Abstract
Background
In elder patients after out‐of‐hospital cardiac arrest, diminished neurologic function as well as reduced neuronal plasticity may cause a low response to targeted temperature management (TTM). Therefore, we investigated the association between TTM (32‐34°C) and neurologic outcome in cardiac arrest survivors with respect to age.
Material and Methods
This retrospective cohort study included patients 18 years of age or older suffering a witnessed out‐of‐hospital cardiac arrest with presumed cardiac cause, which remained comatose after return of spontaneous circulation. Patients were a priori split by age into four groups (<50 years (n = 496); 50‐64 years (n = 714); 65‐74 years (n = 395); >75 years (n = 280)). Subsequently, within these groups, patients receiving TTM were compared to those not treated with TTM.
Results
Out of 1885 patients, 921 received TTM for 24 hours. TTM was significantly associated with good neurologic outcome in patients <65 years of age whereas showing no effect in elders (65‐74 years: OR: 1.49 (95% CI: 0.90‐2.47); > 75 years: OR 1.44 (95% CI 0.79‐2.34)).
Conclusion
In our cohort, it seems that TTM might not be able to achieve the same benefit for neurologic outcome in all age groups. Although the results of this study should be interpreted with caution, TTM was associated with improved neurologic outcome only in younger individuals, patients with 65 years of age or older did not benefit from this treatment.
Keywords: cardiopulmonary resuscitation, induced hypothermia, prognosis, resuscitation, survival
1. INTRODUCTION
Ageing of the population and the consecutively increased need for medical attention are among the most challenging problems of medical systems in industrialized countries. According to the World Health Organization, the proportion of elderlies is growing faster than any other age group.1 Treatment of elder patients, especially in the setting of intensive care medicine, is ethically and medical challenging. Changing in physiology, comorbidities and polypharmacy in elders making intensive care complex.2 Due to underrepresentation of this age group in most prospective randomized trials, scientific evidence is poor. The answer to the question if an individual will actually benefit from specific intensive care measures depends on multiple factors.3
Due to a higher incidence of cardio‐vascular morbidity in higher age and ageing of the population, out‐of‐hospital cardiac arrest (OHCA) is an increasing event and a global health issue.4 Studies have shown that the median age of patients suffering cardiac OHCA ranges between 60 and 70 years.5, 6, 7 Patient age may significantly impact decisions and treatment strategies for post‐resuscitation care.8, 9 The chance of good neurologic outcome and survival after OHCA may be influenced by the increasing age, but cannot be generalised to be bad.10
With respect to modest evidence available, targeted temperature management (TTM) has shown to improve neurologic outcome as well as survival after OHCA.11 However, until now, TTM is used as an “one‐size fits all” therapy. Little attention is paid to individual treatment approaches guided by patient‐specific factors. Growing evidence supports the assumption that the protective effect of TTM is influenced by patient‐specific characteristics or circumstances concerning the cardiac arrest itself.12, 13 In the elders, an altered neurologic function as well as reduced neuronal plasticity may cause a lower chance of recovery after global cerebral ischaemia. Restructuring of the cortical network as well as impaired neurogenesis is apparent in the “old brain”.14, 15, 16, 17 Decreased neuronal plasticity may cause an increased sensitivity to ischaemia/reperfusion injury and therefore might alter the beneficial effect of TTM in OHCA survivors. The aim of this retrospective cohort study was to investigate the association between the TTM with a target temperature of 32‐34°C and outcome in different age groups.
2. MATERIAL AND METHODS
This is a retrospective cohort analysis based on data prospectively collected between June 1992 and May 2015 for the Vienna Clinical Cardiac Arrest Registry. The registry comprises all adult OHCA patients, admitted to‐ and treated at the Department of Emergency Medicine of the Medical University of Vienna, a tertiary care facility. The institutional ethical review board has approved this registry (ethic committee number: 1814/2012). Acquisition and documentation of data were performed according to the Utstein Style.18 Every patient is followed up op to 6 months by a health care professional. Personal visits or standardized telephone interviews, if the patient is discharged from hospital. Initial data, for example “no‐flow” time, are detected carefully through meticulous communications with the dispatch centre, the emergency physicians, the paramedics on scene, bystanders and relatives.
2.1. Study population
The study population comprises patients with witnessed OHCA who remained comatose (Glasgow coma scale ≤8) after return of spontaneous circulation (ROSC). Patients younger than 18 years of age, patients with traumatic or cerebrovascular cause of arrest, a core temperature of <30°C as well as patients with pre‐existing severe neurological or overall deficits [defined as cerebral performance category (CPC) >2 or overall performance category (OPC) >2] were excluded. Performance scores are as follows: 1 (good function); 2 (moderate disability); 3 (severe disability); 4 (a vegetative state); and 5 (death).
2.2. Exposure
The main risk factor was receiving TTM or not. Patients treated with TTM were cooled to a target temperature of 32‐34°C. Target temperature was kept within this range for 24 hours followed by rewarming with 0.5°C/hour.
To assess an association of TTM and outcome with respect to age, patients were a priori split by age into four groups (1st group <50 years; 2nd group 50‐64 years; 3rd group 65‐74 years; and 4th group >75 years). Subsequently, within these groups, patients receiving TTM were compared to those not treated with TTM.
2.3. Outcome
The primary outcome was neurologic function during a follow‐up period of 6 months. Cerebral function was assessed prospectively on arrival at the emergency department and at predefined time points up to 6 months after ROSC and was expressed in terms of CPC categories. A performance score of 1 or 2 was considered as a good neurologic recovery. The best ever achieved CPC within 6 months after ROSC was used for determination of primary outcome. Patients dying during sedation were categorised as unfavourable neurologic outcome. Secondary outcome was overall survival rate assessed at 6 months.
2.4. Statistical analysis
Continuous variables are reported as the median and quartile 1 to quartile 3 (Q1‐Q3). Categorical variables are presented as absolute and relative frequencies. Primary and secondary outcomes were binary, and the chi‐square test was used to compare the outcomes between groups. For normally distributed continuous variables, the Student's t test was performed. To test non‐normally distributed variables, we used the Kruskal‐Wallis test. All effects were calculated using multivariable logistic regression models to estimate the association between the mild therapeutic hypothermia and neurological recovery while allowing for potential confounders and including interaction terms for age groups. Therefore, we initially included all available confounders which, from a clinical point of view, may be associated with the outcomes: sex, bystander basic life support (yes/no), presumed cardiac cause of arrest (yes/no), “no‐flow” time (time from collapse until start of life support [minutes]), “low‐flow” time (time from start of life support until return of spontaneous circulation [minutes]), known coronary heart disease (yes/no), known chronic obstructive pulmonary disease (yes/no), known cerebral vascular insufficiency (yes/no), ventricular fibrillation or pulseless ventricular tachycardia as the first monitored rhythm (yes/no) and the cumulative dosage of epinephrine (mg). To compensate temporal changes in post‐resuscitation care, we included the time of cardiac arrest stratified into three subgroups (1st period 1992‐2002, 2nd period 2003‐2008 and 3rd period 2009‐2015). Continuous variables were examined for any possible linear associations with the outcome. To keep the most parsimonious model, we excluded variables that did not change the effect size of the primary risk factors by more than 10% from the final model and did not significantly change the goodness‐of‐fit model. The goodness‐of‐fit was assessed by the Hosmer‐Lemeshow test. To estimate absolute risks, we calculated “no‐flow” quartile specific baseline risks from contingency tables. We transformed the adjusted age group specific odds ratios and 95% confidence intervals into risk ratios using the specific baseline risks with standard formulas. For data management and analyses, we used MS Excel 2011 for Mac, SPSS software (version 20.0, SPSS Inc., Chicago, Ill) and Stata 11 for Mac (Stata Corp, College Station, Tx). Generally, a two‐sided P‐value <0.05 was considered statistically significant.
3. RESULTS
Out of 4700 registered patients, 1885 fulfilled the inclusion criteria and were analysed. A patient flow chart is presented in Figure 1. The baseline characteristics of patients are presented in Table 1. More women, especially over 75 years of age, did not receive TTM treatment. Basic life support was also performed less in the No‐TTM group. Patients with an initial non‐shockable rhythm more likely received TTM treatment. The “low‐flow” time was a shorter in the No‐TTM groups compared to those patients who received TTM.
Figure 1.
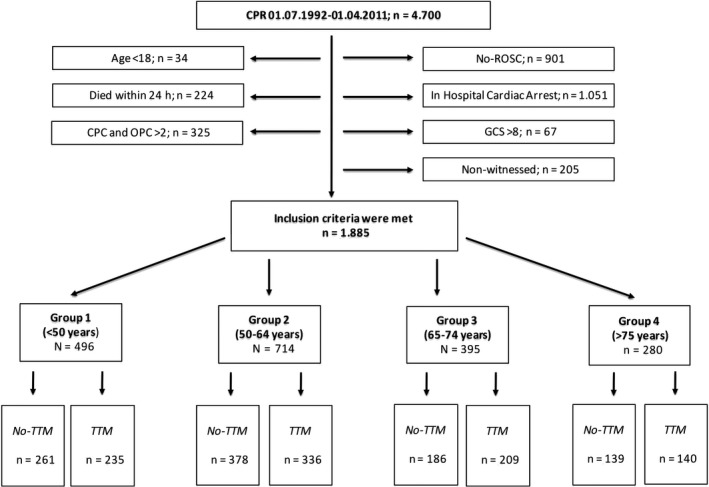
Patient Flow chart; CPR, cardiopulmonary resuscitation; CPC, cerebral performance category; GCS, Glasgow coma scale; OPC, overall performance category; ROSC, return of spontaneous circulation; TTM, targeted temperature management
Table 1.
Baseline characteristics
| Groups of age | No‐TTMa (n = 964) | TTMa (n = 921) | P‐value |
|---|---|---|---|
| Female Sex‐no./total no. (%) | |||
| 1. Group (<50 years) | 97/261 (37) | 69/235 (29) | 0.07 |
| 2. Group (50‐64 years) | 92/378 (24) | 73/336 (22) | 0.42 |
| 3. Group (65‐74 years) | 62/186 (33) | 52/209 (25) | 0.08 |
| 4. Group (> 75 years) | 63/139 (45) | 38/141 (27) | <0.01 |
| Basic Life Support no./total no.l (%) | |||
| 1. Group (<50 years) | 98/261 (38) | 125/235 (53) | <0.01 |
| 2. Group (50‐64 years) | 124/378 (33) | 186/336 (55) | <0.01 |
| 3. Group (65‐74 years) | 65/186 (35) | 91/209 (44) | 0.10 |
| 4. Group (> 75 years) | 44/139 (32) | 72/141 (51) | <0.01 |
| Initial shockable rhythm ‐ no./total no. (%) | |||
| 1. Group (<50 years) | 123/261 (47) | 87/235 (37) | 0.03 |
| 2. Group (50‐64 years) | 155/378 (41) | 104/336 (30) | 0.05 |
| 3. Group (65‐74 years) | 83/186 (44) | 85/209 (41) | 0.48 |
| 4. Group (>75 years) | 74/139 (53) | 63/141 (45) | 0.19 |
| Cardiac Origin ‐ no./total no. (%) | |||
| 1. Group (<50 years) | 144/261 (55) | 148/235 (63) | 0.08 |
| 2. Group (50‐64 years) | 287/378 (76) | 263/337 (78) | 0.54 |
| 3. Group (65‐74 years) | 136/186 (73) | 141/209 (67) | 0.28 |
| 4. Group (>75 years) | 103/139 (74) | 107/141 (76) | 0.89 |
| “No‐flow” time, minutes ‐ median (Q1‐Q3) | |||
| 1. Group (<50 years) | 2 (0‐8) | 2 (0‐5) | 0.14 |
| 2. Group (50‐64 years) | 3 (0‐8) | 2 (0‐6) | 0.11 |
| 3. Group (65‐74 years) | 2 (0‐8) | 2 (0‐7) | 0.99 |
| 4. Group (>75 years) | 2 (0‐8) | 2 (0‐6) | 0.89 |
| “Low‐flow” time, minutes‐median (Q1‐Q3) | |||
| 1. Group (<50 years) | 15 (8‐24) | 20 (11‐35) | <0.01 |
| 2. Group (50‐64 years) | 15 (7‐25) | 19 (12‐31) | <0.01 |
| 3. Group (65‐74 years) | 17 (10‐25) | 21 (14‐36) | <0.01 |
| 4. Group (>75 years) | 15 (6‐23) | 19 (14‐29) | <0.01 |
| Cumulative Adrenalin dose, milligram‐median (Q1‐Q3) | |||
| 1. Group (<50 years) | 2 (0‐5) | 2 (0‐4) | 0.02 |
| 2. Group (50‐64 years) | 2 (1‐5) | 2 (0‐4) | <0.01 |
| 3. Group (65‐74 years) | 3 (2‐5) | 3 (1‐4) | 0.34 |
| 4. Group (>75 years) | 2 (0‐4) | 2 (1‐4) | 0.95 |
Temperature Management with 32‐34°C.
3.1. Outcomes
In our analysis, increasing age was independently associated with worse neurologic outcome (odds ratio [OR]: 098; 95% confidence interval [CI]: 0.97‐0.98) as well as with overall mortality (OR: 0.97; 95% CI: 0.96‐0.98). In spite of showing a significant improvement in favourable neurologic outcome within 6 months after ROSC in all patients treated with TTM (OR: 1.69; 95% CI: 1.35‐2.02), this effect changed with increasing age. A pronounced effect of mild therapeutic hypothermia was evident in patients younger than 65 years with the highest odds ratio in patients under 50 years (OR: 2.11; 95% CI: 1.32‐3.39). On the other hand, we found no significant difference in neurologic outcome in patients 65 years of age or older (Table 2). TTM was associated with an overall survival benefit (OR 1.62; 95% CI: 1.30‐2.02). This association was evident in all subgroups except for patients aged from 65 to 74 years (Table 2).
Table 2.
Outcome analysis
| No ‐ TTMa No./total no. (%) | TTMa No./total no. (%) | Odds ratio (95% CI) crude | Odds ratio (95% CI) adjusted | |
|---|---|---|---|---|
| Good neurological outcome | ||||
| Overall | 363/964 (45%) | 448/921 (55%) | 1.57 (1.31‐1.88) | 1.90 (1.36‐2.67) |
| 1. Group (<50 years) | 113/261 (43%) | 142/235 (60%) | 2.00 (1.40‐2.86) | 2.70 (1.39‐5.25) |
| 2. Group (50‐64 years) | 152/378 (40%) | 188/336 (56%) | 1.89 (1.40‐2.54) | 2.43 (1.32‐4.45) |
| 3. Group (65‐74 years) | 59/186 (32%) | 74/209 (35%) | 1.18 (0.78‐1.79) | 1.15 (0.53‐2.53) |
| 4. Group (>75 years) | 39/139 (28%) | 44/141 (31%) | 1.16 (0.70‐1.94) | 1.63 (0.74‐3.60) |
| Survival | ||||
| Overall | 363/964 (45%) | 448/921 (55%) | 1.59 (1.32‐1.91) | 1.94 (1.40‐2.69) |
| 1. Group (<50 years) | 136/261 (53%) | 155/235 (66%) | 1.78 (1.24‐2.56) | 2.13 (1.12‐4.08) |
| 2. Group (50‐64 years) | 172/378 (46%) | 202/336 (60%) | 1.81 (1.34‐2,43) | 2.66 (1.45‐4.87) |
| 3. Group (65‐74 years) | 58/186 (31%) | 81/209 (39%) | 1.40 (0.92‐2.12) | 1.22 (0.57‐2.62) |
| 4. Group (>75 years) | 30/139 (22%) | 46/141 (33%) | 1.76 (1.03‐3.04) | 2.33 (1.04‐5.20) |
TTM, Targeted Temperature Management with 32‐34°C.
Adjustment for multiple confounders included sex, presumed cardiac cause of arrest, initial shockable rhythm, no‐flow time (time from collapse to initiation of life support), low‐flow time (time from start of life support to return of spontaneous circulation), cumulative dose of epinephrine, time of cardiac arrest.
4. DISCUSSION
In our study, targeted temperature management with a goal temperature of 32‐34°C was associated with an increased rate of good neurological outcome in younger patients (age <64 years), whereas the beneficial effect of TTM was not detectable in elderly patient aged over 65 years. TTM was not associated with improved overall survival in the young elderly (aged from 65 to 74 years), whereas all other subgroups showed a significant improved survival when TTM was applied.
Our database comprises more than 4000 patients and a health care professional continuously followed every patient. Through this intense effort and communication of our staff members, we can ensure the best possible quality of our data.
Although our data originate from a large database, due to the retrospective nature, we have to mention some limitations. Most important, our study results from a single‐centre registry. Therefore, various types of bias (including selection bias), as well as confounding, cannot be excluded and generalization of our study results is limited. As our older cohorts consist of less patients than the younger cohorts, the results of our study should be interpreted with caution as they are as they are at risk of type II error. Likewise, our observational period is long and intensive care treatment, and post‐resuscitation care standards have changed over the past 20 years. Treatment was always performed according to the appropriate guidelines and should not have been dependent on patient age. However, as this will influence all age groups, we therefore do not think that this will have a major impact on our results. Temporal trends in treatment, prognostication, withdrawal of care and inclusion of patients are possible factors influencing our results. We tried to compensate for this by including the era of resuscitation in our regression model.
Generally, and as previously reported, prevalence of favourable neurological outcome declined with increasing patient age. This is comparable to a recently published cohort study in resuscitated elderly patients.19 The pathophysiological mechanisms of brain response to cerebral ischaemia in advanced age are poorly understood and merely derive from models of focal cerebral ischaemia.20 In experimental reversible focal ischaemia, infarct size develops even more rapid in older individuals. Furthermore, scar tissue formation was accelerated and functional recovery was impaired.21, 22, 23 In experimental intracerebral haemorrhage in aged animals, cerebral injury was aggravated by increased in microglial activation.24 A change in cytokine response after cerebral insult increases the risk of neurodegeneration and cognitive decline in aged animal models.25, 26 Therapeutic interventions are rare. Interestingly, long‐term hypothermia of 48 hours reduced infarct volume in aged rats after focal ischaemia.27 Consequently, increased and prolonged neuroinflammation in the elderly might be a reason for the missing neuroprotective effect of TTM in the patients >75 years in our study.
The majority of previous studies focused on elderly patients paid little attention to the neurological outcomes in these highly vulnerable patients.28 Recently, increasing age was shown to be associated with significantly increased mortality and risk of poor neurological outcome after OHCA.29 As a substudy of the “targeted temperature management trial,” results were consistent in terms of showing no influence of different temperature ranges of TTM on neurologic outcome or survival.30 Considering the demographic changes of the western populations, the number of potential elderly OHCA patients needing specialized post‐resuscitation care is increasing.31 Therefore, intensified research in the field of elderly resuscitated patients including prospective randomized trials seems to be mandatory to optimize treatment and outcome.
To the opinion of the authors, this analysis should not be the basis for withholding TTM to elderly cardiac arrest victims. Future studies should focus on specific TTM strategies especially in the elderly.
5. CONCLUSION
In our cohort, it seems that in patients above an age of 65 years, TTM might not be able to achieve the same benefit for neurologic outcome as for survival in the same way than in younger patients. It is time for working on personalized targeted temperature management.
CONFLICT OF INTEREST AND FUNDING
The authors declare that there is no conflict of interest. There is no financial funding/support to declare.
AUTHOR CONTRIBUTION
CW and CT conceived the study idea. PS, PH and ALS conducted the data analysis. CT and ANS implemented statistical analysis. ALS, ANS, PH, FS, CT and CW jointly drafted the manuscript.
ACKNOWLEDGEMENTS
We are indebted to the nurses and staff for their enthusiastic cooperation and to the patients who participated in this study for their trust and support.
Wallmüller C, Spiel A, Sterz F, et al. Age‐dependent effect of targeted temperature management on outcome after cardiac arrest. Eur J Clin Invest. 2018;48:e13026 10.1111/eci.13026
REFERENCES
- 1. Aging and life course. World report on ageing and health. World Health Organization, 2015. (Accessed 12 September 2016, at External Link http://apps.who.int/iris/bitstream/10665/186463/1/9789240694811_eng.pdf?ua=1) [Google Scholar]
- 2. Sprung CL, Artigas A, Kesecioglu J, et al. The Eldicus prospective, observational study of triage decision making in European intensive care units. Part II: intensive care benefit for the elderly. Crit Care Med. 2012;40:132‐138. [DOI] [PubMed] [Google Scholar]
- 3. Sprung CL, Baras M, Iapichino G, et al. The Eldicus prospective, observational study of triage decision making in European intensive care units: part I–European Intensive Care Admission Triage Scores. Crit Care Med. 2012;40:125‐131. [DOI] [PubMed] [Google Scholar]
- 4. Roth GA, Forouzanfar MH, Moran AE, et al. Demographic and epidemiologic drivers of global cardiovascular mortality. N Engl J Med. 2015;372:1333‐1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tagami T, Matsui H, Fushimi K, Yasunaga H. Changes in therapeutic hypothermia and coronary intervention provision and in‐hospital mortality of patients with out‐of‐hospital cardiac arrest: a nationwide database study. Crit Care Med. 2016;44:488‐495. [DOI] [PubMed] [Google Scholar]
- 6. Schewe JC, Kappler J, Heister U, et al. Outcome of out‐of‐hospital cardiac arrest over a period of 15 years in comparison to the RACA score in a physician staffed urban emergency medical service in Germany. Resuscitation. 2015;96:232‐238. [DOI] [PubMed] [Google Scholar]
- 7. Ong ME, Shin SD, De Souza NN, et al. Outcomes for out‐of‐hospital cardiac arrests across 7 countries in Asia: The Pan Asian Resuscitation Outcomes Study (PAROS). Resuscitation. 2015;96:100‐108. [DOI] [PubMed] [Google Scholar]
- 8. Swor RA, Jackson RE, Tintinalli JE, Pirrallo RG. Does advanced age matter in outcomes after out‐of‐hospital cardiac arrest in community‐dwelling adults? Acad Emerg Med. 2000;7:762‐768. [DOI] [PubMed] [Google Scholar]
- 9. Sulzgruber P, Sterz F, Poppe M, et al. Age‐specific prognostication after out‐of‐hospital cardiac arrest ‐ The ethical dilemma between ‘life‐sustaining treatment’ and ‘the right to die’ in the elderly. Eur Heart J Acute Cardiovasc Care. 2017;6:112‐120. [DOI] [PubMed] [Google Scholar]
- 10. Bunch TJ, White RD, Khan AH, Packer DL. Impact of age on long‐term survival and quality of life following out‐of‐hospital cardiac arrest. Crit Care Med. 2004;32:963‐967. [DOI] [PubMed] [Google Scholar]
- 11. Arrich J, Holzer M, Havel C, Müllner M, Herkner H. Hypothermia for neuroprotection in adults after cardiopulmonary resuscitation. Cochrane Database Syst Rev. 2016;15:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wallmüller C, Testori C, Sterz F, et al. Limited effect of mild therapeutic hypothermia on outcome after prolonged resuscitation. Resuscitation. 2016;98:15‐19. [DOI] [PubMed] [Google Scholar]
- 13. Testori C, Sterz F, Holzer M, et al. The beneficial effect of mild therapeutic hypothermia depends on the time of complete circulatory standstill in patients with cardiac arrest. Resuscitation. 2012;83:596‐601. [DOI] [PubMed] [Google Scholar]
- 14. Cabeza R. Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychol Aging. 2002;17:85. [DOI] [PubMed] [Google Scholar]
- 15. Vidal‐Piñeiro D, Valls‐Pedret C, Fernández‐Cabello S, et al. Decreased default mode network connectivity correlates with age‐associated structural and cognitive changes. Front Aging Neurosci. 2014;6:256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Christian KM, Song H, Ming GL. Functions and dysfunctions of adult hippocampal neurogenesis. Ann Rev Neurosci. 2014;37:243‐262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Damoiseaux JS, Beckmann CF, Arigita EJ. Reduced resting‐state brain activity in the “default network” in normal aging. Cereb Cortex. 2008;18:1856‐1864. [DOI] [PubMed] [Google Scholar]
- 18. Jacobs I, Nadkarni V, Bahr J, et al. Cardiac arrest and cardiopulmonary resuscitation outcome reports: update and simplification of the Utstein templates for resuscitation registries. A statement for healthcare professionals from a task force of the international liaison committee on resuscitation (American Heart Association, European Resuscitation Council, Australian Resuscitation Council, New Zealand Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Southern Africa). Resuscitation. Resuscitation. 2004;63:233‐249. [DOI] [PubMed] [Google Scholar]
- 19. Grimaldi D, Dumas F, Perier MC, et al. Short‐ and long‐term outcome in elderly patients after out‐of‐hospital cardiac arrest: a cohort study. Crit Care Med. 2014;42:2350‐2357. [DOI] [PubMed] [Google Scholar]
- 20. Beumer D, Rozeman AD, Lycklama À, et al. The effect of age on outcome after intraarterial treatment in acute ischemic stroke: a MR CLEAN pretrial study. BMC Neurol. 2016;16:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Badan I, Dinca I, Buchhold B, et al. Accelerated accumulation of N‐ and C‐terminal beta APP fragments and delayed recovery of microtubule‐associated protein 1B expression following stroke in aged rats. Eur J Neurosci. 2004;19:2270‐2280. [DOI] [PubMed] [Google Scholar]
- 22. Deng XH, Bertini G, Xu YZ, Yan Z, Bentivoglio M. Cytokine‐induced activation of glial cells in the mouse brain is enhanced at an advanced age. Neuroscience. 2006;141:645‐661. [DOI] [PubMed] [Google Scholar]
- 23. Popa‐Wagner A, Badan I, Walker L, Groppa S, Patrana N, Kessler C. Accelerated infarct development, cytogenesis and apoptosis following transient cerebral ischemia in aged rats. Acta Neuropathol. 2007;113:277‐293. [DOI] [PubMed] [Google Scholar]
- 24. Lee TY, Murphy BD, Aviv RI, et al. Cerebral blood flow threshold of ischemic penumbra and infarct core in acute ischemic stroke: a systematic review. Stroke. 2006;37:2201. [DOI] [PubMed] [Google Scholar]
- 25. Buchanan JB, Sparkman NL, Chen J, Johnson RW. Cognitive and neuroinflammatory consequences of mild repeated stress are exacerbated in aged mice. Psychoneuroendocrinology. 2008;33:755‐765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gavilán MP, Revilla E, Pintado C, et al. Molecular and cellular characterization of the age‐related neuroinflammatory processes occurring in normal rat hippocampus: potential relation with the loss of somatostatin GABAergic neurons. J Neurochem. 2007;103:984‐996. [DOI] [PubMed] [Google Scholar]
- 27. Florian B, Vintilescu R, Balseanu AT, et al. Long‐term hypothermia reduces infarct volume in aged rats after focal ischemia. Neurosci Lett. 2008;438:180‐185. [DOI] [PubMed] [Google Scholar]
- 28. Murphy DJ, Murray AM, Robinson BE, Campion EW. Outcomes of cardiopulmonary resuscitation in the elderly. Ann Intern Med. 1989;111:199‐205. [DOI] [PubMed] [Google Scholar]
- 29. Oh SJ, Kim JJ, Jang JH, et al. Age is related to neurological outcome in patients with out‐of‐hospital cardiac arrest (OHCA) receiving therapeutic hypothermia (TH). Am J Emerg Med. 2018;36:243‐247. [DOI] [PubMed] [Google Scholar]
- 30. Winther‐Jensen M, Pellis T, Kuiper M, et al. Mortality and neurological outcome in the elderly after target temperature management for out‐of‐hospital cardiac arrest. Resuscitation. 2015;91:92‐98. [DOI] [PubMed] [Google Scholar]
- 31. Vandrevala T, Hampson SE, Daly T, Arber S, Thomas H. Dilemmas in decision‐making about resuscitation–a focus group study of older people. Soc Sci Med. 2006;62:1579‐1593. [DOI] [PubMed] [Google Scholar]


