Abstract:
Our high-fidelity simulation model provides a realistic example for health-care professionals to experience cannulation, initiation, and hemodynamic stabilization during extracorporeal membrane oxygenation (ECMO) therapy. This educational experience brings a variety of critical care specialties together, in a controlled simulation setting, to develop, master, and maintain clinical skills. This may include perfusionists, ECMO specialists, surgical technicians, registered nurses, physicians, and students. The simulation component includes a unique vascular access pad that is attached to either a static fluid model or to the Califia perfusion simulator system (Biomed Simulation, Inc., San Diego, CA). This collective high-fidelity simulation model can be surgically cannulated via a cutdown technique using an appropriately sized cannula and connected to an in situ ECMO circuit. This article explains the educational strategy, how the surgical pad is made, and the simulator connections so that any hospital can re-create this experience.
Keywords: ECMO (extracorporeal membrane oxygenation), education, simulation, high-fidelity simulation, surgical instruments, skin, manikin, cannula, cervical, carotid, jugular, polymer, califia perfusion simulator system, extracorporeal life support
Simulation-based medical education is rapidly advancing, offering customized learning experiences for a variety of health-care specialties. Extracorporeal membrane oxygenation (ECMO) is normally an unplanned event which occurs during emergent periods involving clinicians with varying levels of experience. The high-risk, low-volume nature of ECMO necessitated a need to develop a model for continuous education and review. The challenges of training an ECMO team and concurrently demonstrating competency may best be achieved with a simulation focal point that increases the level of fidelity. Our vascular access pad achieved both goals.
Our pediatric ECMO program consists of an in-house team of perfusionists, respiratory therapists, and critical care nurses that support an average of 50 ECMO therapies each year. This team provides 24/7 coverage for planned and unplanned cannulations in any area of the hospital. We follow Extracorporeal Life Support Organization (ELSO) guidelines (1,2) for our team’s credentials and educational statuses. This support team consists of either critical care nurses or pediatric respiratory care therapists. To meet this goal, we have an established an educational program that includes 36 hours of didactic instruction; 20 hours of clinical practicum, some of which is simulation based; and a written/practical examination. This curriculum is followed with 72 hours of mentored bedside monitoring. Our simulation activity is a combination of low- and high-fidelity wet laboratories that incorporate ECMO cannulation with initiation, stabilization, and monitoring experiences. Simulation is the cornerstone for this training.
DESCRIPTION
Educational Strategy
Health-care simulation provides several educational opportunities beyond the realism it offers to the participants. An experienced simulation facilitator can create a safe environment for learning while maintaining some of the stress associated with a scenario. This includes the safety of the simulation environment, which creates a context for learning, with the support of the content and reinforcement process by the facilitators. The active engagement of the learners also supports teamwork and accountability (3). This style of education is intended to improve cognitive aptitude and heighten psychomotor skill sets.
Simulation participants have an opportunity to focus on their specialty in a safe and controlled setting. The role of the facilitator is to identify, with the learners, what is to be learned. With medical simulation, this needs assessment is an essential shared task by the learners. The participants choose which part of the involvement is ideal for them. For example, the surgical technicians participate in prepping, instrument setup, instrument use, and surgical understanding, whereas the physicians focus on surgical technique and cannulation. Perfusionists and ECMO specialists concentrate on the circuit setup, initiation, stabilization, and/or troubleshooting. Participants can also focus on their peer’s tasks, supporting a team approach for care.
Vascular Access Pad
Because our scenarios are generally triggered by the simulated patient’s requirement for ECMO support, our in-house vascular access pad is the initial focus of the scenario. Key elements used with our training include a custom surgical pad, a Califia perfusion simulator system (Biomed Simulation, Inc., San Diego, CA), and various support adjuncts normally used during ECMO therapy. The surgical pad is the primary focal point that initiates the demonstration.
Plastic products used in our vascular access pad are composed of a three-layered silicone polymer model assembled to mimic cervical or femoral vascular access for venoarterial ECMO (Figure 1). Silicon rubber polymer products are skin-safe, latex free, and require a simple process of mixing components A and B together with a coloring product. Blood vessels can be affixed to the mold with a layered approach that mimics the cervical area of a neonate. The degree of hardness of the polymer is unique and requires feedback from skilled practitioners about the realism of the simulated tissue. The solidity or hardness of the specific polymer product is significant.
Figure 1.
Three-layered polymer skin model that mimics a cervical operative field for ECMO cannulation.
There are different Shore hardness scales for measuring the elasticity of different materials. These scales were devised to describe solidity of materials that is associated with a common point of reference. The Shore hardness scale measures rubbers and gels from the spectrum of extra soft to extra hard. Our choice in material further delineated the indentation hardness that mimics the density of jelly candy to the feel of the insole of a gel shoe insert.
Shore hardness becomes an important factor when considering which material to use for the surgical model (4). The Shore hardness of each dermal layer was decided by surgeon preference after multiple simulations using a variety of products offered by Smooth-on (Smooth-On, Inc., Macungie, PA). Models with various color pigmented materials layered with mesh and imbedded blood vessels were fashioned. Through trial and error, we determined the polymer that best represented the tactile sensation of human tissue.
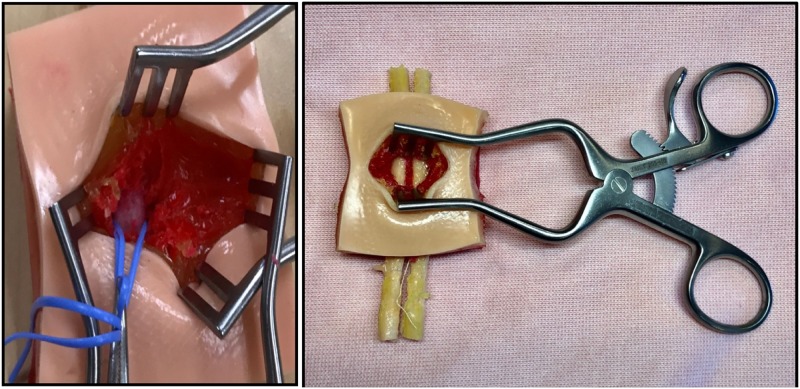
The surgical pad is made using three layers of pigmented Smooth-on Ecoflex material (Smooth-On). The epidermis is composed of 2–3 mm of Ecoflex 30 with a fine plastic matrix material used to simulate the durability of the outmost layer. Based on the pigment of the manikin, the skin tone can be adjusted using Silc Pig Flesh Tone Silicone Pigment (Smooth-On). The subcutaneous tissue is 2–3 mm Ecoflex Gel with Silc Pig Yellow Tone Silicone Pigment (Smooth-On). The muscle and carotid sheath or hypodermis is 1 cm Ecoflex Gel with Silc Pig Blood pigment (Smooth-On). The blood vessels are set to a 5- to 6-mm depth and range in diameter between 4 and 6 mm. The total depth of the hypodermis can extend slightly beyond 1 cm (Figure 2). The depth beyond the outermost blood vessel layer must be >5 mm (Table 1).
Figure 2.
The vascular access pad is a three-layer model. The tan tone at the top of the picture is 2–3 mm deep. The epidermis is 2–3 mm thick with yellow coloring to simulate fat. The hypodermis extends beyond a depth of 1 cm with vessels imbedded in the red polymer.
Table 1.
Smooth-On silicon rubber polymer product is mixed in separate plastic cups with the respective pigment tone for that tissue layer.
| Vascular Access Layer | Silicon Rubber Polymer | Depth | Tissue Tone | Silicon Rubber Polymer |
|---|---|---|---|---|
| Epidermis | Ecoflex 30 | 2–3 mm | <.5 mL of Silc Pig Flesh Tone | 4–5 mL |
| Subcutaneous | Ecoflex Gel | 2–3 mm | <.5 mL of Silc Pig Yellow Pigment | 5 mL |
| Hypodermis | Ecoflex Gel | 5 mm | <1 mL of Silc Pig Blood or Red Pigment | 8–10 mL |
| Blood vessels | Dragon Skin | 4-mm OD within the 1-cm hypodermis | <.5 mL of Silc Pig Flesh Tone | 5 mL per vessel |
| Hypodermis | Ecoflex Gel | >5 mm | <1 mL of Silc Pig Blood or Red Pigment | >10 mL |
The amount needed is poured into a Container Store® plastic box (4 × 2.5 × 4 cm) until the depth is achieved. The approximate milliliter amount of silicon rubber polymer is detailed within this table. The mesh tulle used within the epidermis layer may displace a variable amount of rubber polymer product. The size of the vessels may displace a variable amount of rubber polymer product within the hypodermis layer. Tissue tone is added until the desired color is achieved (very little required). Six to eight molds are prepped and poured with larger batches of respective silicon rubber polymer vascular access layers.
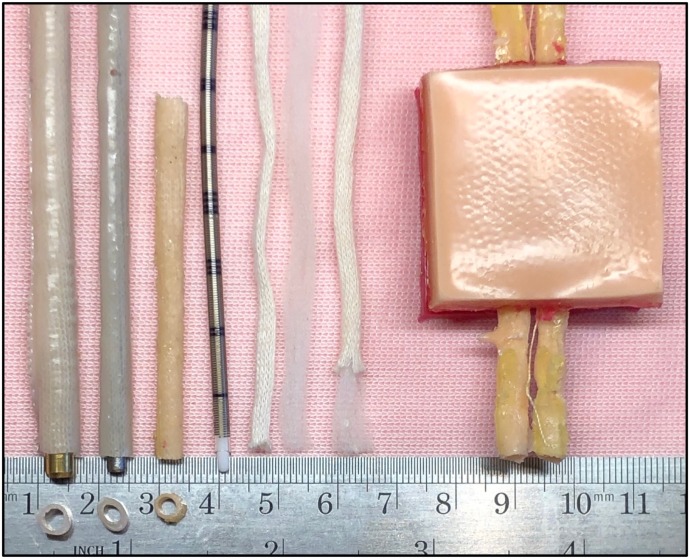
The vessels are made by using Dragon Skin 10 Fast mixed with Silc Pig Flesh Tone Silicone Pigment is used to make the carotid and jugular vessels (Smooth-On). A 3-mm metal rod, capable of accepting an 8-French (F) cannula, is used to cast the blood vessel mold. The Kern mantel cotton knitting material is separated, and the mantel portion is used with a polymer coating around a rod or dilator. Dragon Skin 10 Fast is coated over the mantel, adding strength and durability. The treated mantel is removed and tested for leakages. The degree of blood vessel thickness can be adjusted by adding another layer of Dragon Skin 10 to simulate the arterial vessel. A 4- to 5-mm rod accepts a 10-F cannula. It is feasible using the obturator from cannula two sizes larger than the expected simulated blood vessel. The overall length of each vessel should be >12 cm (Figure 3).
Figure 3.
A cotton mantel is used to add strength and hold the polymer material that mimics the blood vessel elasticity. When cured, the vessel can be removed from either a cannula obturator or a metal dial rod. The vessel diameter is determined by the obturator or rod size. This picture shows three vessel sizes with dial rods, an 8-F arterial cannula, and the cotton knitting material. The outer most layer or mantle is pictured next to the core or kern. Kernmantle cotton string is separated so that the mantle can be coated with the polymer. The completed surgical pad with the vagus nerve in situ is displayed.
Mortensen et al. (5) suggest the right internal jugular and right common carotid appear to be slightly larger than those on the left. The diameter of the carotid artery measures approximately 4 mm and larger depending on the age, body surface area (BSA), and volume status. The diameter of the internal jugular vein is slightly larger with the same variable. Literature searches related to vessel size for specific age and BSA groups are difficult. It seems obvious to correlate cannulation experience at our center with cannula size ranges available within the medical marketplace. Our common practice is basing BSA and visual inspection of the caliber of the anatomy to choose the correct cannula that permits adequate drainage to the circuit and flow away from the circuit.
Anatomical arrangement of the cervical model and the corresponding cannulation techniques were referenced using ELSO’s 4th edition ECMO manual (6). However, suture placement and specific cannulation techniques were institutional directed by the physician authors.
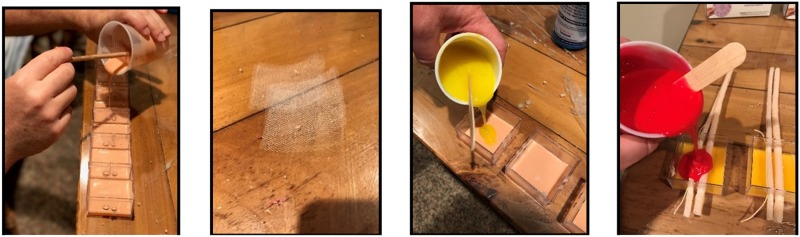
The epidermis layer is poured first and normally takes 4 hours to set. The mesh is placed with the first pour. The second subcutaneous layer is poured with a set period of 2 hours. The blood vessels are placed through holes and set in place for the final layer. A yellow thread can be placed between the blood vessels to simulate the vagus nerve. The third hypodermis layer is poured as the last step. This final step has a set time of 2 hours. The mold is removed from the plastic 4 × 4-cm Container Store® plastic box (Container Store, Inc., Coppell, TX). Holes for the blood vessels are drilled into opposing sides 2–3 mm apart at a depth of 4–6 mm (Figure 4).
Figure 4.
The three polymer layers are made separately using two different Shore hardness products each with colors specific to that layer. The epidermis has a fine mesh product imbedded with the initial pour that provides added strength if sutures are used to close the cannulation site.
The carotid pad is affixed to the right cervical area of a 3B Scientific baby (American 3B Scientific, Tucker, GA) care model (Figure 5). The surgical field can be prepped and draped in the usual fashion.
Figure 5.
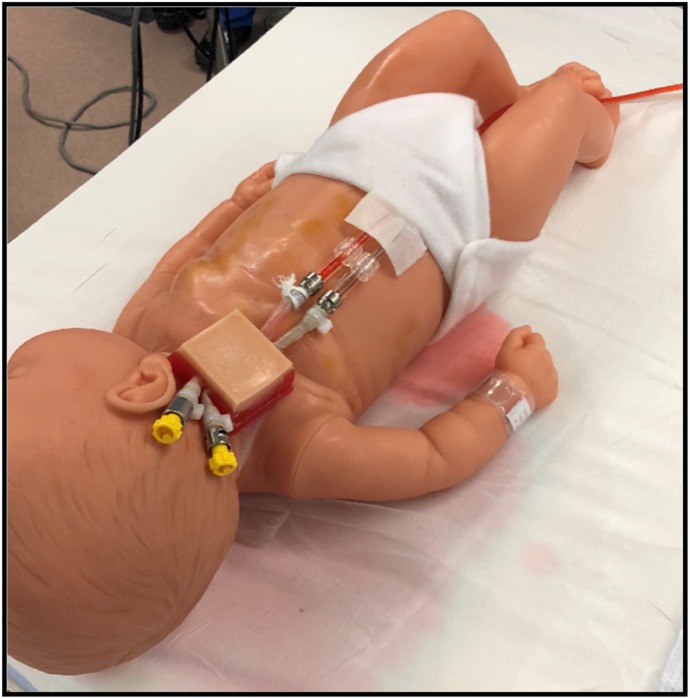
The surgical pad is secured using extra mesh that was imbedded with the epidermis layer. Metal male luer connectors are used with either tape or Panduit tie bands on both sides of the blood vessels. Priming with either the static or Califia model is simplified by removing the male cap distal to the vessel.
A standard surgical instrument tray used for neonatal cervical ECMO cannulation is used with this vascular access pad (Figure 6). The blood vessels are connected using 3/16 × 1/4-in. straight connectors to 3 feet of 1/4 × 1/16-in. tubing. The 1/4-in. tubing is connected to a Califia Perfusion Simulator System. This Califia simulator mimics all of the hemodynamics experienced with ECMO therapies, pulsatile arterial pressures, blood loss monitoring, and venous pressure. Used in concert with the surgical cannulation experience, this supports continued reproductions for other health-care professionals well after cannulation, initiation, and stabilization (Figure 7).
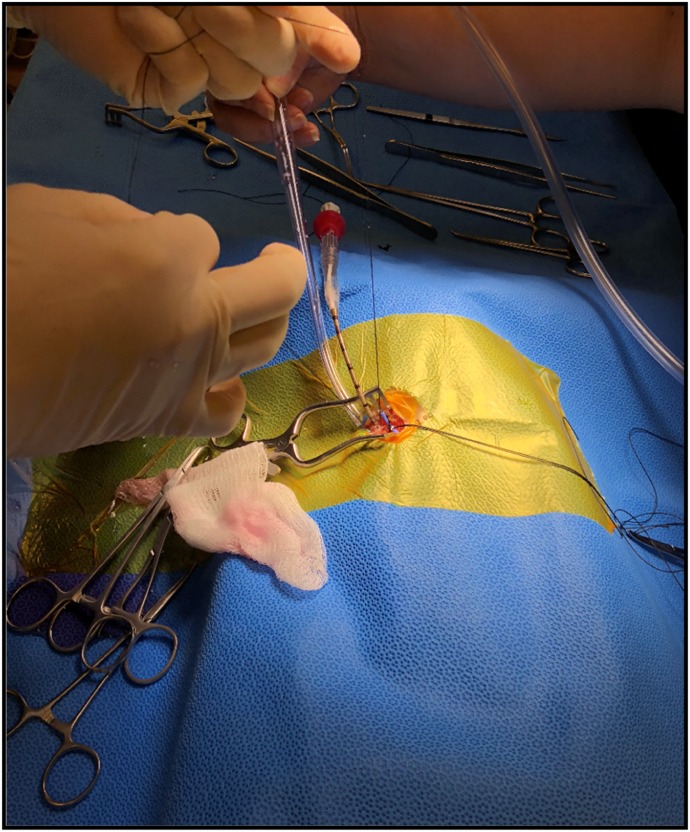
Figure 6.
Typical surgical field with the arterial cannula in situ.
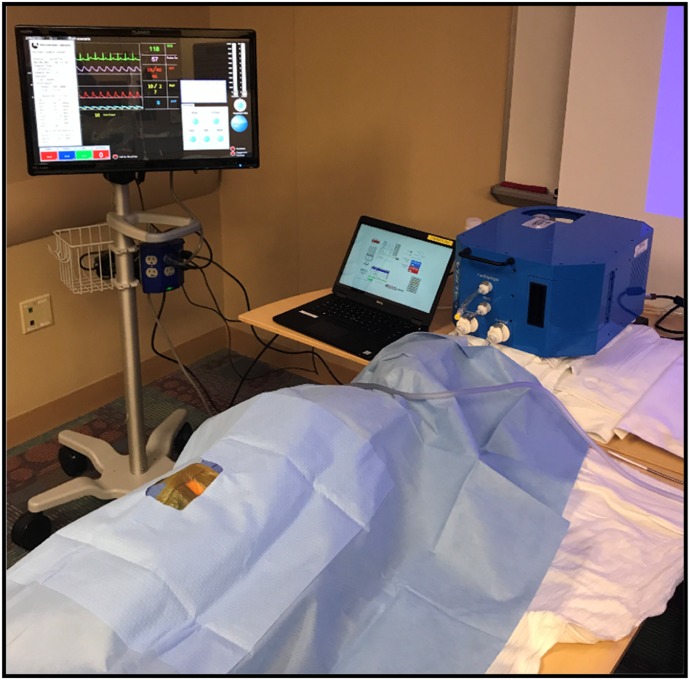
Figure 7.
The Califia simulator is attached using standard connectors with the unit slightly higher than the level of the venous vessel. This height, like the static Buretrol model, mimics the mean venous pressure. Activating the pulsatile mode with the Califia, the arterial mean pressure and pulsation mimic the arterial vessel.
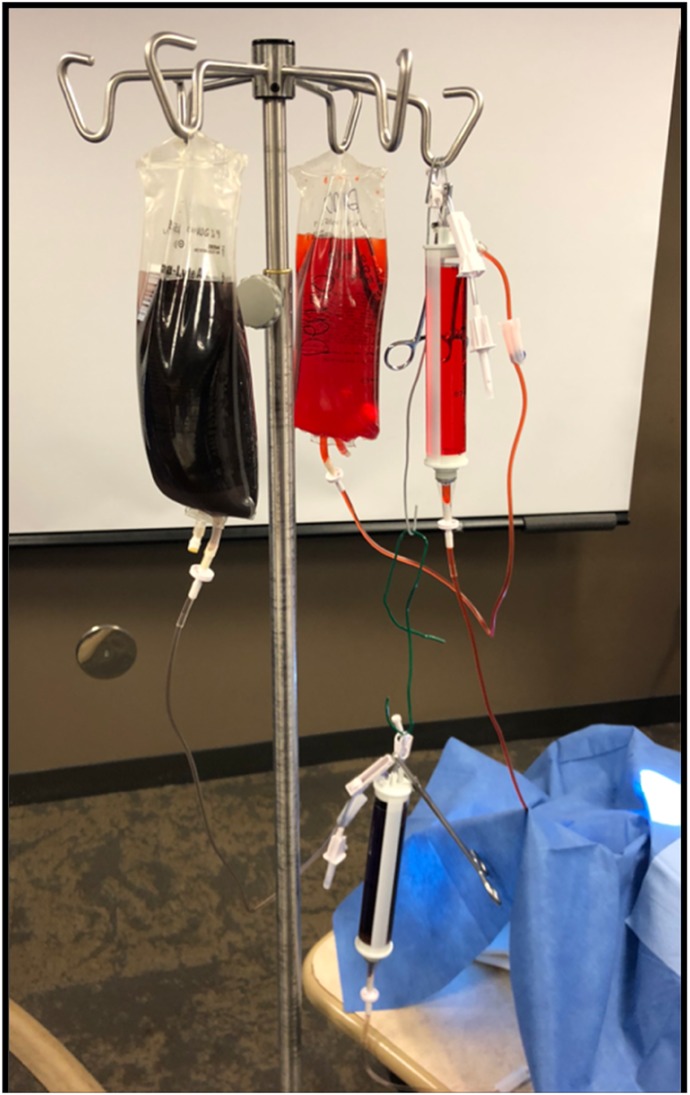
The vascular access pad also can be used in concert with a stand-alone intravenous set and bags attached to Buretrol IV Solution Sets with a 150-mL Clearlink burette (Baxter Healthcare-DMG, Deerfield, IL) to monitor blood loss. Separate intravenous sets attached to the artery and vein, with bright red and dark blue food coloring, respectively, enhance the realism. Changing the height of each intravenous bag establishes a static mean vessel pressure. This can be measured using a standard pressure transducer with modest height adjustments (Figure 8).
Figure 8.
The static model uses two Buretrol sets connected to the skin pad using any available connector. The height is adjusted to regulate a mean pressure normal to the respective for the artery and vein. Incision to cannulation times are recorded along with blood loss.
DISCUSSION
To address the ongoing need for education at our hospital, we developed various models that mimic clinical experiences our team would likely encounter. This method of education is an opportunity to experience a variety of clinical scenarios in a controlled environment while demonstrating clinical routines, minimizing medical errors, and improving overall care. Skill-based performance is a cognitive habit best learned by experience. As such, simulation works to develop this clinical acumen through hands-on realistic training (7).
With this tool, physicians have a reproducible model to demonstrate the cervical surgical cutdown technique and accurate cannulation placement. We have used this mock-up to in-service and provide competency assessments for pediatric general surgeons so that we could widen the physician network offering cannulation beyond our cardiothoracic practitioners. We used the vascular access pad to teach, practice, and develop surgical skills. During these mock-ups, skin incision to cannulation time was recorded and blood loss during the procedure was observed, and these variables offered both the clinician and the facilitators factors related to competency. We hypothesize with practice, the blood loss and cannulation times would both decrease and the clinicians comfort level would improve as indicated by preliminary data collection by our team.
Like most hospitals, we are experiencing a shortage of nurses and surgical technicians. The need to educate and assure competency for new staff requires either caseload or simulated experiences. This simulation model improves the experience level of new team members. Circulating nurses and the surgical technicians learn the prepping, instrument selection and setup, physician cannulation routine, and ECMO circuit line placement. Critical care nurses in any of our intensive care units have an opportunity to practice vital hemodynamic variations, advanced life support requisites, and other clinical duties related to stabilizing the patient before, during, and after ECMO initiation. This can also be an opportunity for physicians in the critical care environment to support the team approach for care. These multidisciplinary simulations provide an opportunity to educate administrators about the clinical requirements for a successful extracorporeal life support program while elucidating what resources are required to sustain such an endeavor.
The future for this type of simulation is endless. For example, decannulation of the model with vessel repair is another teachable occurrence for the team. Cannulation during an active resuscitation (ECMO-CPR) is often challenging for the providers. Simulating a code with active chest compressions and concurrent ECMO cannulation extends the reach of the simulated event. All of the providers can be involved with the resuscitation. In addition, it is possible to set up an active Extracorporeal Life Support mock loop in the intensive care setting, which is ideal for family education. This controlled environment could be a setting to speak about care plans so that the family is aware of the benefits and limitations of extracorporeal life support.
Our requests for simulated experiences continue to develop. The participants are engaged in what they are learning, and the event we create is a context for learning. This approach to learning suggests the adults have enough life experience to be in dialogue with anyone on the team about any subject and will learn new knowledge, attitudes, or skills best in relation to that experience (8). Simulation provides the life experience for the learners and the opportunity to educate through dialogue (3).
REFERENCES
- 1. ELSO Guidelines for Training and Continuing Education of ECMO Specialists. Available at: https://www.elso.org/Resources/Guidelines.aspx. Retrieved February 2010.
- 2.Orino MT, Froehlich CD, Moore EA. Education and training. In: Brogan TV, Lequier L, Lorusso R, et al., eds. Extracorporeal Life Support: The ELSO Red Book. Ann Arbor, MI: Extracorporeal Life Support Organization; 2017:747–61. [Google Scholar]
- 3.Vella J. Learning to Listen Learning to Teach. San Francisco, CA: Jossey-Bass; 2002:4, 149, 115. [Google Scholar]
- 4.Sparks JL, Vavalle NA, Kasting KE, et al. Use of silicone materials to simulate tissue biomechanics as related to deep tissue injury. Adv Skin Wound Care. 2015;28:59–68. [DOI] [PubMed] [Google Scholar]
- 5.Mortensen JD, Talbot S, Burkart JA. Cross-sectional internal diameters of human cervical and femoral blood vessels: Relationship to subject’s sex, age, body size. Anat Rec. 1990;226:115–24. [DOI] [PubMed] [Google Scholar]
- 6.Pranikoff T, Hines MH. Vascular access for extracorporeal support. In: Annich GM, Lynch WR, MacLaren G, et al., eds. ECMO Extracorporeal Cardiopulmonary Support in Critical Care. Ann Arbor, MI: Extracorporeal Life Support Organization; 2012:133–47. [Google Scholar]
- 7.Reason J. The Human Contribution: Unsafe Acts, Accidents and Heroic Recoveries. Burlington, VT: Ashgate; 2008:13. [Google Scholar]
- 8.Knowles M. The Modern Practice of Adult Education: An Autobiographical Journey. New York, NY: Association Press; 1970. [Google Scholar]