Abstract
The high photoluminescence quantum yield, wide color tunability and narrow bandwidth of perovskite nanocrystals make them favorable for light source and display applications. Here, highly transparent green-light-emitting devices (LEDs) using inorganic cesium lead halide perovskite nanocrystal films as the emissive layer are reported. The effect of multilayered nanostructured transparent electrode on optical properties and performance within the LEDs is investigated by fine tuning layer thickness. The results show that the light transmission in visible region can be enhanced with this nanostructured film. These LEDs exhibited a high transmittance (average 73% over 400–700 nm) and high brightness of 2640 and 1572 cd m−2 for indium-doped tin oxide (ITO) cathode and MoOx/Au/MoOx anode sides, respectively.
Keywords: capping layer, charge injection, light-emitting device, perovskite nanocrystal, transparent
In recent years, perovskite materials have become a significant class of optoelectronic materials, widely used in photovoltaics,[1] light-emitting devices (LEDs),[2] and lasers.[3] The excellent performances are mainly attributed to their unique optoelectronic properties, such as high optical absorption coefficient, direct band gap, long carrier-diffusion length, and high carrier mobility.[4] Due to the enhanced stability and radiative recombination efficiency, the all-inorganic cesium lead halide (CsPbX3, X = Cl, Br, and I) perovskite nanocrystals (NCs) have been developed as promising emitters.[5] Notably, the photoluminescence (PL) quantum yields of these inorganic perovskite NCs are characterized up to 90%, also making them appealing for LED applications.[6]
Transparent LEDs (TLEDs) are necessary in realizing new LED applications in many areas, such as integration into screens in electronic equipment or windows in automobiles, and may also be important for the fabrication of vertically stacked device geometry to obtain full color.[7] Therefore, it is significant to investigate perovskite-based TLEDs for future applications. However, perovskite materials have high absorption coefficients, which will hinder the transmission of visible region photons, so the light-emitting layer should be as thin as possible to maximize the light pass through the whole device.
In order to fabricate TLEDs, transparent electrodes for both anode and cathode are required. Typically, indium-doped tin oxide (ITO)-coated glass has been used as transparent electrode for photovoltaic and LEDs because of the high transparency and conductivity.[8] For the other side electrode, several kinds of materials have been developed, such as transparent conductive oxides,[9] ultrathin metal films,[10] metal nanowires,[11] carbon materials,[12] and conducting polymers.[7b] In view of the high sensitivity of perovskite materials to temperature and solvent, ultrathin metal films deposited by thermal evaporation may be the most suitable alternative transparent electrode. However, there is significant light reflection at the metal–air interface because of the refractive index mismatch, leading to reduced transmission of the whole device.[13] To overcome this disadvantage, a high refractive index material (such as MoO3) can be deposited to reduce the light reflection.
Herein, we report transparent cesium lead tribromide (CsPbBr3) NC-LEDs for the first time, with a multilayered MoOx/Au/MoOx (MAM) structure as a transparent electrode. Usually, MoOx/Au film is used as anode in LEDs, where MoOx aims to enhance hole injection. In this paper, an additional MoOx layer was deposited, with the aim to enhance light transmission. By fine-tuning the thickness of the MoOx capping layer, the devices exhibited a high transparency, with an average transmittance of 73% over the visible range (400–700 nm). When applied a driving voltage, the device emitted bright green light, transmitting from both ITO cathode side and MAM anode side. The total peak brightness of the device was 4212 cd m−2, while the maximum luminance and external quantum efficiency (EQE) for ITO and MAM electrode sides were 2640 cd m−2 and 0.35%, 1572 cd m−2 and 0.23%, respectively.
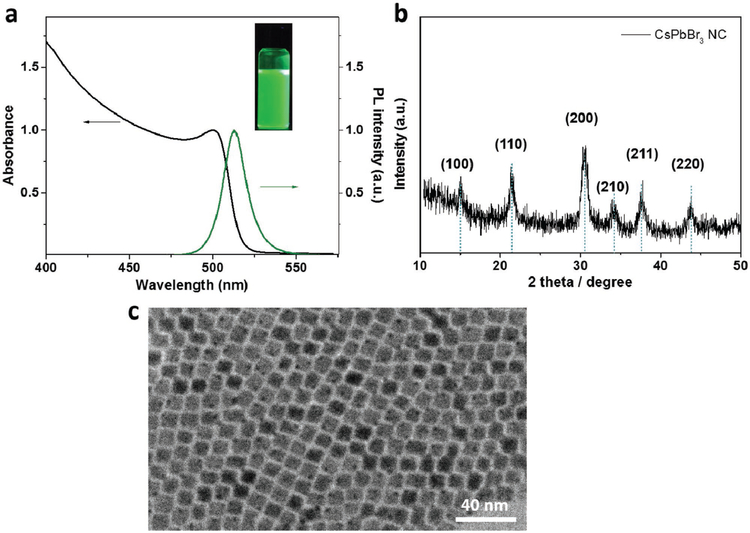
To investigate the optical properties, the absorption and PL spectra of the CsPbBr3 NC solution were recorded at room temperature (Figure 1a, together with a photograph of the NC solution emitting bright green light). A sharp transition is observed at around 500 nm in the absorbance spectrum. The narrow PL spectrum of CsPbBr3 NCs corresponds to excitonic emission is peaked at 517 nm, with a narrow full width at half maximum (FWHM) of 16 nm. The crystallinity of CsPbBr3 NCs was characterized by X-ray diffraction (XRD). The XRD pattern shown in Figure 1b indicates the CsPbBr3 NC powder belonging to a cubic phase. A representative transmission electron microscopy (TEM) image of CsPbBr3 NCs (Figure 1c) shows the monodispersed cubic shape NCs with an average edge length of 10–11 nm.
Figure 1.
a) The absorption and PL spectra of CsPbBr3 NC solution, with an inset showing the photograph of the NC solutions under 365 nm irradiation; b) XRD pattern of the CsPbBr3 NC powder; and c) TEM image of CsPbBr3 NCs.
Since CsPbBr3 NCs intrinsically possess lower valence band maximum (−6.19 eV) energy level as compared with the highest occupied molecular orbital energy level of typical conjugated organic molecules or polymers (≈−5.5 eV), the hole injection was found to be difficult due to the large energy barrier.[14] Herein, we adopted an inverted LED design, which enables the systematic engineering of the hole transport layer (HTL) with conventional organic materials. According to our previous work,[15] the flatband energy level diagram of device can be mapped in Figure 2a. The emitting layer was sandwiched between the hybrid inorganic/organic charge-transport layers. The CsPbBr3 NC-LEDs have a typical multilayer structure consisting of ITO (transparent cathode), ZnO/polyethylenimine (PEI) (electron transport layer), CsPbBr3 NCs (emissive layer), 4,4′-bis(carbazole-9-yl)biphenyl (CBP), 4,4′,4″-tris(carbazol-9-yl)triphenylamine (TCTA) (HTL), and MAM (transparent anode). ZnO NCs are widely employed in LEDs to transport electrons and block holes.[16] Figure 2b shows the absorption spectrum of our ZnO NC solution, with a TEM image. The average size of our ZnO NCs is around 2 nm, which will lead to a higher lowest unoccupied molecular orbital (LUMO) energy, thus provide a more efficient electron injection within the device than larger sizes.
Figure 2.
a) Device energy band diagram of the CsPbBr3 NC-LED. b) The UV–vis absorption spectrum of ZnO NCs, with a TEM image.
In the whole device, ZnO, CBP, and TCTA are all wide-band gap materials, with high transparency in visible region. Therefore, the perovskite NC layer and MAM electrode have important effect on the optical properties within the LEDs. Generally, the emissive layers in NC-LEDs are very thin, making them highly transparent. Therefore, the major factor affecting the light transmission of the device will be the top MAM electrode. One MoOx layer can be used as a hole injection layer (HIL), which is common for inverted optoelectronic devices.[16a] For the other MoOx layer, it will enhance the light transmission and film conductivity as a capping layer.[17]
As the device structure is electron abundant, hole injection will have large effect on device efficiency. According to the literature, MoOx (5.44 eV work function) can shift the work function of Au electrode to higher values and as a result enhances the hole injection.[17c,18] In order to design highly efficient TLEDs, the MoOx layer thickness should be optimized. Figure 3 shows the performance of LEDs with an opaque electrode as a function of MoOx layer thickness. It can be obviously seen that the device performances are strongly dependent on the thickness of MoOx layer. First, the MoOx layer will enhance hole injection, resulting in an improved device performance with 20 nm MoOx. Second, when the thickness of MoOx layer kept increasing, the device luminance, current efficiency, and device EQE all decreased. It may be caused by the increase of series resistance at the MoOx–Au interface, as a result, hole-injection rate will slow down, leading to poorer device performances. By finely tuning the MoOx layer, the best-performed LED with opaque electrode can be obtained, with a luminance of 4550 cd m−2.
Figure 3.
Comparison of a) current density–voltage (J–V) and b) luminance–voltage (L–V) characteristics of CsPbBr3 NC-LED as a function of MoOx layer thickness. c) External quantum efficiency and d) current efficiency versus current density characteristics of CsPbBr3 NC-LED as a function of MoOx layer thickness.
Electrodes with both high transparency and high conductivity are essential in order to realize effective transparent LEDs. As discussed above, we employed transparent ITO and an ultrathin Au film with 10 nm thickness as cathode and anode, respectively. To obtain details about the effect of MoOx capping layer on the transparency of Au film, the resultant MoO3/Au and MAM structures were prepared for optical transmission measurements, where the thicknesses of the first MoOx layer and Au film were fixed at 20 and 10 nm, respectively. Figure 4a shows the optical transmission spectra of these films. It is obvious that the transmittance of Au film is significantly enhanced after capped with an additional MoOx layer. Besides, the transmittance is strongly dependent on the thickness of the capping layer. The enhanced transmission can be attributed to decreased light reflection at the interface of Au/air. However, when the thickness of MoOx capping layer kept increasing, the transparency of MAM films shifted to an opposite trend, which was resulted from stronger light absorption by the MoOx capping layer. Therefore, it is significant to finely tune the thickness of MoOx capping layer to maximize the transparency of MAM. After fine-tuning, the transparency of our electrode reached a highest value when the MoOx layer thickness was about 25 nm. As shown in Figure 4b, the best MAM electrode exhibits a maximum transmittance of 77% (around 530 nm) and an average transmittance of 73% over the visible range (400–700 nm). We measured the resistance of the MAM film using the four-point probe method, and found the value was 13.3 Ω sq.−1 As a result, the MAM films can be served as the device anodes due to their high transparency and conductivity. Also, the transmittance of an ITO film was measured over the visible range, and the average value was found to be around 93% (Figure 4b). For the whole device (including the glass substrate), a high transparency (58% around 520 nm) was obtained. A photograph of our transparent device is given in Figure 4b. The background image can be clearly seen through the transparent LED, demonstrating the device’s high transparency.
Figure 4.
a) Visible transmittance spectra of transparent electrode without any capping layers and with different thicknesses of the MoOx capping layer; b) Transmittance spectra of the three samples with the structure of ITO, MAM, and the whole device, plus a photograph of the transparent LED.
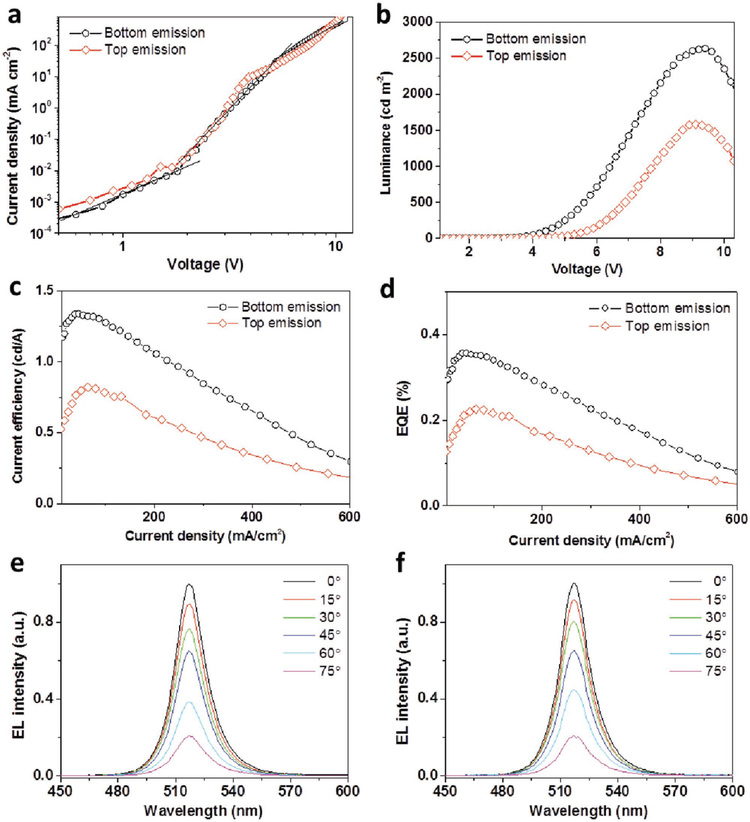
In order to measure the emission performances from both bottom and top sides, we fabricated several devices simultaneously under control conditions. One was used for bottom emission measurement, while another one for top emission. Current density–voltage (J–V) and luminance–voltage (L–V) characteristics are shown in Figure 5a,b. Since the device’s performances are not perfectly the same, there is a small difference between the two J–V curves in Figure 5a.
Figure 5.
a) Current density–voltage (J–V) and b) luminance–voltage (L–V) characteristics of the transparent LED; c) current efficiency and d) external quantum efficiency versus current density characteristics of the LED; EL spectra of the LED for bottom side e) and top side f) with emission angles varying from 0° to 75°.
Noticeably, the maximum luminance of bottom (substrate) and top (MAM film) emissions was 2640 and 1572 cd m−2, respectively. The total emission (the sum of bottom and top emission at the same voltage) reached a peak luminance of 4212 cd m−2. To assess the reproducibility, 24 devices were fabricated, showing that the emitting luminance from bottom side was a little higher than that from the top side. The slightly lower luminance of top emission can be attributed to the relatively lower transmittance of MAM anode. Compared with the above device with opaque electrodes, the transparent devices worked at a little higher voltage. It is similar to some previous reports, which may be due to the higher resistance of the ultrathin Au film[10a] Also, the results are tabulated in Table 1 for easy comparison. As a consequence, the peak current efficiency and EQE for top side of the transparent LED was 0.82 cd A−1 and 0.23%, while the bottom side presented a slightly higher efficiency of 1.38 cd A−1 and 0.35%. Considering the practical application, the electroluminescence (EL) spectra of the transparent device were recorded when varying the emission angles, as shown in Figure 5e,f. Obviously, the EL spectra show no shift at peak position, which demonstrates the high transparency of the device. These results demonstrate that our transparent green LEDs can work efficiently and have a great potential for display.
Table 1.
Device characteristics of the transparent NC-LEDs.
| Side |
Vona) [V] |
Lmax [cd m−2] |
ηEQE[%] peak/at 100 mA cm−2 |
ηP [lm W−1] peak/at 100 mA cm−2 |
ηA [cd A−1] peak/at 100 mA cm−2 |
|---|---|---|---|---|---|
| Bottom | ≈2.4 | 2640 | 0.35/0.33 | 0.91/0.47 | 1.38/0.28 |
| Top | ≈2.5 | 1572 | 0.23/0.21 | 0.6/0.31 | 0.82/0.78 |
Von represents the applied voltage when the device luminance reaches 1 cd m−2.
Figure 6a shows the comparison of EL spectra from both MAM and ITO sides at an operating voltage of 7 V. The device’s EL spectrum peaked at 518 nm with a FWHM of 18 nm, which is consistent with the NC solution PL spectrum with a 517 nm emission peak and a 16 nm FWHM as shown in Figure 1b. The EL emission through the top electrode is almost the same compared with the EL spectrum from the bottom. The symmetric emissions from both sides correspond with Commission Internationale de l’Eclairage (CIE) color coordinates of (0.12 and 0.73), as shown in Figure 6b. The inset of Figure 6a is a photograph of a transparent device emitting bright green light under a driving voltage of 7 V. In Figure 6c, the EL intensity of the ITO side and MAM side exhibited similar but a little faster decrease with the increasing emission angles compared with Lambertian patterns.
Figure 6.
a) EL spectra of the transparent device for both bottom and top sides and a photograph of a working LED at 7 V; b) CIE coordinates of the EL spectra for both bottom and top sides; and c) variations of EL intensity as a function of emission angles.
In conclusion, we successfully fabricated transparent green LEDs based on CsPbBr3 NCs for the first time, with nearly Lambertian emission characteristics. These highly transparent LEDs employed inverted device structures, which helped to reduce the hole-injection barrier. The total emission of these devices achieved a peak luminance of 4212 cd m−2. Full color transparent perovskite NC-LEDs could be fabricated simply by replacing CsPbBr3 NC films with other perovskite NC emissive layers. Our study demonstrates the potential of perovskite nanomaterials for transparent display applications.
Experimental Section
Chemicals:
All chemicals were used directly. PbBr2 (99.999%), Cs2CO3 (99.9%), and oleylamine (OLA, 80–90%) were purchased from Aladdin. Oleic acid (OA, 90%) was obtained from Alfa. 1-Octadecene (ODE, 90%), Zn(Ac)2 (99.999%), and sodium hydroxide (99.99%) were got from Aldrich. Toluene and hexane were acquired from Aldrich.
Synthesis of CsPbBr3 NCs:
The synthesis of CsPbBr3 NCs was carried out as literature described.[19] For the preparation of Cs-oleate precursor, a mixture of Cs2CO3 (0.8 g), oleic acid (OA, 2.5 mL), and 1-octadecene (ODE, 30.0 mL) was added into a three-neck flask. The flask was dried at 120 °C for 1 h and then heated to 150 °C until all Cs2CO3 was dissolved. For the synthesis of CsPbBr3 NCs, ODE (10 mL) and PbBr2 (0.138 g) were added into a 50 mL three-neck flask and dried at 120 °C under vacuum for 1 h. To dissolve PbBr2, dried OA (1 mL) and oleylamine (OLA, 1 mL) were injected into the flask under N2 to obtain a clear solution. The temperature was raised to 180 °C and 1 mL of Cs-oleate solution was injected quickly. After 5 s, the mixture was quickly cooled by an ice-water bath. The reaction mixture was directly centrifuged at 5000 rpm for 10 min; then the precipitate was collected and dispersed in 3 mL toluene. One more centrifugation for 10 min at 12 000 rpm was applied and the precipitate was dissolved in 2 mL of toluene for use.
Synthesis of ZnO NCs:
The synthesis of ZnO NCs followed a published method.[20] A total of 0.4403 g Zn(Ac)2 and 30 mL of ethyl alcohol were loaded in a 100 mL three-neck flask. After continuously flowing N2 for 10 min, the mixture was then heated to boiling point temperature under a reflux condenser. When the mixture turned into clear, the heating mantle was then turned off and removed to cool the solution to room temperature naturally. When the solution temperature was stable, a solution consisting of 0.2 g sodium hydroxide and 10 mL ethanol was injected into the flask rapidly, and the solution was kept stirring for 4 h to grow NCs. To obtain pure products, excess hexane was used to precipitate NC products. Then the precipitate was dissolved in 3 mL ethanol. The purification process was repeated twice. Finally, the solution was filtered through a 0.45 μm filter for further use.
Device Fabrication:
For device fabrication, the ITO glass substrate was washed by deionized water, chloroform, acetone, and isopropanol in sequence and then treated by UV-ozone for 15 min. A ZnO film (≈40 nm) was deposited by spin-coating ZnO NC solution, and the prepared film was annealed at 100 °C for 10 min in air. The substrate was transferred into a glove box for the next layer. A solution of polyethylenimine (PEI) (dissolve in 2-methoxyethanol in 0.2% mass fraction) was spin coated onto the ZnO film at a speed of 3000 rpm and annealed at 110 °C for 10 min. CsPbBr3 NC films (≈20 nm) were deposited by spin coating the prepared NC solution at 1000 rpm. The device was then transferred into a vacuum deposition clamber (1 × 10−7 torr) to deposit the CBP (≈10 nm), TCTA (≈35 nm), MoO3, and Au (≈80 nm) layers by thermal evaporation.
Characterizations:
Optical absorption and transmission measurements were carried out in a PerkinElmer Lambda 950 spectrometer. Powder XRD patterns were collected on a Bruker SMART-CCD diffractometer. PL spectra were recorded by using a Cary Eclipse spectrofluorimeter. The current–voltage characteristics of the device were performed by Keithley 2612B sourcemeter. The luminance was recorded by using a calibrated Newport 1936-R power meter with a 918D-SL-0D3R silicon photodetector. The EQE values were calculated using the modified coefficients since the emitting patterns were a little different to the Lambertian pattern. The EL spectra were measured via a Maya spectrometer (Ocean Optics) coupled to an optical fiber. TEM characterization was done on a FEI Tecnai F20 microscope.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (61475062, 61675086, 11674127), Jilin Province Science Fund for Excellent Young Scholars (20170520129JH), the BORSF RCS/Endowed Professor programs, the Institutional Development Award (P20GM103424), and the National Postdoctoral Program for Innovative Talents (BX201600060).
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
Contributor Information
Hua Wu, State Key Laboratory on Integrated Optoelectronics and College of Electronic Science and Engineering, Jilin University, Changchun 130012, China.
Prof. Yu Zhang, State Key Laboratory on Integrated Optoelectronics and College of Electronic Science and Engineering, Jilin University, Changchun 130012, China, yuzhang@jlu.edu.cn
Dr. Xiaoyu Zhang, State Key Laboratory on Integrated Optoelectronics and College of Electronic Science and Engineering, Jilin University, Changchun 130012, China
Min Lu, State Key Laboratory on Integrated Optoelectronics and College of Electronic Science and Engineering, Jilin University, Changchun 130012, China.
Dr. Chun Sun, State Key Laboratory on Integrated Optoelectronics and College of Electronic Science and Engineering, Jilin University, Changchun 130012, China
Prof. Xue Bai, State Key Laboratory on Integrated Optoelectronics and College of Electronic Science and Engineering, Jilin University, Changchun 130012, China
Prof. Tieqiang Zhang, State Key Laboratory of Superhard Materials, College of Physics, Jilin University, Changchun 130012, China
Dr. Guang Sun, China-Japan Union Hospital, Jilin University, Changchun 130012, China, guangsun2013@163.com
Prof. William W. Yu, State Key Laboratory on Integrated Optoelectronics and College of Electronic Science and Engineering, Jilin University, Changchun 130012, China, Department of Chemistry and Physics, Louisiana State University, Shreveport, LA 71115, USA, wyu6000@gmail.com
References
- [1] a).Saliba M, Matsui T, Domanski K, Seo J-Y, Ummadisingu A, Zakeeruddin SM, Correa-Baena J-P, Tress WR, Abate A, Hagfeldt A, Grätzel M, Science 2016, 354, 206; [DOI] [PubMed] [Google Scholar]; b) Yuan Y, Huang J, Acc. Chem. Res 2016, 49, 286; [DOI] [PubMed] [Google Scholar]; c) McMeekin DP, Sadoughi G, Rehman W, Eperon GE, Saliba M, Hörantner MT, Haghighirad A, Sakai N, Korte L, Rech B, Johnston MB, Herz LM, Snaith HJ, Science 2016, 351, 151; [DOI] [PubMed] [Google Scholar]; d) Kulbak M, Cahen D, Hodes G, J. Phys. Chem. Lett 2015, 6, 2452. [DOI] [PubMed] [Google Scholar]
- [2] a).Cho H, Jeong S-H, Park M-H, Kim Y-H, Wolf C, Lee C-L, Heo JH, Sadhanala A, Myoung N, Yoo S, Im SH, Friend RH, Lee T-W, Science 2015, 350, 1222; [DOI] [PubMed] [Google Scholar]; b) Wang J, Wang N, Jin Y, Si J, Tan Z-K, Du H, Cheng L, Dai X, Bai S, He H, Ye Z, Lai ML, Friend RH, Huang W, Adv. Mater 2015, 27, 2311; [DOI] [PubMed] [Google Scholar]; c) Tan Z-K, Moghaddam RS, Lai ML, Docampo P, Higler R, Deschler F, Price M, Sadhanala A, Pazos LM, Credgington D, Hanusch F, Bein T, Snaith HJ, Friend RH, Nat. Nanotechnol 2014, 9, 687; [DOI] [PubMed] [Google Scholar]; d) Li J, Bade SGR, Shan X, Yu Z, Adv. Mater 2015, 27, 5196; [DOI] [PubMed] [Google Scholar]; e) Cho H, Wolf C, Kim JS, Yun HJ, Bae JS, Kim H, Heo J-M, Ahn S, Lee T-W, Adv. Mater 2017, 29, 1700579; [DOI] [PubMed] [Google Scholar]; f) Kim Y-H, Cho H, Heo JH, Kim T-S, Myoung N, Lee C-L, Im SH, Lee T-W, Adv. Mater 2015, 27, 1248. [DOI] [PubMed] [Google Scholar]
- [3] a).Xing G, Mathews N, Lim SS, Yantara N, Liu X, Sabba D, Grätzel M, Mhaisalkar S, Sum TC, Nat. Mater 2014, 13, 476; [DOI] [PubMed] [Google Scholar]; b) Zhu H, Fu Y, Meng F, Wu X, Gong Z, Ding Q, Gustafsson MV, Trinh MT, Jin S, Zhu XY, Nat. Mater 2015, 14, 636; [DOI] [PubMed] [Google Scholar]; c) Deschler F, Price M, Pathak S, Klintberg LE, Jarausch D-D, Higler R, Hüttner S, Leijtens T, Stranks SD, Snaith HJ, Atatüre M, Phillips RT, Friend RH, J. Phys. Chem. Lett 2014, 5, 1421; [DOI] [PubMed] [Google Scholar]; d) Xu Y, Chen Q, Zhang C, Wang R, Wu H, Zhang X, Xing G, Yu WW, Wang X, Zhang Y, Xiao M, J. Am. Chem. Soc 2016, 138, 3761. [DOI] [PubMed] [Google Scholar]
- [4].Kim YH, Cho H, Lee TW, Proc. Natl. Acad. Sci. USA 2016, 113, 11694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5] a).Sutherland BR, Sargent EH, Nat. Photonics 2016, 10, 295; [Google Scholar]; b) Hu F, Yin C, Zhang H, Sun C, Yu WW, Zhang C, Wang X, Zhang Y, Xiao M, Nano Lett. 2016, 16, 6425; [DOI] [PubMed] [Google Scholar]; c) Protesescu L, Yakunin S, Bodnarchuk MI, Krieg F, Caputo R, Hendon CH, Yang RX, Walsh A, Kovalenko MV, Nano Lett. 2015, 15, 3692; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Li J, Xu L, Wang T, Song J, Chen J, Xue J, Dong Y, Cai B, Shan Q, Han B, Zeng H, Adv. Mater 2017, 29, 1603885. [DOI] [PubMed] [Google Scholar]
- [6] a).Huang H, Zhao F, Liu L, Zhang F, Wu X-G, Shi L, Zou B, Pei Q, Zhong H, ACS Appl. Mater. Interfaces 2015, 7, 28128; [DOI] [PubMed] [Google Scholar]; b) Song J, Li J, Li X, Xu L, Dong Y, Zeng H, Adv. Mater 2015. 27, 7162; [DOI] [PubMed] [Google Scholar]; c) Zhao L, Yeh Y-W, Tran NL, Wu F, Xiao Z, Kerner RA, Lin YL, Scholes GD, Yao N, Rand BP, ACS Nano 2017, 11, 3957; [DOI] [PubMed] [Google Scholar]; d) Sun C, Zhang Y, Ruan C, Yin C, Wang X, Wang Y, Yu WW, Adv. Mater 2016, 28, 10088. [DOI] [PubMed] [Google Scholar]
- [7] a).Bulovic V, Gu G, Burrows PE, Forrest SR, Thompson ME, Nature 1996, 380, 29; [Google Scholar]; b) Levermore PA, Jin R, Wang X, Chen L, Bradley DDC, de Mello JC, J. Mater. Chem 2008, 18, 4414; [Google Scholar]; c) Meyer J, Winkler T, Hamwi S, Schmale S, Johannes H-H, Weimann T, Hinze P, Kowalsky W, Riedl T, Adv. Mater 2008, 20, 3839; [Google Scholar]; d) Kim HY, Park YJ, Kim J, Han CJ, Lee J, Kim Y, Greco T, Ippen C, Wedel A, Ju B-K, Oh MS, Adv. Funct. Mater 2016. 26, 3454. [Google Scholar]
- [8].Wu H, Zhang X, Zhang Y, Yan L, Gao W, Zhang T, Wang Y, Zhao J, Yu WW, ACS Appl. Mater. Interfaces 2015, 7, 21082. [DOI] [PubMed] [Google Scholar]
- [9] a).Wang W, Peng H, Chen S, J. Mater. Chem. C 2016, 4, 1838; [Google Scholar]; b) Song J, Kulinich SA, Li J, Liu Y, Zeng H, Angew. Chem., Int. Ed 2015, 127, 472. [DOI] [PubMed] [Google Scholar]
- [10] a).Kim H-M, bin Mohd Yusoff AR, Kim T-W, Seol Y-G, Kim H-P, Jang J, J. Mater. Chem. C 2014, 2, 2259; [Google Scholar]; b) Yang X, Mutlugun E, Dang C, Dev K, Gao Y, Tan ST, Sun XW, Demir HV, ACS Nano 2014, 8, 8224. [DOI] [PubMed] [Google Scholar]
- [11] a).Kim D, Fu Y, Kim S, Lee W, Lee K-H, Chung HK, Lee H-J, Yang H, Chae H, ACS Nano 2017, 11, 1982; [DOI] [PubMed] [Google Scholar]; b) Jing P, Ji W, Zeng Q, Li D, Qu S, Wang J, Zhang D, Sci. Rep 2015, 5, 12499; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Song J, Li J, Xu J, Zeng H, Nano Lett. 2014, 14, 6298. [DOI] [PubMed] [Google Scholar]
- [12].Seo J-T, Han J, Lim T, Lee K-H, Hwang J, Yang H, Ju S, ACS Nano 2014, 8, 12476. [DOI] [PubMed] [Google Scholar]
- [13] a).Zhang X, Hägglund C, Johansson MB, Sveinbjörnsson K, Johansson EMJ, Adv. Funct. Mater 2016, 26, 1921; [Google Scholar]; b) Zhang X, Hagglund C, Johansson EMJ, Energy Environ. Sci 2017, 10, 216. [Google Scholar]
- [14].Zhang X, Lin H, Huang H, Reckmeier C, Zhang Y, Choy WCH, Rogach AL, Nano Lett. 2016, 16, 1415. [DOI] [PubMed] [Google Scholar]
- [15].Zhang X, Sun C, Zhang Y, Wu H, Ji C, Chuai Y, Wang P, Wen S, Zhang C, Yu WW, J. Phys. Chem. Lett 2016, 7, 4602. [DOI] [PubMed] [Google Scholar]
- [16] a).Kwak J, Bae WK, Lee D, Park I, Lim J, Park M, Cho H, Woo H, Yoon DY, Char K, Lee S, Lee C, Nano Lett. 2012, 12, 2362; [DOI] [PubMed] [Google Scholar]; b) Bae WK, Park Y-S, Lim J, Lee D, Padilha LA, McDaniel H, Robel I, Lee C, Pietryga JM, Klimov VI, Nat. Commun. 2013, 4, 2661; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Yang Y, Zheng Y, Cao W, Titov A, Hyvonen J, MandersJesse R, Xue J, Holloway PH, Qian L, Nat. Photonics 2015, 9, 259; [Google Scholar]; d) Dai X, Zhang Z, Jin Y, Niu Y, Cao H, Liang X, Chen L, Wang J, Peng X, Nature 2014, 515, 96. [DOI] [PubMed] [Google Scholar]
- [17] a).Xue Z, Liu X, Zhang N, Chen H, Zheng X, Wang H, Guo X, ACS Appl. Mater. Interfaces 2014, 6, 16403; [DOI] [PubMed] [Google Scholar]; b) Hong K, Kim K, Kim S, Lee I, Cho H, Yoo S, Choi HW, Lee N-Y, Tak Y-H, Lee J-L, J. Phys. Chem. C 2011, 115, 3453; [Google Scholar]; c) Kim M, Lim C, Jeong D, Nam H-S, Kim J, Lee J, Org. Electron 2016, 36, 61. [Google Scholar]
- [18].Brown PR, Lunt RR, Zhao N, Osedach TP, Wanger DD, Chang L-Y, Bawendi MG, Bulović V, Nano Lett. 2011, 11, 2955. [DOI] [PubMed] [Google Scholar]
- [19] a).Wang P, Bai X, Sun C, Zhang X, Zhang T, Zhang Y, Appl. Phys. Lett 2016, 109, 063106; [Google Scholar]; b) Wang Y, Li X, Song J, Xiao L, Zeng H, Sun H, Adv. Mater 2015, 27, 7101; [DOI] [PubMed] [Google Scholar]; c) Hu F, Zhang H, Sun C, Yin C, Lv B, Zhang C, Yu WW, Wang X, Zhang Y, Xiao M, ACS Nano 2015, 9, 12410. [DOI] [PubMed] [Google Scholar]
- [20] a).Zhang X, Zhang Y, Yan L, Ji C, Wu H, Wang Y, Wang P, Zhang T, Wang Y, Cui T, Zhao J, Yu WW, J. Mater. Chem. A 2015, 3, 8501; [Google Scholar]; b) Wu H, Zhang Y, Zhang X, Lu M, Sun C, Zhang T, Yu WW, Adv. Opt. Mater 2017, 5, 1700377. [Google Scholar]