Abstract
Glioblastoma is an intrinsic brain tumour thought to arise from neuroglial progenitor cells. Its incidence increases steadily with age. Males are moderately more often affected. Genetic predisposition and exposure to irradiation in childhood are the only established risk factors which, however, account only for a very small proportion of glioblastomas. Surgery as safely feasible not only to allow for tissue diagnosis but also to reduce tumour volume is usually the first therapeutic measure. Radiotherapy delivered to the tumour region with a safety margin has been demonstrated to roughly double survival four decades ago. Temozolomide given during radiotherapy followed by six cycles of maintenance chemotherapy was the first and so far only pharmacological treatment shown to prolong survival. Adding tumour-treating fields during maintenance, temozolomide chemotherapy has been reported to prolong survival. There is little evidence that any intervention at relapse improves outcome, but nitrosourea-based chemotherapy, commonly lomustine, is probably the most agreed on standard of care. Bevacizumab prolongs progression-free survival and probably quality of life in the first line or recurrent setting, but not overall survival, and is therefore not approved in the European Union. Immunotherapy remains experimental. Drugs in advanced clinical development include the programmed death 1 antibody, nivolumab, the antibody drug conjugate depatuxizumab directed to the epidermal growth factor receptor and the proteasome inhibitor marizomib.
Keywords: glioblastoma, surgery, radiotherapy, chemotherapy, MGMT
Definition and diagnosis
The diagnosis of glioblastoma requires the histological and molecular genetic study of tumour tissue obtained during open tumour resection or biopsy. Morphologically, glioblastoma is defined as a glial brain tumour with features of malignancy that include angiogenesis and focal necrosis. The new WHO classification recognises, in addition to classical glioblastoma, giant cell glioblastoma, gliosarcoma and epithelioid glioblastoma as histologically defined variants. Half of the rare epithelioid glioblastomas show BRAFV600E mutations.1 So far treatment recommendations do not vary for these glioblastoma variants.
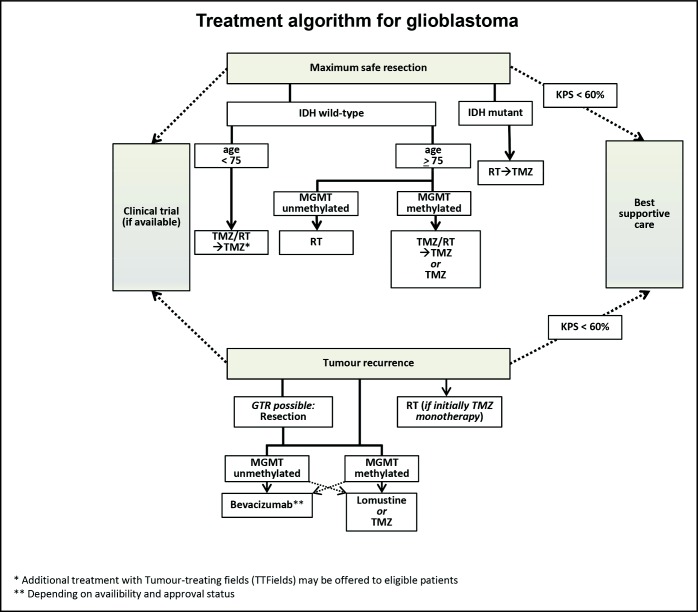
Furthermore, classical glioblastoma does not exhibit isocitrate dehydrogenase (IDH) 1 or 2 mutations; tumours carrying such mutations show a less aggressive clinical course and will probably no longer be labelled glioblastomas in future revisions of the WHO classification. In contrast, it has been proposed to consider glial tumours without IDH 1 or 2 mutations which do not fulfil histological criteria of glioblastoma as tumours equivalent to glioblastoma, for example, in the context of a clinical trial if they fulfill one of the following criteria: epidermal growth factor receptor amplification, whole chromosome 7 gain combined with whole chromosome 10 loss or telomerase reverse transcriptase promoter mutation.2 Specifically in glioblastoma without IDH mutation and thus commonly without CpG island methylator phenotype, methylation of the promoter region of the O6-methylguanine DNA methyltransferase (MGMT) gene, tested by methylation-specific PCR or by pyrosequencing, has been established as a predictive biomarker for benefit from alkylating agent chemotherapy. Extensive efforts have been undertaken to define cut-offs to dissect what extent of MGMT promoter methylation is required to confer benefit from alkylating chemotherapy and which CpG of the promoter region should be assessed. With methylation-specific PCR, there is a grey zone in between truly unmethylated and methylated, resulting in a recommendation of a cut-off of 1.27 (Log2 (1000×(MGMT+1)/ACTB) plus a safety margin of −0.28). With pyrosequencing, the cut-off for being methylated is usually set at 7%–25%.3 Young age and good performance status are therapy-independent positive prognostic factors. Figure 1 provides an algorithm how to approach glioblastoma that is consistent with the 2017 guideline of the European Association of Neuro-Oncology.4
Figure 1.
Therapeutic approach to glioblastoma. GTR, gross total resection; IDH, isocitrate dehydrogenase; KPS, Karnofsky performance score; MGMT, O6-methylguanine DNA methyltransferase; RT, radiation therapy; TMZ, temozolomide.
Newly diagnosed setting
Surgery
Maximum surgical resection using microsurgical techniques as safely feasible is considered standard of care, although the role of surgery has been difficult to define in controlled clinical trials. The proof-of-concept trial for fluorescence-guided resection indicated improved progression-free survival in patients who had a gross total resection.5 The extent of surgery should be verified by contrast-enhanced MRI within 24–72 hours of surgery. Although the volume of residual tumour after surgery negatively correlates with outcome, it has remained impossible to clarify whether extent of resection improves outcome or whether tumours amenable to gross total resection have a different, less malignant course of disease. Still, we support efforts at gross total tumour resection and do not believe that a randomised clinical trial to demonstrate benefit from this intervention can be ethically justified. If microsurgical resection is not feasible due to medical reasons or refusal of the patient, an image-guided stereotactic serial biopsy can provide sufficient material for both histological as well as molecular diagnosis to guide therapeutic management.6 Some centres prefer open biopsies to get more tissue. Treatment decisions without obtaining tissue for a histological diagnosis are strongly discouraged.
Radiotherapy
Involved field radiotherapy has been established as a standard of care for glioblastoma more than 40 years ago. It improves local control and probably roughly doubles survival. Standard radiotherapy is delivered in 1.8–2 Gy fractions to a total dose of 54–60 Gy. Hypofractionated radiotherapy with a biologically equivalent dose of 40 Gy given in 15 fractions of 2.67 Gy has been established as a valid option for older patients and for patients with limited life expectancy because of poor prognostic factors.7 Amino acid positron emission tomography is currently explored as a tool to better delineate the target volume of radiotherapy. Neither novel techniques of administering irradiation nor the combination of radiotherapy with candidate radiosensitising agents have shown superior efficacy over standard fractionated radiotherapy. Radiotherapy may be withheld in elderly patients or patients with poor performance status if the tumour exhibits MGMT promoter methylation; such patients may be treated with temozolomide alone.8
Pharmacotherapy
Temozolomide chemotherapy during radiotherapy (75 mg/m2 daily) followed by six cycles of maintenance chemotherapy (150–200 mg/m2, 5/28 days) is standard of care for the majority of patients with glioblastoma.9 10 Limitations include major comorbidities such as haematological or hepatic disease or infection. Myelosuppression, notably thrombocytopenia, is the most common side effect. There is no evidence that changing the dosing of the temozolomide regimen or prolonging its administration beyond 6 months improve survival.4 Benefit from temozolomide is largely restricted to patients with tumours with MGMT promoter methylation.11
Tumour-treating fields
Tumour-treating fields involve the focal delivery of low-intensity, intermediate-frequency (200 kHz) alternating electrical fields to the tumour-bearing brain that are postulated to inhibit cell cycle progression through metaphase. An open label randomised trial of tumour-treating fields administered from the initiation of maintenance temozolomide reported superior progression-free survival and overall survival compared with temozolomide alone.12 While this treatment is safe and usually well tolerated except for local skin reactions, its acceptance by patients, relatives and healthcare professionals in most parts of Europe is low. Furthermore, reimbursement remains a critically debated issue in most countries.
Best supportive care
This strategy may be selected for patients with extensive or multifocal lesions in poor general or neurological condition and patients who cannot consent to further treatment once the diagnosis of glioblastoma has been established.
Recurrent setting
Standards of care at recurrence are far less well defined than in the newly diagnosed setting. Up to half of patients with glioblastoma may not qualify for salvage treatment.13 Repeat surgery should be offered if a gross total resection seems feasible and if the time from first surgical intervention is longer than approximately 6 months.14 Reirradiation can be offered to patients with circumscribed recurrences. Neither for surgery nor for radiotherapy, a randomised trial to document prolongation of survival has been conducted. Tumour-treating fields were not superior to best physicians’ choice in the recurrent setting.
Most patients with recurrent glioblastoma who are eligible for salvage therapy are treated with a second course of alkylating agent chemotherapy, mostly lomustine (CCNU). The strategy of temozolomide rechallenge is less frequently pursued today than a few years ago. Either alkylating agent is unlikely to provide benefit in patients with tumours without MGMT promoter methylation. Bevacizumab as a single agent was approved in the USA and various other countries, although not the European Union, based on response rates and progression-free survival rates at 6 months interpreted to be superior to historical controls. Yet, the combination of bevacizumab with lomustine prolonged progression-free survival only, but not overall survival, compared with lomustine alone.15 The differential interpretation of the significance and validity of progression-free survival explains the different approval status of bevacizumab in recurrent glioblastoma. Depending on local availability, the combination of bevacizumab and lomustine is a treatment option in particular in patients with rapidly progressing disease. Much more commonly, bevacizumab where available is now used in symptomatic patients at later recurrences. A population-based analysis has not been able to find evidence that the approval of bevacizumab prolonged survival on a population level.13
Follow-up
Neurological evaluation and MRI at least every 3 months are the central tools to assess benefit from therapy and for monitoring during follow-up. Pseudoprogression remains a concern especially within the first 3–6 months after completion of radiotherapy. Advanced MRI and positron emission tomography are explored to provide guidance, but the most important consideration in patients with potential pseudoprogression is not to discontinue a potentially active treatment too early. Throughout the course of disease, control of symptomatic seizures and detection, treatment and secondary prevention of venous thromboembolic events remain important tasks. Primary seizure prophylaxis with antiepileptic drugs is not indicated and steroids should be given to symptomatic patients only, at the lowest dose and for the shortest time feasible.
Current developments
Data from four phase III trials may change clinical practice in the upcoming years. CheckMate 498 (NCT02617589) and CheckMate 548 (NCT02667587) explored nivolumab in newly diagnosed patients with glioblastoma without and with MGMT promoter methylation. Depatuxizumab, an antibody drug conjugate, was tested in the same setting in estimated glomerular filtration rate-amplied glioblastoma (NCT02573324). These trials have completed accrual and data should become available in 2019. The proteasome inhibitor marizomib is examined in newly diagnosed glioblastoma within the European Organisation for Research and Treatment of Cancer trial 1709 (NCT03345095).
Footnotes
Contributors: All authors wrote the article and approved the content.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: MW has received research grants from Abbvie, Adastra, Dracen, Merck, Sharp & Dohme (MSD), Merck (EMD), Novocure, OGD2, Piqur and Roche and honoraria for lectures or advisory board participation or consulting from Abbvie, Basilea, Bristol Meyer Squibb, Celgene, Merck, Sharp & Dohme (MSD), Merck (EMD), Novocure, Orbus, Roche and Tocagen. ELR has received research grants from Mundipharma and Amgen and honoraria for lectures or advisory board participation from Abbvie, Daiichi Sankyo, Mundipharma and Novartis. MP has received honoraria for lectures, consultation or advisory board participation from the following for-profit companies: Bayer, Bristol-Myers Squibb, Novartis, Gerson Lehrman Group (GLG), CMC Contrast, GlaxoSmithKline, Mundipharma, Roche, Astra Zeneca, AbbVie, Lilly, Medahead, Daiichi Sankyo, Merck Sharp & Dome. JCT has received research grants from BrainLab and honoraria for lectures or consulting from BrainLAb and medac. PR has received honoraria for advisory board participation or lectures from Bristol-Myers Squibb, Covagen, Medac, MSD, Novartis, Novocure, Roche and Virometix.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 2016;131:803–20. 10.1007/s00401-016-1545-1 [DOI] [PubMed] [Google Scholar]
- 2.Brat DJ, Aldape K, Colman H, et al. cIMPACT-NOW update 3: recommended diagnostic criteria for "Diffuse astrocytic glioma, IDH-wildtype, with molecular features of glioblastoma, WHO grade IV". Acta Neuropathol 2018;136:805–10. 10.1007/s00401-018-1913-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hegi ME, Genbrugge E, Gorlia T, et al. MGMT promoter methylation cutoff with safety margin for selecting glioblastoma patients into trials omitting temozolomide: a pooled analysis of four clinical trials. Clin Cancer Res 2019;25:1809–16. 10.1158/1078-0432.CCR-18-3181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weller M, van den Bent M, Tonn JC, et al. European association for neuro-oncology (EANO) guideline on the diagnosis and treatment of adult astrocytic and oligodendroglial gliomas. Lancet Oncol 2017;18:e315–29. 10.1016/S1470-2045(17)30194-8 [DOI] [PubMed] [Google Scholar]
- 5.Stummer W, Pichlmeier U, Meinel T, et al. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol 2006;7:392–401. 10.1016/S1470-2045(06)70665-9 [DOI] [PubMed] [Google Scholar]
- 6.Eigenbrod S, Trabold R, Brucker D, et al. Molecular stereotactic biopsy technique improves diagnostic accuracy and enables personalized treatment strategies in glioma patients. Acta Neurochir 2014;156:1427–40. 10.1007/s00701-014-2073-1 [DOI] [PubMed] [Google Scholar]
- 7.Roa W, Brasher PMA, Bauman G, et al. Abbreviated course of radiation therapy in older patients with glioblastoma multiforme: a prospective randomized clinical trial. J Clin Oncol 2004;22:1583–8. 10.1200/JCO.2004.06.082 [DOI] [PubMed] [Google Scholar]
- 8.Wick W, Platten M, Meisner C, et al. Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: the NOA-08 randomised, phase 3 trial. Lancet Oncol 2012;13:707–15. 10.1016/S1470-2045(12)70164-X [DOI] [PubMed] [Google Scholar]
- 9.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005;352:987–96. 10.1056/NEJMoa043330 [DOI] [PubMed] [Google Scholar]
- 10.Perry JR, Laperriere N, O'Callaghan CJ, et al. Short-course radiation plus temozolomide in elderly patients with glioblastoma. N Engl J Med 2017;376:1027–37. 10.1056/NEJMoa1611977 [DOI] [PubMed] [Google Scholar]
- 11.Hegi ME, Diserens A-C, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med 2005;352:997–1003. 10.1056/NEJMoa043331 [DOI] [PubMed] [Google Scholar]
- 12.Stupp R, Taillibert S, Kanner A, et al. Effect of Tumor-Treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: a randomized clinical trial. JAMA 2017;318:2306–16. 10.1001/jama.2017.18718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gramatzki D, Roth P, Rushing EJ, et al. Bevacizumab may improve quality of life, but not overall survival in glioblastoma: an epidemiological study. Ann Oncol 2018;29:1431–6. 10.1093/annonc/mdy106 [DOI] [PubMed] [Google Scholar]
- 14.Suchorska B, Weller M, Tabatabai G, et al. Complete resection of contrast-enhancing tumor volume is associated with improved survival in recurrent glioblastoma-results from the director trial. Neuro Oncol 2016;18:549–56. 10.1093/neuonc/nov326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wick W, Gorlia T, Bendszus M, et al. Lomustine and bevacizumab in progressive glioblastoma. N Engl J Med 2017;377:1954–63. 10.1056/NEJMoa1707358 [DOI] [PubMed] [Google Scholar]