Abstract
Two‐stage revision arthroplasty is the treatment of choice for periprosthetic infection, a serious complication after knee or hip arthroplasty. Our prospective clinical trial aimed to investigate the concentrations of gentamicin and vancomycin in wound exudate and tissue in two‐stage revision arthroplasty. Wound exudate and periprosthetic membrane samples were collected from 18 patients (10 hip and eight knee patients), who were due for two‐stage treatment after a periprosthetic joint infection. Samples were taken during insertion of antibiotic‐impregnated spacers and after their removal. The concentrations of gentamicin and vancomycin in wound exudates and adjacent tissue were analyzed using high‐performance liquid chromatography mass spectrometry. Average time period of spacer implantation was 13.6 weeks (9.3–22.6 weeks). The concentration of vancomycin in wound exudate decreased from a median of 43.28 μg/mL (0.28–261.22) after implantation to 0.46 μg/mL (0.13–37.47) after the removal of the spacer. In the adjacent tissue, vancomycin concentration was mainly undetectable prior to spacer implantation (0.003 μg/g [0.003–0.261]) and increased to 0.318 μg/g [0.024–484.16] at the time of spacer removal. This was also observed for gentamicin in the tissue of patients who previously had cement‐free implants (0.008 μg/g [0.008–0.087] vs. 0.164 μg/g [0.048–71.75]) while in the tissue of patients with previously cemented prosthesis, baseline concentration was already high (8.451 μg/g [0.152–42.926]). Despite the rapid decrease in antibiotics release from spacer cement observed in vitro, in vivo antibiotics are much longer detectable, especially in the adjacent soft tissue. © 2018 The Authors. Journal of Biomedical Materials Research Part B: Applied Biomaterials Published By Wiley Periodicals, Inc. J Biomed Mater Res B Part B, 2019. © 2018 Wiley Periodicals, Inc. J Biomed Mater Res Part B: Appl Biomater 107B: 1587–1597, 2019.
Keywords: antibiotics release, cement spacer, two‐stage treatment, periprosthetic joint infection, vancomycin, gentamicin, revision arthroplasty
INTRODUCTION
Periprosthetic joint infection is the most severe local specific complication associated with total joint replacement (TJR). While the incidence is relatively low, with 0.5–2.0% after primary implantation,1, 2 it still represents a considerable number of cases, especially given the background of the ever rising implantation rates.3 There are two main treatment regimes: either a single stage revision—removal of the infected implant, debridement and re‐implantation of a TJR—or a long‐interval two‐stage spacer procedure. The latter is the more commonly used method, in particular for difficult‐to‐treat pathogens and in patients who had already undergone a previous revision due to infection.4, 5 In the two stage approach, systemic antibiotic therapy is adjusted according to the intraoperatively detected pathogens and is given for 6 weeks after implant removal in order to eradicate the causative pathogens. During this period, spacers made of polymethylmethacrylate (PMMA) cement function as an interposition arthroplasty, which provides mechanical stability, preserves the space needed for the revision implant and when impregnated with antibiotics serves as an antibiotic delivery system.6, 7 The use of antibiotic‐impregnated cement was shown to efficiently prevent implant‐associated infections.8, 9 With the rise of antibiotic‐resistant pathogens, often more than one antibiotic is added to the bone cement to enable broadened prophylactic effects. One common supplementation is the combination of gentamicin and vancomycin, which have been shown to act synergistically.10, 11 While there are a number of studies investigating the in vitro and ex vivo release of antibiotics from bone cement,12, 13, 14, 15, 16, 17 less is known about the concentration of the antibiotics in joint fluid and adjacent soft tissue in vivo, especially for the duration of the cement spacer treatment.18, 19
Therefore, the aim of the present study was to analyze the concentrations of gentamicin and vancomycin in wound exudate and in the adjacent soft tissue (periprosthetic membrane) not only at the time of implantation but also at removal of the PMMA cement spacer. These data were compared to in vitro antibiotics release from prepared test specimens of same cement spacer material.
MATERIALS AND METHODS
Study design
Patients from two prospective, multicenter trials investigating cement spacer treatment of implant‐associated infections after total hip and knee joint replacement, respectively, were included to analyze antibiotics' release from those spacer implants. Both studies were approved by the Local Ethics Committee of Rostock, Germany (registration numbers: A2011‐137 and HV‐2011‐0011). The study design followed a standardized algorithm of cement spacer treatment as described before.20 Briefly, patients who were preoperatively diagnosed with periprosthetic joint infection due to a positive microbiological joint aspiration and elevated inflammation markers were implanted with a custom‐made antibiotic‐loaded PMMA cement spacer after complete removal of the infected endoprosthesis and extensive debridement. Two different bone cements were used in the in vivo study: PALACOS® R+G (Heraeus Medical GmbH, Wehrheim, Germany) a fast‐curing, radiopaque, poly(methyl methacrylate)‐based bone cement which contains the aminoglycoside antibiotic gentamicin and COPAL® spacem, a fast‐curing cement‐like polymer based on poly(methylacrylate, methyl methacrylate) with no added antibiotics which contains calcium carbonate as an X‐ray contrast agent (Heraeus Medical GmbH, Wehrheim, Germany). In all hip patients, PALACOS® R+G cement was combined with vancomycin (Vancomycin hydrochloride, Lyomark Pharma, Oberhaching, Germany), while patients with total knee replacements were randomly allocated to either receiving a spacer made from PALACOS® R+G cement plus vancomycin or a spacer made from COPAL® spacem loaded with gentamicin (gentamicin sulfate) and vancomycin (composition listed in Table 1). The final concentration of the antibiotics in all spacers was 0.5 g gentamicin and 2 g vancomycin per 40 g of spacer cement. The amount of spacer cement differed between 40 and 160 g per patient, depending on the extent of the operation, and was adjusted individually to the patient's joint conditions (Table 2). The shape and stability of spacers were ensured by silicon molds with a metal core (StageOne™ Hip or Knee Cement Spacer Molds, Biomet Inc., Warsaw, IN). After a 4‐ to 6‐week systemic antibiotics therapy and an antibiotics‐free time interval of 2 weeks, control samples that is, joint fluid and a punch biopsy of the soft tissue around the spacer were taken without intra‐articular local anesthesia in order to avoid interactions with the antiseptic properties of local anesthetics. If clinical and laboratory findings as well as histological and microbiological samples showed no signs of ongoing infection after another 2 weeks, the spacer was explanted and the revision endoprosthesis was implanted. If there were continued signs of inflammation or extended wound secretion, an additional cement spacer exchange was performed according to the treatment scheme. Periprosthetic membrane samples and redon‐drainage (i.e., continuous negative drainage) containing wound exudate were taken during implantation as well as during removal of the cement spacers to measure the antibiotic release from the cement spacers.
Table 1.
Composition of Bone Cements
| PALACOS® R+G | COPAL® spacem | COPAL® G+V | |
|---|---|---|---|
| Polymer powder ingredients |
|
|
|
| Monomer liquid ingredients |
|
|
|
| Ingredients additionally mixed in |
|
0.5 g (0.84 g gentamicin sulfate) gentamicin per 40 g powder component.
2.0 g vancomycin per 40 g powder component.
Table 2.
Antibiotics Concentration in Wound Exudate and Tissue Samples and Information About Possible Influencing Factors
| Patient ID | Previous Implant Cemented | Weight of 24 h Wound Exudate (g) Collected at | Spacer Characteristics | Antibiotics Concentration in Wound Exudate (μg/mL) | Antibiotics Concentration in Tissue Samples (μg/g) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IMP | EXP | Spacer Cement | Mold Usedc | Amount (g) of Prepared Cement with [V (g), G (g)] | Period of Implantation (Weeks) | Vancomycin | Gentamicin | Vancomycin | Gentamicin | ||||||
| IMP | EXP | IMP | EXP | IMP | EXP | IMP | EXP | ||||||||
| H1a | No | 186 | Palacos R+G | M1 | 160 [8.0, 2.0] | 12.6 | 17.46 | n.c. | 20.80 | n.c. | 0.00* | 0.09 | |||
| H1 2nd spacer | n.c. | Palacos R+G | n.s. | 40 [2.0, 0.5] | 10.0 | 0.04 | 0.16 | ||||||||
| H2b | Yes | 133 | 182 | Palacos R+G | M3 | 160 [8.0, 2.0] | 11.1 | 27.06 | 17.67 | 6.69 | 12.53 | 0.01 | 39.49 | 0.15 | 1.07 |
| H3 | No | 302 | 123 | Palacos R+G | M3 | 160 [8.0, 2.0] | 11.7 | 10.37 | 11.37 | 3.67 | 9.44 | 0.00* | 0.26 | 0.01* | 0.13 |
| H4b | Yes | 263 | 172 | Palacos R+G | M3 | 160 [8.0, 2.0] | 12.4 | 92.73 | 5.47 | 71.80 | 0.74 | 0.00* | 0.38 | 25.81 | 0.05 |
| H5a | No | n.c. | n.c. | Palacos R+G | M3 | 160 [8.0, 2.0] | 11.6 | n.c. | n.c. | n.c. | n.c. | 0.00* | 484.16 | 0.01* | 71.75 |
| H6 | No | 167 | Palacos R+G | M3 | 80 [4.0, 1.0] | 2.6 | 43.28 | 21.55 | 0.00* | 0.01* | |||||
| H6 2nd spacer | 571 | Palacos R+G | n.s. | 60 [3.0, 0.75] | 15.0 | 0.13* | 53.87 | 0.02 | 0.05 | ||||||
| H7 | No | 213 | 98 | Palacos R+G | M1 | 80 [4.0, 1.0] | 11.1 | 50.25 | 37.47 | 23.01 | 12.77 | 0.00* | 0.12 | 0.01* | 0.12 |
| H8b | No | 552 | Palacos R+G | n.s. | 40 [2.0, 0.5] | 2.1 | 63.24 | 19.49 | 9.36 | 0.01* | |||||
| H8 2nd spacer | 12 | Palacos R+G | M3 | 160 [8.0, 2.0] | 12.3 | 0.44 | 0.01 | 0.23 | 0.70 | ||||||
| H9 | No | 478 | 31 | Palacos R+G | M3 | 140 [7.0, 1.75] | 12.7 | 189.05 | 0.82 | 15.63 | 10.32 | 0.00* | 16.31 | 0.01* | 17.98 |
| H10 | No | 359 | 357 | Palacos R+G | M3 | 160 [8.0, 2.0] | 11.4 | 27.82 | 0.33 | 9.38 | 17.25 | 0.01 | 0.29 | 0.01* | 0.87 |
| K1a | Yes | 425 | n.c. | Palacos R+G | T4+F4 | 160 [8.0, 2.0] | 16.0 | 50.19 | n.c. | 18.73 | n.c. | 0.26 | 0.99 | 42.93 | 0.91 |
| K2a | Yes | 384 | n.c. | Copal spacem | T4+F4 | 160 [8.0, 2.0] | 11.6 | 56.18 | n.c. | 7.06 | n.c. | 0.00* | 26.20 | 1.50 | 3.92 |
| K3 | Yes | 151 | Palacos R+G | T3+F3 | 120 [6.0, 1.5] | 2.0 | 0.28 | 52.54 | 0.01 | 18.83 | |||||
| K3 2nd spacer | 399 | Palacos R+G | T4+F4 | 180 [2.25, 9.0] | 18.4 | 0.62 | 104.02 | 0.37 | 3.84 | ||||||
| K4 | Yes | 174 | 367 | Copal spacem | T2+F2 | 120 [6.0, 1.5] | 9.3 | 93.58 | 0.13* | 5.82 | 9.14 | 0.00* | 0.03 | 1.35 | 0.01* |
| K5 | No | 324 | 180 | Palacos R+G | T3+F3 | 120 [6.0, 1.5] | 9.3 | 261.22 | 0.37 | 15.28 | 17.53 | 0.00* | 0.07 | 0.01* | 0.05 |
| K6 | Yes | 447 | 93 | Palacos R+G | T4+F4 | 160 [8.0, 2.0] | 12.1 | 27.61 | 30.32 | 12.71 | 11.64 | 0.00* | 0.99 | 31.83 | 6.24 |
| K7 | Yes | 193 | 542 | Copal spacem | T4+F4 | 160 [8.0, 2.0] | 13.9 | 33.51 | 0.13* | 14.74 | 59.17 | 0.00* | 0.32 | 1.70 | 0.41 |
| K8 | Yes | 177 | 5 | Palacos R+G | T3+F3 | 160 [8.0, 2.0] | 14.9 | 143.09 | 0.46 | 84.60 | 8.40 | 0.00* | 15.03 | 8.45 | 19.13 |
All values excluded from the statistical analysis and from depiction in Figures 1 and 2 are underlaid in gray.
Abbreviations: H = hip patient; K = knee patient; IMP = spacer implantation; EXP = spacer explantation; V = vancomycin; G = gentamicin; n.c. = not collected.
Patient excluded from analysis of wound exudate due to missing sample at spacer explantation.
Patient excluded from analysis of vancomycin release due to systemic antibiotics therapy with vancomycin at time point of sample collection.
Spacer molds hip with stem size/head size: spacer mold 1 (M1) with 9 × 125 mm/43 mm; spacer mold 2 (M2) 9 × 125 mm/51 mm; spacer mold 3 (M3) 13 × 145 mm/57 mm; spacer mold 4 (M4) 17 × 165 mm/64 mm. Spacer molds knee with tibia and femur: spacer mold tibia 1 (T1) 65 mm M/Lx 39 mm A/P; spacer mold tibia 2 (T2) 70 mm M/Lx 42 mm A/P; spacer mold tibia 3 (T3) 75 mm M/Lx 45 mm A/P; spacer mold tibia 4 (T4) 80 mm M/Lx 48 mm A/P; spacer mold femur 1 (F1) 60 mm M/Lx 43 mm A/P; spacer mold femur 2 (F2) 65 mm M/Lx 48 mm A/P; spacer mold femur 3 (F3) 70 mm M/Lx 52 mm A/P; spacer mold femur 4 (F4) 75 mm M/Lx 57 mm A/P.
Values below the detection limit. Lower limit of quantification (LLOQ) for gentamicin were 0.008 μg/g in periprosthetic membrane samples and 0.004 μg/mL in wound exudates, LLOQs for vancomycin were 0.0025 μg/g in periprosthetic membrane samples and 0.125 μg/mL in wound exudates. Values below the detection limit were set at the value of the LLOQ for depiction and statistical analysis.
Study patients
Between July 2012 and January 2015 18 patients (10 total hip replacement (THR) patients and eight for total knee replacements (TKR)) were recruited at the Department of Orthopedics. Inclusion criteria were 45–85 years of age and the ability to understand the scope and importance of the trial. Patients with an active tumor disease, known allergy to the used materials or participation in other medical trials as well as lactating and pregnant women were excluded from this study. All included patients were adequately informed about the study by trained investigators and had signed the informed consent.
From the 18 recruited patients with periprosthetic infection, nine were men and nine were women with an average age of 67.6 (48.6–79.4) with the majority of patients belonging to the 70–79 age group (Table 3). Most of the patients (83.3%) were either overweight or obese (Table 3). Mean body mass index (BMI) of all 18 patients was 30.4 (19–39). The most common comorbidities were high blood pressure (16 patients), Diabetes mellitus (nine patients) and heart disease including arrhythmia (five patients) followed by metabolic disorders such as hyperlipidaemia (four patients) and hyperuricemia (three patients). Five patients suffered from allergies and two were smokers.
Table 3.
Patient Details and Systemic Antibiotic Therapy at the Time Point of Sample Collection
| Patient ID | Sex | Age | BMI | Previous Revision | Months Between Implantation of Previous TEP and | Systemic Antibiotic Therapy Within 48 h After | ||
|---|---|---|---|---|---|---|---|---|
| Explantation of TEP | Incipient complaints | Spacer Implantation | Spacer Explantation | |||||
| H1a | M | 73 | 21 | No | 21 | 17 | Ce 3 × 1500 mg/d; Mo 1 × 400 mg/d | Ce 3 × 1500 mg/d; Mo 1 × 400 mg/d |
| H2b | M | 68 | 22 | Yesc | 14 | 4 | Ri 2 × 600 mg/d; Va 2 × 1000 mg/d | Ri 2 × 600 mg/d; Va 2 × 1000 mg/d |
| H3 | M | 59 | 32 | No | 172 | 169 | Ce 3 × 1500 mg/d | Ce 3 × 1500 mg/d |
| H4b | F | 79 | 29 | Yesd | 123 | 117 | Ce 3 × 1500 mg/d; Le 1 × 500 mg/d | Ri 1 × 600 mg/d; Va 2 × 1000 mg/d |
| H5a | M | 55 | 33 | No | 179 | 178 | Ri 1 × 600 mg/d; Le 1 × 500 mg/d | Ri 2 × 600 mg/d; Le 2 × 500 mg/d |
| H6 | M | 77 | 29 | No | 72 | 57 | Cl 2 × 600 mg/d | Le 2 × 500 mg/d |
| H7 | F | 78 | 19 | No | 40 | 23 | Ce 3 × 1500 mg/d; Mo 1 × 400 mg/d | Ce 3 × 1500 mg/d; Le 2 × 500 mg/d |
| H8b | M | 58 | 34 | No | 75 | 74 | Ri 2 × 600 mg/d; Va 2 × 1000 mg/d; | Ce 3 × 1500 mg/d; Le 2 × 500 mg/d |
| H9 | M | 75 | 32 | No | 1 | 0 | Ce 3 × 1500 mg/d; Mo 1 × 400 mg/d | Cl 2 × 600 mg/d; Le 2 × 500 mg/d |
| H10 | M | 66 | 34 | No | 98 | 61 | Ce 3 × 1500 mg/d; Le 1 × 500 mg/d | Cl 3 × 600 mg/d; Le 2 × 500 mg/d |
| K1a | F | 49 | 34 | Yesd | 133 | 118 | Ce 3 × 1500 mg/d; Mo 1 × 400 mg/d | Cl 3 × 600 mg/d; |
| K2a | F | 56 | 39 | Yesd | 7 | 1 | Ce 3 × 1500 mg/d; Cl 2 × 600 mg/d | Ce 3 × 1500 mg/d; Cl 2 × 600 mg/d |
| K3 | F | 55 | 30 | Yesd | 52 | 52 | Ce 3 × 1500 mg/d; Le 2 × 500 mg/d | Ce 3 × 1500 mg/d; Le 2 × 500 mg/d |
| K4 | M | 75 | 37 | No | 95 | 81 | Ce 3 × 1500 mg/d; Mo 1 × 400 mg/d | Ce 3 × 1500 mg/d; Mo 1 × 400 mg/d |
| K5 | F | 73 | 27 | No | 178 | 176 | Cl 2 × 600 mg/d | Ce 3 × 1500 mg/d |
| K6 | F | 75 | 27 | Yesd | 29 | 26 | Ce 3 × 1500 mg/d; Mo 1 × 400 mg/d | Ce 3 × 1500 mg/d; Le 2 × 500 mg/d |
| K7 | F | 73 | 31 | Yesd | 8 | 8 | Ce 3 × 1500 mg/d; Le 2 × 500 mg/d | Ce 3 × 1500 mg/d |
| K8 | F | 73 | 37 | Yesc | 78 | 77 | Ri 1 × 600 mg/d; Le 1 × 500 mg/d; Fl 4 × 2000 mg/d | Cl 3 × 600 mg/d; Le 2 × 500 mg/d |
| Mean/total | 9 M/9 F | 67.1 ± 9.4 | 30.4 ± 5.4 | 8 | 76.4 ± 58.9 | 68.8 ± 59.1 | ||
Cumulative data of all patients are either presented as mean ± SD or total number.
H = hip patient; K = knee patient; Antibiotics: Ce = Cefuroxim, Cl = Clindamycin, Fl = Flucloxacillin, Le = Levofloxacin, Mo = Moxifloxacin, Ri = Rifampicin, Va = Vancomycin, all systemic antibiotic treatment within the first 48 h after operation was administered intravenously.
Patient excluded from analysis of wound exudate due to missing sample at spacer explantation.
Patient excluded from analysis of vancomycin release due to systemic antibiotics therapy with vancomycin at time point of sample collection.
Wound revisions and debridements.
Partial or total component exchange surgery.
Quantitative determination of gentamicin and vancomycin release in vivo
After spacer implantation as well as explantation surgery the redon‐drainage containing 24‐h wound exudate was collected in order to measure the antibiotic release of gentamicin and vancomycin. Additionally, a piece of periprosthetic membrane (approx. 10 mm × 10 mm × 10 mm) was acquired during the surgery. The tissue sample was obtained before the implantation of the spacer during initial debridement to eliminate any contact between tissue and spacer. The redon‐drainage and the periprosthetic membrane samples were stored at −20°C and weighed. The weight of the membrane samples was 3.2 ± 2.1 g (mean ± SD) while for the wound exudates an average of 260 ± 161 g (mean ± SD) were collected after 24 h (Table 2). The quantification of antibiotics release was performed by a (GLP)‐certified testing facility (Analytisches Zentrum Biopharm GmbH, Berlin, Germany).
Standardized quantities of 1 g of periprosthetic membrane and 0.5 mL wound exudate were deployed for the analysis of gentamicin components C1a, C2/C2a, C1, and vancomycin. Deproteinization was carried out by sonication of tissue and exudate in 4% (v/v) perchloric acid in water followed by centrifugation. The gentamicin components C1a, C2/C2a, and C1 were derivatized with propyl chloroformate in acetone and extracted in ethyl acetate/hexane (liquid/liquid extraction). After evaporation of the organic phase the samples were dissolved in reconstitution solution and separated on a C18 column (Restek Ultra PFPP, 5 μm, 100 × 2.1 mm, Restek GmbH, Bad Homburg, Germany) under gradient conditions. Vancomycin was separated directly after protein precipitation under gradient conditions using a C18 column (Luna C18(2), 5 μm, 150 × 2.1 mm, Phenomenex, Aschaffenburg, Germany), too. Analysis was carried out using a high performance liquid chromatography Agilent 1200 system (Agilent Technologies, Waldbronn, Germany) and mass selective detection using an API 4000 QTrap (ABSciex, Darmstadt, Germany). Ionization was carried out with an electrospray interface (ESI, positive polarity) and mass selective detection in the multiple reaction monitoring mode (MRM). The calibration range for gentamicin components C1a/C2 + C2a/C1 in membrane and exudate was 0.00128/0.00160/0.00112 μg/mL to 1.600/2.000/1.400 μg/mL, respectively. Quality controls with different concentrations showed that accuracy after separation was 99.87 ± 7.69% (mean ± SD, coefficient of variation [CV] = 7.70%). The ratio of the gentamicin components to each other varied slightly after elution depending on the concentration (CV = 5.60 ± 2.49%), however, the difference was not significant. The sum of the three components was used for statistical analysis since membrane and exudate samples showed a similar distribution of the different components of gentamicin compared to the calibration samples. Calibration range for vancomycin was 0.0025–2.500 μg/mL. Each batch was controlled using quality control samples at three levels and showed good reproducibility. Lower limit of quantification (LLOQ) for gentamicin were 0.008 μg/g in periprosthetic membrane samples and 0.004 μg/mL in wound exudates, LLOQs for vancomycin were 0.0025 μg/g in periprosthetic membrane samples and 0.125 μg/mL in wound exudates.
Quantitative determination of gentamicin and vancomycin release in vitro
Before production of the test specimens, bone cement powders “COPAL® G+V,” “PALACOS® R+G” and “COPAL® spacem” (Heraeus Medical GmbH, Wehrheim, Germany, Table 1) were dried in a desiccator in a vacuum (<20 mbar) for at least 24 h.
COPAL® G+V contains gentamicin and vancomycin and did not require further antibiotics addition. PALACOS® R+G contains gentamicin but lacks vancomycin, therefore 2 g vancomycin hydrochloride (Lyomark Pharma GmbH, Oberhaching, Germany) were added. COPAL® spacem contains neither vancomycin nor gentamicin; accordingly 2 g vancomycin hydrochloride and 0.87 g gentamicin sulfate were added. The final concentrations of antibiotics were 1.17% w/w gentamicin and 4.66% w/w vancomycin, 1.17% w/w gentamicin and 4.67% w/w vancomycin as well as 1.16% w/w gentamicin and 4.65% w/w vancomycin per weight of powder component for COPAL® spacem, PALACOS® R+G and COPAL® G+V, respectively.
Forty grams of bone cement powder, 20 mL liquid component and antibiotics (in the case of PALACOS® R+G and COPAL® spacem) were mixed in a porcelain crucible until the mix was no longer adhesive. It was then manually filled into a cylindrical casting form with 25 mm of diameter and 10 mm of height. The mold was covered with polyester foil and condensed with a manually operated hydraulic press at 1–1.5 bar for 30 min. The resulting cylindrical test specimens were removed from the form and dried in a desiccator with orange gel for 2 days. The discs had an average weight of 5.6 g and a surface area of 17.66 cm2. The specimens were then transferred to a polyethylene tube containing 20 mL of phosphate‐buffered saline solution (PBS) and incubated for 24 h at 37°C. Next, the specimens were removed and transferred into a new polyethylene tube with fresh PBS for a further 24‐h incubation period while the supernatants from the test tubes were stored in a fridge at 4°C. This procedure was repeated for up to 5 days. The supernatants from day one, three, and five were analyzed for gentamicin and vancomycin content.
The concentration of vancomycin was measured via separation in a C18 column (XBridge) under gradient conditions using UV‐detection. Gentamicin concentration analysis was performed using a commercial kit (COBAS INTEGRA GENT, Roche Diagnostics GmbH, Basel, Switzerland) via automatic biochemistry analyser (COBAS INTEGRA® 400, Roche Diagnostics GmbH, Mannheim, Germany).
Statistical analysis
Statistical analysis was performed by GraphPadPRISM version 7.0 (GraphPad Inc. San Diego, CA). In order to include samples with values below the detection limit, these values were set at the value of the LLOQ. Concentrations of vancomycin between implantation and explantation were compared using Wilcoxon signed rank test. Concentrations of gentamicin between implantation and explantation were analyzed with Kruskal‐Wallis test followed by Dunn's multiple comparison as post hoc test since here it was also differentiated whether the explanted prosthesis had been cemented or uncemented. The non‐parametric tests were chosen as Kolmogorow‐Smirnov test revealed that in vivo data were not normally distributed. Therefore, in vivo data are reported as median with the ranges in square brackets. Correlations analyses were performed using Spearman correlation test.
As the in vitro results were normally distributed, they are reported as mean ± standard deviation and compared using two‐way repeated measures ANOVA with time of release and cement type as variables followed by Bonferroni post‐tests.
RESULTS
In vivo data
The most common bacterial species found in intraoperative samples at the time point of spacer implantation were coagulase‐negative staphylococci (nine strains) including Staphylococcus epidermidis (seven strains), Staphylococcus lugdunensis (one strain), and Staphylococcus capitis (one strain) followed by Staphylococcus aureus (three strains). Furthermore, obligatory pathogenic or opportunistic species such as Corynebacterium spp., Bacillus cereus, Finegoldia magna, Bacteroides ureolyticus, Propionibacterium acnes, and Klebsiella pneumoniae were detected. Of note with respect to the antibiotics in the spacer, in three patients the infection‐causing bacteria—three strains of Staphylococcus epidermidis and one strain of Staphylococcus aureus—were resistant to gentamicin. None of the detected strains were resistant to vancomycin.
In the present study, counting from the previous endoprosthetic implant, the mean time to revision was 76.4 months (1–179 months) with clinical signs recorded slightly earlier (68.8 months, 0–178 months). The majority of cases (55.6%) occurred after 5 years and later (Table 1). Following their primary TJR, eight patients (two hips and six knees) had previously undergone revision at the same site. There were no revisions due to septic or aseptic loosening of the primary implant in the hip patients. However, one patient had undergone a debridement of an epifascial cyst and another had an exchange of the acetabular shell due to a fall and resulting breakage within 10 days of primary implantation. Notably, six among eight knee patients (75%) had undergone revision before. Partial or total component exchange surgery was performed in five knee patients; in three of those due to an implant‐associated infection. One knee patient had prior wound revisions and debridements only.
The mean period of implantation of the spacer for all patients was 13.6 weeks, in the 14 patients with only one spacer it was 12.1 weeks while in the four patients (three hips and one knee) who had a spacer exchange operation, total period of implantation for both spacers was 18.8 weeks (Table 2). Delayed wound healing and continued serous wound secretion following spacer implantation was the main reason for spacer exchange and was indicative of the necessity for an exchange in three cases (two hips and one knee) which was performed shortly after the first spacer implantation after 2 weeks. The decision for the spacer exchange in the fourth patient was based on the histological examination of the control biopsy at week 8, which showed signs of a persistent inflammation.
It is noteworthy that only one of the exchanged first spacers was positive in microbiological tests. Moreover, Enterobacter cloacae was only detected in one of the three independent sampling sites of this spacer. None of the patients who had undergone a spacer exchange showed signs of continued infection in the subsequent course of the treatment and up to 1 year follow‐up.
Gentamicin and vancomycin release in vivo
In vivo release of antibiotics was measured in the subfascial wound exudate of the patients collected for 24 h postoperatively while adjacent membrane tissue samples were taken intraoperatively during both spacer implantation and spacer explantation.
Membrane samples were collected from all 18 patients. However, in the case of four patients, wound exudate was only collected from the implantation but not from the explantation of the spacer. In order to allow for pairwise comparison between implantation and explantation samples, those four patients were excluded from the analysis of the wound exudate. Furthermore, patients who received intravenous vancomycin prior and up to 48 h after operations were excluded when analyzing vancomycin release in the wound exudate as well as from the periprosthetic membrane samples. Thus, 11 exudates and 15 membrane samples at each time point were analyzed for vancomycin concentration. As shown in Figure 1(A), vancomycin concentration is significantly reduced in the 24‐h wound exudate at explantation compared to during implantation of the spacer (43.28 μg/mL [0.28–261.22] vs. 0.46 μg/mL [0.13–37.47], n = 11, Wilcoxon signed rank test, p = 0.0137). As expected, the concentration of vancomycin was low in the periprosthetic membrane samples before the implantation of the spacer with 10 out of 15 samples being below the LLOQ. The concentration of vancomycin in the membrane was, however, significantly higher at explantation of the spacer (0.003 μg/g [0.003–0.261] vs. 0.318 μg/g [0.024–484.16], n = 15, Wilcoxon signed rank test, p < 0.0001) [Figure 1(B)].
Figure 1.
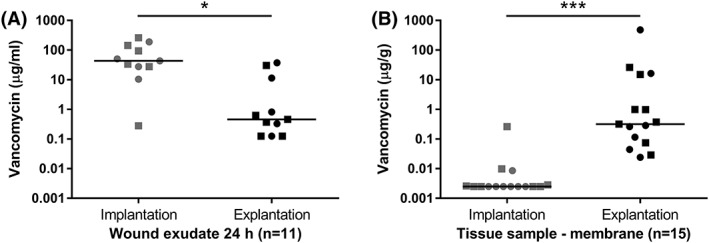
Vancomycin concentrations in wound exudate (A) and periprosthetic membrane samples (B) of patients undergoing two stage treatment of periprosthetic joint infection under exclusion of those patients with systemic vancomycin treatment prior and up to 48 h after operation. Single values are presented with the horizontal bar representing the median. Hip patients are represented by dots while knee patients are shown as squares. Time points of samples: spacer implantation and spacer explantation, n: number of patients, * significant p < 0.05, *** extremely significant p < 0.001 (Wilcoxon signed rank test).
For gentamicin analyses, we differentiated between patients whose previous implant had been cemented (nine patients: two hip and seven knee implants), and those whose removed prosthesis had been uncemented (nine patients: eight hip and one knee implants). There were no differences in the gentamicin concentration in the wound exudates (Kruskal‐Wallis test, p = 0.8814), neither when comparing implantation and explantation of spacer nor when analyzing the cemented (n = 7) and uncemented (n = 7) groups [Figure 2(A)] However, as depicted in Figure 2(B) gentamicin concentrations differed significantly in the periprosthetic membrane samples (Kruskal‐Wallis test, p = 0.0001). Dunn's post hoc test revealed that even before the implantation of the spacer, the concentration of gentamicin in the periprosthetic membrane samples between the cemented and uncemented group differed significantly (8.451 μg/g [0.152–42.926, n = 9] vs. 0.008 μg/g [0.008–0.087, n = 9], p < 0.001). While the concentration of gentamicin increased from implantation to explantation in the uncemented group (0.008 μg/g [0.008–0.087] vs. 0.164 μg/g [0.048–71.75], n = 9, p < 0.05), there was no such change in the cemented group (8.451 μg/g [0.152–42.926] vs. 1.075 μg/g [0.008–19.128], n = 9).
Figure 2.
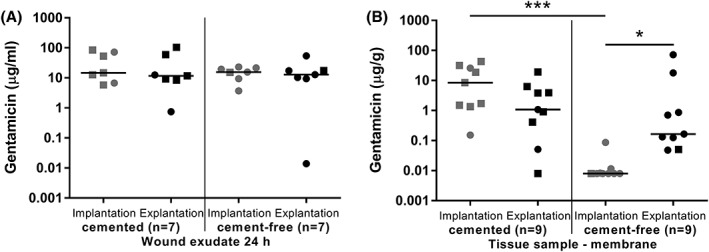
Gentamicin concentrations in wound exudate (A) and periprosthetic membrane samples (B) of patients undergoing two stage treatment of periprosthetic joint infection. Patients were divided in two groups depending on whether the removed infected implant was previously cemented or uncemented. Single values are presented with the horizontal bar representing the median. Total hip patients are represented by dots while total knee patients are shown as squares. Time points of samples: spacer implantation and spacer explantation, n: number of patients, * significant p < 0.05, *** extremely significant p < 0.001 (Kruskal‐Wallis test).
Spearman analyses of correlation revealed that the differences in antibiotics concentration between implantation and explantation in wound exudate or tissue samples of a patient did not correlate with the period of implantation time of the spacer (data not shown). However, the observed increase in vancomycin concentration in the tissue samples was positively correlated to the amount of cement used to create the spacer implant (r = 0.6759; p = 0.0137). There were no further correlations between antibiotics concentration and amount of cement. Patients requiring a second spacer were analyzed separately as the amount of cement differed between first and second spacer. The assumption that the necessity of a second spacer was due to a low antibiotics release after implantation could not be confirmed as concentration in the wound exudate 24 h after implantation of the first spacer did not differ significantly in patients with one spacer versus patients requiring a spacer exchange (vancomycin: 50.25 μg/mL [10.37–261.22, n = 9] vs. 21.78 μg/mL [0.28–43.28, n = 2], p = 0.3273; gentamycin: 14.74 μg/mL [3.67–84.60, n = 11] vs. 21.56 μg/mL [19.49–52.54, n = 3], p = 0.2253; Mann–Whitney test).
Gentamicin and vancomycin release in vitro
In order to compare the in vivo results from the patient study with the in vitro release kinetics of the antibiotics from the spacers, cylindrical specimens were molded from Palacos R+G and Copal Spacem under addition of the respective antibiotics according to the procedures used in the in vivo study. Additionally, Copal G+V which already contained both antibiotics of interest, gentamicin and vancomycin, was tested. This cement was not applied in the clinical study as it was not commercially available when the study commenced.
As expected, there was a significant decline of released antibiotics over time for both antibiotics (p < 0.0001 for time, two‐way repeated measures ANOVA for gentamicin and vancomycin, respectively). The interaction of both variables (time × cement) was also highly significant (p < 0.0001 for time × cement, two‐way repeated measures ANOVA for gentamicin and vancomycin, respectively) indicating significant differences in the release kinetics of the cements over time. The differences between the three cements were most pronounced on day 1. The highest release of gentamicin on day 1 was measured for Palacos R+G followed by Copal G+V (p < 0.001, Bonferroni post test), while Copal Spacem released the least amount of gentamicin compared to Palacos R+G and Copal G+V (p < 0.001 and p < 0.001, Bonferroni post tests, respectively). On day 3, only Palacos R+G and Copal Spacem differed significantly from each other with regard to gentamicin release [Figure 3(A)].
Figure 3.
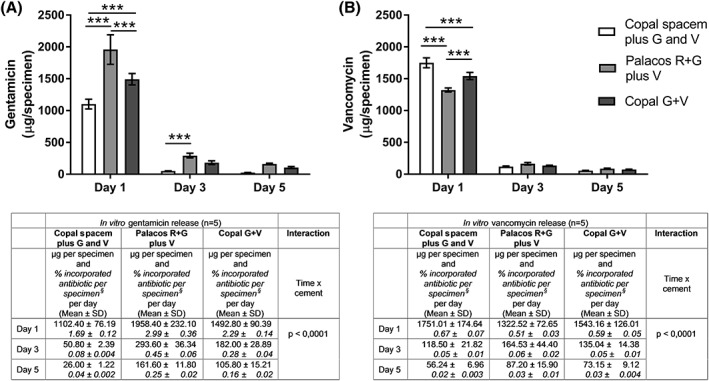
Release of the antibiotics gentamicin (A) and vancomycin (B) from cylindrical test specimens of three different cements in vitro. Values are mean ± standard deviation of released antibiotic per specimen over 24 h on the indicated days. Percentage of released antibiotics per incorporated antibiotic per specimen per day is presented in Italics. §—Values are calculated based on an average weight of 5.6 g per specimen with 1.17% w/w gentamicin and 4.66% w/w vancomycin, 1.17% w/w gentamicin and 4.67% w/w vancomycin as well as 1.16% w/w gentamicin and 4.65% w/w vancomycin per weight of powder component for COPAL® spacem, PALACOS® R+G and COPAL® G+V, respectively. Results were recorded from five independent experiments. In vitro results were compared using two‐way repeated measures ANOVA with time and cement as variables followed by Bonferroni post‐tests. *** extremely significant p < 0.001 (Bonferroni post‐tests) G = gentamicin, V = vancomycin.
Release kinetics of vancomycin from the three types of cement differed from those of gentamicin. On day 1, Copal Spacem released the highest amount of vancomycin with significantly lower amounts set free from Copal G+V followed by Palacos R+G (p < 0.001 and p < 0.001, Bonferroni post‐tests, respectively) [Figure 3(B)].
In general, despite higher concentration of vancomycin in the bone cements the relative release was lower for vancomycin compared to gentamicin (Figure 3).
DISCUSSION
Periprosthetic infection is a severe complication after knee or hip arthroplasty. Over half of the patients in this study were affected by conditions that had been identified as individual risk factors for developing a periprosthetic joint infection such as diabetes, obesity or old age.21
The use of antibiotic impregnated cement has been shown to increase the success rate in two‐stage revision arthroplasty as a treatment of periprosthetic infection.22, 23 The assumed reason is the high initial release of antibiotics locally, which prevents a re‐infection. A systematic review of studies investigating the in vivo release of antibiotics from bone cements reported considerable differences in the released concentration between, but also within the studies.24 This is in line with our observation of high inter‐individual variation for both antibiotics in the 24‐h wound exudate after spacer implantation. The authors speculated that the volume of joint fluid surrounding the spacer might be a limiting factor in antibiotics release.24 Thus, the differences in the amount of wound exudate might be one reason for the high inter‐individual variation. Additionally, the surface area of the spacer might be an important factor. In a recent randomized controlled trial no difference in the concentration of gentamicin in drain fluid between patients with either a thick or a thin cement mantle was observed, that is, the local concentration of antibiotic was independent from the amount of bone cement.25 While this is contrary to our results, as we observed a correlation between the prepared amount of bone cement and the release of vancomycin, our result could be due to the increased size, and thus an increased surface area, of the cement spacers prepared in the standardized molds. Furthermore, systemic vancomycin administration influences the local antibiotic concentration. Roy et al. reported that already a single intravenous dose of vancomycin resulted in therapeutic concentrations of approximately 6.8 μg/mL vancomycin in joint fluid.26 In order to avoid this bias, only data of vancomycin release after exclusion of patients who had been administered vancomycin intravenously were statistically analyzed. The thus observed significant decline of vancomycin release in the 24‐h wound exudate at the time of explantation of the spacer compared to implantation is in agreement with the decreased in vitro release. However, given the sharp decrease in vitro on day 3 and day 5, it was rather unexpected that there was still a considerable release of vancomycin after an average of 90 days in vivo, especially when the spacer had been removed. Such a prolonged release of antibiotics was already described in other studies.27, 28, 29 The accumulation of antibiotics in the adjacent tissue and their release after tissue damage due to the second stage procedure could be a possible reason for the detection of antibiotics in the wound exudate after explantation. This hypothesis is supported by the significantly higher vancomycin concentration in the tissue at the time of explantation. The hypothesis could also explain the effects that we observed when analyzing gentamicin. Here the differentiation between patients with cemented and uncemented endoprosthesis prior to spacer implantation also showed that the use of gentamicin‐containing cement for fixation of TJRs resulted in considerable accumulation of the antibiotic in the tissue even after several years. We measured significantly higher concentrations of gentamicin in the tissue before the implantation of the spacer in patients with previously cemented compared to uncemented TJRs. Our data suggest that especially gentamicin remains in the tissue, as contrary to vancomycin there was no decline in gentamicin in wound exudates after explantation of the spacer.
However, another hypothesis should not be dismissed here. Powles et al. showed that fracturing old cement during revision surgery led to the release of gentamicin and in turn to the “contamination” of tissue and joint fluid with the antibiotic.30 The disruption of the cement opens new surface areas from which enclosed antibiotics can be released. Indeed, several studies found that a high percentage of the antibiotics remained in the cement.18, 31 Thus, the detected antibiotics could derive from the cement after disruption during either the explantation of the previous TJR or the explantation of the spacer.
The importance of the surface area for the release kinetics was mentioned, that is, cements with a microporous structure, high porosity or higher surface roughness, which extend the surface area, showed higher and prolonged antibiotic elution.15, 32 The addition of ingredients such as calcium polyphosphate or mesoporous silica nanoparticles which increase porosity or surface roughness also resulted in improved release kinetics.33, 34 It was reported that Copal spacem is more effective in preventing biofilm formation compared to Palacos® R+G due to better elution kinetics for gentamicin.35 However, that in our in vitro experiments this was only observed for the vancomycin but not for gentamicin could be due to the fact that contrary to our bone cement composition Copal spacem in the aforementioned publication contained clindamycin. Indeed, Boelch et al. reported that the addition of clindamycin improved gentamicin release from test specimens while the addition of vancomycin had no effect.36 In the clindamycin containing Copal cement the weight of gentamicin is with 2.34% higher than in the bone cements tested in our study which have all similar weights of gentamicin and vancomycin. There might be various other reasons why we did not observe the improved release kinetics of Copal spacem for gentamicin. For example, in Palacos R+G and Copal G+V, especially manufactured gentamicin was used, which generally has better release kinetics. Measurement of surface roughness within a previous study37 also showed higher roughness for Palacos® R+G with addition of vancomycin compared to Copal® spacem with gentamicin and vancomycin (R a = 20.89 ± 0.49 μm, R z = 139.54 ± 5.24 μm and R a = 18.93 ± 0.67 μm, R z = 121.86 ± 3.85 μm for Palacos® R+G plus V and Copal® spacem plus G and V, respectively [mean ± SD, n = 3, unpublished data]) which might support the observation that initial release of gentamicin from Palacos® R+G with addition of vancomycin was highest. The importance of the surface was highlighted by the work of Salih et al. who showed that indenting a spacer with a MacDonald dissector more than doubled the gentamicin release.38 In contrast, the test specimens in our in vitro investigation had a smooth surface which was ensured by using a polyester foil. While the release kinetics of the combination of gentamicin and vancomycin are relatively well studied in vitro, its importance in vivo is still not clear. Despite synergistic effects of the combination on the size of the inhibition zone in vitro 39 a recent in vivo study showed that the combination of gentamicin and vancomycin did not offer any advantages regarding clinical outcomes compared to gentamicin alone.40 However, especially considering that four of the infection‐causing strains in our in vivo investigation were identified as gentamicin‐resistant highlights the importance of antibiotic combinations to prevent re‐infection.
Our inability to replicate in vivo the differences that we detected between cements in vitro may be due to the relatively low number of patients who received spacers made from Copal spacem and the observed high inter‐individual variability. The small sample size is a general limitation in our study. This is due to periprosthetic infection occurring in only approximately 1% of all primary TJR patients. We improved patient recruitment by widening the inclusion criteria to allow recruitment of patients who had undergone revision before but numbers remained low in our general patient population.
CONCLUSIONS
Our data allow the conclusion that while antibiotics release from spacer cement decreases rapidly in vitro within the first 24 h, in vivo antibiotics are present much longer at similar concentrations, even after an average of 90 days. This was observed especially in the spacer‐adjacent soft tissue.
CONFLICT OF INTEREST
Co‐authors Dr. Hans Bösebeck and Mr. Tobias Reichel were employees of Heraeus Medical GmbH at the time of the study. There were no conflicts of interest that affected the design and execution of the study protocol or the analysis of the data. None of the other authors have any conflicts of interest to declare.
ACKNOWLEDGMENTS
We would like to thank the Federal Ministry of Education and Research of Germany (BMBF) for funding this research as part of the Health Innovative Care and Regional Economy (HICARE) consortium (01KQ1001B). Dr. rer. nat. Martin Reinsch, Analytisches Zentrum Biopharm GmbH, Berlin, Germany kindly analyzed the in vivo samples of wound exudates and tissue for gentamicin and vancomycin. We would also like to thank Lyomark Pharma GmbH (Oberhaching, Germany) for the donation of vancomycin hydrochloride.
Klinder A, Zaatreh S, Ellenrieder M, Redanz S, Podbielski A, Reichel T, Bösebeck H, Mittelmeier W, Bader R. Antibiotics release from cement spacers used for two‐stage treatment of implant‐associated infections after total joint arthroplasty. J Biomed Mater Res B Part B. 2019:107B:1587–1597.
REFERENCES
- 1. Dale H, Fenstad AM, Hallan G, Havelin LI, Furnes O, Overgaard S, Pedersen AB, Kärrholm J, Garellick G, Pulkkinen P, Eskelinen A, Mäkelä K, Engesæter LB. Increasing risk of prosthetic joint infection after total hip arthroplasty. Acta Orthop 2012;83(5):449–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schrama JC, Espehaug B, Hallan G, Engesaeter LB, Furnes O, Havelin LI, Fevang B‐TS. Risk of revision for infection in primary total hip and knee arthroplasty in patients with rheumatoid arthritis compared with osteoarthritis: A prospective, population‐based study on 108,786 hip and knee joint arthroplasties from the Norwegian Arthroplasty Register. Arthritis Care Res 2010;62(4):473–479. [DOI] [PubMed] [Google Scholar]
- 3. Wengler A, Nimptsch U, Mansky T. Hip and knee replacement in Germany and the USA: Analysis of individual inpatient data from German and US hospitals for the years 2005 to 2011. Dtsch Arzteblatt Int 2014;111(23–24):407–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Moran E, Byren I, Atkins BL. The diagnosis and management of prosthetic joint infections. J Antimicrob Chemother 2010;65(Suppl 3):iii45–iii54. [DOI] [PubMed] [Google Scholar]
- 5. Jiranek WA, Waligora AC, Hess SR, Golladay GL. Surgical treatment of prosthetic joint infections of the hip and knee: Changing paradigms? J Arthroplasty 2015;30(6):912–918. [DOI] [PubMed] [Google Scholar]
- 6. Cohen JC, Hozack WJ, Cuckler JM, Booth RE. Two‐stage reimplantation of septic total knee arthroplasty. J Arthroplasty 1988;3(4):369–377. [DOI] [PubMed] [Google Scholar]
- 7. Kuehn K‐D, Ege W, Gopp U. Acrylic bone cements: Composition and properties. Orthop Clin North Am 2005;36(1):17–28. [DOI] [PubMed] [Google Scholar]
- 8. Parvizi J, Saleh KJ, Ragland PS, Pour AE, Mont MA. Efficacy of antibiotic‐impregnated cement in total hip replacement. Acta Orthop 2008;79(3):335–341. [DOI] [PubMed] [Google Scholar]
- 9. Engesaeter LB, Lie SA, Espehaug B, Furnes O, Vollset SE, Havelin LI. Antibiotic prophylaxis in total hip arthroplasty: Effects of antibiotic prophylaxis systemically and in bone cement on the revision rate of 22,170 primary hip replacements followed 0‐14 years in the Norwegian Arthroplasty Register. Acta Orthop Scand 2003;74(6):644–651. [DOI] [PubMed] [Google Scholar]
- 10. Lowy FD, Chang DS, Lash PR. Synergy of combinations of vancomycin, gentamicin, and rifampin against methicillin‐resistant, coagulase‐negative staphylococci. Antimicrob Agents Chemother 1983;23(6):932–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Houlihan HH, Mercier RC, Rybak MJ. Pharmacodynamics of vancomycin alone and in combination with gentamicin at various dosing intervals against methicillin‐resistant Staphylococcus aureus‐infected fibrin‐platelet clots in an in vitro infection model. Antimicrob Agents Chemother 1997;41(11):2497–2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dall GF, Simpson PMS, Breusch SJ. In vitro comparison of Refobacin‐Palacos R with Refobacin bone cement and Palacos R + G. Acta Orthop 2007;78(3):404–411. [DOI] [PubMed] [Google Scholar]
- 13. Neut D, Kluin OS, Thompson J, van der Mei HC, Busscher HJ. Gentamicin release from commercially‐available gentamicin‐loaded PMMA bone cements in a prosthesis‐related interfacial gap model and their antibacterial efficacy. BMC Musculoskelet Disord 2010;11:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bertazzoni Minelli E, Della Bora T, Benini A. Different microbial biofilm formation on polymethylmethacrylate (PMMA) bone cement loaded with gentamicin and vancomycin. Anaerobe 2011;17(6):380–383. [DOI] [PubMed] [Google Scholar]
- 15. Bitsch RG, Kretzer JP, Vogt S, Büchner H, Thomsen MN, Lehner B. Increased antibiotic release and equivalent biomechanics of a spacer cement without hard radio contrast agents. Diagn Microbiol Infect Dis 2015;83(2):203–209. [DOI] [PubMed] [Google Scholar]
- 16. Goltzer O, McLaren A, Overstreet D, Galli C, McLemore R. Antimicrobial release from prefabricated spacers is variable and the dose is low. Clin Orthop 2015;473(7):2253–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Frew NM, Cannon T, Nichol T, Smith TJ, Stockley I. Comparison of the elution properties of commercially available gentamicin and bone cement containing vancomycin with “home‐made” preparations. Bone Joint J 2017;99‐B(1):73–77. [DOI] [PubMed] [Google Scholar]
- 18. Bertazzoni Minelli E, Benini A, Magnan B, Bartolozzi P. Release of gentamicin and vancomycin from temporary human hip spacers in two‐stage revision of infected arthroplasty. J Antimicrob Chemother 2004;53(2):329–334. [DOI] [PubMed] [Google Scholar]
- 19. Kendoff DO, Gehrke T, Stangenberg P, Frommelt L, Bösebeck H. Bioavailability of gentamicin and vancomycin released from an antibiotic containing bone cement in patients undergoing a septic one‐stage total hip arthroplasty (THA) revision: A monocentric open clinical trial. Hip Int 2016;26(1):90–96. [DOI] [PubMed] [Google Scholar]
- 20. Ellenrieder M, Lenz R, Haenle M, Bader R, Mittelmeier W. Two‐stage revision of implant‐associated infections after total hip and knee arthroplasty. GMS Krankenhhyg Interdiszip 2011;6(1):Doc17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tande AJ, Patel R. Prosthetic joint infection. Clin Microbiol Rev 2014;27(2):302–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Joseph TN, Chen AL, Di Cesare PE. Use of antibiotic‐impregnated cement in total joint arthroplasty. J Am Acad Orthop Surg 2003;11(1):38–47. [DOI] [PubMed] [Google Scholar]
- 23. Kontekakis A, Berghaus M, Gaiser S, Kühn K‐D. Evidence generation for medical devices In: Schulz M, editor. Biofunctional Surface Engineering. Singapore: Pan Stanford Publishing; 2014. pp. 291–314. [Google Scholar]
- 24. Anagnostakos K, Meyer C. Antibiotic elution from hip and knee acrylic bone cement spacers: A systematic review. Biomed Res Int 2017;2017:4657874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. van IJperen W, Van Quickenborne D, Buyl R, Scheerlinck T. Cement mantle thickness does not influence serum or local gentamicin concentrations in hybrid total hip arthroplasty: A randomised controlled trial. Hip Int 2018;28(4):415–421. [DOI] [PubMed] [Google Scholar]
- 26. Roy ME, Peppers MP, Whiteside LA, LaZear RM. Vancomycin concentration in synovial fluid: Direct injection into the knee vs. intravenous infusion. J Arthroplasty 2014;29(3):564–568. [DOI] [PubMed] [Google Scholar]
- 27. Fink B, Vogt S, Reinsch M, Büchner H. Sufficient release of antibiotic by a spacer 6 weeks after implantation in two‐stage revision of infected hip prostheses. Clin Orthop 2011;469(11):3141–3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Neut D, van de Belt H, van Horn JR, van der Mei HC, Busscher HJ. Residual gentamicin‐release from antibiotic‐loaded polymethylmethacrylate beads after 5 years of implantation. Biomaterials 2003;24(10):1829–1831. [DOI] [PubMed] [Google Scholar]
- 29. Masri BA, Duncan CP, Beauchamp CP. Long‐term elution of antibiotics from bone‐cement. J Arthroplasty 1998;13(3):331–338. [DOI] [PubMed] [Google Scholar]
- 30. Powles JW, Spencer RF, Lovering AM. Gentamicin release from old cement during revision hip arthroplasty. J Bone Joint Surg Br 1998;80(4):607–610. [PubMed] [Google Scholar]
- 31. Wroblewski BM, Esser M, Srigley DW. Release of gentamicin from bone cement an ex‐vivo study. Acta Orthop Scand 1986;57(5):413–414. [DOI] [PubMed] [Google Scholar]
- 32. Van de Belt H, Neut D, Uges DRA, Schenk W, van Horn JR, van der Mei HC, Busscher HJ. Surface roughness, porosity and wettability of gentamicin‐loaded bone cements and their antibiotic release. Biomaterials 2000;21(19):1981–1987. [DOI] [PubMed] [Google Scholar]
- 33. Letchmanan K, Shen SC, Ng WK, Kingshuk P, Shi Z, Wang W, Tan RBH. Mechanical properties and antibiotic release characteristics of poly(methyl methacrylate)‐based bone cement formulated with mesoporous silica nanoparticles. J Mech Behav Biomed Mater 2017;72:163–170. [DOI] [PubMed] [Google Scholar]
- 34. Zhou Z, Seta J, Markel DC, Song W, Yurgelevic SM, Yu XW, Ren W. Release of vancomycin and tobramycin from polymethylmethacrylate cements impregnated with calcium polyphosphate hydrogel. J Biomed Mater Res B Appl Biomater 2017. 10.1002/jbm.b.34063. [DOI] [PubMed] [Google Scholar]
- 35. Ensing GT, van Horn JR, van der Mei HC, Busscher HJ, Neut D. Copal bone cement is more effective in preventing biofilm formation than Palacos R‐G. Clin Orthop Relat Res 2008;466(6):1492–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Boelch SP, Jordan MC, Arnholdt J, Rudert M, Luedemann M, Steinert AF. Loading with vancomycin does not decrease gentamicin elution in gentamicin premixed bone cement. J Mater Sci Mater Med 2017;28(7):104. [DOI] [PubMed] [Google Scholar]
- 37. Zaatreh S, Wegner K, Strauß M, Pasold J, Mittelmeier W, Podbielski A, Kreikemeyer B, Bader R. Co‐culture of S. epidermidis and human osteoblasts on implant surfaces: An advanced in vitro model for implant‐associated infections. PLoS One 2016;11(3):e0151534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Salih S, Paskins A, Nichol T, Smith T, Hamer A. The cement spacer with multiple indentations: Increasing antibiotic elution using a cement spacer 'teabag'. Bone Joint J 2015;97‐B(11):1519–1524. [DOI] [PubMed] [Google Scholar]
- 39. Roth KE, Krause B, Siegel E, Maier G, Schoellner C, Rommens PM. Liquid dextran does not increase the elution rate of different antibiotics from bone cement. Eur J Orthop Surg Traumatol 2015;25(1):83–89. [DOI] [PubMed] [Google Scholar]
- 40. Corona PS, Barro V, Mendez M, Cáceres E, Flores X. Industrially prefabricated cement spacers: Do vancomycin‐ and gentamicin‐impregnated spacers offer any advantage? Clin Orthop Relat Res 2014;472(3):923–932. [DOI] [PMC free article] [PubMed] [Google Scholar]


