ABSTRACT
BACKGROUND AND PURPOSE
Post‐traumatic stress disorder is associated with connectivity changes in the default mode, central executive, and salience networks, and other brain regions. This study evaluated changes in network connectivity associated with usage of High‐resolution, relational, resonance‐based electroencephalic mirroring (HIRREM®; Brain State Technologies, Scottsdale, AZ), a closed‐loop, allostatic, acoustic stimulation neurotechnology, for military‐related traumatic stress.
METHODS
Eighteen participants (17 males, mean age 41 years [SD = 7], 15 active duty) enrolled in an IRB approved pilot trial for symptoms of military‐related traumatic stress. Participants received 19.5 (1.1) HIRREM sessions over 12 days. Symptoms, physiological and functional measures, and whole brain resting MRI were collected before and after HIRREM. Six whole brain functional networks were evaluated using summary variables and community structure of predefined networks. Pre to postintervention change was analyzed using paired‐sample statistical tests.
RESULTS
Postintervention, there was an overall increase in connectivity of the default mode network (P = .0094). There were decreases of community structure in both the anterior portion of the default mode (medial prefrontal cortex, P = .0097) and in the sensorimotor (P = .005) network. There were no statistically significant changes at the whole brain level, or in the central executive, salience, or other networks analyzed. Participants demonstrated significant improvements in clinical symptoms, as well as autonomic cardiovascular regulation, which have been reported previously.
CONCLUSIONS
Use of closed‐loop, allostatic, acoustic stimulation neurotechnology (HIRREM) was associated with connectivity changes in the default mode and sensorimotor networks, in directions that may have explained the subjects’ clinical improvements.
Keywords: fMRI, HIRREM, post‐traumatic stress, default mode network, sensorimotor network
Introduction
Resting state MRI is a powerful tool to evaluate brain network connectivity, and network studies have the potential to provide a unified model for psychiatric disorders. Menon1 has proposed that psychopathologies may reflect maladaptive alterations in connectivity within and among three core brain networks: the default mode network (DMN), central executive network (CEN), and salience network (SN). The DMN is associated with spontaneous, resting state brain activity, including self‐referential “mind wandering” and autobiographical memory. The CEN is active during goal‐directed and cognitively demanding tasks. The SN encompasses emotional, interoceptive, and autonomic processes which assign significance to stimuli (internal or external), and has a crucial role for the direction of attention and motivation, as well as switching between the DMN and the CEN. Typically, the CEN and SN are coactivated during outwardly focused attention while the DMN is deactivated, and opposite activity patterns are observed during inwardly focused self‐referential thoughts.1
In recent years, this “triple network” model has been applied to advance understanding of post‐traumatic stress disorder (PTSD).2, 3, 4 From the clinical diagnostic perspective, PTSD is defined by symptom clusters of trauma re‐experiencing, avoidant behaviors, negative cognitions or mood, and increased arousal (DSM‐5).5 In network studies of PTSD, the DMN consistently shows altered connectivity6, 7, 8, 9, 10, 11, 12 with the anterior portion, encompassing the medial prefrontal cortex, being particularly disrupted.8 DMN changes may be related to the dissociative features of PTSD, including the loss of a stable and coherent sense of the self. Altered CEN connectivity is also shown in PTSD2, 13 and may contribute to difficulty for top down tasks of cognitive control including those related to working memory or emotional regulation. SN alterations in PTSD9, 10, 14 may produce perceptions of stimuli (internal or external) that entail maladaptive modes of threat sensitivity, and may interfere with switching between the CEN and DMN,2, 8, 10 leading to inefficient discrimination between task‐relevant and task‐irrelevant behaviors. Resting state MRI may also offer insights regarding the effects of interventions on network connectivity in PTSD, and reports are emerging about the use of methods such as mindfulness‐based exposure therapy.15
The objective of this manuscript is to report brain network connectivity changes in military service members and recent Veterans with symptoms of military‐related traumatic stress who undertook usage of an allostatic, closed‐loop, acoustic stimulation neurotechnology. Reductions in self‐reported symptoms of post‐traumatic stress, insomnia, depression, and anxiety, as well as improvements in functional outcomes and autonomic cardiovascular regulation (heart rate variability and baroreflex sensitivity), in this same cohort, were recently reported.16 Symptom reduction was durable through the final online data collection 6 months after completion of the intervention.
The noninvasive, nonpharmacological intervention, High‐resolution, relational, resonance‐based, electroencephalic mirroring (HIRREMⓇ; Brain State Technologies, Scottsdale, AZ)17 is intended to support autocalibration of neural oscillations by using algorithmic guidance to translate selected brain electrical frequencies into audible tones in real time. Use of HIRREM has been associated with reductions of high‐frequency (23–36 Hz) electrical amplitudes18 and greater hemispheric symmetry.17, 19 As a closed‐loop process, the intervention does not require conscious cognitive activity or training.
The HIRREM approach leverages insights of the paradigm of allostasis (stability through change), which views the brain as the organ of central command. The role of the brain is to orchestrate operations across systems on a top‐down basis, to support optimal anticipatory behaviors at the level of the whole organism, within an environmental context.20 A thesis of the allostasis paradigm is that there is no single form or level of biological function which can be designated as “normal,” and that the brain can learn to interact successfully with the new needs of changing environments. Allostatic therapeutics aim to recalibrate or “de‐rigidify” system behaviors to support dynamic flexibility for adaptive and anticipatory functionality, in contrast to homeostatic modulation toward normative set points. Successful allostatic intervention is predicted to entail a range of adaptive or healthful changes in brain network connectivity. Brain networks of interest for the present analysis included the central executive, default, and salience, and also other regions that have shown alterations in PTSD including the sensorimotor cortex (SMC),7, 9, 21 visual cortex (VC),6, 9 and basal ganglia (BG).9, 21
Methods
A single site, ongoing, IRB approved pilot study (Clinicaltrials.gov registration NCT03230890) is being carried out in the Department of Neurology at Wake Forest School of Medicine, Winston‐Salem, North Carolina, USA. The objective of this study is to evaluate clinical, physiological, physical functional, and brain connectivity changes associated with the use of HIRREM by military service members with traumatic stress symptoms. Funding allowed evaluation of the effect of HIRREM on functional brain networks for the first 18 participants enrolled, before and after the use of HIRREM over a 12‐day period.16
Participants
Eighteen participants (17 males, mean age = 41 years, SD = 6.9) were enrolled for this study. All participants were either active duty military personnel or recent Veterans (15 active‐duty, 3 Veterans, most from special operations), whose military service was primarily since September 11, 2001. Seventeen participants served with the Navy. The mean length of military service was 19.8 years (SD = 8.1), with duration of symptoms for 1–25 years. All were over the age of 18, and had been formally diagnosed with PTSD, were referred by a military medical provider for active symptoms of traumatic stress, or received medical treatment for symptoms of traumatic stress, with or without traumatic brain injury. If contact was established through self‐referral, and in the absence of a formal diagnosis of PTSD, a score of 50 points on a screening PTSD Checklist – Military version (PCL‐M) was required. Participants were recruited through referral from military medical providers, and by word of mouth, posted flyers, and self‐referral. Potential participants were excluded for known seizure disorder, severe hearing impairment, and a need for ongoing treatment with opiates, benzodiazepines, antipsychotics, antidepressants (selective serotonin reuptake inhibitors or serotonin norepinephrine reuptake inhibitors), sleep medications, stimulants, or thyroid hormone. Additionally, they were asked to abstain from recreational drug and alcohol use until 1 month after the active portion of the study. More details regarding the cohort may be found in the recent report on clinical outcomes.16
HIRREM Sessions
Procedures for provision of HIRREM have been described elsewhere.17 Subjects are encouraged to relax while sitting or reclining in a zero gravity chair. Pairs of scalp sensors monitor brain electrical activity, and software algorithms translate specific frequencies into audible tones of variable pitch and timing. Each closed‐loop cycle of monitoring and audible tone delivery occurs within a 4–8 millisecond span. Tones are delivered via earbud headphones, bilaterally. Each session typically consisted of a series of 5–10 protocols (90‐180 minutes each), some received with eyes open, others with eyes closed, defined by a scalp sensor montage in conjunction with a software design selected by the technologist.
Participants received a mean of 19.5 (SD 1.1) HIRREM sessions, over a 12‐day period. The average total intervention time was 2,778.5 minutes (SD 315.0) with a range of 1,692‐3,059 minutes. The intervention was typically administered with two sessions per day separated by a break of at least 30–60 minutes. One weekend session was offered, typically on Sunday afternoon, and there was typically only one session on Friday morning prior to repeat outcome measures and MRI scanning.
MRI Acquisition
Brain imaging was performed using magnetic resonance imaging (MRI) before and after completion of the HIRREM intervention. All participants had the initial scan in the late morning or midday as part of the enrollment visit, prior to the first HIRREM session later in the afternoon. Due to scheduling issues, one participant had the postintervention MRI scan just prior to the final intervention session, but all others had the follow up MRI right after the final HIRREM session. Imaging data were collected in a 3T Siemens Magnetom Skyra using a 32‐channel head coil. An anatomical brain image was collected using a Magnetization Prepared Rapid Acquisition Gradient Echo (MPRAGE) sequence. Functional MRI (fMRI) images based on the blood oxygenation level‐dependent signal22 were collected using a gradient echo, echo planar imaging pulse sequence. At each imaging session, fMRI images were collected at rest with eyes opened focusing on a crosshair in the center of a computer screen followed by a resting scan with eyes closed. For both fMRI scans, the participants were instructed to relax, lie still, and to remain awake. Results that follow focus on the eyes open state since there were no additional significant findings to report with the eyes closed data. The MPRAGE was collected in the sagittal plane with the TR = 2,300 milliseconds, voxel dimensions = .98 × .98 × 1.22 mm, 256 × 256 voxels, 192 slices, and slice thickness = 1 mm. Images were reformatted by the Siemens scanner into the axial plane with 130 slices. The fMRI scan was collected in the axial plane and acquired 187 image volumes with the TR = 2,000 milliseconds, 35 slices, and voxel dimensions 4 × 4 × 4 mm.
Image Preprocessing and Network Generation
All image processing was performed with SPM8 software (http://www.fil.ion.ucl.ac.uk/spm/) and in‐house Matlab scripts. The MPRAGE was converted to the axial plane and warped to Montreal Neurological Institute (MNI) space using the unified segmentation method in SPM8. The fMRI data first had the initial eight volumes removed to allow for signal stabilization. The images were then realigned to the first image of the series and coregistered to the structural image. Images were then normalized to MNI by applying the transformation from the warping procedure for the anatomical image. To limit analyses to gray matter, only voxels which overlap with the gray matter in the Automated Anatomical Labeling atlas23 were retained. A band‐pass filter of .009–.08 Hz was then applied in order to remove physiological noise and low‐frequency drift.24 Finally, confounding signals including the six rigid‐body transformation parameters generated during the realignment process and three global mean time courses—whole brain, white matter, and cerebrospinal fluid were regressed from the functional data.25 Motion correction was performed using the motion scrubbing methodology developed by Power and colleagues.26, 27 For both eyes opened and eyes closed conditions, on average less than <1% of image volumes were removed. There was no significant difference (P = .2) in the number of volumes removed between conditions.
Network analyses were completed using MATLAB scripts. The gray matter voxels from the preprocessed functional data were used in the construction of voxel‐wise networks for each participant. A Pearson correlation was performed with the time series from each voxel being correlated with the time series of every other voxel. The resultant correlation matrices were thresholded using the formula N = KS, where N is the number of nodes, K is the average degree of the network, and S was set to 2.0, 2.5., and 3.028 yielding unweighted, undirected networks with comparable densities across subjects. Whole brain properties were assessed at all thresholds. Regional network properties were assessed using a threshold of 2.5 based on previous research showing that S = 2.5 produces networks with size to density ratios that are comparable to other naturally occurring networks,29 while networks thresholded with an S greater than 3 tend to fragment.28
Network Variables
The topology of the brain networks was evaluated using multiple summary variables that were extracted from the networks. Each variable was computed for each participant across thresholds and conditions. Briefly, degree (k) is a measure of the connectedness of a node. It is computed by totaling the number of edges for any given node. Global efficiency (Eglob) is a measure of distributed connectivity in a network. It is the inverse of the average shortest path length for each given node.30 The resulting value is scaled between 0 and 1, with a value of 1 indicating that a node has direct access to all other nodes in the network. Local efficiency (Eloc) is a measure of regional connectivity in a network. It is the inverse of the average shortest path length between all neighbors of a given node.30 Eloc is scaled between 0 and 1 with a value of 1 indicating that all neighbors of a given node are also neighbors. Modularity (Q) is a statistic that quantifies how well a network can be partitioned into individual communities, or modules, relative to a comparable random network.31 A network community is a collection of network nodes that are more connected to each other than they are to other communities within the network. The Louvain algorithm was used to maximize Q and identify the associated network partitioning in the current study.32 Because each run of a modularity analysis could yield different results, modularity was run 100 times on each network and the run with the highest Q value was chosen for subsequent analyses. The actual network partitions were used for analyses of regional modular structure. Further details about each variable and information concerning additional network variables can be found elsewhere.33
Statistical Analyses
To assess the global topology of the brain networks, each participant's network metrics were averaged across the entire brain for each threshold and condition. The global network properties were compared between pre and postintervention using a 2(pre vs. post) X 3(threshold) analysis of variance (ANOVA). Each network metric was assessed in an independent ANOVA and analyses were performed separately for the eyes opened and eyes closed conditions. Note that K was not assessed at the level of the whole brain because the thresholding procedure ensures that the average degree will be stable across conditions and participants for a given threshold. Statistical significance was set at P = .05 for these and all other analyses.
As opposed to global analyses that are focused on overall network topology, regional analyses are designed to identify the location of key network nodes as defined by the various network metrics. For each network metric (K, Eglob, Eloc), the location of the nodes within the top 20% of the distribution was identified for each participant. The consistency of the location across subjects was determined by overlapping the maps. The resultant maps are scaled from 0% to 100% indicating the percentage of participants who had a top 20% node in each location. Due to the fact that these metrics are typically not normally distributed (particularly at the voxel level), this procedure is commonly used to identify the location of network hubs.28, 34, 35
The regional modular structure of the entire group was determined using a statistical measure called scaled inclusivity (SI). SI measures the consistency of a network community across subjects by quantifying regional overlap while penalizing for disjunction.36, 37 For the current project, SI was computed for predefined functional brain regions and subnetworks. Regions of interest (ROIs) were used to define DMN, CEN, and the SN to evaluate changes in the triple network. In addition, ROIs were defined for the SMC, VC, and the BG as these subnetworks have been shown to be the most consistent across participants.37 The DMN, CEN, and SN were defined according Shirer et al,38 the SMN was defined according to Hugenschmidt et al,39 and the VC and BG were defined according to Moussa et al.37, 38 The ROIs are depicted in Figure 1.
Figure 1.
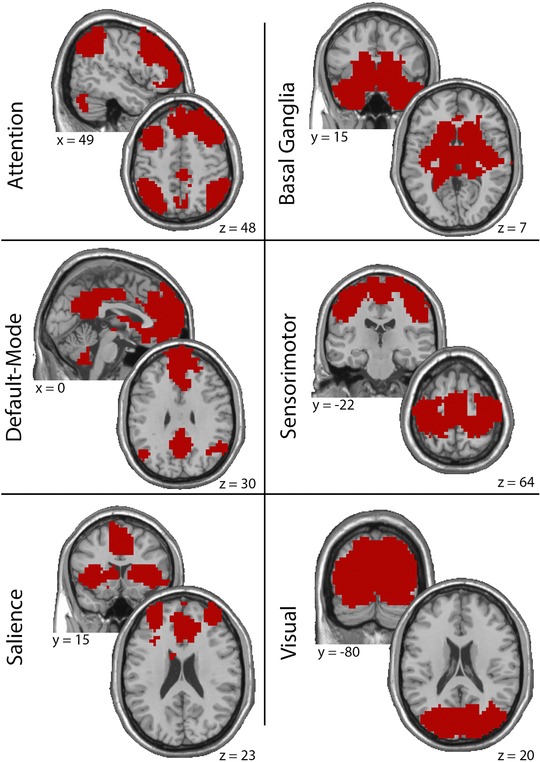
Maps showing location and extent of the ROIs used in this study. Montreal Neurological Institute (MNI) coordinates are listed below each slice.
Statistical comparisons of the regional network properties all used paired‐sample permutation tests.40 For the network metrics (K, Eglob, Eloc), the maps of the top 20% nodes from each participant were included in separate permutation tests. For modularity, a permutation was performed for each ROI using the SI maps from the individual participants. Average SI maps were generated to visually depict the locations and intensities of the key network nodes across the group. To address the significant imbalance in sex of the participants, an additional analysis was performed that removed the one female.
Results
Whole‐Brain Network Properties
The results from the whole‐brain analyses for each of the network metrics are presented in Figure 2. There was an expected significant effect of the threshold for all metrics (Eglob: F(2,16) = 2,327, P < .001; Eloc: F(2,16) = 2,957, P < .001; Q: F(2,16) = 145, P < .001). Across all network thresholds, none of the whole brain metrics exhibited a main effect of the intervention (Eglob: F(1,17) = .46, P = .508; Eloc: F(1,17) = .071, P = .792; Q: F(1,17) = .051, P = .824). None of the network variables exhibited a significant interaction between threshold and intervention (Eglob: F(2,16) = 2.21, P = .143; Eloc: F(2,16) = 1.04, P = .376; Q: F(2,16) = .37, P = .698).
Figure 2.
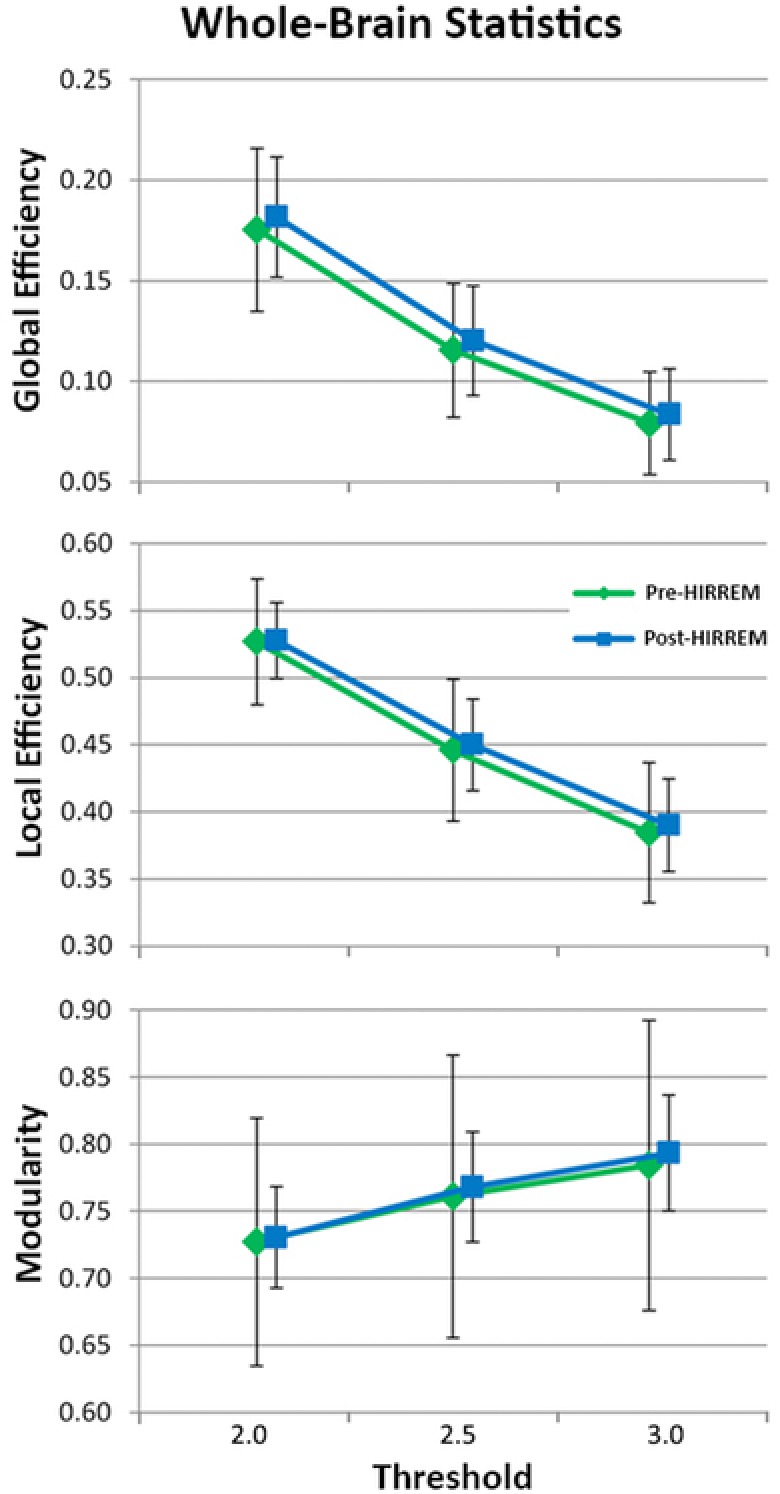
Effect of threshold on network metrics of whole‐brain analyses including global efficiency, local efficiency, and modularity.
Regional Network Properties
Figure 3 shows the spatial location of the top network hubs for (A) degree, (B) global efficiency, and (C) local efficiency pre‐ and post‐HIRREM. The degree maps were significantly different between pre‐ and post‐HIRREM (P = .007). The hubs were consistently localized to the sensorimotor area, VC, and the DMN prior to the intervention. After the intervention, there are clear reductions in the hubs in sensorimotor and visual regions. However, connectivity in the DMN tended to increase.
Figure 3.
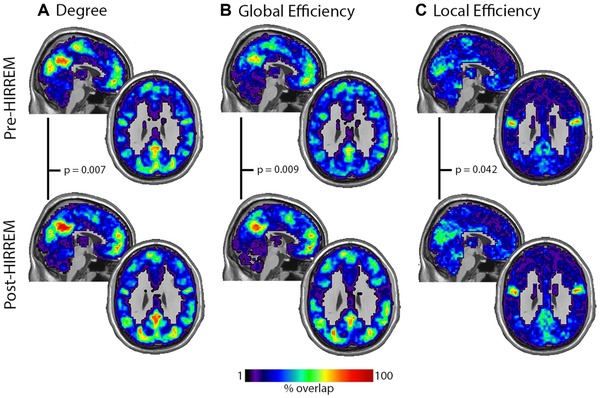
Maps showing spatial location of the top network hubs for (A) degree, (B) global efficiency, and (C) local efficiency pre‐ and post‐HIRREM. P values for the paired comparison between pre and postintervention are presented for each network metric. The calibration bar applies to all images.
Maps of the high global efficiency nodes closely resembled the degree maps. Key nodes were also located in the VC, SMC, and regions of the DMN. Statistical comparisons revealed significant differences (P = .009) between pre‐ and post‐HIRREM treatment. As with the degree, there were reductions of the consistency of high Eglob nodes in the visual and motor areas and increases in the DMN.
Regional maps of local efficiency demonstrated that sensorimotor and visual cortices exhibited high Eloc, but with reduced consistency compared to degree or Eglob. The area of highest consistency of Eloc was noted in the secondary somatosensory cortex. This region was not evident in the degree or global efficiency maps. These maps are also notable for a lack of high Eloc in the DMN. Statistical comparisons between pre‐ and post‐HIRREM intervention revealed a significant difference (P = .042). The change observed was likely driven by a reduction in the clustering of the SMC and an increase in the VC. The clustering in secondary somatosensory cortex did not exhibit notable change.
Maps showing the consistency of community structure across the group for each of the functional brain regions/subnetworks are presented in Figure 4. Statistical comparisons between pre‐ and post‐HIRREM intervention revealed significant differences in the SMC (P = . 005) and differences that approached significance in the DMN (P = .084). Analyses of the remaining communities did not reveal significant differences between pre and postintervention; CEN – P = .238, SN – P = .588, VC – P = .741, BG – P = .847.
Figure 4.
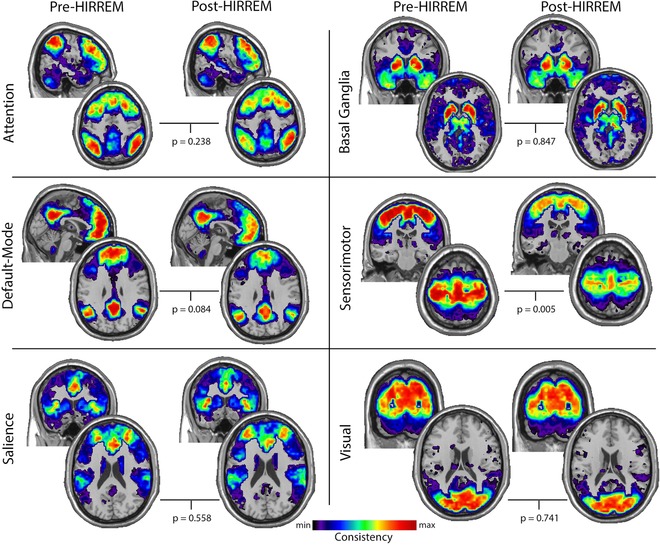
Maps showing consistency of community structure across the group for each of the functional brain regions/subnetworks pre‐ and post‐HIRREM intervention. P values for the paired comparison between pre and postintervention are presented for each subnetwork. The images were scaled to visually depict the spatial variance in the community consistency. Thus, the minimum and maximum consistencies are different across subnetworks. The scaling values (min, max) for each subnetwork are: attention (.01, .13), basal ganglia (.01, .1), default‐mode (.01, .18), sensorimotor (.01, .33), salience (.01, .07), and visual (.01, .25).
It has been suggested that the DMN is not a homogenous network with the anterior (comprised of the medial prefrontal cortex) and posterior (encompassing the precuneus/posterior cingulate and the lateral temporoparietal areas) aspects of this circuit being involved in different neurobiological processes.41 Given this suggestion and the growing documentation of altered medial prefrontal function in PTSD,6, 7, 8, 9 secondary analyses were performed to separately compare pre and postintervention community structure in the anterior and posterior aspects of the DMN. This analysis revealed that the anterior portion of the DMN exhibited highly significant reductions in community structure (P = .010) following HIRREM. The consistency of community structure within the posterior aspect of the DMN did not change with the intervention (P = .668).
Findings in Men Only
Given that this study included 17 males and only one female, all analyses (whole brain and regional) were performed again while excluding the female participant. Without the one female, the significant difference in the regional maps of local efficiency was no longer significant (P = .110) and there was a significant prepost difference in the DMN community (P = .030) that did not reach significance with the female included. No other statistical comparison, either for whole‐brain or regional measures, resulted in a different outcome when excluding the one female. Thus, the overall outcomes of this study did not qualitatively change when the female participant was excluded from the analyses.
Discussion
Convergent data suggest that the neurobiology of PTSD involves disruption of complex brain networks. The current study provides the first data showing changes in brain connectivity on resting‐state MRI among individuals with symptoms of military‐related PTS or PTSD, who undertook usage of an allostatic neurotechnology (HIRREM) for autocalibration of neural oscillations. There were postinterventional changes in connectivity in the DMN, sensorimotor, and visual circuits which may explain clinical benefits reported elsewhere.16 In contrast, the present study did not demonstrate significant changes in connectivity at the whole brain level or in the SN or CEN as a whole.
The network degree and global efficiency analyses revealed a general increase in DMN connectivity, which is consistent with the premise that these subjects may have had relatively low DMN connectivity at their baseline, as shown in other studies of PTSD.42 In addition to the increased connectivity, the DMN exhibited a relative decrease in community structure. Although not directly measured, the findings suggest that the connectivity increase in DMN was actually from an increase in connections from the DMN to other brain circuits. If connectivity had increased within DMN regions, community structure would have increased as well. These changes in DMN connectivity after the intervention may have reflected a therapeutic effect. Optimized communication between the DMN and other brain circuits could facilitate a more coherent sense of self (eg, reduction of dissociative or depersonalizing aspects of PTSD), and might also be associated with improved switching between the DMN and other circuits.26
In contrast to the increased connectivity demonstrated in the DMN overall, other regions showed connectivity reductions. In addition to the changes in overall number of connections and connection efficiency, the anterior portion of the DMN and the sensorimotor network (SMN) showed dramatic decrease in community structure. The change in the anterior DMN community, which encompasses medial prefrontal cortex, is consistent with data showing enhanced anterior DMN connectivity in PTSD, which may contribute to performance impairment.8 For individuals with PTS(D), a decrease in anterior DMN connectivity might indicate therapeutic reduction of self‐referencing or mind‐wandering. The decrease in SMN connectivity may explain the reduction of insomnia symptoms reported by this cohort.16 Non‐rapid eye movement sleep and sleep deprivation are shown to be associated with reduced and increased connectivity of the SMN, respectively,43, 44 raising the possibility that autocalibration of the waking SMN, toward reduction of connectivity, may have helped these highly vigilant and physically trained individuals to transition to sleep states.
The findings indicate both increases and decreases of network connectivity in directions that would be consistent with symptom remediation. By focusing the intervention on the brain, with respect for its role as the organ of central command, successful allostatic therapeutics is predicted to entail optimized reorchestration of functioning across systems.17, 45 The allostatic strategy is to go upstream to the source of pathogenesis, to facilitate adaptive variability of set points across subsystems downstream. Studies have also indicated that this allostatic neurotechnology may be therapeutic for postural orthostatic tachycardia syndrome,46 sport‐related concussion,47 and other conditions.18, 48
Several features or limitations of this study should be noted. Some of the participants did not necessarily endorse a clinical diagnosis of PTSD, and all but one was male. We believe that studies of military traumatic stress should continue to enroll individuals who are not necessarily treatment‐seeking, as has been recommended given the widespread reality of symptom under‐reporting.49 It is also important for future research to evaluate network changes in women, who are increasingly integrated into the armed forces. Because this study did not include a control group, it is not possible to quantify what portion of the network connectivity changes may have been due to usage of the neurotechnology intervention as such, rather than nonspecific treatment factors including subjective expectation and overall participation in a study intervention over 12 days. Nonetheless, the durability of the clinical improvements (to 6 months) as well as the autonomic cardiovascular changes reported elsewhere16 is not consistent with regression to the mean, the natural history of PTS(D) symptomatology, or typical characteristics of the placebo response. The statistical significance for the changes in the DMN (including anterior portion) as well as the SMN, corresponding to data that associate these regions to PTSD symptomatology and sleep respectively, suggests that those changes were due to intervention effects rather than natural variability in network connectivity. Most participants were affiliated with special operations, and their unique training and experiences may have influenced the results. Finally, the majority of participants reported recognized traumatic brain injuries, which can also alter brain networks.
In conclusion, we report changes in brain network connectivity that are consistent with remediation of symptoms of PTS(D), for a cohort of mostly male special operation officers who undertook usage of an allostatic, closed‐loop acoustic stimulation neurotechnology (HIRREM) for autocalibration of neural oscillations. Postinterventional changes in connectivity of the DMN including the anterior portion, as well as the SMN, may explain their clinical improvements. Ongoing study is strongly warranted, with continued and increasing attention to participant characteristics including enrollment of women, the role of nonspecific treatment factors, test‐retest reliability of connectivity measures, and inclusion of additional analyses that will allow correlation to clinical and physiological outcomes.
Acknowledgments and Disclosures: Katelyn McNab for assistance with statistical analyses. Nancy C. Buchheimer assisted with the IRB approval and research grant management. Lindsay J. Howard and Krystal D. Schmidt helped with provision of the study intervention and the organization of data for analysis.
The primary financial support for this study was through the Joint Capability Technology Demonstration Program within the Office of the Under Secretary of Defense (Acquisition, Technology, and Logistics) via a contract with the U.S. Special Operations Command (H92222‐14‐P‐0012). It is also supported by a research grant from The Susanne Marcus Collins Foundation, Inc. Sean L. Simpson was supported by NIBIB K25 EB012236‐01A1. Opinions, interpretations, conclusions, and recommendations are those of the authors and are not necessarily endorsed by the Department of Defense. All coauthors affiliated with Wake Forest School of Medicine have no conflicts to report. Lee Gerdes is an employee, and Dr. Sung W. Lee was previously an employee of Brain State Technologies.
References
- 1. Menon V. Large‐scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci 2011;15:483‐506. [DOI] [PubMed] [Google Scholar]
- 2. Lanius RA, Frewen PA, Tursich M, et al. Restoring large‐scale brain networks in PTSD and related disorders: a proposal for neuroscientifically‐informed treatment interventions. Eur J Psychotraumatol 2015;6:27313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Koch SB, van Zuiden M, Nawijn L, et al. Aberrant resting‐state brain activity in posttraumatic stress disorder: a meta‐analysis and systematic review. Depress Anxiety 2016;33:592‐605. [DOI] [PubMed] [Google Scholar]
- 4. Akiki TJ, Averill CL, Abdallah CG. A network‐based neurobiological model of PTSD: evidence from structural and functional neuroimaging studies. Curr Psychiatry Rep 2017;19:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders: DSM‐5. 5th ed. Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- 6. Kennis M, van Rooij SJ, van den Heuvel MP, et al. Functional network topology associated with posttraumatic stress disorder in veterans. Neuroimage Clin 2016;10:302‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kennis M, Rademaker AR, van Rooij SJ, et al. Resting state functional connectivity of the anterior cingulate cortex in veterans with and without post‐traumatic stress disorder. Hum Brain Mapp 2015;36:99‐109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Daniels JK, McFarlane AC, Bluhm RL, et al. Switching between executive and default mode networks in posttraumatic stress disorder: alterations in functional connectivity. J Psychiatry Neurosci 2010;35:258‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sripada RK, King AP, Welsh RC, et al. Neural dysregulation in posttraumatic stress disorder: evidence for disrupted equilibrium between salience and default mode brain networks. Psychosom Med 2012;74:904‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tursich M, Ros T, Frewen PA, et al. Distinct intrinsic network connectivity patterns of post‐traumatic stress disorder symptom clusters. Acta Psychiatr Scand 2015;132:29‐38. [DOI] [PubMed] [Google Scholar]
- 11. Bluhm RL, Williamson PC, Osuch EA, et al. Alterations in default network connectivity in posttraumatic stress disorder related to early‐life trauma. J Psychiatry Neurosci 2009;34:187‐94. [PMC free article] [PubMed] [Google Scholar]
- 12. Lanius RA, Bluhm RL, Coupland NJ, et al. Default mode network connectivity as a predictor of post‐traumatic stress disorder symptom severity in acutely traumatized subjects. Acta Psychiatr Scand 2010;121:33‐40. [DOI] [PubMed] [Google Scholar]
- 13. Cisler JM, Sigel BA, Kramer TL, et al. Modes of large‐scale brain network organization during threat processing and posttraumatic stress disorder symptom reduction during TF‐CBT among adolescent girls. PLoS One 2016;11:e0159620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Long Z, Duan X, Xie B, et al. Altered brain structural connectivity in post‐traumatic stress disorder: a diffusion tensor imaging tractography study. J Affect Disord 2013;150:798‐806. [DOI] [PubMed] [Google Scholar]
- 15. King AP, Block SR, Sripada RK, et al. Altered default mode network (DMN) resting state functional connectivity following a mindfulness‐based exposure therapy for posttraumatic stress disorder (PTSD) in combat veterans of Afghanistan and Iraq. Depress Anxiety 2016;33:289‐99. [DOI] [PubMed] [Google Scholar]
- 16. Tegeler CL, Gerdes L, Shaltout HA, et al. Successful use of closed‐loop allostatic neurotechnology for post‐traumatic stress symptoms in military personnel: self‐reported and autonomic improvements. Mil Med Res 2017;4:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gerdes L, Gerdes P, Lee SW, et al. HIRREM: a noninvasive, allostatic methodology for relaxation and auto‐calibration of neural oscillations. Brain Behav 2013;3:193‐205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tegeler CH, Kumar SR, Conklin D, et al. Open label, randomized, crossover pilot trial of high‐resolution, relational, resonance‐based, electroencephalic mirroring to relieve insomnia. Brain Behav 2012;2:814‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tegeler CH, Cook JF, Tegeler CL, et al. Clinical, hemispheric, and autonomic changes associated with use of closed‐loop, allostatic neurotechnology by a case series of individuals with self‐reported symptoms of post‐traumatic stress. BMC Psychiatry 2017;17:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sterling P. Allostasis: a model of predictive regulation. Physiol Behav 2012;106:5‐15. [DOI] [PubMed] [Google Scholar]
- 21. Lei D, Li K, Li L, et al. Disrupted functional brain connectome in patients with posttraumatic sress disorder. Radiology 2015;276:818‐27. [DOI] [PubMed] [Google Scholar]
- 22. Ogawa S, Lee TM, Kay AR, et al. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci USA 1990;87:9868‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tzourio‐Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. Neuroimage 2002;15:273‐89. [DOI] [PubMed] [Google Scholar]
- 24. Biswal B, Yetkin FZ, Haughton VM, et al. Functional connectivity in the motor cortex of resting human brain using echo‐planar MRI. Magn Reson Med 1995;34:537‐41. [DOI] [PubMed] [Google Scholar]
- 25. Hayasaka S. Functional connectivity networks with and without global signal correction. Front Hum Neurosci 2013;7:880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Power JD, Barnes KA, Snyder AZ, et al. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 2012;59:2142‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Power JD, Mitra A, Laumann TO, et al. Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage 2014;84:320‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hayasaka S, Laurienti PJ. Comparison of characteristics between region‐and voxel‐based network analyses in resting‐state fMRI data. Neuroimage 2010;50:499‐508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Laurienti PJ, Joyce KE, Telesford QK, et al. Universal fractal scaling of self‐organized networks. Physica A 2011;390:3608‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Latora V, Marchiori M. Efficient behavior of small‐world networks. Phys Rev Lett 2001;87:198701. [DOI] [PubMed] [Google Scholar]
- 31. Newman ME, Girvan M. Finding and evaluating community structure in networks. Phys Rev E Stat Nonlin Soft Matter Phys 2004;69:026113. [DOI] [PubMed] [Google Scholar]
- 32. Vincent DB, Jean‐Loup G, Renaud L, et al. Fast unfolding of communities in large networks. J Stat Mech 2008;2008:P10008. [Google Scholar]
- 33. Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci 2009;10:186‐98. [DOI] [PubMed] [Google Scholar]
- 34. Hagmann P, Cammoun L, Gigandet X, et al. Mapping the structural core of human cerebral cortex. PLoS Biol 2008;6:e159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. van den Heuvel MP, Stam CJ, Boersma M, et al. Small‐world and scale‐free organization of voxel‐based resting‐state functional connectivity in the human brain. Neuroimage 2008;43:528‐39. [DOI] [PubMed] [Google Scholar]
- 36. Steen M, Hayasaka S, Joyce K, et al. Assessing the consistency of community structure in complex networks. Phys Rev E Stat Nonlin Soft Matter Phys 2011;84:016111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Moussa MN, Steen MR, Laurienti PJ, et al. Consistency of network modules in resting‐state FMRI connectome data. PLoS One 2012;7:e44428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shirer WR, Ryali S, Rykhlevskaia E, et al. Decoding subject‐driven cognitive states with whole‐brain connectivity patterns. Cereb Cortex 2012;22:158‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hugenschmidt CE, Burdette JH, Morgan AR, et al. Graph theory analysis of functional brain networks and mobility disability in older adults. J Gerontol B Psychol Sci Soc Sci 2014;69:1399‐406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Simpson SL, Lyday RG, Hayasaka S, et al. A permutation testing framework to compare groups of brain networks. Front Comput Neurosci 2013;7:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Uddin LQ, Kelly AM, Biswal BB, et al. Functional connectivity of default mode network components: correlation, anticorrelation, and causality. Hum Brain Mapp 2009;30:625‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wisco BE, Marx BP, Miller MW, et al. Probable posttraumatic stress disorder in the US veteran population according to DSM‐5: results from the National Health and Resilience in Veterans Study. J Clin Psychiatry 2016;77:1503‐10. [DOI] [PubMed] [Google Scholar]
- 43. Wu CW, Liu PY, Tsai PJ, et al. Variations in connectivity in the sensorimotor and default‐mode networks during the first nocturnal sleep cycle. Brain Connect 2012;2:177‐90. [DOI] [PubMed] [Google Scholar]
- 44. Zhu Y, Feng Z, Xu J, et al. Increased interhemispheric resting‐state functional connectivity after sleep deprivation: a resting‐state fMRI study. Brain Imaging Behav 2016;10:911‐9. [DOI] [PubMed] [Google Scholar]
- 45. Sterling P. Principles of allostasis: optimal design, predictive regulation, pathophysiology, and rational therapeutics In: Schulkin J, ed. Allostasis, Homeostasis, and the Costs of Physiological Adaptation. New York, NY: Cambridge University Press; 2004:17‐64. [Google Scholar]
- 46. Fortunato JE, Tegeler CL, Gerdes L, et al. Use of an allostatic neurotechnology by adolescents with postural orthostatic tachycardia syndrome (POTS) is associated with improvements in heart rate variability and changes in temporal lobe electrical activity. Exp Brain Res 2016;234:791‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tegeler CH, Tegeler CL, Cook JF, et al. A preliminary study of the effectiveness of an allostatic, closed‐loop, acoustic stimulation neurotechnology in the treatment of athletes with persisting post‐concussion symptoms. Sports Med Open 2016;2:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tegeler CH, Tegeler CL, Cook JF, et al. Reduction in menopause‐related symptoms associated with use of a noninvasive neurotechnology for autocalibration of neural oscillations. Menopause 2015;22:650‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ramchand R, Rudavsky R, Grant S, et al. Prevalence of, risk factors for, and consequences of posttraumatic stress disorder and other mental health problems in military populations deployed to Iraq and Afghanistan. Curr Psychiatry Rep 2015;17:37. [DOI] [PubMed] [Google Scholar]


