Abstract
Purpose
To characterize intraocular pressure (IOP) dynamics by identifying the sources of transient IOP fluctuations and quantifying the frequency, magnitude, associated cumulative IOP-related mechanical energy, and temporal distribution.
Methods
IOP was monitored at 500 Hz for periods of 16 to 451 days in nine normal eyes of six conscious, unrestrained nonhuman primates using a validated, fully implanted wireless telemetry system. IOP transducers were calibrated every two weeks via anterior chamber cannulation manometry. Analysis of time-synchronized, high-definition video was used to identify the sources of transient IOP fluctuations.
Results
The distribution of IOP in individual eyes is broad, and changes at multiple timescales, from second-to-second to day-to-day. Transient IOP fluctuations arise from blinks, saccades, and ocular pulse amplitude and were as high as 14 mm Hg (>100%) above momentary baseline. Transient IOP fluctuations occur ∼10,000 times per waking hour, with ∼2000 to 5000 fluctuations per hour greater than 5 mm Hg (∼40%) above baseline. Transient IOP fluctuations account for up to 17% (mean of 12%) of the total cumulative IOP-related mechanical energy that the eye must withstand during waking hours.
Conclusions
Transient IOP fluctuations occur frequently and comprise a large and significant portion of the total IOP loading in the eye and should, therefore, be considered in future studies of cell mechanotransduction, ocular biomechanics, and/or clinical outcomes where transient IOP fluctuations may be important. If IOP dynamics are similar in humans, clinical snapshot IOP measurements are insufficient to capture true IOP.
Keywords: intraocular pressure, transient IOP fluctuation, glaucoma, nonhuman primate, telemetry
Intraocular pressure (IOP), the fluid pressure in the eye, is central to almost every aspect of ocular function. Yet, very little is known about IOP dynamics, the second-to-second, minute-to-minute, hour-to-hour, and day-to-day fluctuations in IOP due to transient events such as blink, saccade, and ocular pulse amplitude (OPA), as well as slower events such as body position changes and nycthemeral rhythm. Homeostasis of IOP is critical in maintaining the precise shape of the globe required for optimal optical transmission and has direct effects on ocular blood flow, corneoscleral biomechanics, and cellular homeostasis throughout the eye.1–6
Elevated IOP is a major risk factor for glaucoma, a leading cause of irreversible blindness affecting more than 70 million people worldwide; lowering IOP is the only proven treatment for the disease.7 Although IOP plays a key role in ocular physiology and pathophysiology, surprisingly little is known about IOP dynamics in living humans because clinicians typically measure IOP using snapshot devices during office examinations. Current snapshot IOP measurement techniques require that the patient's eye remain still and provide only measurements of IOP at a single point in time. Hence, we know relatively little about IOP variation over short- and long-term timescales even though IOP variability affects ocular physiology and could impact glaucoma onset and progression. Studies have shown that IOP is incredibly dynamic,8–10 so current snapshot measurement techniques are not likely to provide an accurate assessment of either mean IOP or its dynamics.
IOP affects ocular physiology through multiple pathways. First, studies on aqueous distribution and outflow, critical variables that regulate IOP homeostasis, show that IOP dynamics play a key role in aqueous outflow dynamics.11,12 Second, ocular arterial blood pressure must be higher than IOP for blood to enter the eye. Hence, IOP and its dynamics are critical to vascular perfusion of ocular tissues, which is essential to the vitality of the eye and maintenance of vision. Ocular perfusion pressure (ophthalmic arterial blood pressure − IOP) has been implicated in clinical studies as a risk factor for glaucoma.13,14 Third, the corneoscleral shell is continuously exposed to dynamic pressure loading, which engenders mechanical strains in the ocular tissues with every transient IOP fluctuation,15 over and above the mechanical strain levels associated with baseline IOP. Hence, transient IOP fluctuations drive strain fluctuations that likely play a significant role in cellular homeostasis in the eye,16 and perturbations in the cyclic strains induced by transient IOP fluctuations could prove injurious to ocular tissues.17
Previous studies have not quantified IOP dynamics for extended periods in either humans or large animal models such as nonhuman primates (NHPs) that have eyes similar enough to humans that reasonable conclusions about human IOP dynamics can be inferred. A recent study using the Triggerfish contact lens sensor (CLS; Sensimed AG, Lausanne, Switzerland) for 24-hour periods showed that larger transient corneolimbal stretch fluctuations are correlated to greater visual field progression in glaucoma.18 It should be noted that larger stretch fluctuations measured by the Triggerfish CLS could be due to either lower corneolimbal tissue stiffness or larger transient IOP fluctuations or some combination. To fill this important gap in current knowledge, this study quantified IOP dynamics over long periods using a novel, fully implanted, radiotelemetry system to measure and record continuous IOP via direct pressure recordings in conscious, unrestrained NHPs.
Methods
Animals
All animal experiments were conducted in accordance with the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research, under Institutional Animal Care and Use Committee approval from the University of Alabama at Birmingham. For this study, we used six rhesus macaques aged 4 to 6 years old, consisting of two females and four males.
IOP Telemetry System
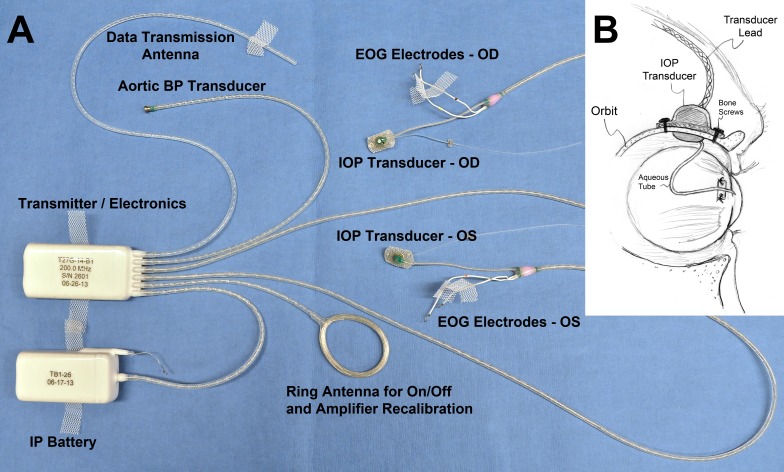
We have developed and validated a fully implanted wireless telemetry system (Konigsberg Instruments, Inc., Pasadena, CA, USA) that allows continuous monitoring of IOP in conscious, unrestrained NHPs (Fig. 1).10 Continuous telemetry data are transmitted wirelessly from a battery-powered transceiver module implanted in the animals' abdominal wall to an antenna in the perch of the cage. Data are transferred from the receiving antenna to an analog base station wherein the digital signals are decoded and stored. There are two iterations of this system used in this study. The first-generation system measures and transmits four data streams, namely, unilateral IOP (500 Hz), blood pressure (250 Hz), electrocardiogram (500 Hz), and body temperature (50 Hz); this system was used in two NHPs (21356 and 18012). The second-generation system measures and transmits six continuous data streams, namely, bilateral IOP (500 Hz), bilateral electrooculogram (500 Hz), temperature (50 Hz), and blood pressure (250 Hz), via a transducer implanted directly in the aorta; this system was used in NHPs 9028, 9140, 9160, and 0804025. NHP 9028 was converted from bilateral to unilateral IOP due to sensor failure in one eye. For this study, we did not use the temperature, blood pressure, or electrocardiogram data streams. Both systems use a barometric pressure sensor at the base station recorder to compensate for atmospheric pressure in real time.
Figure 1.
The second-generation University of Alabama at Birmingham IOP telemetry implant. (A) Photograph of enhanced second-generation Konigsberg Instruments T27G total implant system for continuous monitoring of bilateral IOP, bilateral electrooculogram (EOG), aortic blood pressure, and body temperature. (B) A 23-gauge silicone tube delivers aqueous from the anterior chamber to a fluid reservoir on the intraorbital side of the transducer; the tube (with appropriate slack to allow for eye movement) is trimmed, inserted into the anterior chamber, sutured to the sclera using the integral scleral anchor plate, and covered with a corneal patchgraft (not shown). Adapted from Downs et al.10 with permission.
IOP Transducer Calibration
The IOP transducers were calibrated approximately every 2 weeks as follows. Each animal was anesthetized, and the anterior chamber(s) of the implanted eye(s) were cannulated through the peripheral cornea with a sterile 27-gauge needle connected to a bottle of sterile isotonic saline solution via a sterile infusion set fitted with an in-line, digital pressure gauge (model XP2i; Crystal Engineering, San Luis Obispo, CA, USA) placed level with the needle into the eye. IOP was calibrated in increments of 5 mm Hg from 5 to 40 mm Hg, and the telemetric IOP reading was compared to the in-line pressure gauge at each step after IOP stabilized. The telemetric and gauge IOP were used to quantify IOP transducer signal accuracy and drift.
IOP Transducer Drift Compensation and Signal Loss Filtering
After data collection and real-time barometric pressure compensation, the IOP signal was adjusted according to the IOP calibration tests. The IOP signal was assumed to have drifted linearly in the 2 weeks between transducer calibration tests. The data from each acquisition session were continually offset by the drift calculated for that period. Typically, IOP transducers drifted <1 mm Hg per week.
The continuous IOP radiotelemetry signal is subject to signal transmission loss when the animal moves too far from the in-cage antenna or orients its body in a way that the signal must pass through most of the animal's body to reach the antenna. As a result, the data were digitally filtered using an automated algorithm to eliminate signal loss to ensure maximum data quality. Only 24-hour periods in which at least 75% of the data in all 24 1-hour increments remained after filtering were used for the analyses. The percentage of days excluded due to signal loss ranged from 1% to 20% depending on the animal, with a mean of 10% rejection rate overall.
Primary Data Acquisition
After telemetry system implantation and a heal-in period of at least 4 weeks, telemetry data were acquired continuously in 24-hour blocks until battery or implant failure. IOP transducers were calibrated every 2 weeks via anterior chamber calibration as described above, with subsequent continuous IOP drift compensation via software, and the drift-corrected data were filtered to eliminate signal transmission loss. Data acquired on days in which the anterior chamber of the eye was cannulated for IOP calibration were excluded from the analyses.
Data Acquisition—Transient IOP Fluctuation Source Analysis
To investigate the physiologic cause of transient IOP fluctuations, three young male rhesus macaques were presented with video entertainment to capture their attention, and high-definition video of their faces was simultaneously recorded using a tilt-pan-zoom video camera (Q6035-E; Axis Communications, Lund, Sweden). The video camera has a resolution of 1920 × 1080 pixels and records 30 frames per second.
Telemetric IOP measurements were captured using NOTOCORD-hem (Notocord Systems, Croissy-sur-Seine, France) data acquisition software, and the video was simultaneously recorded. This required time-syncing the data stream with the video so that events recorded in the video corresponded in time to continuous IOP recordings. Transient IOP fluctuations were matched to blinks and saccades by a single observer (DCT), and the troughs immediately before and after transient IOP fluctuations were marked. The two IOP troughs were averaged and subtracted from the IOP transient peak to quantify the magnitude of the transient IOP fluctuation and tagged as a blink or saccade, noting the primary direction of the saccade as either horizontal left, horizontal right, or vertical.
Quantification of Transient IOP Fluctuation Frequency and Magnitude
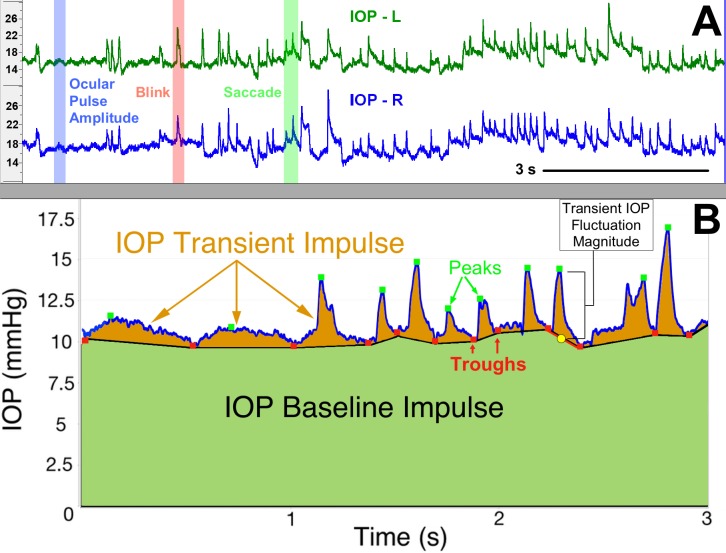
First, the raw IOP data were smoothed slightly with a seven-sample, 14-millisecond running average to reduce signal jitter. We used a dual-band finite impulse response (FIR) filter to identify transient IOP fluctuation peaks and troughs for quantification in the larger dataset. The low-band FIR filter is tuned to identify slower transient IOP fluctuations such as OPA, whereas the high-band FIR filter is tuned to identify higher frequency IOP fluctuations from sources such as blink and saccade.19 The FIR filter was only used to identify the transient IOP fluctuation trough positions in the IOP data stream, with the peaks identified by searching for the largest IOP value between adjacent troughs to avoid false identification of double peaks, such as those shown at time 2.6 seconds in Figure 2B. Transient IOP fluctuations were quantified by identifying each IOP peak surrounded by two troughs (Fig. 2B); the two troughs were averaged into a single baseline IOP value. The peak IOP-to-baseline IOP difference is catalogued and counted as the magnitude of an individual transient IOP fluctuation. Transient IOP magnitude frequency was plotted in a histogram by magnitude bin within each eye. The limits of the smallest magnitude bin were determined by our minimum transient magnitude detection resolution (0.6 mm Hg) and analyzing the OPA (typically less than 2 mm Hg), so we chose to make the lowest bin range 0.6 to 2 mm Hg. The other limits were chosen arbitrarily to represent small (2 to 5 mm Hg), medium (5 to 10 mm Hg), large (10 to 15 mm Hg), and very large (>15 mm Hg) transient IOP fluctuations.
Figure 2.
Identification and quantification of frequency, magnitude, and impulse of transient IOP fluctuations. (A) The top panel shows a screenshot of ∼11 seconds of continuous telemetry data showing IOP signals from the left (L) and right (R) eyes of an animal. Selected, verified blinks, saccades, and OPA events are shown with red, green, and blue shading, respectively. (B) The bottom panel shows the area under the IOP versus time curve, which represents total IOP impulse, a surrogate measure for the cumulative IOP-related mechanical energy the eye must withstand and absorb over time. The green-shaded area represents the IOP baseline impulse, which is a measure of the IOP energy the eye must withstand due to momentary baseline IOP, and the orange-shaded area represents the IOP transient impulse, which is a measure of the IOP energy the eye must withstand due to transient IOP fluctuations alone. Adapted from Markert et al.52 with permission.
Quantification of IOP Impulse
Total IOP impulse, defined as the area under the continuous IOP versus time curve, is an engineering-based metric that serves as a surrogate measure of the total amount of IOP-related energy the eye must withstand over time. IOP transient impulse is quantified as the portion of total IOP impulse associated with transient IOP fluctuations alone (Fig. 2B, orange shaded area), calculated using the IOP troughs and peaks identified in earlier analysis steps. IOP transient impulse is a surrogate measure of the energy the ocular coat must absorb due to transient IOP fluctuations, and the IOP Baseline Impulse (Fig. 2B, green shaded area) is a measure of the amount of IOP energy the eye must withstand due to momentary baseline IOP. The relative contribution of IOP transient impulse, calculated as a percentage of total IOP impulse, was averaged hourly over all days for all NHPs. This metric assesses the amount of transient IOP fluctuation-related energy relative to the total IOP-related energy the eye must absorb over time.
Statistical Analyses
Nested, linear mixed effects models were used to assess the sources of variance and the difference between waking and sleeping hours for two parameters: transient IOP fluctuation frequency and IOP transient impulse. We characterized the variability in these two parameters from the following components in the models: day-to-day, hour-to-hour, NHP-to-NHP, and eye-to-eye within NHPs. Lastly, we used a chi-squared test of independence to determine if the number of transient IOP fluctuations per transient IOP peak amplitude frequency bin (0.6 to 2, 2 to 5, 5 to 10, 10 to 15, and >15 mm Hg) is different between waking and sleeping hours.
Results
The Source of Transient IOP Fluctuations
Transient IOP fluctuations on the order of milliseconds to seconds are the result of blinks, saccades, and OPA (Figs. 2A and 3; Supplementary Video). IOP fluctuations on the order of multiple seconds are due to respiration (observed in anesthetized animals; data not shown) and other factors, such as ocular muscle tone. IOP fluctuations on the order of minutes to hours have been shown to result from changes in aqueous humor inflow and outflow, body position, blood pressure fluctuation, or nycthemeral rhythm (sleep vs. awake).11,20–23
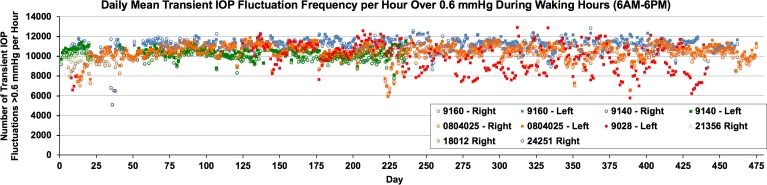
Figure 3.
The daily mean number of transient IOP fluctuations per hour over 0.6 mm Hg in magnitude during waking hours (6AM–6PM) plotted over the entire data acquisition period in each eye. Each data point represents one day of data, presented as the mean number of transient IOP fluctuations per hour during the 12 waking hours. The eye must withstand ∼10,000 transient IOP fluctuations every hour during waking hours.
Validation of Transient IOP Fluctuation Frequency Counts
To validate the detection and frequency quantification of transient IOP fluctuations from OPA, the number of heartbeats calculated by NOTOCORD-hem from the aortic blood pressure data was compared to the number of transient IOP fluctuations (OPA) counted by our FIR algorithm (as shown in Fig. 2) during an hour when NHPs were sleeping and had minimal signal loss. The NOTOCORD data acquisition software has the ability to detect and count heartbeats from the blood pressure data through a separate commercial analysis module used for cardiovascular studies. During sleep, we expect most transient IOP fluctuations to be associated with OPA alone and <3 mm Hg in magnitude, although there are brief periods of waking during which the NHP has blink- and saccade-related transient IOP fluctuations that are larger than 3 mm Hg. In one test in one eye, NOTOCORD-hem's commercial module detected 4799 heartbeats and our software identified 4846 transient IOP fluctuations of 0.6 to 3 mm Hg; 99% of the heartbeats during this time translated to OPA-related transient IOP fluctuations. Similar results were found for all eyes in the other animals (data not shown).
Also, brief random segments of raw IOP data from every eye of awake behaving animals were plotted with automated peak and trough detection markers shown (green and red markers in Fig. 2) to confirm that the FIR algorithm appropriately detected peaks and troughs of larger and more variable transient IOP fluctuations that occur during the day. In all cases, the FIR algorithm accurately identified and counted transient IOP fluctuations of varying magnitude and duration.
Magnitude and Frequency of Transient IOP Fluctuations
Transient IOP fluctuations occur ∼10,000 times per waking hour in NHPs (Fig. 3). Transient IOP fluctuation magnitudes associated with blinks and saccades were as high as 14 mm Hg in individual eyes (Fig. 4). Note both the magnitude and frequency were highly variable between animals but were consistent within eyes and between the fellow eyes in each NHP.
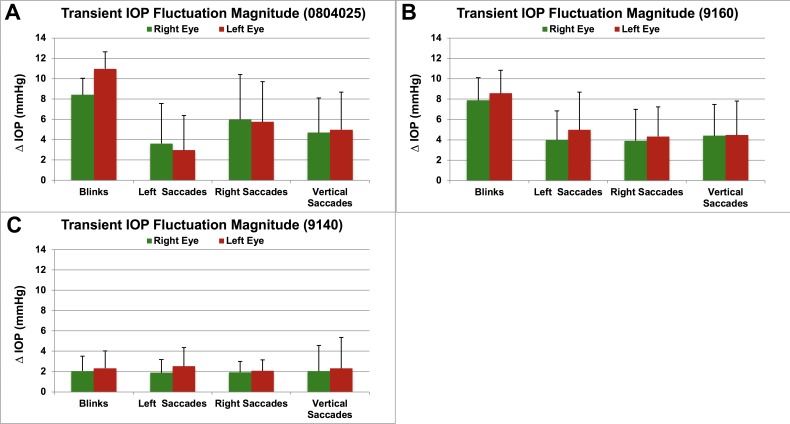
Figure 4.
Mean transient IOP fluctuation magnitude above baseline for blinks and saccades for each NHP (A–C) (ID in parentheses), quantified in ∼12 hours of video (2 hours of video for each of two sessions in each NHP, at least 1 week apart). Error bars represent the standard deviation. Saccades are separated by direction to show that magnitudes are similar for each direction of saccade within eyes and between fellow eyes in each animal. All NHPs were of similar age (4.5 to 5 years old).
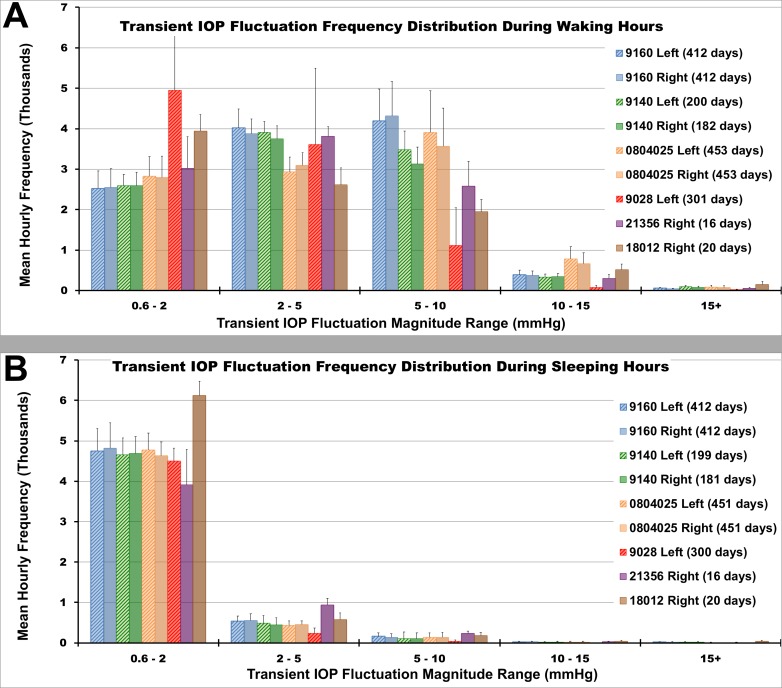
Transient IOP Fluctuation Frequency Distributions
The magnitudes and frequency of transient IOP fluctuations are distributed differently during sleeping and waking hours, and these distributions vary among NHPs (Fig. 5). Some NHPs exhibit a larger number of transient IOP fluctuations in the lower magnitude range (e.g., NHP 9028), while other NHPs exhibit a larger number of fluctuations in the higher magnitude range (e.g., NHP 0804025). As seen in Figure 5, the number of transient IOP fluctuations from 0.6 to 2 mm Hg in magnitude is much larger than those of larger magnitudes, presumably due to OPA, the low magnitude IOP fluctuation that occurs with every heartbeat. Although we observed more transient IOP fluctuations in the low magnitude range of 0.6 to 2 mm Hg during sleeping hours, they also occur during waking hours but are largely obscured by larger transient IOP fluctuations from blinks, saccades, and other sources (Fig. 2A). There are approximately 5000 more transient IOP fluctuations >0.6 mm Hg per hour during waking hours than during sleeping hours (P = 2.2e-16).
Figure 5.
The mean hourly frequency distribution of transient IOP fluctuation magnitude during (A) waking hours (6AM–6PM) and during (B) sleeping hours (6PM–6AM) binned by magnitude range for all data collected (error bars are standard deviation).
A chi-squared test of independence determined that the number of transient IOP fluctuations in each transient IOP fluctuation peak amplitude frequency bin (0.6 to 2, 2 to 5, 5 to 10, 10 to 15, and >15 mm Hg) is significantly different between wake and sleep hours, as well as between NHPs (P values = 0.057, 0.047, 2.5e-8, 7.9e-12, and 0.526 for each bin, respectively). The number of transient IOP fluctuations during sleeping hours are largely in the 0.6- to 2-mm Hg range, whereas those during waking hours exhibit much larger amplitudes (Fig. 5).
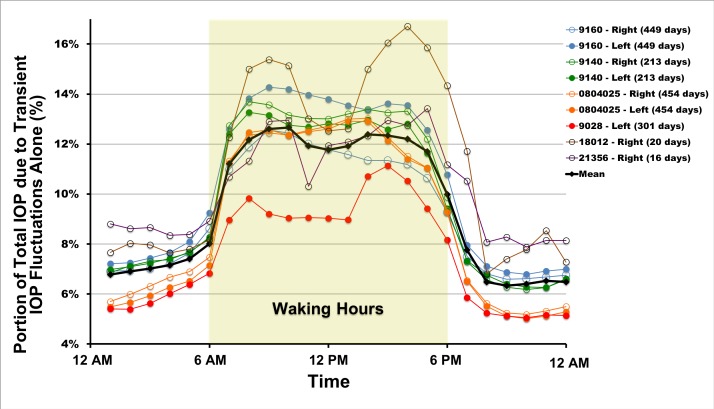
IOP transient impulse quantifies the area under the IOP time curve due to transient IOP fluctuations alone and is a measure of the magnitude of the dynamic or transient portion of IOP over a given period of time. Figure 6 shows the hourly mean IOP transient impulse as a percentage of total IOP impulse, which is the relative contribution of transient IOP fluctuations to total IOP plotted over time of day, averaged both overall and for each eye. These data show that transient IOP fluctuations account for 5% to 17% of the total IOP-related energy the eye must absorb and withstand over time. Furthermore, during waking hours, transient IOP fluctuations account for 9% to 17% of total IOP within animals and from 10% to 13% when averaged across all eyes (black trace, Fig. 6). Overall, transient IOP fluctuations account for an average of 12% of total IOP during the waking period. The bulk of the transient IOP impulse is present during waking hours when the animals are active, blinking, and moving their eyes. These data are consistent with the distributions of transient IOP fluctuation magnitude shown in Figure 5, as greater numbers of larger transient IOP fluctuations should generate more transient IOP impulse. For all animals and eyes, the IOP transient impulse percentage (Fig. 6) and number of transient IOP fluctuations greater than 0.6 mm Hg show clear separation between waking and sleeping hours, with waking hour values of both parameters being significantly higher than those during sleeping hours (P = 2.2e-16 for both parameters).
Figure 6.
Mean hourly IOP transient impulse, plotted as a percentage of total IOP, for all eyes of all animals. The plotted values are a measure of the amount of energy the eye must withstand from transient IOP fluctuations relative to the total IOP energy the eye must absorb. The number of days of data used is indicated in the legend. Waking hours are highlighted (6AM–6PM).
Parameter Variability
The sources of variance in IOP transient impulse percentage and the number of transient IOP fluctuations above 0.6 mm Hg are shown in the Table. For the number of transient IOP fluctuations above 0.6 mm Hg, day-to-day variability accounts for approximately 50% of the variability, whereas the IOP transient impulse variability is driven primarily by animal-to-animal differences. In general, eye-to-eye variability is very low within animals, so variability between eyes can be attributed to the differences in treatment in future studies where the two eyes are treated differently.
Table.
Sources of Variance in IOP Transient Impulse and the Number of Transient IOP Fluctuations >0.6 mm Hg in Magnitude, Separated Into Waking (6AM–6PM) and Sleeping Hour Periods (6PM–6AM).
| Source of Variability |
% of Total Variability |
|
| Waking Hours |
Sleeping Hours |
|
| IOP transient impulse | ||
| NHP | 13 | 18 |
| Eye within NHP | 6 | 0.7 |
| Day within eye within NHP | 50 | 48 |
| Hour within eye within NHP | 32 | 34 |
| Number of transient IOP fluctuations | ||
| NHP | 44 | 45 |
| Eye within NHP | 2 | 0.4 |
| Day within eye within NHP | 15 | 22 |
| Hour within eye within NHP | 39 | 32 |
Discussion
In this study, we identified the sources of transient IOP fluctuations and quantified both their magnitude and frequency by using continuous IOP telemetry in NHPs. IOP transient impulse, a measure of the energy the eye must absorb and withstand due to transient IOP fluctuations, was calculated as a percentage of the total IOP energy. Results demonstrate the truly dynamic nature of IOP in normal eyes. IOP is typically measured with snapshot devices that capture mean values, and so the transient IOP fluctuations characterized herein have been largely disregarded by the clinical community and have only recently been acknowledged as important by the research community. Hence, the most important and striking result of this work is the finding that an average of 12% of the total IOP energy the eye must absorb and withstand over time during waking hours is due to transient IOP fluctuations, driven by 2000 to 5000 fluctuations per hour greater than 5 mm Hg above baseline (Figs. 2, 4–6).
To our knowledge, there have been two studies involving direct continuous pressure measurement in the eye of a healthy living human8,24 and only one assessed the effects of blinks and saccadic eye movement on IOP.8 The latter study was performed in a single subject over a short time period and reported 5- to 10-mm Hg transient IOP increases due to blink and saccade but did not assess how IOP or transient IOP fluctuations change over time or quantify the impact of transient IOP fluctuations in relation to overall IOP. Other studies examining continuous IOP in humans have measured OPA, the fluctuation in IOP associated with the cardiac cycle, by using dynamic contour tonometry25 or recorded the frequency (but not magnitude) of transient IOP fluctuations with a CLS.9,26 The Pascal dynamic contour tonometer (Ziemer Ophthalmic Systems AG, Port, Switzerland)25 can provide accurate measurement of IOP and OPA for the short period of a few seconds that a patient can tolerate corneal contact without blinking but cannot assess the large transient IOP fluctuations from sources such as blink and saccade identified in this study. The Sensimed Triggerfish CLS (Sensimed AG) measures corneal stretch at the corneoscleral junction as a surrogate measure of IOP and can quantify the frequency of transient IOP fluctuations from all sources, but the data are equivocal that the CLS can assess nycthemeral IOP rhythm, and utility is limited because the system output (millivolt equivalent units) cannot be calibrated to true IOP (mm Hg) in patients. Although the Triggerfish cannot measure true IOP per se, the number of large corneolimbal strain peaks measured with the contact lens during a single 24-hour session has been associated with faster visual field deterioration in glaucoma patients,18 suggesting that large transient IOP fluctuations and/or cyclic mechanical stretch levels may play a role in glaucoma progression.
IOP plays a dominant role in the biomechanics of the eye. As with any solid, load-bearing structure, mechanical strain in the ocular tissues will fluctuate with momentary fluctuations in stress (IOP). Hence, transient IOP fluctuations will drive mechanical strain fluctuations, with strain magnitudes dependent on both the transient IOP fluctuation magnitude and stiffness of the corneoscleral shell. Although previous clinical studies on IOP “fluctuation” have analyzed changes in snapshot mean IOP measurements taken from hours27,28 to months apart,29,30 the notion that short-duration transient IOP fluctuations could play a crucial role in ocular physiology and underlie pathophysiologic change in diseases such as glaucoma has only recently been seriously considered.11,31–35
Mean IOP, as measured clinically with snapshot devices, is a major risk factor in glaucoma, although there is a wide range of eye-specific susceptibility to mean IOP.1,36,37 Interestingly, there is also a wide range of transient IOP fluctuation magnitude at normal IOPs (Fig. 5), presumably driven by differences in ocular coat stiffness, blink forces, and ocular muscle forces. The distribution of the magnitude of transient IOP fluctuations is eye-specific, with large differences between animals and less difference between eyes within animals. Some NHPs exhibit a greater number of transient IOP fluctuations of lower magnitude (e.g., NHP 9028), which may indicate a more compliant corneoscleral shell that can viscoelastically deform to absorb IOP-related energy; other NHPs exhibit a greater number of transient IOP fluctuations of higher magnitude (e.g., NHP 0804025), which could suggest a more rigid corneoscleral envelope (Fig. 5). This is consistent with prior in vivo studies in NHPs and in vitro studies of porcine eyes showing that transient IOP fluctuation magnitudes are larger in eyes with stiffer corneoscleral shells.15,32,38 These results show there is a wide range of transient IOP fluctuation behavior in individual eyes of normal NHPs of similar age.
Transient IOP Fluctuations and Cellular Mechanotranduction
Cellular mechanotransduction refers to the conversion of mechanical stimuli to chemical signals at the cellular level. Transient IOP fluctuations are mechanical stimuli to which ocular tissues must constantly react and withstand. On average, the normal NHP eye is subjected to ∼10,000 transient IOP fluctuations >0.6 mm Hg, of which 2000 to 5000 are >5 mm Hg, every hour during waking hours (Fig. 4). Results show that transient IOP fluctuations up to 14 mm Hg above momentary baseline IOP occur frequently, and the principle sources of these transient IOP fluctuations are OPA, blinks, and saccadic eye movements.
The eye is a compliant pressure vessel, so transient IOP fluctuations drive transient mechanical strain, which has significant effects on ocular cell activity.39 Xin et al.11 showed that OPA causes cyclic optic nerve head deformation by using phase-sensitive optical coherence tomography measurements in vivo, indicating that even small transient IOP fluctuations elicit cyclic mechanical strain. Many studies have examined cyclical mechanical strain and cited the various cellular changes that accompany mechanical stimulation, and almost all of these studies examine tissue response to stretch with multiple cycles of uniform applied strain at consistent time intervals, that is monotonous cyclic stretch.33,35,40,41 There is a significant difference between identical, repetitive stretch cycles that have been used in most in vitro studies of mechanotransduction in ocular cells and the more variable cyclic strains induced by transient IOP fluctuations in vivo (Fig. 1). Studies have shown that cyclic stretch on various cell types can have different effects depending on whether the applied cyclic stretch was monotonous (identical amplitude and frequency) or variable (varying amplitude and frequency).16,42 The data presented herein show that variable transient IOP fluctuations occur up to 10,000 times per hour, which should induce concomitant variable strain fluctuations in all ocular tissues. Hence, it is of the utmost importance to fully characterize transient IOP fluctuations and their effects in living eyes, particularly those with differing biomechanic properties due to age or racial heritage43–46 and/or disease-induced changes in ocular coat stiffness, such as those known to occur with glaucoma47,48 and myopia.49 Similarly, in vitro experiments of mechanotransduction should incorporate variable cyclic stretch to best simulate the in vivo environment.
Clinical Implications
The presented results show that there is a wide range of transient IOP fluctuation magnitudes, frequencies, and impulse in normal NHP eyes from animals of similar ages. Also, the posterior sclera has been shown to stiffen with advancing age in eye donors of European descent and stiffens to a greater extent with age in donors of African descent.43–45 Consistent with prior studies32 and biomechanic engineering principles, transient IOP fluctuations should be larger in the elderly and even larger still in elderly persons of African heritage. Given that these two populations are at greater risk for glaucoma1,50 and there is a wide range of eye-specific transient IOP fluctuation magnitudes as shown herein, it is possible that eye-specific variation in transient IOP fluctuations independently confers risk for glaucoma in addition to mean IOP. However, research studies in NHPs equipped with telemetry and clinical studies in patients will be required to determine if transient IOP fluctuations contribute to glaucoma pathophysiology.
If transient IOP fluctuations are shown to contribute to glaucoma pathogenesis and progression, new therapeutic approaches to lower the magnitude of transient IOP fluctuations could be developed. Possible avenues for attenuating transient IOP fluctuation magnitude include using pharmacologic agents to increase corneoscleral shell compliance or implanting a compliant object in the eye, such as gas-filled balloon51 or viscoelastic solid mass. In addition to possible treatment modalities, the clinical quantification of transient IOP fluctuation magnitude may have utility as a biomarker of eye-specific ocular coat stiffness, which could lead to better understanding of eye-specific susceptibility to ocular conditions, such as myopia and glaucoma, and help guide clinical treatment approaches in the future. Future studies will be required to determine the extent to which transient IOP fluctuations contribute to ocular diseases, which will drive development of these approaches.
Limitations
This study has several limitations. First, the study was performed in NHPs, which may not translate directly to human subjects due to differences in body and eye size and other physiologic factors. However, NHPs are considered the most translatable animal model for ocular physiology and glaucoma. Second, although extensive data are presented for most animals, some animals have as few as 16 days of data (NHP 21356), resulting in more variability in those animals monitored for shorter periods. That said, continuous IOP telemetry provides an unprecedented volume of data even over the period of a few days, and so our principle results and conclusions are unlikely to be significantly different even if more data were available. Third, our sample size was small; three NHPs were equipped with unilateral IOP transducers and three others with bilateral IOP transducers. This limited our ability to quantify intra- and intersubject variability, although this was not a primary goal of the study.
Fourth, IOP was measured through a tube into the anterior chamber of the eye connected to a pressure transducer mounted in the superotemporal orbital wall (Fig. 1), rather than with a pressure transducer residing inside the eye itself. One potential concern with this approach is that the aqueous in the transducer reservoir or tube may be subjected to fluid inertia and/or tube flexion as the eye and tube move, creating false pressure readings. To address this concern, we analyzed transient IOP fluctuation data from left and right saccades in fellow eyes. If there were inertial or tube-bending effects, we would expect that when the eye rotates toward the sensor (i.e., right saccade for right eye) and the tube is in flexion, the transient IOP fluctuation magnitude would differ from the left eye during that same right saccade, when the eye is rotating away from the sensor and the tube is in extension. Results show that for each pair of fellow eyes, transient IOP fluctuation magnitudes were similar in both eyes for both left and right saccades. In addition, transient IOP fluctuation magnitudes associated with vertical saccades in which the tubes in fellow eyes bend similarly were similar to those from horizontal saccades in which the aqueous transduction tubes are in flexion and extension, respectively. Although there are small torsional and convergent/divergent movements associated with all saccades, the lack of significant differences between fellow eyes in the three primary saccade directions reported in Figure 3 indicate that tube extension/flexion and fluid inertial effects are minimal.
We also considered the possibility that the tube may be compressed during blinking. The tube is high-stiffness silicone, with an inside lumen diameter of 250 μm and a wall thickness of 125 μm and, hence, is extremely resistant to compression and kinking due to its high wall-thickness-to-lumen-diameter ratio. Also, the tubing is buried in the extraocular tissues and does not protrude, so blink force is likely exerted equally around the circumference of the tubing and along its length. Hence, although we did not test the compression resistance of the aqueous transduction tubing, it seems unlikely that the forces associated with blink would be sufficient to significantly alter lumen diameter. Even if lumen diameter is slightly altered with blink, the tube is open to the eye and the concomitant change in intraocular volume would be negligible and so the resulting transient IOP fluctuation small. For example, near complete compression of a 3 mm length of tube would change intraocular volume by 0.15 μL, which is negligible relative to the total volume of the monkey eye (∼60% of the volume of a human eye). It is important to note that the reference volume considered in this theoretical analysis should be the total volume of the transducer reservoir, the aqueous transduction tube, and the eye combined and not the transducer reservoir alone. The primary reason for this is that the tube cannot be entirely occluded even with tremendous force due to the tubing wall thickness, as there will still be an area along the edges of the full length of the collapse zone that remains patent because the lumen will assume the shape of a figure eight, with full apposition in the center only. Hence, there will be pressure communication between the rigid transducer reservoir and the eye even with severe tube compression, and therefore, the eye/tube/transducer reservoir will act as a single elastic volume system. Also, if tube compression was causing a positive transient pressure artifact, one would expect a similar negative transient pressure artifact to follow immediately afterward due to the negative pressure associated with the lumen reopening and refilling with fluid pulled from the adjacent tubing. We did not observe a negative pressure bounce-back immediately after blink, again suggesting that tube compression artifact is minimal if present. Finally, the transient IOP fluctuation magnitudes associated with blinks and saccades reported herein are consistent with transient IOP increases of 5 to 10 mm Hg with blink and saccade obtained by Coleman and Trokel8 using direct anterior chamber cannulation measurements in a single human patient.
Finally, the possibility of fluid leaking from the eye around tube insertion site has been examined. If there were any leaks in the system, we would expect either mean IOP or the daily number of transient IOP fluctuations >5 mm Hg to decrease or change over time. We did not observe this trend in any eye (data not shown). If leaks did occur, autoregulation of aqueous outflow must have compensated for the increase in outflow to keep mean IOP and transient IOP fluctuation magnitude stable.
Conclusions
IOP is much more dynamic than has previously been appreciated. Over time, the eye must absorb the energy associated with transient IOP fluctuations, which will affect ocular physiology through multiple pathways and could play a role in the onset or progression of ocular diseases, such as keratoconus, myopia, and glaucoma, in which IOP-related deformation and strain are important. Every waking hour, the eye must absorb ∼10,000 transient IOP fluctuations larger than 0.6 mm Hg above momentary baseline, and ∼5000 transient IOP fluctuations greater than 5 mm Hg above baseline. This is the first study to show that transient IOP fluctuations comprise a large and significant portion of the total IOP loading in the eye, and these data should be considered in future studies that use cyclic strain in cells or tissues, models of ocular biomechanical behavior, or clinical data where transient IOP fluctuations may be important.
Supplementary Material
Acknowledgments
The authors thank Claude F. Burgoyne, MD, for his invaluable help in developing the first-generation implant system and the surgical approach, resulting in successful data collection in 2 NHPs; Chester Calvert, for developing software programs to handle all filtering, processing, and quantification of transient IOP fluctuations and impulse; and Lisa Hethcox, LVT, for her invaluable assistance with all NHP experiments and caretaking of animals.
Supported by National Institutes of Health Grants R01-EY024732 (JCD), R01-EY026035 (JCD), and P30-EY003039 (Pittler; UAB Core Facilities Grant); EyeSight Foundation of Alabama, Birmingham, AL; and Research to Prevent Blindness, New York, NY.
Disclosure: D.C. Turner, None; A.M. Edmiston, None; Y.E. Zohner, None; K.J. Byrne, None; W.P. Seigfreid, None; C.A. Girkin, None; J.S. Morris, None; J.C. Downs, None
References
- 1.Gordon MO, Beiser JA, Brandt JD, et al. The ocular hypertension treatment study: Baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:714–720. doi: 10.1001/archopht.120.6.714. [DOI] [PubMed] [Google Scholar]
- 2.Quigley HA, Addicks EM, Green WR, Maumenee AE. Optic nerve damage in human glaucoma. II. The site of injury and susceptibility to damage. Arch Ophthalmol. 1981;99:635–649. doi: 10.1001/archopht.1981.03930010635009. [DOI] [PubMed] [Google Scholar]
- 3.Fechtner RD, Weinreb RN. Mechanisms of optic nerve damage in primary open angle glaucoma. Surv Ophthalmol. 1994;39:23–42. doi: 10.1016/s0039-6257(05)80042-6. [DOI] [PubMed] [Google Scholar]
- 4.Burgoyne CF, Downs JC, Bellezza AJ, Suh JK, Hart RT. The optic nerve head as a biomechanical structure: a new paradigm for understanding the role of IOP-related stress and strain in the pathophysiology of glaucomatous optic nerve head damage. Prog Retin Eye Res. 2005;24:39–73. doi: 10.1016/j.preteyeres.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 5.Friedenwald J. Contribution to the theory and practice of tonometry. Am J Ophthalmol. 1937;20:985–1024. [Google Scholar]
- 6.Cherecheanu AP, Garhofer G, Schmidl D, Werkmeister R, Schmetterer L. Ocular perfusion pressure and ocular blood flow in glaucoma. Curr Opin Pharmacol. 2013;13:36–42. doi: 10.1016/j.coph.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262–267. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coleman DJ, Trokel S. Direct-recorded intraocular pressure variations in a human subject. Arch Ophthalmol. 1969;82:637–640. doi: 10.1001/archopht.1969.00990020633011. [DOI] [PubMed] [Google Scholar]
- 9.Mansouri K, Shaarawy T. Continuous intraocular pressure monitoring with a wireless ocular telemetry sensor: initial clinical experience in patients with open angle glaucoma. Br J Ophthalmol. 2011;95:627–629. doi: 10.1136/bjo.2010.192922. [DOI] [PubMed] [Google Scholar]
- 10.Downs JC, Burgoyne CF, Seigfreid WP, Reynaud JF, Strouthidis NG, Sallee V. 24-hour IOP telemetry in the nonhuman primate: implant system performance and initial characterization of IOP at multiple timescales. Invest Ophthalmol Vis Sci. 2011;52:7365–7375. doi: 10.1167/iovs.11-7955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xin C, Wang RK, Song S, et al. Aqueous outflow regulation: Optical coherence tomography implicates pressure-dependent tissue motion. Exp Eye Res. 2017;158:171–186. doi: 10.1016/j.exer.2016.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnstone M, Martin E, Jamil A. Pulsatile flow into the aqueous veins: Manifestations in normal and glaucomatous eyes. Exp Eye Res. 2011;92:318–327. doi: 10.1016/j.exer.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Oliveira AP, Kasahara N. Correlation between ocular perfusion pressure fluctuation and glaucoma severity. Int Ophthalmol. 2015;35:187–192. doi: 10.1007/s10792-014-9929-5. [DOI] [PubMed] [Google Scholar]
- 14.Leske MC. Ocular perfusion pressure and glaucoma: clinical trial and epidemiologic findings. Curr Opin Ophthalmol. 2009;20:73–78. doi: 10.1097/ICU.0b013e32831eef82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Downs JC. IOP telemetry in the nonhuman primate. Exp Eye Res. 2015;141:91–98. doi: 10.1016/j.exer.2015.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suki B, Parameswaran H, Imsirovic J, Bartolak-Suki E. Regulatory roles of fluctuation-driven mechanotransduction in cell function. Physiology (Bethesda) 2016;31:346–358. doi: 10.1152/physiol.00051.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Resta V, Novelli E, Vozzi G, et al. Acute retinal ganglion cell injury caused by intraocular pressure spikes is mediated by endogenous extracellular ATP. Eur J Neurosci. 2007;25:2741–2754. doi: 10.1111/j.1460-9568.2007.05528.x. [DOI] [PubMed] [Google Scholar]
- 18.De Moraes CG, Jasien JV, Simon-Zoula S, Liebmann JM, Ritch R. Visual field change and 24-hour IOP-related profile with a contact lens sensor in treated glaucoma patients. Ophthalmology. 2016;123:744–753. doi: 10.1016/j.ophtha.2015.11.020. [DOI] [PubMed] [Google Scholar]
- 19.Chandra A, Chattopadhyay S. Design of hardware efficient FIR filter: a review of the state-of-the-art approaches. Int J Eng Sci. 2016;19:212–226. [Google Scholar]
- 20.Turner DC, Samuels BC, Huisingh C, Girkin CA, Downs JC. The magnitude and time course of IOP change in response to body position change in nonhuman primates measured using continuous IOP telemetry. Invest Ophthalmol Vis Sci. 2017;58:6232–6240. doi: 10.1167/iovs.17-22858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carlson KH, McLaren JW, Topper JE, Brubaker RF. Effect of body position on intraocular pressure and aqueous flow. Invest Ophthalmol Vis Sci. 1987;28:6. [PubMed] [Google Scholar]
- 22.Krieglstein GK, Waller WK, Leydhecker W. The vascular basis of the positional influence on the intraocular pressure. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1978;206:99–106. doi: 10.1007/BF00414618. [DOI] [PubMed] [Google Scholar]
- 23.Kiel JW. Choroidal myogenic autoregulation and intraocular pressure. Exp Eye Res. 1994;58:529–544. doi: 10.1006/exer.1994.1047. [DOI] [PubMed] [Google Scholar]
- 24.Hoh H, Schwanengel M. [Continuous intraocular pressure measurement over several days with the Codman Microsensor--a case report] Klin Monbl Augenheildk. 1999;215:186–196. doi: 10.1055/s-2008-1034697. [DOI] [PubMed] [Google Scholar]
- 25.Kaufmann C, Bachmann LM, Robert YC, Thiel MA. Ocular pulse amplitude in healthy subjects as measured by dynamic contour tonometry. Arch Ophthalmol. 2006;124:1104–1108. doi: 10.1001/archopht.124.8.1104. [DOI] [PubMed] [Google Scholar]
- 26.Mottet B, Aptel F, Romanet JP, Hubanova R, Pepin JL, Chiquet C. 24-hour intraocular pressure rhythm in young healthy subjects evaluated with continuous monitoring using a contact lens sensor. JAMA Ophthalmol. 2013;131:1507–1516. doi: 10.1001/jamaophthalmol.2013.5297. [DOI] [PubMed] [Google Scholar]
- 27.Liu JH, Kripke DF, Hoffman RE, et al. Nocturnal elevation of intraocular pressure in young adults. Invest Ophthalmol Vis Sci. 1998;39:2707–2712. [PubMed] [Google Scholar]
- 28.Liu JH, Kripke DF, Twa MD, et al. Twenty-four-hour pattern of intraocular pressure in the aging population. Invest Ophthalmol Vis Sci. 1999;40:2912–2917. [PubMed] [Google Scholar]
- 29.Asrani S, Zeimer R, Wilensky J, Gieser D, Vitale S, Lindenmuth K. Large diurnal fluctuations in intraocular pressure are an independent risk factor in patients with glaucoma. J Glaucoma. 2000;9:134–142. doi: 10.1097/00061198-200004000-00002. [DOI] [PubMed] [Google Scholar]
- 30.Caprioli J, Coleman AL. Intraocular pressure fluctuation a risk factor for visual field progression at low intraocular pressures in the advanced glaucoma intervention study. Ophthalmology. 2008;115:1123–1129.e1123. doi: 10.1016/j.ophtha.2007.10.031. [DOI] [PubMed] [Google Scholar]
- 31.Downs JC, Girkin CA. Lamina cribrosa in glaucoma. Curr Opin Ophthalmol. 2017;28:113–119. doi: 10.1097/ICU.0000000000000354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clayson K, Pan X, Pavlatos E, et al. Corneoscleral stiffening increases IOP spike magnitudes during rapid microvolumetric change in the eye. Exp Eye Res. 2017;165:29–34. doi: 10.1016/j.exer.2017.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qu J, Chen H, Zhu L, et al. High-magnitude and/or high-frequency mechanical strain promotes peripapillary scleral myofibroblast differentiation. Invest Ophthalmol Vis Sci. 2015;56:7821–7830. doi: 10.1167/iovs.15-17848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zou H, Yuan R, Zheng Q, et al. Fluctuations in intraocular pressure increase the trabecular meshwork extracellular matrix. Cell Physiol Biochem. 2014;33:1215–1224. doi: 10.1159/000358691. [DOI] [PubMed] [Google Scholar]
- 35.Quill B, Irnaten M, Docherty NG, et al. Calcium channel blockade reduces mechanical strain-induced extracellular matrix gene response in lamina cribrosa cells. Br J Ophthalmol. 2015;99:1009–1014. doi: 10.1136/bjophthalmol-2014-306093. [DOI] [PubMed] [Google Scholar]
- 36.Leske MC, Heijl A, Hussein M, Bengtsson B, Hyman L, Komaroff E. Factors for glaucoma progression and the effect of treatment: the early manifest glaucoma trial. Arch Ophthalmol. 2003;121:48–56. doi: 10.1001/archopht.121.1.48. [DOI] [PubMed] [Google Scholar]
- 37.The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucoma. Collaborative Normal-Tension Glaucoma Study Group. Am J Ophthalmol. 1998;126:498–505. doi: 10.1016/s0002-9394(98)00272-4. [DOI] [PubMed] [Google Scholar]
- 38.Liu J, He X. Corneal stiffness affects IOP elevation during rapid volume change in the eye. Invest Ophthalmol Vis Sci. 2009;50:2224–2229. doi: 10.1167/iovs.08-2365. [DOI] [PubMed] [Google Scholar]
- 39.Tan JC, Kalapesi FB, Coroneo MT. Mechanosensitivity and the eye: cells coping with the pressure. Br J Ophthalmol. 2006;90:383–388. doi: 10.1136/bjo.2005.079905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kirwan RP, Crean JK, Fenerty CH, Clark AF, O'Brien CJ. Effect of cyclical mechanical stretch and exogenous transforming growth factor-beta1 on matrix metalloproteinase-2 activity in lamina cribrosa cells from the human optic nerve head. J Glaucoma. 2004;13:327–334. doi: 10.1097/00061198-200408000-00011. [DOI] [PubMed] [Google Scholar]
- 41.Morrison JC. Integrins in the optic nerve head: potential roles in glaucomatous optic neuropathy (an American Ophthalmological Society thesis) Trans Am Ophthalmol Soc. 2006;104:453–477. [PMC free article] [PubMed] [Google Scholar]
- 42.Bartolak-Suki E, Imsirovic J, Parameswaran H, et al. Fluctuation-driven mechanotransduction regulates mitochondrial-network structure and function. Nat Mater. 2015;14:1049–1057. doi: 10.1038/nmat4358. [DOI] [PubMed] [Google Scholar]
- 43.Grytz R, Fazio MA, Libertiaux V, et al. Age- and race-related differences in human scleral material properties. Invest Ophthalmol Vis Sci. 2014;55:8163–8172. doi: 10.1167/iovs.14-14029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fazio MA, Grytz R, Morris JS, Bruno L, Girkin CA, Downs JC. Human scleral structural stiffness increases more rapidly with age in donors of African descent compared to donors of European descent. Invest Ophthalmol Vis Sci. 2014;55:7189–7198. doi: 10.1167/iovs.14-14894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fazio MA, Grytz R, Morris JS, et al. Age-related changes in human peripapillary scleral strain. Biomech Model Mechanobiol. 2014;13:551–563. doi: 10.1007/s10237-013-0517-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Girard MJ, Suh JK, Bottlang M, Burgoyne CF, Downs JC. Scleral biomechanics in the aging monkey eye. Invest Ophthalmol Vis Sci. 2009;50:5226–5237. doi: 10.1167/iovs.08-3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Coudrillier B, Tian J, Alexander S, Myers KM, Quigley HA, Nguyen TD. Biomechanics of the human posterior sclera: age- and glaucoma-related changes measured using inflation testing. Invest Ophthalmol Vis Sci. 2012;53:1714–1728. doi: 10.1167/iovs.11-8009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Girard MJ, Suh JK, Bottlang M, Burgoyne CF, Downs JC. Biomechanical changes in the sclera of monkey eyes exposed to chronic IOP elevations. Invest Ophthalmol Vis Sci. 2011;52:5656–5669. doi: 10.1167/iovs.10-6927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grytz R, Siegwart JT., Jr. Changing material properties of the tree shrew sclera during minus lens compensation and recovery. Invest Ophthalmol Vis Sci. 2015;56:2065–2078. doi: 10.1167/iovs.14-15352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tielsch JM, Sommer A, Katz J, Royall RM, Quigley HA, Javitt J. Racial variations in the prevalence of primary open-angle glaucoma. The Baltimore Eye Survey. JAMA. 1991;266:369–374. [PubMed] [Google Scholar]
- 51.Connors KGW, Pintauro WL, Wallin SK, Kilcoyne JT, Cao HH, Nguyen KM, Yurek MT. Implantable self-inflating attenuation device and method for treating ocular pressure spikes. inventors; Cascade Ophthalmics Inc., assignee. US patent 7484510. February 3, 2009.
- 52.Markert JE, Jasien JV, Turner DC, Huisingh C, Girkin CA, Downs JC. IOP. IOP transient impulse, ocular perfusion pressure, and mean arterial pressure relationships in nonhuman primates instrumented with telemetry. Invest Ophthalmol Vis Sci. 2018;59:4496–4505. doi: 10.1167/iovs.18-23802. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.