ABSTRACT
Severe cytopenias (anemia, thrombocytopenia, neutropenia or any combination of these) are common causes of ER visits and hospital admissions. In adults, the etiology of cytopenias has a broad differential diagnosis including vitamin and mineral deficiencies, autoimmune conditions, infections, bone marrow failure disorders, or malignancies. We present a case of severe anemia and thrombocytopenia who was initially diagnosed with myelodysplastic syndrome (MDS) based on the results of a bone marrow biopsy. However, subsequent workup revealed that she had B12 deficiency secondary to pernicious anemia. This case highlights how performing a bone marrow biopsy without investigating secondary causes of cytopenia and bone marrow dysplasia can lead to a false diagnosis of MDS. Confirmation of the appropriate diagnosis spared the patient emotional trauma and unnecessary treatment with hypomethylating agents.
KEYWORDS: B12 deficiency, myelodysplastic syndrome, pernicious anemia
1. Introduction
B12 deficiency can cause bone marrow failure and dysplasia, presenting as severe pancytopenia without the classical neurological findings associated with this condition [1]. Therefore, if B12 deficiency is not considered in the differential diagnosis of cytopenias and bone marrow dysplasia, it can be misdiagnosed as myelodysplastic syndrome [2]. We present a patient with severe anemia, thrombocytopenia, and a bone marrow biopsy consistent with MDS features. However, further work up revealed she had pernicious anemia requiring treatment with B12, opposed to hypomethylating agents for treatment of MDS.
2. Case presentation
A 54-year-old Hispanic female with hypertension presented to the emergency department for fatigue, headache, hematochezia, rash on all extremities, and bleeding from the oral mucosa. She reported that about two weeks prior to admission she went to an outside emergency room (ER) for three days of nausea, vomiting, diarrhea, and abdominal pain, which subsequently improved with antiemetics and hydration. Therefore, she was discharged from the ER with antiemetics. Two days later during a follow up visit with her primary care physician, she complained of a cough and flulike symptoms for which she was prescribed Keflex. However, the patient discontinued the antibiotic two days later when she developed hemoptysis, hematochezia, and a rash on her extremities.
During the current ER visit, the physical exam was significant for tachycardia, pallor, oral wet purpura, and petechiae on the lower extremities. CBC revealed a WBC 16.9x103/µL, RBC count of 2.29x1012/L, Hgb of 6.2 g/dL, mean corpuscular volume of 88.6 fL, reticulocyte percent of 10.54%, platelets 2000/µL, absolute neutrophil count of 11x109/L. LDH was elevated at 623 U/L but direct and indirect coombs were negative, and there was no elevated bilirubin or AST, thus ruling out hemolysis. Creatinine, PT, and PTT were all in the normal range as well. B12 level was 337 pg/ml (within the reference range of the laboratory test manufacturer) and folic acid was 19.1 ng/ml. Stool studies including shigatoxin, and urinalysis were all normal. However, stool hemoccult was weakly positive. Analysis of the peripheral smear revealed slight anisocytosis, polychromasia, dyserythropoiesis, dysplastic neutrophils, absence of schistocytes, and left shifted myeloid cell lines with few blasts (Figures 1, 2). Serology for HCV, HBV, and HIV were negative. Ebstein Barr Virus (EBV) titer was found to be 1344 U/l and cytomegalovirus (CMV) was undetectable by PCR. Given the patient’s headache in the setting of severe thrombocytopenia, a CT head without contrast completed in the ER ruled out intracranial bleeding. A CT abdomen/pelvis showed mild thickening of the distal esophagus, antrum, and pyloric area. An abdominal ultrasound showed gallbladder polyps but was otherwise unremarkable. However, given the low platelet counts and increased risk of bleeding, endoscopy and colonoscopy were postponed until after the platelet count recovery.
Figure 1.
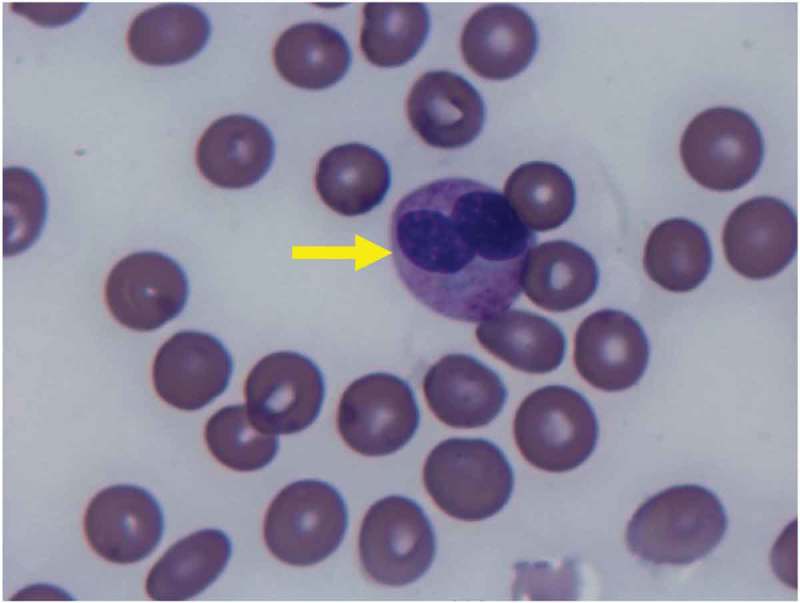
Peripheral smear H&E stain. Pseudo Pelger-Huet neutrophil: a finding seen in MDS, can also be seen in B12 deficiency. This finding is characterized by a bilobed nucleus, and markedly reduced granulation.
Figure 2.
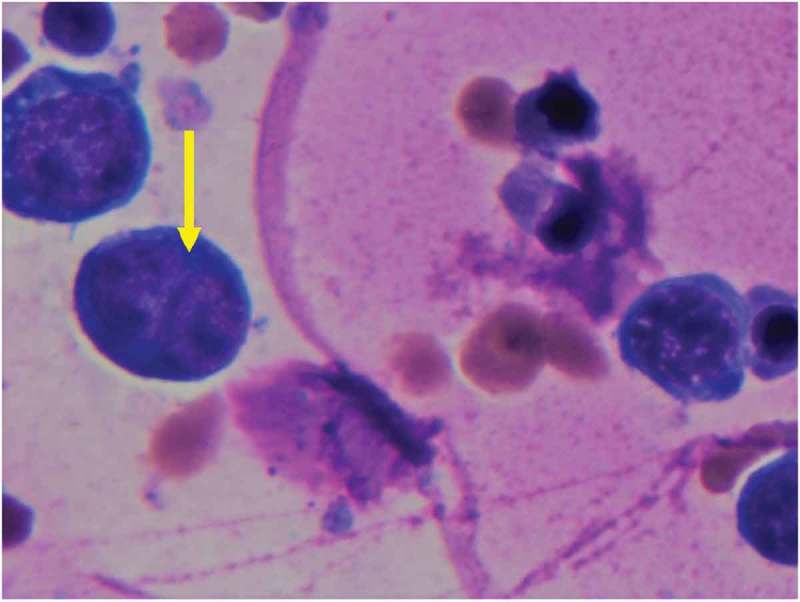
Peripheral smear H&E stain. (a) Dyserythropoiesis Giemsa stain 1000x. Nuclear budding is evident in image.
Given the presence of blasts in the peripheral blood smear and her transfusion requirements, the patient was admitted to the medicine service to rule out malignancy. She was transfused several units of platelets and packed red blood cells to maintain the platelet count >10x103/µL and Hgb>7 g/dL. The patient subsequently developed hypoxia and chest imaging revealed bilateral infiltrates. She was treated with IV furosemide, which resulted in resolution of the hypoxia and volume overload.
On day two of the hospitalization a bone marrow biopsy was performed (Figure 3). On day five, examination of the bone marrow was reported to be consistent with the diagnosis of myelodysplastic syndrome without evidence of EBV in the bone marrow, therefore hematology was consulted for management of MDS. At this time, she was treated with an injection of 1000 µg Vitamin B12 due to her borderline B12 levels (B12 < 400 pg/ml), after which her Hgb remained above 8 g/dl without blood transfusions. Given the response, empiric treatment with high dose oral B12 was continued. She was also treated with folic acid and iron to empower effective hematopoiesis. Methylmalonic acid (MMA) was ordered to confirm B12 deficiency, and blood was collected to rule out deficiencies of zinc and copper (which returned normal). However, her serum ferritin was considered low at 66.7 ng/ml (given the elevated inflammatory markers of ESR at 79 mm/hr, CRP 3.17 mg/L, and equivocal ANA at 1/40 and homogenous).
Figure 3.
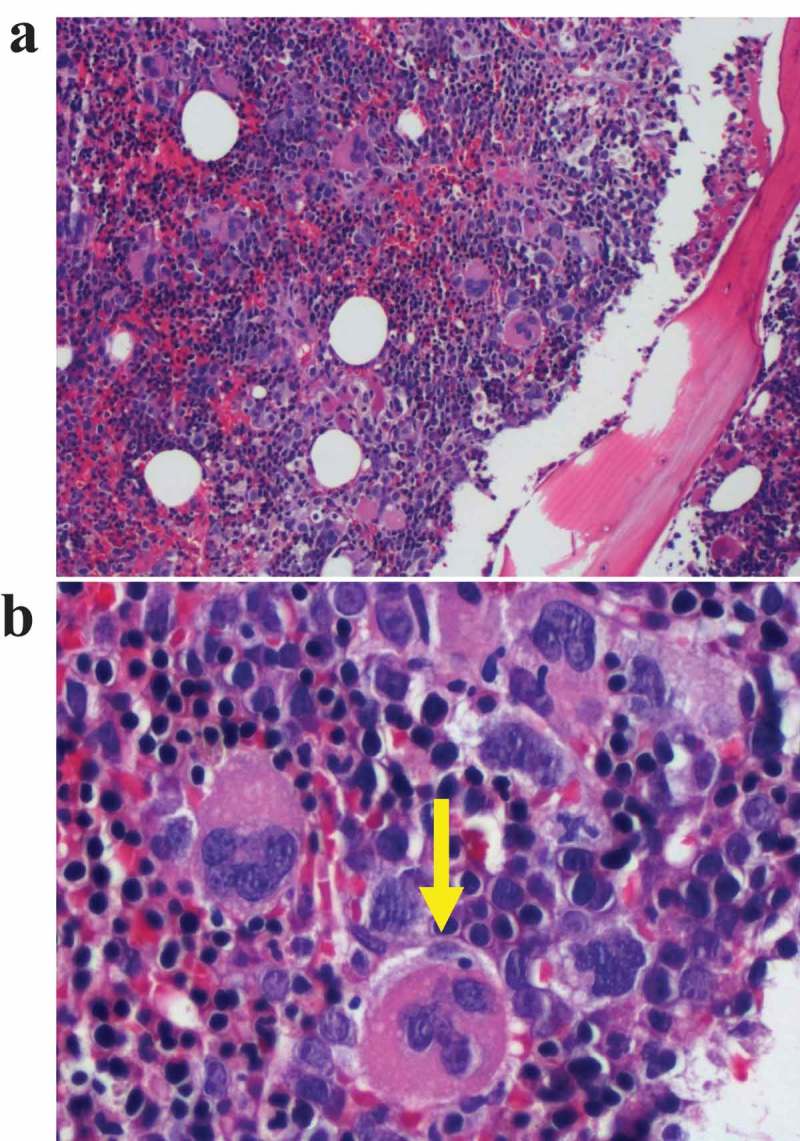
Bone marrow aspirate one day after admission. (a) Erythroid and megakaryocyte hyperplasia: The bone marrow is hypercellular with ineffective erythropoiesis. (b) Megakaryocyte dysplasia. The megakaryocytes are abnormal with multiple small lobes seemingly disconnected from one another.
On day twelve, her MMA resulted elevated at 666 nmol/L, confirming B12 deficiency. Therefore, the patient was discharged with high dose oral cyanocobalamin, iron, and folic acid with a hemoglobin of 9.5 g/dl and a platelet count of 8000/µL. As outpatient, she only required two more platelet transfusions in the week of discharge. Two weeks after discharge, she was seen in the hematology clinic for a follow up visit. At this time her Hb was 11.4 g/dl, platelet count was at 49,000/µL, and reticulocyte count decreased to 5.29%. Her MMA and B12 both normalized. She was tested for intrinsic factor antibody, which was positive confirming pernicious anemia. Her EBV titers became undetectable. Three weeks after discharge from the hospital, her platelets and hemoglobin normalized.
3. Discussion
Cases have been previously reported of having a classical presentation of B12 deficiency (macrocytic anemia, low B12 on labs, neurological symptoms, and hypersegmented neutrophils) who still underwent a bone marrow biopsy [3]. The case described herein demonstrates that B12 deficiency can also present without the classical symptoms, further complicating the clinical picture. In this case, one confounding factor that also confused the clinical picture was her iron deficiency anemia, which likely occurred due to a GI bleed that began prior to admission.
Clinicians may order a bone marrow biopsy on patients with pancytopenia to rule out malignancies [1,3]. However, this case highlights that the evaluation of a bone marrow biopsy without considering secondary causes of pancytopenia in their differential diagnosis may complicate the scenario. The presence of blasts in the peripheral smear in this case is a sign of increased bone marrow activity due to ineffective erythropoiesis, which can be misinterpreted as a malignant process. Thus, a hematology consult is appropriate for an unclear etiology of cytopenia, to guide the workup and assist with the interpretation of findings.
The first step to narrowing the differential diagnosis is to gather a thorough history from the patient. The clinician should consider the patient’s medication list, comorbidities, occupational exposure to solvents, recent viral infections, and any history of alcohol abuse [1,4,5]. In this case, the history revealed hematochezia, which likely caused her iron deficiency and lack of macrocytosis in the presence of B12 deficiency [1].
Important to the case described herein is Vitamin B12 deficiency. If a patient has an unclear cause of pancytopenia, B12 deficiency should be suspected. The presence of macrocytic anemia and hypersegmented neutrophils suggests B12 deficiency, however in this case these classic features where likely absent secondary to a concomitant iron deficiency. Additionally, definitive cut-off points to define clinical and subclinical B12 deficiency are still controversial due to the various methods used to test cobalamin levels, as well as the existing potential technical errors [6,7]. Studies agree that a diagnosis of B12 deficiency requires a value of <200pg/ml. There is also a consensus that the clinical picture of the patient is of utmost importance in assessing for B12 deficiency [7].
The case reported in this article demonstrates that patients with a B12 value at the lower end of the reference range of the laboratory test manufacturer, in this case337pg/ml, can still be considered B12 deficient. Therefore, if B12 deficiency is suspected, it is critical to order a methylmalonic acid (MMA) even if the B12 value appears to be within the reference range of the laboratory test manufacturer. An elevated MMA level is diagnostic of vitamin B12 deficiency [1,7]. Elevated MMA can also occur in patients with renal insufficiency due to impaired glomerular filtration, possibly leading to a false diagnosis of B12 deficiency. However, existing data suggests that MMA>500 nmol/L in the setting of renal insufficiency may be suggestive of B12 deficiency [1].
When there is an unclear reason as to why the patient would be B12 deficient, the clinician should suspect pernicious anemia, which is the most common cause of B12 deficiency [1]. Pernicious anemia is an autoimmune cause of B12 deficiency [6,8,9]. Testing for anti-intrinsic factor antibodies is a specific means to confirm the patient has pernicious anemia [1,6]. If the clinical picture and labs do not point to the same diagnosis, or the results of specific diagnostic tests are not available in a timely manner (as in this case) it is safe to empirically administer Vitamin B12 and assess the bone marrow response, evident by an increasing reticulocyte count and hemoglobin level [10]. If a patient receives empiric B12 treatment, anti-intrinsic factor antibodies should be checked at least one week after holding B12 supplementation to avoid a false positive result [1]. Also, it is important to consider that patients with anti-intrinsic factor antibodies can have falsely elevated B12 levels due to the autoantibodies’ ability to interfere with the test reagents [11–13], which possibly occurred in this case.
MDS comprises a heterogeneous group of disorders characterized by dysplastic and ineffective hematopoiesis with some risk of transformation to leukemia [14]. Both the quality and the quantity of different cell lines can be affected [14]. Patients with MDS also present with cytopenias. A bone marrow biopsy of a patient with B12 deficiency may resemble that of a patient with MDS because both processes can lead to ineffective hematopoiesis and bone marrow failure [2,15]. Since the treatments for these two conditions are drastically different, it is important to distinguish between them to avoid unnecessarily treating a patient with hypomethylating agents [2].
4. Conclusion
Cytopenias and bone marrow dysplasia can be due to primary MDS or secondary to more common causes, including B12 deficiency. If a bone marrow biopsy is performed without investigating secondary causes of cytopenia, a false diagnosis of MDS may be made, causing the patient emotional trauma and unnecessary treatment with hypomethylating agents. In pernicious anemia, B12 levels can be falsely elevated in the presence of anti-intrinsic factor antibodies, therefore, checking methylmalonic acid and anti-intrinsic factor antibodies can establish the diagnosis. B12 supplementation can also affect the anti-intrinsic factor antibody assays leading to false positive results. B12 measurements should be completed before starting supplementation or delayed to at least one week after stopping B12 supplementation.
Funding Statement
This article has been independently funded.
Acknowledgments
Authors would like to thank Dr. Ronadlo Gnass for providing us with the histopathology images for this manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Stabler SP.Clinical practice. Vitamin B12 deficiency. N Engl J Med. 2013;368:149. [DOI] [PubMed] [Google Scholar]
- [2].Steensma DP.Dysplasia has a differential diagnosis: distinguishing genuine myelodysplastic syndromes (MDS) from mimics, imitators, copycats and impostors. Curr Hematol Malig Rep. 2012;7:310–320. [DOI] [PubMed] [Google Scholar]
- [3].Randhawa J, Ondrejka SL, Setrakian S, et al. What should I know before ordering a bone marrow aspiration/biopsy in patients with vitamin B12 deficiency? Case Rep. 2013;2013:bcr2013010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Jain A, Naniwadekar M. An etiological reappraisal of pancytopenia - largest series reported to date from a single tertiary care teaching hospital. BMC Hematol. 2013;13:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Pascutti MF, Erkelens MN, Nolte MA. Impact of viral infections on hematopoiesis: from beneficial to detrimental effects on bone marrow output. Front Immunol. 2016;7:364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Green R. Vitamin B12 deficiency from the perspective of a practicing haematologist. Blood. 2017May11;129(19):2603–2611. [DOI] [PubMed] [Google Scholar]
- [7].Devalia V, Hamilton MS, Molloy AM. The British committee for standards in haematology. Guidelines for the diagnosis and treatment of cobalamin and folate disorders. Brit J Haematol. 2014;166:496–513. [DOI] [PubMed] [Google Scholar]
- [8].Kapadia CR, Donaldson RM Jr.. Disorders of cobalamin (vitamin B12) absorption and transport. Annu Rev Med. 1985;36:93–110. [DOI] [PubMed] [Google Scholar]
- [9].Irvine WJ, Davies SH, Teitelbaum S, et al. The clinical and pathological significance of gastric parietal cell antibody. Ann N Y Acad Sci. 1965;124(2):657–691. [DOI] [PubMed] [Google Scholar]
- [10].Kim M, Lee SE, Park J, et al. Vitamin B 12 responsive pancytopenia mimicking myelodysplastic syndrome. Acta Haematol. 2011;125:198–201. [DOI] [PubMed] [Google Scholar]
- [11].Yang DT, Cook RJ. Spurious elevations of vitamin B12 with pernicious anemia. N Engl J Med. 2012;366(18):1742–1743. [DOI] [PubMed] [Google Scholar]
- [12].Hamilton MS, Blackmore S, Lee A. Possible cause of false normal B-12 assays. Bmj. 2006;333:654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Carmel R, Agrawal YP. Failures of cobalamin assays in pernicious anemia. N Engl J Med. 2012;367:385. [DOI] [PubMed] [Google Scholar]
- [14].Pang WW, Pluvinage JV, Price EA, et al. Hematopoietic stem cell and progenitor cell mechanisms in myelodysplastic syndromes. Proc Natl Acad Sci U S A. 2013;110(8):3011–3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Weinzierl EP, Arber DA. Bone marrow evaluation in new-onset pancytopenia. Hum Pathol. 2013;44:1154. [DOI] [PubMed] [Google Scholar]


