Abstract
Objectives
Primate social systems are remarkably diverse, and thus play a central role in understanding social evolution, including the biological origin of human societies. Although baboons have been prominently featured in this context, historically little was known about the westernmost member of the genus, the Guinea baboon (Papio papio).
Material and Methods
Here, we summarize the findings from the first years of observations at the field site CRP Simenti in the Niokolo Koba National Park in Senegal.
Results
Guinea baboons reveal a nested multi‐level social organization, with reproductive units comprising one “primary” male, one to several females, young, and occasionally “secondary” males at the base of the society. Three to five units form “parties,” which team up with other parties to form a “gang.” Different gangs have largely overlapping home ranges and agonistic interactions between different parties or gangs are rare. Some but not all strongly socially bonded males are highly related, and population genetic and behavioral evidence indicate female‐biased dispersal. Females play an important role in intersexual bond formation and maintenance, and female tenure length varies between a few weeks to several years.
Discussion
While the social organization resembles that of hamadryas baboons (P. hamadryas), the social structure differs considerably, specifically in terms of low male aggressiveness and female freedom. Despite substantial differences in social organization and social structure, the acoustic structure of Guinea baboon vocalizations does not differ substantially from that of other baboon taxa. With its multi‐level organization, stable bonds between males and females, as well as a high‐degree of male‐male cooperation and tolerance, Guinea baboons constitute an intriguing model for reconstructing human social evolution.
Keywords: female dispersal, Guinea baboons, human evolution, nested multi‐level society, Papio papio, primate evolution, social system
1. Primate social evolution and the origin of human societies
Primate social systems are remarkably diverse, ranging from a mostly solitary life‐style to pair living, and a variety of different forms of group living. A key question in the field of social evolution is to understand the drivers that give rise to this variation (Crook & Gartlan, 1966; Dunbar, 1988; Grueter, Chapais, & Zinner, 2012; Kappeler & van Schaik, 2002; Mitani, Call, Kappeler, Palombit, & Silk, 2012; Swedell & Plummer, 2012). Insights from comparative studies of nonhuman primate social systems contribute to disentangling human social evolution and thus to our understanding of human societies (Chapais, 2011). Because extant human societies vary markedly, several lines of evidence need to be considered to reconstruct what Bernard Chapais termed the “deep social structure” of humankind (Chapais, 2011, p. 1276). Comparative studies on closely related species, or species that evolved under similar ecological conditions as early humans, are valuable sources to identify selective pressures as well as evolutionary constraints that play a role in social evolution, including the origins of human societies (Chapais, 2008). Following Kappeler and van Schaik (2002), it has proven helpful to distinguish between different components of the social system, namely the social organization (grouping and dispersal patterns), the mating system, and the social structure or social relationships. At the same time, it is obvious that these components clearly influence each other (Chapais, 2008; Dunbar, 1988).
Early human societies are assumed to have been relatively fluid, consisting of several family groups that form stable communities (Chapais, 2011; Rodseth, Wrangham, Harrigan, & Smuts, 1991). Within these communities, individual members may form new temporary aggregations on a short‐term basis. The remarkable degree of cooperation, male‐male tolerance, and reduced male‐male aggression within human societies have been linked to this multi‐level organization, which gives rise to alliances at different levels (Boyd & Silk, 2011; Grueter et al., 2012). The mating pattern in these societies has been assumed to be monogamous (Marlowe, 2004; Rodseth et al., 1991). A detailed study of the residence and kinship patterns in present‐day human foraging societies fits these assumptions in that individuals of both sexes may disperse from their family groups or stay, resulting in co‐residence of both brothers and sisters (Hill et al., 2011). Members of these societies maintain lifetime bonds with their natal kin, irrespective of spatial proximity, resulting in long‐term alliances between family groups within the community (Chapais, 2011; Rodseth et al., 1991; Rodseth & Novak, 2000). From an evolutionary perspective, the link between grouping and dispersal as well as mating patterns are particularly interesting because dispersal patterns shape the composition and kin structure of the group and hence the degree of kin competition and cooperation through kin selection (Foley & Gamble, 2009).
Attempts to model human social evolution based on studies of nonhuman primates heavily rely on data from our closest living relatives, chimpanzees (Pan troglodytes) and bonobos (P. paniscus) (Boesch, Hohmann, & Marchant, 2002; Susman, 1987). Chimpanzees live in a “fission‐fusion” society. Within a given “community,” temporary subgroups or “parties” can be found that vary in size, composition and time of association (Boesch & Boesch‐Achermann, 2000; Mitani, Watts, & Muller, 2002; Nishida & Hiraiwa‐Hasegawa, 1987). Community males defend their territory (Watts & Mitani, 2001). Males are philopatric, while females tend to disperse (Foerster et al., 2015). Although female intrasexual competition may be intense (Pusey & Schroepfer‐Walker, 2013), females have preferred female social partners (Lehmann & Boesch, 2004). When they have the choice, they are more likely to associate with kin than with non‐kin partners (Foerster et al., 2015). Females mate with multiple males, though there is considerable skew in favor of the high‐ranking males (Boesch, Kohou, Nene, & Vigilant, 2006; Newton‐Fisher, Thompson, Reynolds, Boesch, & Vigilant, 2010; Wroblewski et al., 2009).
A similar social organization also occurs in bonobos, but compared with chimpanzees, bonobo parties tend to be larger (Hohmann & Fruth, 2002; Nishida & Hiraiwa‐Hasegawa, 1987; Stumpf, 2011). Party size in chimpanzees increases with presence of estrous females (Anderson, Nordheim, Boesch, & Moermond, 2002; Goodall, 1986; Newton‐Fisher, 2002), whereas in bonobos party size seems to be independent of the number of estrous females (Hohmann & Fruth, 2002). As in chimpanzees, bonobo males are philopatric, but there is anecdotal evidence of occasional male transfer (Hohmann & Fruth, 2002). Although female bonobos travel together more often than female chimpanzees, data on social relationships among females do not support closer female‐female bonding compared with chimpanzees (Hohmann & Fruth, 2002).
There are thus some similarities between members of the genus Pan and presumed early human societies, notably female‐biased dispersal and perhaps also group fissioning into smaller foraging parties, although, in contrast to early humans, these parties are mostly instable in composition. There are also some notable differences (Marlowe, 2005), such as the lack of a nested multi‐level organization (Aureli et al., 2008). Reconstructions of the evolutionary history, including the identification of the driving forces in the social evolution of Pan and Homo, are clearly hampered by the small sample size and the limited variation in social organization within the genus Pan, which renders the identification of selective forces difficult. Therefore, other models have been considered to aid in the reconstruction of the evolution of social systems.
2. Baboons as a model for social evolution
Species that likely evolved in similar habitats and on a similar trophic level as early humans constitute important analogue models to reconstruct the evolution of different societies (Jolly, 2009). Members of the genus Papio (baboons) have played an important role in this context because they reveal considerable variation in ecology and social organization and thus provide a quasi‐experimental approach to study the effect of environmental conditions and phylogeny on social evolution (Barrett & Henzi, 2008; Jolly, 2009). The fossil record as well as phylogeographic analyses indicate that baboons of the genus Papio originated in southern Africa and dispersed over large parts of sub‐Saharan Africa during the Pleistocene (Newman, Jolly, & Rogers, 2004; Zinner, Buba, Nash, & Roos, 2011; Zinner, Groeneveld, Keller, & Roos, 2009). Baboons are still widely distributed through sub‐Saharan Africa and southwestern Arabia, excluding most parts of the west and central African rainforest and extreme arid regions. Currently, and according to the phylogenetic species concept (Cracraft, 1989) six species are recognized in the genus (Zinner et al., 2009; Zinner, Wertheimer, Liedigk, Groeneveld, & Roos, 2013): chacma (P. ursinus), olive (P. anubis), yellow (P. cynocephalus), Kinda (P. kindae), Guinea (P. papio), and hamadryas baboons (P. hamadryas) (Figure 1). Where ranges of species overlap, gene flow is observed and hybrid zones are formed (Alberts & Altmann, 2001; Burrell, 2008; Jolly, Burrell, Phillips‐Conroy, Bergey, & Rogers, 2011; Nagel, 1973; Phillips‐Conroy, Jolly, Nystrom, & Hemmalin, 1992).
Figure 1.
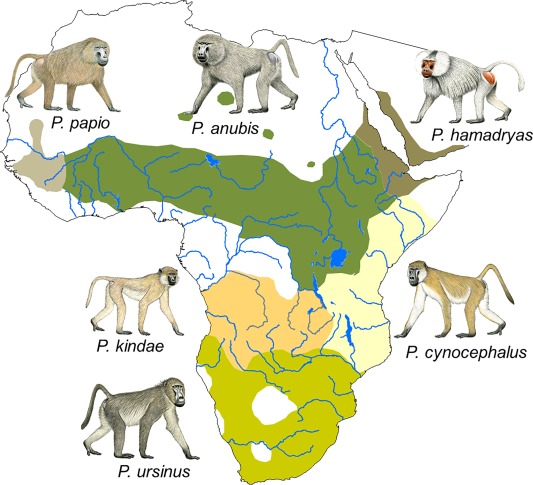
Distribution of the six Papio species. Species distributions are modified from Zinner et al. (2013). Baboon drawings by Stephen Nash
Phylogenetic analyses of the genus Papio indicate a clear split between a southern and a northern clade that occurred about 2.1 mya (as inferred from mitochondrial sequence information Zinner et al., 2009, 2013). Based on 13 noncoding nuclear sequences Boissinot, Alvarez, Giraldo‐Ramirez, and Tollis (2014) estimated the first split among chacma baboons and the more northerly taxa at around 1.5 mya. The analysis of mitochondrial genomes indicates several incongruences among the mitochondrial phylogeny and morphology, suggesting multiple para‐ and polyphyletic relationships. The current notion is that baboons originated in southern Africa and that the phylogenetic relationships among extant baboons are a result of range expansion into both northern and southern savanna habitats, recurrent fragmentation and isolation of populations, local adaptation, and introgressive hybridization after secondary contact of populations, most prominently by dispersing males (Jolly, 2007; Zinner et al., 2011).
The different species vary in their social organization and mating systems, including the identity of the dispersing sex, resulting in variation of genetic relatedness and familiarity between females as well as between males within groups. This may have affected the evolution of social bonds and the characteristics of dominance hierarchies among males and females (e.g., Kappeler, 2008). Traditionally, two main social systems have been contrasted: firstly, more or less stable female‐bonded social groups as found in “savanna baboons” (chacma, olive, and yellow baboons) (Barrett & Henzi, 2008); secondly, the multi‐level system of hamadryas baboons. Savanna baboons live in multi‐male multi‐female groups with female philopatry and male dispersal. Their mating system is classified as polygynandrous. Mating success and reproduction are skewed in favor of high‐ranking males (Alberts, Buchan, & Altmann, 2006; Alberts, Watts, & Altmann, 2003, Bulger, 1993; Hausfater, 1975; Packer, 1979). Associations between males and females are generally restricted to specific periods during the reproductive cycle (see e.g., Smuts, 1985). During such consortships, males stay in close proximity to estrous females (Swedell, 2011). In addition, lactating females with dependent infants establish affiliative “friendships” with specific males. These associations are mediated by the presence of the infant and are assumed to protect against harassment (of the female) and infanticide risk (Lemasson, Palombit, & Jubin, 2008; Moscovice et al., 2010; Palombit, 2009). Note, however, that most of the knowledge on savanna baboons was collected in eastern and southern Africa, and comparatively little is known about West‐African representatives of this taxon (Kunz & Linsenmair, 2008).
Hamadryas baboons, in contrast to savanna baboons, live in a multilevel social system in which females are associated with specific males, regardless of female reproductive state. Such associations of males with one or several females are known as one‐male‐units (OMUs). OMUs form the social core of the hamadryas baboon society (Kummer, 1968). Females are spatially segregated from other OMUs, partly through male enforcement (Kummer, 1968; Swedell & Schreier, 2009). Despite their name, some OMUs may have “follower males” (Chowdhury, Pines, Saunders, & Swedell, 2015; Kummer, 1968; Pines, Saunders, & Swedell, 2011), who are less likely to interact sexually with females than leader males (Swedell, 2006). Multiple OMUs aggregate into clans, and several clans and additional bachelor males form a band (Kummer, 1968; Swedell & Plummer, 2012). Finally, hamadryas baboon males are seen as predominantly philopatric although both sexes disperse (Städele, Van Doren, Pines, Swedell, & Vigilant, 2015; Swedell et al., 2011).
The Kinda baboon (Papio kindae) is the least studied of the baboon species. Previously it was regarded as a subspecies of the yellow baboon and its social organization and social behavior is not well documented (Phillips‐Conroy, Jolly, Burrell, Rogers, & Weyher, 2009; Weyher & Chiou, 2013; Weyher, Phillips‐Conroy, Fourrier, & Jolly, 2014). Genetic data suggest that females are philopatric and males disperse (Burrell, Jolly, Phillips‐Conroy, & Disotell, 2011). However, first behavioral observations suggest significant differences compared with other baboon species. Kinda baboons are smaller and more gracile than other baboon species and it is the least sexually dimorphic in body size (Weyher & Chiou, 2013). Like other savanna baboons they live in multi‐male‐multi‐female groups and do not form spatially coherent one‐male units. Adult males initiate and maintain proximity to adult females in all reproductive states and, most often, when they have a small infant. Males frequently groom females. Male‐female relationships and female‐female relationships differ substantially from those seen in yellow, chacma, and olive baboons, suggesting that Kinda baboons constitute a further variant of baboon social systems (Phillips‐Conroy et al., 2009; Weyher & Chiou, 2013; Weyher et al., 2014).
3. Early studies on guinea baboons
Until recently, comparatively little attention had been paid to the westernmost member of the genus, the Guinea baboon. One of the earliest studies (Bert, Ayats, Martino, & Collomb, 1967) observed that the aggregations of Guinea baboons appeared to be “unstructured and anarchic.” In the ensuing years, authors aired diverging views regarding the social organization of the species. Dupuy and Gaillard (1969) noted homogenous aggregations without subgrouping (Dupuy & Gaillard, 1969 cf. Sharman, 1981), while Dunbar and Nathan (1972) observed some substructuring; these authors also found that females were able to roam relatively independently. Gilbert Boese, who had studied Guinea baboons in the Brookfield Zoo in Chicago, also spent several weeks in Senegal. His sightings of Guinea baboons suggested that within the society, reproductive units consisting of one adult male with one or more females and associated young could be identified (Boese, 1973, 1975).
Notably, until recently, only one longer field study had been conducted. Martin Sharman studied the feeding ecology of two “troops” of Guinea baboons near Mt. Assirik in the Niokolo Koba National Park (PNNK) in Senegal over a 19‐month period in 1977 and 1978. He found that season had a significant effect on activity patterns; the baboons spent more time moving and foraging in the dry season compared with the wet season, where they socialized more (Sharman, 1981). The home ranges of his two study troops were estimated at 45–50 km2 and 18–20 km2, respectively. Interestingly, the mean distance travelled per day did not vary with season, despite seasonal variation in activity patterns. The location of the sleeping sites greatly affected the ranging patterns, as the troops spent disproportionate amounts of time near the sleeping trees, which were located near permanent water. The baboons fed not only on a wide variety of fruits and seeds, but also on invertebrates and some vertebrates (Sharman, 1981).
In terms of understanding the social organization and mating patterns of the species, Sharman had to rely on indirect inferences from the observation of foraging parties, as he was not able to individually identify most of the subjects. He observed that adult males were found together in small foraging parties more frequently than expected, and that the composition of social groups differed from those of foraging groups (Sharman, 1981). Sharman thus suspected that the Guinea baboon system was considerably different from the OMUs observed in hamadryas baboons, and that the system as a whole was more similar to that found in savanna baboons. Yet, given the lack of individual identification, he also noted that some caution was in order (Sharman, 1981).
The majority of authors found that individuals aggregated in larger groups when travelling and at sleeping sites, while foraging and resting seemed to take place in smaller groups (Boese, 1973; Dunbar & Nathan, 1972; Galat‐Luong, Galat, Hagell, & Tuttle, 2006; Sharman, 1981, but see Bert et al., 1967; Dupuy & Gaillard, 1969). Moreover, a number of authors reported OMU‐like subgroups as the smallest entities (Boese, 1973; Galat‐Luong et al., 2006; Sharman, 1981); however, it remained unclear whether these OMUs represent reproductive units as in hamadryas baboons (Kummer, 1968). Based on observations collected from captive Guinea baboons, Maestripieri, Mayhew, Carlson, Hoffman, and Radtke (2007) assumed the existence of OMUs, while Sharman (1981) conducted nearest‐neighbor analyses and found that females tended to associate most frequently with juveniles and other females. Sharman considered this to be consistent with the idea of female‐bonded kin groups, as in savanna baboons. Moreover, he reported that Guinea baboon females were in the company of an adult male less frequently than expected by chance (given the adult sex‐ratio in the troops), which also spoke against the idea that the Guinea baboon society consisted of female social groups bonded to a specific adult male, as in geladas (Theropithecus gelada). Some authors noted aggressive herding in Guinea baboons, as in hamadryas baboons (Boese, 1973, 1975; Maestripieri et al., 2007), while others did not (Galat‐Luong et al., 2006; Sharman, 1981). Yet, several authors reported that females moved freely between subgroups (Boese, 1973; Dunbar & Nathan, 1972; Galat‐Luong et al., 2006; Maestripieri et al., 2007; Sharman, 1981). Boese (1973) speculated that the Guinea baboon social organization represents an evolutionary precursor to the more rigid multi‐layered social organization of hamadryas baboons, while Sharman (1981) suggested that the Guinea baboons are more similar to savanna baboons. In light of the considerable divergence among different researchers, the scarcity of available data, and the importance of the genus Papio in the context of social evolution, we embarked on a long‐term study of this species.
4. Study site and habitat
We established the “Centre de Recherche de Primatologie Simenti” as a field station of the German Primate Center (DPZ) near Simenti (13°01′34″N, 13°17′41″W) in the PNNK in 2007. According to the Köppen‐Geiger climate classification system (Kottek, Grieser, Beck, Rudolf, & Rubel, 2006; Peel, Finlayson, & McMahon, 2007), the PNNK is situated in the tropical savanna zone (Code: Aw). The climate is highly seasonal with a dry season from November until May and a rainy season from June until October (Figure 2a,b). The annual rainfall ranges from 800 to 1100 mm (Madsen, Dione, Traoré, & Sambou, 1996; Mbow, Goı¨ta, & Bénié, 2004) and is mostly concentrated in the rainy season. Annual average temperatures are around 30 °C, but can reach local maxima of 48 °C during peaks in dry season. At Simenti, the vegetation types range from a variety of different savanna types to dry deciduous forest according to edaphic conditions (Hejcmanova‐Nežerková & Hejcman, 2006; Tappan, Sall, Wood, & Cushing, 2004), and are characterized by Sahelo‐Sudanian and Sudanian plant species (Arbonnier, 2004). Our study site is located close to the Gambia River, with luxuriant gallery forest limited to the riverbanks. Senegal bushbabies (Galago senegalensis), West African green monkeys (Chlorocebus sabaeus), patas monkeys (Erythrocebus patas), and Temminck's red colobus (Piliocolobus temminckii) can be found sympatrically with Guinea baboons in the region. Chimpanzees (P. troglodytes) also still occur in the national park, but not in our study area. Despite a dramatic decrease in large mammal population sizes during the last decades (Howard, Wangari, & Rakotoaisoa, 2007; Renaud, Gueye, Hejcmanová, Antonínová, & Samb, 2006), potential predators such as lions (Panthera leo), leopards (Panthera pardus), African wild dogs (Lycaon pictus), spotted hyenas (Crocuta crocuta), and West African crocodiles (Crocodylus suchus) can still be found in the area (Ndao & Henschel, 2011).
Figure 2.
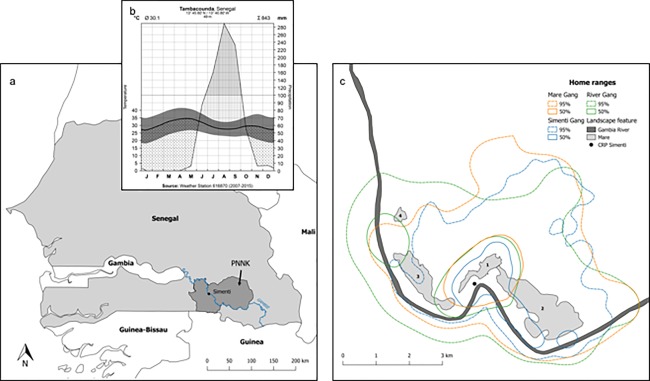
(a) Map of Senegal with the location of the Niokolo Koba National Park. The field site CRP Simenti lies next to the Gambia river (b). Hygrothermic climograph of the Tambacounda region according to Walter and Lieth (1967). Depicted are monthly temperatures (mean, min, max) in relation to monthly precipitation. Based on the assumed dependency between evaporation and temperature (e.g., monthly Ø 10 °C evaporates 20 mm, and Ø 20 °C evaporates 40 mm), the climograph demarcates periods of aridity, i.e., dry season and humidity, i.e. wet season. Meteorological data was recorded at the Tambacounda Weather Station (ID: GHCND:SG000061687) and derived from the National Oceanic and Atmospheric Administration (NOAA; http://www.ncdc.noaa.gov/cdo-web/). (c) Home range overlap of three of the study gangs at Simenti. Location data was collected by means of GPS‐enabled collars over a three‐year period (2010–2012). Kernel home ranges (fixed KDE) were modeled using the rule‐based ad‐hoc method (Kie, 2013). Dashed lines depict the home ranges (95% contour) and solid lines the core areas (50% contour). The numbers represent the most important wetlands (Mare) in our study region: (1) Mare Simenti, (2) temporary Mare, (3) Mare Kountadala, (4) Mare Nanaka
Near our field station is the “Mare de Simenti,” a seasonal flood plain of about 3.8 ha, with an additional “Mare temporaire” of 23.4 ha (see Figure 2c). The Mare is surrounded by savanna and riparian vegetation, including plant species such as the Palmyra palm tree (Borassus akeassii), African peach tree (Sarcocephalus latifolius), woody vines (e.g., Saba senegalensis), as well as various shrub species (e.g., Guiera senegalensis, Mimosa pigra). It is covered with mainly herbaceous plants (e.g., Echinochloa spp., Panicum spp.) and represents an important resource for wildlife (Dupuy, 1971). Grazers such as warthogs (Phacochoerus africanus), kob (Kobus kob), and waterbuck (Kob defassa), as well as numerous bird species utilize the Mare. Water cover varies considerably between years. In dry years, a muddy pool remains in the center during the dry season and is used for wallowing by warthogs and for drinking by other animals, including the baboons.
5. First steps
Observations began in 2007, and it proved unexpectedly difficult to habituate the animals to our presence. Partly, this was due to the high spatial tolerance between groups, and the high population density near our field site, which initially rendered encountering the same individuals difficult. During this time, we thus mainly collected observations from animals that were not yet individually identified when they came to drink at their most important water source, the Mare de Simenti, in the mornings and evenings (Patzelt et al., 2011).
During this time, we focused on whether we would find evidence for stable groups, which would be shown by a unimodal distribution of group size with only small variation over the observation period, or a multi‐level system, which would be reflected by multiple peaks in the frequency distribution of observed numbers of animals traveling together (Patzelt et al., 2011). Data were collected on 78 days between April and August of 2007, until the Mare flooded and the baboons no longer crossed it. Days before the first heavy rain in mid‐June were classified as “dry season,” thereafter as “wet season.” Subgroups were distinguished based on spatio‐temporal cohesion. More specifically, they were defined as aggregations of 4 or more individuals that came from or left in the same direction but were separated by an interval of at least 5 min, or that came from or left in different directions (>45°), even if they arrived or left at the same time (Patzelt et al., 2011).
The observations revealed neither clear unimodal nor multimodal peaks. Instead, we observed a continuous distribution. Moreover, subgroup sizes varied with time of day and with season. In the mornings, parties were larger (median: 25, interquartile range IQR 11.5–78.5) than in the afternoons (median 19, IQR 11.5–32). Irrespective of daily variation, the subgroup size in the dry season was 16 (IQR 8.5–24) and 30 in the rainy season (IQR 16–58). This variation in party size translated into corresponding variation in the number of parties, with smaller party sizes associated with a larger number of parties, and vice versa (Patzelt et al., 2011). The most striking observation was that parties would fission, fuse or mingle with other parties. For 366 arriving parties, we found that in 198 cases, the composition remained stable. In 18 cases, fissioning into subgroups was observed, while a total of 92 of arriving parties left the Mare as larger cohesive groups (35 cases). Fifty‐eight of the arriving parties would mingle with other subjects, and leave in novel compositions (partly fused). Overall, the observations confirmed Sharman's earlier report that the animals were highly spatially tolerant, and few aggressive interactions were observed between subgroups.
6. Morphometric measures
To facilitate the tracking of specific individuals, and to obtain a better understanding of their whereabouts and association patterns, we equipped several individuals with GPS or radio collars. Further, captured individuals were used to obtain morphometric measures and small skin biopsies for genetic analyses. We trapped animals in cages (1 m3) baited with peanuts; the sliding doors were operated from a hide. Once the group had left the area, caged individuals were anaesthetized with the aid of a blowpipe. Body mass was determined with a hanging scale. The length of the canines as well as length and width of the testes were measured with a vernier caliper. Further body measures were taken with a measuring tape. For six individuals, body measurements were repeated twice, revealing a rather moderate measurement error with a mean (±SE) 3.7 ± 5.1% (Maciej, 2013). The capture was approved by the Senegalese authorities and was conducted in collaboration with a local veterinarian.
Adult males weighed 20.2 kg on average, adult females 11.8 kg, with considerable variation among individuals (see Figure 3a, Table 1). The ratio of the average body mass of males and females (i.e., sexual dimorphism in body size) was 1.7. Males appear to experience a growth spurt of the upper canines when they reach about 14 kg, presumably indicating the transition from adolescence to adulthood (Figure 3b). While the lower canines were longer than the upper canines in females and subadult males, this ratio changed in adult males, who had longer upper than lower canines. The relative testicular volume was calculated as (π/6) × testicular breadth2 × testicular length following Jolly and Phillips‐Conroy (2006). There was no significant correlation between absolute testicular volume and body mass (Pearson r = 0.19, N = 37, p > .05; Figure 3c). The relative values in adult males varied between 0.45 and 1.2 mm3/kg body mass, with a mean of 0.8 mm3/kg body mass. Our measurements of the testicular volume revealed a substantially smaller relative volume compared with other baboon species; on average, it is also smaller than previously published measurements (Jolly & Phillips‐Conroy, 2006; Phillips‐Conroy & Jolly, 1981; Smith & Jungers, 1997; Thorén, Lindenfors, & Kappeler, 2006; the large relative testes size for Guinea baboons reported in Harcourt, Harvey, Larson, & Short, 1981 was most likely erroneously taken from a mandrill (Mandrillus sphinx); see Schultz, 1938). The sexual dimorphism in body mass and the relative canine size are comparable with other baboons, however (Table 2).
Figure 3.
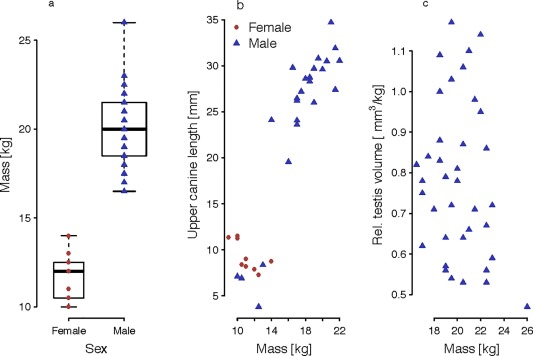
Boxplot with median, IQR, and minimum and maximum values (a). Adult female and adult male body mass. (b) Correlation between maxillary canine height and body mass. (c) Correlation between testicular volume and adult male body mass
Table 1.
Morphological measures from Guinea baboons near the CRP Simenti. Given is the mean with (SD)
| Measure | Adult female | Subadult male | Adult male |
|---|---|---|---|
| Body mass [kg] | 11.8 (1.4) | 13.8 (2.8) | 20.2 (2.2) |
| Arm length [cm] | 46.9 (1.4) | 49.2 (3.2) | 54.3 (1.7)* |
| Leg Length [cm] | 40.0 (1.5) | 43.3 (2.3) | 46.8 (1.4)* |
| Body length [cm] | 49.1 (5.9) | 49.0 (2.4) | 57.3 (4.6) |
| Chest circumf. [cm] | 45.6 (2.5) | 48.3 (5.0) | 58.2 (3.3) |
| Hip circumf. [cm] | 39.8 (2.1) | 39.4 (4.6) | 46.4 (3.1) |
| Skull length [cm] | 9.9 (0.7) | 10.4 (0.7) | 10.9 (0.9) |
| Skull width [cm] | 8.9 (0.6) | 9.8 (0.8) | 10.9 (1.0) |
| Snout length [cm] | 7.0 (0.6) | 8.1 (1.1) | 9.9 (0.7) |
| Snout width [cm] | 3.2 (0.2) | 4.0 (0.3) | 4.4 (0.4) |
| Maxillary canine [mm] | 9.3 (1.7) | 15.2 (8.4) | 29.2 (2.2) |
| Mandibular canine [mm] | 11.3 (1.4) | 17.5 (4.6) | 21.0 (2.2) |
| Rel. test. vol. [mm3/kg] | – | 0.63 (0.16) | 0.78 (0.19) |
Measures were taken from N = 17 adult females, N = 8 subadult males, and N = 37 adult male Guinea baboons, except where indicated (*N = 36). Canine measurements were confined to subjects with unchipped teeth in good condition (N = 9 adult females, N = 8 subadult males, N = 18 adult males). Mean values from left and right limbs and teeth were calculated for each subject. Body measurements followed Pfefferle and Fischer (2006). Canine Height is measured from the apex to the gum.
Table 2.
Traits associated with intra‐sexual selection in different baboon species
| Species | N | Male body mass [kg] | Sex dimorph. | Rel. Test. Vol. | Rel. Can. Size |
|---|---|---|---|---|---|
| P. papio 1 | 38 | 20.2 | 1.7 | 0.8 | 1.5 |
| P. papio 2 | 7 | 23.5 | 1.7 | 1.4 | |
| P. hamadryas 2 | 34 | 20.8 | 1.8 | 1.4 | 1.6 |
| P. hamadryas 3 | N/A | 19.0 | 1.8 | 1.6 | |
| P. hamadryas 4 | 41 | 16.9 | 1.7 | ||
| P. cynocephalus 2 | 35 | 22.8 | 2.0 | 1.7 | 2.2 |
| P. cynocephalus 3 | N/A | 17.2 | 1.5 | ||
| P. cynocephalus 4 | 37 | 21.8 | 1.8 | ||
| P. anubis 2 | 148 | 22.7 | 1.8 | 2.2 | 1.5 |
| P. anubis 3 | N/A | 23.2 | 1.9 | 1.5 | |
| P. ursinus 2 | N/A | 1.8 | 1.6 | ||
| P. ursinus 3 | N/A | 29.8 | 2.0 | 1.6 |
Sources: 1This study, 2Jolly and Phillips‐Conroy (2006), 3Thorén et al. (2006), 4Phillips‐Conroy and Jolly (1981), and Smith and Jungers (1997). Sex dimorphism given as the ratio of male/female body mass. Rel. Test. Vol.: relative testicular volume [mm3/kg]; Rel. Can. Size: Ratio of Canine height/body mass [mm/kg].
Overall, our data suggest that in terms of relative canine size, the Guinea baboons in Senegal compare with other baboon species, despite the fact that their testis volume is substantially smaller. The male baboons in Simenti overall had a smaller mass than other baboon species, yet some males reached a body mass that compared with olive or chacma baboons. Given the lack of strong intra‐sexual selection in this species (see below), this suggests that male canine size may play a role in predator defense, or may signal male quality and hence play a role in female mate choice (the simplest explanation being that it constitutes an ancestral trait that has not been selected against). Kummer (1995) assumed that the cape of the hamadryas baboon is a result of sexual selection; this might also be true for the pelage of male Guinea baboons.
Relative testis size is regarded as an indicator for degree of sperm competition and hence mating system. Males of species where females have multiple mating partners within an estrus period often have large relative testes, whereas males in monandric mating systems tend to have smaller relative testes (Jolly & Phillips‐Conroy, 2003). Given the relative small testis size of Guinea baboons, even smaller than in the monandrous hamadryas baboon, one can speculate that the mating system is similarly monandric (see also below: male–female relationships).
7. Male association patterns
First insights into the association patterns of specific individuals were obtained from the animals that were equipped with GPS collars (Tellus GPS, Televilt, Lindesberg, Sweden). The collars were programmed to take synchronous fixes every 2 h between 06:00 and 18:00, and at 21:00, 00:00, and 03:00 h. From these fixes (N > 110,000), we calculated dyadic association indices for a total of 83 dyads. Specifically, we assessed how frequently two individuals were found within 100 m of each other (Patzelt et al., 2014). Using a change‐point analysis, we identified three structural levels. Dyads that spent more than 68% of the time together (n = 12) were classified as belonging to the same “party.” Dyads that spent between 12% and 68% of the time together were classified as belonging to the same “gang.” The remaining dyads (65 out of 83) associated rarely, and were referred to as belonging to the same “community.” Across all data points, the average distance of individuals that belonged to the same party was 143 m (median, interquartile range (IQR) 41–301), while subjects from the same gang, but different parties maintained a median distance of 757 m (IQR 327–1028). Individuals from different gangs were on average 2246 m apart (IQR 1930–2695). At this time, we had equipped males from three different gangs with collars. Remarkably, the home ranges of the three gangs were overlapping to a large degree (Figure 2b), although the subjects in the different gangs spent on average less than 5% within 100 m of each other (Patzelt et al., 2014). More detailed analyses of home range use and overlap are currently under way (Klapproth et al., in preparation). From our observations of 5 parties in 2 gangs between the years 2012 and 2016, we estimated an average party size of around 28 subjects (range 9–40), with 11.4 adults (range 3–21). There was considerable variation in sex ratio between parties across the years, ranging from 0.54 to 1.96 (female to male ratio; mean: 1.3). The average gang size was 71.2 in the “Mare gang” and 70.4 in the “Simenti gang.” Note however that the estimation of juveniles was only approximate, because not all juvenile individuals are identified, resulting in a considerable margin of error for the total party size.
Systematic observations of the behavior of individually identified males from the so‐called “Mare gang” began in 2010, and from males from the “Simenti gang” in 2011. Observations on foot of association patterns confirmed the pattern extracted from the GPS data. We used a hierarchical cluster analysis on dyadic association indices extracted from proximity measures taken during group scans (Figure 4). On average, males of the same party were observed 65% of the time within 20 m distance of another male of the same party, while males in the same gang, but different parties were found within 20 m 24% of the time. Strikingly, males spent considerable amounts of time in close proximity (≤1 m) of other males. Focal males were observed within 1 m of another subject in 17% of 1,480 instances. In almost a third of these cases, this individual was another adult male. Notably, in about a fifth of the instances when a male was sitting near another male, that individual belonged to another party of the same gang (Patzelt et al., 2014). In sum, males were highly spatially tolerant of each other, irrespective of party membership. Overall, this corroborated Sharman's earlier reports of a high degree of gregariousness and male spatial tolerance (Sharman, 1981).
Figure 4.
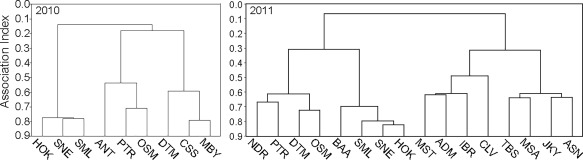
Dendrograms derived from a hierarchical cluster analysis of the association indices among nine adult males during the 2010 observation period (n = 36 dyads) and among 16 adult males during the 2011 observation period (n = 120 dyads). Letter codes represent individual males (from Patzelt et al., 2014)
To test whether male Guinea baboons would distinguish between males from their own gang from males in neighboring gangs, despite the high overlap of territory, as well as from strangers, we conducted playback experiments in which we presented males with grunts from males from their own gang, from a neighboring gang, and from unknown individuals residing more than 50 km from our study site (Maciej, Patzelt, Ndao, Hammerschmidt, & Fischer, 2013). Our initial reasoning was that males might be responding strongly to calls from unknown (“stranger”) animals, while they considered neighboring subjects as familiar individuals that did not warrant a strong response. Furthermore, we predicted that they would not respond strongly to males from their own gang. Much to our surprise, the males mainly attended to the calls of members of their own gang, while they ignored both calls from other males residing in the same area, or strangers (Maciej, Patzelt, et al., 2013). Apparently, males took a great interest in the social manoeuvres in their own social unit. Although it remained unclear whether they were simply unmotivated or unable to keep track of individual identities outside their own gang, the experiments still suggested that the levels identified by our observations are salient to the baboons (Maciej, Patzelt, et al., 2013).
8. Male‐male social relationships
In terms of social interactions, males mostly interacted with males of their own gang. Affiliative interactions were observed more frequently within parties than between parties, while severe aggression occurred more frequently between rather than within parties. We then checked whether more differentiated relationships could be found among males. To this end, we calculated dyadic association indices as (AB + BA)/2 with AB being the proportion of scans for male A in which he was seen with male B, and BA being the proportion of scans for male B in which he was seen with male A. We then divided the observed proportion by the mean proportion and defined as preferred partners dyads with an association index >1, i.e. higher than average. Most males had a limited number of preferred partners (mean 2.4); yet, some affiliative interaction was observed among a third of all possible male‐male dyads. Except two dyads, all preferred partners were found within the same party. Also, 28 out of 30 observed coalitions were formed between males of the same party, and all of these 28 coalitions occurred between males that were preferred partners. The comparison of the association patterns across 2010 and 2011 furthermore revealed that many of the preferred partnerships were retained over two years. Follow‐up observations (unpublished data) indicate that a number of these preferred partnership lasted several years.
Data from our field study reveal that among male Guinea baboons greeting interactions are one of the most frequent social interactions (Dal Pesco, 2013). Greetings are defined as a ritualized exchange of affiliative signals between two individuals (adapted from Peláez, 1982) that can be intense, such as genital manipulation and mount, or less intense, such as hip‐touches and embraces (Whitham & Maestripieri, 2003). Prior to our field studies, greeting behavior in Guinea baboons was investigated in one study in captivity. Whitham and Maestripieri (2003) found that in captivity greeting occurrence correlates with the number of grooming bouts and the proportion of time spent in contact or close proximity, indicating that greetings in this species serves as a social bond maintenance mechanism, by allowing males to test the strength and the commitment of their relationship (Whitham & Maestripieri, 2003). However, observations conducted in our field site revealed that greeting interactions occurred among 80% of male‐male dyads, while only 1/3 engaged in affiliative interactions (Patzelt, 2013; Patzelt et al., 2014), indicating that greetings are not confined to preferred partners. An ongoing study at our field site is investigating the function of greetings to attempt to resolve this discrepancy (Dal Pesco et al., in preparation).
In sum, we found several pieces of evidence that males maintained friendly and enduring relationships with other males. These relationships were characterized by high spatial tolerance, support in agonistic interactions, and occasional grooming sessions between males. Agonistic interactions were observed rarely. During more than 460 h of observation (Patzelt et al., 2014), only 93 agonistic interactions were observed. In two thirds of these cases (N = 64), the outcome was decided with a clear winner and loser. Because of the low number of agonistic interactions, we were not able to establish a significant linear rank hierarchy, despite the fact that agonistic interactions were mostly unidirectional. We did not observe any intransitive dominance relationships. In this initial observation period, a sizeable proportion of males never engaged in any agonistic interactions. Ongoing observations (unpublished data), however, suggest a more intricate pattern, with counter aggression occurring, and subtler forms of agonistic interactions such as threat stares between a larger share of adult males.
A pressing question was whether preferred partners would be more highly related to each other than non‐preferred partners. Analyses of genetic relatedness based on 25 autosomal microsatellite markers indicated that males who belonged to the same gang were significantly more closely related than males belonging to different gangs, while there was no significant difference in relatedness for males that belonged either to the same or to different parties within the same gang. Overall, we detected 17 dyads that appeared to be highly related. In 3 out of 5 parties, we identified one closely related dyad each, but also males that were not highly related. One highly related dyad was found in the same gang, but not the same party. Finally, five highly related dyads comprised individuals that were members of different gangs. The remaining eight highly related dyads included males that we could not assign to any party or gang, since we did not observe them again after taking the samples for genetic analyses. Overall, these first insights into the kinship pattern suggest that relatedness may facilitate the formation of a preferred partnership, but it is not a necessary precondition. Further data will be needed to corroborate, or perhaps correct, this initial notion of the limited role of relatedness for the formation of social bonds among males. Long‐term data will be required to assess the factors that promote the formation and maintenance of affiliative relationships across the life span. Of particular interest is the question of whether bonds are already established relatively early among juveniles or adolescents, as Boese (1973) suggested.
9. Female‐biased dispersal
To further disentangle the Guinea baboon social system and to tackle the role of kin selection in shaping their high spatial tolerance, we used skin samples of identified individuals and non‐invasively collected fecal samples of unidentified individuals for genetic analyses. Genotypes and mitochondrial sequence data of 376 samples from their whole distribution of the species (including 165 individuals from the PNNK) and comparison with samples from other baboon taxa provided evidence for female‐biased dispersal (Kopp, Fischer, Patzelt, Roos, & Zinner, 2015; Kopp et al., 2014). Specific individuals were repeatedly sampled together, pointing to a stable composition of groups (Kopp et al., 2015). Average relatedness decreased from the gang to the community to the population level. Strikingly, male philopatry did not translate into higher mean relatedness between males within the different levels of the society, raising questions about the role of kinship in the formation of high tolerance among Guinea baboon males (Kopp et al., 2015), but also the question whether the mean relatedness is in fact a good measure when there are many unrelated and only few highly related dyads.
10. Male‐female relationships
After we had developed a first idea about male‐male relationships and social organization, we aimed to clarify whether female‐male association patterns conformed to the savanna baboon model, where intersexual relationships are largely confined to the estrous period and lactation, or whether females associated with males throughout their reproductive cycle, as in hamadryas baboons (Goffe, Zinner, & Fischer, 2016). For this first analysis, we focused on 16 females of one gang. We assessed spatial proximity from instantaneous scans, as well as relationship quality from focal observations. At least one male was found within 5 m of a female in almost half of the instances, and within 2 m in about a fifth of the time. Yet, this also meant that in slightly more than half of the time, no male was found within 5 m of the female, pointing to more relaxed relationships between males and females than in hamadryas baboons. We also used the proximity data to construct social networks for 15 females from 2 parties. The 5 m proximity data did not prove to be very informative as there was a high level of connectivity among individuals resulting in a relatively cohesive structure, supporting the notion that the Guinea baboons can be highly gregarious (Sharman, 1981). At the 2 m level, however, a clearer picture emerged and the parties within the gang were well distinguishable. A network based on proximity data from a different gang depicts our current understanding of the Guinea baboon social organization, with reproductive units nested within parties, and parties within gangs (Figure 5). From our demographic records for the years 2012–2016, we found that on average, each male was associated with 1.53 females (range 0–5, for N = 35 individually identified males and 106 male years).
Figure 5.
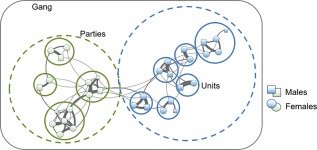
Social organization within a gang of Guinea baboons, based on proximity scans (unpublished data) collected during a 2‐month period in 2013 from the Simenti gang. One‐male‐units, some with secondary males (small squares), team up to form “parties,” which in turn form larger aggregations (“gangs”). The number of females per male varies considerably, as does female tenure length (Goffe et al., 2016). Females may maintain relations with females outside their own unit or party (Goffe et al. in preparation)
When we investigated the female‐male relationships in more detail (Goffe et al., 2016), we found that each female was mainly found in close proximity (≤2 m) of one specific male. Based on these data, we classified males as “primary” male when they were the top partner, as “secondary” male when they were sometimes in proximity of the female, and otherwise as “unaffiliated.” Grooming between females and males was mostly confined to the primary male (1.26 min/h), with some occasional grooming with the secondary male (0.16 min/h). Grooming with secondary males occurred in full view of primary males and did not elicit aggression by primary males. Typically, females had the active part (76% of all grooming observed). Ritualized greetings, involving touches, embraces, hip touches, genital manipulations, and mounting were mainly restricted to the primary male. Aggressive behaviors between males and females occurred at a rate of 0.1 events/h; they mostly occurred between the primary male and the female and consisted of male aggression against the female. We observed counter aggression by females in 20% of the cases. Primary males also handled infants more frequently than secondary or unaffiliated males. Importantly, copulations were largely confined to the primary males. Of 493 copulations observed ad libitum, 98.6% occurred between females and their respective primary male (Goffe et al., 2016). Overall, primary males were slightly more inclined to initiate interactions than females were, as they initiated 60% of all interactions, while secondary males initiated 76% of all interactions.
Our observations showed that male‐female interaction patterns were not strongly affected by female reproductive state (Goffe et al., 2016). Neither grooming nor aggression patterns changed with female reproductive state; only the likelihood of observing greetings was significantly lower when the female was lactating. In these cases, infant handling appeared to replace the occurrence of greeting. The majority of females shared their primary male with at least one, and up to four, other females. Thus, our data suggest that females maintain rather exclusive relationships with a specific male during any point in time, while any given male may be affiliated with a varying number of females. As a result, we concluded that “reproductive units” consisting of a primary male, one or several females, and occasionally an affiliated secondary male (see below) constitute the core of the Guinea baboon society.
To track variation in male‐female association, we used ad libitum data collected over 507 study days (Goffe et al., 2016). Changes in female‐primary male affiliation, based on the occurrence of grooming, greeting and copulations, were immediately obvious. We found that females transferred to other males both between and within parties. Females were not observed to transfer to their secondary males, but rather to bachelors or already established primary males. Changes occurred irrespective of reproductive state (lactating, pregnant and cycling) and no infanticide was observed. Eight females remained with the same primary male throughout the study period, while the other eight females transferred between primary males at least once. Transfers occurred “overnight”; i.e. the female was seen with one male on one day and with another on the next. There was no clear pattern predicting female transfer, and no obvious fighting of males over females; the few available observations tentatively suggest that within generally stable periods, shorter instable phases of multiple transfers occur. Female tenure time with any single male varied from 15 to 507 days. Median female tenure length was 200 days. However, this value is certainly a conservative estimate, as some females continued to affiliate with the same male beyond the end of the study period. Indeed, personal observations indicated that some dyads lasted more than 4 years (Goffe et al., 2016). Long‐term data will be needed to assess the true variability in tenure length, and the determinants of female fidelity.
Intriguingly, we found that males who had captured and killed small antelope allowed females of their own reproductive units to take scraps of the kill (Goffe & Fischer, 2016). Females in various reproductive states were observed to eat meat, suggesting that estrus state did not have an immediately obvious influence on meat sharing. However, males did preferentially share meat with females within their units. Whether or not meat sharing influenced the likelihood of securing the bond remained unclear. Female tenure at the time of sharing varied from four months to over two years (Goffe & Fischer, 2016).
11. Male aggressiveness in comparative perspective
As noted above, baboon species show considerable variation in behavior and our research supports the idea that Guinea baboons reveal distinct male reproductive strategies. While male savanna baboons compete over high rank to obtain access to proceptive females, male hamadryas baboons form stable associations with several females (OMUs), and males aggressively herd females and therefore force them to stay close to them (Pines & Swedell, 2011). Male Guinea baboons form relatively stable relationships with one or several females, but these relationships appear to be much looser than in hamadryas baboons. These differences in male reproductive strategies correlate with variation in male aggressiveness. Male Guinea baboons are less often involved in agonistic interactions than male chacma baboons, the savanna baboon species with the most intense level of contest competition (Kalbitzer, Heistermann, Cheney, Seyfarth, & Fischer, 2015). Intensity of male competition is predicted to be higher if many males compete over access to few females, but differences in the ratio of males and cycling females could not explain the observed differences in male aggressiveness. In fact, male Guinea baboons were generally less aggressive than male chacma baboons, not only toward other males but also toward females (Kalbitzer et al., 2015). The same study also confirmed that, in contrast to male chacma baboons, male Guinea baboons do not form linear dominance hierarchies and dominance relationships are generally less consistent. Interestingly, these differences in reproductive strategies and male aggressiveness seem to have consequences for relative stress levels related to consortships. In chacma baboons, males show higher glucocorticoid (or stress hormone) levels during consort with estrous females, while primary male Guinea baboons do not show higher glucocorticoid levels while one of their females is in estrous (Bergman, Beehner, Cheney, Seyfarth, & Whitten, 2005; Cheney, Crockford, Engh, Wittig, & Seyfarth, 2015; Kalbitzer et al., 2015).
The observed differences in male aggressiveness seem to be true interspecific differences and not related to, for example, variation in the availability of mating partners. These findings inspired us to conduct a study investigating the genetics underlying this interspecific variation in aggressiveness. Two genetic polymorphisms (5‐HTTLPR and MAOALPR), which are both involved in the functionality of the serotonin neurotransmitter system, have been associated with interspecific differences in aggressiveness and tolerance in humans and other primates (see Kalbitzer et al., 2016 and references therein). Therefore, we obtained genetic samples from five of the six baboons species (all but Kinda baboons) and compared the two polymorphisms among the different baboon species (Kalbitzer et al., 2016). Surprisingly, subjects in our sample were almost monomorphic in 5‐HTTLPR; only some hamadryas baboons carried an additional allele. However, we found three distinct alleles in MAOALPR, the so‐called “warrior gene,” although there was as much variation within species (i.e., among different populations of the same species) as among species. Therefore, behavioral variation among species cannot be linked to these two genetic polymorphisms, though differences in the metabolism of neurotransmitter levels linked to the MAOALPR genotype might be important for behavioral differences among individuals of the same population or behavioral differences among populations.
12. Vocal communication
Despite the fact that the social organization and the quality of the social interactions differs quite considerably among different baboon species, the structure of the vocalizations does not differ substantially. A qualitative comparison of the Guinea baboon loud calls (Byrne, 1981) and chacma baboon loud calls (Fischer, Hammerschmidt, Cheney, & Seyfarth, 2001, 2002) already suggested that the call types are largely similar. For a quantitative analysis of the Guinea baboon vocal repertoire, we used two‐step cluster analyses and identified 6 different call types (clusters) in adults, namely two types of screams, barks, wahoos (a two syllable bark), grunts, and roar grunts (Figure 6). Infant calls and juvenile calls were not considered, and female copulation calls were too faint to be included in a meaningful acoustic analysis (Maciej, Ndao, Hammerschmidt, & Fischer, 2013). Nevertheless, the call types identified above have been described in a number of studies on other baboon species, including chacma baboons from Southern Africa (Fischer et al., 2001, 2002; Meise, Keller, Cowlishaw, & Fischer, 2011; Palombit, Seyfarth, & Cheney, 1997; Rendall, Seyfarth, Cheney, & Owren, 1999). Yet, in terms of usage of the two most common call types in males (barks and grunts), we found considerable differences between male Guinea and chacma baboons. The significantly higher rate of affiliative interaction resulted in a higher rate of grunts in Guinea baboons; chacma baboons, on the other hand, used wahoos not only in response to predators and when they had lost contact to the rest of the group, but also as display signals in male‐male contests (Maciej, Ndao, et al., 2013). In sum, this study corroborated the view that the call structure of primates is highly conserved (Fischer, 2016) and largely unaffected by the species’ social organization. In contrast, the usage of calls is closely tied to the quality of interactions, driving the differential usage of calls that signal “benign intent” (Cheney, Seyfarth, & Silk, 1995) vs. those that signal the willingness to escalate a fight (Fischer, Kitchen, Seyfarth, & Cheney, 2004; Kitchen, Seyfarth, Fischer, & Cheney, 2003).
Figure 6.
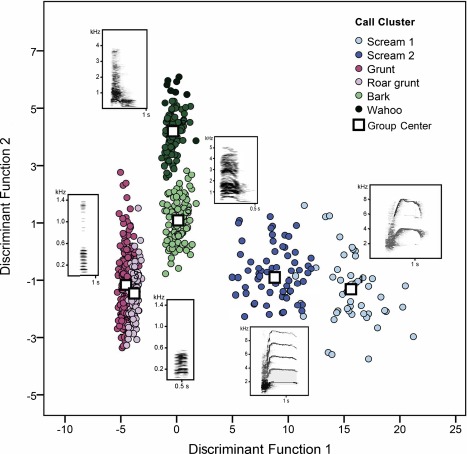
Vocal Repertoire of Guinea baboons. Discriminant function analyses using the six identified call clusters as grouping variable. The classification procedure revealed a high overall classification success with 99.2% of calls correctly assigned to the respective call type. For each call‐cluster a representative spectrogram is shown (modified from Maciej, Ndao, et al., 2013)
13. Summary and outlook
Our studies provide strong evidence that Guinea baboons live in a nested multi‐level society. Females maintain exclusive social and mating relationships with one male at a time, while males may be affiliated with a variable number of females, ranging from 1 to 5. Some males are not affiliated sexually with any female. The stability of male‐female relationships varies considerably. Overall, despite occasional fights, relationships among males are generally relatively relaxed, with high spatial and feeding tolerance. Some males maintain bonds with other males over several years. Surprisingly, kinship among males did not emerge as the primary determinant of male relationships. Furthermore, we found evidence for female‐biased dispersal.
Although we now have a good idea about the social system of the species, numerous questions remain, such as the feeding ecology and the effects of food availability on association patterns and home range usage, the determinants of male mating success, the role of female choice in this species, and the quality of female‐female relationships. Long‐term data will be needed to fully describe the social dynamics, i.e. the formation and maintenance of social bonds over time. What is also unclear is the variation in social organization among different populations of Guinea baboons. Population genetic analyses suggest that the general pattern of female‐biased dispersal can be found across the entire distribution of the species (Ferreira da Silva, Casanova, & Gondinho, 2013; Kopp et al., 2014).
On the assumption that the nested multi‐level organization and female‐biased dispersal apply to the entire range of Guinea baboons, the findings suggest that this species mirrors presumed early human societies in terms of their multi‐level social organization, strong male‐male bonds, as well as female‐biased dispersal pattern. Until recently, the hamadryas baboon was considered to be the only nonhuman primate species having these features in common with humans (Swedell & Plummer, 2012); this species will now have to share that title with the Guinea baboon.
Ultimately, we aim to put the Guinea baboon system into a broader context in terms of understanding the drivers of social evolution. One primary determinant in this context is the ecology, and more specifically the food distribution (Clutton‐Brock & Harvey, 1977; Crook & Gartlan, 1966). On the assumption that the social organization and female‐biased dispersal patterns are an ancestral trait shared between Guinea and hamadryas baboons (Kopp, 2015), and the fact that Guinea baboons retained these traits even in habitats with a high carrying capacity such as the Niokolo Koba National Park, this discounts the idea that the hamadryas baboon system is an adaptation to present‐day arid conditions (Schreier & Swedell, 2012). More generally, ecological conditions appear to be of little explanatory power in this genus (Henzi & Barrett, 2003; Jolly, 2011), despite the success of socio‐ecological models in primate evolution (but see Janson, 2000).
The genus reveals a gradient of decreasing male aggressiveness and increasing male spatial tolerance from southern to northern species (Barrett & Henzi, 2008; Kalbitzer et al., 2015). There are, however, also some discontinuities: of particular importance is the question of transition from female to male philopatry. This may have occurred during a phase of range expansion into the north/west (Jolly, 2009). Range expansion has been recognized as an important factor in evolution (Excoffier, Foll, & Petit, 2009). Demographic patterns presumably also played an important role. More specifically, when the population density is high, inbreeding depression is alleviated, which may favor males that do not disperse and are more likely to cooperate with other males. Furthermore, increased male tenure length promotes female dispersal (Clutton‐Brock & Lukas, 2012; Lukas & Clutton‐Brock, 2012). Remarkably, several primate species that live in multi‐level or fission‐fusion societies also reveal female‐biased dispersal (see Clutton‐Brock & Lukas, 2012; Grueter et al., 2012). This may be due to the fact that these two patterns emerge due to similar ecological or evolutionary forces, or alternatively, that one feature is a catalyst for the other (Kopp, 2015). Possibly, in nested societies, the dispersal costs for females are reduced, because they are able to disperse to familiar groups within the higher‐level grouping (Kopp, 2015).
In addition to the interplay between ecological drivers and phylogenetic inertia (Dunbar, 1988; Koenig, Scarry, Wheeler, & Borries, 2013), models of human evolution need to consider the feedback mechanisms between demographic factors, social organization and dispersal patterns in more detail, as well as the nonlinear effects that may be observed at the frontier of expanding populations (Hallatschek & Nelson, 2008; Ray & Excoffier, 2010). Although the genus baboon does not yet hold the clue to these factors that may have shaped human evolution, we suggest that it constitutes a valuable test case to assess the effects and interactions of these factors. Together with arising genomic information and concerted modeling efforts, such studies may contribute to a deeper understanding of the causes and effects of nested multi‐level social organization on mating and cooperation patterns in humans (Dyble et al., 2016).
Acknowledgments
We thank the Diréction des Parcs Nationaux and Ministère de l'Environnement et de la Protéction de la Nature de la République du Sénégal for permission to work in the Niokolo‐Koba National Park (Attestation 0383/24/03/2009 and 0373/10/3/2012). We particularly thank the conservators of the park for their support, as well as all the field assistants and volunteers who put in many hours in scorching heat to follow the baboons. Holger Sennhenn‐Reulen and Rebecca Jürgens helped with preparation of the figures and the bibliography. The studies presented in this paper were supported by the Leibniz Association, the German Science Foundation DFG Fi707/9‐1, the German Initiative of Excellence, the Leibniz Graduate School for the Foundations of Primate Social Behaviour (Göttingen, Germany), the Leakey Foundation, and the German Academic Exchange Service (DAAD).
How to Cite this Article: Fischer J, Kopp GH, Dal Pesco F, Goffe A, Hammerschmidt K, Kalbitzer U, Klapproth M, Maciej P, Ndao I, Patzelt A, Zinner D. Charting the neglected West: The social system of Guinea baboons. Am. J. Phys. Anthropol. 2017;162:15–31. doi: 10.1002/ajpa.23144.
References
- Alberts, S. C. , & Altmann, J. (2001). Immigration and hybridization patterns of yellow and anubis baboons in and around Amboseli, Kenya. American Journal of Primatology, 53, 139–154. [DOI] [PubMed] [Google Scholar]
- Alberts, S. C. , Buchan, J. C. , & Altmann, J. (2006). Sexual selection in wild baboons: From mating opportunities to paternity success. Animal Behaviour, 72, 1177–1196. [Google Scholar]
- Alberts, S. C. , Watts, H. E. , & Altmann, J. (2003). Queuing and queue‐jumping: Long‐term patterns of reproductive skew in male savannah baboons, Papio cynocephalus . Animal Behaviour, 65, 821–840. [Google Scholar]
- Anderson, D. P. , Nordheim, E. V. , Boesch, C. , & Moermond, T. C. (2002). Factors influencing fission‐fusion grouping in chimpanzees in the Tai National Park, Cote d'Ivoire In Boesch C., Hohmann G., & Marchant L. F. (Eds.), Behavioural diversity in chimpanzees and bonobos (pp. 90–101). Cambridge: Cambridge University Press. [Google Scholar]
- Arbonnier, M. (2004). Trees, shrubs and lianas of West African dry zones (CIRAD, editor), Montpellier: Margraf Publishers. [Google Scholar]
- Aureli, F. , Schaffner, C. M. , Boesch, C. , Bearder, S. K. , Call, J. , Chapman, C. A. , … van Schaik, C. P. (2008). Fission‐fusion dynamics: New research frameworks. Current Anthropology, 49, 627–654. [Google Scholar]
- Barrett, L. , & Henzi, S. P. (2008). Baboons. Current Biology, 18, R404–R406. [DOI] [PubMed] [Google Scholar]
- Bergman, T. J. , Beehner, J. C. , Cheney, D. L. , Seyfarth, R. M. , & Whitten, P. L. (2005). Correlates of stress in free‐ranging male chacma baboons, Papio hamadryas ursinus. Animal Behaviour, 70, 703–713. [Google Scholar]
- Bert, J. , Ayats, H. , Martino, A. , & Collomb, H. (1967). Note sur l'organisation de la vigilance sociale chez le babouin Papio papio dans l'est Sénégalais. Folia Primatologica, 6, 44–47. [DOI] [PubMed] [Google Scholar]
- Boesch, C. , & Boesch‐Achermann, H. (2000). The chimpanzees of the Taï forest: Behavioral ecology and evolution. Oxford: Oxford University Press. [Google Scholar]
- Boesch, C. , Hohmann, G. , & Marchant, L. (2002). Behavioural diversity in chimpanzees and bonobos. Cambridge: Cambridge University Press. [Google Scholar]
- Boesch, C. , Kohou, G. , Nene, H. , & Vigilant, L. (2006). Male competition and paternity in wild chimpanzees of the Taï forest. American Journal of Physical Anthropology, 130, 103–115. [DOI] [PubMed] [Google Scholar]
- Boese, G. K. (1973). Behavior and Social Organization of the Guinea Baboon (Papio papio) (PhD dissertation). Johns Hopkins University, Baltimore, USA.
- Boese, G. K. (1975). Social behavior and ecological considerations of West African baboons (Papio papio) In: Tuttle R. H. (Ed.), Socioecology and psychology of primates (pp. 205–230). The Hague: Mouton. [Google Scholar]
- Boissinot, S. , Alvarez, L. , Giraldo‐Ramirez, J. , & Tollis, M. (2014). Neutral nuclear variation in baboons (Genus Papio) provides insights into their evolutionary and demographic histories. American Journal of Physical Anthropology, 155, 621–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd, R. , & Silk, J. B. (2011). How humans evolved. 6th ed. New York, NY: W. W. Norton & Company. [Google Scholar]
- Bulger, J. B. (1993). Dominance rank and access to estrous females in male savanna baboons. Behaviour, 124, 89–122. [Google Scholar]
- Burrell, A. S. (2008). Phylogenetics and population genetics of central African baboons (PhD dissertation). New York University, USA. [Google Scholar]
- Burrell, A. S. , Jolly, C. J. , Phillips‐Conroy, J. E. , & Disotell, T. R. (2011). Inferring the dispersal behavior of the Kinda baboon (Papio kindae) from multilocus genetic data. American Journal of Physical Anthropology, 144 (S52), 99. [Google Scholar]
- Byrne, R. W. (1981). Distance vocalisations of Guinea baboons (Papio papio) in Senegal: An analysis of function. Behaviour, 78, 283–313. [Google Scholar]
- Chapais, B. (2008). Primeval kinship: How pair bonding gave birth to human society. Cambridge, MA: Harvard University Press. [Google Scholar]
- Chapais, B. (2011). The deep social structure of humankind. Science, 331, 1276–1277. [DOI] [PubMed] [Google Scholar]
- Cheney, D. L. , Crockford, C. , Engh, A. L. , Wittig, R. M. , & Seyfarth, R. M. (2015). The costs of parental and mating effort for male baboons. Behavioral Ecology and Sociobiology, 69, 303–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheney, D. L. , Seyfarth, R. M. , & Silk, J. B. (1995). The role of grunts in reconciling opponents and facilitating interactions among adult female baboons. Animal Behaviour, 50, 249–257. [Google Scholar]
- Chowdhury, S. , Pines, M. , Saunders, J. , & Swedell, L. (2015). The adaptive value of secondary males in the polygynous multi‐level society of hamadryas baboons. American Journal of Physical Anthropology, 158, 501–513. [DOI] [PubMed] [Google Scholar]
- Clutton‐Brock, T. H. , & Harvey, P. H. (1977). Primate ecology and social‐organization. Journal of Zoology, 183, 1–39. [Google Scholar]
- Clutton‐Brock, T. H. , & Lukas, D. (2012). The evolution of social philopatry and dispersal in female mammals. Molecular Ecology, 21, 472–492. [DOI] [PubMed] [Google Scholar]
- Cracraft, J. (1989). Speciation and its ontology: The empirical consequences of alternative species concepts for understanding patterns and processes of differentiation In Otte D., & Endler J. A., (Eds.), Speciation and its consequences (pp. 28–59) Sunderland, MS: Sinauer. [Google Scholar]
- Crook, J. H. , & Gartlan, J. S. (1966). Evolution of primate societies. Nature, 210, 1200–1203. [DOI] [PubMed] [Google Scholar]
- Dal Pesco, F. (2013). Greeting behaviour in wild Guinea baboons (Papio papio) in the Niokolo‐Koba National Park, Senegal (MSc thesis). Freie Univerität zu Berlin, Germany.
- Dunbar, R. I. M. (1988). Primate social systems. New York, NY: Cornell University Press. [Google Scholar]
- Dunbar, R. I. M. , & Nathan, M. F. (1972). Social organization of the Guinea baboon (Papio papio). Folia Primatologica, 17, 321–334. [DOI] [PubMed] [Google Scholar]
- Dupuy, A. R. (1971). Le Niokolo Koba - premier grand parc national de la République du Sénégal. Dakar: G.I.A.
- Dupuy, A. R. , & Gaillard, D. R. (1969). Capture d'un cynocephales presentant une anomalié de coloration. Mammalia, 33, 732–733. [Google Scholar]
- Dyble, M. , Thompson, J. , Smith, D. , Salali, G. D. , Chaudhary, N. , Page, A. E. , … Migliano, A. B. (2016). Networks of food sharing reveal the functional significance of multilevel sociality in two hunter‐gatherer groups. Current Biology, 26, 1–5. [DOI] [PubMed] [Google Scholar]
- Excoffier, L. , Foll, M. , & Petit, R. J. (2009). Genetic consequences of range expansions. Annual Review of Ecology, Evolution, and Systematics, 40, 481–501. [Google Scholar]
- Ferreira da Silva, M. , Casanova, C. , & Gondinho, R. (2013). On the western fringe of baboons distribution: Mitochondrial D‐loop diversity of Guinea baboons (Papio papio, Desmarest 1820) (Primates: Cercopithecidae) in Coastal Guinea‐Bissau, Western Africa. Journal of Threatened Taxa, 5, 4441–4450. [Google Scholar]
- Fischer, J. (2016). Primate vocal production and the riddle of language evolution. Psychonomic Bulletin & Review [Epub ahead of print] doi: 10.3758/s13423-016-1076-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer, J. , Hammerschmidt, K. , Cheney, D. L. , & Seyfarth, R. M. (2001). Acoustic features of female chacma baboon barks. Ethology, 107, 33–54. [Google Scholar]
- Fischer, J. , Hammerschmidt, K. , Cheney, D. L. , & Seyfarth, R. M. (2002). Acoustic features of male baboon loud calls: Influences of context, age, and individuality. The Journal of the Acoustical Society of America, 111, 1465–1474. [DOI] [PubMed] [Google Scholar]
- Fischer, J. , Kitchen, D. M. , Seyfarth, R. M. , & Cheney, D. L. (2004). Baboon loud calls advertise male quality: Acoustic features and their relation to rank, age, and exhaustion. Behavioral Ecology and Sociobiology, 56, 140–148. [Google Scholar]
- Foerster, S. , McLellan, K. , Schroepfer‐Walker, K. , Murray, C. M. , Krupenye, C. , Gilby, I. C. , & Pusey, A. E. (2015). Social bonds in the dispersing sex: Partner preferences among adult female chimpanzees. Animal Behaviour, 105, 139–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley, R. , & Gamble, C. (2009). The ecology of social transitions in human evolution. Philosophical Transactions of the Royal Society B Biological Sciences, 364, 3267–3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galat‐Luong, A. , Galat, G. , Hagell, S. , & Tuttle, R. H. (2006). The social and ecological flexibility of Guinea baboons: Implications for Guinea baboon social organization and male strategies In Swedell L., & Leigh S. R. (Eds.), Reproduction and fitness in baboons. Behavioral, ecological, and life history perspectives (pp. 105–121). New York, NY: Springer. [Google Scholar]
- Goffe, A. S. , & Fischer, J. (2016). Meat sharing between male and female Guinea baboons (Papio papio). Primate Biology, 3, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goffe, A. S. , Zinner, D. , & Fischer, J. (2016). Sex and friendship in a multilevel society: Behavioural patterns and associations between female and male Guinea baboons. Behavioral Ecology and Sociobiology, 70, 323–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodall, J. (1986). The chimpanzees of Gombe: Patterns of behavior. Cambridge, MA: Harvard University Press. [Google Scholar]
- Grueter, C. C. , Chapais, B. , & Zinner, D. (2012). Evolution of multilevel social systems in nonhuman primates and humans. International Journal of Primatology, 33, 1002–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallatschek, O. , & Nelson, D. R. (2008). Gene surfing in expanding populations. Theoretical Population Biology, 73, 158–170. [DOI] [PubMed] [Google Scholar]
- Harcourt, A. H. , Harvey, P. H. , Larson, S. G. , & Short, R. V. (1981). Testis weight, body weight and breeding system in primates. Nature, 293, 55–57. [DOI] [PubMed] [Google Scholar]
- Hausfater, G. (1975). Dominance and reproduction in baboons (Papio cynocephalus) – Quantitative‐analysis. Contributions to Primatology, 7, 2–150. [PubMed] [Google Scholar]
- Hejcmanova‐Nežerková, P. , & Hejcman, M. (2006). A canonical correspondence analysis (CCA) of the vegetation‐environment relationships in Sudanese savannah, Senegal. The South African Journal of Botany, 72, 256–262. [Google Scholar]
- Henzi, S. P. , & Barrett, L. (2003). Evolutionary ecology, sexual conflict, and behavioral differentiation among baboon populations. Evolutionary Anthropology, 12, 217–230. [Google Scholar]
- Hill, K. R. , Walker, R. S. , Bozicević, M. , Eder, J. , Headland, T. , Hewlett, B. , … Wood, B. (2011). Co‐residence patterns in hunter‐gatherer societies show unique human social structure. Science, 331, 1286–1289. [DOI] [PubMed] [Google Scholar]
- Hohmann, G. , & Fruth, B. (2002). Dynamics in social organization of bonobos (Pan paniscus) In Boesch C., Hohmann G., & Marchant L. F. (Eds.), Behavioural diversity in chimpanzees and bonobos (pp. 138–150) Cambridge: Cambridge University Press. [Google Scholar]
- Howard, W. , Wangari, E. , & Rakotoaisoa, N. (2007). Mission report ‐ UNESCO/IUCN joint monitoring mission to Niokolo Koba National Park, Senegal Available from: http://whc.unesco.org/document/8951
- Janson, C. H. (2000). Primate socio‐ecology: The end of a golden age. Evolutionary Anthropology, 9, 73–86. [Google Scholar]
- Jolly, C. J. (2007). Baboons, mandrills, and mangabeys: Afro‐Papionin socioecology in a phylogenetic perspective In Campbell C., Fuentes A., MacKinnon K., Panger M., & Bearder S. K. (Eds.) Primates in perspective (pp. 240–251). New York, NY; Oxford: Oxford University Press. [Google Scholar]
- Jolly, C. J. (2009). Fifty years of looking at human evolution: Backward, forward, and sideways. Current Anthropology, 50, 187–199. [DOI] [PubMed] [Google Scholar]
- Jolly, C. J. (2011). Rainfall is not a genus‐wide predictor of mean body mass in baboon populations. Journal of Zoology, 286, 185–193. [Google Scholar]
- Jolly, C. J. , Burrell, A. S. , Phillips‐Conroy, J. E. , Bergey, C. , & Rogers, J. (2011). Kinda baboons (Papio kindae) and grayfoot chacma baboons (P. ursinus griseipes) hybridize in the Kafue river valley, Zambia. American Journal of Primatology, 73, 291–303. [DOI] [PubMed] [Google Scholar]
- Jolly, C. J. , & Phillips‐Conroy, J. E. (2003). Testicular size, mating system, and maturation schedules in wild anubis and hamadryas baboons. International Journal of Primatology, 24, 125–142. [Google Scholar]
- Jolly, C. J. , & Phillips‐Conroy, J. E. (2006). Testicular size, developmental trajectories, and male life history strategies in four baboon taxa In Swedell L., & Leigh S. R. (Eds.), Reproduction and fitness in baboons: Behavioral, ecological, and life history perspectives. (pp 257–275) New York, NY: Springer. [Google Scholar]
- Kalbitzer, U. , Heistermann, M. , Cheney, D. L. , Seyfarth, R. M. , & Fischer, J. (2015). Social behavior and patterns of testosterone and glucocorticoid levels differ between male chacma and Guinea baboons. Hormones and Behavior, 75, 100–110. [DOI] [PubMed] [Google Scholar]
- Kalbitzer, U. , Roos, C. , Kopp, G. H. , Butynski, T. M. , Knauf, S. , Zinner, D. , & Fischer, J. (2016). Insights into the genetic foundation of aggression in Papio and the evolution of two length‐polymorphisms in the promoter regions of serotonin‐related genes (5‐HTTLPR and MAOALPR) in Papionini. BMC Evolutionary Biology, 16, 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappeler, P. M. (2008). Genetic and ecological determinants of primate social systems In Korb J., & Heinze J. (Eds.), Ecology of social evolution (pp. 225–243) Berlin: Springer. [Google Scholar]
- Kappeler, P. M. , & van Schaik, C. P. (2002). Evolution of primate social systems. International Journal of Primatology, 23, 707–740. [Google Scholar]
- Kie, J. G. (2013). A rule‐based ad hoc method for selecting a bandwidth in kernel home‐range analyses. Animal Biotelemetry, 1, 13. [Google Scholar]
- Kitchen, D. M. , Seyfarth, R. M. , Fischer, J. , & Cheney, D. L. (2003). Loud calls as indicators of dominance in male baboons (Papio cynocephalus ursinus). Behavioral Ecology and Sociobiology, 53, 374–384. [Google Scholar]
- Koenig, A. , Scarry, C. J. , Wheeler, B. C. , & Borries, C. (2013). Variation in grouping patterns, mating systems and social structure: What socio‐ecological models attempt to explain. Philosophical Transactions of the Royal Society B Biological Sciences, 368, 20120348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp, G. H. (2015). Gene flow dynamics in baboons – The influence of social systems (PhD dissertation). Georg‐August‐University Göttingen, Germany.
- Kopp, G. H. , Fischer, J. , Patzelt, A. , Roos, C. , & Zinner, D. (2015). Population genetic insights into the social organization of Guinea baboons (Papio papio): Evidence for female‐biased dispersal. American Journal of Primatology, 77, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp, G. H. , Silva, M. J. F. , Fischer, J. , Brito, J. C. , Regnaut, S. , Roos, C. , & Zinner, D. (2014). The influence of social systems on patterns of mitochondrial DNA variation in baboons. International Journal of Primatology, 35, 210–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kottek, M. , Grieser, J. , Beck, C. , Rudolf, B. , & Rubel, F. (2006). World map of the Köppen‐Geiger climate classification updated. Meteorologische Zeitschrift, 15, 259–263. [Google Scholar]
- Kummer, H. (1968). Social organization of hamadryas baboons: A field study. 1st ed. Chicago, IL: The University of Chicago Press. [Google Scholar]
- Kummer, H. (1995). In quest of the sacred baboon. Princeton, NJ: Princeton University Press. [Google Scholar]
- Kunz, B. K. , & Linsenmair, K. E. (2008). The disregarded west: Diet and behavioural ecology of olive baboons in the Ivory Coast. Folia Primatologica, 79, 31–51. [DOI] [PubMed] [Google Scholar]
- Lehmann, J. , & Boesch, C. (2004). To fission or to fusion: Effects of community size on wild chimpanzee (Pan troglodytes verus) social organisation. Behavioral Ecology and Sociobiology, 56, 207–216. [Google Scholar]
- Lemasson, A. , Palombit, R. A. , & Jubin, R. (2008). Friendships between males and lactating females in a free‐ranging group of olive baboons (Papio hamadryas anubis): Evidence from playback experiments. Behavioral Ecology and Sociobiology, 62, 1027–1035. [Google Scholar]
- Lukas, D. , & Clutton‐Brock, T. (2012). Cooperative breeding and monogamy in mammalian societies. Proceedings of the Royal Society B: Biological Sciences, 279, 2151–2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciej, P. (2013). Vocal communication in a tolerant multi‐level society: insights from signallers and receivers in Guinea baboons (PhD dissertation). Georg‐August‐University Göttingen, Germany.
- Maciej, P. , Ndao, I. , Hammerschmidt, K. , & Fischer, J. (2013). Vocal communication in a complex multi‐level society: Constrained acoustic structure and flexible call usage in Guinea baboons. Frontiers in Zoology, 10, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciej, P. , Patzelt, A. , Ndao, I. , Hammerschmidt, K. , & Fischer, J. (2013). Social monitoring in a multilevel society: A playback study with male Guinea baboons. Behavioral Ecology and Sociobiology, 67, 61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen, J. E. , Dione, D. , Traoré, A. , & Sambou, B. (1996). Flora and vegetation of Niokolo‐Koba National Park, Senegal In van der Maesen L., van der Burgt X. M., & van Medenbach de Rooy J. M. (Eds.), The biodiversity of African Plants: Proceedings XIVth AETFAT Congress (pp. 214–219). 22–27 August 1994, Wageningen, The Netherlands. Dordrecht: Springer Netherlands. [Google Scholar]
- Maestripieri, D. , Mayhew, J. , Carlson, C. L. , Hoffman, C. L. , & Radtke, J. M. (2007). One‐male harems and female social dynamics in Guinea baboons. Folia Primatologica, 78, 56–68. [DOI] [PubMed] [Google Scholar]
- Marlowe, F. W. (2004). Mate preferences among Hadza hunter‐gatherers. Human Nature, 15, 365–376. [DOI] [PubMed] [Google Scholar]
- Marlowe, F. W. (2005). Hunter‐gatherers and human evolution. Evolutionary Anthropology, 14, 54–67. [Google Scholar]
- Mbow, C. , Goı¨Ta, K. , & Bénié, G. (2004). Spectral indices and fire behavior simulation for fire risk assessment in savanna ecosystems. Remote Sensing of Environment, 91, 1–13. [Google Scholar]
- Meise, K. , Keller, C. , Cowlishaw, G. , & Fischer, J. (2011). Sources of acoustic variation: Implications for production specificity and call categorization in chacma baboon (Papio ursinus) grunts. The Journal of the Acoustical Society of America, 129, 1631–1641. [DOI] [PubMed] [Google Scholar]
- Mitani J. C., Call J., Kappeler P. M., Palombit R. A., Silk J. B. Eds. (2012). The evolution of primate societies. Chicago, IL: University of Chicago Press. [Google Scholar]
- Mitani, J. C. , Watts, D. P. , & Muller, M. N. (2002). Recent developments in the study of wild chimpanzee behavior. Evolutionary Anthropology, 11, 9–25. [Google Scholar]
- Moscovice, L. R. , Di Fiore, A. , Crockford, C. , Kitchen, D. M. , Wittig, R. M. , Seyfarth, R. M. , & Cheney, D. L. (2010). Hedging their bets? Male and female chacma baboons form friendships based on likelihood of paternity. Animal Behaviour, 79, 1007–1015. [Google Scholar]
- Nagel, U. (1973). Comparison of Anubis baboons, hamadryas baboons and their hybrids at a species border in Ethiopia. Folia Primatologica, 19, 104–165. [DOI] [PubMed] [Google Scholar]
- Ndao, I. , & Henschel, P. (2011). Rapport de l’étude sur la population des lions au Parc National Niokolo Koba. Tambacounda, Senegal: DPN.
- Newman, T. K. , Jolly, C. J. , & Rogers, J. (2004). Mitochondrial phylogeny and systematics of baboons (Papio). American Journal of Physical Anthropology, 124, 17–27. [DOI] [PubMed] [Google Scholar]
- Newton‐Fisher, N. E. (2002). Relationships of male chimpanzees in the Budongo Forest, Uganda In Boesch C., Hohmann G., & Marchant L. (Eds.), Behavioural diversity in chimpanzees and bonobos (pp. 125–137). Cambridge University Press. [Google Scholar]
- Newton‐Fisher, N. E. , Thompson, M. E. , Reynolds, V. , Boesch, C. , & Vigilant, L. (2010). Paternity and social rank in wild chimpanzees (Pan troglodytes) from the Budongo Forest, Uganda. American Journal of Physical Anthropology, 142, 417–428. [DOI] [PubMed] [Google Scholar]
- Nishida, T. , & Hiraiwa‐Hasegawa, M. (1987). Chimpanzees and bonobos: Cooperative relationships among males In Smut B. B., Cheney D., Seyfarth R. M., & Wrangham R. W. (Eds.), Primate societies (pp. 165–177). Chicago, IL: University of Chicago Press. [Google Scholar]
- Packer, C. (1979). Male dominance and reproductive activity in Papio anubis . Animal Behaviour, 27, 37–45. [DOI] [PubMed] [Google Scholar]
- Palombit, R. A. (2009). “Friendship” with males: A female counterstrategy to infanticide in chacma baboons of the Okavango Delta In Muller M. N., & Wrangham R. W. (Eds.), Sexual coercion in primates and humans: An evolutionary perspective on male aggression against females (pp. 377–409). Cambridge, MA: Harvard University Press. [Google Scholar]
- Palombit, R. A. , Seyfarth, R. M. , & Cheney, D. L. (1997). The adaptive value of “friendships” to female baboons: Experimental and observational evidence. Animal Behaviour, 54, 599–614. [DOI] [PubMed] [Google Scholar]
- Patzelt, A. (2013). The social system of Guinea baboons (Papio papio) with a focus on male‐male relationships (PhD dissertation). Georg‐August‐University Göttingen, Germany.
- Patzelt, A. , Kopp, G. H. , Ndao, I. , Kalbitzer, U. , Zinner, D. , & Fischer, J. (2014). Male tolerance and male–male bonds in a multilevel primate society. Proceedings of the National Academy of Sciences of the United States of America, 111, 14740–14745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patzelt, A. , Zinner, D. , Fickenscher, G. , Diedhou, S. , Camara, B. , Stahl, D. , & Fischer, J. (2011). Group composition of Guinea baboons (Papio papio) at a water place suggests a fluid fission‐fusion social organisation. International Journal of Primatology, 32, 652–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peel, M. C. , Finlayson, B. L. , & McMahon, T. (2007). Updated world map of the Köppen‐Geiger climate classification. Hydrology and Earth System Sciences, 11, 1633–1644. [Google Scholar]
- Peláez, F. (1982). Greeting Movements among adult males in a colony of Baboons: Papio hamadryas, P. cynocephalus and their hybrids. Primates, 23, 233–244. [Google Scholar]
- Pfefferle, D. , & Fischer, J. (2006). Sounds and size: Identification of acoustic variables that reflect body size in hamadryas baboons, Papio hamadryas . Animal Behaviour, 72, 43–51. [Google Scholar]
- Phillips‐Conroy, J. E. , & Jolly, C. J. (1981). Sexual dimorphism in two subspecies of Ethiopian baboons (Papio hamadryas) and their hybrids. American Journal of Physical Anthropology, 56, 115–129. [DOI] [PubMed] [Google Scholar]
- Phillips‐Conroy, J. E. , Jolly, C. J. , Burrell, A. , Rogers, J. , & Weyher, A. H. (2009). Genetic and behavioral observations of “Kinda” baboons (Papio cynocephalus kindae) in Zambia. American Journal of Physical Anthropology, 138 (S48), 211. [Google Scholar]
- Phillips‐Conroy, J. E. , Jolly, C. J. , Nystrom, P. , & Hemmalin, H. A. (1992). Migration of male hamadryas baboons into anubis groups in the Awash National Park, Ethiopia. International Journal of Primatology, 13, 455–476. [Google Scholar]
- Pines, M. , Saunders, J. , & Swedell, L. (2011). Alternative routes to the leader male role in a multi‐level society: Follower vs. solitary male strategies and outcomes in hamadryas baboons. American Journal of Primatology, 73, 679–691. [DOI] [PubMed] [Google Scholar]
- Pines, M. , & Swedell, L. (2011). Not without a fair fight: Failed abductions of females in wild hamadryas baboons. Primates, 52, 249–252. [DOI] [PubMed] [Google Scholar]
- Pusey, A. E. , & Schroepfer‐Walker, K. (2013). Female competition in chimpanzees. Philosophical Transactions of the Royal Society B Biological Sciences, 368, 20130077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray, N. , & Excoffier, L. (2010). A first step towards inferring levels of long‐distance dispersal during past expansions. Molecular Ecology Resources, 10, 902–914. [DOI] [PubMed] [Google Scholar]
- Renaud, P. C. , Gueye, M. B. , Hejcmanová, P. , Antonínová, M. , & Samb, M. (2006). Inventaire aérien et terrestre de la faune et relevé des pressions au Parc National du Niokolo Koba: Plan of Urgence, Report Annex A. Ministère de l'Environnement et de la Protection de la Nature Protection – African Parks Foundation, Dakar.
- Rendall, D. , Seyfarth, R. M. , Cheney, D. L. , & Owren, M. J. (1999). The meaning and function of grunt variants in baboons. Animal Behaviour, 57, 583–592. [DOI] [PubMed] [Google Scholar]
- Rodseth, L. , & Novak, S. A. (2000). The social modes of men. Human Nature, 11, 335–366. [DOI] [PubMed] [Google Scholar]
- Rodseth, L. , Wrangham, R. W. , Harrigan, A. , & Smuts, B. B. (1991). The human community as a primate society. Current Anthropology, 32, 221–254. [Google Scholar]
- Schreier, A. L. , & Swedell, L. (2012). Ecology and sociality in a multilevel society: Ecological determinants of spatial cohesion in hamadryas baboons. American Journal of Physical Anthropology, 148, 580–588. [DOI] [PubMed] [Google Scholar]
- Schultz, A. H. (1938). The relative weight of the testes in primates. Anatomical Record, 72, 387–394. [Google Scholar]
- Sharman, M. J. (1981). Feeding, ranging and the social organisation of the Guinea baboon (PhD dissertation). University of St. Andrews, St. Andrews, UK.
- Smith, R. J. , & Jungers, W. L. (1997). Body mass in comparative primatology. Journal of Human Evolution, 32, 523–559. [DOI] [PubMed] [Google Scholar]
- Smuts, B. B. (1985). Sex and friendship in baboons. New York, NY: Aldine Publishing Company. [Google Scholar]
- Städele, V. , Van Doren, V. , Pines, M. , Swedell, L. , & Vigilant, L. (2015). Fine‐scale genetic assessment of sex‐specific dispersal patterns in a multilevel primate society. Journal of Human Evolution, 78, 103–113. [DOI] [PubMed] [Google Scholar]
- Stumpf, R. M. (2011). Chimpanzees and bonobos: Inter‐ and intraspecies diversity In Campbell C. J., Fuentes A., MacKinnon K. C., Bearder S. K., & Stumpf R. M. (Eds.), Primates in perspective (pp. 340–356). New York, NY: Oxford University Press. [Google Scholar]
- Susman, R. L. (1987). Pygmy chimpanzees and common chimpanzees In Kinzey W. G. (Ed.), The evolution of human behavior: Primate models (pp. 73–86). Albany, NY: SUNY University Press. [Google Scholar]
- Swedell, L. (2006). Strategies of sex and survival hamadryas baboons: Through a female lens. Upper Saddle River, NJ: Pearson Prentice Hall. [Google Scholar]
- Swedell, L. (2011). African Papionins: Diversity of social organization and ecological flexibility In Campbell C. J. (Ed.), Primates in perspective (2nd ed., pp. 241–277). New York, NY: Oxford University Press. [Google Scholar]
- Swedell, L. , & Plummer, T. (2012). A Papionin multilevel society as a model for hominin social evolution. International Journal of Primatology, 33, 1165–1193. [Google Scholar]
- Swedell, L. , Saunders, J. , Schreier, A. , Davis, B. , Tesfaye, T. , & Pines, M. (2011). Female “dispersal” in hamadryas baboons: Transfer among social units in a multilevel society. American Journal of Physical Anthropology, 145, 360–370. [DOI] [PubMed] [Google Scholar]
- Swedell, L. , & Schreier, A. (2009). Male aggression towards females in hamadryas baboons: Conditioning, coercion, and control In Muller M. N., & Wrangham R. W. (Eds.), Sexual coercion in primates and humans: An evolutionary perspective on male aggression against females (pp. 244–268). Cambridge, MA: Harvard University Press. [Google Scholar]
- Tappan, G. , Sall, M. , Wood, E. , & Cushing, M. (2004). Ecoregions and land cover trends in Senegal. Journal of Arid Environments, 59, 427–462. [Google Scholar]
- Thorén, S. , Lindenfors, P. , & Kappeler, P. M. (2006). Phylogenetic analyses of dimorphism in primates: Evidence for stronger selection on canine size than on body size. American Journal of Physical Anthropology, 130, 50–59. [DOI] [PubMed] [Google Scholar]
- Walter, H. , & Lieth, H. (1967). Klimadiagramm Weltatlas. Jena: Gustav Fischer. [Google Scholar]
- Watts, D. P. , & Mitani, J. C. (2001). Boundary patrols and intergroup encounters in wild chimpanzees. Behaviour, 138, 299–327. [Google Scholar]
- Weyher, A. H. , & Chiou, K. L. (2013). Adult kinda baboon (Papio kindae) behavior: Preliminary results from a two year study. American Journal of Physical Anthropology, 150 (S56), 289. [Google Scholar]
- Weyher, A. H. , Phillips‐Conroy, J. E. , Fourrier, M. S. , & Jolly, C. J. (2014). Male‐driven grooming bouts in mixed‐sex dyads of Kinda baboons (Papio kindae). Folia Primatologica, 85, 178–191. [DOI] [PubMed] [Google Scholar]
- Whitham, J. C. , & Maestripieri, D. (2003). Primate rituals: The function of greetings between male Guinea baboons. Ethology, 109, 847–859. [Google Scholar]
- Wroblewski, E. E. , Murray, C. M. , Keele, B. F. , Schumacher‐Stankey, J. C. , Hahn, B. H. , & Pusey, A. E. (2009). Male dominance rank and reproductive success in chimpanzees, Pan troglodytes schweinfurthii . Animal Behaviour, 77, 873–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinner, D. , Buba, U. , Nash, S. , & Roos, C. (2011). Pan‐African voyagers. The phylogeography of baboons In Sommer V., Ross C. (Eds.), Primates of Gashaka. Socio‐ecology and Conservation in Nigeria's Biodiversity Hotspot (Vol. 35, pp. 319–358). Series: Developments in Primatology: Progress and Prospects. New York, NY: Springer. [Google Scholar]
- Zinner, D. , Groeneveld, L. F. , Keller, C. , & Roos, C. (2009). Mitochondrial phylogeography of baboons (Papio spp.) – Indication for introgressive hybridization? BMC Evolutionary Biology, 9, 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinner, D. , Wertheimer, J. , Liedigk, R. , Groeneveld, L. F. , & Roos, C. (2013). Baboon phylogeny as inferred from complete mitochondrial genomes. American Journal of Physical Anthropology, 150, 133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]


