ABSTRACT
AL amyloidosis is due to deposition of protein derived from immunoglobulin light chain fragments. It is a systemic disorder in which deposition of plasma proteins can adversely affect function of the heart, liver, kidneys, and peripheral nerves. Deposition in the heart results in a decrease in the amplitude of the electrical activity of the heart and can be an early clue to the diagnosis.
A 63-year-old male admitted for volume overload was found to have nephrotic range proteinuria, progressive renal insufficiency (Creatinine 4.0 increased from his baseline 0.9), and hypoalbuminemia. On exam, he had diffuse anasarca and peripheral neuropathy. A renal biopsy showed AL amyloidosis, lambda related, involving the glomeruli, interstitium, and arterial walls. Bone marrow biopsy showed 30% plasma cells with lambda light chain predominance. Serum free light chains were elevated. Lamda was 11.50 mg/dL and kappa was 5.12 mg/dL. In retrospective review of his chart, an EKG with low voltage and anterior pseudo-infarct pattern was first apparent on an admission for stroke two years prior. Echocardiogram showed mild concentric left ventricular hypertrophy. The patient was started on chemotherapy with Bortezomib.
The differential of a low-voltage EKG includes many common pulmonary and chest wall (COPD, obesity) as well as pericardial diseases (effusions), but also important rarer infiltrative diseases including sarcoidosis and amyloidosis. Amyloidosis of the heart can cause progressive irreversible heart failure, but its progress can be altered if identified early. Physicians should consider amyloidosis when faced with a low-voltage EKG along with systemic symptoms including nephrotic range proteinuria, peripheral neuropathy, hepatosplenomegaly, and macroglossia.
KEYWORDS: Cardiac amyloidosis, electrocardiography
1. Introduction
AL amyloidosis is a rare systemic disease resulting from tissue accumulation of amyloid fibrils derived from monoclonal immunoglobulin light chains. These light chains can deposit in various organs, resulting in varying clinical presentations of the disease.
Amyloidosis frequently involves the heart when amyloid fibrils are deposited in myocardial tissue resulting in reduced ventricular compliance with impairment of relaxation[1]. Amyloid fibrils may also deposit in myocardial vessels and cause local ischemia and can also cause fibrosis of conduction tissue, resulting in conduction abnormalities and arrhythmias[2]. Cardiac amyloidosis (CA) is associated with a variable but generally poor prognosis, and early recognition may lead to improved outcomes.
We discuss two particular findings on ECG in our case, low voltage on limb leads and pseudo-infarct pattern, that can help provide early identification and diagnosis of CA.
2. Case Description
A 63-year-old male with a past medical history of COPD, CVA with residual left-sided weakness, and hypertension presented with dyspnea on exertion, lower extremity swelling, abdominal distention, and weight gain over the course of six months. He was admitted for anasarca. Initial laboratory evaluation revealed nephrotic range proteinuria (urine protein creatinine ratio 5.14), progressive renal insufficiency (Creatinine 2.5 mg/dL increased from his baseline 0.9 mg/dL), and hypoalbuminemia (albumin 2.0 g/dL). On exam, he had diffuse anasarca and peripheral neuropathy. A renal biopsy showed AL amyloidosis, lambda related, involving the glomeruli, interstitium, and arterial walls. During this admission, the patient unfortunately left against medical advice.
He presented to the hospital three months later with the same complaints of dyspnea on exertion, abdominal distention, weight gain, and difficulty walking from the lower extremity swelling. His renal function worsened to creatinine of 3.4 mg/dL. Serum free light chains revealed elevated lambda light chains 11.50 mg/dL, elevated kappa light chains 5.12 mg/dL, but a normal kappa/lambda ratio 0.45. Hemoglobin and calcium were within normal limits at 14.4 g/dL and 8.8 mg/dL. Skeletal x-ray did not show bone lesions.
Hematology-Oncology was consulted who recommended a bone marrow biopsy. Bone marrow biopsy showed 30% plasma cells with lambda light chain predominance. A CD 138 immunohistochemical stain identified plasma cells (see Figure 1). The bone marrow touch imprints (see Figure 2) identify plasma cells. Congo red stain was performed and the apple green birefringence characteristic of amyloid was not identified. FISH analysis was positive for 13q deletion, monosomy 13, and additional copies of chromosomes 1q and 9. Cytogenetics revealed normal male karyotype.
Figure 1.
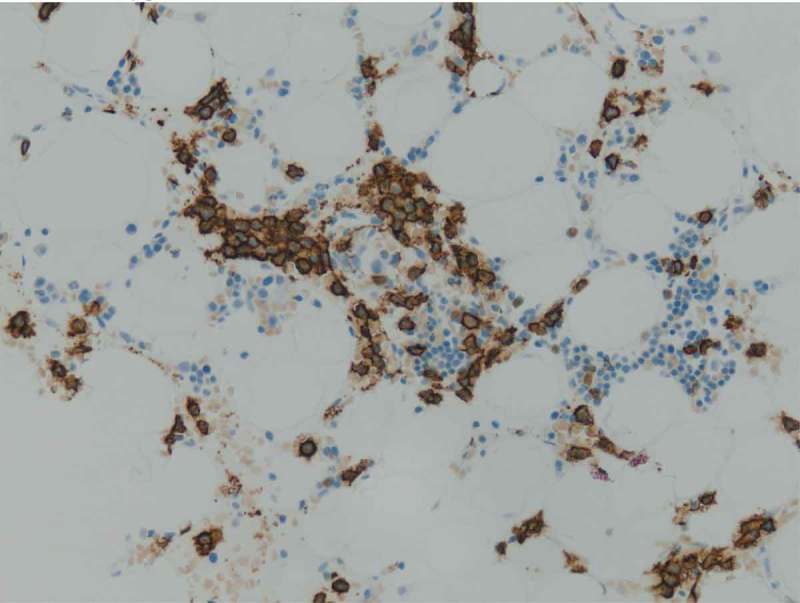
During normal B-cell development, cells acquire expression of CD 138 which is a marker highly specific for terminally differentiated normal plasma cells. Since CD 138 is a specific surface antigen for plasma cells in the bone marrow, a CD 138 immunohistochemical stain can identify plasma cells as seen in our case.
Figure 2.
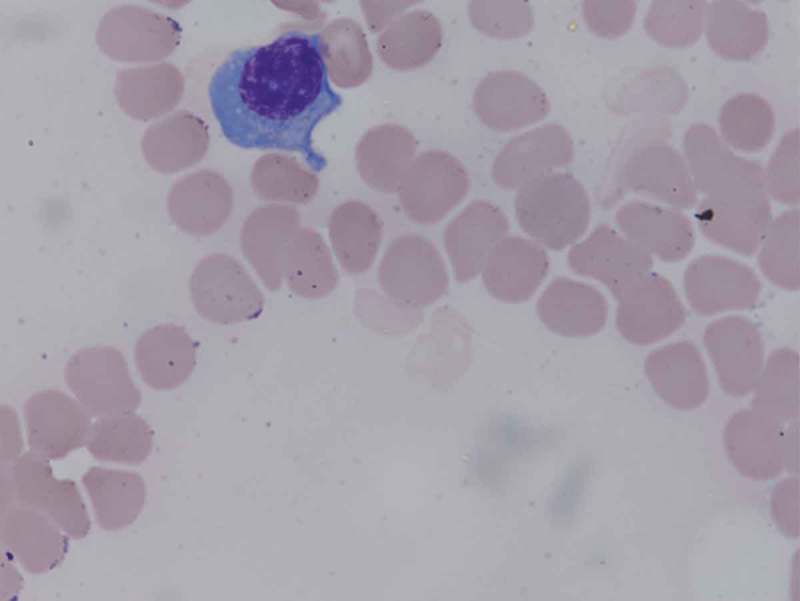
Plasma cells are large lymphocytes with a considerable nucleus-to-cytoplasm ratio. They have an eccentric nucleus with chromatin in a characteristic clock-face arrangement. This plasma cell in our patient is seen from the bone marrow touch imprints.
In retrospective review of his chart, there were signs of cardiac amyloidosis on his admission for a stroke two years prior. ECG at that time showed low voltage criteria and an anterior pseudo-infarct pattern with Q waves in V1, V2, and V3 (see Figure 3). Echocardiogram showed mild concentric left ventricular hypertrophy. The patient was started on chemotherapy with Bortezomib but after one cycle, decided to go on home hospice.
Figure 3.
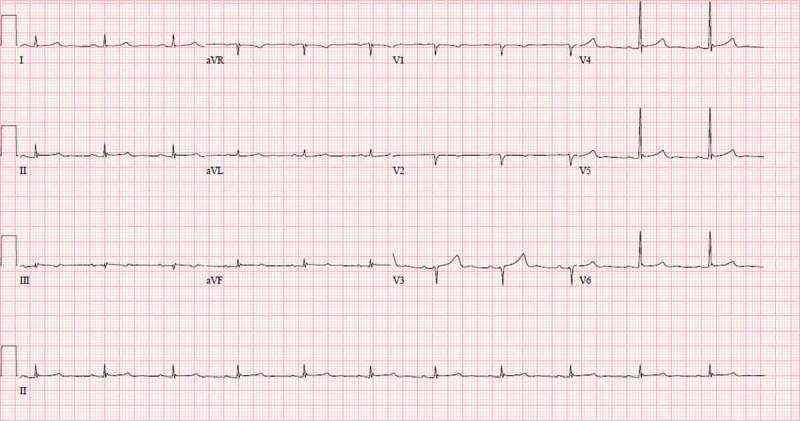
EKG shows low voltage criteria in the limb leads. EKG is also significant for anterior pseudo-infarct pattern with Q waves in anterior leads V1 – V3. These two findings have high specificity and high predictive value for cardiac amyloidosis.
3. Discussion
AL amyloidosis is the most frequent form of amyloidosis (70%)[3]. Amyloidosis constitutes a group of diseases in which misfolding of extracellular protein generates insoluble, toxic protein aggregates that are deposited in tissues in bundles of β-sheet fibrillary protein[4]. The clinical manifestations of the disease are extremely variable depending on the organs involved and the extent of tissue deposition. Three quarters of the patients have involvement of multiple organs at presentation with, in order of frequency, kidneys (46%), heart (30%), liver (9%), gastrointestinal tract (7%), peripheral nervous system (5%), and soft tissues (3%)[4].
Renal amyloidosis usually manifests as proteinuria, often resulting in nephrotic syndrome, as seen in our case. Development of end-stage renal disease is common [3,5]. Cardiac involvement may lead to cardiomegaly, congestive heart failure, and different rhythm disturbances. The electrocardiographic findings include low voltage and, often, a pattern of myocardial infarction. Echocardiography usually reveals a concentric thickening of the left ventricle [3,5]. Increased myocardial echogenicity with ‘granular sparkling’ appearance is very specific of the disease[5]. In this patient, there was renal amyloidosis present with nephrotic range proteinuria, progressive renal insufficiency, and hypoalbuminemia. Renal amyloidosis was confirmed with the renal biopsy. Two years prior to the diagnosis of renal amyloidosis, low voltage and anterior pseudo-infarct pattern on ECG were clinical clues for cardiac amyloidosis (CA). Cheng et al. concluded that the combination of low voltage on limb leads and pseudo-infarct pattern on ECG has high specificity and positive predictive value for the diagnosis of CA[6]. The results of the Cheng et al. study were similar with previous reports [7–9]. Murtagh et al. [7]. from the Mayo Clinic reported that in 127 patients with biopsy-proven proven CA, 46% had low voltage and 47% had pseudo-infarct pattern. Rahman et al. [8]. reported that the low voltage was in 56% and pseudo-infarct pattern was in 60% of patients. Austin et al. [9]. reported that 45 patients with biopsy-proven CA, the low voltage was seen in 27% of patients.
The aforementioned studies implicate there were multiple organs involved at the time of our patient’s diagnosis with renal and cardiac involvement. During the admission when our patient was diagnosed with renal amyloidosis, he also complained of numbness and tingling in his legs. Sensory neuropathy can be present in AL amyloidosis, usually as a distal to proximal and symmetric pattern[5]. However, without electromyography and nerve conduction studies, there cannot be a definitive diagnosis of peripheral nervous system involvement.
The clinical history of AL amyloidosis is an interesting illustration of a multi-systemic disease. The prognosis of amyloidosis is poor with a median survival of 13 months in untreated patients[10]. Survival of patients with AL undergoing autologous stem-cell transplantation is prolonged to 54 months[11]. Prognosis is also dependent on the number of organs involved and the extent of tissue deposition. Our patient likely had renal and cardiac amyloidosis at the time of diagnosis. Our retrospective chart review revealed the low voltage and anterior pseudo-infarct pattern on ECG that preceded the diagnosis by two years. When evaluating low voltage, one must consider that other conditions such as pericardial disease, pleural effusions, obesity, and anasarca may contribute to the presence of low voltage on the ECG [12–14]. The combination of low voltage and anterior pseudo-infarct pattern on ECG, however, have high specificity and positive predictive value for the diagnosis of CA[1]. Our case highlights the role that electrocardiography can play for early diagnosis and treatment of AL amyloidosis.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Cheng Z, Zhu K, Tian Z, et al. The Findings of Electrocardiography in Patients with Cardiac Amyloidosis. Ann Noninvasive Electrocardiol. 2013;18:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Gertz MA, Comenzo R, Falk RH, et al. Definition of organ involvement and treatment response in immunoglobulin light chain amyloidosis (AL): A consensus opinion from the 10th International Symposium on Amyloid and Amyloidosis, Tours, France, 18–22 April 2004. Am J Hematol. 2005;79:319–328. [DOI] [PubMed] [Google Scholar]
- [3].Falk RH, Comenzo RL, Skinner M.. The systemic amyloidosis. N Engl J Med. 1997;337:898–909. [DOI] [PubMed] [Google Scholar]
- [4].Merlini G, Bellotti V. Molecular mechanisms of amyloidosis. N Engl J Med. 2003;349:583–596. [DOI] [PubMed] [Google Scholar]
- [5].Pascali E. Diagnosis and treatment of primary amyloidosis. Critic Rev Oncol Hemato. 1995;19:149–181. [DOI] [PubMed] [Google Scholar]
- [6].Cheng Z, Kang L, Tian Z, et al. Utility of combined indexes of electrocardiography and echocardiography in the diagnosis of biopsy proven primary cardiac amyloidosis. Ann Noninvasive Electrocardiol. 2011;16:25–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Murtagh B, Hammill SC, Gertz MA, et al. Electrocardiographic findings in primary systemic amyloidosis and biopsy-proven cardiac involvement. Am J Cardiol. 2005;95:535–537. [DOI] [PubMed] [Google Scholar]
- [8].Rahman JE, Helou EF, Gelzer-Bell R, et al. Noninvasive diagnosis of biopsy-proven cardiac amyloidosis. J Am Coll Cardiol. 2004;43:410–415. [DOI] [PubMed] [Google Scholar]
- [9].Austin BA, Duffy B, Tan C, et al. Comparison of functional status, electrocardiographic, and echocardiographic parameters to mortality in endomyocardial-biopsy proven cardiac amyloidosis. Am J Cardiol. 2009;103:1429–1433. [DOI] [PubMed] [Google Scholar]
- [10].Rysava R, Merta M, Spicka I, et al. Current therapeutic possibilities in primary and secondary amyloidosis and our experience with 31 patients. Nephrol Dial Transplant. 2003;18(Suppl 5):v38–v40. [DOI] [PubMed] [Google Scholar]
- [11].Martha S, Vaishali S, David C, et al. High-dose melphalan and autologous stem-cell transplantation in patients with AL amyloidosis: an 8-year study. Ann Intern Med. 2004;140:85–93. [DOI] [PubMed] [Google Scholar]
- [12].Carroll JD, Gaasch WH, McAdam KP. Amyloid cardiomyopathy: characterization by a distinctive voltage/mass relation. Am J Cardiol. 1982;49:9–13. [DOI] [PubMed] [Google Scholar]
- [13].Nizet PM, Marriott HJL. The electrocardiogram and pericardial effusion. Jama. 1966;198:169. [PubMed] [Google Scholar]
- [14].Madias JE, Bazaz R, Agarwal H, et al. Anasarca-mediated attenuation of the amplitude of electrocardiogram complexes: a description of heretofore unrecognized phenomenon. J Am Coll Cardiol. 2001;38:756–764. [DOI] [PubMed] [Google Scholar]


