Abstract
Aim: Renal replacement therapy was primary treatment for end stage kidney (ESRD) patients. Numbers of studies comparing peritoneal dialysis (PD) and hemodialysis (HD) yielded inconsistent results. The aim of this study was to assess the mortality risk between diabetic PD patients and those in HD.
Methods: We included cohort studies comparing the risk of death among diabetic ESRD patients who receiving peritoneal dialysis or hemodialysis by searching Medline and Embase. Overall estimates were calculated using the random-effects model.
Results: Seventeen studies were included in the meta-analyses. Mortality comparison between PD and HD in the diabetic ESRD patients showed PD significantly increased mortality rate (hazard ratio (HR) 1.20; 95% confidence interval (CI) 1.10–1.30; I2 = 89.1%). The overall HR using an intention-to-treat analysis was 1.23 with 95% CI (1.13 to 1.34). Meta-regression demonstrated PD patients from Asian country were associated with increase in mortality risk (coefficient = 0.270, SE = 0.112, p = .033).
Limitation: The high heterogeneity in our meta-analyses undermined the robustness of the findings.
Conclusion: ESRD patients with diabetes may benefit more from HD than PD.
Keywords: Hemodialysis, peritoneal dialysis, chronic kidney disease, diabetes, survival, meta-analysis
Introduction
Diabetes has become the most common cause of end-stage renal disease (ESRD) [1]. Over the past few decades, patients requiring renal replacement therapy have been rapidly increasing worldwide, which imposed a tremendous burden on both family and society. Compared to normal population, ESRD patients have significant higher mortality rate [2]. Renal transplantation is the optimal therapy for ESRD and limited by the available organs. The most common treatments for ESRD are hemodialysis (HD) and peritoneal dialysis (PD). It is estimated that more than 2.2 million patients receive dialysis globally in 2020 [3]. However, the comparison of survival rates between PD and HD are still controversial.
One randomized controlled trial (RCT) has been published so far to compare outcomes in PD or HD patients [4]. The 3-year mortality rate was comparable between the two groups. However, the fact that only 38 patients finally recruited in the trial probably gave it insufficient statistic power to identify the survival difference for PD versus HD. The recruitment problem implied the difficulties in conducting such RCT in the future, as most patients prefer to make their own decision instead of being randomized to a modality.
Increased risk of cardiovascular events was observed in patients who had both ESRD and diabetes [5]. Numbers of studies indicated that ESRD patients with diabetes suffer higher death risk than ESRD patients without diabetes [6–8]. Vascular access may difficult to achieve because of diabetes-related atherosclerotic calcification in HD while continuous exposure to high glucose load might contribute to cardiac compromise and glucose imbalance in PD. It is vital to address which modality is better regarding the impact on mortality of ESRD patients especially in patients with diabetes and to synthesize existing knowledge to inform clinical practice and health policy. In the absence of extensive RCT data to compare survival outcomes associated with HD versus PD, observational studies of preexisting cohorts have had to suffice. Therefore, in the present work, we performed a comprehensive meta-analysis to compare the mortality of PD and HD in ESRD patients with diabetes.
Materials and methods
Data source and search strategy
Medline, Embase databases were searched for relevant articles through search strategies provided by a university librarian with retrieval deadline of April 2019. Keywords and corresponding medical subject headings were terms describing ‘mortality’ or ‘survival’ or ‘death’, ‘diabetes’, ‘dialysis modality’, ‘hemodialysis’, and ‘peritoneal dialysis’. The reference lists of all eligible articles and recent reviews on the subject were scanned to identify further potential studies. No language restriction was applied in the search. The object was carried out according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and the Meta-analysis of Observational Studies in Epidemiology (MOOSE) [9,10]. The protocol for this meta-analysis was registered with PROSPERO (Website: https://www.crd.york.ac.uk/PROSPERO; Registration number: CRD42018085852).
Study selection and data extraction
We included cohort studies comparing the risk of death among ESRD patients with diabetes who underwent peritoneal dialysis or hemodialysis. The outcome we focused was mortality. Diabetes mellitus was considered either as being the cause of ESRD or a comorbidity. The outcome of interest was all-cause mortality after the initiation of dialysis therapy.
Studies were excluded if they: (1) reported no hazard ratio (HR) on mortality; (2) were supplement, abstract, comments, editorials or letters; (3) reported estimates on mortality with the same or overlapping data; (4) included only home HD patients in the HD group; (5) included only automated peritoneal dialysis (APD) patients or patients using icodextrin in the PD group.
The suitable studies with the largest number of cases or latest publication were selected to avoid duplication. No follow-up duration restrictions were applied. Two authors independently selected all relevant studies based on inclusion and exclusion criteria (W.H. Chen and J. Xue). The articles with discrepancies were resolved by consensus or reviewed by a third author (Q.L. Zhou). Afterward, the following data was extracted independently using standardized data extraction forms: the first author’s name, year of publication, country, the source of data or name of cohort, number of patients in both PD and HD groups, follow up duration, stratifying factors, survival estimates (hazard ratio). Discrepancies were solved by discussion.
Quality assessment
The Newcastle-Ottawa scale was used for quality appraisal of included studies by two authors (W.H. Chen and J. Xue) independently [11]. The scale had three main domains with quality score: the selection of the study groups (0–4 points), the comparability of the groups (0–2 points), and the ascertainment of outcome (0–3 points). A ‘high’ or ‘unclear’ risk of bias was scored ‘0’ while a ‘low’ risk of bias was scored ‘1’. A score above five points (including 5) was deemed as high quality.
Statistical analysis
Meta-analyses were conducted using STATA (STATA version 13.0, StataCorp LP, College Station, TX, USA). All survival or mortality estimates for stratifications in individual studies were collected. A random-effects model was applied considering the confounding in observational studies. Overall HRs and 95% confidence intervals for mortality rates were calculated. For studies that only provided relevant data of subgroups (i.e., separate estimates for women and men), we combined within-study survival estimates using random-effects method for further meta-analysis. Between-study heterogeneity across studies was estimated by the I2 statistic [12]. The estimates corresponding to the longest follow up duration were selected for meta-analysis of comparison between HD and PD dialysis. We conducted meta-regression analyses and subgroup analyses to explore the heterogeneity. We performed sensitivity analyses by omitting one single study from the overall pooled analysis each time to evaluate the stability of the results. Publication bias was assessed by Egger’s test [13].
Results
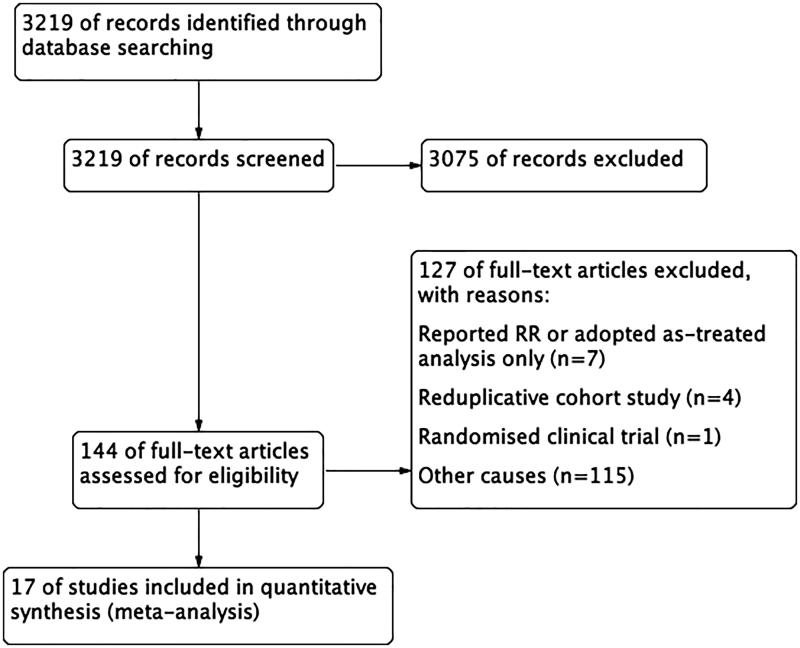
The flow chart of the literature search and study selection was presented in Figure 1. A total of 3219 potential relevant studies without duplication were searched from both Medline and Embase databases. After title and abstract evaluation, 3075 irrelevant studies were excluded. The left 144 publications underwent further review while 127 of them were removed. Among the studies being removed, seven studies reported RR only [14–20], four were reduplicative cohort study [21–24], one was randomized clinical trial [4], the remain were excluded for other causes, such as no comparison groups, no relevant outcomes or no results of interest reported. Finally, a total of 17 studies were included in our analyses [25–41].
Figure 1.
Study flow diagram.
Characteristics of included studies
The characteristics of the studies and basic information of the subjects were listed in Table 1. Among them, four studies were from North America [33,35,37,41], six from Europe [26,27,31,32,36,39], six from Asia [25,28–30,38,40], and one from Oceania [34]. The studies were published between 2007 and 2019 and included a total of 504 304 dialysis patients among whom 62 462 patients were treated with peritoneal dialysis, 441 842 patients underwent hemodialysis. The number of patients enrolled in studies ranged from 230 to 340 280. The follow up duration was 15 months to as long as ten years.
Table 1.
Characteristics of included studies.
| Author | Publication year | Location | Database | PD patients | HD patients | Enrollment period (year) | Follow-up duration (year) |
|---|---|---|---|---|---|---|---|
| Chang | 2013 | Korea | Gachon University Gil Hospital | 118 | 112 | 2000–2009 | 10 |
| Couchoud | 2007 | France | The French Renal Epidemiology and Information Network (REIN) registry | NR | NR | 2002–2005 | 2 |
| Heaf | 2014 | Danmark | The Danish Nephrology Registry (DNR) | 916 | 1822 | 1990–2010 | 6 |
| Huang | 2008 | China, Taiwan | Taiwan Renal Registry | 761 | 16388 | 1995–2002 | 10 |
| Kim | 2017 | Korea | The Korean Health Insurance Review and Asessment Service (HIRA) database | 3996 | 12190 | 2005–2008 | 2 |
| Lee | 2009 | China, Taiwan | Chang Gung Memorial Hospital, Keelung, Taiwan | 79 | 437 | 1991–2005 | 15 |
| Liem | 2007 | Netherlands | The Dutch End-Stage Renal Disease Registry (RENINE) | 928 | 1615 | 1987–2002 | 1.3 |
| Luijtgaarden | 2016 | Europe | The European Renal Association–European Dialysis and Transplant Association (ERA-EDTA) | 6769 | 24594 | 1993–2007 | 5 |
| Lukowsky | 2013 | USA | The United States Renal Data System (USRDS), the DaVita database | 747 | 13863 | 2001–2006 | 1.7 |
| Marshall | 2014 | New Zealand | The Australian and New Zealand Dialysis and Transplant Registry (ANZDATA) Registry | 404 | 246 | 1997–2011 | 3 |
| Mehrotra | 2011 | USA | The United States Renal Data System (USRDS) | 30270 | 310010 | 1996–2004 | 5 |
| Mircescu | 2014 | Romania | The Romanian Renal Registry | 194 | 1246 | 2008–2011 | 3 |
| Nesrallah | 2016 | USA | The United States Renal Data System (USRDS) | 768 | 768 | 2004–2011 | 1.9 |
| Sung Woo Lee | 2019 | Korea | The National Health Insurance Service database (NHIS) | 10370 | 44809 | 2004–2015 | 5 |
| Waldum-Grevbo | 2015 | Norway | The Norwegian Renal Registry | 200 | 209 | 2005–2012 | 5 |
| Wang | 2016 | China, Taiwan | The National Health Insurance Research Database (NHIRD) of Taiwan | 327 | 328 | 2000–2010 | 2.6–2.8 |
| Yeates | 2012 | Canada | The Canadian Organ Replacement Register (CORR) | 5615 | 13205 | 1991–2007 | 5 |
NR: Non-reported.
Study quality
The assessment of risk of bias in the included studies is summarized in Supporting Information table. The overall quality of included studies was high with quality score from 6 to 9. However, the included studies may have high risk of allocation bias or selection bias due to their observational design.
Mortality comparison between HD and PD in diabetic ESRD patients
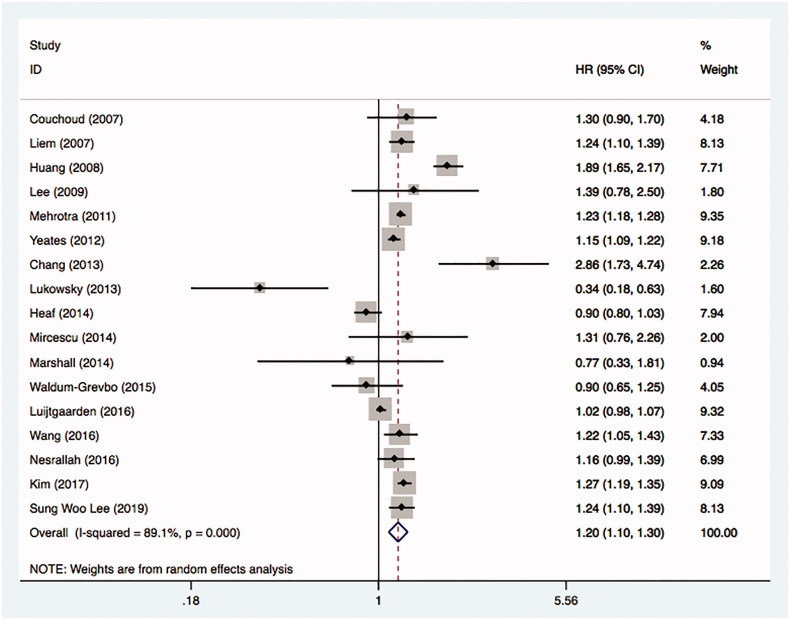
A total of 17 studies were included in the analysis. Figure 2 was the forest plot showed the combined results. The overall HR was 1.20 with 95% CI (1.10 to 1.30), which indicated hemodialysis had lower mortality risk than peritoneal dialysis in ESRD patients with diabetes. However, the heterogeneity between studies was high with the estimate for I2 equals to 89.1%.
Figure 2.
Comparative mortality of diabetic ESRD patients treated with HD and PD.
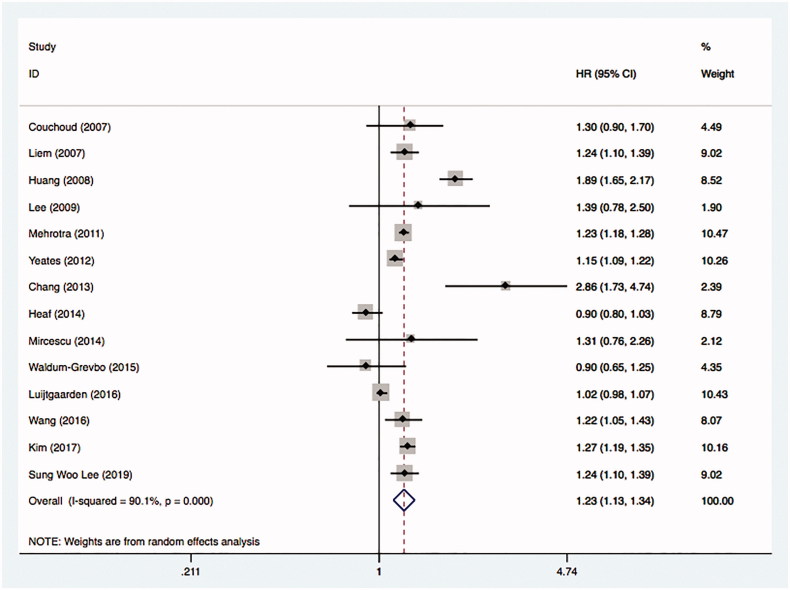
A total of 14 studies using an intention-to-treat framework were included in the analysis [25–32,35,36,38–41]. The comparison showed the overall HR was 1.23 with 95% CI (1.13 to 1.34). The heterogeneity between studies was significant with the estimate for I2 equals to 90.1% (Figure 3).
Figure 3.
Comparative mortality of diabetic ESRD patients treated with HD and PD by intention-to-treat principle.
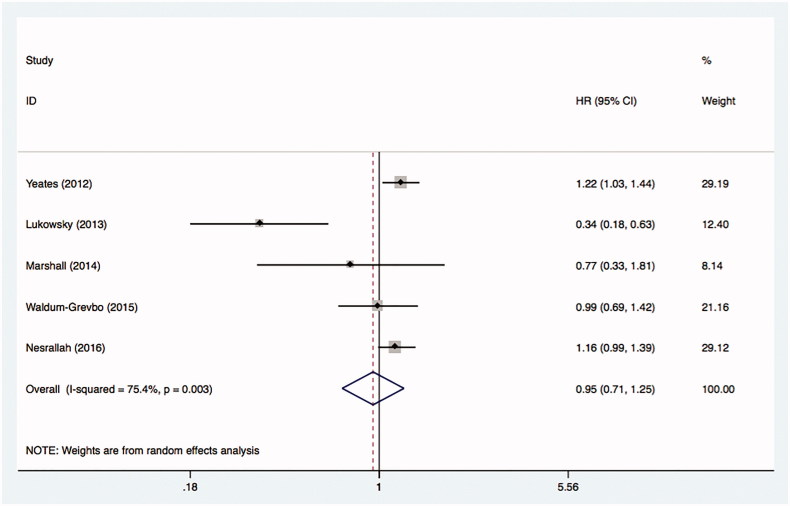
A total of five studies using an as-treated framework were included in the analysis [33,34,37,39,41]. Figure 4 demonstrated the combined results was 0.95 with 95% CI (0.71 to 1.25). The estimate for I2 equals to 75.4%.
Figure 4.
Comparative mortality of diabetic ESRD patients treated with HD and PD by as-treated principle.
Meta-regression and subgroup analyses
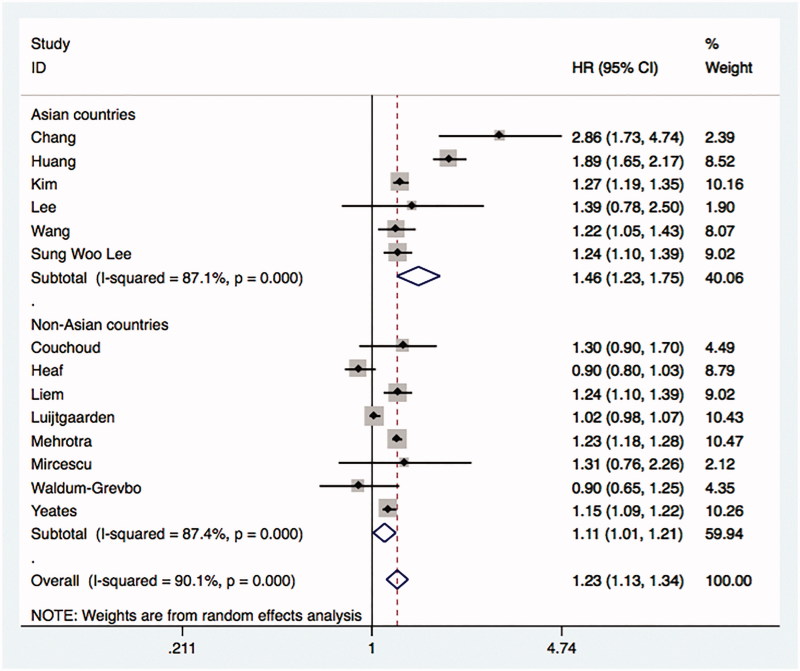
The high heterogeneity was presented in our meta-analysis. Meta-regression analyses were performed. Comparing with patients from non-Asian countries, PD patients from Asian countries were associated with increased mortality risk (coefficient = 0.270, SE = 0.112, p = .033). No significant association was observed in publication year (p = .245), duration of follow up period (p = .125).
We did the subgroup analyses by regions to explore the potential sources of heterogeneity. The pooled HR of PD compared with HD was 1.46 (95% CI 1.23 to 1.75) in Asian countries while the HR was 1.11 (95% CI 1.01 to 1.21) in non-Asian countries. The heterogeneity was still significant in both two subgroups (Figure 5). The subgroup analyses by publication year and duration of follow up were seen in Supporting Information.
Figure 5.
The summary estimates of subgroups by regions.
Sensitivity analysis and publication bias
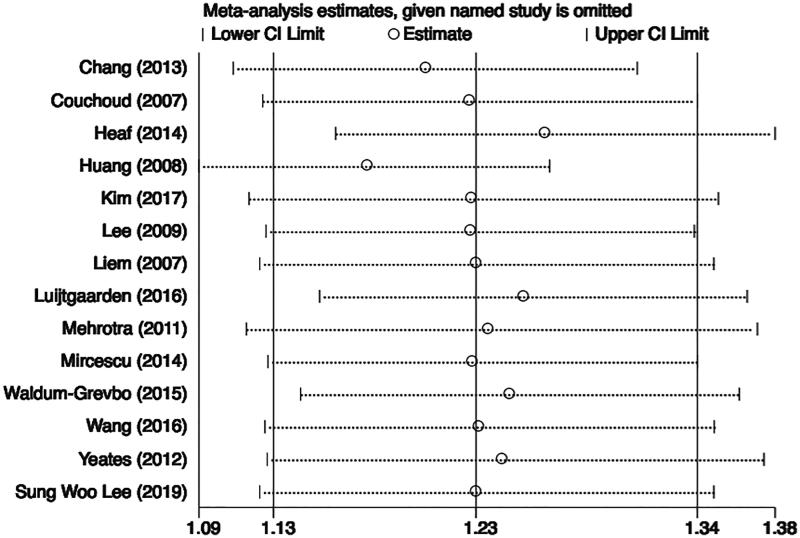
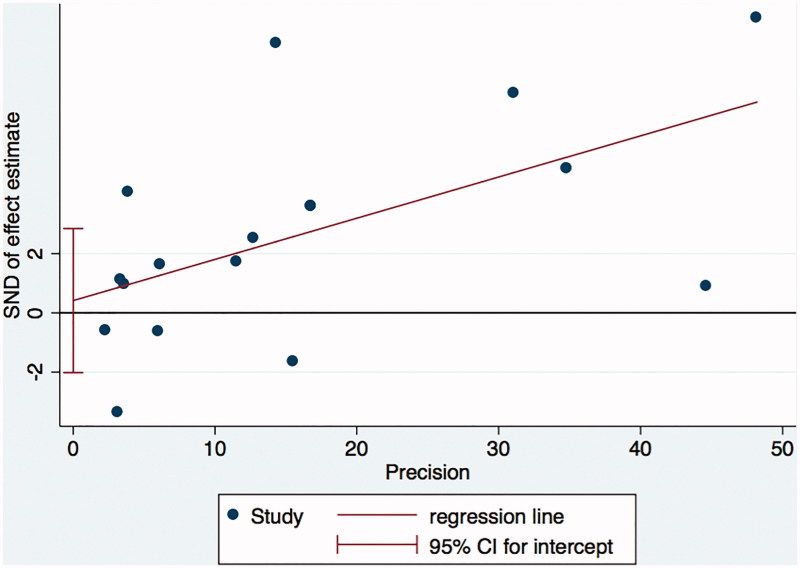
Sensitivity analysis showed the meta-analysis was low sensitivity and the overall results were stable and reliable (Figure 6). Egger test showed no publication bias (p = .722) (Figure 7).
Figure 6.
The sensitivity analysis.
Figure 7.
Publication bias using Egger test.
Discussion
The present systematic review and meta-analyses of 17 cohort studies found that HD dialysis might be superior to PD dialysis in diabetic ESRD patients. PD patients from Asian countries were probably associated with higher mortality risk comparing to PD patients from non-Asian countries.
NECOSAD study, which is the only RCT to date, failed to demonstrate a difference in mortality between PD and HD [4]. Difficulties in recruiting patients impede researchers to conduct a RCT with enough statistical power. Considering this, observational studies need to suffice. A previous meta-analysis by Han et al compared PD with HD in elderly ESRD patients [42]. The survival benefit from HD was significant in subgroup with diabetes. The pooled estimates comparing PD and HD for mortality was 1.26 (95% CI, 1.13–1.40) in elderly diabetic patients. The result was similar with ours. Furthermore, our studies indicate that HD showed lower mortality rate in ESRD patients with diabetes regardless of age. A review by Couchoud systematically discussed the available evidence concerning the modality comparison in diabetic patients with ESRD. The author argued it is not convincing to support a particular dialysis modality as first choice treatment in dialysis patients with diabetes [2]. Even though heterogeneity exists between studies, the results of meta-analyses could still provide us evidence to choose between HD and PD in diabetic patients.
In the subgroup analyses, we found diabetic PD patients from Asian countries had increased mortality risk comparing patients from non-Asian countries, which were mostly western countries. Studies showed no significant difference in survival between Asian and Caucasian PD patients [43,44]. A study by Cheng et al. compared mortality rate among HD patients in China and the US. The authors found Chinese HD patients had a survival benefit compared with patients in the US [45]. We assumed the higher HR of PD compared with HD in Asia could be the explained by the better survival benefit of HD in Asian. However due to the lack of related data in our analyses, we cannot explore this subject further.
When the data was pooled by analysis type (‘intention-to-treat’ or ‘as-treated’ analysis), we observed that HD shows a significant survival benefit over PD using intention-to-treat analysis while no difference in mortality was observed between the two groups by as-treated analysis. The reason for that maybe modality changes from PD to HD. A ‘PD first’ approach has been suggested for ESRD patients in many areas. Numbers of patients who initially went through PD turned to HD for various causes such as infections. These patients experienced increased mortality rate. Modality turnover and related increased death risk would lower the statistical power using as-treated analysis to detect the difference between the two groups. Death that ought to be in the PD group was assign to the HD group in as-treated analysis. Observing such phenomenon, we prefer the intention-to-treat analysis principle over as-treated analysis.
Those who need to start renal replacement therapy opt to consider the advantages, drawbacks and contradictions for each modality. PD needs no vascular access and provides continuous slow ultrafiltration. Some reports have suggested that residual renal function and urine output is better preserved among patients treated with PD as compared to HD [46,47], but it is still on debate. From economical view, PD costs approximately 30–40% less than HD and eases the burden on health care system [48]. As a result, ‘PD-First’ or ‘PD-Favored’ strategy was implemented for the ESRD patients in many areas [49–51]. However, PD might be associated with higher prevalence of infection, inadequate dialysis, inadequate volume control and catheter problems. All of the PD patients received mainly traditional glucose-based solutions, high in glucose degradation products (GDPs). GDPs were thought to induce apoptosis of peritoneal mesothelial cells [52]. Poor glycemic control is thought to associate with poor outcomes in dialysis patients [53,54]. Factors like inflammation, solution bioincompatibility, acidosis, or hyperglycemia were also affected by PD outcomes [55,56]. The diabetic population was reported to have higher risk of peritonitis, ultrafiltration failure, insulin resistance, worse glycemic control, and lower survival rate compared to non-diabetic patient on PD [57,58]. Above all, these mechanisms might explain our results, which suggested the higher mortality risk in diabetic PD patients compared with those in HD.
Limitations
To the best of our knowledge, this is the first meta-analysis to assess the effect of dialysis modality on mortality in the diabetic ESRD patients. However, our study has some limitations. First, the included studies were observational design instead of randomized clinical trials. Patients were non-randomly assigned to either peritoneal dialysis or hemodialysis. Substantial confounders from selection and attrition bias may have impact on the outcomes. Even though most studies attempt to minimize the adverse effects through adjustment, unknown confounders or unmeasured confounders may still undermine the robustness of the outcomes. Second, the between study heterogeneity include patients’ profile, study year, statistical approach, follow up duration, and specific dialysis modality. There was great advance in PD therapy for diabetic patients after the availability of icodextrins, APD and biocompatible fluids [59,60]. Intensive hemodialysis or home hemodialysis had also been proposed with potential survival advantage over conventional hemodialysis [61]. However, most included studies compared all PD patients with HD patients irrespective of specific dialysis techniques. As a consequence, no subgroup analysis of specific dialysis techniques was performed. The majority of dialysis patients still using dextrose dialysate or underwent conventional hemodialysis, especially in developing countries. It is reasonable to believe that the conclusion we derived from the meta-analysis were mainly the comparisons between PD using dextrose dialysate with conventional hemodialysis. Third, medical and social context changed in the last decade. With the emergence of new dialysates, such as icodextrins or solutions using amino acid as osmotic agent, ultrafiltration capacity improved, the cardiovascular risk may be decreased in PD patients. Due to better patients’ education, related technology improved, new type of dialysis modality introduced (such as short daily hemodialysis), the mortality rate of either PD or HD may have changed over time. Considering factors above existed, it is explainable that some studies shows better survival benefit of HD while some studies may show opposite outcome.
In conclusion, HD might have a survival benefit in diabetic ESRD patients compared with PD. Without a large scale and well-organized randomized controlled trial exit, this meta-analysis provided comprehensive evidence for the patients or health care provider to choose between peritoneal dialysis and hemodialysis considering mortality. Further studies should focus on conducting prospective cohorts minimizing bias to the best extent possible.
Supplementary Material
Funding Statement
This work was supported by the National Youth Science Foundation of China [number 81600536] and the Natural Science Foundation of Hunan Province of China [number 2018JJ3833]
Research involving human participants and/or animals
For this type of study formal consent is not required.
Informed consent
For this type of study formal consent is not required.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- 1.Assogba FG, Couchoud C, Hannedouche T, et al. Trends in the epidemiology and care of diabetes mellitus-related end-stage renal disease in France, 2007-2011. Diabetologia. 2014;57:718–728. [DOI] [PubMed] [Google Scholar]
- 2.Couchoud C, Bolignano D, Nistor I, et al. Dialysis modality choice in diabetic patients with end-stage kidney disease: a systematic review of the available evidence. Nephrol Dial Transplant. 2015;30:310–320. [DOI] [PubMed] [Google Scholar]
- 3.Sichart JM, Moeller S. Utilization of hemodiafiltration as treatment modality in renal replacement therapy for end-stage renal disease patients-a global perspective. Contrib Nephrol. 2011;175:163–169. [DOI] [PubMed] [Google Scholar]
- 4.Korevaar JC, Feith GW, Dekker FW, et al. Effect of starting with hemodialysis compared with peritoneal dialysis in patients new on dialysis treatment: a randomized controlled trial. Kidney Int. 2003;64:2222–2228. [DOI] [PubMed] [Google Scholar]
- 5.Chang YT, Wu JL, Hsu CC, et al. Diabetes and end-stage renal disease synergistically contribute to increased incidence of cardiovascular events: a nationwide follow-up study during 1998-2009. Diabetics Care. 2014;37:277–285. [DOI] [PubMed] [Google Scholar]
- 6.Icks A, Claessen H, Morbach S, et al. Time-dependent impact of diabetes on mortality in patients with stroke: survival up to 5 years in a health insurance population cohort in Germany. Diabetes Care. 2012;35:1868–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tien KJ, Lin ZZ, Chio CC, et al. Epidemiology and mortality of new-onset diabetes after dialysis: Taiwan national cohort study. Diabetes Care. 2013;36:3027–3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sattar A, Argyropoulos C, Weissfeld L, et al. All-cause and cause-specific mortality associated with diabetes in prevalent hemodialysis patients. BMC Nephrol. 2012;13:3027–3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. [DOI] [PubMed] [Google Scholar]
- 10.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–1012. [DOI] [PubMed] [Google Scholar]
- 11.Wells G, Shea B, O’connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Oxford: Ottawa Hospital Research Institute; 2014. [Google Scholar]
- 12.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 14.Mircescu G, Garneata L, Florea L, et al. The success story of peritoneal dialysis in Romania: analysis of differences in mortality by dialysis modality and influence of risk factors in a national cohort. Perit Dial Int. 2006;26:266–275. [PubMed] [Google Scholar]
- 15.Garcia-Canton C, Rufino-Hernandez JM, Vega-Diaz N, et al. A comparison of medium-term survival between peritoneal dialysis and haemodialysis in accordance with the initial vascular access. Nefrologia. 2013;33:629–639. [DOI] [PubMed] [Google Scholar]
- 16.Collins AJ, Weinhandl E, Snyder JJ, et al. Comparison and survival of hemodialysis and peritoneal dialysis in the elderly. Semin Dial. 2002;15:98–102. [DOI] [PubMed] [Google Scholar]
- 17.Vonesh EF, Snyder JJ, Foley RN, et al. The differential impact of risk factors on mortality in hemodialysis and peritoneal dialysis. Kidney Int. 2004;66:2389–2401. [DOI] [PubMed] [Google Scholar]
- 18.Jaar BG, Coresh J, Plantinga LC, et al. Comparing the risk for death with peritoneal dialysis and hemodialysis in a national cohort of patients with chronic kidney disease. Ann Intern Med. 2005;143:174–183. [DOI] [PubMed] [Google Scholar]
- 19.Ganesh SK, Hulbert-Shearon T, Port FK, et al. Mortality differences by dialysis modality among incident ESRD patients with and without coronary artery disease. J Am Soc Nephrol. 2003;14:415–424. [DOI] [PubMed] [Google Scholar]
- 20.Termorshuizen F, Korevaar JC, Dekker FW, et al. Hemodialysis and peritoneal dialysis: comparison of adjusted mortality rates according to the duration of dialysis: analysis of The Netherlands Cooperative Study on the Adequacy of Dialysis 2. J Am Soc Nephrol. 2003;14:2851–2860. [DOI] [PubMed] [Google Scholar]
- 21.Fenton SS, Schaubel DE, Desmeules M, et al. Hemodialysis versus peritoneal dialysis: a comparison of adjusted mortality rates. Am J Kidney Dis. 1997;30:334–342. [DOI] [PubMed] [Google Scholar]
- 22.Kim H, Kim KH, Park K, et al. A population-based approach indicates an overall higher patient mortality with peritoneal dialysis compared to hemodialysis in Korea. Kidney Int. 2014;86:991–1000. [DOI] [PubMed] [Google Scholar]
- 23.van de Luijtgaarden MW, Noordzij M, Stel VS, et al. Effects of comorbid and demographic factors on dialysis modality choice and related patient survival in Europe. Nephrol Dial Transplant. 2011;26:2940–2947. [DOI] [PubMed] [Google Scholar]
- 24.Wang IK, Lin CL, Yen TH, et al. Comparison of survival between hemodialysis and peritoneal dialysis patients with end-stage renal disease in the era of icodextrin treatment. Eur J Intern Med. 2018;50:69–74. [DOI] [PubMed] [Google Scholar]
- 25.Chang JH, Sung JY, Ahn SY, et al. Hemodialysis leads to better survival in patients with diabetes or high comorbidity, compared to peritoneal dialysis. Tohoku J Exp Med. 2013;229:271–277. [DOI] [PubMed] [Google Scholar]
- 26.Couchoud C, Moranne O, Frimat L, et al. Associations between comorbidities, treatment choice and outcome in the elderly with end-stage renal disease. Nephrol Dial Transplant. 2007;22:3246–3254. [DOI] [PubMed] [Google Scholar]
- 27.Heaf JG, Wehberg S. Relative survival of peritoneal dialysis and haemodialysis patients: effect of cohort and mode of dialysis initiation. PLoS One. 2014;9:e90119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang CC, Cheng KF, Wu HD. Survival analysis: comparing peritoneal dialysis and hemodialysis in Taiwan. Peritoneal dialysis international. J Int Soc Perit Dial. 2008;28(Suppl 3):S15–S20. [PubMed] [Google Scholar]
- 29.Kim HJ, Park JT, Han SH, et al. The pattern of choosing dialysis modality and related mortality outcomes in Korea: a national population-based study. Korean J Intern Med. 2017;32:699–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee CC, Sun CY, Wu MS. Long-term modality-related mortality analysis in incident dialysis patients. Perit Dial Int. 2009;29:182–190. [PubMed] [Google Scholar]
- 31.Liem YS, Wong JB, Hunink MG, et al. Comparison of hemodialysis and peritoneal dialysis survival in The Netherlands. Kidney Int. 2007;71:153–158. [DOI] [PubMed] [Google Scholar]
- 32.van de Luijtgaarden MW, Jager KJ, Segelmark M, et al. Trends in dialysis modality choice and related patient survival in the ERA-EDTA Registry over a 20-year period. Nephrol Dial Transplant. 2016;31:120–128. [DOI] [PubMed] [Google Scholar]
- 33.Lukowsky LR, Mehrotra R, Kheifets L, et al. Comparing mortality of peritoneal and hemodialysis patients in the first 2 years of dialysis therapy: a marginal structural model analysis. CJASN. 2013;8:619–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marshall MR, Walker RC, Polkinghorne KR, et al. Survival on home dialysis in New Zealand. PLoS One. 2014;9:e96847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mehrotra R, Chiu YW, Kalantar-Zadeh K, et al. Similar outcomes with hemodialysis and peritoneal dialysis in patients with end-stage renal disease. Arch Intern Med. 2011;171:110–118. [DOI] [PubMed] [Google Scholar]
- 36.Mircescu G, Ştefan G, Gârneaţă L, et al. Outcomes of dialytic modalities in a large incident registry cohort from Eastern Europe: the Romanian Renal Registry. Int Urol Nephrol. 2014;46:443–451. [DOI] [PubMed] [Google Scholar]
- 37.Nesrallah GE, Li L, Suri RS. Comparative effectiveness of home dialysis therapies: a matched cohort study. Can J Kidney Health Dis. 2016;3:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee SW, Lee NR, Son SK, et al. Comparative study of peritoneal dialysis versus hemodialysis on the clinical outcomes in Korea: a population-based approach. Sci Rep. 2019;9:5905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Waldum-Grevbo B, Leivestad T, Reisaeter AV, I O. Impact of initial dialysis modality on mortality: a propensity-matched study. BMC Nephrol. 2015;16:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang IK, Liang WM, Lin CL, et al. Impact of dialysis modality on the survival of patients with end-stage renal disease and prior stroke. Int Urol Nephrol. 2016;48:139–147. [DOI] [PubMed] [Google Scholar]
- 41.Yeates K, Zhu N, Vonesh E, et al. Hemodialysis and peritoneal dialysis are associated with similar outcomes for end-stage renal disease treatment in Canada. Nephrol Dial Transplant. 2012;27:3568–3575. [DOI] [PubMed] [Google Scholar]
- 42.Han SS, Park JY, Kang S, et al. Dialysis modality and mortality in the elderly: a meta-analysis. Clin J Am Soc Nephrol. 2015;10:983–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chung SH, Heimburger O, Lindholm B, et al. Peritoneal dialysis patient survival: a comparison between a Swedish and a Korean centre. Nephrol Dial Transplant. 2005;20:1207–1213. [DOI] [PubMed] [Google Scholar]
- 44.Fang W, Qian J, Lin A, et al. Comparison of peritoneal dialysis practice patterns and outcomes between a Canadian and a Chinese centre. Nephrol Dial Transplant. 2008;23:4021–4028. [DOI] [PubMed] [Google Scholar]
- 45.Cheng X, Nayyar S, Wang M, et al. Mortality rates among prevalent hemodialysis patients in Beijing: a comparison with USRDS data. Nephrol Dial Transplant. 2013;28:724–732. [DOI] [PubMed] [Google Scholar]
- 46.Rottembourg J. Residual renal function and recovery of renal function in patients treated by CAPD. Kidney Int Suppl. 1993;40:S106–S110. [PubMed] [Google Scholar]
- 47.Lameire N, Van Biesen W. The impact of residual renal function on the adequacy of peritoneal dialysis. Perit Dial Int. 1997;17(Suppl 2):S102–S110. [PubMed] [Google Scholar]
- 48.Lee H, Manns B, Taub K, et al. Cost analysis of ongoing care of patients with end-stage renal disease: the impact of dialysis modality and dialysis access. Am J Kidney Dis. 2002;40:611–622. [DOI] [PubMed] [Google Scholar]
- 49.Li PK, Szeto CC. Success of the peritoneal dialysis programme in Hong Kong. Nephrol Dial Transplant. 2008;23:1475–1478. [DOI] [PubMed] [Google Scholar]
- 50.Dhanakijcharoen P, Sirivongs D, Aruyapitipan S, et al. The. "PD First. " policy in Thailand: three-years experiences (2008-2011). J Med Assoc Thai. 2011;94(Suppl 4):S153–S161. [Google Scholar]
- 51.Qiao B, Liu L, Liu J, editors, et al. A study on the attitude toward kidney transplantation and factors among hemodialysis patients in China. Transplant Proc. 2016;48:2601–2607. [DOI] [PubMed] [Google Scholar]
- 52.Kim YL, Cho JH, Choi JY, et al. Systemic and local impact of glucose and glucose degradation products in peritoneal dialysis solution. J Ren Nutr. 2013;23:218–222. [DOI] [PubMed] [Google Scholar]
- 53.Drechsler C, Krane V, Ritz E, et al. Glycemic control and cardiovascular events in diabetic hemodialysis patients. Circulation. 2009;120:2421–2428. [DOI] [PubMed] [Google Scholar]
- 54.Yoo DE, Park JT, Oh HJ, et al. Good glycemic control is associated with better survival in diabetic patients on peritoneal dialysis: a prospective observational study. PLoS One. 2012;7:e30072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang F, Liu H, Gong X, et al. Risk factors for mortality in Chinese patients on continuous ambulatory peritoneal dialysis. Perit Dial Int. 2015;35:199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu Y, Dong Z, Liu H, et al. Transition of mesothelial cell to fibroblast in peritoneal dialysis: EMT, stem cell or bystander? Perit Dial Int. 2015;35:14–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ozener C, Arikan H, Karayaylali I, et al. The impact of diabetes mellitus on peritoneal dialysis: the Turkey Multicenter Clinic Study. Ren Fail. 2014;36:149–153. [DOI] [PubMed] [Google Scholar]
- 58.Park MS, Lee HA, Chu WS, et al. Peritoneal accumulation of AGE and peritoneal membrane permeability. Perit Dial Int. 2000;20:452–460. [PubMed] [Google Scholar]
- 59.Shu Z-J, Peng Y-M, Sun L, et al. Maltose, a promising osmotic agent in peritoneal dialysis solution. Med Hypotheses. 2010;75:645–647. [DOI] [PubMed] [Google Scholar]
- 60.Jian-Hui Z, Zhao-Hui N, Chang-Lin M, et al. Efficacy and safety of Changfu peritoneal dialysis solution: a multi-center prospective randomized controlled trial. Chin Med J. 2013;126:4204–4209. [PubMed] [Google Scholar]
- 61.Nesrallah GE, Lindsay RM, Cuerden MS, et al. Intensive hemodialysis associates with improved survival compared with conventional hemodialysis. JASN. 2012;23:696–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.