ABSTRACT
Objective: Our study aimed to determine the national estimates of the 30-day all-cause readmission rate among patients with spontaneous pneumothorax and to investigate the burden of these readmissions in terms of mortality, length of stay and hospitalization costs in the USA.
Methods: We utilized the Nationwide Readmission Database for 2013–2014 and identified adults with a primary diagnosis of spontaneous pneumothorax. We analyzed and reported patient- and hospital-level variables of the study cohort. Our primary outcome was 30-day readmission rate, including the reasons for readmission. Our secondary outcomes included all-cause mortality, resources utilization and predictors of readmissions.
Results: We identified 47,108 index admissions with spontaneous pneumothorax. The 30-day readmission rate was 13.6%. The most common reason for admission was recurrent pneumothorax. In index admissions, the in-hospital mortality rate was 3.1%; whereas, in readmissions, the mortality was higher (4.6%, p < 0.001). Both age group 45–64 (HR: 1.31, 95% CI: [1.15–1.49], p < 0.001) and history of cancer (HR: 1.34, 95% CI: [1.17–1.53], p < 0.001) were found to predict the risk of 30-day readmission.
Conclusion: The 30-day readmission rate in patients with spontaneous pneumothorax was 13.6%, and a recurrent event was the most likely cause. The 30-day readmissions were associated with higher mortality and hospitalization charges. Middle age and history of cancer increase likelihood of 30-day readmission.
KEYWORDS: Pneumothorax, readmission, mortality, NRD, AHRQ database
1. Introduction
Pneumothorax refers to the entry of air or gas into the pleural space with resultant alteration in the negative intrapleural pressure and subsequent lung collapse. Spontaneous pneumothorax (SP) refers to pneumothorax in the absence of any traumatic or iatrogenic causes [1]. SP is further subdivided clinically based on the absence or presence of underlying lung disease into primary SP (PSP) and secondary SP (SSP). SP is a prevalent diagnosis; its estimated incidence ranges from 17–24/100,000 and 1–6/100,000 among males and females, respectively, and recurrence rates ranging from 17% to 54% within 1–6 years [1,2].
Spontaneous pneumothorax is an under-recognized cause of frequent readmissions. With the current trend of increasing health-care costs and unplanned readmissions with overall estimated annual costs of $17.4 billion in the US; we sought to investigate the burden of 30-day readmissions in SP in terms of mortality, length of stay (LOS) and hospitalization costs [3].
2. Patients and methods
2.1. Data source
This is a retrospective analysis based on the 2013–2014 Nationwide Readmissions Database (NRD) which is maintained by the Agency for Healthcare Research and Quality (AHRQ). In the NRD, each year includes approximately 36 million discharges from more than 20 States. These States are geographically dispersed and account for roughly half of the total US resident population and nearly half of all US hospitalizations [4].
The NRD incorporates both patient- and hospital-level information. Discharge diagnoses and procedures are reported for each discharge using the International Classification of Diseases – 9th Revision – Clinical Modification (ICD-9-CM). These data are de-identified and publicly available and thus deemed to be exempt from institutional review board approval.
2.2. Study cohort
We queried the NRD using ICD-9-CM codes for hospital discharges with a primary diagnosis of SP (ICD-9-CM: 512.0, and 512.8x) or emphysematous bleb (ICD-9-CM: 492.0). We excluded patients <18 years of age and elective admissions. Index admissions in December were also excluded, as it would be unknown if a patient were readmitted in January of the following year.
2.3. Study measures and outcomes
We utilized variables that are part of the NRD, including patient-level and hospital-level data. Patients’ comorbidities were evaluated individually and combined utilizing Elixhauser’s comorbidity score, which was calculated by using the 31 weighted Elixhauser’s comorbidity variables and excluding the primary diagnosis for each discharge.
The primary outcome of this study was to determine the rate of 30-day unplanned all-cause readmissions, which was defined as hospitalization for any cause within 30 days of discharge after the index admission. We only considered the first readmission if a patient had multiple readmissions within 30 days. Moreover, we analyzed the primary diagnoses of these readmissions, and several diagnoses were combined to formulate clinically relevant groups.
The secondary outcomes of this study included (a) in-hospital all-cause mortality rates of index admissions; (b) in-hospital all-cause mortality rate during readmissions; (c) resources utilization related to readmissions, including LOS and total hospitalization costs and charges; and (d) independent predictors of readmission.
2.4. Statistical analyses
Statistical analyses were performed utilizing Stata 15.1 for Windows (StataCorp, College Station, Texas). The NRD is based on a sophisticated sampling design that includes stratification, clustering, and weighting. This software permits analyses to produce nationally representative results.
We used weights provided by AHRQ to calculate the national estimates of discharges throughout the US Descriptive statistics were provided for all weighted variables and presented as mean ± standard deviation for continuous variables and as count and percentage for categorical variables. Statistical tests of significance (Student’s t-test for continuous variables and Fischer’s Exact test for categorical variables) were conducted to assess between-group differences. Two-tailed p-values ≤0.05 were considered statistically significant.
Univariate Cox regression analysis was conducted to calculate unadjusted hazard ratios for predictors of readmission, and multivariate Cox regression was used to adjust the results for potential confounders. Multivariate Cox regression models were built by including all confounders that had a p-value of ≤0.20 in univariate analysis.
3. Results
There were approximately 71 million discharges included in the 2013–2014 NRD. We identified 62,171 index admissions with a primary diagnosis of SP. From these; we excluded 4,043 patients aged <18 years, 6,273 elective admissions, and 4,747 index admissions in December of both years. Our final study cohort included 47,108 index admissions (Figure 1), with an annual incidence rate of 33/100,000.
Figure 1.
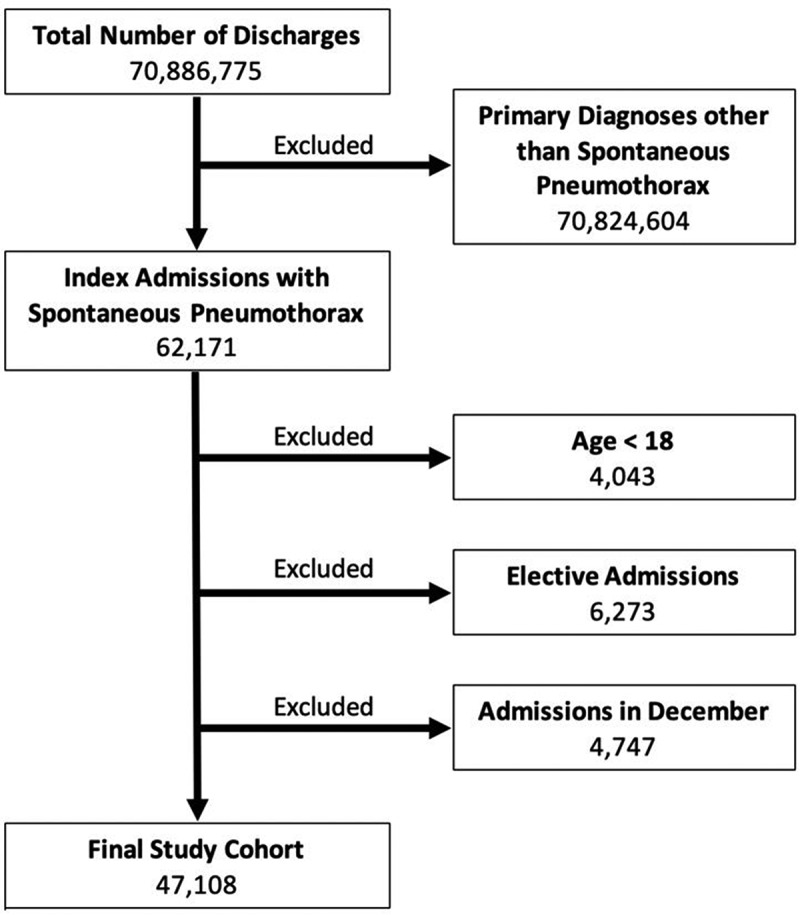
Flow diagram of patients’ selection.
3.1. Baseline characteristics of index admissions
Table 1 demonstrates the baseline characteristics of index admissions; the age distribution is shown in Figure 2; in general, the mean age was 51.7 ± 22.1 years; 70% were males. Medicare was the most common insurance provider (37%) followed closely by private insurances (35%). Fifty-five percent had an Elixhauser’s score of ≥2, with a mean score of 2.2 ± 2.0. The most common comorbidity was chronic pulmonary disease accounting for 53%, followed by hypertension (35%). Approximately 32% were smokers. The patients were roughly equally distributed among the four income quartiles, but most (89%) lived in a metropolitan area. More than half of the patients (57%) were treated in teaching hospitals, and more than two-thirds of the hospitals (68%) were in urban areas. Chest tube placement was performed in 65%, 39% underwent chemical or surgical pleurodesis, and less than 1% had EBV placement. Approximately 19% had an acute respiratory failure, but fewer than 5% required ventilatory support. Less than 1% were transferred to a rehabilitation facility on discharge.
Table 1.
Baseline characteristics of index admissions.
| Characteristics | n (%) |
|---|---|
| Age (years) | |
| Mean ± SD | 51.7 ± 22.1 |
| 18–44 | 18,184 (38.6) |
| 45–64 | 13,379 (28.4) |
| ≥65 | 15,545 (33.0) |
| Gender | |
| Male | 32,881 (69.8) |
| Female | 14,227 (30.2) |
| Insurance provider | |
| Medicare | 17,336 (36.8) |
| Medicaid | 6,360 (13.5) |
| Private | 16,299 (34.6) |
| Self-payer/uninsured | 7,113 (15.1) |
| Comorbidities | |
| Smoking | 15,263 (32.4) |
| Chronic pulmonary disease | 24,920 (52.9) |
| Diabetes | 4,522 (9.6) |
| Hypertension | 16,346 (34.7) |
| Heart failure | 3,439 (7.3) |
| Renal failure | 2,685 (5.7) |
| Liver disease | 1,130 (2.4) |
| HIV/AIDS | 141 (0.3) |
| Obesity | 1,837 (3.9) |
| Anemia | 895 (1.9) |
| Cancer | 4,287 (9.1) |
| Elixhauser’s comorbidity score (points) | |
| Mean ± SD | 2.2 ± 2.0 |
| <2 | 21,010 (44.6) |
| ≥2 | 26,098 (55.4) |
| Median income in patient’s ZIP code | |
| $1–$39,999 | 12,984 (28.0) |
| $40,000–$50,999 | 12,984 (28.0) |
| $51,000–$65,999 | 10,943 (23.6) |
| ≥$66,000 | 9,459 (20.4) |
| Patient’s residence | |
| Large metropolitan areas with ≥1 million residents | 24,449 (51.9) |
| Small metropolitan areas with <1 million residents | 17,524 (37.2) |
| Micropolitan areas | 3,957 (8.4) |
| Not metropolitan or micropolitan (non-urban) | 1,178 (2.5) |
| Hospital bed size | |
| Small | 6,360 (13.5) |
| Medium | 12,012 (25.5) |
| Large | 28,736 (61.0) |
| Teaching hospital | 26,852 (57.0) |
| Urban hospital | 31,986 (67.9) |
| Chest tube placement | 30,573 (64.9) |
| Chemical/surgical pleurodesis | 18,513 (39.3) |
| EBV placement | 188 (0.4) |
| Acute respiratory failure | 8,809 (18.7) |
| Ventilatory support | 2,308 (4.9) |
| Discharge to a rehabilitation facility | 141 (0.3) |
Figure 2.
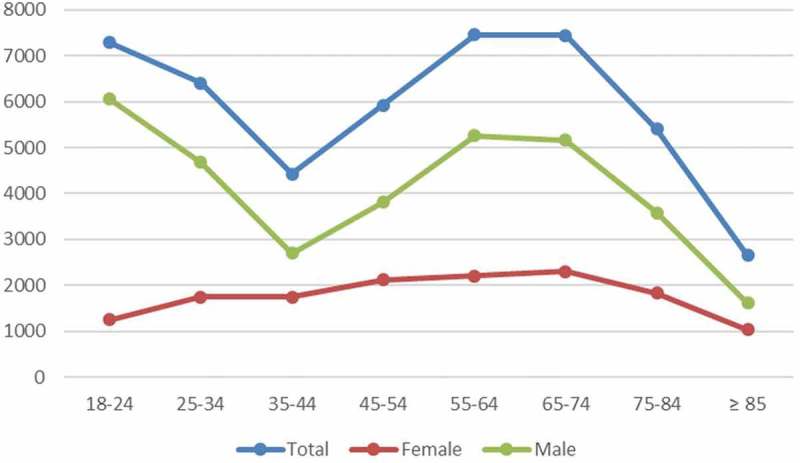
Age distribution at index admission by gender.
3.2. Thirty-day readmission rate
Among index admissions, 45,642 were discharged alive; From these; 6,214 patients were readmitted within 30 days, leading to a 30-day all-cause readmission rate of 13.6%. The average time to readmission was 17.9 ± 10.5 days. The highest rate of 30-day readmissions was observed in the age >45 years, p < 0.001. The majority were males (68% vs. 32%, p = 0.028); However, the readmission rates among males and females were 13.3% and 14.6%, respectively (p < 0.001).
3.3. Most common reasons for readmission
The most common five reasons for readmission are listed in Table 2. These collectively accounted for 69% of all readmissions. Respiratory failure was among the most common primary diagnoses; however, most cases of respiratory failure were due to acute COPD exacerbation, pneumonia or septicemia.
Table 2.
The most common reasons for 30-day readmission.
| R | Diagnosis | n (%) |
|---|---|---|
| 1 | Pneumothorax | 3,435 (55.3) |
| 2 | Acute exacerbation of COPD | 270 (4.3) |
| 3 | Pneumonia | 260 (4.2) |
| 4 | Septicemia | 206 (3.3) |
| 5 | Pleural effusion | 90 (1.4) |
3.4. Mortality among index admissions and readmissions
There were 1,466 patients admitted with an SP who died during the index hospitalization. Consequently, the in-hospital all-cause mortality rates were 3.1%. Whereas in readmissions, 286 patients died during the hospitalization, resulting in a mortality rate of 4.6%. The mortality rate during readmissions was significantly higher than that of the index admissions (4.6% vs. 3.1%, p < 0.001).
3.5. Health-care resources utilization
The mean LOS was almost similar between index admissions and readmissions and did not show a statistically significant difference (6.6 ± 7.0 days vs. 6.4 ± 6.3 days, respectively; p = 0.145). The mean total cost of hospitalization was slightly higher in readmitted patients but did not reach statistical significance ($13,961 ± $15,789 vs. $13,420 ± $18,880, respectively; p = 0.112). However, the mean total charges, which were higher in readmitted patients compared to index admissions, were statistically significant ($52,118 ± $69,490 vs. $48,947 ± $74,254, respectively; p = 0.028).
Fewer chest tubes were placed during readmissions compared to index admissions (36% vs. 65%, respectively; p < 0.001). On the other hand, the frequency of chemical/surgical pleurodesis was higher in readmissions (44% vs. 39%, respectively; p < 0. 011). There was no statistically significant difference in endobronchial valve placement between index admissions and readmissions (0.4% vs. 0.6%, respectively; p = 0.113).
In readmissions, the total annual hospitalization costs were $42.6 million, and total yearly hospitalization charges were $159 million.
3.6. Predictors of 30-day readmission
On multivariate analysis (Figure 3), independent predictors associated with increased odds of 30-day readmission included age 45–64 years (HR: 1.31, 95% CI [1.15–1.49], p < 0.001), and cancer (HR: 1.34, 95% CI [1.17–1.53], p < 0.001). Factors associated with decreased odds of 30-day readmission were self-payer/uninsured (HR: 0.73, 95% CI [0.61–0.88], p = 0.001), smoking (HR: 0.75, 95% CI [0.67–0.82], p < 0.001), residency in a small metropolitan area (HR: 0.90, 95% CI [0.85–0.99], p = 0.023), chemical/surgical pleurodesis during index admission (HR: 0.632, 95% CI [0.57–0.70], p < 0.001).
Figure 3.
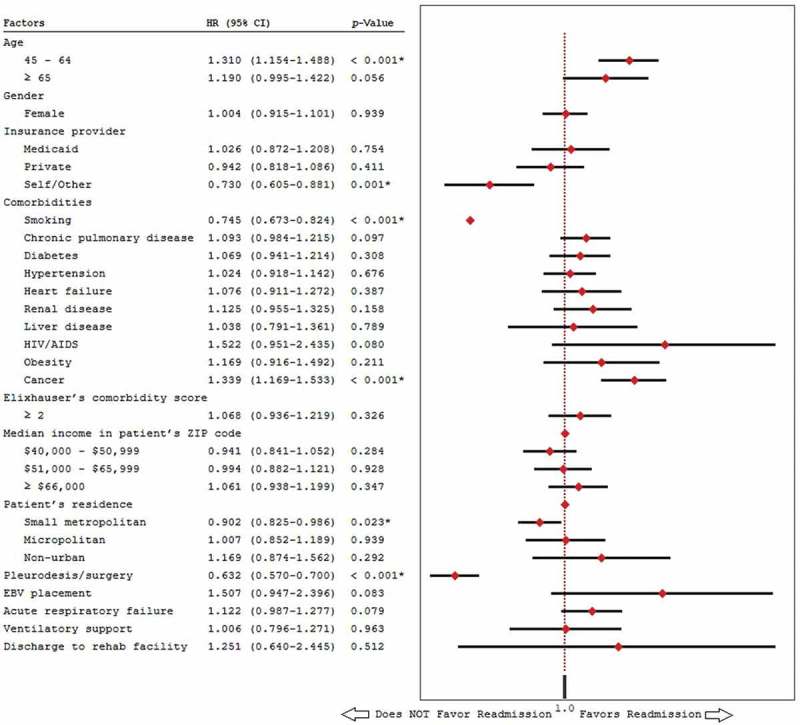
Multivariate analysis of predictors of 30-day readmission.
4. Discussion
Spontaneous pneumothorax carries a significant impact on morbidity, mortality, and cost which is further complicated by a high risk of recurrence [2]. To our knowledge, our analysis is the first to offer a nationally representative readmission data for patients with SP from a large readmission database in the US.
4.1. Epidemiology of index admissions
We identified three large-scale epidemiological studies on SP: one from England (Gupta et al., n = 22,749) [5], one from France (Bobbio et al., n = 59,637) [6] and one from Germany (Schnell et al., n = 52,738) [7]. Compared to these studies, we also observed bimodal age distribution in males similar to previous studies, with the first peak incidence at 18–24 and a second peak at 55–74. However, our study population had the highest mean age and likely due to the high prevalence of chronic lung diseases (53%) and subsequent higher incidence of SSP. With an annual incidence of 33/100,000, we also report the highest incidence of SP among all these studies. Our female-to-male ratio of 1:2.3 supports the previous finding of male predominance in SP [5–7].
4.2. Readmissions and recurrence
It is challenging to predict lifetime recurrence rates, but it is known to be higher in SSP compared to PSP and is most common among smokers, COPD and AIDS patients [1,8]. With recurrence rates, in the literature, ranging from 17%-54% within 1–6 years; it becomes difficult to ascertain the proper management [2,9]. If the recurrence rate is indeed above 50%, then the treatment approach would significantly vary and would make clinicians inclined towards aggressive interventions at an early stage which was shown to have fewer recurrences [10]. In our study, the 30-day readmission rate of 13.6%, and >50% were readmitted with recurrent pneumothorax. Since our study was conducted in a readmission database, we were unable to obtain the recurrence rate directly. However, we can conclude that the recurrence rate in our study was 7.5%. Additionally, it showed that patients were more likely to undergo pleurodesis during readmission than index admission.
4.3. In-hospital mortality
Mortality with SP is generally considered low. Schnell et al. reported the disease-specific death at 0.094/100,000 (0.7%) [7,11]. The overall mortality rate from 2011 to 2015 was 3%, which is similar to our mortality rate in index admissions.
Takahashi F. et al. revealed that pneumonia and respiratory/heart failure were significant causes of death within 90 days of SSP in the elderly [12]. In our study, the in-hospital mortality rate was 4.6% in readmissions with the most readmission being a recurrence, COPD exacerbation, pneumonia, septicemia, or respiratory failure.
4.4. Predictors of readmission
There are limited studies on predictors of readmission for SP. Previous studies reported factors like female sex, low BMI, and dystrophic lungs on radiology as a risk for recurrences while smoking cessation was associated with lower recurrence rates [10]. Current literature lacks studies to predict outcomes and establish a risk stratification system.
Our study identified middle-age (45–64) and cancer leading to an increased risk of 30-day readmission. Surprisingly, despite the evident relation between smoking and high incidence and recurrent of pneumothorax, smoking in our study was associated with a decreased risk of 30-day readmission, which concurs with the finding of Steger et al. [13]. Whether the inspiratory efforts during smoking help prevent early recurrence or not is difficult to postulate. Moreover, there are reports of SP secondary to deep breathing, including inceptive spirometry use [14]. We additionally noted that patients who underwent pleurodesis are less likely to be readmitted within 30 days, which was also observed in other studies [15,16]. Early pleurodesis at the first presentation is currently under evaluation, given the high recurrence rates of SP [17].
There is a need for validated outcome predictors and clear evidence on the optimal approach to reduce recurrences and re-hospitalizations. For the time being, it is difficult to recommend aggressive intervention at first presentation, in the absence of other indications for pleurodesis, without considering the outcomes of these studies.
4.5. Study limitations
When interpreting the findings of the study, one should consider these limitations: The use of an administrative database is limited and does not allow for adjustment of all possible confounders. Generally, rates are likely to be underestimated because the NRD does not capture out-of-hospital deaths, neither it tracks out-of-state admissions nor readmissions across the years. We were not able to isolate PSP from SSP, nor we could distinguish chemical vs. surgical pleurodesis using reported ICD-9-CM codes. Despite these limitations, this study describes a real-world experience that complements and confirms the findings of previous studies.
5. Conclusion
The 30-day readmission rate in SP was 13.6%, with a recurrent event being the most likely cause. Readmissions were associated with higher mortality and additional total annual hospitalization costs of $42.6 million. Close observation and strict management of the identified causes such as COPD and infections might result in a reduction in readmissions.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Baumann MH, Strange C, Heffner JE, et al. Management of spontaneous pneumothorax: an American College of Chest Physicians Delphi consensus statement. CHEST. 2001;119(2):590–602. DOI: 10.1378/chest.119.2.590. [DOI] [PubMed] [Google Scholar]
- [2].Savitsky E, Oh SS, Lee JM.. The evolving epidemiology and management of spontaneous pneumothorax. Jama. 2018October9;320(14):1441–1443. . PubMed PMID: 30304415; eng. [DOI] [PubMed] [Google Scholar]
- [3].Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418–1428. DOI: 10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- [4].The Agency for Healthcare Research and Quality Nationwide Readmissions Database (NRD) website. [cited 2019 April7]. Available from:https://www.hcup-us.ahrq.gov/nrdoverview.jsp
- [5].Gupta D, Hansell A, Nichols T, et al. Epidemiology of pneumothorax in England. Thorax. 2000August;55(8):666–671. PubMed PMID: 10899243; PubMed Central PMCID: PMCPMC1745823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bobbio A, Dechartres A, Bouam S, et al. Epidemiology of spontaneous pneumothorax: gender-related differences. THORAX. 2015;70(7):653–658. DOI: 10.1136/thoraxjnl-2014-206577. [DOI] [PubMed] [Google Scholar]
- [7].Schnell J, Koryllos A, Lopez-Pastorini A, et al. Spontaneous pneumothorax: epidemiology and treatment in Germany between 2011 and 2015. DTSCH AERZTEBL INT. 2017;114(44):739 DOI: 10.3238/arztebl.2017.0739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Costumbrado J, Ghassemzadeh S. Pneumothorax, Spontaneous. StatPearls [Internet], StatPearls Publishing; 2017. [PubMed] [Google Scholar]
- [9].Schramel F, Postmus P, Vanderschueren R. Current aspects of spontaneous pneumothorax. Eur Respir J. 1997;10(6):1372–1379. DOI: 10.1183/09031936.97.10061372. [DOI] [PubMed] [Google Scholar]
- [10].Walker SP, Bibby AC, Halford P, et al. Recurrence rates in primary spontaneous pneumothorax: a systematic review and meta-analysis. Eur Respir J. 2018September;52(3). PubMed PMID: 30002105; eng DOI: 10.1183/13993003.00864-2018. [DOI] [PubMed] [Google Scholar]
- [11].Schnell J, Beer M, Eggeling S, et al. Management of spontaneous pneumothorax and postinterventional pneumothorax: German S3-guideline. Am J Respir Crit Care Med 2018;143(S 01):S12–S43. [DOI] [PubMed] [Google Scholar]
- [12].Takahashi F, Takihara T, Nakamura N, et al. Prognosis of secondary pneumothorax in the elderly. Am J Respir Crit Care Med. 2018. Available from: https://www.atsjournals.org/doi/pdf/10.1164/ajrccm-conference.2018.197.1_MeetingAbstracts.A1531 [Google Scholar]
- [13].Steger V, Sostheim U, Leistner M, et al. Recurrence of spontaneous pneumothorax is not associated with allegedly risk-prone lifestyle conduct. Ann Thorac Cardiovasc Surg. 2018February20;24(1):25–31. PubMed PMID: 29279462; PubMed Central PMCID: PMCPMC5833137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kenny JE, Kuschner WG. Pneumothorax caused by aggressive use of an incentive spirometer in a patient with emphysema. Respir Care. 2013July;58(7):e77–9. . PubMed PMID: 23232741. [DOI] [PubMed] [Google Scholar]
- [15].Carson-Chahhoud KV, Wakai A, van Agteren JE, et al. Simple aspiration versus intercostal tube drainage for primary spontaneous pneumothorax in adults. Cochrane Database Syst Rev. 2017September7;9:Cd004479 PubMed PMID: 28881006; eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kim MJ, Park I, Park JM, et al. Systematic review and meta-analysis of initial management of pneumothorax in adults: intercostal tube drainage versus other invasive methods. PLoS One. 2017;12(6):e0178802 PubMed PMID: 28640890; PubMed Central PMCID: PMCPMC5480863. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Plojoux J, Froudarakis M, Janssens JP, et al. New insights and improved strategies for the management of primary spontaneous pneumothorax. Clin Respir J. 2019April;13(4):195–201. PubMed PMID: 30615303; eng. [DOI] [PubMed] [Google Scholar]


