Abstract:
The 1/2″ venous line has long been the drainage tubing diameter of choice for adult patients undergoing cardiac surgery. However, several programs use a smaller diameter venous line when used in conjunction with kinetic-assisted venous drainage or vacuum-assisted venous drainage. In 2014, our perfusion team made an institution-wide effort to miniaturize the cardiopulmonary bypass (CPB) circuit for children. One of our changes was the transition to a 3/8″ diameter venous line for drainage, even in our larger patients (up to 80 kg). We reviewed the current literature on this topic and delineated the various parameters required to be able to use the 3/8″ venous line with gravity drainage with the aim of using it on patients up to 115 kg with the appropriate venous reservoir. We have successfully used the 3/8″ venous line in more than 40 of our larger patients (35–90 kg) without the need for assisted venous drainage. We were able to reduce CPB prime from 625 ± 118 to 425 ± 52 mL before retrograde autologous priming (RAP)/venous autologous priming (VAP). The prime was further reduced to 325 ± 66 mL after RAP/VAP. Homologous blood utilization was reduced from 217 ± 311 mL to 27 ± 77 mL. Both results were statistically significant. We hypothesize that taking into account two of the parameters of Poiseuille’s law, namely length and diameter, it is possible to safely drain large children and mid-size adults via gravity venous drainage and the 3/8″ venous line. This technique allows reducing prime volume, simplifies CPB circuits with increased safety and potentially reduces the need for homologous blood transfusion.
Keywords: Cardiopulmonary bypass, kinetic assisted venous drainage, vacuum assisted venous drainage, Poiseuille’s law
The pediatric perfusion strategy at our institution is focused on miniaturization of the cardiopulmonary bypass (CPB) circuit. This allows us to reduce CPB prime volume, thereby decreasing the amount of hemodilution. Although most of our surgical cases fall into the neonatal and pediatric age group, we do operate on a number of larger children and adult congenital patients.
Before November 2014, the average prime volume was 600 mL in our adult cases despite using centrifugal pump technology for kinetic assist venous drainage (KAVD) with a flow range of 0–7 L per minute (LPM). A transition was made to a roller pump with gravity venous drainage, which resulted in a 66% reduction in prime volume for our neonatal and pediatric circuits (1). We intended to achieve the same for our larger patients. We do not adhere to a custom pack philosophy. The extracorporeal circuit (ECC) comprises raceway tubing and an oxygenator with integrated arterial filter and an arteriovenous (AV) loop. The cardioplegia pack is separate, with two choices for hemofiltration. The separation of these components allows us the flexibility to mix and match tubing sizes and devices based on weight, flow ranges, complexity of the operation, and operative times.
METHODS
Approval was obtained for a retrospective chart review from our institutional review board (Ref. #: 1149055-1). We compared data from two groups. Group CA (centrifugal pump with assisted venous drainage, n = 21) and Group RG (roller pump with gravity venous drainage, n = 25).
A standard “adult” ECC at our institution comprises 1/2″ × 3/8″ raceway tubing with an oxygenator and integrated arterial filter. In those patients who weigh 35–80 kg, we use the Capiox® FX15 oxygenator (Terumo, Shibuya, Japan) with a 3/8″ × 3/8″ AV loop, with a flow range of 0–5 LPM. For patients who weigh >80 kg, we use the Medtronic Fusion integrated oxygenator (Medtronic, Minneapolis, MN) with a 3/8″ × 3/8″ AV loop.
For flows ranging from 0 to 7 LPM, we use the Medtronic Fusion® integrated oxygenator (2). The curved inlet, 105 micron venous screen, 1/2″–5/8″ bottom outlet, and the ability to bypass the defoamer reduces resistance and augments venous drainage.
We use a 24-Fr DLP (Medtronic) right-angled metal tip for the superior vena cava and 28-Fr DLP (Medtronic) right-angled metal tip for the inferior vena cava for patients weighing 50–100 kg. For dual-stage single cannulation, we use the two-stage Medtronic 29 × 29-Fr cannula for patients weighing <70 kg or 34 × 46-Fr Medtronic cannula for patients weighing >70 kg.
RESULTS
Before November of 2014, pediatric patients were placed on bypass using a centrifugal pump with KAVD. These patients formed a historical control group (centrifugal with assisted venous drainage [CA]). The CA group was compared with the study cohort (roller pump and gravity venous drainage [RG]).
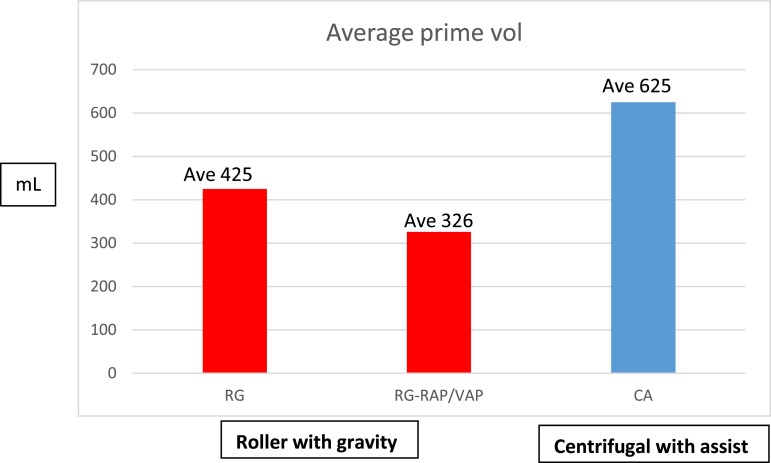
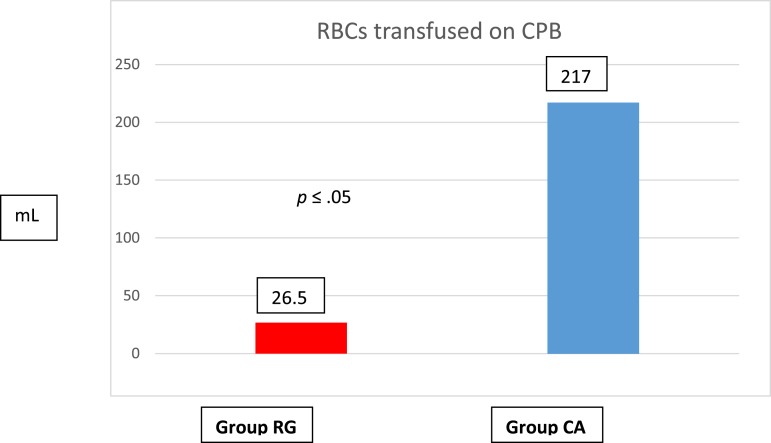
Static prime volume in Group CA was 625 ± 118 mL compared with 425 ± 52 mL in Group RG (Figure 1). The incidence of red blood cell (RBC) transfusion was significantly less in Group RG, 12/21 (57%) compared with 18/20 (90%) in Group CA (p < .05), as was the average volume of RBCs transfused during CPB: 26 ± 77 mL in Group RG vs. 217 ± 311 mL Group CA (p < .05). The average hematocrit (Hct) at the initiation of CPB was similar for both groups of patients, 29 ± 3% vs. 28 ± 5%; Group RG vs. Group CA, respectively (Table 1). The static prime volumes quoted are before retrograde autologous priming (RAP) and venous autologous priming (VAP). These techniques allowed us to further reduce our static prime volumes, making our effective static prime a little more than 325 ± 66 mL. RAP or VAP was not performed with the CA group. Before November of 2014, when the centrifugal pump with KAVD was the perfusion technique being used, there was no concerted effort to minimize prime, miniaturize the CPB circuit, or conserve the use of blood products. This change in prime volume was statistically significant (Figure 1). All values are represented as mean ± SD.
Figure 1.
Static prime comparison.
Table 1.
Data comparison between two groups.
| Wt (kg) | BSA (m2) | CPB Time (minutes) | RBC Prime (mL) | Starting HCT (%) | |
|---|---|---|---|---|---|
| RG (n = 20) | 53.8 ± 16 | 1.54 ± .26 | 158 ± 78 | 0 | 35 ± 2 |
| CA (n = 21) | 65 ± 19 | 1.72 ± .09 | 85 ± 100 | 0 | 37 ± 2 |
BSA, body surface area. Group RG: Roller pump with gravity drainage. Group CA: Centrifugal pump with assisted drainage. Data expressed as mean ± SD.
Average venous saturations as measured by CDI 500 were 73 ± 8 in the CA group and 77 ± 4 in the RG group, suggesting adequate perfusion. Average calculated flows in the RG group was 3.99 ± .7 and average flows delivered was 3.5 ± .6, with an average low temperature of 28 ± 3°C. Average calculated flow in the RG group was 3.7 ± .6 and average flows delivered was 3.22 ± .5, with an average low temperature of 30.7 ± 2.7. Lactate values were missing in four patients of 25 in the RG group due to a transition in our data management systems. Average peak lactate in the RG group was 1.38 ± .57 and average post CPB lactate was 1.46 ± .6.
DISCUSSION
In our efforts to miniaturize our CPB circuitry (Figure 2), we critically analyzed all lengths and diameters of tubing in use, and it became apparent that downsizing the venous drainage length was one possible option among other modifications. Our current choice of venous line (1/2 inch) is based on Galetti et al. (3), originally published in 1962.
Figure 2.
Adult CPB circuit.
In contrast to the arterial circulation, a high-pressure/low-compliance system, the venous circulation is a low-pressure/high-compliance system. Physiologically, venous return toward the heart is dependent on the pressure difference between the mean circulatory filling pressure and the right atrial pressure, usually 7–10 mmHg. On initiation of CPB, venous drainage by gravity is governed by the principles of Poiseuille’s law (Figure 3), namely the height of the table, the distance of the venous reservoir from the floor, the internal diameter (ID) and length of the venous line, the architecture of the venous reservoir, and the viscosity of the fluid being drained. Venous lines with large diameters or greater lengths increase priming volumes and hemodilution. In addition, vacuum-assisted drainage does not necessarily translate to greater venous return. The extensive use of assist techniques can mask poor cannulation, increase hemolysis and gaseous microemboli, and the potential for adverse events (4,5). Assisted venous drainage should only be used if it contributes toward minimizing extracorporeal circuitry and prime volume. At our institution, use of KAVD (before November of 2014) was not used for this purpose.
Figure 3.
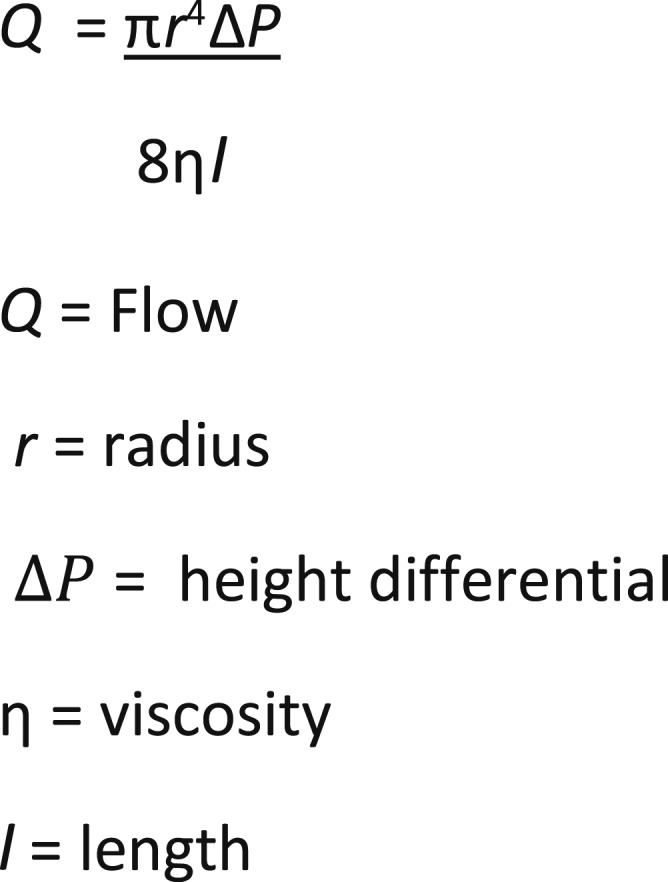
Poseuille’s law.
A 6.6-foot, 3/8″ venous line can drain up to 3.68 L/min, whereas a 1/2″ venous line can drain 7.55 L/min (6). Rarely is a return of 7 L/min required to support arterial flow of 4–5.5 L/min, the arterial flow most adults require. We are able to increase the venous drainage between four and five LPM by reducing the length to a four-foot 3/8″ venous line and a Capiox FX-15 venous reservoir. For venous drainages of higher than five LPM, we use the Fusion venous reservoir and optimize the height differential between the operating room (OR) table and the venous reservoir, closer to 30 cm.
A height differential between the OR table and venous reservoir of 30 cm can generate a negative pressure of 20–25 mmHg (7). This negative pressure can be increased by adding assisted drainage (vacuum-assisted venous drainage [VAVD] or KAVD), increasing the height differential or increasing the diameter of the venous tubing. Although assisted venous drainage techniques can be used, they add complexity to the CPB circuitry. There are multiple steps that can be taken in the clinical setting to miniaturize the ECC and prime volume without the need for KAVD or VAVD.
As stated in Poiseuille’s Law, flow is directly proportional to the fourth power of diameter and inversely proportional to length. When applied to the venous drainage component of a CPB circuit, by reducing the diameter (and therefore the flow), an appropriate reduction in length should maintain a constant flow. Reducing the venous tubing length from eight to four feet can theoretically double venous drainage through the venous reservoir. The venous line at our institution was reduced from six feet (not including the venous cannulae) to four feet, inclusive of the length of the venous cannulas.
An article published by Ni et al. (8) combined a bench top study with clinical data. Using a predictive equation, they calculated the ideal cross-sectional area for venous tubing to be 1 cm2. The ID for 1/2-inch tubing is 1.27 cm2, whereas the ID for 3/8 inch tubing is .71 cm2. In 138 adult CPB patients, they were able to generate flows up to 6 L/min with a 6.6-foot venous line and a pressure gradient of 50 cm H2O, with a tubing ID of 1.0 cm2.
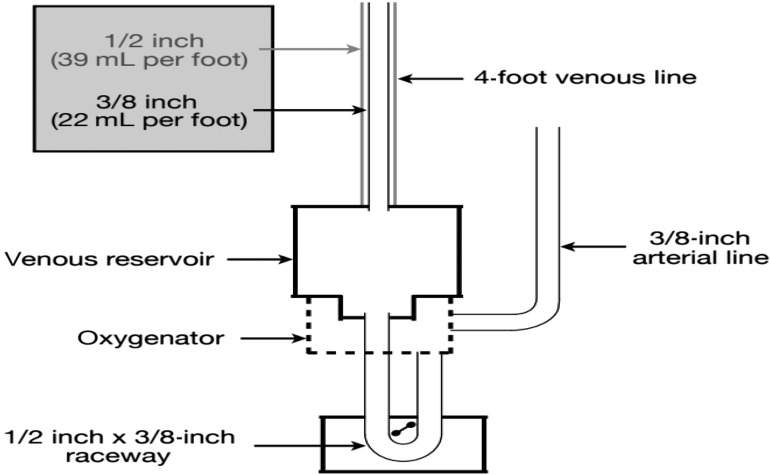
The top of the venous reservoir on our ECC is approximately 15 inches from the sterile field and the bottom of our venous reservoir is approximately 10 inches from the floor (deliberately no lower to keep the venous lines as short as possible). Combining a short venous line (four feet) with 3/8″ diameter tubing, we are able to generate flows of 5–5.5 L/min despite a venous line (Figure 4) with a cross-sectional area of <1 cm2 and no VAVD. The height of the venous reservoir and the distance of the top from the sterile field can be adjusted to generate adequate venous drainage for most patients during circuit set up. If the required flow ranges are >5 LPM, the reservoir is placed on the lower end of the height differential at 30 cm. Alternatively, if the predicted flow is <4 LPM, the reservoir can be placed with a height differential of 20 cm. This change in our circuit configuration reduced our static prime volume by approximately 62% in this small cohort of patients (Figure 1).
Figure 4.
Venous line circuitry schematic.
Although arterial pump flows at our institution were recorded up to 5.5 L/min, the range of flow generally fell between 4 and 5.2 L/min. CPB flows were determined on the basis of a mean arterial pressure of >50 mmHg, a venous saturation of >70 and adequate urine output >.5 mL/kg/h. We hypothesize that lower than calculated flow range were possible due to low circuit prime volume and consequently higher oxygen-carrying capacity/delivery (higher hematocrits) on CPB.
The adoption of this new protocol formed a baseline for reduced intraoperative homologous blood utilization (Figure 5). The reduction in blood utilization was statistically significant. No RBC’s were utilized as a part of the prime in either group. The target transfusion trigger remained the same, a Hb of 7 g/dL for two ventricle patients and a Hb of 8 g/dL for single ventricle patients. The target was a nadir Hct of 24% in both groups, this was easier to achieve in the RG group because of the substantially lower CPB primes.
Figure 5.
Homologous blood utilization comparison.
The routine use of VAVD in pediatric perfusion practice has increased in the last two decades from 24% in 1999 to 64% in 2011 (9,10). With the advent of integrated oxygenators, a staggered pump configuration and shorter venous lines, it may be possible to reduce the diameter of the venous line without the need to routinely use VAVD and risk potential complications.
CONCLUSION
We have shown that contrary to routine practice, it is possible to modify the ECC in an effort to reduce prime volumes. We were able to shorten the venous line, reduce its diameter from 1/2″ to 3/8″, and with appropriate integrated oxygenators, achieve adequate venous drainage in patients up to 90 kg without the need for assisted venous drainage. This has important clinical implications, in that with a smaller prime volume, there is less hemodilution and a reduction in the need for homologous blood transfusion. A less complex bypass circuitry and the use of gravity venous drainage may also reduce the potential for perfusion accidents.
ACKNOWLEDGMENT
We would like to thank Alicia Kube, RN, research coordinator, for assistance with the institutional review board process.
REFERENCES
- 1.Datt B, Nguyen MB, Plancher G, et al. The impact of roller pump vs centrifugal pump on homologous blood transfusion in pediatric cardiac surgery. J Extra Corpor Technol. 2017;49:36–43. [PMC free article] [PubMed] [Google Scholar]
- 2.Hamilton C, Marin D, Weinbrenner F, et al. A new method to measure oxygenator oxygen transfer performance during cardiopulmonary bypass: Clinical testing using the medtronic fusion oxygenator. Perfusion. 2017;32:133–40. [DOI] [PubMed] [Google Scholar]
- 3.Galetti PM, Brecher GA. Heart Lung Bypass: Principles and Techniques of Extra-Corporeal Circulation. New York, NY: Grune and Stratton; 1962:317. [Google Scholar]
- 4.Wang S, Undar A. Vacuum assisted venous drainage and gaseous microemboli in cardiopulmonary bypass. J Extra Corpor Technol. 2008;40:249–56. [PMC free article] [PubMed] [Google Scholar]
- 5.La Pietra A, Grossi EA, Pua BB, et al. Assisted venous drainage presents the risk of undetected air microembolism. J Thorac Cardiovasc Surg. 2000;120:856–62. [DOI] [PubMed] [Google Scholar]
- 6.De Somer F. Venous drainage-gravity or assisted? Perfusion. 2011;26(Suppl):15–9. [DOI] [PubMed] [Google Scholar]
- 7.Corno AF. Systemic venous drainage: Can we help Newton? Eur J Cardiothorac Surg. 2007;31:1044–51. [DOI] [PubMed] [Google Scholar]
- 8.Ni YM, Leskosek B, Shi LP, et al. Optimization of venous return tubing diameter for cardiopulmonary bypass. Eur J Cardiothorac Surg. 2001;20:614–20. [DOI] [PubMed] [Google Scholar]
- 9.Groom RC, Froebe S, Martin J, et al. Update on pediatric perfusion practice in North America: 2005 survey. J Extra Corp Technol. 2005;37:343–50. [PMC free article] [PubMed] [Google Scholar]
- 10.Harvey B, Shann KG, Fitzgerald D, et al. International pediatric perfusion practice: 2011 survey results. J Extra Corpor Technol. 2012:44:186–93. [PMC free article] [PubMed] [Google Scholar]