fabricated using temperature-responsive culture dishes, microfluidic flat channels or sacrificial layers125–127. Stacking enables the assembly of cell-laden sheets, for example, to produce macroscopic hepatic tissues, in which hepatocyte-laden sheets are sandwiched between endothelial-laden sheets128 (FIG. 5e). Alternatively, rolling can be used to form tubular tissues. A cellular sheet containing precise arrangements of endothelial cells, smooth muscle cells and fibroblasts can be rolled up into a tube, resulting in a millimetre-sized hierarchical model of a vascular tube129,130 (FIG. 5d). Such plane-shaped cell-laden structures can be applied for the construction of the cellular tissues with simple shapes.
Tissue and organ regeneration
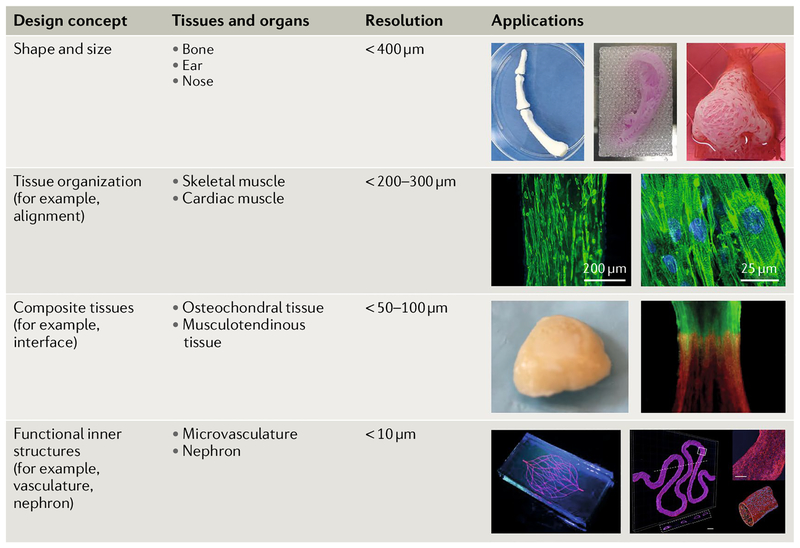
Biofabrication strategies can be applied to create clinically applicable tissue constructs that can be implanted in the body. Biofabrication has the potential to engineer heterogeneous tissue structures, including shape-based tissues such as bone, cartilage, skin and cornea; organized tissues such as skeletal muscle and cardiac and neural tissues; composite tissues such as osteochondral and musculotendinous tissues; and whole organs with vasculature and functional inner structures such as the kidney and heart (FIG. 6). Biofabrication offers the opportunity to reconstruct the structural and ultimately the functional complexity of human tissues through incorporating materials, cells, biochemical and biophysical cues to specifically design tissue shape, organization, structure and integration.
Fig. 6 |. 3D bioprinting of tissues and organs.
Biomedical applications based on design concept and printing resolution. Constructs of various shapes and sizes can be made: human-scale bone, ear-shaped and nose-shaped structures can be fabricated. At the level of tissue organization, cellular alignment can be achieved for skeletal and cardiac muscle constructs. Composite tissues, such as osteochondral (bone–cartilage) and musculotendinous (muscle–tendon), can be fabricated by sequentially patterning multiple components. Functional inner structures, such as microvasculature and nephrons, are required for whole organ bioprinting. The ear and skeletal muscle images are reproduced from REF138, Macmillan Publishers Limited. The osteochondral tissue image is reproduced by permission from authors Francois Berthiaume and Jeffrey Morgan, 3D tissue engineering, Norwood, MA: Artech House, Inc., (2010). Copyright 2010 by Artech House, Inc. The musculotendinous tissue image is reproduced with permission from REF163, IOP Publishing. The microvasculature image is reproduced with permission from REF31, John Wiley and Sons. The nephron image is reproduced from REF8, CC-BY-4.0.
Shape
Additive manufacturing technologies, such as direct metal laser sintering or electron beam melting, are already clinically in use, for example, for the fabrication of patient-specific metal implants131. Bioprinting technologies can be used to engineer patient-specific implants, for example, for bone grafting, and can take into account anatomic differences, defect size and patient-specific morphology of bone pathologies132–134. Furthermore, medical imaging techniques, such as computed tomography and magnetic resonance imaging can be applied to inform the customized design of personalized engineered tissues, for example, to fabricate bone constructs composed of osteoconductive materials such as hydroxyapatite and β-tricalcium phosphate as well as osteogenic cell types135–138. Similarly, heart valves, intervertebral discs and menisci can be constructed using biofabrication techniques139–141. The possibility to design personalized constructs constitutes a major strength of biofabrication technologies.
Current cartilage tissue engineering approaches can only partially recreate healthy and functional cartilage142 owing to difficulties in engineering the zonal differences in articular cartilage that have distinct cellular compositions143. Bioprinting can be applied to fabricate stratified cartilage constructs by regenerating the patient-specific size and shape of individual lesions144–148. Bioprinted cartilage exhibits similar biomechanical and biochemical properties to native cartilage and is well integrated with surrounding cartilage tissue as assessed by ECM deposition in vitro146. Auricular cartilage, for example, as found in the ear, can be reconstructed by an extrusion-based bioprinting process using sodium alginate, silver nanoparticles and chondrocytes arranged in an ear-shaped geometry around a conductive, sound-translating coil. This bionic ear can translate sound waves into an electrical output149. Furthermore, an extrusion-based bio printing system has been applied to fabricate a human ear-shaped cartilage tissue construct138. The shape of the ear was well maintained for 2 months in a subcutaneous mouse model, as confirmed by glycosaminoglycan and collagen type II expression. Alternatively, chondrocyte-laden alginate beads can be used for the construction of cartilage tissues. For example, chondrocyte-laden beads that are assembled with the shape of the cartilage defects in the knee can serve as grafts150,151. Skin is composed of thin layers of epidermis and dermis. In situ 3D bioprinting approaches can be applied to construct tissue replacements for large-scale skin wounds and burns, which have been successfully tested in mice152. In this approach, amniotic fluid-derived stem cells in a fibrinogen and collagen hydrogel are bioprinted over the wound area by the use of extrusion-based printing. This in situ skin bioprinting approach delivers cell-laden hydrogels directly onto the wound, achieving uniform wound coverage, thus providing an effective treatment for large-scale skin wounds.
Cornea also has a plane-shaped tissue structure composed of three layers: epithelium, stroma and endothelium. Corneal epithelial disorders caused by severe disease or trauma can result in corneal opacity and thus loss of visual acuity. Corneal epithelial cell sheets prepared by culturing limbal stem cells on a temperature-responsive culture dish can be directly placed onto the damaged tissue to reconstruct the corneal epithelium and to recover the transparency of the cornea153,154. Alternatively, oral mucosal epithelial cell laden sheets can be used for the treatment of bilateral corneal deficiency153,154.
Tissue organization
Skeletal muscle accounts for ~40% of the human body weight155 and is composed of highly aligned muscle fibres. Fibre organization is essential for muscle contraction and force generation156. Extrusion-based printing can be applied to recreate the spatial organization of skeletal muscle fibres through the fabrication of micrometre-scale muscle-like bundles (~400 μm in diameter)138. The bioprinted muscle construct then matures into functional muscle tissue once integrated with the host nerves in vivo.
Similarly, cardiac tissue features complex myocardial organization to enable contractility97. Laser-induced forward transfer cell bioprinting can be applied to construct a cardiac patch exhibiting spatially organized patterns of human mesenchymal stromal cells (hMSCs) and endothelial cells on a poly(ester urethane) urea matrix157. Implantation of the cardiac patch into an infarcted rat heart promotes vascularization and improves cardiac function. A bioprinted, porous half heart structure containing primary feline adult and H1 cardiomyocytes in alginate can be produced using a modified inkjet printing method158. The deposited cells retain their viability in the tissue construct and exhibit contractility upon electric stimulation in vitro. Human cardiac-derived cardiomyocyte progenitor cells can also be bioprinted, resulting in ~90% cell viability and cardiac tissue maturation for 7 days in culture159. Multiple cell types, including hiPSC-derived cardi-omyocytes, human smooth muscle cells and human endothelial cells, can be seeded into a bioprinted uniaxially oriented gelatin scaffold to produce an hiPSC-derived cardiac muscle patch160. Similarly, bioassembly approaches can be applied for cardiac regeneration, for example, cardiomyocyte-laden, skeletal myoblast-laden or hMSC-laden sheets can be implanted into damaged rat hearts to improve cardiac performance153.
Composite tissues
Simple-shaped tissue constructs offer the opportunity to replace parts of damaged tissue. However, such engineered scaffolds cannot recreate complex composite tissue types161. An anatomically correct osteochondral tissue construct composed of PCL and hydroxyapatite can be engineered using bioprinting. The bioprinted osteochondral tissue can be implanted to repair the entire articular surface of a synovial joint in a rabbit model162. The regeneration of musculotendinous tissue represents a different challenge because of its mechanical function. To recreate the composite nature of the tissue and to enable mechanical function, four different tissue components are bioprinted into an integrated muscle–tendon unit (MTU) construct163. The MTU construct is composed of mechanically heterogeneous polymeric materials that are elastic on the muscle side and stiffer on the tendon side. Additionally, cells are distributed in a tissue-specific manner, with myoblasts on the muscle side and fibroblasts on the tendon side. The cells maintain high cell viability and orientation and express genes associated with musculotendinous junctional tissue in vitro, demonstrating the possibility to bioprint a 3D heterogeneous tissue construct with localized biological and biomechanical characteristics.
Inner structures
The incorporation of microvascular networks and functional inner structures in bioengineered constructs is crucial for whole organ bioprinting164. The limit of oxygen and nutrient diffusion for cells to survive in vivo is 100–200 μm (REFS165–167). Building a functional vasculature within 3D tissue constructs remains challenging. Microtubular structures can be created using micro-fluidics and patterns to guide tissue invasion and vascularization in vivo168,169. Bioprinting can be employed to create microchannels that contain layers of endothelial cells. A sacrificial material, such as carbohydrate glass, can be used to provide a template for printing. Once removed, a microchannel remains, which mimics vascular tissue; however, this tissue is limited in size owing to difficulties associated with direct perfusion36.
Alternatively, 3D tissue constructs can be prefabricated with vasculature and printed using multiple cell types and ECM proteins170,171. Human microvascular endothelial cells self-align inside printed biomaterial-based microchannels and form a confluent microvascular lining. Engineered vascular tubes fabricated by bioassembly of cell-laden sheets have been used for arterial bypass through anastomosis of the tubes172. However, connecting the vasculature to the host circulatory system remains a challenge173.
Functional inner structures, such as the nephron and hepatic lobules, can also be fabricated by bioprinting. For example, 3D human renal proximal tubular structures containing proximal tubular epithelial cells can be generated8. The tubule-like structure is circumscribed by proximal tubule epithelial cells and actively perfused through the open lumen. The bioprinted epithelial barrier can be disrupted by introducing nephrotoxin or ciclosporin A.
The integration of nerves is essential to render bio-engineered tissues functional in vivo. Neuron-laden collagen fibres can be used for the formation of neural tissues exhibiting pathways of aligned neurons in spatially distinct areas174. The neuron-laden collagen fibres can then be used to connect different brain regions, for example, the hippocampal-prefrontal and visual pathways.
Tissue encapsulation
Advances in biofabrication techniques and materials science have enabled the fabrication of complex tissue structures in vitro; however, translation to the clinic still faces challenges, such as the fibrotic encapsulation of implanted constructs due to the foreign body response, which results in protein deposition and often failure of the implant. Alginate-based and PEG-based hydrogels have the potential to mitigate the foreign body response175–177. These hydrogels are semipermeable with a diffusion cut-off that shields the implant from the immune cells but enables nutrients, waste and cell-secreted products to pass through; for example, embedded hydrogel beads can be simply prepared and injected. Beads with diameters >1.5 mm (REF178) or triazole–thiomorpholine dioxide modifications of alginate enable the suppression of the host immune response176,179.
Using this method, cells in the islets of Langerhans, which secrete insulin in response to the glucose concentration in the blood, can be combined with hydrogel beads and implanted to replace diabetic pancreatic islets177–179. The hydrogel beads are used to protect the islet cells from the host immune response, and thus the tissue constructs maintain viable and secrete insulin for a long period of time in the body of the patient, even in case of xenotransplantation180,181. Alginate beads can also be integrated with hepatic tissue and implanted to provide continuous secretion of albumin in the body182,183. Alternative to beads, alginate gel fibres and sheets containing pancreatic tissue have been proposed as retrievable grafts owing to their single-unit and connected structures121,122,184,185. Pancreatic islets can also be combined with alginate gel fibres and sheets and can secrete insulin to regulate blood glucose levels.
Challenges and opportunities
Conventional bioprinting and bioassembly spur from additive manufacturing and self-assembly processes15. Despite the many advantages, there are still a few limitations that need to be addressed to achieve complex tissue regeneration and ultimately organ regeneration.
Tissue complexity
Progress in software design to control additive manufacturing systems has led to the implementation of scripts to calculate complex pore network architectures and multimaterial deposition. However, the majority of fabricated biological constructs are still characterized by simple architectures, which do not resemble the complexity of human tissues. Complex pore network architectures enable the recreation of functional mechanical properties, mimicking the mechanical behaviour of human tissues. For example, structures with variable Poisson ratios186 lead to better vascular tissue regeneration than constructs with homogeneous contraction:relative expansion ratios54·187. Such engineered structures could be designed to enable large deformations188, storage of energy189 or magnetic responsiveness190, endowing tissues with extended functionalities. Moreover, modelling can be applied to predict and design network organization of tissues and organs; for example, the vascular tree network can be modelled191,192 on the basis of theoretical cell aggregate fusion mechanisms193,194. Informing the experimental design of a given tissue construct through computational modelling will certainly improve the properties and function of biofabricated tissues.
Scalability and manufacturing time
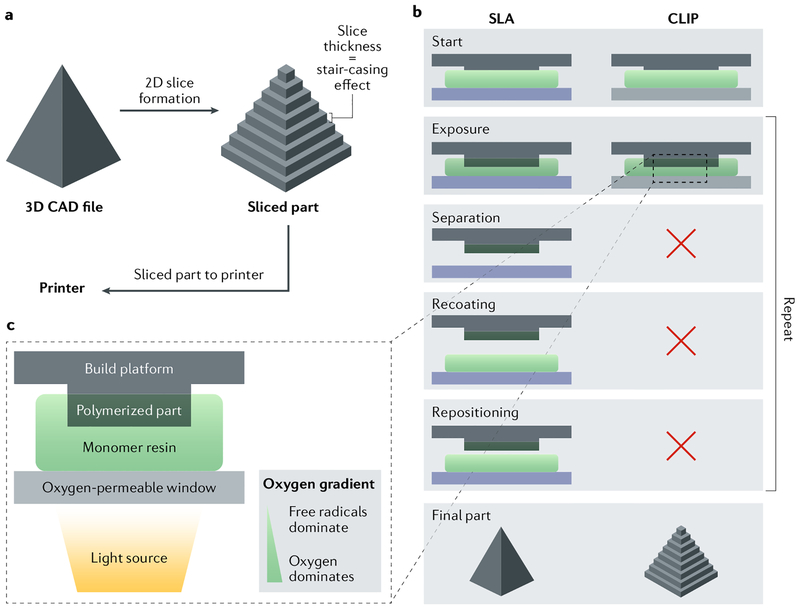
Scalability and long manufacturing times of bioprinted constructs remain issues for clinical translation. Vat photopolymerization printing methods, such as ste reolithography, digital light processing and continuous liquid interface production (CLIP), are additive manufacturing technologies based on photosensitive materials, which enable shorter lead fabrication times than other biofabrication technologies195–200. For example, CLIP allows for the fabrication of cubic centimetresized objects in minutes by controlling the amount of oxygen present at the interface between the photosensitive polymer and the light projector (FIG. 7). Therefore, the printing process is dependent only on the curing rate and viscosity of the polymer. The development of biomedical-grade photopolymers will pave the way for the bioprinting of large biological constructs using these additive manufacturing technologies.
Fig. 7 |. Stereolithography and continuous liquid interface production.
a | A 3D computer-aided design (CAD) file is first created for a given structure and then sliced. b | Continuous liquid interface production (CLIP) requires fewer steps than stereolithography (SLA) to assemble the designed structure. c The fabrication process includes placement of the build elevator on the resin, subsequent UV exposure to selectively cure the resin, separation of the cured resin from the oxygen-impermeable window, mechanical recoating of the resin and, finally, repositioning of the build elevator to repeat the process until the part is fully printed. CLIP uses a constant liquid interface enabled by an oxygen-permeable window, which eliminates the need for the last three steps. Adapted with permission from REF197, Proceedings of the National Academy of Sciences.
Levitation
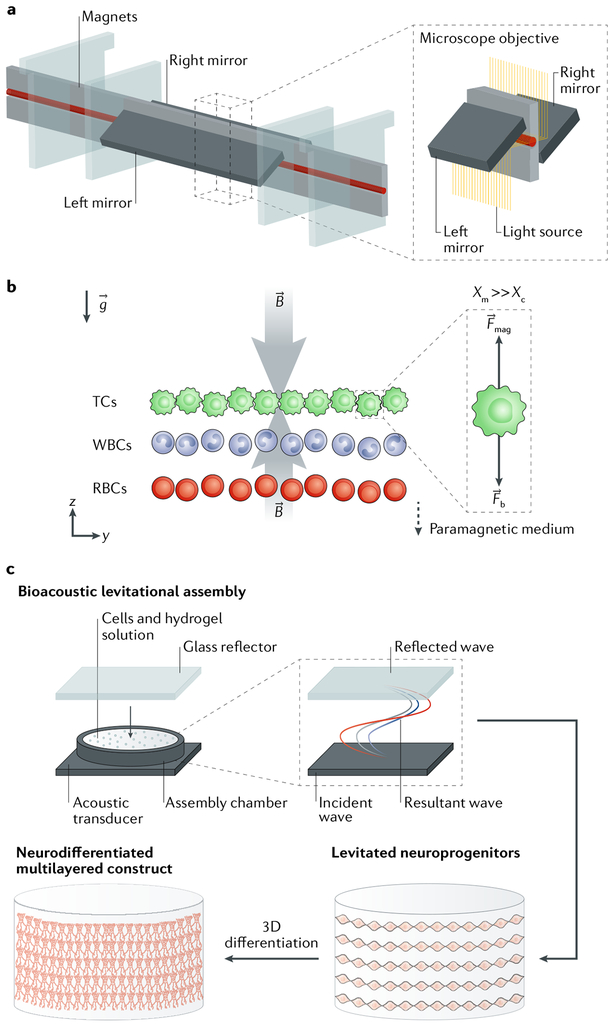
Bioacoustic levitation can be used to assemble cell laden constructs at high speed (FIG. 8). Bioacoustic printing enables the patterning of cell-laden hydrogels by applying Faraday waves201. The waves, when coherently interfering with each other, can be used to initiate cell levitation in a resonant chamber containing a cell-laden hydrogel, enabling the deposition of cell layers in <10 seconds and thus the fabrication of constructs with high cell density (FIG. 8a). The deposited cells remain viable and proliferate for up to 7 days. However, fabrication times are an order of magnitude slower than those of conventional bioprinting technologies202. Moreover, bioacoustic levitation is limited by the use of one single cell population and has thus far been used only with fibrin hydrogels201. Alternatively, magnetic levitation assembly can be used to manipulate different cell types203. Using this method, cells can be sorted by specific density in 3D through levitation in an equilibrium plane between magnetic and buoyancy forces. Cells remain viable for up to 5 days in vitro following magnetic levitation sorting. Levitation technologies could potentially be extended to enable the manipulation of hydrogels, which, in combination with other bioprinting approaches, would facilitate the fabrication of integrated systems, such as gel-in-gel and nanocolloidal systems32,33. However, validation of levitation technologies in longer in vitro studies and in animal models is necessary to assess the potential for therapeutic applications.
Fig. 8 |. Bioacoustic levitation.
a | A densitometry platform for the bioacoustic levitation of cells is shown. b | Owing to magnetic induction (B) and gravity (g), cells are levitated in the channel and focused in a plane in which magnetic forces (Fmag) and buoyancy forces (Fb) are in equilibrium. The magnetic susceptibility of the medium (Xm) has to be substantially larger than the magnetic susceptibility of the cells (Xc), such that different cell types with different densities (tumour cells (TCs), white blood cells (WBCs) and red blood cells (RBCs)) can be separated. c A bioacoustic levitation bioprinting process to construct 3D neural constructs is shown. Neuroprogenitor cells in a fibrin hydrogel are placed in the levitation chamber. An acoustic ceramic generates incident waves, which coherently interfere with the waves reflected from the glass reflector, which is placed on top of the chamber. The resultant standing waves induce cells to levitate, resulting in 3D multilayer constructs of differentiated neural cells. Panels a and b are reproduced with permission from REF.203, Proceedings of the National Academy of Sciences. Panel c is adapted with permission from REF202, John Wiley and Sons.
Levitation could further be used to assemble cellular aggregates or cell-laden microgels for the engineering of biological building blocks (FIG. 8b). Microgels provide a flexible platform because they can be synthesized with different built-in biological cues and cell types204. Furthermore, microgels allow for the fabrication of materials that have proved challenging to be processed, such as silicone, which might be relevant for fields such as soft robotics205. Cellular or cell-laden microgel building blocks can be self-assembled through cell-cell206 or secondary material interactions207. For example, DNA modification of these biological building blocks can trigger and program self-assembly a priori208,209. Alternatively, cell-laden microgels can be assembled into more complex structures by the use of magnetically actuated microrobotic systems210. Such microrobots contain magnetic materials that can be actuated by electromagnets, which are controlled by high-level user algorithms. The robots can apply forces of up to 70 nN to pick and place PEG and GelMA cell-laden hydrogels, and the incorporated cells maintain viability for up to 7 days after processing.
Future perspective and conclusions
Despite the rapid pace with which biofabrication strategies are being developed in different laboratories and companies worldwide, fabricating a fully functional tissue or organ is still beyond reach. Several challenges remain to produce functional organs for clinical applications and as therapeutic 3D models. Vascularization and innervation of engineered tissues will be key milestones for the construction and engraftment of functional constructs. Several strategies have already been explored to enable vascularization37,54, but only limited progress has been made in the design of innervation. Furthermore, the role of the immune system and the foreign bodyresponse needs to be fully elucidated to ensure functional engraftment upon implantation of biofabricated constructs176,211,212. For the recreation of whole organs, a detailed biological understanding of tissue-specific cell populations and phenotypes is required to replicate the anatomy and physiology ofthe organ, including cell–cell and cell–ECM communication, as well as morphogenesis. For example, complex artificial niche-like environments in combination with two-photon polymerization enable fundamental studies of cell–ECM interactions at a submicrometre resolution213–215. Biological studies need to be accompanied by technological development to ultimately achieve a high degree of similarity between native tissues and organs and biofabricated constructs. Biomaterials need to be developed that mimic the dynamics of the ECM, for example, by exploiting hydrophilic and hydrophobic interactions216, electrical properties217 or molecular self-assembly218. Experimental observations need to be coupled with multiparametric models of bioink viscosity to understand biomaterial behaviour during tissue fabrication. The fabrication of constructs comprising multiple materials, for example, by exploring microfluidic technologies219,220, will allow for the integration of different cell types and properties within one engineered tissue. Ultimately, the aim is to fabricate constructs that mimic native tissue organization221,222.
The deposition into or onto support materials enables bioprinting of materials that rapidly flow after extrusion. For example, a co-printed thermoplastic support framework into which soft materials can be deposited138,223 facilitates the fabrication of organ-scale constructs from soft materials. Liquid support systems, for example, the deposition of aqueous droplets into an oil reservoir216, can be applied for the bioprinting of materials with cells224. The immiscibility of the bioprinted ink with the oil support and the stabilization of the droplet by lipids in the oil phase enable the formation of stable, self-supported, droplet-based structures. By integrating low-gelling temperature agarose and cells in the aqueous ink, cooling can be used to trigger gelation, stabilizing the structure upon removal from the oil224.
The fabrication of macroscopic tissues and organs further requires a large number of cells, which are often challenging to obtain or produce. For generating large numbers of specialized and patient-specific cells, hiP-SCs hold great potential, and biomaterials can provide an adequate, controlled environment for cell expansion and differentiation by dynamically displaying the correct biochemical and biophysical cues and by promoting cell viability225,226.
Simple tissue defects can already be treated with biofabricated scaffolds exhibiting hierarchical structural properties or engineered surface properties to steer cell activity. However, complex biofabricated constructs require maturation in bioreactors and cannot yet be directly implanted in patients after fabrication. Technologies need to be developed to monitor and control the behaviour of processed cells, the maturation of assembled tissues and physicochemical changes, such as shrinking, swelling, deformation and degradation, that occur in the supporting biomaterials during the fabrication and maturation process. Adhesion, migration, proliferation, differentiation and apoptosis of cells also need to be monitored and, ideally, controlled, and the biological building blocks need to be correctly combined, fixed and connected in 3D. For example, impedance measurements of integrated carbon nanotubes allow for the monitoring of cell adhesion, spreading and density in 3D227. Similarly, sol-gel formulations, fabricated by inkjet printing, enable the integration of pH sensors into cell-laden hydrogels228. Tissue maturation can be followed by assessing functional markers in real time, for example, by integrating biofabrication technologies with electronically active biomaterials or materials with intrinsic optoelectronic properties229,230.
Tissues can also be regenerated in situ by the use of biomaterials that actively trigger the regeneration process, for example, for musculoskeletal231,232 and cardiovascular233,234 applications, possibly in combination with biofabrication strategies235. The development of dynamic materials (for example, shape memory materials or supramolecular hydrogels) compatible with bioprinting will facilitate 4D printing236, exploiting temporal external stimuli during processing or after implantation237.
The biofabrication and medical application of a fully functional organ will depend on fruitful collaborations across many disciplines, encompassing engineering, materials science, biology, medicine and business administration, to ensure not only progress in fundamental science but also clinical translation through commercialization. The biofabrication community has already developed tissue constructs for preclinical models and is working towards upscaling to human-scale tissues. We envision that tissues such as biofabricated skin, cartilage and vascular and cardiac patches, as well as peripheral neural grafts, have the potential to reach the clinic within the next 5–10 years.
Acknowledgements
L.M. acknowledges the Dutch Province of Limburg and the European Research Council (grant #637308) for funding. J.A.B. thanks the AO foundation for funding. S.J.L. and J.J.Y. were supported by the US National Institutes of Health (1P41EB023833-01). S.T. and Y.M. thank A. Shima, S. Nagata and F. Ozawa for valuable discussion.
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Gomes ME, Rodrigues MT, Domingues RMA & Reis RL Tissue engineering and regenerative medicine: new trends and directions-a year in review. Tissue Eng. Part B Rev 23, 211–224 (2017). [DOI] [PubMed] [Google Scholar]
- 2.Tschugg A et al. A prospective randomized multicenter phase I/II clinical trial to evaluate safety and efficacy of NOVOCART disk plus autologous disk chondrocyte transplantation in the treatment of nucleotomized and degenerative lumbar disks to avoid secondary disease: safety results of Phase I-a short report. Neurosurg. Rev 40, 155–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin I et al. The survey on cellular and engineered tissue therapies in Europe in 2013. Tissue Eng. A 22, 5–16 (2016). [DOI] [PubMed] [Google Scholar]
- 4.Groll J et al. Biofabrication: reappraising the definition of an evolving field. Biofabrication 8, 013001 (2016). [DOI] [PubMed] [Google Scholar]
- 5.Lind JU et al. Instrumented cardiac microphysiological devices via multimaterial three dimensional printing. Nat. Mater 16, 303–308 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dai X et al. Coaxial 3D bioprinting of self-assembled multicellular heterogeneous tumor fibers. Sci. Rep 7, 1457 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Faulkner-Jones A et al. Bioprinting of human pluripotent stem cells and their directed differentiation into hepatocyte-like cells for the generation of mini-livers in 3D. Biofabrication 7, 044102 (2015). [DOI] [PubMed] [Google Scholar]
- 8.Homan KA et al. Bioprinting of 3D convoluted renal proximal tubules on perfusable chips. Sci. Rep 6, 34845 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jeon H et al. Generation of multilayered 3D structures of HepG2 cells using a bio-printing technique. Gut Liver 11, 121–128 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burdick JA & Murphy WL Moving from static to dynamic complexity in hydrogel design. Nat. Commun 3, 1269 (2012). [DOI] [PubMed] [Google Scholar]
- 11.Gobaa S et al. Artificial niche microarrays for probing single stem cell fate in high throughput. Nat. Methods 8, 949–955 (2011). [DOI] [PubMed] [Google Scholar]
- 12.Lutolf MP & Hubbell JA Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat. Biotechnol 23, 47 (2005). [DOI] [PubMed] [Google Scholar]
- 13.Tibbitt MW & Anseth KS Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol. Bioeng 103, 655–663 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khetan S & Burdick JA Patterning hydrogels in three dimensions towards controlling cellular interactions. Soft Matter 7, 830–838 (2011). [Google Scholar]
- 15.Moroni L et al. Biofabrication: a guide to technology and terminology. Trends Biotechnol 36, 384–402 (2018). [DOI] [PubMed] [Google Scholar]
- 16.Jungst T, Smolan W, Schacht K, Scheibel T & Groll J Strategies and molecular design criteria for 3D printable hydrogels. Chem. Rev 116, 1496–1539 (2016). [DOI] [PubMed] [Google Scholar]
- 17.Guvendiren M, Molde J, Soares RM & Kohn J Designing biomaterials for 3D printing. ACS Biomater. Sci. Eng 2, 1679–1693 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ligon SC, Liska R, Stampfl J, Gurr M & Mulhaupt R Polymers for 3D printing and customized additive manufacturing. Chem. Rev 117, 10212–10290 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi W et al. Structurally and functionally optimized silk-fibroin-gelatin scaffold using 3D printing to repair cartilage injury in vitro and in vivo. Adv. Mater 29, 1701089 (2017). [DOI] [PubMed] [Google Scholar]
- 20.Levato R et al. The bio in the ink: cartilage regeneration with bioprintable hydrogels and articular cartilage-derived progenitor cells. Acta Biomater 61, 41–53 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Compaan AM, Christensen K & Huang Y Inkjet bioprinting of 3D silk fibroin cellular constructs using sacrificial alginate. ACS Biomater. Sci. Eng (2016). [DOI] [PubMed] [Google Scholar]
- 22.Colosi C et al. Microfluidic bioprinting of heterogeneous 3D tissue constructs using low-viscosity bioink. Adv. Mater 28, 677–684 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ouyang L, Highley CB, Sun W & Burdick JAA Generalizable strategy for the 3D bioprinting of hydrogels from nonviscous photo-crosslinkable inks. Adv. Mater 29, 1604983 (2017). [DOI] [PubMed] [Google Scholar]
- 24.Liu W et al. Rapid continuous multimaterial extrusion bioprinting. Adv. Mater 29, 1604630 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schacht K et al. Biofabrication of cell-loaded 3D spider silk constructs. Angew. Chem. Int. Ed 54, 2816–2820 (2015). [DOI] [PubMed] [Google Scholar]
- 26.Ouyang LL, Highley CB, Rodell CB, Sun W & Burdick JA 3D printing of shear-thinning hyaluronic acid hydrogels with secondary cross-linking. ACS Biomater. Sci. Eng 2, 1743–1751 (2016). [DOI] [PubMed] [Google Scholar]
- 27.Li C et al. Rapid formation of a supramolecular polypeptide-dna hydrogel for in situ three-dimensional multilayer bioprinting. Angew. Chem. Int. Ed 54, 3957–3961 (2015). [DOI] [PubMed] [Google Scholar]
- 28.Dubbin K, Hori Y, Lewis KK & Heilshorn SC Dual-stage crosslinking of a gel-phase bioink improves cell viability and homogeneity for 3D bioprinting. Adv. Healthc. Mater 5, 2488–2492 (2016). [DOI] [PubMed] [Google Scholar]
- 29.Loo Y & Hauser CAE Bioprinting synthetic self-assembling peptide hydrogels for biomedical applications. Biomed. Mater 11, 014103 (2015). [DOI] [PubMed] [Google Scholar]
- 30.Landers R, Hübner U, Schmelzeisen R & Mülhaupt R Rapid prototyping of scaffolds derived from thermoreversible hydrogels and tailored for applications in tissue engineering. Biomaterials 23, 4437–4447 (2002). [DOI] [PubMed] [Google Scholar]
- 31.Wu W, DeConinck A & Lewis JA Omnidirectional printing of 3D microvascular networks. Adv. Mater 23, H178–H183 (2011). [DOI] [PubMed] [Google Scholar]
- 32.Hinton TJ et al. Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels. Sci. Adv 1, e1500758 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bhattacharjee T et al. Writing in the granular gel medium. Sci. Adv 1, e1500655 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Highley CB, Rodell CB & Burdick JA Direct 3D printing of shear-thinning hydrogels into self-healing hydrogels. Adv. Mater 27, 5075–5079 (2015). [DOI] [PubMed] [Google Scholar]
- 35.Shi L et al. Dynamic coordination chemistry enables free directional printing of biopolymer hydrogel. Chem. Mater 29, 5816–5823 (2017). [Google Scholar]
- 36.Miller JS et al. Rapid casting of patterned vascular networks for perfusable engineered 3D tissues. Nat. Mater 11, 768 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kolesky DB, Homan KA, Skylar-Scott MA & Lewis JA Three-dimensional bioprinting of thick vascularized tissues. Proc. Natl Acad. Sci. USA 113, 3179–3184 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bhattacharjee T et al. Liquid-like solids support cells in 3D. ACS Biomater. Sci. Eng 2, 1787–1795 (2016). [DOI] [PubMed] [Google Scholar]
- 39.Mironov V et al. Organ printing: tissue spheroids as building blocks. Biomaterials 30, 2164–2174 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sego TJ, Kasacheuski U, Hauersperger D, Tovar A & Moldovan NI A heuristic computational model of basic cellular processes and oxygenation during spheroid-dependent biofabrication. Biofabrication 9, 024104 (2017). [DOI] [PubMed] [Google Scholar]
- 41.Bakirci E, Toprakhisar B, Zeybek M, Ozaydin IG & Koc B Cell sheet based bionk for 3D bioprinting applications. Biofabrication 9, 024105 (2017). [DOI] [PubMed] [Google Scholar]
- 42.Owaki T, Shimizu T, Yamato M & Okano T Cell sheet engineering for regenerative medicine: current challenges and strategies. Biotechnol. J 9, 904–914 (2014). [DOI] [PubMed] [Google Scholar]
- 43.Roh S, Parekh DP, Bharti B, Stoyanov SD & Velev OD 3D printing by multiphase silicone/water capillary inks. Adv. Mater 29, 1701554 (2017). [DOI] [PubMed] [Google Scholar]
- 44.Rutz AL, Hyland KE, Jakus AE, Burghardt WR & Shah RN A multimaterial bioink method for 3D printing tunable, cell-compatible hydrogels. Adv. Mater 27, 1607–1614 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosales AM & Anseth KS The design of reversible hydrogels to capture extracellular matrix dynamics. Nat. Rev. Mater 1, 15012 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fairbanks BD et al. A versatile synthetic extracellular matrix mimic via thiol-norbornene photopolymerization. Adv. Mater 21, 5005–5010 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stichler S et al. Thiol-ene clickable poly(glycidol) hydrogels for biofabrication. Ann. Biomed. Eng 45, 273–285 (2017). [DOI] [PubMed] [Google Scholar]
- 48.Stichler S, Bertlein S, Tessmar J, Jungst T & Groll J Thiol-ene cross-linkable hydrogels as bioinks for biofabrication. Macromol. Symp 372, 102–107 (2017). [Google Scholar]
- 49.Bertlein S et al. Thiol-ene clickable gelatin: a platform bioink for multiple 3D biofabrication technologies. Adv. Mater 29, 1703404 (2017). [DOI] [PubMed] [Google Scholar]
- 50.Yeh Y-C, Ouyang L, Highley CB & Burdick JA Norbornene-modified poly (glycerol sebacate) as a photocurable and biodegradable elastomer. Polym. Chem 8, 5091–5099 (2017). [Google Scholar]
- 51.Mondschein RJ, Kanitkar A, Williams CB, Verbridge SS & Long TE Polymer structure-property requirements for stereolithographic 3D printing of soft tissue engineering scaffolds. Biomaterials 140, 170–188 (2017). [DOI] [PubMed] [Google Scholar]
- 52.Vitale A et al. Oxygen-inhibition lithography for the fabrication of multipolymeric structures. Adv. Mater 27, 4560–4565 (2015). [DOI] [PubMed] [Google Scholar]
- 53.Zhang AP et al. Rapid fabrication of complex 3D extracellular microenvironments by dynamic optical projection stereolithography. Adv. Mater 24, 4266–4270 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhu W et al. Direct 3D bioprinting of prevascularized tissue constructs with complex microarchitecture. Biomaterials 124, 106–115 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brandenberg N & Lutolf MP In situ patterning of microfluidic networks in 3D cell-laden hydrogels. Adv. Mater 28, 7450–7456 (2016). [DOI] [PubMed] [Google Scholar]
- 56.Arakawa CK, Badeau BA, Zheng Y & DeForest CA Multicellular vascularized engineered tissues through user-programmable biomaterial photodegradation. Adv. Mater 29, 1703156 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mouser VH et al. Development of a thermosensitive HAMA-containing bio-ink for the fabrication of composite cartilage repair constructs. Biofabrication 9, 015026 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rhee S, Puetzer JL, Mason BN, Reinhart-King CA & Bonassar LJ 3D bioprinting of spatially heterogeneous collagen constructs for cartilage tissue engineering. ACS Biomater. Sci. Eng 2, 1800–1805 (2016). [DOI] [PubMed] [Google Scholar]
- 59.Diamantides N et al. Correlating rheological properties and printability of collagen bioinks: the effects of riboflavin photocrosslinking and pH. Biofabrication 9, 034102 (2017). [DOI] [PubMed] [Google Scholar]
- 60.Bolaños RAV et al. The use of a cartilage decellularized matrix scaffold for the repair of osteochondral defects: the importance of long-term studies in a large animal model. Osteoarthritis Cartilage 25, 413–420 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pati F et al. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat. Commun 5, 3935 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim BS, Kim H, Gao G, Jang J & Cho DW Decellularized extracellular matrix: a step towards the next generation source for bioink manufacturing. Biofabrication 9, 034104 (2017). [DOI] [PubMed] [Google Scholar]
- 63.Levato R et al. The bio in the ink: cartilage regeneration with bioprintable hydrogels and articular cartilage-derived progenitor cells. Acta Biomaterialia (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shi W et al. Structurally and functionally optimized silk-fibroin-gelatin scaffold using 3D printing to repair cartilage injury in vitro and in vivo. Adv. Mater 29, 1701089 (2017). [DOI] [PubMed] [Google Scholar]
- 65.Klotz BJ, Gawlitta D, Rosenberg AJWP, Malda J & Melchels FPW Gelatin-methacryloyl hydrogels: towards biofabrication-based tissue repair. Trends Biotechnol 34, 394–407 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Khalil S & Sun W Bioprinting endothelial cells with alginate for 3D tissue constructs. J. Biomech. Eng 131, 111002 (2009). [DOI] [PubMed] [Google Scholar]
- 67.Duan B, Hockaday LA, Kang KH & Butcher JT 3D bioprinting of heterogeneous aortic valve conduits with alginate/gelatin hydrogels. J. Biomed. Mater. Res. A 101, 1255–1264 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jose RR, Brown JE, Polido KE, Omenetto FG & Kaplan DL Polyol-silk bioink formulations as two-part room-temperature curable materials for 3D printing. ACS Biomater. Sci. Eng 1, 780–788 (2015). [DOI] [PubMed] [Google Scholar]
- 69.Sommer MR, Schaffner M, Carnelli D & Studart AR 3D printing of hierarchical silk fibroin structures. ACS Appl. Mater. Inter 8, 34677–34685 (2016). [DOI] [PubMed] [Google Scholar]
- 70.Badylak SF, Taylor D & Uygun K Whole-organ tissue engineering: decellularization and recellularization of three-dimensional matrix scaffolds. Annu. Rev. Biomed. Eng 13, 27–53 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cheng J et al. Rheological properties of cell-hydrogel composites extruding through small-diameter tips. J. Manuf. Sci. Eng 130, 021014 (2008). [Google Scholar]
- 72.Xu CX et al. Study of droplet formation process during drop-on-demand inkjetting of living cell-laden bioink. Langmuir 30, 9130–9138 (2014). [DOI] [PubMed] [Google Scholar]
- 73.Zhang ZY, Xu CX, Xiong RT, Chrisey DB & Huang Y Effects of living cells on the bioink printability during laser printing. Biomicrofluidics 11, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nussinovitch A Resemblance of immobilized trichoderma-viride fungal spores in an alginate matrix to a composite-material. Biotechnol. Progr 10, 551–554 (1994). [Google Scholar]
- 75.Zhang XZ & Chu CC Fabrication and characterization of microgel-impregnated, thermosensitive PNIPAAm hydrogels. Polymer 46, 9664–9673 (2005). [Google Scholar]
- 76.Lim CT, Zhou EH & Quek ST Mechanical models for living cells — a review. J. Biomech 39, 195–216 (2006). [DOI] [PubMed] [Google Scholar]
- 77.Mekhileri NV et al. Automated 3D bioassembly of micro-tissues for biofabrication of hybrid tissue engineered constructs. Biofabrication 10, (2018). [DOI] [PubMed] [Google Scholar]
- 78.Yeo M, Lee JS, Chun W & Kim GH An innovative collagen-based cell-printing method for obtaining human adipose stem cell-laden structures consisting of core sheath structures for tissue engineering. Biomacromolecules 17, 1365–1375 (2016). [DOI] [PubMed] [Google Scholar]
- 79.Levato R et al. Biofabrication of tissue constructs by 3D bioprinting of cell-laden microcarriers. Biofabrication 6, (2014). [DOI] [PubMed] [Google Scholar]
- 80.Kamperman T et al. Single cell microgel based modular bioinks for uncoupled cellular micro- and macroenvironments. Adv. Healthc. Mater 6, (2017). [DOI] [PubMed] [Google Scholar]
- 81.Bhatia SN & Ingber DE Microfluidic organs-on-chips. Nat. Biotechnol 32, 760–772 (2014). [DOI] [PubMed] [Google Scholar]
- 82.Cohen DL, Malone E, Lipson H & Bonassar LJ Direct freeform fabrication of seeded hydrogels in arbitrary geometries. Tissue Eng 12, 1325–1335 (2006). [DOI] [PubMed] [Google Scholar]
- 83.Pati F, Gantelius J & Svahn HA 3D bioprinting of tissue/organ models. Angew. Chem. Int. Ed 55, 4650–4665 (2016). [DOI] [PubMed] [Google Scholar]
- 84.Min D et al. Bioprinting of biomimetic skin containing melanocytes. Exp. Dermatol 10.1111/exd.13376 (2017). [DOI] [PubMed] [Google Scholar]
- 85.Lee V et al. Design and fabrication of human skin by three-dimensional bioprinting. Tissue Eng. C 20, 473–484 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lee W et al. Multi-layered culture of human skin fibroblasts and keratinocytes through three dimensional freeform fabrication. Biomaterials 30, 1587–1595 (2009). [DOI] [PubMed] [Google Scholar]
- 87.Mandrycky C, Wang Z, Kim K & Kim DH 3D bioprinting for engineering complex tissues. Biotechnol. Adv 34, 422–434 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ozbolat IT, Peng W & Ozbolat V Application areas of 3D bioprinting. DrugDiscov. Today 21, 1257–1271 (2016). [DOI] [PubMed] [Google Scholar]
- 89.Arslan-Yildiz A et al. Towards artificial tissue models: past, present, and future of 3D bioprinting. Biofabrication 8, 014103 (2016). [DOI] [PubMed] [Google Scholar]
- 90.Vyas D et al. Self-assembled liver organoids recapitulate hepatobiliary organogenesis in vitro. Hepatology 67, 750–761 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bhise NS et al. A liver-on-a-chip platform with bioprinted hepatic spheroids. Biofabrication 8, 014101 (2016). [DOI] [PubMed] [Google Scholar]
- 92.Knowlton S & Tasoglu SA Bioprinted liver-on-a-chip for drug screening applications. Trends Biotechnol 34, 681–682 (2016). [DOI] [PubMed] [Google Scholar]
- 93.Konar D, Devarasetty M, Yildiz DV, Atala A & Murphy SV Lung-on-a-chip technologies for disease modeling and drug development. Biomed. Eng. Comput. Biol 7, 17–27 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Doryab A, Amoabediny G & Salehi-Najafabadi A Advances in pulmonary therapy and drug development: lung tissue engineering to lung-on-a-chip. Biotechnol. Adv 34, 588–596 (2016). [DOI] [PubMed] [Google Scholar]
- 95.Horvath L et al. Engineering an in vitro air-blood barrier by 3D bioprinting. Sci. Rep 5, 7974 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Huh D et al. Reconstituting organ-level lung functions on a chip. Science 328, 1662–1668 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang Z, Lee SJ, Cheng HJ, Yoo JJ & Atala A 3D bioprinted functional and contractile cardiac tissue constructs. Acta Biomater 10.1016/j.actbio.2018.02.007 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang YS et al. Bioprinting 3D microfibrous scaffolds for engineering endothelialized myocardium and heart-on-a-chip. Biomaterials 110, 45–59 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.King SM et al. 3D proximal tubule tissues recapitulate key aspects of renal physiology to enable nephrotoxicity testing. Front. Physiol 8, 123 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Abaci HE & Shuler ML Human-on-a-chip design strategies and principles for physiologically based pharmacokinetics/pharmacodynamics modeling. Integr. Biol 7, 383–391 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Skardal A et al. Multi-tissue interactions in an integrated three-tissue organ-on-a-chip platform. Sci. Rep 7, 8837 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Weiswald LB, Bellet D & Dangles-Marie V Spherical cancer models in tumor biology. Neoplasia 17, 1–15 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Skardal A, Devarasetty M, Rodman C, Atala A & Soker S Liver-tumor hybrid organoids for modeling tumor growth and drug response in vitro. Ann. Biomed. Eng 43, 2361–2373 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Friedrich J, Ebner R & Kunz-Schughart LA Experimental anti-tumor therapy in 3D: spheroids — old hat or new challenge? Int. J. Radiat. Biol 83, 849–871 (2007). [DOI] [PubMed] [Google Scholar]
- 105.Xu F et al. A three-dimensional in vitro ovarian cancer coculture model using a high-throughput cell patterning platform. Biotechnol. J 6, 204–212 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.King SM, Presnell SC & Nguyen DG Development of 3D bioprinted human breast cancer for in vitro drug screening. Cancer Res 74 (Suppl), 2034 (2014). [Google Scholar]
- 107.Morimoto Y, Hsiao AY & Takeuchi S Point-, line-, and plane-shaped cellular constructs for 3D tissue assembly. Adv. Drug Deliv. Rev 95, 29–39 (2015). [DOI] [PubMed] [Google Scholar]
- 108.Morimoto Y, Onuki M & Takeuchi S Mass production of cell-laden calcium alginate particles with centrifugal force. Adv. Healthc. Mater 6, 1601375 (2017). [DOI] [PubMed] [Google Scholar]
- 109.Tan WH & Takeuchi S Monodisperse alginate hydrogel microbeads for cell encapsulation. Adv. Mater 19, 2696–2701 (2007). [Google Scholar]
- 110.Headen DM, Aubry G, Lu H & Garcia AJ Microfluidic-based generation of size-controlled, biofunctionalized synthetic polymer microgels for cell encapsulation. Adv. Mater 26, 3003–3008 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Griffin DR, Weaver WM, Scumpia PO, Di Carlo D & Segura T Accelerated wound healing by injectable microporous gel scaffolds assembled from annealed building blocks. Nat. Mater 14, 737–744 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Matsunaga YT, Morimoto Y & Takeuchi S Molding cell beads for rapid construction of macroscopic 3D tissue architecture. Adv. Mater 23, H90–H94 (2011). [DOI] [PubMed] [Google Scholar]
- 113.Hong SM, Hsu HJ, Kaunas R & Kameoka J Collagen microsphere production on a chip. Lab. Chip 12, 3277–3280 (2012). [DOI] [PubMed] [Google Scholar]
- 114.Kato-Negishi M, Morimoto Y, Onoe H & Takeuchi S Millimeter-sized neural building blocks for 3D heterogeneous neural network assembly. Adv. Healthc. Mater 2, 1564–1570 (2013). [DOI] [PubMed] [Google Scholar]
- 115.Luo HY et al. Fabrication of viable centimeter-sized 3D tissue constructs with microchannel conduits for improved tissue properties through assembly of cell-laden microbeads. J. Tissue Eng. Regen. Med 8, 493–504 (2014). [DOI] [PubMed] [Google Scholar]
- 116.Dutta D, Heo I & Clevers H Disease modeling in stem cell-derived 3D organoid systems. Trends Mol. Med 23, 393–410 (2017). [DOI] [PubMed] [Google Scholar]
- 117.Chung SE, Park W, Shin S, Lee SA & Kwon S Guided and fluidic self-assembly of microstructures using railed microfluidic channels. Nat. Mater 7, 581–587 (2008). [DOI] [PubMed] [Google Scholar]
- 118.Yang W, Yu H, Li G, Wang Y & Liu L High-throughput fabrication and modular assembly of 3D heterogeneous microscale tissues. Small 13, 1602769 (2017). [DOI] [PubMed] [Google Scholar]
- 119.Kang E et al. Digitally tunable physicochemical coding of material composition and topography in continuous microfibres. Nat. Mater 10, 877–883 (2011). [DOI] [PubMed] [Google Scholar]
- 120.Wei D et al. Continuous fabrication and assembly of spatial cell-laden fibers for a tissue-like construct via a photolithographic-based microfluidic chip. ACS Appl. Mater Inter 9, 14606–14617 (2017). [DOI] [PubMed] [Google Scholar]
- 121.Onoe H et al. Metre-long cell-laden microfibres exhibit tissue morphologies and functions. Nat. Mater 12, 584–590 (2013). [DOI] [PubMed] [Google Scholar]
- 122.Jun Y et al. Microfluidics-generated pancreatic islet microfibers for enhanced immunoprotection. Biomaterials 34, 8122–8130 (2013). [DOI] [PubMed] [Google Scholar]
- 123.Sakai S, Yamaguchi S, Takei T & Kawakami K Oxidized alginate-cross-linked alginate/gelatin hydrogel fibers for fabricating tubular constructs with layered smooth muscle cells and endothelial cells in collagen gels. Biomacromolecules 9, 2036–2041 (2008). [DOI] [PubMed] [Google Scholar]
- 124.Lee KH, Shin SJ, Park Y & Lee SH Synthesis of cell-laden alginate hollow fibers using microfluidic chips and microvascularized tissue-engineering applications. Small 5, 1264–1268 (2009). [DOI] [PubMed] [Google Scholar]
- 125.Yang J et al. Cell sheet engineering: recreating tissues without biodegradable scaffolds. Biomaterials 26, 6415–6422 (2005). [DOI] [PubMed] [Google Scholar]
- 126.Leng L, McAllister A, Zhang BY, Radisic M & Gunther A Mosaic hydrogels: one-step formation of multiscale soft materials. Adv. Mater 24, 3650–3658 (2012). [DOI] [PubMed] [Google Scholar]
- 127.Yan J, Chen F & Amsden BG Cell sheets prepared via gel-sol transition of calcium RGD-alginate. Acta Biomaterialia 30, 277–284 (2016). [DOI] [PubMed] [Google Scholar]
- 128.Kim K, Utoh R, Ohashi K, Kikuchi T & Okano T Fabrication of functional 3D hepatic tissues with polarized hepatocytes by stacking endothelial cell sheets in vitro. J. Tissue Eng. Regen. Med 11, 2071–2080 (2017). [DOI] [PubMed] [Google Scholar]
- 129.Cheng S et al. Self-adjusting, polymeric multilayered roll that can keep the shapes of the blood vessel scaffolds during biodegradation. Adv. Mater 29, 1700171 (2017). [DOI] [PubMed] [Google Scholar]
- 130.Ito A et al. Novel methodology for fabrication of tissue-engineered tubular constructs using magnetite nanoparticles and magnetic force. Tissue Eng 11, 1553–1561 (2005). [DOI] [PubMed] [Google Scholar]
- 131.Hsu AR & Ellington JK Patient-specific 3-dimensional printed titanium truss cage with tibiotalocalcaneal arthrodesis for salvage of persistent distal tibia nonunion. Foot Ankle Spec 8, 483–489 (2015). [DOI] [PubMed] [Google Scholar]
- 132.Jeong CG & Atala A 3D printing and biofabrication for load bearing tissue engineering. Adv. Exp. Med. Biol 881, 3–14 (2015). [DOI] [PubMed] [Google Scholar]
- 133.Bose S, Vahabzadeh S & Bandyopadhyay A Bone tissue engineering using 3D printing. Mater. Today 16, 496–504 (2013). [Google Scholar]
- 134.McBeth C et al. 3D bioprinting of GelMA scaffolds triggers mineral deposition by primary human osteoblasts. Biofabrication 9, 015009 (2017). [DOI] [PubMed] [Google Scholar]
- 135.Yao Q et al. Design, construction and mechanical testing of digital 3D anatomical data-based PCL-HA bone tissue engineering scaffold. J. Mater. Sci. Mater. Med 26, 5360 (2015). [DOI] [PubMed] [Google Scholar]
- 136.Wang MO, Piard CM, Melchiorri A, Dreher ML & Fisher JP Evaluating changes in structure and cytotoxicity during in vitro degradation of three dimensional printed scaffolds. Tissue Eng. A 21, 1642–1653 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Pati F et al. Ornamenting 3D printed scaffolds with cell-laid extracellular matrix for bone tissue regeneration. Biomaterials 37, 230–241 (2015). [DOI] [PubMed] [Google Scholar]
- 138.Kang HW et al. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat. Biotechnol 34, 312–319 (2016). [DOI] [PubMed] [Google Scholar]
- 139.Ballyns JJ et al. Image-guided tissue engineering of anatomically shaped implants via MRI and micro-CT using injection molding. Tissue Eng. A 14, 1195–1202 (2008). [DOI] [PubMed] [Google Scholar]
- 140.Hockaday LA et al. Rapid 3D printing of anatomically accurate and mechanically heterogeneous aortic valve hydrogel scaffolds. Biofabrication 4, 035005 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bowles RD, Gebhard HH, Hartl R & Bonassar LJ Tissue-engineered intervertebral discs produce new matrix, maintain disc height, and restore biomechanical function to the rodent spine. Proc. Natl Acad. Sci. USA 108, 13106–13111 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Makris EA, Gomoll AH, Malizos KN, Hu JC & Athanasiou KA Repair and tissue engineering techniques for articular cartilage. Nat. Rev. Rheumatol 11, 21–34 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tatman PD et al. Multiscale biofabrication of articular cartilage: bioinspired and biomimetic approaches. Tissue Eng. B 21, 543–559 (2015). [DOI] [PubMed] [Google Scholar]
- 144.Di Bella C, Fosang A, Donati DM, Wallace GG & Choong PF 3D bioprinting of cartilage for orthopedic surgeons: reading between the lines. Front. Surg 2, 39 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gruene M et al. Laser printing of stem cells for biofabrication of scaffold-free autologous grafts. Tissue Eng. C 17, 79–87 (2011). [DOI] [PubMed] [Google Scholar]
- 146.Cui X, Breitenkamp K, Finn MG, Lotz M & D’Lima DD Direct human cartilage repair using three-dimensional bioprinting technology. Tissue Eng. A 18, 1304–1312 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Cui X, Breitenkamp K, Lotz M & D’Lima D Synergistic action of fibroblast growth factor-2 and transforming growth factor-beta1 enhances bioprinted human neocartilage formation. Biotechnol. Bioeng 109, 2357–2368 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Xu T et al. Hybrid printing of mechanically and biologically improved constructs for cartilage tissue engineering applications. Biofabrication 5, 015001 (2013). [DOI] [PubMed] [Google Scholar]
- 149.Mannoor MS et al. 3D printed bionic ears. Nano Lett 13, 2634–2639 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Almqvist KF et al. Treatment of cartilage defects in the knee using alginate beads containing human mature allogenic chondrocytes. Am. J. Sport Med 37, 1920–1929 (2009). [DOI] [PubMed] [Google Scholar]
- 151.Dhollander AAM et al. Midterm results of the treatment of cartilage defects in the knee using alginate beads containing human mature allogenic chondrocytes. Am. J. Sport Med 40, 75–82 (2012). [DOI] [PubMed] [Google Scholar]
- 152.Skardal A et al. Bioprinted amniotic fluid-derived stem cells accelerate healing of large skin wounds. Stem Cells Transl Med 1, 792–802 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Yang J et al. Cell delivery in regenerative medicine: the cell sheet engineering approach. J. Control. Release 116, 193–203 (2006). [DOI] [PubMed] [Google Scholar]
- 154.Iwata T et al. Cell sheet engineering and its application for periodontal regeneration. J. Tissue Eng. Regen. Med 9, 343–356 (2015). [DOI] [PubMed] [Google Scholar]
- 155.Frontera WR & Ochala J Skeletal muscle: a brief review of structure and function. Calcified Tissue Int 96, 183–195 (2015). [DOI] [PubMed] [Google Scholar]
- 156.Ostrovidov S et al. Skeletal muscle tissue engineering: methods to form skeletal myotubes and their applications. Tissue Eng. Part B Rev 20, 403–436 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Gaebel R et al. Patterning human stem cells and endothelial cells with laser printing for cardiac regeneration. Biomaterials 32, 9218–9230 (2011). [DOI] [PubMed] [Google Scholar]
- 158.Xu T, Baicu C, Aho M, Zile M & Boland T Fabrication and characterization of bio-engineered cardiac pseudo tissues. Biofabrication 1, 035001 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Gaetani R et al. Cardiac tissue engineering using tissue printing technology and human cardiac progenitor cells. Biomaterials 33, 1782–1790 (2012). [DOI] [PubMed] [Google Scholar]
- 160.Gao L et al. Myocardial tissue engineering with cells derived from human-induced pluripotent stem cells and a native-like, high-resolution, 3-dimensionally printed scaffold. Circ. Res 120, 1318–1325 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Atala A, Kasper FK & Mikos AG Engineering complex tissues. Sci. Transl Med 4, 160rv12 (2012). [DOI] [PubMed] [Google Scholar]
- 162.Lee CH et al. Regeneration of the articular surface of the rabbit synovial joint by cell homing: a proof of concept study. Lancet 376, 440–448 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Merceron TK et al. A 3D bioprinted complex structure for engineering the muscle-tendon unit. Biofabrication 7, 035003 (2015). [DOI] [PubMed] [Google Scholar]
- 164.Novosel EC, Kleinhans C & Kluger PJ Vascularization is the key challenge in tissue engineering. Adv. DrugDeliv. Rev 63, 300–311 (2011). [DOI] [PubMed] [Google Scholar]
- 165.Jain RK, Au P, Tam J, Duda DG & Fukumura D Engineering vascularized tissue. Nat. Biotechnol 23, 821–823 (2005). [DOI] [PubMed] [Google Scholar]
- 166.Gross BC, Erkal JL, Lockwood SY, Chen C & Spence DM Evaluation of 3D printing and its potential impact on biotechnology and the chemical sciences. Anal. Chem 86, 3240–3253 (2014). [DOI] [PubMed] [Google Scholar]
- 167.Ozbolat IT & Yu Y Bioprinting toward organ fabrication: challenges and future trends. IEEE Trans. Biomed. Eng 60, 691–699 (2013). [DOI] [PubMed] [Google Scholar]
- 168.Zheng Y et al. Microstructured templates for directed growth and vascularization of soft tissue in vivo. Biomaterials 32, 5391–5401 (2011). [DOI] [PubMed] [Google Scholar]
- 169.Choi NW et al. Microfluidic scaffolds for tissue engineering. Nat. Mater 6, 908–915 (2007). [DOI] [PubMed] [Google Scholar]
- 170.Kolesky DB et al. 3D bioprinting of vascularized, heterogeneous cell-laden tissue constructs. Adv. Mater 26, 3124–3130 (2014). [DOI] [PubMed] [Google Scholar]
- 171.Attalla R, Ling C & Selvaganapathy P Fabrication and characterization of gels with integrated channels using 3D printing with microfluidic nozzle for tissue engineering applications. Biomed. Microdevices 18, (2016). [DOI] [PubMed] [Google Scholar]
- 172.LHeureux N et al. Human tissue-engineered blood vessels for adult arterial revascularization. Nat. Med 12, 361–365 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Schubert C, van Langeveld MC & Donoso LA Innovations in 3D printing: a 3D overview from optics to organs. Br. J. Ophthalmol 98, 159–161 (2014). [DOI] [PubMed] [Google Scholar]
- 174.Kato-Negishi M, Onoe H, Ito A & Takeuchi S Rod-shaped neural units for aligned 3D neural network connection. Adv. Healthc. Mater 6, 1700143 (2017). [DOI] [PubMed] [Google Scholar]
- 175.de Vos P, Faas MM, Strand B & Calafiore R Alginate-based microcapsules for immunoisolation of pancreatic islets. Biomaterials 27, 5603–5617 (2006). [DOI] [PubMed] [Google Scholar]
- 176.Vegas AJ et al. Combinatorial hydrogel library enables identification of materials that mitigate the foreign body response in primates. Nat. Biotechnol 34, 345–352 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Rengifo HR, Giraldo JA, Labrada I & Stabler CL Long-term survival of allograft murine islets coated via covalently stabilized polymers. Adv. Healthc. Mater 3, 1061–1070 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Veiseh O et al. Size- and shape-dependent foreign body immune response to materials implanted in rodents and non-human primates. Nat. Mater 14, 643–651 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Vegas AJ et al. Long-term glycemic control using polymer-encapsulated human stem cell-derived beta cells in immune-competent mice. Nat. Med 22, 306–311 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Dufrane D, Goebbels RM, Saliez A, Guiot Y & Gianello P Six-month survival of microencapsulated pig islets and alginate biocompatibility in primates: proof of concept. Transplantation 81, 1345–1353 (2006). [DOI] [PubMed] [Google Scholar]
- 181.Elliott RB et al. Live encapsulated porcine islets from a type 1 diabetic patient 9.5 yr after xenotransplantation. Xenotransplantation 14, 157–161 (2007). [DOI] [PubMed] [Google Scholar]
- 182.Capone SH et al. Impact of alginate composition: from bead mechanical properties to encapsulated HepG2/C3A cell activities for in vivo implantation. PLOS ONE 8, e62032 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Song W et al. Engraftment of human induced pluripotent stem cell-derived hepatocytes in immunocompetent mice via 3D co-aggregation and encapsulation. Sci. Rep 5, 16884 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Qi M et al. PVA hydrogel sheet macroencapsulation for the bioartificial pancreas. Biomaterials 25, 5885–5892 (2004). [DOI] [PubMed] [Google Scholar]
- 185.Veriter S et al. The impact of hyperglycemia and the presence of encapsulated islets on oxygenation within a bioartificial pancreas in the presence of mesenchymal stem cells in a diabetic Wistar rat model. Biomaterials 32, 5945–5956 (2011). [DOI] [PubMed] [Google Scholar]
- 186.Fozdar DY, Soman P, Lee JW, Han LH & Chen S Three-dimensional polymer constructs exhibiting a tunable negative poisson’s ratio. Adv. Funct. Mater 21, 2712–2720 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Lee H & Cho DW One-step fabrication of an organ-on-a-chip with spatial heterogeneity using a 3D bioprinting technology. Lab. Chip 16, 2618–2625 (2016). [DOI] [PubMed] [Google Scholar]
- 188.Clausen A, Wang F, Jensen JS, Sigmund O & Lewis JA Topology optimized architectures with programmable Poisson’s ratio over large deformations. Adv. Mater 27, 5523–5527 (2015). [DOI] [PubMed] [Google Scholar]
- 189.Shan S et al. Multistable architected materials for trapping elastic strain energy. Adv. Mater 27, 4296–4301 (2015). [DOI] [PubMed] [Google Scholar]
- 190.Martin JJ, Fiore BE & Erb RM Designing bioinspired composite reinforcement architectures via 3D magnetic printing. Nat. Commun 6, 8641 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Han X, Bibb R & Harris R Engineering design of artificial vascular junctions for 3D printing. Biofabrication 8, 025018 (2016). [DOI] [PubMed] [Google Scholar]
- 192.Sun Y, Yang X & Wang Q In-silico analysis on biofabricating vascular networks using kinetic Monte Carlo simulations. Biofabrication 6, 015008 (2014). [DOI] [PubMed] [Google Scholar]
- 193.Yang X, Mironov V & Wang Q Modeling fusion of cellular aggregates in biofabrication using phase field theories. J. Theor. Biol 303, 110–118 (2012). [DOI] [PubMed] [Google Scholar]
- 194.McCune M, Shafiee A, Forgacs G & Kosztin I Predictive modeling of post bioprinting structure formation. Soft Matter 10, 1790–1800 (2014). [DOI] [PubMed] [Google Scholar]
- 195.Zhu W et al. 3D printing of functional biomaterials for tissue engineering. Curr. Opin. Biotechnol 40, 103–112 (2016). [DOI] [PubMed] [Google Scholar]
- 196.Tumbleston JR et al. Additive manufacturing. Continuous liquid interface production of 3D objects. Science 347, 1349–1352 (2015). [DOI] [PubMed] [Google Scholar]
- 197.Janusziewicz R, Tumbleston JR, Quintanilla AL, Mecham SJ & DeSimone JM Layerless fabrication with continuous liquid interface production. Proc. Natl Acad. Sci. USA 113, 11703–11708 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Shanjani Y, Pan CC, Elomaa L & Yang Y A novel bioprinting method and system for forming hybrid tissue engineering constructs. Biofabrication 7, 045008 (2015). [DOI] [PubMed] [Google Scholar]
- 199.Hoffmann A et al. New stereolithographic resin providing functional surfaces for biocompatible three-dimensional printing. J. Tissue Eng 8, 2041731417744485 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Morris VB, Nimbalkar S, Younesi M, McClellan P & Akkus O Mechanical properties, cytocompatibility and manufacturability of chitosan:PEGDA hybrid-gel scaffolds by stereolithography. Ann. Biomed. Eng 45, 286–296 (2017). [DOI] [PubMed] [Google Scholar]
- 201.Serpooshan V et al. Bioacoustic-enabled patterning of human iPSC-derived cardiomyocytes into 3D cardiac tissue. Biomaterials 131, 47–57 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Bouyer C et al. A bio-acoustic levitational (BAL) assembly method for engineering of multilayered, 3D brain-like constructs, using human embryonic stem cell derived neuro-progenitors. Adv. Mater 28, 161–167 (2016). [DOI] [PubMed] [Google Scholar]
- 203.Durmus NG et al. Magnetic levitation of single cells. Proc. Natl Acad. Sci. USA 112, E3661–E3668 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Li Y et al. Rapid assembly of heterogeneous 3D cell microenvironments in a microgel array. Adv. Mater 28, 3543–3548 (2016). [DOI] [PubMed] [Google Scholar]
- 205.O’Bryan CS et al. Self-assembled micro-organogels for 3D printing silicone structures. Sci. Adv 3, e1602800 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Vrij E et al. Directed Assembly and Development of Material-Free Tissues with Complex Architectures. Adv. Mater 28, 4032–4039 (2016). [DOI] [PubMed] [Google Scholar]
- 207.Fernandez JG & Khademhosseini A Micro-masonry: construction of 3D structures by microscale self-assembly. Adv. Mater 22, 2538–2541 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Qi H et al. DNA-directed self-assembly of shape-controlled hydrogels. Nature Commun 4, 2275 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Todhunter ME et al. Programmed synthesis of three-dimensional tissues. Nat. Methods 12, 975–981 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Tasoglu S, Diller E, Guven S, Sitti M & Demirci U Untethered micro-robotic coding of three-dimensional material composition. Nat. Commun 5, 3124 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Dondossola E et al. Examination of the foreign body response to biomaterials by nonlinear intravital microscopy. Nat. Biomed. Eng 1, 0007 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Doloff JC et al. Colony stimulating factor-1 receptor is a central component of the foreign body response to biomaterial implants in rodents and non-human primates. Nat. Mater 16, 671–680 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Gullo MR, Takeuchi S & Paul O Multicellular biohybrid materials: probing the interplay of cells of different types precisely positioned and constrained on 3D wireframe-like microstructures. Adv. Healthc. Mater 6, 1601053 (2017). [DOI] [PubMed] [Google Scholar]
- 214.Nava MM, Zandrini T, Cerullo G, Osellame R & Raimondi MT 3D stem cell niche engineering via two-photon laser polymerization. Methods Mol. Biol 1612, 253–266 (2017). [DOI] [PubMed] [Google Scholar]
- 215.Richter B et al. Guiding cell attachment in 3D microscaffolds selectively functionalized with two distinct adhesion proteins. Adv. Mater 29, 1604342 (2017). [DOI] [PubMed] [Google Scholar]
- 216.Villar G, Graham AD & Bayley H A tissue-like printed material. Science 340, 48–52 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Chiang MY, Hsu YW, Hsieh HY, Chen SY & Fan SK Constructing 3D heterogeneous hydrogels from electrically manipulated prepolymer droplets and crosslinked microgels. Sci. Adv 2, e1600964 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Inostroza-Brito KE et al. Cross-linking of a bio polymer-peptide co-assembling system. Acta Biomaterialia 50, 80–89 (2017). [DOI] [PubMed] [Google Scholar]
- 219.Hardin JO, Ober TJ, Valentine AD & Lewis JA Microfluidic printheads for multimaterial 3D printing of viscoelastic inks. Adv. Mater 27, 3279–3284 (2015). [DOI] [PubMed] [Google Scholar]
- 220.Snyder J, Son AR, Hamid Q, Wu H & Sun W Hetero-cellular prototyping by synchronized multimaterial bioprinting for rotary cell culture system. Biofabrication 8, 015002 (2016). [DOI] [PubMed] [Google Scholar]
- 221.Duchi S et al. Handheld co-axial bioprinting: application to in situ surgical cartilage repair. Sci. Rep 7, 5837 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Mogas-Soldevila L, Duro-Royo J & Oxman N Water-based robotic fabrication: large-scale additive manufacturing of functionally graded hydrogel composites via multichamber extrusion. 3D Print. Addit. Manuf 1, 141–151 (2014). [Google Scholar]
- 223.Schuurman W et al. Bioprinting of hybrid tissue constructs with tailorable mechanical properties. Biofabrication 3, 021001 (2011). [DOI] [PubMed] [Google Scholar]
- 224.Graham AD et al. High-resolution patterned cellular constructs by droplet-based 3D printing. Sci. Rep 7, 7004 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Gu Q, Tomaskovic-Crook E, Wallace GG & Crook JM 3D bioprinting human induced pluripotent stem cell constructs for in situ cell proliferation and successive multilineage differentiation. Adv. Healthc. Mater 6, 1700175 (2017). [DOI] [PubMed] [Google Scholar]
- 226.Zujur D et al. Three-dimensional system enabling the maintenance and directed differentiation of pluripotent stem cells under defined conditions. Sci. Adv 3, e1602875 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Whulanza Y, Ucciferri N, Domenici C, Vozzi G & Ahluwalia A Sensing scaffolds to monitor cellular activity using impedance measurements. Biosens. Bioelectron 26, 3303–3308 (2011). [DOI] [PubMed] [Google Scholar]
- 228.Orsi G et al. Combining inkjet printing and sol-gel chemistry for making pH-sensitive surfaces. Curr. Top. Med. Chem 15, 271–278 (2015). [DOI] [PubMed] [Google Scholar]
- 229.Muskovich M & Bettinger CJ Biomaterials-based electronics: polymers and interfaces for biology and medicine. Adv. Healthc. Mater 1, 248–266 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Minev IR et al. Biomaterials. Electronic dura mater for long-term multimodal neural interfaces. Science 347, 159–163 (2015). [DOI] [PubMed] [Google Scholar]
- 231.Tatara AM et al. Reconstruction of large mandibular defects using autologous tissues generated from in vivo bioreactors. Acta Biomater 45, 72–84 (2016). [DOI] [PubMed] [Google Scholar]
- 232.Emans PJ et al. Autologous engineering of cartilage. Proc. Natl Acad. Sci. USA 107, 3418–3423 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Kluin J et al. In situ heart valve tissue engineering using a bioresorbable elastomeric implant — from material design to 12 months follow-up in sheep. Biomaterials 125, 101–117 (2017). [DOI] [PubMed] [Google Scholar]
- 234.Rothuizen TC et al. Development and evaluation of in vivo tissue engineered blood vessels in a porcine model. Biomaterials 75, 82–90 (2016). [DOI] [PubMed] [Google Scholar]
- 235.Di Bella C et al. In-situ handheld 3D Bioprinting for cartilage regeneration. J. Tissue Eng. Regen. Med 12, 611–621 (2017). [DOI] [PubMed] [Google Scholar]
- 236.Gladman AS, Matsumoto EA, Nuzzo RG, Mahadevan L & Lewis JA Biomimetic 4D printing. Nat. Mater 15, 413–418 (2016). [DOI] [PubMed] [Google Scholar]
- 237.Hendrikson WJ et al. Towards 4D printed scaffolds for tissue engineering: exploiting 3D shape memory polymers to deliver time-controlled stimulus on cultured cells. Biofabrication 9, 031001 (2017). [DOI] [PubMed] [Google Scholar]
- 238.Peltola SM et al. A review of rapid prototyping techniques for tissue engineering purposes. Ann. Med 40, 268–280 (2008). [DOI] [PubMed] [Google Scholar]
- 239.Malda J et al. 25 th anniversary article: Engineering hydrogels for biofabrication. Adv. Mater 25, 5011–5028 (2013). [DOI] [PubMed] [Google Scholar]