Abstract
Objectives: To improve the mouse model of relief for unilateral ureteral obstruction (RUUO) and explore the pathological process of renal fibrosis after the obstruction was relieved.
Methods: C57BL/6 mice in model group were randomly divided into RUUO group, improved RUUO group, and UUO group. After leaving Unilateral Ureteral Obstruction (UUO) for 3 days, the obstruction was released by reimplantation way in RUUO group and in reimplantation + catheter way in improved RUUO group. C57BL/6 mice in observation group were randomly divided into 1d RUUO group, 3d RUUO group, 7d RUUO group, and 14d RUUO group. Three days after UUO, the obstruction was released by reimplantation + catheter in four groups. We detected the renal volume, H&E, Masson staining, and immunohistochemistry of kidney pathology on the seventh day after RUUO in model group and on the 1st, 3rd, 7th, and 14th day after RUUO in observation group.
Results: Comparing with mice in RUUO group, mice in improved RUUO group had lower renal volume, tubular damage score, and collagen area percentage. After the obstruction was relieved, the renal volume decreased gradually within 2 weeks. The tubular damage score in 7d RUUO group was lower than that in 1d RUUO and 3d RUUO group. However, the tubular damage score in 14d RUUO group was higher than that in 7d RUUO group. The tendency of collagen area percentage and α-SMA IOD value were consistent with the tubular damage score.
Conclusions: Using the method of reimplantation + catheter, a reliable mice model of RUUO can be got. After RUUO, the de-obstructed kidneys are still in damage and fibrosis state.
Keywords: Relief of unilateral ureteral obstruction, catheter, renal fibrosis, pathology
Introduction
In recent years, the incidence of end-stage renal disease caused by ON has been increasing year-by-year, resulting in an increase in dialysis costs. The ideal animal model is helpful to study the pathogenesis of disease and drug intervention [1]. UUO model has been experimented to explore the pathological features of ON for many years [2]. However, the UUO model could not effectively simulate the pathological process after the clinical recanalization, the second operation was not utilized to remove the obstruction. In contrast, the relief for unilateral ureteral obstruction (RUUO) model is an appropriate simulation of clinical recanalization of ureteral obstruction, which is more conducive to the study of renal damage and repair after the release of obstruction. At present, there are three main methods to establish the mouse model of RUUO. Among which, the method using vascular and using the ureter folding device to build model [3–5] are relatively simple to operate. However, there is no guarantee of complete patency of the ureter after recanalization and unable to measure kidney function longitudinally. Due to these two drawbacks, these two methods are not widely used. The RUUO model in which the obstructed ureter was reimplanted into the bladder after UUO [6] is the closest to the clinical ON. Whereas, due to its technical difficulty, there is less corresponding research. In our experiments, we explored this method and found that there were still some problems existed in this method, such as low success rate of recanalization and incomplete recanalization of ureter, so we made some improvements on the basis of this method. By using the modified RUUO model, we explored the pathological changes of kidney in mice after obstruction was relieved.
Materials and methods
Materials
Animals
Adult male C57BL/6 mice, aged 6 weeks and weighting 20 g, were obtained from Nanjing Medical University (Production license number: SCXK (Su 2014–0002)) and kept in Nanjing Drum Tower Hospital Experimental Animal Center (Use the license number (Su 2014–0052)). Animals were adapted for 1 week before surgery.
Experimental reagents
Hematoxylin-Eosin (H&E) staining; Masson staining; α-SMA antibody; Collagen I antibody; 10% chloral hydrate.
Experimental equipment
Surgical microscopes; Micro tweezers; Micro shear; With needle suture (model 4–0, 7–0, 10–0, round needle); Syringe (1 mL, 5 mL, 20 mL, insulin needle); Microvascular clamp (6 mm, medium); Teflon tube (inner diameter 0.3 mm, outer diameter 0.6 mm); Digital display vernier caliper (KORLOY, range 100 mm, resolution 0.1 mm); Computer Microscope (OLYMPUS, U-TVQ5XO-3); OLYMPUS cellsens Entry camera system.
Methods
Grouping of animals
C57BL/6 mice in model group were randomly divided into RUUO group (n = 10), improved RUUO group (n = 10), and UUO group (n = 10). After leaving UUO for 3 days, the obstruction was released by reimplantation way in RUUO group and in reimplantation + catheter way in improved RUUO group. The standard of successful RUUO used in our study was marked no hydronephrosis, and no expansion of the ureter. Otherwise it is not complete recanalization.
C57BL/6 mice in observation group were randomly divided into 1d RUUO group (n = 10), 3d RUUO group (n = 10), 7d RUUO group (n = 10), and 14d RUUO group (n = 10). Three days after UUO, the obstruction was released by reimplantation + catheter in four groups.
Comparison of two methods to build mouse model of RUUO
Procedure of UUO model
The mouse was anesthetized by using 10% chloral hydrate, the limbs were fixed on a heated surgical pad while the abdominal cavity was open. A midline laparotomy and an incision of the avascular linea alba were made by using tissue separating scissors to gain access to the peritoneal cavity. Sterilized cotton buds were used to expose the left ureter by displacing the intestines toward the right side of the abdominal cavity. Angled forceps were used to isolate and lift the left ureter. To create a ureteric obstruction, the left ureter was ligated twice with 7/0 black braided silk suture anywhere between the bladder and renal pelvis. The top of the line to retain a certain length of the thread. The ureter was divided between the two sutures in order to isolate the bladder from the ureter. At last, the intestines were replaced into the peritoneal cavity carefully, the incision was closed and the anesthesia was reversed.
Procedure of RUUO model
The methods in RUUO group
Three days after UUO, the obstruction was released by reimplantation way in RUUO group. Preoperative preparation was the same as the former. The abdominal cavity was opened from the original incision. Identification of the black 7/0 silk tie used to tie the left ureter in the first operation usually aimed to locate the obstructed end. The ureter is inserted into the bladder [6]: A 20-mL syringe was pushed through into the bladder from the left-hand side, into its cavity, and out again through the right-hand side. A pair of fine forceps was used to hold the sharp end of the needle. The needle and forceps were then pushed through into the bladder and out again in a right-to-left manner. The forceps were adopted to grasp the distal end of the ligated ureter which enabled the ureter to be pulled into the cavity of the bladder and out again through the other side. A vascular clamp was taken to secure the distal end of the ureter and prevent it from slipping back into the bladder cavity. Using 10/0 nylon monocryl wire, four or five evenly spaced interrupted sutures were placed to stitch the ureter to the wall of the bladder on the left (Figure 1(A–C)).
Figure 1.
Surgery pictures. (A) The end of the left enlarged ureter was found and isolated under the identification of the black 7/0 silk tie used to tie the left ureter in the first operation. The solid arrow indicates the enlarged ureter. The dashed arrow indicates the filled bladder. (B) A 20-mL syringe was pushed through into the bladder from the left-hand side, into its cavity, and out again through the right-hand side. The solid arrow indicates the enlarged ureter. The dashed arrow indicates the bladder. (C) A vascular clamp was taken to secure the distal end of the ureter and prevent it from slipping back into the bladder cavity. Using 10/0 monocryl wire, four or five evenly spaced interrupted sutures were placed to stitch the ureter to the wall of the bladder on the left. The solid arrow indicates the enlarged ureter. The dashed arrow indicates a vascular clamp. (D) Under the guidance of the insulin needle, a diameter of 6–9 mm PTFE tube was pushed into the ureteral cavity with tube mouth exposed 1–2 mm. The solid arrow indicates the PTFE tube. The dashed arrow indicates the ureteral wall. (E) The details of the figure (D) under the microscope. (F) The anterior wall of the bladder was sutured. Arrows indicate the anterior wall of the bladder. (G, P) The comparison of kidney at 10 days post-UUO (figure right) and CUK (figure left). (H, Q) The comparison of kidney at 7 days post RUUO by reimplantation way (figure right) and CUK (figure left). (I, R) The comparison of kidney at 7 days post RUUO by reimplantation + catheter way (figure right) and CUK (figure left). (J) The kidney at 7 days post RUUO by reimplantation way. The solid arrow indicates hydronephrosis. The dashed arrow indicates the bladder. (K) The details of the figure (J) under the microscope. (L) The catheterization section of the ureter was surrounded by connective tissue, when the bladder is cut open. There was no dye flow out of the ureter when the brown dye was injected into the renal pelvis. The arrow indicates the segment of the ureter. (M) The kidney at 7 days post RUUO by reimplantation + catheter way. The solid arrow indicates the no hydronephrosis. The dashed arrow indicates the bladder. (N) The catheter was seen in the bladder wall and smoothly, when the bladder in figure (M) was cut open. The arrow indicates the mouth of the catheter. (O) The details of the figure (M) under the microscope. The arrow indicates the mouth of the catheter.
Methodology in improved RUUO group
The procedure for inserting the ureter into the bladder and suturing the ureter and bladder was the same as that in RUUO group. After suturing the ureter and bladder, the half of the ureteral wall in the 7/0 silk tied up the top 5 mm was cut and a gush of urine could be seen. Under the guidance of the insulin needle, a diameter of 6–9 mm PTFE tube was pushed into the ureteral cavity with tube mouth exposed 1–2 mm. The end of the ureter was cut off and repaired. The end of the ureter and polytetrafluoroethylene tube was put back to the bladder as a whole. The anterior wall of the bladder was sutured and the abdominal cavity was rinsed with the raw salt water. The incision was closed and then the anesthesia was reversed (Figure 1(A–F)).
Collection and treatment of specimen
The mice were killed at 1st, 3rd, 7th, and 14th day post-RUUO and the bilateral kidneys were removed. The half of the kidney was fixed at 10% formalin and the other half of the kidney was stored in a refrigerator at –80 °C.
Renal volume and conventional staining of kidney tissue
The kidney tissue fixed with 10% formalin solution for 24 h was made into 3 μm paraffin sections. H&E staining and Masson staining were performed according to conventional methods. The kidney histological sections were quantitative analyzed according to Image Pro Plus 6.0.1. H&E staining of renal tubular injury score. The percentages of histological changes in the kidney tissue were scored using a semi-quantitative scale designed to evaluate the degree of tubular necrosis as follows [7]: 0 = normal kidney; 1 = minimal necrosis (5% involvement); 2 = mild necrosis (5–25% involvement); 3 = moderate necrosis (25–50% involvement); 4 = severe necrosis (50–75% involvement); and 5 = most severe necrosis (>75% involvement). Semi-quantitative analysis of Masson staining was calculated according to the percentage of collagen positive area. Each section was randomly selected from the 10 non-overlapping skin at the junction of the visual field (Magnification: 400×) to take the average. The renal volume is estimated according to the following equation: V = πLTA/6.
Immunohistochemistry
The expression level of α-SMA and collagen I in kidney were assessed in paraffin-embedded kidney sections (4-µm-thick). Immunohistochemical staining was performed as described previously [8,9]. The results were conducted quantitatively by using Image Pro Plus 6.0.-1 system and ten high-power fields (400× magnification) were analyzed separately for each immunohistochemical reaction in renal tissue.
Statistical analysis
SPSS25.0 software was used for statistical analysis. The measurement data are demonstrated as mean ± standard deviation (x ± s). Comparisons between multiple groups were performed by a one-way analysis of variance test followed by the Kruskal–Wallis test where appropriately. p < 0.05 was considered statistically different.
Results
Results in model group
The rate of successful reversal of obstruction
On the seventh day after RUUO, all mice in model group were dissected and we found that the ureters of two mice in RUUO group were completely blocked by the blood clots. The catheter of one mouse in improved RUUO group was completely blocked by blood clots, the catheters of two mice were incompletely blocked by blood clots, and the rest of the mice were completely recanalized. The rate of successful reversal of obstruction in improved RUUO group was 70%. All mice in RUUO group were incompletely recanalized. Mice with re-canalization failure were discarded.
Renal volume
We compared the degree of hydronephrosis in RUUO and improved RUUO group on seventh day post-RUUO and tenth day post-UUO in UUO group. The results showed that obstructed kidneys in UUO group were characterized by hydronephrosis, thinning of renal parenchyma and significantly expanded renal pelvis (Figure 1(G,P)). Hydronephrotic in RUUO group is not obvious compared with that in UUO group. A certain degree of hydronephrosis in RUUO group suggested that the ureter still has some degree of obstruction (Figure 1(H,J,Q)). The opening of the ureter was blurred and sticky in RUUO group when the bladder is cut open (Figure 1(I)), which suggested the ureter is incompletely recanalized. When the obstructed kidneys were released by reimplantation + catheter way, the volume of de-obstructed kidneys were smaller than that in contralateral unobstructed kidney (CUK), and there was no obvious hydronephrosis in the renal pelvis (Figure 1(I,M,R)). When the mouse bladder is cut, the catheter was seen in place smoothly (Figure 1(N,O)), which suggested the ureter is completely recanalized. The catheter of one mouse in improved RUUO group was completely blocked by blood clots and its performance was consistent with that in UUO group, two mice whose catheter were incompletely blocked by the blood clots showed minor hydronephrosis, similar to that in RUUO group. The renal volume in RUUO group (139.793 ± 2.78 mm3) was smaller than that in UUO group (412.536 ± 31.12 mm3, p < 0.05), which was higher than that in improved RUUO group (85.019 ± 12.36 mm3, p < 0.05) (Figure 2(A)).
Figure 2.
Renal volume, tubular damage score and collagen area percentage in different groups. (A) The length of the kidney L, the width T, and the depth A were measured by the vernier caliper. The renal volume is estimated according to the following equation: V = πLTA/6. The renal volume in RUUO group was smaller than in UUO group (139.793 ± 2.78 vs.412.536 ± 31.12 mm3, p < 0.05), which was larger than that in improved RUUO group (139.793 ± 2.78 vs. 85.019 ± 12.36 mm3, p < 0.05). *p < 0.05 for the indicated comparison. (B) The tubular damage score in RUUO and improved RUUO group was 4.38 ± 0.74 (n = 8) and 3.14 ± 0.69 (n = 7). *p < 0.05 for the indicated comparison. (C) The proportion of collagen area in RUUO and improved RUUO group was 0.55 ± 0.19% (n = 8) and 0.26 ± 0.11% (n = 7). *p < 0.05 for the indicated comparison.
Renal histopathology
De-obstructed kidneys in RUUO group at 7 days post-RUUO showed renal tubular disorder, brush border loss, lumen expansion, tube formation and the damage and fibrosis still can be observed. Masson staining presented that the de-obstructed kidneys in RUUO group showed a distinct blue staining area, which was mainly distributed in the renal interstitium. The renal histopathology in improved RUUO group was better than that in RUUO group (Figure 3). The tubular damage score in RUUO group (4.38 ± 0.74) was higher than that in improved RUUO group (3.14 ± 0.69) (p < 0.05, Figure 2(B)). The tendency of collagen area percentage was consistent with the tubular damage score (Figure 2(C)).
Figure 3.
Representative histological sections (magnification: 200×). De-obstructed kidneys at 7 days post-RUUO showed renal tubular disorder, brush border loss, lumen expansion, tube formation in RUUO group, and the damage and fibrosis still can be observed. The renal histopathology in improved RUUO group was better than that in RUUO group, which was still more serious than the CUK.
Results in observation group
The rate of successful reversal of obstruction
The obstruction was released by reimplantation + catheter way in observation group. The catheters of two mice in 1d RUUO group were completely blocked by blood clots resulting in recanalization failure. The catheters of three mice in 3d RUUO group were completely blocked by blood clots. One catheter of mouse in 7d RUUO group was completely blocked by blood clots and one catheter detached causing incomplete recanalization. In 14d RUUO group, one catheter of mouse detached and one mouse died before the experiment stopped. We dissected the dead mouse and found that there was a significant abdominal infection. The rate of successful reversal of obstruction in observation group was 77.5%. Mice with re-canalization failure were discarded.
Renal volume
We compared the renal volume of four groups and found that all the de-obstructed kidneys in 1d RUUO group were characterized by hydronephrotic, thinning of renal parenchyma, and the renal volume was obviously larger than the CUK (Figure 4(A)). The hydronephrotic in 3d RUUO group was relieved compared with that in 1d RUUO group, and the renal volume was slightly smaller than CUK (Figure 4(A)), meaning that the obstruction was successfully relieved. The hydronephrotic in 7d RUUO group was relieved compared with that in 3d RUUO group, and the renal volume was significantly smaller than that in 3d RUUO group and CUK (p < 0.05, Figure 4). The renal volume in 14d RUUO group was smaller than that in 1d, 3d, and 7d RUUO group (p < 0.05, Figure 4). However, as can be clearly seen from the color of the kidneys, the degree of hydronephrotic in 14d RUUO group was deeper than the other three groups and CUK (Figure 4(A)), implying that the kidneys shrunk.
Figure 4.
Appearance of kidneys and renal volume in four groups. (A) 1d RUUO: The comparison of RUUO kidney on 1st day post RUUO (figure right) and CUK (figure left). 3d RUUO: The comparison of RUUO kidney on 3rd day post RUUO (figure right) and CUK (figure left). 7d RUUO: The comparison of RUUO kidney on 7th day post RUUO (figure right) and CUK (figure left). 14d RUUO: The comparison of RUUO kidney on 14th day post RUUO (figure right) and CUK (figure left). (B) The renal volume in 1d RUUO, 3d RUUO, 7d RUUO, and 14d RUUO group was 135.525 ± 2.054 mm3 (n = 8), 100.174 ± 2.209 mm3 (n = 7), 85.530 ± 1.534 mm3 (n = 8), and 43.204 ± 1.208 mm3 (n = 8) respectively. *p < 0.05 for the indicated comparison.
Renal histopathology
The pathology of kidneys undergoing 3 days UUO was considerable tubular injury after 1 day of RUUO, as manifested by tubular dilatation and epithelial cell flattening (and loss of brush border in the proximal segments). As the time of de-obstruction is prolonged, the degree of renal fibrosis was gradually reduced, accompanied by the recovery of normal histological appearance within a week. On seventh day after RUUO, decreased renal tubular damage and a gradual return toward normal histology were apparent in kidneys. However, on day 14 post-RUUO, the degree of kidney damage was aggravated, manifested as inflammation, tubular atrophy and interstitial fibrosis (Figure 5). Tubular damage score in 1d, 3d, 7d, and 14d RUUO group was 4.88 ± 0.35, 4.43 ± 0.79, 3.25 ± 0.71 and 4.75 ± 0.47, respectively. There were statistical differences among four groups (p < 0.05, Figure 6).
Figure 5.
Representative histological sections of four groups. The pathology of kidneys undergoing 3 days UUO was considerable tubular injury after 1 day of RUUO, as manifested by tubular dilatation and epithelial cell flattening. As the time of de-obstruction was prolonged, the degree of renal fibrosis was gradually reduced, accompanied by the recovery of normal histological appearance within a week. At day 14 post-RUUO, the degree of kidney damage was aggravated, manifested as inflammation, tubular atrophy, and interstitial fibrosis. Masson staining showed less and less blue areas within a week, while at 14th day post-RUUO, the blue-stained areas increased significantly. Immunohistochemistry showed that with prolong of de-obstruction time, the expression of α-SMA decreased. At 14th day post-RUUO, the expression of α-SMA increased. The immunohistochemical results of collagen-I were consistent with the results of Masson staining.
Figure 6.
The tubular damage score by H&E staining. The tubular damage score in 1d RUUO, 3d RUUO, 7d RUUO, and 14d RUUO group was 4.88 ± 0.35 (n = 8), 4.43 ± 0.79 (n = 7), 3.25 ± 0.71 (n = 8), and 4.75 ± 0.47 (n = 8) respectively. *p < 0.05 for the indicated comparison.
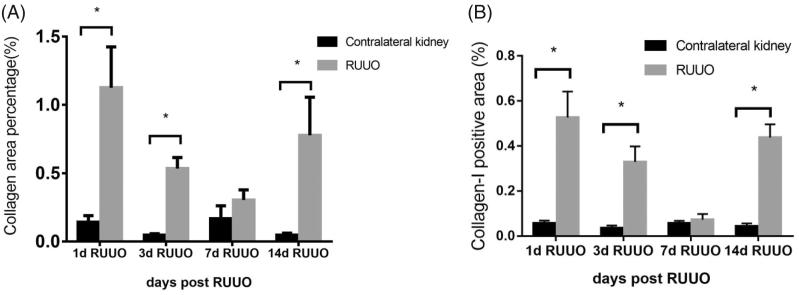
As for Masson, there was obvious blue staining areas in 1d, 3d, and 14d RUUO group, mainly distributed in renal interstitium. 7d RUUO group had reduced blue areas compared with 1d, 3d RUUO group (Figure 5). The proportion of collagen area of CUK and de-obstructed kidneys in 1d, 3d, 7d, and 14d RUUO group was 0.14 ± 0.13% vs 1.13 ± 0.84% (p < 0.05), 0.05 ± 0.03% vs 0.53 ± 0.21% (p < 0.05), 0.17 ± 0.27% vs 0.30 ± 0.21% (p > 0.05), and 0.05 ± 0.04% vs 0.78 ± 0.79% (p < 0.05, Figure 7(A)). The immunohistochemical results of collagen-I were consistent with the results of Masson staining (Figure 5). The expression of collagen-I of CUK and de-obstructed kidneys in 1d, 3d, 7d, and 14d RUUO group was 0.06 ± 0.01% vs 0.53 ± 0.12% (p < 0.05), 0.04 ± 0.01% vs 0.33 ± 0.07% (p < 0.05), 0.06 ± 0.01% vs 0.07 ± 0.03% (p > 0.05), and 0.04 ± 0.01% vs 0.44 ± 0.06% (p < 0.05) (Figure 7(B)).
Figure 7.
The proportion of collagen area by Masson staining and Immunohistochemistry. (A) The proportion of collagen area of contralateral kidneys and de-obstructed kidneys in 1d RUUO, 3d RUUO, 7d RUUO, and 14d RUUO group was 0.14 ± 0.13% vs 1.13 ± 0.84% (n = 8), 0.05 ± 0.03% vs 0.53 ± 0.21% (n = 7), 0.17 ± 0.27% vs 0.30 ± 0.21% (n = 8), and 0.05 ± 0.04% vs 0.78 ± 0.79% (n = 8). *p < 0.05 for the indicated comparison. (B) The proportion of collagen-I area of contralateral kidneys and de-obstructed kidneys in 1d RUUO, 3d RUUO, 7d RUUO, and 14d RUUO group was 0.06 ± 0.01% vs 0.53 ± 0.12% (n = 8), 0.04 ± 0.01% vs 0.33 ± 0.07% (n = 7), 0.06 ± 0.01% vs 0.07 ± 0.03% (n = 8), and 0.04 ± 0.01% vs 0.44 ± 0.06% (n = 8). *p < 0.05 for the indicated comparison.
Immunohistochemical staining of renal α-SMA showed that the expression of α-SMA in 1d, 3d, and 14d RUUO group was significantly increased. The expression of α-SMA in 7d RUUO group was low and mainly expressed in the small vessel wall as well as in the periphery of glomerulus and renal tubule (Figure 5). The expression of α-SMA of CUK and de-obstructed kidneys in 1d, 3d, 7d, and 14d RUUO group was 16582.90 ± 4114.84 vs 44499.41 ± 7928.23 (p < 0.05), 22892.77 ± 14315.49 vs 38907.00 ± 7337.67 (p < 0.05), 17585.28 ± 7203.37 vs 19644.91 ± 8942.41 (p > 0.05), and 17793.53 ± 10832.55 vs 59355.14 ± 14360.67 (p < 0.05, Figure 8).
Figure 8.
The average optical density of α-SMA of contralateral kidneys and de-obstructed kidneys in 1d RUUO, 3d RUUO, 7d RUUO, and 14d RUUO group was 16582.90 ± 4114.84 vs 44499.41 ± 7928.23 (n = 8), 22892.77 ± 14315.49 vs 38907.00 ± 7337.67 (n = 7), 17585.28 ± 7203.37 vs 19644.91 ± 8942.41 (n = 8), and 17793.53 ± 10832.55 vs 59355.14 ± 14360.67 (n = 8). *p < 0.05 for the indicated comparison.
Discussion
ON is a common cause for CKD. In the pathological process of CDK, the ureteral obstruction resulted in various pathological alterations such as increased pressure of ureter, decreased blood flow of kidney. These pathological alterations effectively activate the renin-angiotensin system which will eventually upregulate the expression of angiotensin II (Ang II). The increased expression of Ang II aroused numerous of pathological process such as upregulated the expression of transforming growth factor β1 (TGF-β1), renal interstitial cell infiltration and renal tubular apoptosis. All of the above lead to the progressive development of ON eventually [10–13]. The in-depth study of many mechanisms benefits from the mature and stable UUO model. Many studies have exhibited that renal function will continue to deteriorate until the terminal stage after the obstruction was relieved and the history of renal obstruction is an independent risk factor for CKD [14]. However, few researches were conducted on the intervention after release of obstruction. There is no recognized and stable RUUO model allowed for study of longitudinal changes because the renal parenchyma is lost completely in a few weeks. It has been more than 10 years since the first report of RUUO model in mice [3]. However, no more than ten articles have been reported in this decade and there was no recognized surgical procedure to date. Further research on ON depends on a reliable, standardized, repeatable mouse model of RUUO.
The main problem with mouse model of RUUO was that ureteral injury due to ligation or clipping can lead to ureteral adhesions, which causes reversible failure. The study by Puri in 2010 [4] and by Chaabane et al. in 2012 [5] have referred to the success rate of the reversal procedure. Using a model of ureteral clamping, Puri et al. found the success rate of recanalization was less than 20%. Chaabane et al. described a simple, inexpensive, and reproducible mouse model of RUUO accompanying the rate of 62% ureteral patency by using silicone catheter. Obviously, the rate of successful reversal of obstruction of these two methods was not high. The RUUO model in which the obstructed ureter was reimplanted into the bladder after UUO [6,15] is the closest to the clinical ON. But their study did not report the success rate of ureteral recanalization. In our experiments, we explored this method and found that there are still some problems existing in this method. So, we made some improvements to this method.
The criterion for successful recanalization was that the kidneys show no expansion and no effusion of the renal pelvis. The failed recanalization included complete obstruction and incomplete obstruction. Complete obstruction of the kidneys was characterized by thin kidney parenchyma, kidney water-like severe hydronephrosis, and significant expansion of ureteropelvic ureter. The degree of renal hydronephrosis in incomplete obstruction was lower than UUO, and the renal volume could be reduced, but a small amount of effusion was still visible. We used the method of Tapmeier et al. [6] in our experiments. The distinction between the puncture bladder and traction ureter were completed by 20 mL syringe needle while other instruments were used in Tapmeier’s study. And the obstruction time in our study was 3 days not 7 days. But we noted the renal pelvis still has a certain degree of expansion at 7 days post-RUUO. When the bladder was cut open, we found that the ureteral segment was wrapped in connective tissue. The renal pelvis injected with dye confirmed that the obstruction was at the incision of the ureter. It can be speculated that the change was brought by injury which was caused by cutting the ureter. In order to solve this problem, the clinical practice that the catheter placed after ureteral surgery to prevent ureteral strictures was referred in present study. Therefore, an appropriate catheter was placed in the ureteral bladder connection to prevent ureteral stenosis. The results showed that in RUUO group, except for two mice whose ureters were completely blocked by the blood clots causing failure recanalization, the rest of the mice had a certain degree of hydronephrosis, all of which were incompletely recanalized. Comparing with mice in RUUO group, mice in improved RUUO group had lower renal volume, tubular damage score and collagen area percentage. In our study, 50 mice were modeled using the improved RUUO method. The catheters of seven mice were completely blocked by blood clots, two mice were incompletely blocked by blood clots. Two catheters of mice detached and one mouse died before the experiment stopped. The rest of the mouse were completely recanalized with no effusion in renal pelvis. The rate of successful reversal of obstruction in our study was 76%.
The RUUO model in Hesketh’s [15] study is an improved model based on Tapmeier’s study [6]. The difference is that in the first obstruction surgery, the silicone catheter cut longitudinally was wrapped around the ureter and ligation fixed in front of the ureteral ligation. They thought that the silicone catheter could restrain the ureter to prevent its excessive expansion and make the anastomosis to the bladder easily. We adopted the method of Hesketh et al. in our experiments; however, we found that silicone catheter placed in the abdominal cavity had caused severe adipose adhesion. The ureteral segment was surrounded by the adipose tissue which mainly around the testis. Separation of adipose tissue caused bleeding and ureteral injury. Tipu et al. [6] have noted that abdominal adipose tissue is an important cause of surgical failure. The conclusions of Tapmeier et al. [6] and the results of our study both presented that the expansion of the ureter does not impede the anastomosis to the bladder. Thus, one of the shortcomings of RUUO model in Hesketh’s [15] study is the adipose adhesion. The advantage of using insulin needle as a guide wire during catheterization process in our study is the easy access to operate and the increased success rate of catheterization. In addition, we can draw urine from the ureter during catheterization to detect urine biochemical markers.
Using our improved mouse model of RUUO, we have found that after ureteral recanalization, the degree of renal fibrosis gradually alleviated within a week. On day 7 after RUUO, the indexes of renal fibrosis were significantly lower than 1d, 3d RUUO group with the increase of blood flow and the disappearance of tissue edema after the obstruction was relieved. These results are consistent with the previous researches on RUUO models [6,15]. However, at 14 days after RUUO, the degree of renal fibrosis began to gradually increase, suggesting that the obstructed renal tissue was not completely repaired and progressed to chronic lesions. This is consistent with the conclusion reached by Tipu et al. and Chan et al. [4,16]. They have found that renal function will deviate away from the baseline when the date of obstruction is longer than or equal to 3 days, which may degenerate into the pathological process of CKD in the later stage. Inflammation is appreciated as a highly regulated process that plays an important role in pathogenesis of CKD. Of the inflammatory cells, F4/80+ cells of monocytic lineage were the most prominent in the process of kidney injury [4]. Macrophages are well recognized for their pathogenic role in kidney inflammation and fibrosis [17]. Research by Chaves et al. demonstrated that depletion of mononuclear macrophages using liposomal clodronate during injury and repair altered the susceptibility of C57BL/6 mice to development of CKD in their RUUO model [18]. So, we speculated that the deterioration of renal fibrosis may be associated with infiltrating mononuclear macrophages in kidney tissue. In our next study, we will further study the specific mechanisms by depleting and transfer mononuclear macrophages using improved RUUO model.
In summary, surgery to relieve the obstruction is not the end of ON. To promote renal repair to the maximum extent while renal obstruction is relieved, which is undoubtedly an important step in delaying the progression of renal disease.
Funding Statement
This study was funded by Nanjing Municipal Health and Family Planning Commission (ZKX17018), Nanjing Health Youth Talent (QRX17045).
Disclosure statement
The authors declare that they have no conflict of interest.
References
- 1.Rabe M, Schaefer F. Non-transgenic mouse models of kidney disease. Nephron. 2016;133:53–61. [DOI] [PubMed] [Google Scholar]
- 2.Ucero AC, Benito-Martin A, Izquierdo MC, et al. Unilateral ureteral obstruction: beyond obstruction. Int Urol Nephrol. 2014;46:765–776. [DOI] [PubMed] [Google Scholar]
- 3.Cochrane AL, Kett MM, Samuel CS, et al. Renal structural and functional repair in a mouse model of reversal of ureteral obstruction. J Am Soc Nephrol JASN. 2005;16:3623–3630. [DOI] [PubMed] [Google Scholar]
- 4.Puri TS, Shakaib MI, Chang A, et al. Chronic kidney disease induced in mice by reversible unilateral ureteral obstruction is dependent on genetic background. Am J Physiol Renal Physiol. 2010;298:F1024–F1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaabane W, Praddaude F, Buleon M, et al. Renal functional decline and glomerulotubular injury are arrested but not restored by release of unilateral ureteral obstruction (UUO). Am J Physiol Renal Physiol. 2013;304:F432–F439. [DOI] [PubMed] [Google Scholar]
- 6.Tapmeier TT, Brown KL, Tang Z, et al. Reimplantation of the ureter after unilateral ureteral obstruction provides a model that allows functional evaluation. Kidney Int. 2008;73:885–889. [DOI] [PubMed] [Google Scholar]
- 7.Jun C, Qingshu L, Ke W, et al. Protective effect of CXCR3(+)CD4(+)CD25(+)Foxp3(+) regulatory T cells in renal ischemia-reperfusion injury. Mediators Inflamm. 2015;2015:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mei Y, Yangyang Z, Shuai L, et al. Breviscapine prevents downregulation of renal water and sodium transport proteins in response to unilateral ureteral obstruction. Iran J Basic Med Sci. 2016;19:573–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ji J, He L. Effect of Kangxianling decoction on expression of TGF-β1/Smads and extracellular matrix deposition. Evid Based Compl Alternat Med. 2019;2019:5813549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klahr S, Morrissey J. Obstructive nephropathy and renal fibrosis. Am J Physiol Renal Physiol. 2002;283:F861–F875. [DOI] [PubMed] [Google Scholar]
- 11.Nishida M, Hamaoka K. Macrophage phenotype and renal fibrosis in obstructive nephropathy. Nephron Exp Nephrol. 2008;110:e31–e36. [DOI] [PubMed] [Google Scholar]
- 12.Bani-Hani AH, Campbell MT, Meldrum DR, et al. Cytokines in epithelial-mesenchymal transition: a new insight into obstructive nephropathy. J Urol. 2008;180:461–468. [DOI] [PubMed] [Google Scholar]
- 13.Grande MT, Lopez-Novoa JM. Fibroblast activation and myofibroblast generation in obstructive nephropathy. Nat Rev Nephrol. 2009;5:319–328. [DOI] [PubMed] [Google Scholar]
- 14.Wu AK, Tran TC, Sorensen MD, et al. Relative renal function does not improve after relieving chronic renal obstruction. BJU Int. 2012;109:1540–1544. [DOI] [PubMed] [Google Scholar]
- 15.Hesketh EE, Vernon MA, Ding P, et al. A murine model of irreversible and reversible unilateral ureteric obstruction. J Vis Exp JoVE 2014;94:52559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan W, Krieg RJ Jr., Ward K, et al. Progression after release of obstructive nephropathy. Pediat Nephrol. (Berlin, Germany). 2001;16:238–244. [DOI] [PubMed] [Google Scholar]
- 17.Cao Q, Harris DC, Wang Y. Macrophages in kidney injury, inflammation, and fibrosis. Physiology (Bethesda, MD). 2015;30:183–194. [DOI] [PubMed] [Google Scholar]
- 18.Chaves LD, Mathew L, Shakaib M, et al. Contrasting effects of systemic monocyte/macrophage and CD4+ T cell depletion in a reversible ureteral obstruction mouse model of chronic kidney disease. Clin Dev Immunol. 2013;2013:836989. [DOI] [PMC free article] [PubMed] [Google Scholar]