Abstract
Background and purpose
Juvenile myoclonic epilepsy (JME) is a common epilepsy syndrome for which treatment response is generally assumed to be good. We aimed to determine the prevalence and prognostic risk factors for refractoriness of JME.
Methods
We systematically searched PubMed and EMBASE and included 43 eligible studies, reporting seizure outcome after antiepileptic drug (AED) treatment in JME cohorts. We defined refractory JME as persistence of any seizure despite AED treatment and performed a random‐effects meta‐analysis to assess the prevalence of refractory JME and of seizure recurrence after AED withdrawal in individuals with well‐controlled seizures. Studies reporting potential prognostic risk factors in relation to seizure outcome were included for subsequent meta‐analysis of risk factors for refractoriness.
Results
Overall, 35% (95% confidence interval, 29–41%) of individuals (n = 3311) were refractory. There was marked heterogeneity between studies. Seizures recurred in 78% (95% confidence interval, 52–94%) of individuals who attempted to withdraw from treatment after a period of seizure freedom (n = 246). Seizure outcome by publication year suggested that prognosis did not improve over time. Meta‐analysis suggested six variables as prognostic factors for refractoriness, i.e. having three seizure types, absence seizures, psychiatric comorbidities, earlier age at seizure onset, history of childhood absence epilepsy and praxis‐induced seizures.
Conclusion
One‐third of people with JME were refractory, which is a higher prevalence than expected. Risk factors were identified and can be used to guide treatment and counselling of people with JME.
Keywords: epilepsy, Janz syndrome, meta‐analysis, pharmacoresistance, systematic review
Introduction
Juvenile myoclonic epilepsy (JME) is the most common form of genetic generalized epilepsy, affecting 5–10% of all people with epilepsy, with a prevalence of 0.1–0.2/100 000 1. JME typically manifests during adolescence and is characterized by arrhythmic myoclonic seizures, particularly occurring on awakening, and electroencephalography that shows generalized spike and polyspike waves 2. Although not required for diagnosis, people with JME often also experience generalized tonic‐clonic seizures and, less often, absence seizures 2. According to its definition ‘response to appropriate drugs is good’ 2. This could lead to optimistic counselling by physicians. Seizures, however, continue despite adequate treatment with antiepileptic drugs (AEDs) in a proportion of patients and this impacts on quality of life 3, 4. Once an individual becomes seizure‐free on AEDs, it is usually recommended to continue life‐long therapy, given the high risk of relapse following drug withdrawal 5, 6. Some studies have suggested that a subset of individuals remains seizure‐free after drug withdrawal 7, 8. It is important to establish how often individuals are refractory and how frequently AEDs can be safely withdrawn to allow reliable prognostic counselling.
Several studies have explored risk factors for refractory JME but individual studies are limited by relatively small sample sizes and there are inconsistencies between studies. Prediction of refractoriness is of value for individualized management, e.g. by considering higher drug doses, polytherapy, experimental AEDs or non‐pharmacological treatment options earlier in those at risk 9, 10, 11, 12.
We aimed to provide a systematic overview of refractory JME and its prognostic risk factors. By meta‐analysing available studies, we estimated the proportion of refractory JME and, at the other end of the spectrum, the proportion of individuals remaining seizure‐free after drug withdrawal. Lastly, we assessed which clinical variables may predict refractory JME.
Methods
Search strategy and study selection
Procedures were consistent with PRISMA guidelines 13. A literature search in PubMed and EMBASE identified articles describing treatment outcome in people with JME (see Tables S1 and S2 for search terms). We did not adopt a registered pre‐specified protocol.
We included all retrospective and prospective studies reporting seizure outcome after AED treatment in observational cohorts of individuals with a diagnosis of JME, regardless of the diagnostic criteria used by the study (see Table S3 for an overview), which may vary 14. We excluded articles that specifically recruited refractory individuals or those in remission. Drug‐trial reports were not included as they could be biased towards individuals with a refractory condition. We contacted authors of articles describing multiple generalized epilepsy syndromes to provide stratified data of individuals with JME, if not available in the publication. We only included articles describing seizure freedom from all seizure types and excluded those with ambiguous definitions (e.g. ‘good outcome’) without specifying seizure freedom. When the same cohort was included in multiple reports, we included the most recent report, except in cases where an older article provided data on potential risk factors of refractory JME. Articles in English, Dutch and German were included.
Definitions of seizure freedom and refractory JME varied between articles, primarily regarding the length of the seizure‐free follow‐up period. Only two articles used the definition of drug‐resistant epilepsy proposed by the International League Against Epilepsy in 2010 15. We defined refractory JME as persistence of any seizure (i.e. myoclonic, absence or generalized tonic‐clonic seizures) despite AED treatment, regardless of the length of the seizure‐free follow‐up period. We assessed 1‐year seizure freedom when multiple time points were described within the same study. Where possible, individual cases of ‘pseudo‐refractory’ individuals (i.e. those who had seizures due to non‐compliance, inadequate treatment or other factors not related to therapy) were excluded.
Studies reporting potential prognostic risk factors stratified by seizure outcome were included for subsequent meta‐analysis of risk factors for refractoriness.
All search results were reviewed based on title and abstract, and the full text was reviewed in potentially eligible articles. Reference lists were checked for additional eligible articles.
Data extraction
Study selection and data extraction were performed by R.S. A standardized data extraction form was created that contained the number of individuals who were seizure‐free and those who were refractory, seizure outcome after drug withdrawal, mean follow‐up duration, country, prospective or retrospective design, type of AED used and definition of seizure freedom.
Data of prognostic risk factors from articles reporting clinical variables stratified by seizure outcome were also extracted. To reduce publication bias, raw data of potential risk factors were extracted from all articles, regardless of whether the variable was tested for association with seizure outcome. We analysed only potential risk factors that were reported in at least two articles, regardless of whether they were significantly associated with outcome.
Statistical analyses
A random‐effects meta‐analysis was performed using the R package Metafor (v2.0‐0) to assess the prevalence of refractoriness. The I 2 statistic was assessed as a measure to quantify heterogeneity, where values between 50% and 75% are considered to represent moderate heterogeneity and those >75% represent high heterogeneity 16. We used a random‐effects model to account for heterogeneity between studies 17. Secondary analyses stratified by definition of refractory JME and by study design (prospective or retrospective) were performed to assess whether this increased homogeneity. Differences by publication year and differences between 1‐, 2‐ and 5‐year seizure freedom were assessed with a mixed‐effects meta‐regression, using Metafor. A random‐effects meta‐analysis was performed using Metafor to assess the prevalence of individuals who remained seizure‐free after AED withdrawal.
Random‐effects meta‐analyses of potential risk factors were performed using Review Manager (v5.3) for all potential risk factors reported in at least two articles. We assessed the odds ratio as outcome measure for dichotomous variables and the mean difference for continuous variables.
Quality and bias assessment
The Newcastle–Ottawa quality assessment scale for cohort studies was used to assess the methodological quality of all studies included in the meta‐analysis of risk factors 18. This scale is used to assess three major components, i.e. cohort selection, comparability and assessment of outcome, and ranges from 0 to 9, where studies are considered to have a high quality when scoring ≥5 and a low quality when scoring <5.
Funnel plots were generated as a measure to assess potential publication bias and were visually inspected for asymmetry 19. Considering the small number of studies included per risk factor, we did not perform statistical tests for asymmetry of the funnel plot, as it is only recommended when including >10 studies per analysis 19.
Results
The literature search was last performed on 1 March 2018 and yielded 1362 articles (see Fig. 1 for flowchart). After removing duplicates and applying inclusion and exclusion criteria, 43 articles were included, describing treatment outcomes for a total of 3311 subjects (Table S4).
Figure 1.
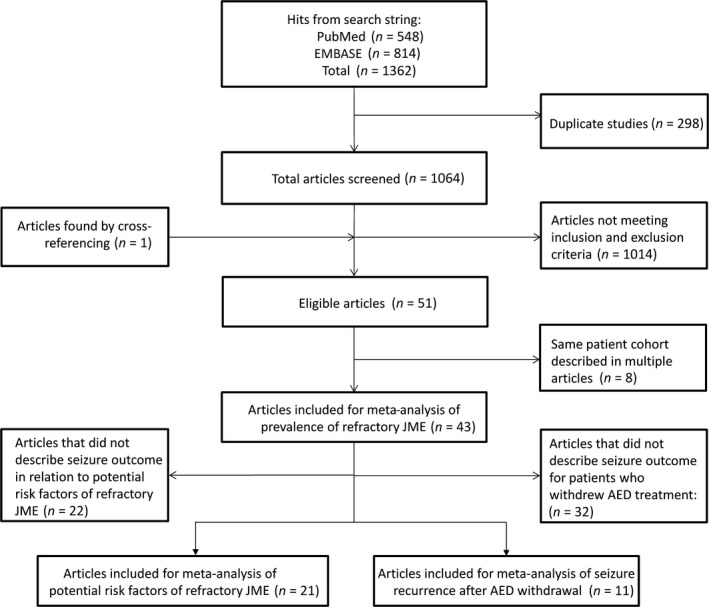
Flowchart of search strategy and study selection. AED, antiepileptic drug; JME, juvenile myoclonic epilepsy.
Prevalence of refractory juvenile myoclonic epilepsy
Meta‐analysis showed that 35% [95% confidence interval (CI), 29–41%] of individuals with JME were refractory to treatment (Fig. 2). The proportion of refractory subjects varied between 7% and 75%, and heterogeneity between studies was high (I 2 = 91%). As the definition of seizure freedom varied between studies, we also performed analyses stratified by definition, which made little difference to the estimate of refractory JME or the amount of heterogeneity (Fig. 3). A meta‐regression analysis showed no significant difference between 1‐, 2‐ and 5‐year seizure freedom (P = 0.41). The proportion of refractory patients was comparable between prospective (36%; 95% CI, 18–56%) and retrospective (35%; 95% CI, 29–42%) studies.
Figure 2.
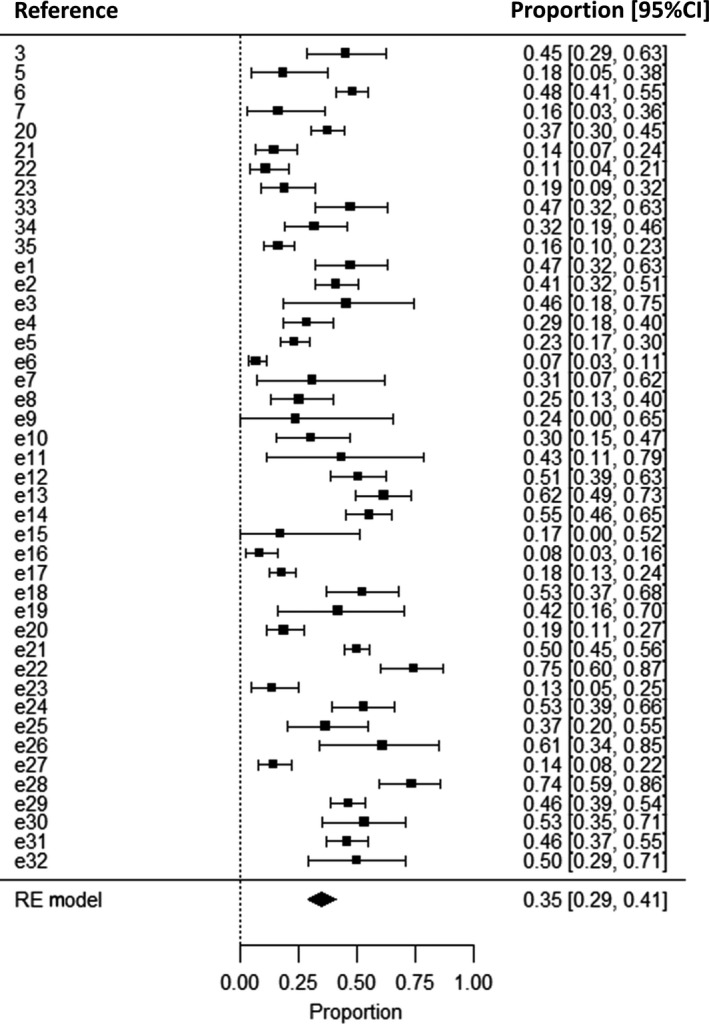
Meta‐analysis of the prevalence of refractory juvenile myoclonic epilepsy (JME). The proportion of subjects who were refractory is displayed on the x‐axis. A total of 43 studies describing seizure outcome in 3311 individuals with JME were included. CI, confidence interval; RE, random‐effects. References denoted as ‘e’ are available in the Supporting Information.
Figure 3.
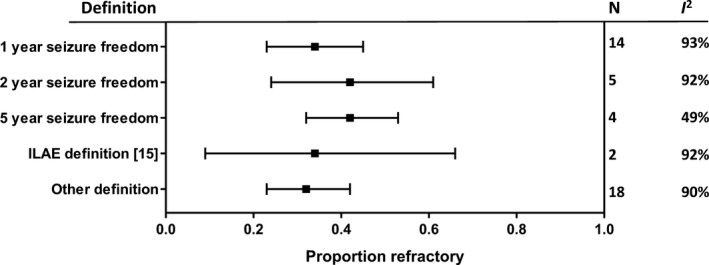
Meta‐analyses of the prevalence of refractory juvenile myoclonic epilepsy stratified by definition of seizure freedom. ILAE, International League Against Epilepsy; N, number of studies; I 2, heterogeneity.
We next assessed whether the proportion of seizure‐free individuals has changed over time (Fig. 4). A meta‐regression analysis showed no significant association between publication year and percentage of refractoriness (mixed‐effects meta‐regression: P = 0.61).
Figure 4.
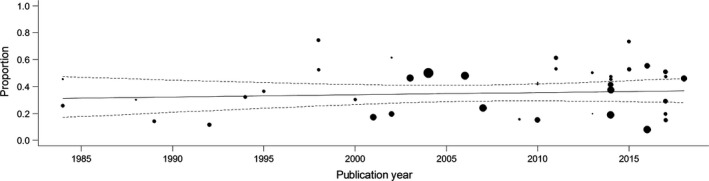
Meta‐regression of refractory juvenile myoclonic epilepsy by publication year. The proportion of refractory subjects per study is plotted by publication year. Each study is represented by a circle whose size is proportional to the sample size. A meta‐regression trend line with 95% confidence interval (dotted lines) is plotted as a solid line.
Seizure recurrence after antiepileptic drug withdrawal
A total of 11 articles described a subset of 246 subjects who attempted AED withdrawal. Some studies had specific criteria for subjects to withdraw (e.g. at least 3‐year seizure freedom), but most did not. Meta‐analysis showed that seizures recurred in 78% (95% CI, 58–94%) of subjects after withdrawal (Fig. 5), although estimates varied widely and heterogeneity was high (I 2 = 84%).
Figure 5.
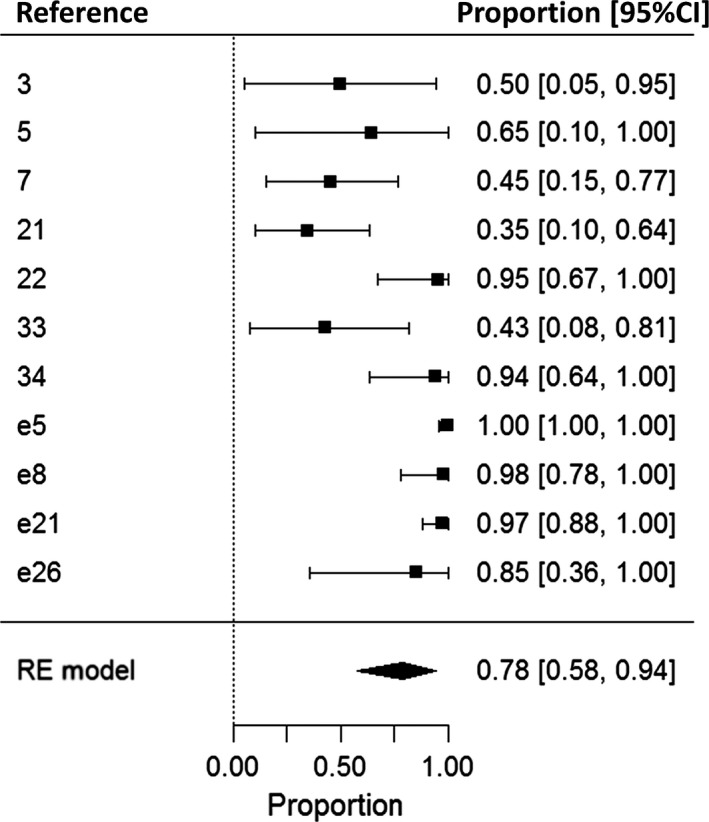
Meta‐analysis of seizure recurrence after antiepileptic drug (AED) withdrawal. The proportion of well‐controlled subjects who experienced recurrence of seizures after AED withdrawal is displayed on the x‐axis. A total of 11 studies describing 246 subjects were included. CI, confidence interval; RE, random‐effects. References denoted as ‘e’ are available in the Supporting Information.
Risk factors for refractory juvenile myoclonic epilepsy
A total of 21 studies reported seizure outcome in relation to potential risk factors for refractory JME. Univariate meta‐analyses were performed for 10 risk factors (Table 1; see Figs S1–S10 for forest plots). Having three seizure types, absence seizures, psychiatric comorbidities, a history of childhood absence epilepsy (CAE) progressing to JME, praxis‐induced seizures (seizures and epileptiform electroencephalographic discharges precipitated by complex, cognition‐guided tasks, such as playing chess, writing or drawing) and early age at epilepsy onset were each significant risk factors for refractory JME. Heterogeneity between studies was mild to moderate. Scores on the Newcastle–Ottawa quality assessment scale (Table S5) ranged between 2 and 7 (mean 4.1) [13 studies were assessed as low (score ≤4) and 8 as high (score ≥5) quality]. Funnel plots, inspected as a measure of publication bias, did not show asymmetry (Figs S1–S10).
Table 1.
Risk factors for refractory juvenile myoclonic epilepsy (JME) assessed with random‐effects meta‐analysis
| Risk factor | No. of studies | No. of subjects | Test statistic (95% CI) | P‐value | Heterogeneity (I 2) (%) |
|---|---|---|---|---|---|
| Three seizure types (myoclonic + GTCS + absences) | 11 | 864 | OR, 2.97 (1.87 to 4.71) | <0.00001 | 19 |
| Absence seizures | 13 | 961 | OR, 2.81 (1.77 to 4.45) | <0.0001 | 42 |
| Psychiatric comorbidities | 8 | 802 | OR, 3.78 (2.46 to 5.81) | <0.00001 | 9 |
| Female gender | 10 | 855 | OR, 1.19 (0.85 to 1.66) | 0.32 | 0 |
| Epileptiform asymmetries on EEG | 7 | 622 | OR, 1.66 (0.71 to 3.92) | 0.24 | 54 |
| Photoparoxysmal response | 5 | 395 | OR, 0.89 (0.49 to 1.62) | 0.70 | 0 |
| Family history of epilepsy | 9 | 782 | OR, 1.03 (0.72 to 1.49) | 0.86 | 0 |
| History of childhood absence epilepsy progressing to JME | 4 | 360 | OR, 5.11 (1.36 to 19.22) | 0.02 | 55 |
| Praxis‐induced seizures | 2 | 110 | OR, 3.73 (1.44 to 9.68) | 0.007 | 0 |
| Early age at epilepsy onset | 8 | 517 | MD, −1.60 (−2.81 to −0.40) | 0.009 | 47 |
CI, confidence interval; EEG, electroencephalography; GTCS, generalized tonic‐clonic seizures; MD, mean difference; OR, odds ratio. Significant associations, defined as a meta‐analysis P‐value <0.05, are highlighted in bold.
Discussion
One‐third of the described subjects with JME were refractory (Fig. 2). The estimates of refractoriness were comparable when assessing 1‐, 2‐ and 5‐year seizure freedom (Fig. 3), suggesting that people who are seizure‐free for at least 1 year are likely to remain so. This is consistent with studies that reported 1‐ and 2‐year or 1‐ and 5‐year seizure freedom in the same subjects, which showed minor differences between outcomes at different follow‐up intervals 20, 21.
We found no evidence for a decrease in the proportion of refractory JME over the last decades. Valproate, marketed as an AED since 1967, is still considered the most effective drug for people with JME 9, 22, 23. Thus, there is still much room for improvement.
In contrast to the International League Against Epilepsy definition (1989) of JME, describing the treatment response to ‘appropriate drugs’ as ‘good’, our results suggest that the proportion of refractoriness is not much different from the overall proportion of refractoriness in people with epilepsy, which is estimated between 16% and 37% 24, 25, 26. Physicians should be careful when counselling people with JME that their prognosis is particularly good. It is possible, however, that we overestimated refractoriness in JME. Individuals in the included studies were mainly treated at tertiary centres and are likely to have more severe or difficult‐to‐treat epilepsy than those at secondary centres. Conversely, it has been shown that seizure control improves after referral to tertiary care 27. It is also possible that some were misdiagnosed, as other conditions may mimic JME 28. There is also the possibility of selection bias and selective loss to follow‐up of people with a more benign course, who might be less inclined to return to the clinic or agree to inclusion in a study. Our estimate, however, could be an underestimation of refractoriness of myoclonic seizures, which are difficult to objectify and can be under‐reported. Another limitation is that study selection and data extraction were performed by a single author. Statistical heterogeneity between studies was substantial, but definition of seizure freedom, publication year or retrospective versus prospective study design did not seem to play a major role in heterogeneity. Other potential causes of heterogeneity could not be assessed, such as ethnic origins, different treatment regimens and different diagnostic criteria. Determining seizure freedom is subjective and a recent study established that inter‐observer variability (using the same criteria and the same individual records) was relatively high, with kappa values ranging between 0.56 and 0.77 29. It is likely that intra‐observer variability would be even higher when the same individual records are not used. Thus, intra‐observer variability is likely to have played a role in heterogeneity between studies.
About one‐fifth of subjects are reported to remain seizure‐free after treatment withdrawal (Fig. 5), which is substantially less than the overall estimate of two‐thirds for all types of epilepsy 30, 31. Estimates between studies, however, varied widely. A potential cause of heterogeneity is age at withdrawal and therefore duration of seizure freedom, as these variables are predictors of seizure recurrence in the general epilepsy population 30 and JME has been shown to subside with age 32. Age at AED withdrawal was rarely reported, but the three studies reporting a good prognosis mostly included people over 40 years of age 7, 21, 33, whereas the two studies reporting that all subjects had seizure recurrence included mainly people in their twenties 22, 34. It is possible that the actual proportion of seizure freedom after AED withdrawal is higher for older subjects. Insufficient information about individuals who attempted AED withdrawal was available to allow identification of potential prognostic factors. Future studies are needed to evaluate which subjects are most likely to remain seizure‐free after treatment withdrawal.
Our meta‐analyses revealed six significant risk factors for refractoriness, but did not provide evidence for the other four clinical variables to be significantly associated (Table 1). It is likely that these variables are inter‐related. For example, a history of CAE relates to having absence seizures and to an earlier age at epilepsy onset 6, and most people with JME who have absence seizures had three seizure types 35.
Cause and effect cannot be established due to the cross‐sectional nature of the studies. We cannot rule out that psychiatric comorbidities are due to AED side‐effects or to having prolonged refractory seizures, rather than being the cause. It is also possible that people with psychiatric comorbidities are less adherent to treatment rather than being non‐responsive to AEDs.
It remains uncertain whether the risk factors for refractory JME represent a lack of response to treatment or a higher disease burden. People with early disease onset, multiple seizure types and psychiatric comorbidities may have more severe brain disease, which makes it more difficult to control all seizure types. Conversely, someone with only occasional seizures can be well controlled even when the medication is only mildly effective. It has also been suggested that people with CAE progressing into JME represent a distinct clinical entity, with a different inheritance pattern and seizure outcome 6. They rarely become completely free of all seizures. Most described individuals, however, do become free of myoclonic seizures and generalized tonic‐clonic seizures, with only absences persisting 6. This suggests the possibility that different seizure types respond differently to treatment. A genetic study comparing drug‐responsive individuals with those who are refractory could unravel a distinct genetic basis of treatment response, higher genetic overlap with CAE or higher polygenic burden of JME‐associated risk alleles.
Further studies using individual data are required to assess which variables are independent predictors of refractory JME to allow for an individualized prediction of seizure outcome to be used to guide treatment.
Disclosure of conflicts of interest
The authors declare no financial or other conflicts of interest. J.W.S. reports grants and personal fees from Eisai, grants and personal fees from UCB, grants from WHO, grants from NEF, personal fees from Eisai, and grants and personal fees from UCB, outside the submitted work. His current position is endowed by the Epilepsy Society and he is a member of the Editorial Board of the Lancet Neurology and receives research support from the Marvin Weil Epilepsy Research Fund.
Supporting information
Figure S1. Presence of all three seizure types as risk factor for refractory juvenile myoclonic epilepsy. Forest plot is displayed on the left and funnel plot is displayed on the right.
Figure S2. Presence of absence seizure types as risk factor for refractory juvenile myoclonic epilepsy. Forest plot is displayed on the left and funnel plot is displayed on the right.
Figure S3. Psychiatric comorbidities as risk factor for refractory juvenile myoclonic epilepsy. Forest plot is displayed on the left and funnel plot is displayed on the right.
Figure S4. Female gender as risk factor for refractory juvenile myoclonic epilepsy. Forest plot is displayed on the left and funnel plot is displayed on the right.
Figure S5. Epileptiform asymmetries on electroencephalography as risk factor for refractory juvenile myoclonic epilepsy. Forest plot is displayed on the left and funnel plot is displayed on the right.
Figure S6. Photoparoxysmal response as risk factor for refractory juvenile myoclonic epilepsy. Forest plot is displayed on the left and funnel plot is displayed on the right.
Figure S7. Family history of epilepsy as risk factor for refractory juvenile myoclonic epilepsy. Forest plot is displayed on the left and funnel plot is displayed on the right.
Figure S8. History of childhood absence epilepsy progressing to juvenile myoclonic epilepsy (JME) as risk factor for refractory JME. Forest plot is displayed on the left and funnel plot is displayed on the right.
Figure S9. Praxis‐induced seizures as risk factor for refractory juvenile myoclonic epilepsy. Forest plot is displayed on the left and funnel plot is displayed on the right.
Figure S10. Early age at epilepsy onset as risk factor for refractory epilepsy. Forest plot is displayed on the left and funnel plot is displayed on the right.
Table S1. PubMed search string. The search was performed on 1 March 2018 and yielded 548 hits. Subsequent rows were linked with AND.
Table S2. EMBASE search string. The search was performed on 1 March 2018 and yielded 814 hits. Subsequent rows were linked with AND. Publications were filtered on the publication type ‘Article’.
Table S3. Overview of the definitions used for the diagnosis of juvenile myoclonic epilepsy in the included studies.
Table S4. Details of all 43 included studies. VPA, valproic acid.
Table S5. Risk of bias assessment using the Newcastle –Ottawa quality assessment scale for cohort studies. Studies can be attributed a maximum of one star (*) for each item. The total score is calculated as the sum of stars. A higher score indicates a better quality of the study.
Online Only E‐extra References.
Acknowledgements
We are grateful to the Ming Fund for supporting this project. We thank Dr Pierre Genton for valuable discussions and advice on the analyses and interpretations. We also thank Dr Christoph Beier, Dr Bernd Vorderwülbecke, Dr Philine Senf, Dr Giorgi Japaridze and Dr Katie Holland for providing stratified data.
References
- 1. Camfield CS, Striano P, Camfield PR. Epidemiology of juvenile myoclonic epilepsy. Epilepsy Behav 2013; 28(Suppl. 1): S15–S17. [DOI] [PubMed] [Google Scholar]
- 2. Commission on Classification and Terminology of the International League Against Epilepsy . Proposal for revised classification of epilepsies and epileptic syndromes. Epilepsia 1989; 30: 389–399. [DOI] [PubMed] [Google Scholar]
- 3. Schneider‐von Podewils F, Gasse C, Geithner J, et al Clinical predictors of the long‐term social outcome and quality of life in juvenile myoclonic epilepsy: 20‐65 years of follow‐up. Epilepsia 2014; 55: 322–330. [DOI] [PubMed] [Google Scholar]
- 4. Leidy NK, Elixhauser A, Vickrey B, Means E, Willian MK. Seizure frequency and the health‐related quality of life of adults with epilepsy. Neurology 1999; 53: 162–166. [DOI] [PubMed] [Google Scholar]
- 5. Calleja S, Salas‐Puig J, Ribacoba R, Lahoz CH. Evolution of juvenile myoclonic epilepsy treated from the outset with sodium valproate. Seizure 2001; 10: 424–427. [DOI] [PubMed] [Google Scholar]
- 6. Martínez‐Juárez IE, Alonso MEME, Medina MT, et al Juvenile myoclonic epilepsy subsyndromes: family studies and long‐term follow‐up. Brain 2006; 129: 1269–1280. [DOI] [PubMed] [Google Scholar]
- 7. Camfield CS, Camfield PR. Juvenile myoclonic epilepsy 25 years after seizure onset: a population‐based study. Neurology 2009; 73: 1041–1045. [DOI] [PubMed] [Google Scholar]
- 8. Senf P, Schmitz B, Holtkamp M, et al Prognosis of juvenile myoclonic epilepsy 45 years after onset: seizure outcome and predictors. Neurology 2013; 81: 2128–2133. [DOI] [PubMed] [Google Scholar]
- 9. Nicolson A, Marson AG. When the first antiepileptic drug fails in a patient with juvenile myoclonic epilepsy. Pract Neurol 2010; 10: 208–218. [DOI] [PubMed] [Google Scholar]
- 10. Mantoan L, Walker M, Mantoan L, et al Treatment options in juvenile myoclonic epilepsy. Curr Treat Options Neurol 2011; 13: 355–370. [DOI] [PubMed] [Google Scholar]
- 11. Kossoff EH, Henry BJ, Cervenka MC. Efficacy of dietary therapy for juvenile myoclonic epilepsy. Epilepsy Behav 2013; 26: 162–164. [DOI] [PubMed] [Google Scholar]
- 12. Jenssen S, Sperling MR, Tracy JI, et al Corpus callosotomy in refractory idiopathic generalized epilepsy. Seizure 2006; 15: 621–629. [DOI] [PubMed] [Google Scholar]
- 13. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med 2009; 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kasteleijn‐Nolst Trenité DGA, Schmitz B, Janz D, et al Consensus on diagnosis and management of JME: from founder's observations to current trends. Epilepsy Behav 2013; 28: S87–S90. [DOI] [PubMed] [Google Scholar]
- 15. Kwan P, Arzimanoglou A, Berg AT, et al Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia 2010; 51: 1069–1077. [DOI] [PubMed] [Google Scholar]
- 16. Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Stat Med 2002; 21: 1539–1558. [DOI] [PubMed] [Google Scholar]
- 17. Hunter JE, Schmidt FL. Fixed effects vs. random effects meta‐analysis models: implications for cumulative research knowledge. Int J Sel Assess 2000; 8: 275–292. [Google Scholar]
- 18. Wells G, Shea B, O'Connell D, et al The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp0 (accessed 15/03/2018).
- 19. Sterne JAC, Sutton AJ, Ioannidis JPA, et al Recommendations for examining and interpreting funnel plot asymmetry in meta‐analyses of randomised controlled trials. BMJ 2011; 343: d4002. [DOI] [PubMed] [Google Scholar]
- 20. Höfler J, Unterberger I, Dobesberger J, Kuchukhidze G, Walser G, Trinka E. Seizure outcome in 175 patients with juvenile myoclonic epilepsy ‐ A long‐term observational study. Epilepsy Res 2014; 108: 1817–1824. [DOI] [PubMed] [Google Scholar]
- 21. Vorderwulbecke BJ, Kowski AB, Kirschbaum A, et al Long‐term outcome in adolescent‐onset generalized genetic epilepsies. Epilepsia 2017; 58: 1244–1250. [DOI] [PubMed] [Google Scholar]
- 22. Canevini MP, Mai R, Di Marco C, et al Juvenile myoclonic epilepsy of Janz: clinical observations in 60 patients. Seizure 1992; 1: 291–298. [DOI] [PubMed] [Google Scholar]
- 23. Gesche J, Khanevski M, Solberg C, Beier C. Resistance to valproic acid as predictor of treatment resistance in genetic generalized epilepsies. Epilepsia 2017; 58: e64–e69. [DOI] [PubMed] [Google Scholar]
- 24. Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med 2000; 342: 314–319. [DOI] [PubMed] [Google Scholar]
- 25. Picot M‐C, Baldy‐Moulinier M, Daurès J‐P, Dujols P, Crespel A. The prevalence of epilepsy and pharmacoresistant epilepsy in adults: a population‐based study in a Western European country. Epilepsia 2008; 49: 1230–1238. [DOI] [PubMed] [Google Scholar]
- 26. Chen Z, Brodie MJ, Liew D, Kwan P. Treatment outcomes in patients with newly diagnosed epilepsy treated with established and new antiepileptic drugs. JAMA Neurol 2018; 75: 279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Szaflarski JP, Rackley AY, Lindsell CJ, Szaflarski M, Yates SL. Seizure control in patients with epilepsy: the physician vs. medication factors. BMC Health Serv Res 2008; 8: 264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. De Haan GJ, Halley DJJ, Doelman JC, et al Univerricht‐Lundborg disease: underdiagnosed in the Netherlands. Epilepsia 2004; 45: 1061–1063. [DOI] [PubMed] [Google Scholar]
- 29. Téllez‐Zenteno JF, Hernández‐Ronquillo L, Buckley S, Zahagun R, Rizvi S. A validation of the new definition of drug‐resistant epilepsy by the International League Against Epilepsy. Epilepsia 2014; 55: 829–834. [DOI] [PubMed] [Google Scholar]
- 30. Lamberink HJ, Otte WM, Geerts AT, et al Individualised prediction model of seizure recurrence and long‐term outcomes after withdrawal of antiepileptic drugs in seizure‐free patients: a systematic review and individual participant data meta‐analysis. Lancet Neurol 2017; 16: 523–531. [DOI] [PubMed] [Google Scholar]
- 31. Lamberink HJ, Otte WM, Geleijns K, Braun KPJ. Antiepileptic drug withdrawal in medically and surgically treated patients: a meta‐analysis of seizure recurrence and systematic review of its predictors. Epileptic Disord 2015; 17: 211–228. [DOI] [PubMed] [Google Scholar]
- 32. Baykan B, Altindag EA, Bebek N, et al Myoclonic seizures subside in the fourth decade in juvenile myoclonic epilepsy. Neurology 2008; 70: 2123–2129. [DOI] [PubMed] [Google Scholar]
- 33. Syvertsen MR, Thuve S, Stordrange BS, et al Clinical heterogeneity of juvenile myoclonic epilepsy: follow‐up after an interval of more than 20 years. Seizure 2014; 23: 344–348. [DOI] [PubMed] [Google Scholar]
- 34. Panayiotopoulos CP, Obeid T, Tahan AR. Juvenile myoclonic epilepsy: a 5‐year prospective study. Epilepsia 1994; 35: 285–296. [DOI] [PubMed] [Google Scholar]
- 35. Gelisse P, Genton P, Thomas P, Rey M, Samuelian J, Dravet C. Clinical factors of drug resistance in juvenile myoclonic epilepsy. J Neurol Neurosurg Psychiatry 2001; 70: 240–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Presence of all three seizure types as risk factor for refractory juvenile myoclonic epilepsy. Forest plot is displayed on the left and funnel plot is displayed on the right.
Figure S2. Presence of absence seizure types as risk factor for refractory juvenile myoclonic epilepsy. Forest plot is displayed on the left and funnel plot is displayed on the right.
Figure S3. Psychiatric comorbidities as risk factor for refractory juvenile myoclonic epilepsy. Forest plot is displayed on the left and funnel plot is displayed on the right.
Figure S4. Female gender as risk factor for refractory juvenile myoclonic epilepsy. Forest plot is displayed on the left and funnel plot is displayed on the right.
Figure S5. Epileptiform asymmetries on electroencephalography as risk factor for refractory juvenile myoclonic epilepsy. Forest plot is displayed on the left and funnel plot is displayed on the right.
Figure S6. Photoparoxysmal response as risk factor for refractory juvenile myoclonic epilepsy. Forest plot is displayed on the left and funnel plot is displayed on the right.
Figure S7. Family history of epilepsy as risk factor for refractory juvenile myoclonic epilepsy. Forest plot is displayed on the left and funnel plot is displayed on the right.
Figure S8. History of childhood absence epilepsy progressing to juvenile myoclonic epilepsy (JME) as risk factor for refractory JME. Forest plot is displayed on the left and funnel plot is displayed on the right.
Figure S9. Praxis‐induced seizures as risk factor for refractory juvenile myoclonic epilepsy. Forest plot is displayed on the left and funnel plot is displayed on the right.
Figure S10. Early age at epilepsy onset as risk factor for refractory epilepsy. Forest plot is displayed on the left and funnel plot is displayed on the right.
Table S1. PubMed search string. The search was performed on 1 March 2018 and yielded 548 hits. Subsequent rows were linked with AND.
Table S2. EMBASE search string. The search was performed on 1 March 2018 and yielded 814 hits. Subsequent rows were linked with AND. Publications were filtered on the publication type ‘Article’.
Table S3. Overview of the definitions used for the diagnosis of juvenile myoclonic epilepsy in the included studies.
Table S4. Details of all 43 included studies. VPA, valproic acid.
Table S5. Risk of bias assessment using the Newcastle –Ottawa quality assessment scale for cohort studies. Studies can be attributed a maximum of one star (*) for each item. The total score is calculated as the sum of stars. A higher score indicates a better quality of the study.
Online Only E‐extra References.


