Abstract
The use of a template as a linchpin motif in directed remote C–H functionalization is a versatile yet relatively underexplored strategy. We have developed a template-directed approach to realizing one-pot sequential palladium-catalyzed meta-selective C–H olefination of phenols, and nickel-catalyzed ipso-C–O activation and arylation. Thus, this bifunctional template converts phenols to synthetically useful 1,3-disubstituted arenes.
Graphical Abstract
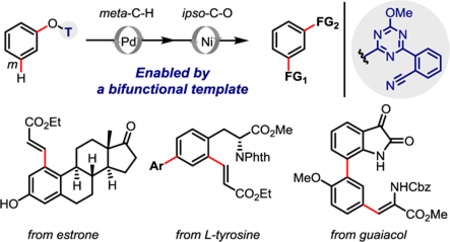
Realizing remote selective C–H functionalization is essential for broad application of directed C–H activation reactions in the presence of multiple C–H bonds.1 Over the past two decades, transition-metal-catalyzed arene ortho-C–H functionalization has been extensively exploited via σ-chelation-assisted directing effect.2 More recently, directed remote meta- and para-C–H functionalization of arenes has also been achieved using distance and geometry as the key recognition parameters.3–5 However, the installation and removal of these templates for remote C–H activation reduces the step economy in synthetic applications. Therefore, a one-pot procedure that can both directly remove the directing group and install additional desired functionality would greatly improve the synthetic efficacy of directed arene C–H activation. In this context, Chatani and others have demonstrated a commendable example with the ortho-selective directing group 2-pyridyloxy (OPy) (Figure 1a).6 Herein we report the development of a palladium-catalyzed remote meta-C–H olefination of phenols enabled by a novel bifunctional template, which can also be directly converted into other functional groups, i.e. an aryl group (vide infra), via the catalytic cleavage of the ipso-C–O bonds in the meta-functionalized phenols (Figure 1b). Notably, this template is easily installed in a single step from the commercially available 2,4-dichloro-6-methoxy-1,3,5-triazine (Figure 1c).
Figure 1.
Development of new bifunctional templates for sequential meta-C–H/ipso-C–O functionalization of phenols.
It is well established that the positional selectivity of template-assisted remote C–H activation is highly associated with the template geometry.3,4 An appropriate template facilitates the assembly of a conformationally favored macrocyclic metallocycle that enables the metal catalyst to activate the remote C–H bond of the arene through an agnostic interaction.7 Moreover, a rigid backbone in the template readily forms a large yet less strained macrocyclic pre-transition state, and therefore provides the palladacycle intermediate with higher stability and a longer lifetime.3f For example, we3f,8 and others9 have found that the use of a relatively rigid template could functionalize remote meta-C–H bonds in long-chain aromatic alcohols. Bearing in mind that the unreactive C–O bonds in phenols may be directly functionalized via transition-metal-catalyzed C–O cleavage of aryl ethers, such as 2-pyridyloxy,6,10 and 2-triazinyloxy groups11, we strove to develop a bifunctional template that could both direct the meta-C–H functionalization of phenols and be subsequently removed through a transition-metal-catalyzed ipso-C–O cleavage, consisting of a 1,3,5-triazine-derived rigid biaryl scaffold (Figure 1b).
Initially, the biaryl templates T1–T3 were synthesized via a Suzuki coupling from the commercially available 2,4-dichloro-6-methoxy-1,3,5-triazine (Figure 1c), and the latter can also be prepared from inexpensive cyanuric chloride in almost quantitative yield (see the SI). The substrate bearing the template T1 was subjected to the previously established meta-C–H olefination conditions.3,8 Much to our delight, the C–H olefination occurred exclusively at the meta-position with meta:others > 20:1 regioselectivity. Ortho-C–H functionalization directed by the donor heteroatom of 1,3,5-triazine was not observed under the reaction conditions.12 Not surprisingly, using templates T2 and T3 also afforded the products in >20:1 meta-selectivity. Increasing the electron density of the phenyl rings in the templates T2 and T3 did not significantly improve the reactivity, but led to a lower ratio of mono- to di-olefinated products (Scheme 1). With the template T1, however, the desired meta-C–H olefination product could be obtained in 82% yield after an elongated reaction time (36 h). Thus, T1 was selected as the optimal template.
Scheme 1. Design of New Template for meta-C-H Olefination of Phenola.
aYield and regioselectivity were determined by 1H NMR with CH2Br2 as an internal standard. bReaction time: 36 h.
Having identified the optimal template, we further investigated the generality of the template-assisted meta-C–H olefination (Table 1). Both electron-donating and -withdrawing groups are well tolerated with predominant meta-selectivity. Excluding alkyl and alkoxyl groups, a variety of functional groups such as halogen (2e, 2f, 2j, 2n and 2o), ester (2g), and acetyl (2p) groups afforded the meta-olefinated products in isolated yields of 49–89%. Substrates bearing two groups in different substitution patterns are also compatible with the present protocol (2q–s). Compared with meta- and para-substituted phenols, substrates derived from ortho-substituted phenols delivered the meta-C–H olefination products in higher yields (2b–g). The presence of an ortho-substituent significantly improves the mono-selectivity as the di-meta-C–H olefination will be subjected to steric hindrance from the adjacent ortho-substituent. It is noteworthy that, using template T1, naturally occurring L-tyrosine (2t) and estrone (2u) also yielded the corresponding olefination products in exclusive meta-selectivity. The synthetically valuable meta-olefinated phenols could be readily obtained through removing the template (See SI for details). In addition, the olefin coupling partner scope was examined. Various α,β-unsaturated esters, amide, sulfone, and phosphonate olefins were reactive, affording the meta-olefinated products in good yields (2v1–2v6). Under the reaction conditions, α- or β-substituted olefins proved to be compatible substrates (2v7 and 2v8). Moreover, C–H olefination with cyclic α,β-unsaturated esters and 2-amidoacrylates also provided the desired meta-products with exclusive Z-selectivity in satisfactory yields (2v9–2v12). The stereochemistry of the double bond is determined by the two competing transition state energy of the β-hydride elimination, analogous to the Heck Coupling. The transition state with the electron-withdrawing ester group being anti to the phenyl group is generally favored which accounts for the observed Z-selectivity of meta-C–H olefinated products (for stereochemistry assignment, see the SI).
Table 1.
Pd-Catalyzed Template-Directed meta-C–H Olefination of Phenolsa
Isolated yield. Regioselectivity was determined to be meta:others > 20:1 by 1H NMR using CH2Br2 as an internal standard.
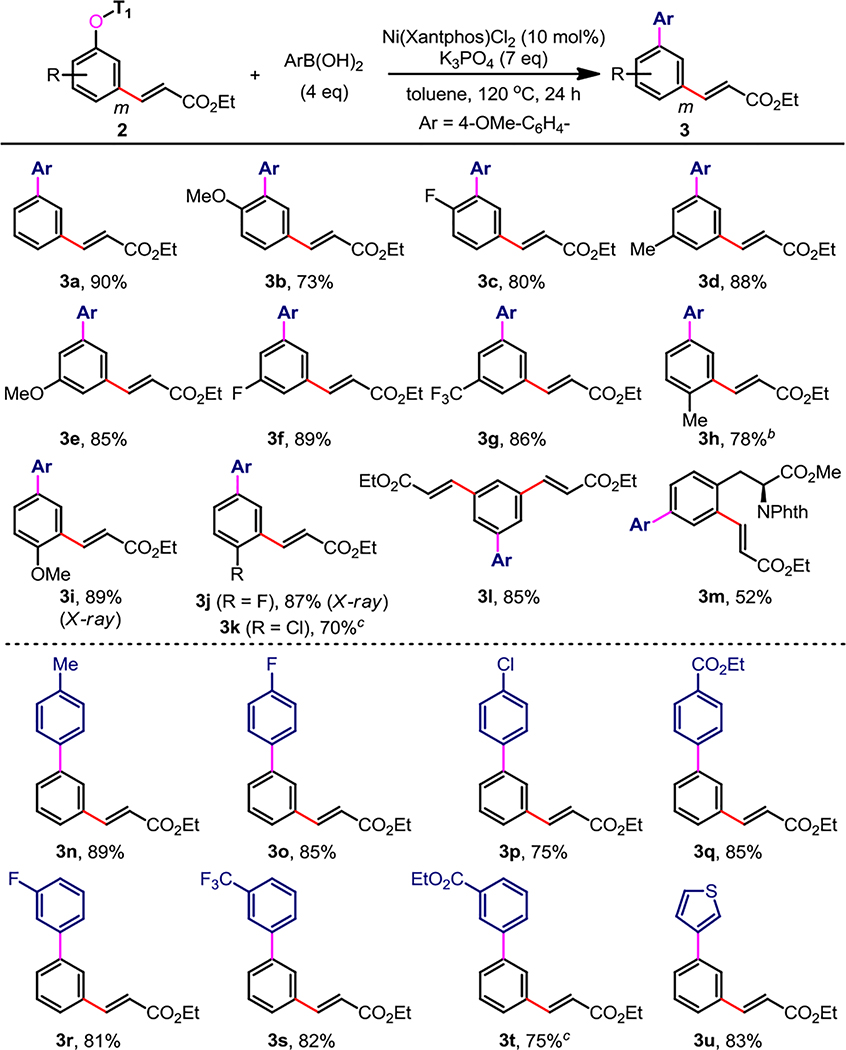
In template-directed C–H functionalization, an additional step is typically required to remove the template and release the C–H functionalized product.3–5 We wonder whether the template could serve as a linchpin to enable further elaboration of the product via C–O activation. Undoubtedly, direct functionalization via template-induced ipso-C–O bond cross-coupling will not only reduce the cost of the process, but also improve the synthetic efficacy. Therefore, transition-metal-catalyzed direct ipso-C–O cross-coupling of the meta-C–H olefinated phenol 2a with arylboronic acid was investigated. Reaction parameters such as metal catalysts, ligand, base, solvent, and temperature were examined (see SI for details), showing that Ni(xantphos)Cl2 (10 mol%), K3PO4 (7.0 eq), in toluene at 120 °C comprised the optimal reaction conditions.
Next, the substrate scope and the functional group tolerance of this nickel-catalyzed transformation were also surveyed (Table 2). Using p-methoxyl phenylboronic acid as a coupling partner, Ni-catalyzed C–O cross-coupling with various o-, m-, and p-substituted phenol substrates provided the biaryl products 3 in good to excellent yields under the optimized reaction conditions (3b–k). Various functional groups, such as alkyl, alkoxyl, halogen, trifluoromethyl, and ester groups, are tolerated in this transformation. Moreover, m-, m′-diolefinated phenol substrates successf-ully underwent the reaction in good yield (3l). Using the meta-C–H olefinated product of N-protected L-tyrosine, it was also possible to synthesize an unnatural amino acid (3m). Single crystal X-ray diffraction analyses of compounds 3i (p-OMe) and 3j (p-F) validated the exclusive meta-selectivity of C–H functionalization and ipso-C–O cross-coupling (see SI for details). The scope of organoboron reagents was also evaluated: both m- and p-substituents on arylboronic acids including alkyl, halogen, trifluoromethyl, and ester groups were tolerated (3n–t). Notably, heteroarylboronic acid (3u) afforded the biaryl product in 83% yield. Regrettably, o-substituted arylboronic acids give the target products in poor yields, probably due to the steric congestion.
Table 2.
Ni-Catalyzed C–O Cross-Coupling of meta-Olefinated Phenolsa
Isolated yield.
110 °C, 36 h.
110 °C.
To improve the synthetic utility of this strategy and avoid the tedious separation of C–H olefination products, a one-pot meta-C–H/ipso-C–O functionalization procedure was pursued (Table 3). Following palladium-catalyzed meta-C–H olefination, the unpurified intermediate was subjected to nickel-catalyzed ipso-C–O cross-coupling reaction with an arylboronic acid. Gratifyingly, this one-pot procedure furnished the desired m-substituted biaryls in good overall yields (3a, 4a–e). These results further demonstrated the synthetic applicability of this template-assisted transformation. More importantly, template T1 can be recovered and regenerated after nickel-catalyzed C–O coupling in excellent yield (Scheme 2), which further enhances the synthetic efficacy and atom-economy of the strategy.
Table 3.
Template-Assisted Tandem meta-C–H/ipso-C–O Functionalization of Phenolsa
Overall yield for two steps.
Scheme 2.
Recovery and Regeneration of Template
Finally, this template-directed remote C–H functionalization strategy was tested in the synthesis of key building blocks of natural products. Template-assisted meta-C–H olefination of the substrate derived from guaiacol afforded the olefinated product 6 in 68% yield. While direct C–O coupling of compound 6 with arylboronate 9 under the present conditions gave the biaryl scaffold 10 in low yield (18%), an alternative route featuring quantitative removal of the template T1, borylation, and palladium-catalyzed Suzuki coupling with bromide 8 efficiently delivered the left-hand moiety 10 of TMC-95 A–D (Scheme 3), a small class of naturally occurring selective proteasome inhibitors with IC50 values of 5–60 nM.13 The synthetic strategy allows for facile modification of the biaryl units and the amino acid residue, for such purposes as a structure-activity relationship (SAR) study for this family of natural products.
Scheme 3. Application to Synthesis of the Central Scaffold of Natural Antibiotic TMC-95a.
aConditions: (1) T1 (1 eq), KOH (1 eq), THF, rt, 95%. (2) olefin (3 eq), Pd(OAc)2 (10 mol%), Ac-Gly-OH (20 mol%), AgOAc (1.5 eq), HFIP (0.2 M), 80 °C, 36 h, 68%. (3) morpholine (1 eq), dioxane, 100 °C, 12 h, 99%. (4) Tf2O (1.1 eq), Pyridine (2.5 eq), CH2Cl2, 0 °C to rt, 3 h, 75%. (5) B2(pin)2 (2 eq), Pd(OAc)2 (10 mol%), DPEphos (20 mol%), TEA (4 eq), dioxane, 80 °C, 16 h, 90 %. (6) ArBr 8 (1.1 eq), Pd(dppf)Cl2 (20 mol%), K2CO3 (4 eq), DME, 80 °C, 14 h, 92%. (7) ArBpin 9 (4 eq), Ni(xantphos)Cl2 (10 mol%), K3PO4 (7 eq), toluene, 120 °C, 24 h, 18%.
In summary, a robust bifunctional template has been developed for the palladium-catalyzed remote meta-C–H olefination of phenols. The template can be synthesized concisely in a two-step sequence from inexpensive cyanuric chloride, and is easily installed and removed. In addition, this bifunctional template is amenable to nickel-catalyzed ipso-C–O cross-coupling. This template-assisted one-pot sequential meta-C–H/ipso-C–O functionalization methodology allows for the expedited synthesis of multiply substituted arenes from simple phenol substrates. Further applications of this template-assisted strategy are under investigation in our laboratory.
Supplementary Material
ACKNOWLEDGMENT
We thank the NSF of China (20502012, 21172111 and 21672116) for financial support of this work. We gratefully acknowledge The Scripps Research Institute, the NIH ((National Institute of General Medical Sciences grant 5R01GM102265).
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website.
Experimental procedures, characterization data, NMR spectra for new compounds (PDF)
Crystal data for compounds 3i and 3j (CIF)
The authors declare no competing financial interests.
REFERENCES
- (1).(a) Breslow R Biomimetic control of chemical selectivity. Acc. Chem. Res 1980, 13, 170. [Google Scholar]; (b) Das S; Incarvito CD; Crabtree RH; Brudvig GW Molecular recognition in the selective oxygenation of saturated C–H bonds by a dimanganese catalyst. Science 2006, 312, 1941. [DOI] [PubMed] [Google Scholar]; (c) Li J-J; Giri R; Yu J-Q Remote C–H bond functionalization reveals the distance-dependent isotope effect. Tetrahedron 2008, 64, 6979. [Google Scholar]
- (2).(a) Seregin IV; Gevorgyan V Direct transition metal-catalyzed functionalization of heteroaromatic compounds. Chem. Soc. Rev 2007, 36, 1173. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Daugulis O; Do HQ; Shabashov D Palladium- and copper-catalyzed arylation of carbon-hydrogen bonds. Acc. Chem. Res 2009, 42, 1074. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Lyons TW; Sanford MS Palladium-catalyzed ligand-directed C–H functionalization reactions. Chem. Rev 2010, 110, 1147. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Colby DA; Tsai AS; Bergman RG; Ellman JA Rhodium catalyzed chelation-assisted C–H bond functionalization reactions. Acc. Chem. Res 2012, 45, 814. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Rouquet G; Chatani N Catalytic functionalization of C(sp2)–H and C(sp3)–H bonds by using bidentate directing groups. Angew. Chem., Int. Ed 2013, 52, 11726. [DOI] [PubMed] [Google Scholar]; (f) Engle KM; Mei T-S; Wasa M; Yu J-Q Weak coordination as a powerful means for developing broadly useful C–H functionalization reactions. Acc. Chem. Res 2012, 45, 788. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Patureau FW; Wencel-Delord J; Glorius F Cp*Rh-catalyzed C–H activations: Versatile dehydrogenative cross-coupling of Csp2 C–H positions with olefins, alkynes, and arenes. Aldrichimica Acta 2012, 45, 31. [Google Scholar]; (h) Ackermann L Carboxylate-assisted ruthenium-catalyzed alkyne annulations by C–H/Het–H bond functionalizations. Acc. Chem. Res 2014, 47, 281. [DOI] [PubMed] [Google Scholar]
- (3).(a) Leow D; Li G; Mei T-S; Yu J-Q Activation of remote meta-C–H bond assisted by an end-on template. Nature 2012, 486, 518. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Dai H-X; Li G; Zhang X-G; Stepan AF; Yu J-Q Pd(II)-catalyzed ortho- or meta-C–H olefination of phenol derivatives. J. Am. Chem. Soc 2013, 135, 7567. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Lee S; Lee H; Tan KL Meta-selective C–H functionalization using a nitrile-based directing group and cleavable Si-tether. J. Am. Chem. Soc 2013, 135, 18778. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Tang R; Li G; Yu J-Q Conformation-induced remote meta-C–H activation of amines. Nature 2014, 507, 215. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Bera M; Maji A; Sahoo SK; Maiti D Pd(II)-catalyzed meta-C–H olefination: Constructing multi-substituted arenes through homo-diolefination and sequent hetero-diolefination. Angew. Chem., Int. Ed 2015, 54, 8515. [DOI] [PubMed] [Google Scholar]; (f) Chu L; Shang M; Tanaka K; Chen Q; Pissarnitski N; Streckfuss E; Yu J-Q Remote meta-C–H activation using a pyridine-based template: achieving site-selectivity via the recognition of distance and geometry. ACS Cent. Sci 2015, 1, 394. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Li S; Cai L; Ji H; Yang L; Li G Pd(II)-catalysed meta-C–H functionalizations of benzoic acid derivatives. Nat. Commun 2016, 7, 10443. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Bag S; Jayarajan R; Mondal R; Maiti D Template-assisted meta-C–H alkylation and alkenylation of arenes. Angew. Chem., Int. Ed 2017, 56, 3182. [DOI] [PubMed] [Google Scholar]; (i) Zhang Z; Tanaka K; Yu J-Q Remote site-selective C–H activation directed by a catalytic bifunctional template. Nature 2017, 543, 538. [DOI] [PMC free article] [PubMed] [Google Scholar]; Template-directed meta-C–H functionalization:
- (4).(a) Bag S; Patra T; Modak A; Deb A; Maity S; Dutta U; Dey A; Kancherla R; Maji A; Hazra A; Bera M; Maiti D Remote para-C–H functionalization of arenes by a D-shaped biphenyl template-based assembly. J. Am. Chem. Soc 2015, 137, 11888. [DOI] [PubMed] [Google Scholar]; (b) Patra T; Bag S; Kancherla R; Mondal A; Dey A; Pimparkar S; Agasti S; Modak A; Maiti D Palladium-catalyzed directed para-C–H functionalization of phenols. Angew. Chem., Int. Ed 2016, 55, 7751. [DOI] [PubMed] [Google Scholar]; Examples of template-directed para-C–H functionalization:
- (5).(a) Cho J-Y; Tse MK; Holmes D; Maleczka RE Jr.; Smith MR III. Remarkably selective iridium catalysts for the elaboration of aromatic C–H bonds. Science 2002, 295, 305. [DOI] [PubMed] [Google Scholar]; (b) Ishiyama T; Takagi J; Ishida K; Miyaura N; Anastasi NR; Hartwig JF Mild iridium-catalyzed borylation of arenes. High turnover numbers, room temperature reactions, and isolation of a potential intermediate. J. Am. Chem. Soc 2002, 124, 390. [DOI] [PubMed] [Google Scholar]; (c) Zhang Y-H; Shi B-F; Yu J-Q Pd(II)-catalyzed olefination of electron-deficient arenes using 2,6-dialkyl pyridine ligands. J. Am. Chem. Soc 2009, 131, 5072. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Phipps RJ; Gaunt MJ A meta-selective copper-catalyzed C–H bond arylation. Science 2009, 323, 1593. [DOI] [PubMed] [Google Scholar]; (e) Saidi O; Marafie J; Ledger AEW; Liu PM; Mahon MF; Kociok-Koehn G; Whittlesey MK; Frost CG Ruthenium-catalyzed meta sulfonation of 2-phenylpyridines. J. Am. Chem. Soc 2011, 133, 19298. [DOI] [PubMed] [Google Scholar]; (f) Hofmann N; Ackermann L meta-Selective C–H bond alkylation with secondary alkyl halides. J. Am. Chem. Soc 2013, 135, 5877. [DOI] [PubMed] [Google Scholar]; (g) Cheng C; Hartwig JF Rhodium-catalyzed intermolecular C–H silylation of arenes with steric regiocontrol. Science 2014, 343, 853. [DOI] [PubMed] [Google Scholar]; (h) Kuninobu Y; Ida H; Nishi M; Kanai M A meta-selective C–H borylation directed by a secondary interaction between ligand and substrate. Nat. Chem 2015, 7, 712. [DOI] [PubMed] [Google Scholar]; (i) Yang Y; Li R; Zhao Y; Zhao D; Shi Z Cu-catalyzed direct C6-arylation of indoles. J. Am. Chem. Soc 2016, 138, 8734. [DOI] [PubMed] [Google Scholar]; (j) Wang P; Verma P; Xia G; Shi J; Qiao JX; Tao S; Cheng PTW; Poss MA; Farmer ME; Yeung K-S; Yu J-Q Ligand-accelerated non-directed C–H functionalization of arenes. Nature 2017, 551, 489. [DOI] [PMC free article] [PubMed] [Google Scholar]; (k) Wang X; Leow D; Yu J-Q Pd(II)-catalyzed para-selective C–H arylation of monosubstituted arenes. J. Am. Chem. Soc 2011, 133, 13864. [DOI] [PMC free article] [PubMed] [Google Scholar]; (l) Luan Y-X; Zhang T; Yao W-W; Lu K; Kong L-Y; Lin Y-T; Ye M Amide-ligand-controlled highly para-selective arylation of monosubstituted simple arenes with arylboronic acids. J. Am. Chem. Soc 2017, 139, 1786. [DOI] [PubMed] [Google Scholar]; Examples of non-directed meta- or para-C–H functionalization:
- (6).(a) Kinuta H; Tobisu M; Chatani N Rhodium-catalyzed borylation of aryl 2-pyridyl ethers through cleavage of the carbon-oxygen bond: Borylative removal of the directing group. J. Am. Chem. Soc 2015, 137, 1593. [DOI] [PubMed] [Google Scholar]; (b) Tobisu M; Zhao J; Kinuta H; Furukawa T; Igarashi T; Chatani N Nickel-catalyzed borylation of aryl and benzyl 2-pyridyl ethers: A method for converting a robust ortho-directing group. Adv. Synth. Catal 2016, 358, 2417. [Google Scholar]
- (7).Brookhart M; Green MLH; Parkin G Agostic interactions in transition metal compounds. Proc. Natl. Acad. Sci. U.S.A 2007, 104, 6908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Zhang L; Zhao C; Liu Y; Xu J; Xu X; Jin Z Activation of remote meta-C–H bonds in arenes with tethered alcohols: A salicylonitrile template. Angew. Chem., Int. Ed 2017, 56, 12245. [DOI] [PubMed] [Google Scholar]
- (9).Jayarajan R; Das J; Bag S; Chowdhury R; Maiti D Diverse meta-C–H functionalization of arenes across different linker lengths. Angew. Chem., Int. Ed 2018, 57, 7659. [DOI] [PubMed] [Google Scholar]
- (10).(a) Li J; Wang Z-X Nickel-catalyzed amination of aryl 2-pyridyl ethers via cleavage of the carbon-oxygen bond. Org. Lett 2017, 19, 3723. [DOI] [PubMed] [Google Scholar]; (b) Li J; Wang Z-X Nickel-catalyzed C–O bond reduction of aryl and benzyl 2-pyridyl ethers. Chem. Commun 2018, 54, 2138. [DOI] [PubMed] [Google Scholar]
- (11).(a) Li X-J; Zhang J-L; Geng Y; Jin Z Nickel-catalyzed Suzuki-Miyaura coupling of heteroaryl ethers with arylboronic acids. J. Org. Chem 2013, 78, 5078. [DOI] [PubMed] [Google Scholar]; (b) Iranpoor N; Panahi F Direct nickel-catalyzed amination of phenols via C–O activation using 2,4,6-trichloro-1,3,5-triazine (TCT) as reagent. Adv. Synth. Catal 2014, 356, 3067. [Google Scholar]
- (12).Peng Z; Yu Z; Li T; Li N; Wang Y; Song L; Jiang C Catalytic regioselective C–H acetoxylation of arenes using 4,6-dimethoxy-1,3,5-triazin-2-yloxy as a removable/modifiable directing group. Organometallics 2017, 36, 2826. [Google Scholar]
- (13).Koguchi Y; Kohno J; Nishio M; Takahashi K; Okuda T; Ohnuki T; Komatsubara S TMC-95A, B, C, and D, novel proteasome inhibitors produced by Apiospora montagnei Sacc. TC 1093. J. Antibiot 2000, 53, 105. [DOI] [PubMed] [Google Scholar]; (a) Lin S; Danishefsky SJ The total synthesis of proteasome inhibitors TMC-95A and TMC-95B: Discovery of a new method to generate cis-propenyl amides. Angew. Chem., Int. Ed 2002, 41, 512. [DOI] [PubMed] [Google Scholar]; (b) Inoue M; Sakazaki H; Furuyama H; Hirama M Total synthesis of TMC-95A. Angew. Chem., Int. Ed 2003, 42, 2654. [DOI] [PubMed] [Google Scholar]; (c) Albrecht BK; Williams RM A concise, total synthesis of the TMC-95A/B proteasome inhibitors. Proc. Natl. Acad. Sci. U.S.A 2004, 101, 11949. [DOI] [PMC free article] [PubMed] [Google Scholar]; For total synthesis of TMC-95:
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.