Abstract
Excessive gestational weight gain (GWG) is a risk factor for several adverse pregnancy outcomes, including macrosomia. Diet is one of the few modifiable risk factors identified. However, most dietary assessment methods are impractical for use in maternal care. This study evaluated whether a short dietary screening questionnaire could be used as a predictor of excessive GWG in a cohort of Icelandic women. The dietary data were collected in gestational weeks 11–14, using a 40‐item food frequency screening questionnaire. The dietary data were transformed into 13 predefined dietary risk factors for an inadequate diet. Stepwise backward elimination was used to identify a reduced set of factors that best predicted excessive GWG. This set of variables was then used to calculate a combined dietary risk score (range 0–5). Information regarding outcomes, GWG (n = 1,326) and birth weight (n = 1,651), was extracted from maternal hospital records. In total, 36% had excessive GWG (Icelandic criteria), and 5% of infants were macrosomic (≥4,500 g). A high dietary risk score (characterized by a nonvaried diet, nonadequate frequency of consumption of fruits/vegetables, dairy, and whole grain intake, and excessive intake of sugar/artificially sweetened beverages and dairy) was associated with a higher risk of excessive GWG. Women with a high (≥4) versus low (≤2) risk score had higher risk of excessive GWG (relative risk = 1.23, 95% confidence interval, CI [1.002, 1.50]) and higher odds of delivering a macrosomic offspring (odds ratio = 2.20, 95% CI [1.14, 4.25]). The results indicate that asking simple questions about women's dietary intake early in pregnancy could identify women who should be prioritized for further dietary counselling and support.
Keywords: dietary habits, dietary screening, food frequency questionnaire, gestational weight gain, macrosomia, maternal nutrition
Key messages.
There is a lack of practical methods to assess dietary intake in the clinical setting.
This study used a short dietary screening questionnaire, answered by pregnant women early in pregnancy. Women with poor dietary habits (with a high dietary risk score) had higher risk of excessive GWG and higher odds of delivering a newborn with an excessive birth weight (≥4,500 g) compared with women with the lowest scores.
By asking simple questions about dietary habits early in pregnancy, it might be possible to identify women who should be prioritized for further dietary counselling. This procedure could translate to more cost‐effective strategies.
1. INTRODUCTION
Gestational weight gain (GWG), that is, the amount of weight gained between conception until the time of birth, is an essential aspect of pregnancy (Institute of Medicine and National Research Council, 2009). However, excessive GWG is associated with pregnancy and birth complications as well as macrosomia (Goldstein et al., 2017; Tian et al., 2016). Excessive GWG is also the primary factor contributing to increased postpartum weight retention (Rong et al., 2015), and high weight gain from first to second pregnancy is related to increased risk of stillbirth and infant mortality (Cnattingius & Villamor, 2016). Excessive GWG has also been associated with long‐term implications for both maternal and offspring health, that is, later adiposity and cardio‐metabolic risk (Fraser et al., 2011; Mamun, Mannan, & Doi, 2014; Perez‐Morales, Bacardi‐Gascon, & Jimenez‐Cruz, 2015; Tie et al., 2014; Walter et al., 2015).
Results from observational studies have repeatedly shown that dietary habits characterized by high consumption of fruits and vegetables, wholegrain, fish, healthy fat, and low consumption of food with little nutritional value are associated with lower risk of excessive GWG and pregnancy complications (Brantsaeter et al., 2009; Chen et al., 2016; Englund‐Ogge et al., 2014; Hillesund, Bere, Haugen, & Overby, 2014; Knudsen, Orozova‐Bekkevold, Mikkelsen, Wolff, & Olsen, 2008; Martin, Sotres‐Alvarez, & Siega‐Riz, 2015; Renault et al., 2015; Shin, Lee, & Song, 2016; Tielemans et al., 2015; Tryggvadottir, Medek, Birgisdottir, Geirsson, & Gunnarsdottir, 2016; Uusitalo et al., 2009). Results from intervention studies, aiming at improving diet, physical activity, or both, have shown that reduction in GWG can be achieved. However, the effect size reported in these studies has been modest or around 0.70 kg (95% confidence interval, CI [0.48, 0.92 kg]) on average according to a recent meta‐analysis (Rogozinska et al., 2017). The clinical relevance of such a modest reduction on other maternal and birth outcomes is unclear (Rogozinska et al., 2017). The mean reduction in GWG reported in various intervention studies has been considerably smaller than associations from previous observational studies have indicated (Knudsen, Heitmann, Halldorsson, Sorensen, & Olsen, 2013; Maslova, Halldorsson, Astrup, & Olsen, 2015; Renault et al., 2015). Apart from problems with compliance, one reason for this difference and the modest effect size seen in intervention studies might be that many interventions recruit their subjects based on weight and do not take into consideration the actual dietary habits of those recruited (Dodd et al., 2014; Guelinckx, Devlieger, Mullie, & Vansant, 2010; Poston et al., 2015). Using this inclusion criteria has its limitations, as far from all overweight or obese women have suboptimal diets (Tryggvadottir et al., 2016).
The combined evidence from observational, experimental, and intervention studies strongly suggests that a healthy diet is important for the short‐ and long‐term health of the mother and child (World Health Organization, 2016). Still, transfer of existing knowledge into clinical practice has been relatively slow. One reason for this is the lack of methods to assess dietary intake, as the use of detailed questionnaires or dietary interviews designed to cover the whole diet is time‐consuming and impractical for use in clinical practice (Shim, Oh, & Kim, 2014). There is a need for a simple dietary screening tool in the clinical setting. It might be more purposeful and cost‐effective to target dietary counselling at more vulnerable groups, based on the background diet. This study aimed to examine whether a short dietary screening questionnaire, answered by pregnant Icelandic women in their first trimester of pregnancy, can give reliable indications of the risk of excessive GWG and macrosomia.
2. PARTICIPANTS AND METHODS
2.1. PREgnant Women of ICEland (The PREWICE cohort)
Between October 1, 2015, and September 30, 2016, pregnant women in gestational weeks 11–14 who came for an ultrasound at the prenatal diagnostic unit at Landspitali University Hospital were offered to take part in the study. At that timepoint, a dietary screening questionnaire was administered. About 75% of all pregnant women living in the metropolitan area use the clinic's services. Women who did not understand Icelandic and could therefore not answer the questionnaire were not invited to take part in the study. A total of 2,113 (77%) out of 2,734 eligible women participated in the study. The ethics committee of Landspitali University Hospital approved the study protocol (21/2015), and written consent was obtained from all participants.
2.2. Subjects included in the analyses
Out of the 2,113 women answering the dietary screening questionnaire, maternal care records were missing for 417 (~20%); the majority probably due to births outside Landspitali University Hospital. Our ethical approval was gathered from the local ethical committee at Landspitali National University Hospital, only allowing us to record information from the maternal care records for women giving birth at Landspitali. Additional 26 women who had multiple pregnancies and another 19 who had missing dietary data were excluded, resulting in 1,651 women being eligible for the analyses. Of these, 313 (19%) had missing data on total GWG, and 12 had missing data on prepregnancy body mass index (BMI) status. The final dataset, therefore, consisted of 1,326 women with data on GWG and 1,651 with data on offspring birth weight. There were no significant differences in general baseline characteristics, that is, marital status, parity, smoking status, or dietary measures (i.e., the dietary risk factors for inadequate diet) among those who were included in the analyses and those who were excluded because of missing data. However, small differences were found for prepregnancy BMI status and education level. Women with missing data had a higher prepregnancy BMI status (24.6 vs. 24.1 kg m−2, P = 0.01) and were less likely to have a university education (52% vs. 59%, P = 0.01) compared with women included in the analyses.
2.3. The dietary assessment
The dietary screening questionnaire (Data S1) was designed to give a snapshot of the participant's general diet in comparison with food‐based dietary guidelines, but at the same time to predict low consumption of key nutrients for fetal development (such as omega‐3 fatty acid, vitamin D, and iodine) based on Icelandic diet. Women could answer the dietary screening questionnaire in 5–10 min. It consisted of a 40‐item list of common foods for which frequency of consumption was recorded (i.e., times per day, daily, times per week, weekly, times per month, monthly, or less than monthly). Women were asked about their diet in the previous 4 weeks, corresponding to the first trimester of pregnancy (enrolled in 11–14 week of pregnancy).
Prior to use in this study, the questionnaire was pilot tested in a group of 25 pregnant women and compared with a 4‐day weighed food record, with acceptable correlation (Spearman's correlation > +0.3) for most food groups/items.
2.4. Covariates and outcomes
Information on maternal lifestyle and socioeconomic factors, prepregnancy weight, and height were recorded at recruitment. Self‐reported prepregnancy weight and height were also available from maternal records, and those measures were used if the information at recruitment was missing. Information about maternal age, gestational length, and GWG was retrieved from the maternal hospital records as women were weighed in antenatal visits. Total GWG was calculated as the difference between the highest recorded weight (≥week 36 in pregnancy) and prepregnancy weight; this information was used to define GWG. Icelandic recommendations on weight gain in pregnancy determined the definition of excessive GWG in this study. Optimal weight gain was 12.1–18.0 kg for prepregnant normal‐weight women and 7.1–12.0 kg for overweigth or obese women (Thorsdottir & Birgisdottir, 1998; Thorsdottir, Torfadottir, Birgisdottir, & Geirsson, 2002). Offspring birth weight was measured at birth by medical staff and collected from the medical records. Macrosomia was defined as a birth weight of 4,500 g or higher (Chatfield, 2001).
2.5. The dietary risk score
The data gathered with the frequency questions were transformed into predefined 13 dietary risk factors for inadequate diet (see Figure 1). These factors are based on the Nordic (Nordic Nutrition Recommendations, 2014) and Icelandic dietary recommendations (Embætti landlæknis, 2016), as well as evidence on the association between diet, nutrient intake, and the health of the mother and child (Englund‐Ogge et al., 2012; Gunnarsdottir et al., 2016; Hrolfsdottir et al., 2016; Olafsdottir, Skuladottir, Thorsdottir, Hauksson, & Steingrimsdottir, 2006; Olsen et al., 2007; Renault et al., 2015; Zhu et al., 2017). Stepwise backward elimination (logistic regression) was used to identify the best combination of these factors for predicting excessive GWG. Model performance was assessed by Nagelkerke's R 2. The following six dietary risk factors (predictors) were included in the final model: not eating a varied diet, fruits/vegetables <5 times per day, dairy <2 times per day, whole grain products <2 times per day, sugar/artificially sweetened beverages ≥5 times per week, and dairy ≥5 times per day. To construct a total dietary risk score, each participant got 1 for fulfilling the risk criteria and 0 for not fulfilling the risk criteria. The scores of the six dietary risk factors were then summed up, ranging from scores of 0 to 5 as it was not possible to be in both milk risk groups (too low/too high).
Figure 1.
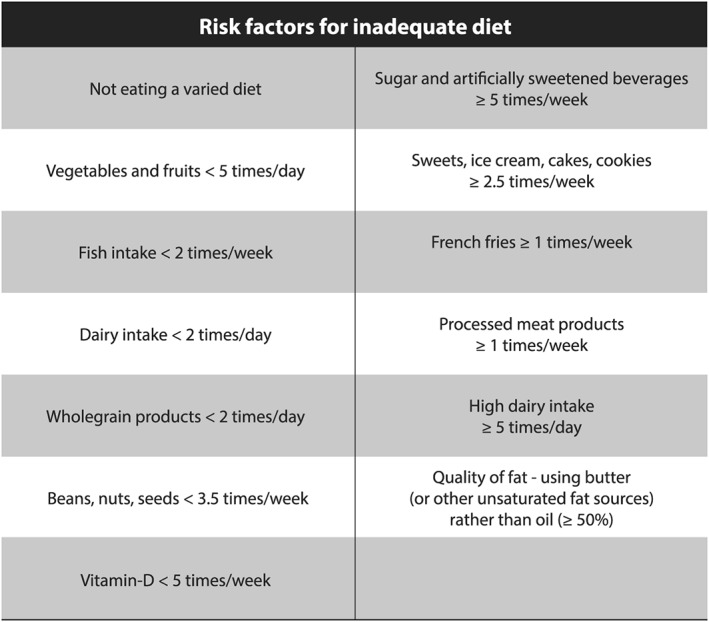
Risk factors for inadequate diet. The frequency of consumption was recorded in the list. This information was converted to frequency per week for all food groups, which was then transformed into 13 predefined dietary risk factors for inadequate diet. The risk factors were mainly based on the Icelandic Food‐Based Dietary Recommendations (Embætti landlæknis, 2016), which are based on the Nordic Nutrition Recommendations (2014). If the women excluded/avoided any of the main food groups (cereal, vegetables/fruits, fish, meat, eggs, high‐fat foods, or dairy), they were categorized to the group not eating a varied diet. Cut‐offs for sugar/artificially sweetened beverages and high dairy intake were set in line with Nordic studies. They show that high intake of these products is associated with high GWG (Hrolfsdottir et al., 2016; Olafsdottir et al., 2006; Renault et al., 2015) and adverse birth outcomes (Englund‐Ogge et al., 2012; Olsen et al., 2007; Zhu et al., 2017)
2.6. Statistical analyses
Assumptions of normality of continuous variables were checked, using histograms and quantile‐quantile (Q‐Q) plots. Normally distributed variables were described by their mean and standard deviation (SD), non‐normally distributed continuous variables by their median and 75–25th percentile, and categorical variables using frequencies (percentages). Students t tests were used to compare normally distributed continuous variables, whereas for skewed and categorical variables, Mann–Whitney U test and Chi‐square tests were used, respectively.
As excessive GWG is a relatively prevalent outcome (~36% in our sample), associations with excessive GWG in terms of relative risk (RR) were assessed using multivariable Poisson log‐linear regression (Zou, 2004). However, for macrosomia, which is relatively rare (~5% in our samples), associations were quantified in terms of odds ratios using logistic regression, as odds ratios and RR would be comparable for this outcome and a logistic regression was more robust in terms of convergence when adjusting for covariates. Associations were stratified by prepregnancy BMI status, as high prepregnancy weight is a well‐known risk factor of excessive GWG and stratifications by prepregnancy weight is commonly reported in studies reporting on predictors of GWG (Hillesund et al., 2014; Maslova et al., 2015; Olafsdottir et al., 2006). Moreover, stratification by nausea and/or vomiting in pregnancy (NVP) was also done as NVP may influence dietary habits and weight gain during pregnancy (Crozier, Inskip, Godfrey, Cooper, & Robinson, 2017; Temming et al., 2014).
We selected covariates in our multivariable models a priori, on the basis of their potential influence on dietary habits and GWG (Gaillard et al., 2012; Olafsdottir et al., 2006; Restall et al., 2014; Rogozinska et al., 2017; Stuebe, Oken, & Gillman, 2009). When examining the association between the dietary risk score and GWG, we included: maternal prepregnancy BMI (continuous), maternal age (quartiles), parity (nulliparous vs. multiparous), education (elementary schooling, high sch. and technical sch., university education, and higher academic), maternal smoking during pregnancy (yes/no), gestational length when the highest weight was recorded (quartiles), and experience of nausea (no nausea, mild [nauseous but not throwing up], moderate [nauseous and throwing up infrequently], and severe nausea [throwing up daily]). When examining the association between the dietary risk score and macrosomia, the following covariates were included: offspring sex, maternal prepregnancy BMI, maternal age, parity, education, maternal smoking during pregnancy, total gestational length, and experience of nausea.
Missing values for covariates (maternal prepregnancy BMI [0.5%], parity [1.2%], educational level [0.9%], maternal smoking during pregnancy [1.5%], and total gestational length [0.8%]) were assumed to be “missing at random” and were imputed using multiple imputation (m = 10) as implemented in SPSS (MCMC algorithm). Statistical significance was accepted at P < 0.05.
3. RESULTS
Anthropometric and demographic characteristics of the study population are presented in Table 1. For prepregnancy BMI, the mean (SD) was 24.1 (6.5) kg m−2 with 4% of subjects being underweight (BMI < 18.5 kg m−2), 55% normal weight (BMI 18.5–24.9 kg m−2), 24% overweight (BMI ≥ 25.0–30.0 kg m−2), and 18% obese (BMI ≥ 30.0 kg m−2). The mean (SD) total GWG was 14.0 (6.3) kg. In total, 36% of the mothers were defined with excessive GWG, and 5% gave birth to a macrosomic infant. The mean (SD) and range of weeks for the last measured weight was 38.5 (1.4) and 36–42 weeks, respectively. Mothers who experienced excessive GWG had a slightly longer pregnancy duration (39.8 vs. 39.6 weeks), and their offspring had a higher mean birth weight (3,844 g vs. 3,689 g) compared with women with optimal weight gain. The percentage of women gaining optimal, suboptimal, and excessive weight gain during pregnancy, by prepregnancy BMI status, is presented in Table 2. Overweight and obese women had a lower mean GWG when compared with those of normal weight or underweight. However, overweight and obese women were more likely to have excessive GWG.
Table 1.
Birth outcomes and characteristics of mothers at baseline in relation to maternal gestational weight gain
| Alla | Optimal GWGb | Suboptimal GWGc | P valuee | Exc. GWGd | P valuef | |
|---|---|---|---|---|---|---|
| (n = 1,326) | (n = 517; 39%) | (n = 333; 25%) | (n = 476; 36%) | |||
| Maternal age (years) | 30.2 ± 5.2 | 30.0 ± 5.1 | 30.1 ± 5.4 | 0.60g | 30.0 ± 5.3 | 0.20g |
| Height (cm) | 167.4 ± 6.0 | 167.3 ± 5.9 | 167.0 ± 6.0 | 0.55g | 167.8 ± 6.1 | 0.20g |
| Birth weight (g) | 3,721 ± 491 | 3,689 ± 477 | 3,596 ± 471 | 0.01g | 3,844 ± 494 | <0.01g |
| Gestational age (weeks) | 39.6 ± 1.2 | 39.6 ± 1.1 | 39.4 ± 1.3 | 0.02g | 39.8 ± 1.2 | <0.01g |
| Prepregnancy weight (kg) | 68.0 (19.0) | 64.0 (16.0) | 68.0 (27.0) | <0.01h | 72.0 (16.0) | <0.01h |
| Prepregnancy BMI (kg/m2) | 24.1 (6.5) | 22.7 (5.1) | 24.2 (9.9) | <0.01h | 25.6 (5.2) | <0.01h |
| Prepregnancy BMI (groups) | <0.01i | <0.01i | ||||
| Underweight (%)j | 4 | 4 | 4 | 3 | ||
| Normal weight (%)k | 55 | 68 | 55 | 40 | ||
| Overweight (%)l | 24 | 15 | 11 | 43 | ||
| Obese (%)m | 18 | 13 | 30 | 14 | ||
| Nulliparous (%) | 39 | 37 | 35 | 0.52i | 42 | 0.10i |
| Single (%) | 6 | 4 | 6 | 0.15i | 7 | 0.02i |
| Smoked before pregnancy (%) | 16 | 12 | 11 | 0.76i | 23 | <0.01i |
| Smoking during pregnancy (%) | 7 | 5 | 6 | 0.29i | 9 | <0.01i |
| Education (%) | <0.01i | <0.01i | ||||
| Elementary schooling | 13 | 10 | 14 | 15 | ||
| High sch. and technical sch. | 29 | 28 | 27 | 31 | ||
| University education | 35 | 31 | 39 | 36 | ||
| Higher academic | 24 | 30 | 20 | 19 | ||
| NVP experience (%) | 0.12i | 0.58i | ||||
| No nausea | 10 | 10 | 11 | 9 | ||
| Mild (not throwing up) | 48 | 48 | 42 | 51 | ||
| Moderate (throwing up infrequently) | 33 | 33 | 33 | 33 | ||
| Severe (throwing up daily) | 10 | 9 | 14 | 7 |
Note. BMI: body mass index; Exc: excessive; GWG: gestational weight gain, NVP: nausea and/or vomiting in pregnancy.
Values are mean ± standard deviation or median (interquartile range) for continuous variables and percentages for categorical variables.
Optimal GWG was determined in accordance with the Icelandic recommendations, that is, underweight and normal‐weight women 12–18 kg total GWG and overweight and obese women 7–12 kg total GWG.
Suboptimal GWG was determined in accordance with the Icelandic recommendations, that is, underweight and normal‐weight women <12 kg total GWG and overweight and obese women <7 kg total GWG.
Excessive GWG was determined in accordance with the Icelandic recommendations, that is, underweight and normal‐weight women >18 kg total GWG and overweight and obese women >12 kg total GWG.
Differences between optimal and suboptimal GWG groups.
Differences between optimal and excessive GWG groups.
F test (Type III) of differences among groups.
Mann–Whitney U test of differences among groups.
Chi‐square test of differences among group.
Underweight, BMI <18.5 kg m−2.
Normal weight, BMI 18.5–24.99 kg m−2.
Overweight, BMI ≥25 kg m−2.
Obesity, BMI ≥30 kg m−2.
Table 2.
The percentage of women gaining suboptimal, optimal,and excessive weight during pregnancy by prepregnancy weight status
| Suboptimal | Optimal | Excessive | ||
|---|---|---|---|---|
| All (n = 1,326) | 25% | 39% | 36% | |
| GWG mean ± std. (kg) | 14.0 ± 6.3 | 6.8 ± 4.3 | 13.6 ± 2.8 | 19.4 ± 4.6 |
| Suboptimal (≤12.0 kg) | Optimal (12.1–18.0 kg) | Excessive (>18.0 kg) | ||
| Prepregnancy BMI <25 (n = 772) | 25% | 48% | 26% | |
| GWG mean ± std. (kg) | 15.4 ± 5.1 | 9.4 ± 2.1 | 15.0 ± 1.7 | 21.9 ± 3.7 |
| Suboptimal (≤7.0 kg) | Optimal (7.1–12.0 kg) | Excessive (>12.0 kg) | ||
| Prepregnancy BMI ≥25 (n = 554) | 25% | 26% | 49% | |
| GWG mean ± std. (kg) | 12.0 ± 7.1 | 3.0 ± 3.6 | 9.8 ± 1.4 | 17.6 ± 4.4 |
Note. BMI: body mass index; GWG: gestational weight gain; std.: standard deviation.
Stepwise backward elimination was used to identify a reduced set of the 13 predefined dietary risk factors for inadequate diet (Figure 1) that best predicted excessive GWG. After elimination, the remaining factors were a nonvaried diet, a nonadequate intake of fruits/vegetables, dairy, and whole grain intake, as well as an excessive intake of sugar/artificially sweetened beverages and dairy. Table 3 shows the percent of women who fulfilled the risk criteria for each of these dietary risk factors. In total, 20% reported that they avoided or excluded some food groups (a nonvaried diet), with dairy being most commonly excluded (9%). Moreover, most women did not meet public recommendations for fruits and vegetables (87%), whole grain (92%), and dairy intake (77%). In total, 28% reported that they drank sugar and/or artificially sweetened beverages more than five times per week, and 2% drank milk products five times or more per day. A higher proportion of women gaining excessive GWG fulfilled the risk criteria for sugar and artificially sweetened beverages (≥5 times per week), compared with women with optimal GWG (P = 0.02). The other dietary risk factors did not differ significantly between women gaining excessive versus optimal GWG (Table 3). The frequency of intake of main food groups by adherence to the recommendation of gestational weight can be seen in Table S1.
Table 3.
Percent of women fulfilling the predefined risk criteria by gestational weight gain (GWG)
| Risk factors | All (n = 1,326) | Optimal GWGa (n = 517; 39%) | Suboptimal GWGb (n = 333; 25%) | P d | Excessive GWGc (n = 476; 36%) | P e |
|---|---|---|---|---|---|---|
| Not eating a varied diet | 20% | 18% | 22% | 0.16 | 21% | 0.20 |
| Vegetables and fruits <5 times per day | 87% | 87% | 85% | 0.26 | 89% | 0.55 |
| Dairy intake <2 times per day | 77% | 78% | 75% | 0.42 | 78% | 0.82 |
| Wholegrain products <2 times per day | 92% | 90% | 91% | 0.64 | 93% | 0.15 |
| Sugar and artificially sweetened beverages ≥5 times per week | 28% | 24% | 29% | 0.07 | 31% | 0.02 |
| Dairy intake ≥5 times per day | 1% | 1% | 2% | 0.09 | 2% | 0.06 |
Optimal GWG was determined in accordance with the Icelandic recommendations, that is, for underweight and normal‐weight women 12–18 kg total GWG and overweight and obese women 7–12 kg total GWG.
Suboptimal GWG was determined in accordance with the Icelandic recommendations; that is, underweight and normal‐weight women <12 kg total GWG and overweight and obese women <7 kg total GWG.
Excessive GWG was determined in accordance with the Icelandic recommendations; that is, underweight and normal‐weight women >18 kg total GWG and overweight and obese women >12 kg total GWG.
Chi‐square test of differences among groups (optimal vs. suboptimal GWG groups).
Chi‐square test of differences among groups (optimal vs. excessive GWG groups).
In Table 4, the results for the multivariable association between the dietary risk score, GWG, and offspring macrosomia are presented. When dichotomizing the exposure, women with the highest risk score (≥4 scores) had 23% higher risk of excessive GWG (95% CI [1.002, 1.50]) and 120% higher odds of offspring being born macrosomic (≥4,500 g; 95% CI [1.14, 4.25]), compared with women with the lowest scores (≤2 scores; Table 4). In stratified analyses (Table 5), the association between the dietary risk score and excessive GWG tended to be stronger among obese women. With macrosomia as the outcome, the association was significant only for normal‐ and underweight women. For obese women, a nonsignificant inverse association was, however, observed. However, exclusion of women with gestational diabetes (GDM; n = 264) resulted in a similar trend among all the BMI groups (Table S2). Moreover, when we stratified for NVP experience, similar results were found between the dietary risk score and excessive GWG among all groups. We, however, noted a more prominent association between the dietary risk score and macrosomia among women with mild NVP experience (nauseous but not throwing up; Table 5). We formally tested effect modification by prepregnancy BMI by including the dietary risk score (continuous variable), BMI (continuous variable), and an interaction term between the two in the regression model, along with the remaining covariates. The same was done for NVP experience (binary variable). Statistically significant interactions (P = 0.03) were observed for macrosomia when including the interaction term between the dietary risk score and BMI, but this was not observed for the other interactions terms.
Table 4.
The association between low, medium, and high dietary risk scores, excessive GWG, and macrosomia
| Excessive GWG | Macrosomia | |||||
|---|---|---|---|---|---|---|
| RR [95% CI]a | OR [95% CI]b | |||||
| Cases (%)/n | Crude | Adjustedc | Cases (%)/n | Crude | Adjustedd | |
| Low scores (≤2) | 99 (32%)/305 | Ref | Ref | 14 (4%)/377 | Ref | Ref |
| Medium scores (3) | 217 (34%)/632 | 1.06 [0.87, 1.28] | 1.04 [0.86, 1.26] | 40 (5%)/766 | 1.43 [0.77, 2.66] | 1.39 [0.73, 2.62] |
| High scores (≥4) | 160 (41%)/389 | 1.27 [1.04, 1.55]e | 1.23 [1.002, 1.50]e | 37 (7%)/508 | 2.04 [1.09, 3.83]e | 2.20 [1.14, 4.25]e |
Note. CI: confidence interval; GWG: gestational weight gain. OR: odds ratio; RR: relative risk.
Poisson log‐linear regression model, reflecting the risk of excessive GWG. Excess GWG was determined in accordance with the Icelandic recommendations,i.e., for underweight and normal‐weight women >18 kg and overweight and obese women >12 kg total GWG.
Logistic regression model, reflecting the odds of giving birth to a macrosomic infant (birthweight ≥4500 g).
Adjusted for maternal prepregnancy BMI, age, parity, smoking during pregnancy, educational level, gestational length when the highest weight was recorded and NVP experience.
Adjusted for maternal prepregnancy BMI, age, parity, smoking during pregnancy, educational level, total gestational length and offspring sex.
indicates significant associations.
Table 5.
The association between the dietary risk score, excessive GWG, and macrosomia (stratified analyses)
| Excessive GWG | Macrosomia | |||||
|---|---|---|---|---|---|---|
| RR [95% CI]a | OR [95% CI]b | |||||
| Cases (%)/n | Crude | Adjustedc | Cases (%)/n | Crude | Adjustedd | |
| Continuous risk scoree | 476 (36%)/1,326 | 1.12 [1.03, 1.22]h | 1.10 [1.01, 1.19]h | 91 (6%)/1,651 | 1.36 [1.06, 1.73]h | 1.41 [1.09, 1.83]h |
| BMI < 25f | 202 (26%)/772 | 1.13 [0.99, 1.29] | 1.08 [0.95, 1.23] | 38 (4%)/950 | 1.56 [1.07, 2.28]h | 1.62 [1.10, 2.40]h |
| BMI 25–30f | 206 (64%)/320 | 1.05 [0.96, 1.16] | 1.05 [0.95, 1.15] | 33 (8%)/395 | 1.45 [0.96, 2.19] | 1.53 [0.95, 2.48] |
| BMI ≥ 30f | 68 (29%)/234 | 1.21 [0.93, 1.57] | 1.25 [0.96, 1.64] | 20 (7%)/306 | 0.78 [0.45, 1.36] | 0.82 [0.43, 1.56] |
| NVP experience (no nausea)f , g | 42 (24%)/127 | 1.01 [0.73, 1.43] | 0.94 [0.65, 1.35] | 4 (2%)/173 | 1.11 [0.30, 4.07] | 1.00 [0.21, 4.68] |
| NVP experience (mild)f , g | 244 (32%)/633 | 1.13 [1.002, 1.27] | 1.11 [0.99, 1.25] | 48 (6%)/772 | 1.70 [1.19, 2.44]h | 1.81 [1.23, 2.66]h |
| NVP experience (moderate)f , g | 156 (36%)/438 | 1.14 [0.99, 1.31] | 1.09 [0.95, 1.25] | 29 (5%)/557 | 1.01 [0.67, 1.51] | 1.04 [0.67, 1.61] |
| NVP experience (severe)f , g | 34 (23%)/128 | 1.15 [0.88, 1.49] | 1.28 [0.91, 1.80] | 10 (7%)/149 | 1.26 [0.65, 2.43] | 1.30 [0.63, 2.54] |
Note. BMI: body mass index; CI: confidence interval; GWG: gestational weight gain; NVP: nausea and/or vomiting in pregnancy; OR: odds ratio; RR: relative risk.
Poisson log‐linear regression model, reflecting the risk of excessive GWG. Excess GWG was determined in accordance with the Icelandic recommendations, that is, for underweight and normal‐weight women >18 kg and overweight and obese women >12 kg total GWG.
Logistic regression model, reflecting the odds of giving birth to a macrosomic infant (birthweight ≥ 4,500 g).
Adjusted for maternal prepregnancy BMI, age, parity, smoking during pregnancy, educational level, gestational length when the highest weight was recorded, and NVP experience.
Adjusted for maternal prepregnancy BMI, age, parity, smoking during pregnancy, educational level, total gestational length, and offspring sex.
Reflecting the risk of excessive GWG or odds macrosomia per one unit increase in risk score.
When stratified by prepregnancy BMI or NVP experience these variables were not included in the models as covariates.
NVP experience: no nausea, mild (nauseous but not throwing up), moderate (nauseous and throwing up infrequently), and severe nausea (throwing up daily).
indicates significant associations.
As use of backward elimination for selecting factors that predict GWG involves some arbitrary decisions in terms of where to stop the elimination process, we examined the stability of our findings by creating standardized risk scores based on fewer and more dietary factors being retained in the model (Table S3). The combination of the six dietary risk factors resulted in the most significant association.
4. DISCUSSION
This study aimed to evaluate whether a short dietary screening questionnaire, answered by pregnant women in their first trimester of pregnancy, could give reliable indications of risk for excessive GWG and macrosomia. We found that a risk score, including a nonvaried diet, a nonadequate intake of fruits/vegetables, dairy, and whole grain, as well as an excessive intake of sugar/artificially sweetened beverages and dairy, was associated with a higher risk of excessive GWG and macrosomia. Our results suggest that by asking simple questions about women's dietary intake early in pregnancy, we might be able to identify women who should be prioritized for further dietary counselling and support.
Results from numerous observational studies have shown that a healthy dietary pattern during pregnancy is associated with a decrease in the odds of excessive GWG and various pregnancy complications (Brantsaeter et al., 2009; Chen et al., 2016; Englund‐Ogge et al., 2014; Hillesund et al., 2014; Knudsen et al., 2008; Martin et al., 2015; Renault et al., 2015; Shin et al., 2016; Tielemans et al., 2015; Tryggvadottir et al., 2016; Uusitalo et al., 2009). However, these results have not been mirrored in nutritional intervention studies (Rogozinska et al., 2017). One reason might be the “one size fits all” approach most commonly used, where researchers recruit and test a specific dietary intervention, independent of the participant's background diet. To change this approach, a practical method to assess dietary intake is needed.
Associations between dietary intake in pregnancy and pregnancy outcomes have commonly been evaluated using detailed food frequency questionnaires or face‐to‐face interviews which might take up to an hour to answer if it is designed to cover the whole diet (Shim et al., 2014). The short dietary screening questionnaire used in this study was, however, designed to give a snapshot of a participant's general diet in comparison with food‐based dietary guidelines. From this, we identified a reduced set of variables that best predicted excessive GWG.
The results of our study are in line with previous studies using more detailed dietary assessment. Soft drinks and intake of foods high in sugar have been linked to a higher risk of excessive GWG in several studies (Hrolfsdottir et al., 2016; Olafsdottir et al., 2006; Renault et al., 2015; Stuebe et al., 2009). Previous Icelandic (Olafsdottir et al., 2006) and Danish (Hrolfsdottir et al., 2016) studies have also found high milk intake in pregnancy to be associated with excessive GWG; which is potentially related to insulin‐like growth factor‐1 mediated growth‐promoting effects (Olsen et al., 2007; Qin, He, & Xu, 2009; Sferruzzi‐Perri, Owens, Pringle, & Roberts, 2011). Dietary pattern analyses have, however, shown that healthy dietary patterns that include dairy may be protective for excessive GWG (Hillesund et al., 2014; Shin et al., 2016). In the Norwegian MoBa cohort (Hillesund et al., 2014), adherence to a diet including dairy, fruits/vegetables, whole grains, potatoes, fish, and regular meals was associated with a lower risk of gaining excessive GWG among women with prepregnancy BMI < 25 kg m−2. In the National Health and Nutrition Examination Survey data (Shin et al., 2016), a similar posteriori‐derived dietary pattern was also inversely associated with GWG. These results harmonize with our findings and indicate that specific food patterns may play a role in weight management during pregnancy.
Macrosomia is a known risk factor for offspring obesity and metabolic syndrome later in life (Boney, Verma, Tucker, & Vohr, 2005; Hermann, Dallas, Haskell, & Roghair, 2010; Rasmussen & Johansson, 1998). Interventions focused on women at higher risk may, therefore, represent a significant strategy to tackle obesity from a population health perspective. Maternal GWG (Tian et al., 2016) and diet seem to be important risk factors for macrosomia, with a recent systematic review and meta‐analysis of nine randomized controlled trials (n = 7,458) reporting that personalized maternal nutrition guidance can significantly reduce the rate of offspring macrosomia (RR 0.29; 95% CI [0.18, 0.45]; Ge, Wang, & Fan, 2015). The current study showed that women with poor dietary habits, that is, with a high dietary risk score (≥4 scores), had ~2 higher odds of giving birth to a macrosomic infant compared with women with the lowest scores (≤2 scores). This association was, however, not observed among obese women. This may be related to the fact that about half of the obese women (49%) received treatment for GDM, which may have resulted in improved dietary habits and lower GWG (Brown et al., 2017). Importantly, our results indicate that prevention efforts should not only target heavy women because more than 40% of the macrosomic cases were among women with a prepregnancy normal BMI. When stratified by prepregnancy BMI status, the association between the dietary risk score and macrosomia was the strongest among lean women (prepregnancy BMI < 25). Today, limited attention is given to the diet quality of normal weight women receiving maternal health care in Iceland, and the situation might be similar in other countries.
GWG is a complex biological phenomenon; maternal physiological and metabolic changes may influence it, along with placental metabolism (Catalano & deMouzon, 2015; Institute of Medicine and National Research Council, 2009). In addition to dietary behaviour, genetic vulnerability (Andersson et al., 2015), gut microbiota composition (Collado, Isolauri, Laitinen, & Salminen, 2008), and the rate of physical activity (Olson & Strawderman, 2003) may all influence women's weight gain. Further development of the dietary screening questionnaire and the dietary risk score presented in this study might include harmonization with other known modifiable risk factors, such as low level of physical activity (Olson & Strawderman, 2003). However, it could simply be used in the present form as a first screening tool for maternal dietary counselling. Standard maternity care could very easily include this procedure, as it only takes 5–10 min to answer the dietary screening questionnaire. Focusing more on maternal diet, prioritized by urgency and expected impact, might translate into more cost‐effective strategies within the primary care.
The high rate of participation, prospective data collection, and information from medical records are the main strength of this study. It is a limitation to our study that the short dietary screening questionnaire has only been validated against 4‐day weighed food records in a pilot study among 25 pregnant women. However, the correlation for most food groups was acceptable (Spearman's correlation >+0.3). Moreover, prepregnancy weight and height were based on self‐reported data, possibly leading to bias due to under‐reporting. Former studies have, however, demonstrated a relatively strong correlation between self‐reported prepregnancy weight and measured weights before pregnancy (Phelan et al., 2011). Even though our covariate adjustments had minimal influence on our effect estimates compared with unadjusted models, we cannot exclude residual confounding or confounding by unmeasured covariate(s). We used the Icelandic recommendations for GWG, and the methodology was based on both predefined (dietary recommendations) and data‐driven (stepwise backward elimination) methods, tested in an Icelandic population. The results might therefore not apply to other populations. Testing this methodology and the dietary risk score in other datasets, as well as examining other important outcomes, for example, GDM and risk of deficiency of nutrients important for fetal growth, will allow for more rigorous conclusions.
In summary, we found that a dietary risk score, including a nonvaried diet, a nonadequate intake of fruits/vegetables, dairy, and whole grain, as well as an excessive intake of sugar/artificially sweetened beverages and dairy, was associated with a higher risk of excessive GWG and macrosomia. Our results stress that dietary counselling to promote healthy GWG and diet during pregnancy should focus not only on targeting overweight and obese women but also women of normal weight. Today, the latter get limited attention regarding this matter in the primary health care setting. By asking simple questions about women's dietary habits early in pregnancy, we might be able to identify women in more need of support and counselling to meet the GWG recommendations and to find women at higher risk of giving birth to macrosomic infants. This procedure could translate to more cost‐effective strategies in the clinical setting.
CONFLICTS OF INTEREST
IG is the owner of a non‐profit company behind the website http://www.nmb.is that includes the questionnaire used in the present study. The website is run as a non‐profit NGO. LH, TIH, BEB, IH, and HH have no conflicts of interest to declare.
CONTRIBUTIONS
IG, LH, and TIH designed the research. IG designed the questionnaire and, with colleagues, initiated the data gathering. LH was responsible for the collection of the web‐based data. LH performed the statistical analyses with guidance and feedback from TIH. LH wrote the first draft of the paper and had primary responsibility for final content. All authors contributed to and critically reviewed the manuscript. They have all approved the final manuscript.
Supporting information
Data S1. Supporting information
Table S1. Food intake of main food groups (per week) by adherence to the recommended gestational weight gain (GWG) (n=1326)
Table S2. The association between the dietary risk score, GWG, and macrosomia (GDM cases excluded).
Table S3. Different combinations of the dietary risk score and the risk of excessive gestational weight gain (GWG)a
ACKNOWLEDGMENTS
We are particularly grateful to the women who participated in the study. We would also like to acknowledge the work of the PREWICE staff and the great staff at the Ultrasound Department at Landspitali National University Hospital who made the study possible.
Hrolfsdottir L, Halldorsson TI, Birgisdottir BE, Hreidarsdottir IT, Hardardottir H, Gunnarsdottir I. Development of a dietary screening questionnaire to predict excessive weight gain in pregnancy. Matern Child Nutr. 2019;15:e12639 10.1111/mcn.12639
REFERENCES
- Andersson, E. S. , Silventoinen, K. , Tynelius, P. , Nohr, E. A. , Sorensen, T. I. , & Rasmussen, F. (2015). Heritability of gestational weight gain—A Swedish register‐based twin study. Twin Research and Human Genetics, 18(4), 410–418. 10.1017/thg.2015.38 [DOI] [PubMed] [Google Scholar]
- Boney, C. M. , Verma, A. , Tucker, R. , & Vohr, B. R. (2005). Metabolic syndrome in childhood: Association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics, 115(3), e290–e296. 10.1542/peds.2004-1808 [DOI] [PubMed] [Google Scholar]
- Brantsaeter, A. L. , Haugen, M. , Samuelsen, S. O. , Torjusen, H. , Trogstad, L. , Alexander, J. , … Meltzer, H. M. (2009). A dietary pattern characterized by high intake of vegetables, fruits, and vegetable oils is associated with reduced risk of preeclampsia in nulliparous pregnant Norwegian women. The Journal of Nutrition, 139(6), 1162–1168. 10.3945/jn.109.104968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, J. , Alwan, N. A. , West, J. , Brown, S. , McKinlay, C. J. , Farrar, D. , & Crowther, C. A. (2017). Lifestyle interventions for the treatment of women with gestational diabetes. Cochrane Database of Systematic Reviews, 5. Cd011970. doi: 10.1002/14651858.CD011970.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalano, P. , & deMouzon, S. H. (2015). Maternal obesity and metabolic risk to the offspring: Why lifestyle interventions may have not achieved the desired outcomes. International Journal of Obesity, 39(4), 642–649. 10.1038/ijo.2015.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatfield, J. (2001). ACOG issues guidelines on fetal macrosomia. American College of Obstetricians and Gynecologists. American Family Physician, 64(1), 169–170. [PubMed] [Google Scholar]
- Chen, X. , Zhao, D. , Mao, X. , Xia, Y. , Baker, P. N. , & Zhang, H. (2016). Maternal dietary patterns and pregnancy outcome. Nutrients, 8(6). 10.3390/nu8060351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cnattingius, S. , & Villamor, E. (2016). Weight change between successive pregnancies and risks of stillbirth and infant mortality: A nationwide cohort study. Lancet, 387(10018), 558–565. 10.1016/s0140-6736(15)00990-3 [DOI] [PubMed] [Google Scholar]
- Collado, M. C. , Isolauri, E. , Laitinen, K. , & Salminen, S. (2008). Distinct composition of gut microbiota during pregnancy in overweight and normal‐weight women. The American Journal of Clinical Nutrition, 88(4), 894–899. [DOI] [PubMed] [Google Scholar]
- Crozier, S. R. , Inskip, H. M. , Godfrey, K. M. , Cooper, C. , & Robinson, S. M. (2017). Nausea and vomiting in early pregnancy: Effects on food intake and diet quality. Maternal & Child Nutrition, 13(4), e12389 10.1111/mcn.12389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd, J. M. , Turnbull, D. , McPhee, A. J. , Deussen, A. R. , Grivell, R. M. , Yelland, L. N. , … Robinson, J. S. (2014). Antenatal lifestyle advice for women who are overweight or obese: LIMIT randomised trial. BMJ, 348, g1285 10.1136/bmj.g1285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Embætti landlæknis (2016). Grundvöllur ráðlegginga um mataræði og ráðlagðir dagskammtar næringarefna. Iceland: Reykjavík. [Google Scholar]
- Englund‐Ogge, L. , Brantsaeter, A. L. , Haugen, M. , Sengpiel, V. , Khatibi, A. , Myhre, R. , … Jacobsson, B. (2012). Association between intake of artificially sweetened and sugar‐sweetened beverages and preterm delivery: A large prospective cohort study. The American Journal of Clinical Nutrition, 96(3), 552–559. 10.3945/ajcn.111.031567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englund‐Ogge, L. , Brantsaeter, A. L. , Sengpiel, V. , Haugen, M. , Birgisdottir, B. E. , Myhre, R. , … Jacobsson, B. (2014). Maternal dietary patterns and preterm delivery: Results from large prospective cohort study. BMJ, 348, g1446 10.1136/bmj.g1446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser, A. , Tilling, K. , Macdonald‐Wallis, C. , Hughes, R. , Sattar, N. , Nelson, S. M. , & Lawlor, D. A. (2011). Associations of gestational weight gain with maternal body mass index, waist circumference, and blood pressure measured 16 y after pregnancy: The Avon Longitudinal Study of Parents and Children (ALSPAC). The American Journal of Clinical Nutrition, 93(6), 1285–1292. 10.3945/ajcn.110.008326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard, R. , Durmus, B. , Hofman, A. , Mackenbach, J. , Steegers, E. , & Jaddoe, V. (2012). OS021. Risk factors and outcomes of maternal obesity and excessive weight gain during pregnancy. Pregnancy Hypertens, 2(3), 186 10.1016/j.preghy.2012.04.022 [DOI] [PubMed] [Google Scholar]
- Ge, J. , Wang, D. , & Fan, L. (2015). Effect of personalized nutrition guidance on the birth rate of fetal macrosomia in Chinese population: A meta‐analysis of nine randomized controlled trials. Cell Biochemistry and Biophysics, 72(3), 669–674. 10.1007/s12013-015-0512-0 [DOI] [PubMed] [Google Scholar]
- Goldstein, R. F. , Abell, S. K. , Ranasinha, S. , Misso, M. , Boyle, J. A. , Black, M. H. , … Teede, H. J. (2017). Association of gestational weight gain with maternal and infant outcomes: A systematic review and meta‐analysis. JAMA, 317(21), 2207–2225. 10.1001/jama.2017.3635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guelinckx, I. , Devlieger, R. , Mullie, P. , & Vansant, G. (2010). Effect of lifestyle intervention on dietary habits, physical activity, and gestational weight gain in obese pregnant women: A randomized controlled trial. The American Journal of Clinical Nutrition, 91(2), 373–380. 10.3945/ajcn.2009.28166 [DOI] [PubMed] [Google Scholar]
- Gunnarsdottir, I. , Tryggvadottir, E. A. , Birgisdottir, B. E. , Halldorsson, T. I. , Medek, H. , & Geirsson, R. T. (2016). Diet and nutrient intake of pregnant women in the capital area in Iceland. Læknablađiđ, 102(9), 378–384. 10.17992/lbl.2016.09.95 [DOI] [PubMed] [Google Scholar]
- Hermann, G. M. , Dallas, L. M. , Haskell, S. E. , & Roghair, R. D. (2010). Neonatal macrosomia is an independent risk factor for adult metabolic syndrome. Neonatology, 98(3), 238–244. 10.1159/000285629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillesund, E. R. , Bere, E. , Haugen, M. , & Overby, N. C. (2014). Development of a New Nordic Diet score and its association with gestational weight gain and fetal growth—A study performed in the Norwegian Mother and Child Cohort Study (MoBa). Public Health Nutrition, 17(9), 1909–1918. 10.1017/s1368980014000421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrolfsdottir, L. , Schalkwijk, C. G. , Birgisdottir, B. E. , Gunnarsdottir, I. , Maslova, E. , Granstrom, C. , … Halldorsson, T. I. (2016). Maternal diet, gestational weight gain, and inflammatory markers during pregnancy. Obesity (Silver Spring), 24, 2133–2139. 10.1002/oby.21617 [DOI] [PubMed] [Google Scholar]
- Institute of Medicine and National Research Council (2009). Weight gain during pregnancy: Reexamining the guidelines. Washington, DC: National Academic Press. [PubMed] [Google Scholar]
- Knudsen, V. K. , Heitmann, B. L. , Halldorsson, T. I. , Sorensen, T. I. , & Olsen, S. F. (2013). Maternal dietary glycaemic load during pregnancy and gestational weight gain, birth weight and postpartum weight retention: A study within the Danish National Birth Cohort. The British Journal of Nutrition, 109(8), 1471–1478. 10.1017/s0007114512003443 [DOI] [PubMed] [Google Scholar]
- Knudsen, V. K. , Orozova‐Bekkevold, I. M. , Mikkelsen, T. B. , Wolff, S. , & Olsen, S. F. (2008). Major dietary patterns in pregnancy and fetal growth. European Journal of Clinical Nutrition, 62(4), 463–470. 10.1038/sj.ejcn.1602745 [DOI] [PubMed] [Google Scholar]
- Mamun, A. A. , Mannan, M. , & Doi, S. A. (2014). Gestational weight gain in relation to offspring obesity over the life course: A systematic review and bias‐adjusted meta‐analysis. Obesity Reviews, 15(4), 338–347. 10.1111/obr.12132 [DOI] [PubMed] [Google Scholar]
- Martin, C. L. , Sotres‐Alvarez, D. , & Siega‐Riz, A. M. (2015). Maternal dietary patterns during the second trimester are associated with preterm birth. The Journal of Nutrition, 145(8), 1857–1864. 10.3945/jn.115.212019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslova, E. , Halldorsson, T. I. , Astrup, A. , & Olsen, S. F. (2015). Dietary protein‐to‐carbohydrate ratio and added sugar as determinants of excessive gestational weight gain: A prospective cohort study. BMJ Open, 5(2), e005839 10.1136/bmjopen-2014-005839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordic Nutrition Recommendations (2014). Integrating nutrition and physical activity. Copenhagen: Nordic Council of Ministers. [Google Scholar]
- Olafsdottir, A. S. , Skuladottir, G. V. , Thorsdottir, I. , Hauksson, A. , & Steingrimsdottir, L. (2006). Maternal diet in early and late pregnancy in relation to weight gain. International Journal of Obesity, 30(3), 492–499. 10.1038/sj.ijo.0803184 [DOI] [PubMed] [Google Scholar]
- Olsen, S. F. , Halldorsson, T. I. , Willett, W. C. , Knudsen, V. K. , Gillman, M. W. , Mikkelsen, T. B. , & Olsen, J. (2007). Milk consumption during pregnancy is associated with increased infant size at birth: Prospective cohort study. The American Journal of Clinical Nutrition, 86(4), 1104–1110. [DOI] [PubMed] [Google Scholar]
- Olson, C. M. , & Strawderman, M. S. (2003). Modifiable behavioral factors in a biopsychosocial model predict inadequate and excessive gestational weight gain. Journal of the American Dietetic Association, 103(1), 48–54. 10.1053/jada.2003.50001 [DOI] [PubMed] [Google Scholar]
- Perez‐Morales, M. E. , Bacardi‐Gascon, M. , & Jimenez‐Cruz, A. (2015). Association of excessive GWG with adiposity indicators and metabolic diseases of their offspring: Systematic review. Nutrición Hospitalaria, 31(4), 1473–1480. 10.3305/nh.2015.31.4.8297 [DOI] [PubMed] [Google Scholar]
- Phelan, S. , Phipps, M. G. , Abrams, B. , Darroch, F. , Schaffner, A. , & Wing, R. R. (2011). Randomized trial of a behavioral intervention to prevent excessive gestational weight gain: The Fit for Delivery Study. The American Journal of Clinical Nutrition, 93(4), 772–779. 10.3945/ajcn.110.005306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poston, L. , Bell, R. , Croker, H. , Flynn, A. C. , Godfrey, K. M. , Goff, L. , … Briley, A. L. (2015). Effect of a behavioural intervention in obese pregnant women (the UPBEAT study): A multicentre, randomised controlled trial. The Lancet Diabetes and Endocrinology, 3(10), 767–777. 10.1016/s2213-8587(15)00227-2 [DOI] [PubMed] [Google Scholar]
- Qin, L. Q. , He, K. , & Xu, J. Y. (2009). Milk consumption and circulating insulin‐like growth factor‐I level: A systematic literature review. International Journal of Food Sciences and Nutrition, 60 Suppl 7, 330–340. 10.1080/09637480903150114 [DOI] [PubMed] [Google Scholar]
- Rasmussen, F. , & Johansson, M. (1998). The relation of weight, length and ponderal index at birth to body mass index and overweight among 18‐year‐old males in Sweden. European Journal of Epidemiology, 14(4), 373–380. [DOI] [PubMed] [Google Scholar]
- Renault, K. M. , Carlsen, E. M. , Norgaard, K. , Nilas, L. , Pryds, O. , Secher, N. J. , … Halldorsson, T. I. (2015). Intake of sweets, snacks and soft drinks predicts weight gain in obese pregnant women: Detailed analysis of the results of a randomised controlled trial. PLoS One, 10(7), e0133041 10.1371/journal.pone.0133041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restall, A. , Taylor, R. S. , Thompson, J. M. , Flower, D. , Dekker, G. A. , Kenny, L. C. , … McCowan, L. M. (2014). Risk factors for excessive gestational weight gain in a healthy, nulliparous cohort. Journal of Obesity, 2014, 148391–148399. 10.1155/2014/148391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogozinska, E. , Marlin, N. , Jackson, L. , Rayanagoudar, G. , Ruifrok, A. E. , Dodds, J. , … Thangaratinam, S. (2017). Effects of antenatal diet and physical activity on maternal and fetal outcomes: Individual patient data meta‐analysis and health economic evaluation. Health Technology Assessment, 21(41), 1–158. 10.3310/hta21410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong, K. , Yu, K. , Han, X. , Szeto, I. M. , Qin, X. , Wang, J. , … Ma, D. (2015). Pre‐pregnancy BMI, gestational weight gain and postpartum weight retention: A meta‐analysis of observational studies. Public Health Nutrition, 18(12), 2172–2182. 10.1017/s1368980014002523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sferruzzi‐Perri, A. N. , Owens, J. A. , Pringle, K. G. , & Roberts, C. T. (2011). The neglected role of insulin‐like growth factors in the maternal circulation regulating fetal growth. The Journal of Physiology, 589(Pt 1), 7–20. 10.1113/jphysiol.2010.198622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim, J. S. , Oh, K. , & Kim, H. C. (2014). Dietary assessment methods in epidemiologic studies. Epidemiol Health, 36, e2014009 10.4178/epih/e2014009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin, D. , Lee, K. W. , & Song, W. O. (2016). Dietary patterns during pregnancy are associated with gestational weight gain. Maternal and Child Health Journal, 20(12), 2527–2538. 10.1007/s10995-016-2078-x [DOI] [PubMed] [Google Scholar]
- Stuebe, A. M. , Oken, E. , & Gillman, M. W. (2009). Associations of diet and physical activity during pregnancy with risk for excessive gestational weight gain. American Journal of Obstetrics and Gynecology, 201(1), 58.e51–58.e58. 10.1016/j.ajog.2009.02.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temming, L. , Franco, A. , Istwan, N. , Rhea, D. , Desch, C. , Stanziano, G. , & Joy, S. (2014). Adverse pregnancy outcomes in women with nausea and vomiting of pregnancy. The Journal of Maternal‐Fetal & Neonatal Medicine, 27(1), 84–88. 10.3109/14767058.2013.806473 [DOI] [PubMed] [Google Scholar]
- Thorsdottir, I. , & Birgisdottir, B. E. (1998). Different weight gain in women of normal weight before pregnancy: Postpartum weight and birth weight. Obstetrics and Gynecology, 92(3), 377–383. [DOI] [PubMed] [Google Scholar]
- Thorsdottir, I. , Torfadottir, J. E. , Birgisdottir, B. E. , & Geirsson, R. T. (2002). Weight gain in women of normal weight before pregnancy: Complications in pregnancy or delivery and birth outcome. Obstetrics and Gynecology, 99(5 Pt 1), 799–806. [DOI] [PubMed] [Google Scholar]
- Tian, C. , Hu, C. , He, X. , Zhu, M. , Qin, F. , Liu, Y. , & Hu, C. (2016). Excessive weight gain during pregnancy and risk of macrosomia: A meta‐analysis. Archives of Gynecology and Obstetrics, 293(1), 29–35. 10.1007/s00404-015-3825-8 [DOI] [PubMed] [Google Scholar]
- Tie, H. T. , Xia, Y. Y. , Zeng, Y. S. , Zhang, Y. , Dai, C. L. , Guo, J. J. , & Zhao, Y. (2014). Risk of childhood overweight or obesity associated with excessive weight gain during pregnancy: A meta‐analysis. Archives of Gynecology and Obstetrics, 289(2), 247–257. 10.1007/s00404-013-3053-z [DOI] [PubMed] [Google Scholar]
- Tielemans, M. J. , Erler, N. S. , Leermakers, E. T. , van den Broek, M. , Jaddoe, V. W. , Steegers, E. A. , … Franco, O. H. (2015). A priori and a posteriori dietary patterns during pregnancy and gestational weight gain: The Generation R Study. Nutrients, 7(11), 9383–9399. 10.3390/nu7115476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tryggvadottir, E. A. , Medek, H. , Birgisdottir, B. E. , Geirsson, R. T. , & Gunnarsdottir, I. (2016). Association between healthy maternal dietary pattern and risk for gestational diabetes mellitus. European Journal of Clinical Nutrition, 70(2), 237–242. 10.1038/ejcn.2015.145 [DOI] [PubMed] [Google Scholar]
- Uusitalo, U. , Arkkola, T. , Ovaskainen, M. L. , Kronberg‐Kippila, C. , Kenward, M. G. , Veijola, R. , … Virtanen, S. M. (2009). Unhealthy dietary patterns are associated with weight gain during pregnancy among Finnish women. Public Health Nutrition, 12(12), 2392–2399. 10.1017/s136898000900528x [DOI] [PubMed] [Google Scholar]
- Walter, J. R. , Perng, W. , Kleinman, K. P. , Rifas‐Shiman, S. L. , Rich‐Edwards, J. W. , & Oken, E. (2015). Associations of trimester‐specific gestational weight gain with maternal adiposity and systolic blood pressure at 3 and 7 years postpartum. American Journal of Obstetrics and Gynecology, 212(4), 499.e491–499.e412. 10.1016/j.ajog.2014.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (2016). Good maternal nutrition the best start in life. Copenhagen, Denmark: WHO Reginal Office for Europe. [Google Scholar]
- Zhu, Y. , Olsen, S. F. , Mendola, P. , Halldorsson, T. I. , Rawal, S. , Hinkle, S. N. , … Zhang, C. (2017). Maternal consumption of artificially sweetened beverages during pregnancy, and offspring growth through 7 years of age: A prospective cohort study. International Journal of Epidemiology, 46(5), 1499–1508. 10.1093/ije/dyx095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou, G. (2004). A modified poisson regression approach to prospective studies with binary data. American Journal of Epidemiology, 159(7), 702–706. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting information
Table S1. Food intake of main food groups (per week) by adherence to the recommended gestational weight gain (GWG) (n=1326)
Table S2. The association between the dietary risk score, GWG, and macrosomia (GDM cases excluded).
Table S3. Different combinations of the dietary risk score and the risk of excessive gestational weight gain (GWG)a


