Abstract
The term movement disorders encompasses all disorders hypokinetic and hyperkinetic, which were previously known as extrapyramidal syndromes. With the definition of movement disorders and their diagnostic criteria and classifications, new studies for therapeutics could be performed. New drugs were launched, functional neurosurgery was developed, and the introduction of botulinum toxin (BoNT) for hyperkinesias was introduced. BoNT is an important therapy for dystonia, tics, myoclonus, and tremors. The aim of this review is to present the new and well-established uses of BoNT for movement disorders.
Keywords: botulinum toxin, dystonia, hyperkinesias, movement disorders, Parkinson’s disease, spasticity
Introduction
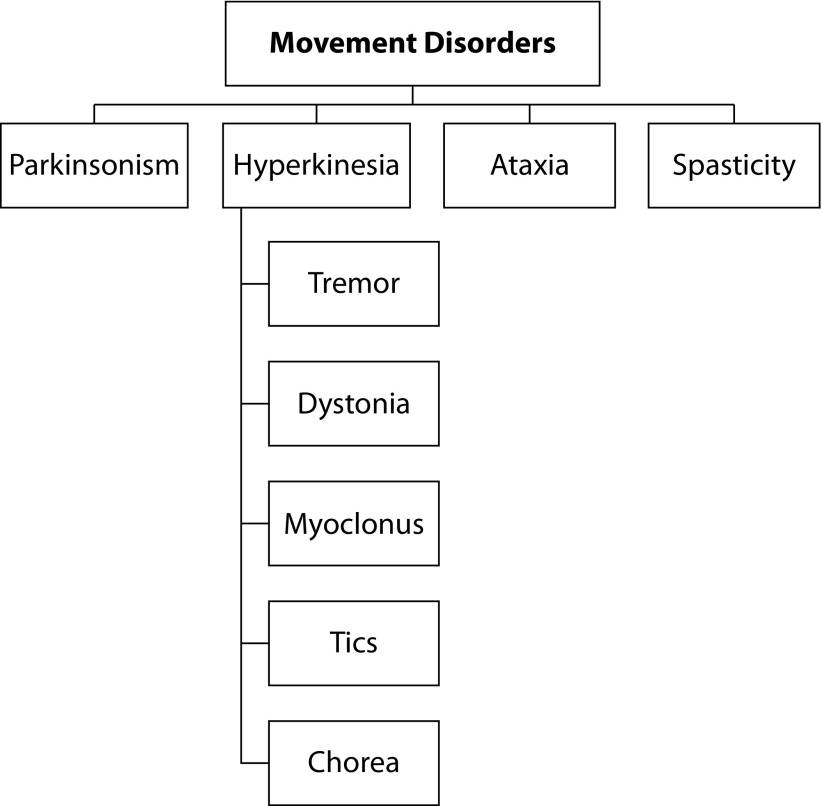
In 1985, Professors Stanley Fahn and David Marsden created a new society, now known as the International Parkinson and Movement Disorders Society (MDS).1,2 Since then, there has been a remarkable development of the subspecialty of neurology, defined today as ‘movement disorders’. This term was suggested by Professor Lewis Rowland to Professor Fahn, to encompass all disorders hypokinetic and hyperkinetic, which were previously known as extrapyramidal syndromes.2 It should also be remembered that Dr. Derek Denny-Brown, professor of neurology at Harvard Medical School – in his classic book, published in 1962, entitled The Basal Ganglia and Its Relation to the Disorders of Movement – was one of the first researchers to draw attention to this subspecialty.3 The main movement disorders are shown in Figure 1.
Figure 1.
Simplified classification of movement disorders.
Design by authors based on data available at https://www.movementdisorders.org/MDS/About.htm
With the definition of movement disorders and their diagnostic criteria and classifications, new studies for therapeutics could be performed. New drugs were launched, functional neurosurgery was developed, and the introduction of botulinum toxin (BoNT) for hyperkinesias was introduced. The United States Food and Drug Administration (FDA) approved BoNT in 1989 for the treatment of strabismus, blepharospasm, and hemifacial spasm. Since then, the use of BoNT has continued to expand within and outside movement disorders and beyond neurology.4,5
The aim of this review is to present the new and well-established uses of BoNT for movement disorders.
Methods
Our review followed a search strategy that included articles published in either English, Portuguese, or Spanish in both MEDLINE and LILACS databases, from the oldest articles available until the most recent ones published online in March 2019, by using an association of the keywords movement disorders, parkinsonism, Parkinson’s disease, tremor, dystonia, chorea, tics, myoclonus, and spasticity with any of the other following: botulinum toxin, BTX-A, BTX-B, BoNT-A, BoNT-B, Botox, Dysport, Xeomin, Myobloc, and Neurobloc. We broadened our search by including the references cited in review articles.
Inclusion criteria for this review were as follows: (1) relevant articles related to efficacy, safety, tolerability, or mechanism of action; (2) articles limited to human beings; (3) articles limited to experimental clinical studies. Articles were excluded if (1) articles with BoNT types and brands were not approved by the FDA or (2) articles were not available/could not be read in their entirety. The recommendations for the treatments were made based on the ranking of the articles chosen. Articles were then ranked based on therapeutical evidence (I, II, III, and IV) and on the strength of recommendation according to levels of evidence (A, B, C, and U) defined by the guideline of the American Academy of Neurology.6
Botulinum toxin
Christian Andreas Justinus Kerner published the first case of botulism in 1817. After clinical follow-up of his patients, he correctly concluded that the substance ‘sausage poison’ or ‘fatty acid’ paralyzed the skeletal muscle function and the parasympathetic nervous system and proposed its use as a therapeutic agent in neurological diseases characterized by involuntary movements.7–9 In 1870, the name sausage poison was replaced by botulinum toxin (from the Latin word botulus, meaning ‘sausage’).7 Kerner is remembered as the intellectual founder of modern BoNT therapy; however, BoNT was only first purified in 1945, and its therapeutic use was initiated in the 1980s by Alan B Scott in patients with strabismus.7–11
BoNT is a complex mixture of proteins that include a botulinum neurotoxin and several nontoxic proteins, produced by Clostridium botulinum.9,11 The functional portion of the neurotoxin is a peptide composed of a 100-kDa heavy chain and a 50-kDa light chain, which blocks the release of acetylcholine at the nerve terminals, causing the denervation of the motor terminals.2,10,12 This neurotoxin action occurs in terminals of cholinergic neurons at the neuromuscular junction. The neurotransmission of acetylcholine is a complex process resulting from a cascade of physiological and biochemical changes. The change in membrane potential increases the intracellular concentration of calcium. Intracellular calcium catalyzes the reaction between a group of proteins necessary for the fusion of the acetylcholine vesicles with the cell membrane allowing the release of the neurotransmitter at the synapse. This group of proteins, known as SNARE complex (soluble N-ethylmaleimide-sensitive factor attachment receptor), consists of synaptobrevin, SNAP-25 (25 kD synaptosomal-associated protein) and membrane-bound syntaxin.10,12 The effect BoNT is temporary, as axonal sprouting on the nerve terminal of the neuromuscular junction reestablishes acetylcholine release to the synaptic gap, which leads to restored muscle contraction. This resprouting phenomenon makes it necessary to periodically administer BoNT to maintain the therapeutic effect.10,12
There are eight different serotypes of BoNT: A, B, C, D, E, F, G, and H. Even though all of them inhibit acetylcholine release on the nerve terminal, their target proteins, intrinsic mode of action, and potency vary substantially. The types A and B were first designated by Georgenia Burke in 1919 and are the only ones approved by the United States FDA currently for therapeutic use.10,12,13 The type H is the deadliest toxin available that was discovered by Stephen Arnon in 2009.14 Although they all inhibit the action of acetylcholine on nerve terminals, their target proteins, action, and potency characteristics vary substantially.10,15,16 BoNT-A and BoNT-B are similar in number of amino acids and molecular weight. BoNT-A acts on SNAP-25, whereas BoNT-B acts on synaptobrevin.10
Over the last decades, several studies have proved the safety and efficacy of different formulations of BoNT used in neurological practice. In 2009, new generic names were associated with BoNT in the FDA. Commercially available formulations are listed in Table 1. As the methodology of studies varies significantly among industrial formulations, a direct equivalence of dosage should not be warranted; therefore, the choice of a specific brand of toxin and dosage to be administered is a medical decision tailored individually for each patient.
Table 1.
Presentations of botulinum toxin.
| Botulinum toxin type | Brand name |
|---|---|
| Botulinum toxin A | |
| OnabotulinumtoxinA* | Botox (Vistabel, Vistabex, Vista) Allergan, Inc., Irvine, CA, USA |
| AbobotulinumtoxinA* | Dysport (Azzalure, Reloxin) Ipsen Biopharm Ltd., Wrexham, UK |
| IncobotulinumtoxinA* | Xeomin (Bocouture, Xeomeen) Merz Pharmaceuticals GmbH, Frankfurt, Germany |
| LetibotulinumtoxinA | Botulax (Zentox, Regenox, Botulim, Reage, Botoshot) Hugel Inc., Chuncheon, South Korea |
| PrabotulinumtoxinA | Nabota Daewoong Pharmaceutical Co., Ltd, Seoul, South Korea |
| – | Prosigne (BTAX, Lantox, Lanzox, Liftox, Redux) Lanzhou Biological Products, Lanzhou, Gansu, China |
| – | Meditoxin (Neuronox, Botulift, Siax) Medy-Tox Inc., Seoul, South Korea |
| Botulinum toxin B | |
| RimabotulinumtoxinB* | Myobloc/Neurobloc US WorldMeds, LLC., Louisville, KY, USA |
Food and Drug Administration (USA) approved.
Table compiled using data made publicly available by manufacturers.
The improvement of the symptoms, after the injection of the BoNT in the muscles, usually occurs from 1 to 14 days. The peak effect is expected for 2–6 weeks, and loss of effect starts within 10–12 weeks. Some degree of muscular atrophy is observed after 1–2 weeks of treatment and a return to about 70–80% of muscle mass is noted after 3 months.17
Use of BoNT for hyperkinetic disorders
Dystonia
The term dystonia was coined by Hermann Oppenheim in 1911.18 In 2013, an international consensus committee proposed the following revised definition: “Dystonia is a movement disorder characterized by sustained or intermittent muscle contractions causing abnormal, often repetitive, movements, postures, or both. Dystonic movements are typically patterned, twisting, and may be tremulous. Dystonia is often initiated or worsened by voluntary action and associated with overflow muscle activation”.19 Dystonias vary greatly in clinical presentation, affecting almost any area of the skeletal muscle. BoNT is the standard treatment for focal and segmental dystonias. The recommended levels for the treatment of dystonia and other movement disorders with BoNTs are in Table 2.
Table 2.
Evidence-based recommendations for the efficacy of FDA-approved botulinum toxins.6
| Indication | Onabot-A (Botox) | Abobot-A (Dysport) | Incobot-A (Xeomin) | Rimabot-B (Myobloc) |
|---|---|---|---|---|
| Blepharospasm | B | C | B | U |
| Cervical dystonia | B | A | B | A |
| Writer’s cramp | C | C | – | – |
| Spasmodic dysphonia | B | – | – | – |
| Oromandibular dystonia | U | U | – | – |
| Hemifacial spasm | C | C | C | U |
| Palatal myoclonus (tremor) | U | – | – | – |
| Tics | C | – | – | – |
| Essential tremor | C | U | B | – |
| PD rest tremor | C | – | C | – |
| PD camptocormia | U | U | – | – |
| PD levodopa complications | U | U | U | U |
| Restless legs syndrome | U | U | C | – |
| Upper limbs spasticity | A | A | A | B |
| Lower limbs spasticity | A | A | – | U |
OnabotulinumtoxinA (Allergan, Inc., Irvine, CA, USA); abobotulinumtoxinA (Ipsen Biopharm Ltd., Wrexham, UK); incobotulinumtoxinA (Merz Pharmaceuticals GmbH, Frankfurt, Germany); rimabotulinumtoxinB (US WorldMeds, LLC., Louisville, KY, USA).
FDA, Food and Drug Administration; PD, Parkinson’s disease. Level A: effective; Level B: probably effective; Level C: possibly effective; Level U: insufficient evidence.
Table compiled by the authors based on the articles referenced in this article. See Methods section for more detail.
Blepharospasm
In 1989, Onabotulinum toxin A (Onabot-A) was approved by the FDA for use in blepharospasm. Previously, there was no effective clinical or surgical treatment for this condition.20 Onabot-A, abobotulinumtoxinA (Abobot-A), and incobotulinumtoxinA (Incobot-A) can be considered as therapeutic options. Initial studies with Onabot-A, at doses between 25 and 50 U, demonstrated efficacy with few adverse effects (visual blurring and ptosis).21,22 A Cochrane systematic review has concluded that although there is no high-quality, randomized, controlled efficacy data to support the use of Onabot-A for blepharospasm, other studies suggest that Onabot-A is highly effective and safe for treating blepharospasm and support its use. The effect size (90% of patients benefit) seen in open studies makes it very difficult and probably unethical to perform new placebo-controlled trials of efficacy of BoNT-A for blepharospasm.23 A double-blind, randomized, placebo-controlled study at a dose of 50 U per eye of Incobot-A24 and a comparative study with the same doses of Incobot-A and Onabot-A25 showed similar efficiency and safety between the two drugs. In a study evaluating 212 patients using a 4:1 ratio of Abobot-A to Onabot-A26 and in another study evaluating 42 patients at a ratio of 4:1.6 to toxins,27 there were similar results in duration as well as efficacy and adverse effects.
Cervical dystonia
Cervical dystonia is the most common of the focal dystonias.28 The classification of cervical dystonia includes torticollis (rotation or turning of head toward one side), anterocollis (flexion of head and neck), laterocollis (head tilting toward one side), and retrocollis (extension of head and neck). A combination of these movements is commonly seen in clinical practice. Recently, combinations of complex cervical dystonias have been classified depending on the neck (collis) and head (caput) movements.29
BoNT is the first choice of treatment for cervical dystonia.30,31 Approximately 50–90% of the patients presented improvement of the dystonic symptoms together to improve dystonia-dependent pain.32–34
The muscles selected for injection vary according to the type of dystonia. For example, for torticollis, the BoNT is injected into the contralateral sternocleidomastoid muscle and ipsilateral splenius muscles; for laterocollis, into the ipsilateral sternocleidomastoid, splenius, trapezius, and scalene muscles; for bilateral retrocollis, into the splenius and trapezius muscles; and for bilateral anterocollis, into the sternocleidomastoid muscles.32,33 Injection may be added to the ipsilateral or contralateral trapezius in cases of torticollis and also in the contralateral trapezius in cases of laterocollis.33,34
Some studies have been conducted to verify the efficacy of BoNT.33–39 Abobot-A, Onabot-A, and Incobot-A should be considered as treatment options. The total dose of BoNT used for cervical dystonia depends on the serotype and trademark. The recommended dose for initiation with Abobot-A is 500 U resulting in a significant benefit for the majority of patients with minimal adverse effects. With Onabot-A, studies have demonstrated efficacy at a suggested dose of between 100 and 300 U.40 A placebo-controlled study using Incobot-A showed improved scores from the 4th week of the 120 and 240 U doses.39 For Rimabotulinum toxin B (Rimabot-B), doses may range from 2500 to 10,000 U to achieve safety effectiveness.40 BoNT-A and BoNT-B have both been approved for treatment of cervical dystonia, and it was seen in studies that there was no significant difference between their efficacies.41
Task-specific dystonia
Task-specific dystonia (TSD) is characterized by abnormal motor activation during the performance of specific, repetitive actions. They are a diverse group of focal dystonias affecting an isolated body part and are triggered, at least initially, by a specific action. In the 1860s, Samuel Solly labeled the difficulty with writing of some clerks ‘scrivener’s palsy’ and it is now called writer’s cramp. TSD may affect musicians, typists, hairdressers, painters, shoemakers, and tailors. Sport-related TSDs have also been described in golfers, pistol shooters, and ping-pong players, among others.42
Writer’s cramp may start with torsion posture or as tremor associated with the dystonic posture. In 1993, Tsui et al.43 published a randomized controlled trial that showed improvement in motor control scores in 20 patients treated with Onabot-A. In 1995, Cole and colleagues44 published a nonrandomized, placebo-controlled clinical study. The dose was from 25 to 40 U, in 10 patients in the Onabot-A group. There was improvement in 80% of patients using Onabot-A through a subjective assessment scale, and 4 of 6 patients reported improvement in writing speed.44 In 2007, Kruisdijk and colleagues45 published a randomized, placebo-controlled, 1-year study showing the use of Abobot-A in 20 patients (mean dose of 100 U/application). Moreover, 30% of patients refused a second application, referring to pain at the site of application and objective or subjective weakness.45 Despite the efficacy and safety of treatment with these BoNTs, there is no consensus on doses, site of application, or application technique. The clinical responses in the studies were very varied and sometimes the improvement in movement scores did not correspond to the patients’ clinical satisfaction. Moreover, there is a delicate balance between reducing dystonic symptoms without inducing concurrent residual weakness resulting in loss of motor function.42
In other TSDs, as in musicians, with treatment many affected are no longer able to play professionally, due to the high level of fine motor skill required for continued professional performance.40 Therefore, the use of BoNTs in patients with TSDs should be indicated and performed by experienced neurologists.
Spasmodic dysphonia
Spasmodic dysphonia is an inappropriate glottic closure or opening due to spasm of intrinsic laryngeal muscles. Symptoms include hoarseness and strangled speech breaks (adductor type) or hypophonia and breathy voice (abductor type).46 In 1991, Truong and colleagues47 published a randomized, placebo-controlled trial using Onabot-A at the mean dose of 5 U/patient with unilateral application in thyroarytenoid muscle. The results showed an excellent response with a low incidence of adverse effects at the doses used. We related the treatment of 12 patients with adductor laryngeal dystonia. Onabot-A was injected in the cricothyreoid membrane, directed toward the thyreoarytenoid muscle, with the aid of electromyography needles. Most of the patients who underwent Onabot-A injection had a significant improvement of their symptoms (83%), with effects lasting for 4 months on average and without important side effects.48 Two meta-analyses confirmed the safety and effectiveness of Onabot-A for the treatment of laryngeal dystonia.49,50
Oromandibular dystonia and bruxism
Oromandibular dystonia (OMD) is a rare focal form of dystonia, characterized by repetitive involuntary jaw movements, subdivided in jaw-opening (JO) or jaw-closing (JC) types. Symptoms may interfere with essential daily living activities, such as feeding, chewing, swallowing, and speaking.51–53 This type of dystonia is often associated with blepharospasm (defining Meige’s syndrome), and cervical dystonia (referred to as cranial-cervical dystonia). JC-OMD is caused by dystonic spasms of the masseter and temporalis muscles, leading to trismus and bruxism. JO-OMD is caused by dystonic contractions of lateral pterygoids, anterior belly of the digastric muscle, and submentalis (geniohyoid, digastric, mylohyoid) muscles.51,54 OMD, especially JO dystonia, remains one of the most challenging forms of dystonia to treat.50
JO-OMD, using an intraoral approach for injections of lateral pterygoid muscles, may require the use of electromyography,53 and the use of BoNT-A in the submentalis complex can improve the results.51–53 In our series of patients with Wilson’s disease and JO-OMD, the use of Onabot-A was partially effective (mild-to-moderate efficacy) in improving the movements, and the most common side effect was transient mild dysphagia, which occurred in 60% of patients.55
Tan and Jankovic49 evaluated 162 patients who received 2529 Onabot-A treatments into the jaw muscles (masseters, submental muscle complex, or both). The mean muscle dose for masseter (per side) was from 25 to 100 U, and for the submentalis complex was 28.6±16.7 U (range, 10–200 U). Definite functional improvement (global score ≥3) in chewing and speaking was reported in 110 patients (67.9%). A significantly higher number of patients with JC-OMD reported functional improvement (global rating ≥3) compared with JO-OMD (80%×57.1%). The most common complications included dysarthria (44 patients/124 visits) and dysphagia (7 patients/11 visits). Patients categorized as JO-OMD and mixed had significantly higher complication rates.51
The frequency of bruxism is significantly higher in the dystonic patients, and its clinical features overlap with JC-OMD.56 Small controlled studies have provided evidence that BoNT is a safe and effective treatment of bruxism.57–59 Two of them were performed with Onabot-A.55,56 Guarda-Nardini and colleagues57 injected BoNT into the masseter muscles, and there was reduction in pain and improvement in range of mandibular movements. Ondo and colleagues58 used Onabot-A 200 U/patient injecting into the masseter and temporal muscles, also demonstrating improvement of pain and movements. A study with Abobot-A, 80 U/patient in masseter muscles, also showed similar results.59
Myoclonus
Hemifacial spasm
The hemifacial spasm is a segmental myoclonus of the face, which affects the muscles innervated by the ipsilateral facial nerve. It may be associated with vascular compression of the facial nerve in its emergence, in the brainstem.60
Onabot-A appears to be an effective and safe method of therapy for hemifacial spasm. Yoshimura and colleagues61 investigated the effectiveness of Onabot-A injections in 11 patients with hemifacial spasm in a prospective placebo-controlled blinded study. Objective improvement was seen after 84% of injections with Onabot-A. The most frequent side effect was facial weakness, seen after 97% of injections of BoNT. Facial bruising (20%), diplopia (13%), ptosis (7%), and various other mild side effects were seen less frequently. Another study compared the results with Incobot-A in the same group of patients (n=14) treated with Onabot-A first.60 The mean dose was 43.3±15 U of Incobot-A/application. The conclusion was that the presentations were similar in effectiveness; however the study had a number of methodological problems.62 A 7-year follow-up open-label study of 175 patients with hemifacial spasm found a clinically significant response at Abobot-A.63 The mean dose used was 92 U (28–220 U). The authors reported a reduction in the incidence of ptosis (from 27 to 9%) with the change of application point in the upper eyelid.63
Palatal myoclonus (tremor)
Palatal myoclonus is now reclassified as a tremor, owing to its electrophysiology and clinical features.64 Palatal myoclonus is characterized by involuntary palatal contractions, causing clicking tinnitus due to the action of soft palate muscles on the membranous Eustachian tube. For palatal myoclonus, Onabot-A can be injected in the soft palate under EMG guidance.65 Injections into the tensor-veli-palatini muscle usually improve clicking sound (2.5 U injected transorally at the level of the pterygoid hamulus/lateral soft palate), whereas an injection into the levator-veli-palatini muscle (2.5 U placed medially on either side of the uvula) controls palatal movements. Risk of dysphagia associated with Onabot-A injections can be minimized by starting with a very low dose and, if no adverse effects are encountered, gradually increasing it in subsequent visits. It should be noted that some cases of palatal myoclonus might be of psychogenic (functional) origin, requiring insight-oriented psychotherapy.66,67
Spinal myoclonus
Most causes of spinal myoclonus have a spinal cord structural etiology.64 There are only case reports of the use of Onabot-A for spinal myoclonus. If the myoclonus is limited to a certain myotome, Onabot-A injections can be helpful with limit systemic side effects.68,69
Essential tremor
Tremor is an involuntary, rhythmic, oscillatory movement of a body part.70 Essential tremor (ET) is among the most common movement disorders, and the most prevalent tremor disorder. Therefore, its importance as a clinical entity should not be underestimated. Although for many years ET was viewed as a monosymptomatic condition, it is known that clinical features are richer, including both motor and nonmotor features.71
BoNT-A has been tested in several studies for ET.72 In 1991, Jankovic and Schwartz73 first reported the effect of Onabot-A in 34 patients with ET in an open observation. Tremor improved in 67% of the patients, but 60% demonstrated hand weakness following injection into arm flexors and extensors. Jankovic74 reduced or eliminated the injections into the extensor muscles and found an appreciable reduction of weakness with comparable tremor control. Only a single study with 20 patients with Abobot-A was performed. The results demonstrated efficacy of medication, but there were also finger weakness in 15% of patients.75 Samotus and colleagues76 used a cinematically guided, individualized, multi-joint upper limb injection approach to determine the efficacy and safety of Incobot-A for ET. Kinematics provided an objective and significant decrease in tremor amplitude. Eight participants (40%) self-reported mild weakness in injected muscles but had no interference in arm function. Mittal and colleagues77 performed a randomized, double-blind, placebo-controlled, cross-over study, with Onabot-A injection pattern (dose and muscle selection) customized to each of 15 patients, and done under EMG guidance. Incobot-A group had a significant improvement compared to placebo group at 4 weeks and 8 weeks (p=0.003 and p=0.002, respectively). One patient withdrew from the study due to unacceptable hand weakness. Mild hand weakness lasting for few days to 4 weeks was seen in six patients in Incobot-A group and four patients in the placebo group (p=0.728).77
Recent studies have shown that the weakness of the hands with BoNT-A application can be minimized by the appropriate and individualized choice of the muscles applied. With the correct indication, the use of BoNT-A may be a recommended therapeutic option in the treatment of ET.72
Tics
Tics are sudden, rapid, recurrent, nonrhythmic motor movements (motor tics) or sound (phonic tics). Motor tics can be classified as myoclonic (jerk-like), dystonic (sustained), tonic (isometric contraction), and blocking (cessation of movement).4,67,78 In addition to its brief, intermittent, and repetitive nature, other features of tics include suggestibility, suppressibility, and distractibility, which may lead to a wrong diagnosis of a tic as a ‘psychogenic’ movement disorder.67,78–81
Focal or segmental motor tics and phonic tics may be successfully treated with Onabot-A injections in the affected muscles according to several small studies.4,80 However, there is only a randomized, placebo-controlled trial showing that Onabot-A injections are efficacious in reducing the frequency and urge of vocal and motor tics (n=18), but the patients did not report an overall benefit from the treatment.79
Use of BoNT for Parkinson’s disease
BoNT has been used for motor symptoms related to Parkinson’s disease (PD) including apraxia of eyelid opening, dystonias, levodopa-induced dyskinesias and freezing, and also for nonmotor manifestations, that is, sialorrhoea, dysphagia, hyperhidrosis, constipation, and urinary problems.5,13,82,83
Rest tremor
Tremor in PD may be more refractory to standard pharmacologic treatments such as levodopa. The published data on the tremor of PD with BoNTs are limited to a few studies indicating that injection of Onabot-A and Incobot-A into forearm muscles may diminish the amplitude of resting and postural PD tremor. However, transient finger and hand weakness in the substantial number of patients and a modest but nonsignificant improvement in these patients dampened the enthusiasm to popularize the use of toxin for PD tremor.82–85 A first randomized, double-blind, placebo-controlled clinical trial, with 34 patients, evaluating the efficacy of Incobot-A in PD tremor. There was a statistically significant improvement in clinical rating scores of rest tremor and tremor severity 4 and 8 weeks after the Incobot-A injection and of action/postural tremor at 8 weeks. There was no statistically significant difference in grip strength at 4 weeks between the two groups.84 Therefore, in patients with poorly controlled PD limb tremor, BoNT injections can be considered, but limited by side effects even with individualized injection patterns.82–85
Camptocormia
Camptocormia is defined as an axial dystonia, comprising 45 degrees of forward flexion, which reverses on sitting, lying down, walking with support, and standing against a wall. It affects the paraspinal muscles and can be considered secondary to the disease pathophysiology or sometimes also secondary to dopaminergic drugs used to treat the disease.86 Thus, BoNT injections in the paraspinal muscles can probably help to alleviate these symptoms.86 Studies related to this use of the BoNT are too few and have recruited less patients to provide any conclusive evidence related to this stated benefit of the toxin. The muscles usually injected are the rectus abdominis, iliopsoas, and external and internal obliques. Although ultrasound-guided injection of BoNT into iliopsoas muscle has been suggested for patients with camptocormia, injections of BoNT into the rectus abdominis and the external abdominal oblique muscles yield the most robust improvement of dystonic camptocormia.5,87 Variable results in the small studies may be related to difficult access of muscles for injection versus appropriateness of muscle selection. This suggests complexity of this syndrome and central control of abnormal posture that is difficult to treat with any form of therapy.83
Levodopa-induced dyskinesias and freezing of gait
Levodopa-induced dyskinesias can affect 45–85% of patients. The most common dyskinesias occur when the patients are under the effect of levodopa, and they have a choreo-dystonic nature. There has not been sufficient data to support BoNT injections for effective treatment of dyskinesias. However, BoNT can be helpful in selected patients with painful off period foot dystonia or other types of predictable phenomena such as blepharospasm or JC-OMD.5,88,89
Freezing of gait may be a dystonic manifestation and is considered to be unresponsive to medical therapy with dopaminergics. Small sample studies with Onabot-A and Rimabot-B injections into gastrocnemius-soleus complex or into tensor fascia latae muscle showed inconsistent benefit for freezing of gait.90–93
Use of BoNT for restless legs syndrome
Restless leg syndrome (RLS) is clinically diagnosed according to the five essential criteria defined by the International Restless Legs Syndrome Study Group (IRLSSG):94
An urge to move the legs usually but not always accompanied by, or felt to be caused by, uncomfortable and unpleasant sensations in the legs.
The urge to move the legs and any accompanying unpleasant sensations begin or worsen during periods of rest or inactivity such as lying down or sitting.
The urge to move the legs and any accompanying unpleasant sensations are partially or totally relieved by movement, such as walking or stretching, at least as long as the activity continues.
The urge to move the legs and any accompanying unpleasant sensations during rest or inactivity only occur or are worse in the evening or night than during the day.
The occurrence of the earlier features is not solely accounted for as symptoms primary to another medical or a behavioral condition.
It is known that RLS is a disease involving the dopaminergic system, but there are no complete studies demonstrating that it is on the same spectrum as PD.95 The involvement of other dopaminergic pathways other than the nigrostriatal pathway of PD is suggested.96 There is physiological evidence for hyperexcitability of the cortical–striatal–thalamic–cortical network in RLS leading to hyperexcitability of spinal motor neurons.97 There is a higher prevalence of PD in men with RLS. SPI may precede the onset of PD motor symptoms, suggesting the possibility that it may be a premotor symptom.95 Levodopa is currently recommended only for the treatment of intermittent RLS, whereas dopaminergic agonists are the most commonly used drugs for the treatment of RLS.98
Three open label studies and two randomized double-blind studies have evaluated the efficacy of BoNTs in RLS.99–103 Nahab and colleagues99 conducted a double-blind placebo-controlled study in six patients from whom three were injected with Onabot-A and three were injected with placebo. The Onabot-A dose was 90 units injected under EMG guidance into each leg muscle (quadriceps femoris, tibialis anterior, gastrocnemius, and soleus). There was no significant improvement of the IRLS score or clinical global improvement score in the Onabot-A group compared to placebo.99 Two other small open studies (three and eight patients) showed improvement in the control of leg movements with Onabot-A.100,101 Ghorayeb and colleagues,102 in another open-label study, assessed the efficacy of intradermal injection of Abobot-A (250 units to the anterior and posterior thigh muscles) in 26 patients with severe RLS. All patients had improvement in IRLS score. Mittal and colleagues103 presented a double-blinded placebo-controlled study assessing the efficacy and safety Incobot-A in refractory RLS patients. There was significant improvement from a severe (IRLS >21) to a mild/moderate (IRLS 20) score at 4 weeks (p=0.0036) and 6 weeks (p=0.0325) following Incobot-A administration compared to placebo. Additionally, there was significant improvement in pain score by VAS at 4 weeks (p=0.01) and in the Johns Hopkins QoL questionnaire at 6 weeks (p=0.04) in the Incobot-A group.103 Future studies may confirm the use of BoNT-A for severe cases of RLS.
Use of BoNT for spasticity
Spasticity can be defined as an abnormal increase in muscle tone resulting from a lesion to the central nervous system compromising the pyramidal pathways (corticothalamic tract, corticospinal tract, or the spine), specifically the upper motor neuron, thus leading to an imbalance of the action of agonists and antagonists muscles resulting in an increase in the resistance to passive movement, reduction of the amplitude of active movements, and functional compromise.104,105
Two BoNT-A formulations (Abobot-A and Onabot-A) are effective and safe for the treatment of spasticity of upper and lower limbs. Incobot-A is a formulation that is effective and safe for the treatment of spasticity of the upper limbs in adults. Rimabot-B is also probably safe and effective in treating upper limb spasticity.
The modified Ashworth scale for spasticity was used as a parameter in nine Class I studies and corroborated the efficacy of Abobot-A, showing a significant reduction of the injected upper limbs muscle tone.106–116 However, this efficacy in decreasing spasticity does not necessarily leads to functional improvement in some patients. Side effects are rare and have been reported in some studies, which include mostly weakness or pain on the injected muscles, or flu-like reactions.106–109 Class I studies with Onabot-A also showed efficacy and safety in the reduction of spasticity of upper limbs in adults, as observed with Abobot-A. Adverse effects most commonly seen were muscle weakness, pain, and hematoma at the site of injection.115–120 Efficacy and safety of Incobot-A for the treatment of spasticity of upper limbs in adults were assessed and confirmed in two Class I studies. Both studies found functional improvement, as there was an enhancement in all the domains of the Disability Assessment Scale.121,122
Class I studies with Abobot-A123–125 and Onabot-A115,126–128 also confirmed the security and efficacy of both formulations for the treatment of spasticity of lower limbs in adults. Adverse events, similar to those related in the upper limbs, were not significant when compared to the ones reported by patients in the control group treated with placebo.123,126 Most authors note a functional improvement, beyond that self-reported by patients. Nevertheless, no clear sustained proof of this effect has been documented. Therefore, there is still a need to devise or establish more adequate tools for assessment of the effect of BoNT on functional improvement.
BoNTs were rarely used and tested in studies for spasticity in progressive diseases. Ineffectiveness for motor enhancement in hereditary spastic paraplegics in a study, for example, is not enough to define the indication status for BoNTs in these patients.129
Conclusions
BoNT was a milestone in the treatment of movement disorders. Over the years, many studies have been done proving the efficacy and safety of this drug in neurological diseases. Today, some movement disorders have BoNT as standard treatment.
Acknowledgements
None.
Footnotes
Contributions: We confirm that the manuscript has been read and approved by both named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by both of us. Both named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosure and potential conflicts of interest: The authors declare that they have no conflicts of interest. The International Committee of Medical Journal Editors (ICMJE) Potential Conflicts of Interests form for the authors is available for download at http://www.drugsincontext.com/wp-content/uploads/2019/05/dic.212586-COI.pdf
Funding declaration: There was no funding associated with the preparation of this article.
Correct attribution: Copyright © 2019 Camargo CHF, Teive HAG. Published by Drugs in Context under Creative Commons License Deed CC BY NC ND 4.0.
Provenance: invited; externally peer reviewed.
Drugs in Context is published by BioExcel Publishing Ltd. Registered office: Plaza Building, Lee High Road, London, England, SE13 5PT.
BioExcel Publishing Limited is registered in England Number 10038393. VAT GB 252 7720 07.
For all manuscript and submissions enquiries, contact the Editor-in-Chief gordon.mallarkey@bioexcelpublishing.com
For all permissions, rights and reprints, contact David Hughes david.hughes@bioexcelpublishing.com
Peer review comments to author: 17 May 2019
References
- 1.Lang AE. In Memoriam. Neurology. 1999;52(1):14. doi: 10.1212/WNL.52.1.14. [DOI] [Google Scholar]
- 2.Hallett M. The rise of movement disorders. Neurol India. 2018 Mar-Apr;66(Supplement):S10–S11. doi: 10.4103/0028-3886.226445. [DOI] [PubMed] [Google Scholar]
- 3.Denny-Brown D. The Basal Ganglia and Their Relation to Disorders of Movement. London, UK: Oxford University Press; 1962. [Google Scholar]
- 4.Jankovic J. An update on new and unique uses of botulinum toxin in movement disorders. Toxicon. 2018;147:84–88. doi: 10.1016/j.toxicon.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 5.Simpson M, Blitzer A, Brashear A, et al. Botulinum neurotoxin for the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Assessment: botulinum neurotoxin for the treatment of movement disorders (an evidence-based review): report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2008;70:1699–1706. doi: 10.1212/01.wnl.0000311389.26145.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Groseth GS, Woodroffe LM, Getchius TS. Clinical Practice Guidelines Process Manual. 2011 ed. St Paul, MN: The American Academy of Neurology; 2011. [Accessed September 20, 2016]. http://tools.aan.com/globals/axon/assets/9023.pdf. [Google Scholar]
- 7.Erbguth FJ, Naumann M. Historical aspects of botulinum toxin: Justinus Kerner (1786–1862) and the ‘sausage poison’. Neurology. 1999 Nov 10;53(8):1850–1853. doi: 10.1212/WNL.53.8.1850. [DOI] [PubMed] [Google Scholar]
- 8.Pearce JM. A note on the use of botulinum toxin. J Neurol Neurosurg Psychiatry. 1999 Aug;67(2):230. doi: 10.1136/jnnp.67.2.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goetz CG, Chmura TA, Lanska DJ. Seminal figures in the history of movement disorders: Gilles de la Tourette, Oppenheim, the Vogts, von Economo, Wilson, and Marsden. Part 12 of the MDS-sponsored History of Movement Disorders exhibit, Barcelona, June 2000. Mov Disord. 2001 Sep;16(5):940–946. doi: 10.1002/mds.1189. [DOI] [PubMed] [Google Scholar]
- 10.Thakker MM, Rubin PA. Pharmacology and clinical applications of botulinum toxins A and B. Int Ophthalmol Clin. 2004 Summer;44(3):147–163. doi: 10.1097/00004397-200404430-00014. [DOI] [PubMed] [Google Scholar]
- 11.Scott AB, Kennedy RA, Stubbs HA. Botulinum A toxin injection as a treatment for blepharospasm. Arch Ophthalmol. 1985 Mar;103(3):347–350. doi: 10.1001/archopht.1985.01050030043017. [DOI] [PubMed] [Google Scholar]
- 12.Wenzel RG. Pharmacology of botulinum neurotoxin serotype A. Am J Health Syst Pharm. 2004 Nov 15;61(22 Suppl 6):S5–S10. doi: 10.1093/ajhp/61.suppl_6.S5. [DOI] [PubMed] [Google Scholar]
- 13.Tater P, Pandey S. Botulinum toxin in movement disorders. Neurol India. 2018;66(Suppl S1):79–89. doi: 10.4103/0028-3886.226441. [DOI] [PubMed] [Google Scholar]
- 14.Truong D, Dressler D, Hallet M, Zachary C. Manual of Botulinum Toxin Therapy. 2nd ed. Cambridge, UK: Cambride University Press; 2013. [Google Scholar]
- 15.Aoki KR. Pharmacology and immunology of botulinum toxin serotypes. J Neurol. 2001 Apr;248(Suppl 1):3–10. doi: 10.1007/PL00007816. [DOI] [PubMed] [Google Scholar]
- 16.Dressler D, Saberi FA, Barbosa ER. Botulinum toxin: mechanisms of action. Arq Neuropsiquiatr. 2005 Mar;63(1):180–185. doi: 10.1590/S0004-282X2005000100035. [DOI] [PubMed] [Google Scholar]
- 17.Tsui JK. Botulinum toxin as a therapeutic agent. Pharmacol Ther. 1996;72(1):13–24. doi: 10.1016/S0163-7258(96)00091-5. [DOI] [PubMed] [Google Scholar]
- 18.Oppenheim H. Über eine eigenartige Krampfkrankheit des kindlichen und jugendlichen Alters (Dysbasia lordotica progressiva, Dystonia musculorum deformans) Neurol Centralbl. 1911;30:1090–1107. [Google Scholar]
- 19.Albanese A, Bhatia K, Bressman SB, et al. Phenomenology and classification of dystonia: a consensus update. Mov Disord. 2013 Jun 15;28(7):863–873. doi: 10.1002/mds.25475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simpson M, Blitzer A, Brashear A, et al. Botulinum Neurotoxin for the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Assessment: botulinum neurotoxin for the treatment of movement disorders (an evidence-based review): report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2008;70:1699–1706. doi: 10.1212/01.wnl.0000311389.26145.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Girlanda P, Quartarone A, Sinicropi S, Nicolosi C, Messina C. Unilateral injection of botulinum toxin in blepharospasm: single fiber electromyography and blink reflex study. Mov Disord. 1996;11:27–31. doi: 10.1002/mds.870110107. [DOI] [PubMed] [Google Scholar]
- 22.Jankovic J, Orman J. Botulinum. A toxin for cranialcervical dystonia: a double blind, placebo-controlled study. Neurology. 1987;37:616–623. doi: 10.1212/WNL.37.4.616. [DOI] [PubMed] [Google Scholar]
- 23.Costa J, Espírito-Santo CC, Borges AA, Ferreira J, Coelho MM, Moore P, Sampaio C. Botulinum toxin type A therapy for blepharospasm. Cochrane Database Syst Rev. 2004;2:CD004900. doi: 10.1002/14651858.CD004900.pub2. [DOI] [PubMed] [Google Scholar]
- 24.Jankovic J, Comella C, Hanschmann A, Grafe S. Efficacy and safety of incobotulinumtoxinA (NT 201, Xeomin) in the treatment of blepharospasm: a randomized trial. Mov Disord. 2011;26:1521–1528. doi: 10.1002/mds.23658. [DOI] [PubMed] [Google Scholar]
- 25.Roggenkamper P, Jost WH, Bihari K, Comes G, Grafe S. Efficacy and safety of a new botulinum toxin type A free of complexing proteins in the treatment of blepharospasm. J Neural Transm. 2006;113:303–312. doi: 10.1007/s00702-005-0323-3. [DOI] [PubMed] [Google Scholar]
- 26.Nüβgens Z, Roggenkämper P. Comparison of two botulinum-toxin preparations in the treatment of essential blepharospasm. Graefes Arch Clin Exp Ophthalmol. 1997;235:197–199. doi: 10.1007/BF00941758. [DOI] [PubMed] [Google Scholar]
- 27.Sampaio C, Ferreira JJ, Simoes F, et al. DYSBOT: a single-blind, randomized parallel study to determine whether any differences can be detected in the efficacy and tolerability of two formulations of botulinum toxin type A—Dysport and Botox—assuming a ratio of 4:1. Mov Disord. 1997;12:1013–1018. doi: 10.1002/mds.870120627. [DOI] [PubMed] [Google Scholar]
- 28.Chan J, Brin MF, Fahn S. Idiopathic cervical dystonia: clinical characteristics. Mov Disord. 1991;6:119–126. doi: 10.1002/mds.870060206. [DOI] [PubMed] [Google Scholar]
- 29.Pandey S, Singh AS. The double-chin posture: posterior sagittal shift in cervical dystonia. Neurol India. 2016;64:556–558. doi: 10.4103/0028-3886.181543. [DOI] [PubMed] [Google Scholar]
- 30.Albanese A, Barnes MP, Bhatia KP, et al. A systematic review on the diagnosis and treatment of primary (idiopathic) dystonia and dystonia plus syndromes: report of an EFNS/MDS-ES Task Force. Eur J Neurol. 2006 May;13(5):433–444. doi: 10.1111/j.1468-1331.2006.01537.x. [DOI] [PubMed] [Google Scholar]
- 31.Brans JW, Lindeboom R, Snoek JW, et al. Botulinum toxin versus trihexyphenidyl in cervical dystonia: a prospective, randomized, double-blind controlled trial. Neurology. 1996 Apr;46(4):1066–1072. doi: 10.1212/WNL.46.4.1066. [DOI] [PubMed] [Google Scholar]
- 32.Berardelli A, Abbruzzese G, Bertolasi L, et al. Guidelines for the therapeutic use of botulinum toxin in movement disorders. Italian Study Group for Movement Disorders, Italian Society of Neurology. Ital J Neurol Sci. 1997 Oct;18(5):261–269. doi: 10.1007/BF02083302. [DOI] [PubMed] [Google Scholar]
- 33.Camargo CH, Teive HA, Becker N, Baran MH, Scola RH, Werneck LC. Cervical dystonia: clinical and therapeutic features in 85 patients. Arq Neuropsiquiatr. 2008 Mar;66(1):15–21. doi: 10.1590/S0004-282X2008000100005. [DOI] [PubMed] [Google Scholar]
- 34.Camargo CH, Cattai L, Teive HA. Pain relief in cervical dystonia with botulinum toxin treatment. Toxins (Basel) 2015 Jun 23;7(6):2321–2335. doi: 10.3390/toxins7062321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singer C. Indicaciones y manejo de la toxina botulínica. Rev Neurol. 1999 Jul 16–31;29(2):157–162. doi: 10.33588/rn.2902.98505. [DOI] [PubMed] [Google Scholar]
- 36.Greene P, Kang U, Fahn S, Brin M, Moskowitz C, Flaster E. Double-blind, placebo-controlled trial of botulinum toxin injections for the treatment of spasmodic torticollis. Neurology. 1990;40:1213–1218. doi: 10.1212/WNL.40.8.1213. [DOI] [PubMed] [Google Scholar]
- 37.Poewe W, Deuschl G, Nebe A, et al. What is the optimal dose of botulinum toxin A in the treatment of cervical dystonia? Results of a double blind, placebo controlled, dose ranging study using Dysport. German Dystonia Study Group. J Neurol Neurosurg Psychiatry. 1998;64:13–17. doi: 10.1136/jnnp.64.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Truong D, Duane DD, Jankovic J, et al. Efficacy and safety of botulinum type A toxin (Dysport) in cervical dystonia: results of the first US randomized, double-blind, placebo-controlled study. Mov Disord. 2005;20:783–791. doi: 10.1002/mds.20403. [DOI] [PubMed] [Google Scholar]
- 39.Comella CL, Jankovic J, Truong DD, Hanschmann A, Grafe S U.S. XEOMIN Cervical Dystonia Study Group. Efficacy and safety of incobotulinumtoxinA (NT 201, XEOMIN, botulinum neurotoxin type A, without accessory proteins) in patients with cervical dystonia. J Neurol Sci. 2011;308:103–109. doi: 10.1016/j.jns.2011.05.041. [DOI] [PubMed] [Google Scholar]
- 40.Comella CL, Thompson PD. Treatment of cervical dystonia with botulinum toxins. Eur J Neurol. 2006 Feb;13(Suppl 1):16–20. doi: 10.1055/s-0035-1571210. [DOI] [PubMed] [Google Scholar]
- 41.Walker FO. Botulinum toxin therapy for cervical dystonia. Phys Med Rehabil Clin N Am. 2003;14:749–766. doi: 10.1016/S1047-9651(03)00045-7. [DOI] [PubMed] [Google Scholar]
- 42.Stahl CM, Frucht SJ. J Neurol. 2017 Jul;264(7):1536–1541. doi: 10.1007/s00415-016-8373-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsui JK, Bhatt M, Calne S, Calne DB. Botulinum toxin in the treatment of writer’s cramp: a double-blind study. Neurology. 1993 Jan;43(1):183–185. doi: 10.1212/wnl.43.1_part_1.183. [DOI] [PubMed] [Google Scholar]
- 44.Cole R, Hallett M, Cohen LG. Double-blind trial of botulinum toxin for treatment of focal hand dystonia. Mov Disord. 1995 Jul;10(4):466–471. doi: 10.1002/mds.870100411. [DOI] [PubMed] [Google Scholar]
- 45.Kruisdijk JJ, Koelman JH, Ongerboer de Visser BW, de Haan RJ, Speelman JD. Botulinum toxin for writer’s cramp: a randomised, placebo-controlled trial and 1-year follow-up. J Neurol Neurosurg Psychiatry. 2007 Mar;78(3):264–270. doi: 10.1136/jnnp.2005.083170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rosenfield DB, Donovan DT, Sulek M, Viswanath NS, Inbody GP, Nudelman HB. Neurologic aspects of spasmodic dysphonia. J Otolaryngol. 1990;19:231–236. [PubMed] [Google Scholar]
- 47.Truong DD, Rontal M, Rolnick M, Aronson AE, Mistura K. Double-blind controlled study of botulinum toxin in adductor spasmodic dysphonia. Laryngoscope. 1991;101(6):630–634. doi: 10.1288/00005537-199106000-00010. [DOI] [PubMed] [Google Scholar]
- 48.Teive HAG, Scola RH, Werneck LC, et al. O uso da toxina botulínica no tratamento da distonia laríngea (disfonia espasmódica) Arq Neuropsiquiatr. 2001;59(1):97–100. doi: 10.1590/S0004-282X2001000100020. [DOI] [PubMed] [Google Scholar]
- 49.Watts CCW, Whurr R, Nye C. Botulinum toxin injections for the treatment of spasmodic dysphonia (Review) Cochrane Database Syst Rev. 2004;3:CD004327. doi: 10.1002/14651858.CD004327.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Watts C, Nye C, Whurr R. Botulinum toxin for treating spasmodic dysphonia (laryngeal dystonia): a systematic Cochrane review. Clin Rehabil. 2006;20:112–122. doi: 10.1191/0269215506cr931oa. [DOI] [PubMed] [Google Scholar]
- 51.Tan EK, Jankovic J. Botulinum toxin A in patients with oromandibular dystonia: long-term follow-up. Neurology. 1999;53:2102–2107. doi: 10.1212/WNL.53.9.2102. [DOI] [PubMed] [Google Scholar]
- 52.Singer C, Papapetropoulos S. A comparison of jaw-closing and jaw-opening idiopathic oromandibular dystonia. Parkinsonism Relat Disord. 2006;12:115–118. doi: 10.1016/j.parkreldis.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 53.Moller E, Bakke M, Dalager T, Werdelin LM. Oromandibular dystonia involving the lateral pterygoid muscles: four cases with different complexity. Mov Disord. 2007;22:785–790. doi: 10.1002/mds.21304. [DOI] [PubMed] [Google Scholar]
- 54.Cardoso F, Jankovic J. Oromandibular dystonia. In: Tsui JKC, Calne DB, editors. Handbook of Dystonia. New York, USA: Marcel Dekker, Inc; 1995. pp. 181–190. [Google Scholar]
- 55.Teive HA, Klüppel LE, Munhoz RP, Becker N, Müller PR, Werneck LC. Jaw-opening oromandibular dystonia secondary to Wilson’s Disease treated with botulinum toxin type A. Arq Neuropsiquiatr. 2012 Jun;70(6):407–409. doi: 10.1590/S0004-282X2012000600005. [DOI] [PubMed] [Google Scholar]
- 56.Borie L, Langbour N, Guehl D, Burbaud P, Ella B. Bruxism in craniocervical dystonia: a prospective study. Cranio. 2016 Sep;34(5):291–295. doi: 10.1080/08869634.2015.1120473. [DOI] [PubMed] [Google Scholar]
- 57.Guarda-Nardini L, Manfredini D, Salamone M, Salmaso L, Tonello S, Ferronato G. Efficacy of botulinum toxin in treating myofascial pain in bruxers: a controlled placebo pilot study. Cranio. 2008 Apr;26(2):126–135. doi: 10.1179/crn.2008.017. [DOI] [PubMed] [Google Scholar]
- 58.Ondo WG, Simmons JH, Shahid MH, Hashem V, Hunter C, Jankovic J. Onabotulinum toxin-A injections for sleep bruxism: a double-blind, placebo-controlled study. Neurology. 2018 Feb 13;90(7):e559–e564. doi: 10.1212/WNL.0000000000004951. [DOI] [PubMed] [Google Scholar]
- 59.Lee SJ, McCall WD, Jr, Kim YK, Chung SC, Chung JW. Effect of botulinum toxin injection on nocturnal bruxism: a randomized controlled trial. Am J Phys Med Rehabil. 2010 Jan;89(1):16–23. doi: 10.1097/PHM.0b013e3181bc0c78. [DOI] [PubMed] [Google Scholar]
- 60.Elston JS. Botulinum toxin treatment of hemifacial spasm. J Neurol Neurosurg Psychiatry. 1986;49:827–829. doi: 10.1136/jnnp.49.7.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yoshimura DM, Aminoff MJ, Tami TA, Scott AB. Treatment of hemifacial spasm with botulinum toxin. Muscle Nerve. 1992;15:10451049. doi: 10.1002/mus.880150909. [DOI] [PubMed] [Google Scholar]
- 62.Dressler D. Routine use of Xeomin in patients previously treated with Botox: long term results. Eur J Neurol. 2009 Dec;16(Suppl 2):2–5. doi: 10.1111/j.1468-1331.2009.02877.x. [DOI] [PubMed] [Google Scholar]
- 63.Jitpimolmard S, Tiamkao S, Laopaiboon M. Long term results of botulinum toxin type A (Dysport) in the treatment of hemifacial spasm: a report of 175 cases. J Neurol Neurosurg Psychiatry. 1998;64:751–757. doi: 10.1136/jnnp.64.6.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Levy A, Chen R. Myoclonus: pathophysiology and treatment Options. Curr Treat Options Neurol. 2016 May;18(5):21. doi: 10.1007/s11940-016-0404-7. [DOI] [PubMed] [Google Scholar]
- 65.Conill Tobias N, Paula Vernetta CD, Garcia Callejo FJ, Marco Algarra J. Objective tinnitus from palatal myoclonus. Use of botulinum toxin: a case report. Acta Otorrinolaringol Esp. 2012;63:391–392. doi: 10.1016/j.otoeng.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 66.Sinclair CF, Gurey LE, Blitzer A. Palatal myoclonus: algorithm for management with botulinum toxin based on clinical disease characteristics. Laryngoscope. 2014;124:1164–1169. doi: 10.1002/lary.23485. [DOI] [PubMed] [Google Scholar]
- 67.Jankovic J. Therapeutic developments for tics and myoclonus. Mov Disord. 2015;30(11):1566–1573. doi: 10.1002/mds.26414. [DOI] [PubMed] [Google Scholar]
- 68.Lagueny A, Tison F, Burbaud P, et al. Stimulus-sensitive spinal segmental myoclonus improved with injections of botulinum toxin type A. Mov Disord. 1999;14:182–185. doi: 10.1002/1531-8257(199901)14:1<182::AID-MDS1040>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 69.Polo KB, Jabbari B. Effectiveness of botulinum toxin type A against painful limb myoclonus of spinal cord origin. Mov Disord. 1994;9:233–235. doi: 10.1002/mds.870090221. [DOI] [PubMed] [Google Scholar]
- 70.Bhatia KP, Bain P, Bajaj N, et al. Consensus Statement on the classification of tremors from the task force on tremor of the International Parkinson and Movement Disorder Society. Mov Disord. 2018;33:7587. doi: 10.1002/mds.27121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Benito-León J. Essential tremor: from a monosymptomatic disorder to a more complex entity. Neuroepidemiology. 2008;31(3):191–192. doi: 10.1159/000154933. [DOI] [PubMed] [Google Scholar]
- 72.Mittal SO, Lenka A, Jankovic J. Botulinum toxin for the treatment of tremor. Parkinsonism Relat Disord. 2019 Jan 26; doi: 10.1016/j.parkreldis.2019.01.023. pii: S1353-8020(19)30023-9. [DOI] [PubMed] [Google Scholar]
- 73.Jankovic J, Schwartz K. Botulinum toxin treatment of tremors. Neurology. 1991 Aug;41(8):1185–1188. doi: 10.1212/WNL.41.8.1185. [DOI] [PubMed] [Google Scholar]
- 74.Jankovic J. The use of botulinum toxin in tic disorders and essential hand and head tremor. In: Truong D, Dressler D, Hallett M, editors. Manual of Botulinum Toxin Therapy. Cambridge, UK: Cambridge University Press; 2013. pp. 160–167. [Google Scholar]
- 75.Pacchetti C, Mancini F, Bulgheroni M, et al. Botulinum toxin treatment for functional disability induced by essential tremor. Neurol Sci. 2000;21:349–353. doi: 10.1007/s100720070049. [DOI] [PubMed] [Google Scholar]
- 76.Mittal SO, Machado D, Richardson D, Dubey D, Jabbari B. Botulinum toxin in essential hand tremor: a randomized double-blind placebo-controlled study with customized injection approach. Parkinsonism Relat Disord. 2018 Nov;56:65–69. doi: 10.1016/j.parkreldis.2018.06.019. [DOI] [PubMed] [Google Scholar]
- 77.Samotus O, Rahimi F, Lee J, Jog M. Functional ability improved in essential tremor by incobotulinumtoxinA injections using kinematically determined biomechanical patterns: a new future. PLoS One. 2016;11(4):e0153739. doi: 10.1371/journal.pone.0153739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Thenganatt MA, Jankovic J. Recent advances in understanding and managing Tourette syndrome. F1000Res. 2016;5(F1000 Faculty Rev):152. doi: 10.12688/f1000research.7424.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Baizabal-Carvallo JF, Jankovic J. The clinical features of psychogenic movement disorders resembling tics. J Neurol Neurosurg Psychiatry. 2014 May;85(5):573–575. doi: 10.1136/jnnp-2013-305594. [DOI] [PubMed] [Google Scholar]
- 80.Persaud R, Garas G, Silva S, Stamatoglou C, Chatrath P, Patel K. An evidence-based review of botulinum toxin (Botox) applications in non-cosmetic head and neck conditions. JRSM Short Rep. 2013;4:10. doi: 10.1177/2042533312472115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Marras C, Andrews D, Sime E, Lang AE. Botulinum toxin for simple motor tics: a randomized, double-blind, controlled clinical trial. Neurology. 2001;56:605–610. doi: 10.1212/WNL.56.5.605. [DOI] [PubMed] [Google Scholar]
- 82.Sheffield JK, Jankovic J. Botulinum toxin in the treatment of tremors, dystonias, sialorrhea and other symptoms associated with Parkinson’s disease. Expert Rev Neurother. 2007;7:637–647. doi: 10.1586/14737175.7.6.637. [DOI] [PubMed] [Google Scholar]
- 83.Jocson A, Lew M. Use of botulinum toxin in Parkinson’s disease. Parkinsonism Relat Disord. 2018 Dec 11; doi: 10.1016/j.parkreldis.2018.12.002. pii: S1353-8020(18)30528-5. [DOI] [PubMed] [Google Scholar]
- 84.Mittal SO, Machado D, Richardson D, Dubey D, Jabbari B. Botulinum toxin in Parkinson disease tremor: a randomized, double-blind, placebo-controlled study with a customized injection approach. Mayo Clin Proc. 2017 Sep;92(9):1359–1367. doi: 10.1016/j.mayocp.2017.06.010. [DOI] [PubMed] [Google Scholar]
- 85.Rahimi F, Samotus O, Lee J, Jog M. Effective management of upper limb Parkinsonian tremor by incobotulinumtoxinA injections using sensor-based biomechanical patterns. Tremor Other Hyperkinet Mov (N Y) 2015 Oct 30;5:348. doi: 10.7916/D8BP0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Srivanitchapoom P, Hallett M. Camptocormia in Parkinson’s disease: definition, epidemiology, pathogenesis and treatment modalities. J Neurol Neurosurg Psychiatry. 2016;87:75–85. doi: 10.1136/jnnp-2014-310049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Von Coelln R, Raible A, Gasser T, Asmus F. Ultrasound-guided injection of the iliopsoas muscle with botulinum toxin in camptocormia. Mov Disord. 2008;23:889–892. doi: 10.1002/mds.21967. [DOI] [PubMed] [Google Scholar]
- 88.Pacchetti C, Albani G, Martignoni E, Godi L, Alfonsi E, Nappi G. “Off” painful dystonia in Parkinson’s disease treated with botulinum toxin. Mov Disord. 1995;10:333–336. doi: 10.1002/mds.870100317. [DOI] [PubMed] [Google Scholar]
- 89.Cardoso F. Botulinum toxin in parkinsonism: the when, how, and which for botulinum toxin injections. Toxicon. 2018 Jun 1;147:107–110. doi: 10.1016/j.toxicon.2017.08.018. [DOI] [PubMed] [Google Scholar]
- 90.Giladi N, Gurevich T, Shabtai H, Paleacu D, Simon ES. The effect of botulinum toxin injections to the calf muscles on freezing of gait in parkinsonism: a pilot study. J Neurol. 2001 Jul;248(7):572–576. doi: 10.1007/s004150170134. [DOI] [PubMed] [Google Scholar]
- 91.Gurevich T, Peretz C, Moore O, Weizmann N, Giladi N. The effect of injecting botulinum toxin type A into the calf muscles on freezing of gait in Parkinson’s disease: a double blind placebo controlled pilot study. Mov Disord. 2007 Apr 30;22(6):880–883. doi: 10.1002/mds.21396. [DOI] [PubMed] [Google Scholar]
- 92.Fernandez HH, Lannon MC, Trieschmann ME, Friedman JH. Botulinum toxin type B for gait freezing in Parkinson’s disease. Med Sci Monit. 2004 Jul;10(7):CR282–CR284. [PubMed] [Google Scholar]
- 93.Vastik M, Hok P, Hlustik P, Otruba P, Tüdös Z, Kanovsky P. Botulinum toxin treatment of freezing of gait in Parkinson’s disease patients as reflected in functional magnetic resonance imaging of leg movement. Neuro Endocrinol Lett. 2016;37(2):147–153. [PubMed] [Google Scholar]
- 94.Allen RP, Picchietti DL, Garcia-Borreguero D, et al. International Restless Legs Syndrome Study Group. Restless legs syndrome/Willis-Ekbom disease diagnostic criteria: updated International Restless Legs Syndrome Study Group (IRLSSG) consensus criteria—history, rationale, description, and significance. Sleep Med. 2014 Aug;15(8):860–873. doi: 10.1016/j.sleep.2014.03.025. [DOI] [PubMed] [Google Scholar]
- 95.Wong JC, Li Y, Schwarzschild MA, Ascherio A, Gao X. Restless legs syndrome: an early clinical feature of Parkinson disease in men. Sleep. 2014;37(2):369–372. doi: 10.5665/sleep.3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Garcia-Borreguero D, Odin P, Serrano C. Restless legs syndrome and PD: a review of the evidence for a possible association. Neurology. 2003;61(6 Suppl 3):S49–S55. doi: 10.1212/wnl.61.6_suppl_3.s49. [DOI] [PubMed] [Google Scholar]
- 97.Ferre S, Garcia-Borreguero D, Allen RP, Earley CJ. New insights into the neurobiology of restless legs syndrome. Neuroscientist. 2019;25(2):113–125. doi: 10.1177/107385841879176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Salminen AV, Winkelmann J. Restless legs syndrome and other movement disorders of sleep-treatment update. Curr Treat Options Neurol. 2018 Nov 9;20(12):55. doi: 10.1007/s11940-018-0540-3. [DOI] [PubMed] [Google Scholar]
- 99.Nahab FB, Peckham EL, Hallett M. Double-blind, placebo-controlled, pilot trial of botulinum toxin A in restless legs syndrome. Neurology. 2008;71:950–951. doi: 10.1212/01.wnl.0000325994.93782.a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Agarwal P, Sia C, Vaish N, Roy-Faderman I. Pilot trial of onabotulinumtoxina (Botox) in moderate to severe restless legs syndrome. Int J Neurosci. 2011;121:622–625. doi: 10.3109/00207454.2011.602774. [DOI] [PubMed] [Google Scholar]
- 101.Rotenberg JS, Canard K, Difazio M. Successful treatment of recalcitrant restless legs syndrome with botulinum toxin type-A. J Clin Sleep Med. 2006;2:275–278. [PubMed] [Google Scholar]
- 102.Ghorayeb I, Benard A, Vivot A, Tison F, Burbaud P. A phase II, open-label, non-comparative study of botulinum toxin in restless legs syndrome. Sleep Med. 2012;13:1313–1316. doi: 10.1016/j.sleep.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 103.Mittal SO, Machado D, Richardson D, Dubey D, Jabbari B. Botulinum toxin in restless legs syndrome: a randomized double-blind placebo-controlled crossover study. Toxins (Basel) 2018 Sep 29;10(10) doi: 10.3390/toxins10100401. pii: E401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Teive HA, Zonta M, Kumagai Y. Treatment of spasticity: an update. Arq Neuropsiquiatr. 1998;56(4):852–858. doi: 10.1590/S0004-282X1998000500025. [DOI] [PubMed] [Google Scholar]
- 105.Simpson DM, Hallett M, Ashman EJ, et al. Practice guideline update summary: botulinum neuro- toxin for the treatment of blepharospasm, cervical dystonia, adult spasticity, and headache: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2016;86(19):1818–1826. doi: 10.1212/WNL.0000000000002560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Smith SJ, Ellis E, White S, et al. A double-blind placebo-controlled study of botulinum toxin in upper limb spasticity after stroke or head injury. Clin Rehabil. 2000;14(1):5–13. doi: 10.1191/026921500666642221. [DOI] [PubMed] [Google Scholar]
- 107.Bhakta BB, Cozens JA, Chamberlain MA, Bamford JM. Impact of botulinum toxin type A on disability and carer burden due to arm spasticity after stroke: a randomised double blind placebo controlled trial. J Neurol Neurosurg Psychiatry. 2000 Aug;69(2):217–221. doi: 10.1136/jnnp.69.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bakheit AM, Pittock S, Moore AP, et al. A randomized, double-blind, placebo-controlled study of the efficacy and safety of botulinum toxin type A in upper limb spasticity in patients with stroke. Eur J Neurol. 2001;8(6):559–565. doi: 10.1046/j.1468-1331.2001.00277.x. [DOI] [PubMed] [Google Scholar]
- 109.Gracies JM, Brashear A2, Jech R3, et al. International AbobotulinumtoxinA Adult Upper Limb Spasticity Study Group. Safety and efficacy of abobotulinumtoxinA for hemiparesis in adults with upper limb spasticity after stroke or traumatic brain injury: a double-blind randomised controlled trial. Lancet Neurol. 2015;14(10):992–1001. doi: 10.1016/S1474-4422(15)00216-1. [DOI] [PubMed] [Google Scholar]
- 110.Bakheit AM, Thilmann AF, Ward AB, et al. A randomized, double-blind, placebo-controlled, dose-ranging study to compare the efficacy and safety of three doses of botulinum toxin type A (Dysport) with placebo in upper limb spasticity after stroke. Stroke. 2000;31(10):2402–2406. doi: 10.1161/01.STR.31.10.2402. [DOI] [PubMed] [Google Scholar]
- 111.Suputtitada A, Suwanwela NC. The lowest effective dose of botulinum A toxin in adult patients with upper limb spasticity. Disabil Rehabil. 2005;27(4):176–184. doi: 10.1080/09638280400009360. [DOI] [PubMed] [Google Scholar]
- 112.McCrory P, Turner-Stokes L, Baguley IJ, et al. Botulinum toxin A for treatment of upper limb spasticity following stroke: a multicentre randomized placebo-controlled study of the effects on quality of life and other person-centred outcomes. J Rehabil Med. 2009;41(7):536–544. doi: 10.2340/16501977-0366. [DOI] [PubMed] [Google Scholar]
- 113.Shaw LC, Price CI, van Wijck FM, et al. BoTULS Investigators. Botulinum toxin for the upper limb after stroke (BoTULS) trial: effect on impairment, activity limitation, and pain. Stroke. 2011;42(5):1371–1379. doi: 10.1161/STROKEAHA.110.582197. [DOI] [PubMed] [Google Scholar]
- 114.Rosales RL, Kong KH, Goh KJ, et al. Botulinum toxin injection for hypertonicity of the upper extremity within 12 weeks after stroke: a randomized controlled trial. Neurorehabil Neural Repair. 2012;26(7):812–821. doi: 10.1177/1545968311430824. [DOI] [PubMed] [Google Scholar]
- 115.Simpson DM, Alexander DN, O’Brien CF, et al. Botulinum toxin type A in the treatment of upper extremity spasticity: a randomized, double-blind, placebo-controlled trial. Neurology. 1996;46(5):1306–1310. doi: 10.1212/WNL.46.5.1306. [DOI] [PubMed] [Google Scholar]
- 116.Richardson D, Sheean G, Werring D, et al. Evaluating the role of botulinum toxin in the management of focal hypertonia in adults. J Neurol Neurosurg Psychiatry. 2000;69(4):499–506. doi: 10.1136/jnnp.69.4.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Brashear A, Gordon MF, Elovic E, et al. Botox Post-Stroke Spasticity Study Group. Intramuscular injection of botulinum toxin for the treatment of wrist and finger spasticity after a stroke. N Engl J Med. 2002;347(6):395–400. doi: 10.1056/NEJMoa011892. [DOI] [PubMed] [Google Scholar]
- 118.Childers MK, Brashear A, Jozefczyk P, et al. Dose-dependent response to intramuscular botulinum toxin type A for upper-limb spasticity in patients after a stroke. Arch Phys Med Rehabil. 2004;85(7):1063–1069. doi: 10.1016/j.apmr.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 119.Kaji R, Osako Y, Suyama K, et al. GSK1358820 Spasticity Study Group. Botulinum toxin type A in post-stroke upper limb spasticity. Curr Med Res Opin. 2010;26(8):1983–1992. doi: 10.1185/03007995.2010.497103. [DOI] [PubMed] [Google Scholar]
- 120.Marciniak CM, Harvey RL, Gagnon CM, et al. Does botulinum toxin type A decrease pain and lessen disability in hemiplegic survivors of stroke with shoulder pain and spasticity?: a randomized, double-blind, placebo-controlled trial. Am J Phys Med Rehabil. 2012;91(12):1007–1019. doi: 10.1097/PHM.0b013e31826ecb02. [DOI] [PubMed] [Google Scholar]
- 121.Kanovský P, Slawek J, Denes Z, et al. Efficacy and safety of botulinum neurotoxin NT 201 in poststroke upper limb spasticity. Clin Neuropharmacol. 2009;32(5):259–265. doi: 10.1097/WNF.0b013e3181b13308. [DOI] [PubMed] [Google Scholar]
- 122.Elovic EP, Munin MC, Kaňovský P, et al. Randomized, placebo-controlled trial of incobotulinumtoxinA for upper-limb post-stroke spasticity. Muscle Nerve. 2016;53(3):415–421. doi: 10.1002/mus.24776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Pittock SJ, Moore AP, Hardiman O, et al. A double-blind randomised placebo-controlled evaluation of three doses of botulinum toxin type A (Dysport) in the treatment of spastic equinovarus deformity after stroke. Cerebrovasc Dis. 2003;15(4):289–300. doi: 10.1159/000069495. [DOI] [PubMed] [Google Scholar]
- 124.Hyman N, Barnes M, Bhakta B, et al. Botulinum toxin (Dysport) treatment of hip adductor spasticity in multiple sclerosis: a prospective, randomised, double blind, placebo controlled, dose ranging study. J Neurol Neurosurg Psychiatry. 2000;68(6):707–712. doi: 10.1136/jnnp.68.6.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gusev YI, Banach M, Simonow A, et al. Efficacy and safety of botulinum type A toxin in adductor spasticity due to multiple sclerosis. J Musculoskel Pain. 2008;16:175–188. doi: 10.1080/10582450802161952. [DOI] [Google Scholar]
- 126.Kaji R, Osako Y, Suyama K, et al. GSK1358820 Spasticity Study Group. Botulinum toxin type A in post-stroke lower limb spasticity: a multicenter, double-blind, placebo-controlled trial. J Neurol. 2010;257(8):1330–1337. doi: 10.1007/s00415-010-5526-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Dunne JW, Gracies JM, Hayes M, et al. Multicentre Study Group. A prospective, multicentre, randomized, double-blind, placebo-controlled trial of onabotulinumtoxinA to treat plantarflexor/invertor overactivity after stroke. Clin Rehabil. 2012;26(9):787–797. doi: 10.1177/0269215511432016. [DOI] [PubMed] [Google Scholar]
- 128.Maanum G, Jahnsen R, Stanghelle JK, et al. Effects of botulinum toxin A in ambulant adults with spastic cerebral palsy: a randomized double-blind placebo controlled-trial. J Rehabil Med. 2011;43(4):338–47. doi: 10.2340/16501977-0672. [DOI] [PubMed] [Google Scholar]
- 129.Servelhere KR, Faber I, Martinez A, et al. Botulinum toxin for hereditary spastic paraplegia: effects on motor and non-motor manifestations. Arq Neuropsiquiatr. 2018 Mar;76(3):183–188. doi: 10.1590/0004-282x20180013. [DOI] [PubMed] [Google Scholar]