Abstract
AKI is associated with increased risk of death, prolonged length of stay and development of de-novo chronic kidney disease. The aim of our study is the development and validation of prediction models to identify the risk of AKI in ICU patients up to 7 days. We retrospectively recruited 692 consecutive patients admitted to the ICU at San Bortolo Hospital (Vicenza, Italy) from 1 June 2016 to 31 March 2017: 455 patients were treated as the derivation group and 237 as the validation group. Candidate variables were selected based on a literature review and expert opinion. Admission eGFR< 90 ml/min /1.73 mq (OR 2.78; 95% CI 1.78–4.35; p<0.001); SOFAcv ≥ 2 (OR 2.23; 95% CI 1.48–3.37; p<0.001); lactate ≥ 2 mmol/L (OR 1.81; 95% CI 1.19–2.74; p = 0.005) and (TIMP-2)•(IGFBP7) ≥ 0.3 (OR 1.65; 95% CI 1.08–2.52; p = 0.019) were significantly associated with AKI. For the q-AKI score, we stratified patients into different AKI Risk score levels: 0–2; 3–4; 5–6; 7–8 and 9–10. In both cohorts, we observed that the proportion of AKI patients was higher in the higher score levels.
Introduction
Acute Kidney Injury (AKI) occurs in approximately 50% of patients admitted to an Intensive Care Unit (ICU). Increasing severity of AKI is associated with increased risk of death, prolonged length of stay, increased Intensive Therapy Unit utilisation, and the development of de-novo chronic kidney disease [1–5].
Currently, more than 200 different definitions of AKI are recorded in the literature worldwide [6]. In March 2012 the “KDIGO acute kidney injury clinical practice guidelines” [7] redefined RIFLE and AKIN criteria, and subsequent studies showed a better prediction performance of KDIGO compared to AKIN or RIFLE classifications in critically ill patients [8–12].
Due to kinetics, a significant rise of serum creatinine (SCr) or a reduction in urinary output (UO) occur 48–72 hrs after a kidney injury, and factors such as hydration, nutrition and lean tissue status further confound the diagnosis [7, 13]. Therefore, imprecise early identification of AKI depends on the definition itself of AKI, which is based on an increase in SCr or a decline in UO, both late and non-specific markers [7,13].
Furthermore, a grey-zone exists, as stage 0/A of Acute Kidney Disease (AKD), when no apparent residual injury is present, but the kidney might be vulnerable for some time after an episode of AKI [14]. AKI is a risk factor for the future loss of kidney function: the delay of approximately 24–48 h in elevating creatinine after AKI could promote iatrogenic injuries or lack of the monitoring of the renal function.
The primary goal for dealing effectively with AKI is to recognize its onset early to allow for timely appropriate interventions.
The aim of our study is the development and validation of prediction models to identify the risk of AKI in ICU patients up to 7 days.
Material and methods
Study design, setting and study population
This is a retrospective analysis of the (TIMP-2)•(IGFBP7) Vicenza registry. This registry has been enrolling consecutive critically ill patients admitted to the multidisciplinary ICU at San Bortolo Hospital since 1 June 2016. The registry inclusion criteria are: patients admitted to ICU who were over 18 and were fitted with a urinary catheter for at least 48 hrs, (TIMP-2)•(IGFBP7) ICU admission measurement, whereas the exclusion criteria are: advanced (stage 5) chronic kidney disease (CKD) [15], patients in anuria or with diuresis less than 30 ml within 24 hrs from ICU admission.
Study approval was obtained from the local Human Research Ethics Committee of the San Bortolo Hospital in Vicenza (protocol number 03/17), and the study complied with the Declaration of Helsinki. Informed consent was obtained under Italian laws (S1 File).
We recruited 692 consecutive patients admitted to ICU from 1 June 2016 to 31 March 2017: 455 patients were treated as the determination group and 237 as the validation group. A flow chart of the study population selection and research process is shown in S1 Fig.
AKI was staged each ICU stay day using the ‘Kidney Disease: Improving Global Outcomes’ (KDIGO) SCr criteria and urine output criteria [7]. For this retrospective study, to assess the baseline creatinine, we used the pre-morbid SCr measured 90–180 days before ICU admission [14].
Laboratory data
Blood and urine samples were immediately collected at ICU admission. Urinary (TIMP-2)•(IGFBP7) was analysed with the Nephocheck Test (Astute Medical, San Diego CA, USA). All values for [TIMP-2]*[IGFBP7] are reported in units of (ng/ml)2/1000. Serum creatinine was measured by the enzymatic method (IL testTM, Instrumentation Laboratory SpA, Milano, Italy) on an ILab650 analyser (Instrumentation Laboratory, Werfen Group, Barcelona, Spain). All laboratory data were analysed by technicians who were blinded to the clinical data.
Data collection
Patient data, demographic characteristics, body weight, height, body mass index (BMI). Comorbidities recorder from baseline included hypertension and diabetes mellitus along with previous use of insulin. Data collected at the time of ICU admission included the main reason for admission, severity of illness using the Simplified Acute Physiology Score II (SAPS II) and Sequential Organ Failure Assessment (SOFA), Mean Arterial Pressure (MAP), and in those patients with use of mechanical ventilation (MV), the Positive End-Expiratory Pressure (PEEP) and PaO2/FiO2 (PF) ratio were collected. Laboratory parameters measured included blood gas analysis, lactate and haemoglobin, as well as, within 24 hours of ICU admission, urine output (UO), cumulative fluid balance (CFB), maximum diuretic dose, higher vasopressors/inotropic drug dose(s), cardiovascular SOFA score, PEEP, Partial Pressure arterial Oxigen (PaO2) and Oxigen Inspired Fraction of Inspired Oxigen (FiO2) ratio (PaO2/FiO2), volume of concentrated red blood cell concentrated, and platelets transfused, along with blood gas analysis, lactate, haemoglobin and procalcitonin.
All Registry data was collected from the electronic health records (Digistat) of the ICU and the clinical laboratory using an Excel-based tool. The export of records for further processing and analysis has been anonymised.
Candidate variables and their cut-off were selected based on the literature review, expert opinion (an intensivist, two nephrologists) and availability in the dataset. The final set of predictor variables was determined incrementally for each increasingly complex model via bootstrapped backwards elimination analysis. S2 File shows the anonymized dataset of the study.
Statistical analysis
To describe the sample, we used summary statistics expressed as means, standard deviations, median and IQR for continuous variables or percentages for qualitative ones. Normality distribution was assessed using the Shapiro-Wilk test. The ICU database sample was randomly split into two cohorts: derivation (66%; n = 455) and validation (34%; n = 237) cohorts.
To test the homogeneity of two groups we used T-test and chi square test or non-parametric equivalent test. Any variable with significant univariate test or clinical relevance was selected as a candidate for multivariate analysis.
After performing a multivariable logistic regression on derivation cohorts, stepwise forward process was used to select predictor variables. We subsequently checked for possible interactions and collinearity among predictors. The Hosmer-Lemeshow goodness of fit statistic was used on the best model, determined using both clinical and statistical criteria, to test the calibration. Discrimination performance of risk index was assessed using the area under the ROC curve, and the best cut-off point that maximized both sensitivity (Se) and specificity (Sp) was chosen. The integrated discrimination improvement (IDI) was reported.
Regression coefficients were used to derive an integer score for the development of an easy-to-use AKI risk score. Afterwards, the final AKI risk score model was assessed in the validation cohort using the area under the ROC curve and the Hosmer-Lemeshow goodness of fit test.
Data analysis was performed using STATA/SE for Windows, version 14 (StataCorp, college Station, TX, U.S.A.). Statistical significance was defined as p < 0.05.
Results and discussion
Six hundred ninety-two patients were enrolled, 61% of whom were male, with a mean age of 65.4 ±16.9 years. Table 1 shows the mean characteristics of all patients and of the derivation and validation cohorts. AKI occurrence within 7 days was 38.7% in the derivation cohort and 36.3% in the validation cohort. Table 2 shows how AKI stages were distributed in the two cohorts. There was no significant difference between the two groups (p>0.05).
Table 1. Characteristics of patients, derivation and validation cohorts.
There was no significant difference between the two groups (p>0.05).
| Variables | All patient (n = 692) |
Derivation cohort (n = 455) |
Validation cohort (n = 237) |
|---|---|---|---|
| Male, n (%) | 421(60.8) | 279 (61.3) | 142 (59.9%) |
| Age (years), mean±SD | 65.4 ±16.9 | 65.9±16.8 | 64.4±17.0 |
| Weight (kg), median (IQR) | 75.0 (65.0–85.0) | 75.0 (65.0–85.0) | 71.5 (65.0–85.0) |
| Height (cm), median (IQR) | 170 (165.-175.0) | 170 (165.-175.0) | 170.0 (168.0–177.0) |
| Obese, n (%) | 111(16.0) | 68 (14.9) | 43 (18.1) |
| Hypertension, n (%) | 352 (51.2) | 233 (51.5) | 119 (50.4) |
| Diabetes mellitus type2, n (%) | 123 (17.9) | 81 (17.8) | 42 (17.9) |
| eGFR ≥ 90 (ml/min/1.73 mq), n (%) | 428 (61.8) | 282 (62.0) | 146 (61.6) |
| SOFA cv ≥2, n (%) | 276 (39.9) | 191 (42.0) | 85 (35.9) |
| Lactate ≥2 (mmol/L), n (%) | 309 (44.7) | 212 (46.6) | 97 (40.9) |
| PEEP (cmH2O), median(IQR) | 7.0 (5.0–8.0) | 7.0 (5.0–8.0) | 7 (5.0–8.0) |
| PaCO2 (mmHg), median(IQR) | 39.0 (34.0–46.0) | 39.0 (34.0–46.0) | 38.4 (33.0–46.1) |
| Surgery, n (%) | 282 (40.9) | 185 (40.7) | 97 (41.1%) |
| AKI presence within 7 days, n (%) | 262 (37.9) | 176 (38.7) | 86 (36.3) |
| (TIMP-2)•(IGFBP7) ((ng/ml)2/1000) ≥0.3, n (%) | 370 (53.5) | 246 (54.1) | 124 (52.3) |
| Nephrotoxic drugsa, n (%) | 140 (20.3) | 102 (22.5) | 38 (16.1%) |
aNephrotoxic drugs included aminoglycosides, non-steroidal anti-inflammatory drugs, and vancomycin.
eGFR = estimated glomerular filtration rate; PEEP = positive end-expiratory pressure; PaCO2 = partial pressure of carbon dioxide in arterial blood SOFAcv = cardiovascular sequential organ failure assessment; TIMP-2, tissue inhibitor of metalloproteinases 2.; IGFBP7, insulin-like growth factor-binding protein 7
Table 2. Distribution of the AKI stages in the derivation and validation cohorts.
There was no significant difference between the two groups (p>0.05).
| AKI occurrence patients, n (%) |
All patient 262 (37.9) |
Derivation cohort 176 (38.7) |
Validation cohort 86 (36.3) |
|---|---|---|---|
| Stage 1 | 158 (60.3) | 105 (23.1) | 53 (22.4) |
| Stage 2 | 66 (25.2) | 45 (9.9) | 21 (8.9) |
| Stage 3 | 38 (14.5) | 26 (5.7) | 12 (5.1) |
Obesity (Body Mass Index (BMI > 30), admission eGFR, cardiovascular SOFA (SOFAcv), Lactate and (TIMP-2)•(IGFBP7) were significantly associated with AKI in a multivariate logistic regression (Table 3). More specifically, these variables were associated with an increased risk of AKI: eGFR< 90 ml/min /1.73 mq (OR 2.78; 95% CI 1.78–4.35; p<0.001); SOFAcv ≥ 2 (OR 2.23; 95% CI 1.48–3.37; p<0.001); lactate ≥ 2 mmol/L (OR 1.81; 95% CI 1.19–2.74; p = 0.005) and (TIMP-2)•(IGFBP7) ≥0.3 (OR 1.65; 95% CI 1.08–2.52; p = 0.019). In this model, the area under the ROC curve was 0.73 (95%CI 0.68–0.78); goodness of fit was p = 0.5232; Se = 57.4 and Sp = 80, IDI 0.012 p = 0.0139. We converted the OR into integer single risk scores. By summing the component variables, the total score can range from a minimum of 0 to a maximum of 10 points (Table 4).
Table 3. Predictors of AKI in derivation cohorts (n = 455).
| OR | 95% CI | p-value | ||
|---|---|---|---|---|
| Lower | Upper | |||
| Obese vs non-obese | 1.28 | 0.72 | 2.26 | 0.399 |
| eGFR < 90 (ml/min/1.73 mq) | 2.78 | 1.78 | 4.35 | <0.001 |
| SOFA cv ≥2 | 2.23 | 1.48 | 3.37 | <0.001 |
| Lactate ≥2 (mmol/L) | 1.81 | 1.19 | 2.74 | 0.005 |
| (TIMP-2)•(IGFBP7) ≥ 0.3 ((ng/ml)2/1000) | 1.65 | 1.08 | 2.52 | 0.019 |
eGFR = estimated glomerular filtration rate SOFAcv = cardiovascular sequential organ failure assessment; TIMP-2, tissue inhibitor of metalloproteinases 2.; IGFBP7, insulin-like growth factor-binding protein 7
Table 4. AKI risk score of the final model.
| Risk factors | Points |
|---|---|
| Obese vs non-obese | 1 |
| eGFR < 90 (ml/min/1.73 mq) | 3 |
| SOFA cv ≥2 | 2 |
| Lactate ≥2(mmol/L) | 2 |
| (TIMP-2)•(IGFBP7) ≥0.3 ((ng/ml)2/1000) | 2 |
eGFR = estimated glomerular filtration rate SOFAcv = cardiovascular sequential organ failure assessment; TIMP-2, tissue inhibitor of metalloproteinases 2.; IGFBP7, insulin-like growth factor-binding protein 7
After that, we performed the final logistic regression model on the validation cohort.
In this “validation” model, the area under the ROC curve was 0.76 (95%CI 0.69–0.82); goodness of fit was p = 0.3204; Se = 48.8 and Sp = 88.7. Finally, through a sensitivity analysis and medical considerations, we defined a low-risk of developing AKI as a score below the cut-off value 5.
Regarding the AKI Risk Score, both cohorts showed similar results: in the derivation cohort, 44% of patients had a Risk Score of less than 5 points compared to 45% in the validation cohort.
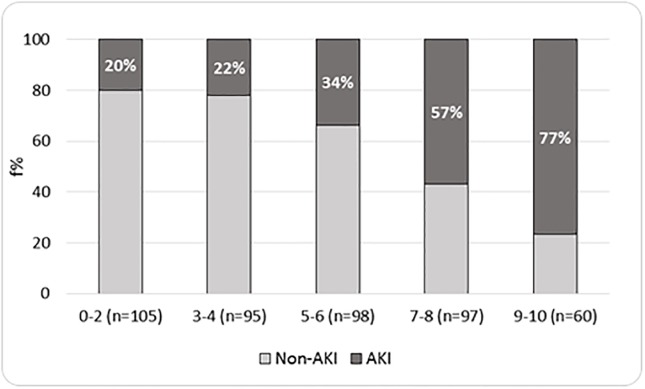
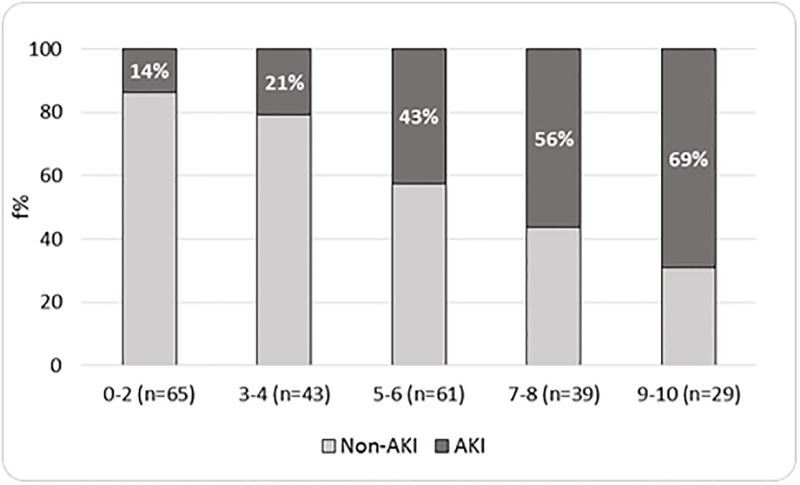
For an easy-to-use AKI risk score, we stratified patients into different AKI Risk Score levels: 0–2; 3–4; 5–6; 7–8 and 9–10 (Figs 1 and 2). In both cohorts, as shown in Figs 1 and 2, we observed that the proportion of AKI patients was higher in the higher score levels. More precisely, in the derivation and validation cohorts, AKI was present in, respectively, 20% and 14% of participants belonging to the lowest risk group, 22% and 21% to the 3–4 risk level group, 34% and 43% to the 5–6 risk level group, 57% and 56% to the 7–8 risk level group, and 77% and 69% to the highest risk level group. In both cohorts, the risk of developing AKI was proportional to the increase in the level of risk (p for trend <0.0001).
Fig 1. Distribution of AKI by AKI risk score levels—Derivation cohort (n = 455).
Fig 2. Distribution of AKI by AKI risk score levels—Validation cohort (n = 237).
In the past 25 years, the incidence of AKI has increased by at least 20 times [16]. Recently, Bellomo et al. and Palevsky et. al. have shown that the mortality rate of critically ill patients with AKI was 40%~70% [17,18]. AKI is not only a medical problem but has also become a major public health concern.
The Annual AKI-associated costs represent a substantial component of the National Health System budget, even when the patients recover renal function. In fact, AKI diagnosis is associated with a length of stay 2.57 times higher than that for admission without AKI; and 59.89% of the critical care bed days are for people with AKI [19]. Pannu et al. found that, among the patients who had developed severe AKI (KDIGO stage 2 or 3), 30.8% died and 2.1% progressed to CKD stage 5, requiring dialysis within a 34-month period [20].
Therefore, early recognition of AKI is relevant for critical care physicians to improve the quality of interventions in order to avoid or limit the progression of renal disease.
Since the treatment is largely supportive, AKI prevention becomes mandatory in critically ill patients [21].
Our study has established a new simple prediction score to quickly predict AKI at any stage up to 7 days.
Previously, we demonstrated that the development of AKI at any stage during the first week of ICU stay can be quickly predicted based on clinical information and (TIMP-2)•(IGFBP7) urine measurement collected up to 24 hours after ICU admission (Ferrari F, submitted to Scientific Reports, 2018). To speed up the risk evaluation, in this study we considered variables available by one hour.
The final model included eGFR< 90 ml/min /1.73 mq; SOFAcv ≥ 2; lactate ≥ 2 mmol/L and (TIMP-2)•(IGFBP7) ≥ 0.3 ((ng/ml)2/1000).
While eGFR gives information on renal function at admission, including anthropometric characteristics, SOFAcv and lactate levels mirror a modified perfusion that might affect renal autoregulation.
Recently, perfusion pressure has been considered as an indicator for the prevention of AKI. In terms of perfusion pressure, diastolic perfusion pressure (DPP) and mean perfusion pressure (MPP) should be pointed out [22]. Two observational studies revealed that lower DAP or decreased MPP were associated with septic AKI [23,24].
Therefore, since decreased DAP is associated with AKI, catecholamines may be effective in preventing AKI [25].
Instead, obesity is associated with an increased risk for AKI in critically ill patients admitted to a medical or surgical ICU [26] after cardiac [27,28] and bariatric surgery [29].
Obesity leads to both venous congestion and poor arterial organ perfusion: in effect, obese patients show an impaired diastolic function due to left ventricular hypertrophy and adipocytes infiltration of the myocardium [30] and an elevated intra-abdominal pressure, which needs a higher Positive End Expiratory Pressure (PEEP) in mechanically ventilated patients [31, 32].
Furthermore, obesity impacts many pharmacokinetic factors: the weight-based dose of hydrophilic drugs might reach the nephrotoxic threshold [33]; on the other hand, adipocytes are involved in the secretion of inflammatory mediators that can lead to kidney damage [34].
Contrasting results regarding interventions to prevent AKI have led to disappointment regarding the use of biomarkers alone [35,36]. Nevertheless, (TIMP-2)•(IGFBP7) improved the predictive performance at ICU admission of a clinical model for AKI at any stage (Ferrari F, submitted to Scientific Reports 2018, [37], even if its best performance is to identify patients at risk of developing severe (stage 2–3) AKI [38–40].
Including a biomarker admission measurement improves the predictive ability of the logistic model and allows for speedier diagnoses, even if glomerular filtration has not yet decreased [13].
The strength of our score is that it quickly identifies patients with a high-risk of developing AKI at any stage through simple and quick information available at ICU admission. Compared to previous studies [38–40], a combined approach could improve the diagnostic prediction of the biomarker alone to identify less severe AKI as well.
However, our study still has some limitations: it was a retrospective study which limits the generalization of its findings and a single-centre study which may not be directly applicable to other patient populations. Furthermore, (TIMP-2)•(IGFBP7) was measured only at ICU admission. There are no studies that evaluate whether serial measurements might improve predictive performance.
Conclusion
We have shown that AKI development within the first week of an ICU stay, as defined by the KDIGO criteria [7], might be identified from a prediction model that uses data routinely available one hour after admission.
Furthermore, (TIMP-2)•(IGFBP7) improves the prediction ability of the model and might allow for speedier diagnoses, even if glomerular filtration has not yet decreased.
Supporting information
(TIF)
(DOCX)
(CSV)
Acknowledgments
The authors wish to thank the physicians and nurses at the ICU and Nephrology Department for their hard work. Without their support, this work would not have been possible.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
CR received compensation and consulted for Astute Medical, OCD, Asahi Medical, Baxter, and Toray Medical. AB is an employee and received salary from abcGO s.r.l. The RTC Colombia subsidiary of Baxter International provided support for this study in the form of a salary for AMT. The specific roles of these authors are articulated in the ‘author contributions’ section. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Lewington AJ, Cerda J, Mehta R L. Raising awareness of acute kidney injury: a global perspective of a silent killer. Kidney Int. 2013; 84:457–467. 10.1038/ki.2013.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoste EA, Bagshaw SM, Bellomo R, Cely CM, Colman R, Cruz DN et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. 2015;41:1411–1423. 10.1007/s00134-015-3934-7 [DOI] [PubMed] [Google Scholar]
- 3.Wald R, Quinn RR., Luo J, Li P, Scales DC, Mamdani MM. et al. Chronic dialysis and death among survivors of acute kidney injury requiring dialysis. JAMA. 2009; 302:1179–1185. 10.1001/jama.2009.1322 [DOI] [PubMed] [Google Scholar]
- 4.Wu VC, Wu CH, Huang TM, Wang CY, Lai CF, Shiao CC et al. Long-term risk of coronary events after AKI. J. Am. Soc. Nephrol. 2014;25 (3):595–605. 10.1681/ASN.2013060610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bedford M, Stevens PE, Wheler TWK, Farmer CK. What is the real impact of acute kidney injury? BMC Nephrology. 2014; 15:95 10.1186/1471-2369-15-95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ricci Z, Cruz DN, Ronco C. Classification and staging of acute kidney injury: beyond the RIFLE and AKIN criteria. Nat Rev Nephrol. 2011; 7:201–208. 10.1038/nrneph.2011.14 [DOI] [PubMed] [Google Scholar]
- 7.KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int Suppl. 2012;2(1):1–138. [Google Scholar]
- 8.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P and the ADQI workgroup. Acute renal failure definition, outcome measures, animal models, fluid therapy and information technology needs: The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care.2004; 8:R204 10.1186/cc2872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007;11(2):R31 10.1186/cc5713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xiong J, Tang X, Hu Z, Nie L, Wang Y, Zhao J. The RIFLE versus AKIN classification for incidence and mortality of acute kidney injury in critical ill patients: A meta-analysis. Sci Rep 2015. 7;5:17917 10.1038/srep17917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pan HC, Chien YS, Jenq CC, Tsai MH, Fan PC, Chan CH et al. Acute Kidney Injury Classification for Critically Ill Cirrhotic Patients: A Comparison of the KDIGO, AKIN, and RIFLE Classifications. Sci Rep. 2016; 17;6:23022 10.1038/srep23022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Z, Cai L, Liang X, Du Z, Chen Y, An S et al. Identification and predicting short-term prognosis of early cardiorenal syndrome type 1: KDIGO is superior to RIFLE or AKIN. PloS One. 2014. 26;9(12):e114369 10.1371/journal.pone.0114369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ronco C. Acute kidney injury: from clinical to molecular diagnosis. Crit Care. 2016; 20: 201 10.1186/s13054-016-1373-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chawla LS, Bellomo R, Bihorac A, Goldstein, Siew ED, Bagshaw SM et al. Acute kidney disease and renal recovery: consensus report ofthe Acute Disease Quality Initiative(ADQI) 16 Workgroup. Nat Rev Nephrol. 2017;13(4):241–257. 10.1038/nrneph.2017.2 [DOI] [PubMed] [Google Scholar]
- 15.Kidney Disease Improving Global Outcomes. Chapter 1: Definition and classification of CKD. Kidney Int Suppl. 2012; 3(1):19–62. 10.1038/kisup.2012.64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murugan R, Kellum JA. Acute kidney injury: what’s the prognosis? Nature reviews Nephrology. 2011; 7, 209–217, 10.1038/nrneph.2011.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bellomo R, Cass A, Cole L, Finfer S, Gallagher M, Lo S et al. Investigators, R. R. T. S. Intensity of continuous renal-replacement therapy in critically ill patients. NEJM. 2009; 361, 1627–1638, 10.1056/NEJMoa0902413 [DOI] [PubMed] [Google Scholar]
- 18.VA/NIH Acute Renal Failure Trial Network, Palevsky PM, Zhang JH, O’Connor TZ, Chertow GM, Crowley ST, Choudhury D et al. Intensity of renal support in critically ill patients with acute kidney injury. NEJM. 2008; 359, 7–20, 10.1056/NEJMoa0802639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kerr M, Bedford M, Matthews B et al. The economic impact of acute kidney injury in England. Nephrol Dial Transplant. 2014;29(79):1362–1368. 10.1093/ndt/gfu016 [DOI] [PubMed] [Google Scholar]
- 20.Pannu N, James M, Hemmelgarn B, Klarenbach S. for the Alberta Kidney Disease Network. Association between AKI, Recovery of Renal Function, and Long-Term Outcomes after Hospital Discharge. Clinl J Am Soc Nephrol.2013; 8(2):194–202. 10.2215/CJN.06480612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joannidis M, Druml W, Forni LG, Groeneveld AB, Honore P, Oudemans-van Straaten HM, Ronco C, Schetz MR, Woittiez AJ; Critical Care Nephrology Working Group of the European Society of Intensive Care Medicine. Prevention of acute kidney injury and protection of renal function in the intensive care unit. Intensive Care Med. 2010; 36:392–411. 10.1007/s00134-009-1678-y [DOI] [PubMed] [Google Scholar]
- 22.Sato R, Luthe SK, Nasu M. Blood pressure and acute kidney injury. Crit Care. 2017;10;21(1):28 10.1186/s13054-017-1611-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Legrand M, Dupuis C, Simon C, Gayat E, Mateo J, Lukaszewicz AC, Payen D. et al. Association between systemic hemodynamics and septic acute kidney injury in critically ill patients: a retrospective observational study. Crit Care. 2013;17(6):R278 10.1186/cc13133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wong BT, Chan MJ, Glassford NJ, Mårtensson J, Bion V, Chai SY, Oughton C, Tsuji IY, Candal CL, Bellomo R. Mean arterial pressure and mean perfusion pressure deficit in septic acute kidney injury. J Crit Care; 2013; 30(5):975–81. [DOI] [PubMed] [Google Scholar]
- 25.Lamia B, Chemla D, Richard C, Teboul JL. Clinical review: interpretation of arterial pressure wave in shock states. Crit Care. 2005;9(6):601–6. 10.1186/cc3891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Danzinger J, Chen KP, Lee J, Feng M, Mark RG, Celi LA, Mukamal KJ. et al. Obesity, acute kidney injury, and mortality in critical illness. Crit Care Med. 2016; 44:328–334. 10.1097/CCM.0000000000001398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yap CH, Mohajeri M, Yii M. Obesity and early complications after cardiac surgery. Med J Aust. 2007;186: 350–354. [DOI] [PubMed] [Google Scholar]
- 28.Billings F, Pretorius M, Schildcrout J, Mercaldo ND, Byrne JG, Ikizler TA, Brown NJ. et al. Obesity and oxidative stress predict AKI after cardiac surgery. J Am Soc Nephrol. 2012; 23:1221–1228. 10.1681/ASN.2011090940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thakar C, Kharat V, Blanck S, Leonard AC. Acute kidney injury after gastric bypass surgery. Clin J Am Soc Nephrol. 2007; 2:426–430. 10.2215/CJN.03961106 [DOI] [PubMed] [Google Scholar]
- 30.McGavoc J, Victor R, Unger R, Szczepaniak LS; American College of Physicians and the American Physiological Society. Adiposity of the heart, revisited. Ann Intern Med. 2006;144:517–524. [DOI] [PubMed] [Google Scholar]
- 31.Mullens W, Abrahams Z, Francis GS, Sokos G, Taylor DO, Starling RC, Young JB, Tang WHW. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol. 2009;53:589–596. 10.1016/j.jacc.2008.05.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim IB, Prowle J, Baldwin I, Bellomo R. Incidence, risk factors and outcome associations of intra-abdominal hypertension in critically ill patients. Anaesth Intensive Care. 2012;40:79–89. 10.1177/0310057X1204000107 [DOI] [PubMed] [Google Scholar]
- 33.Medico CJ, Walsh P. Pharmacotherapy in the critically ill obese patient. Crit Care Clin. 2010;26:679–688. 10.1016/j.ccc.2010.07.003 [DOI] [PubMed] [Google Scholar]
- 34.Nakamuro K, Fuster J, Walsh K. Adipokines: a link between obesity and cardiovascular disease. J Cardiol. 2014;63:250–259. 10.1016/j.jjcc.2013.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meersch M, Schmidt C, Hofmeier A, Van Aken H, Wempe C, Gerss J, Zarbock A. Prevention of cardiac surgery-associated AKI by implementing the KDIGO guidelines in high risk patients identified by biomarkers: the PrevAKI randomized controlled trial. Intensive Care Medicine. 2017;43(11):1551–1561. 10.1007/s00134-016-4670-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moritz Schanz, Christoph Wasser, Sebastian Allgaeuer, Schricker S, Dippon J, Alscher MD, Kimmel M. Urinary [TIMP-2]·[IGFBP7]-guided randomized controlled intervention trial to prevent acute kidney injury in the emergency department, Nephrology Dialysis Transplantation, gfy186, 10.1093/ndt/gfy186 [DOI] [PubMed]
- 37.Di Leo L, Nalesso F, Garzotto F, Xie Y, Yang B. Virzì GM et al. Predicting Acute Kidney Injury in Intensive Care Unit Patients: The Role of Tissue Inhibitor of Metalloproteinases-2 and Insulin-Like Growth Factor-Binding Protein-7 Biomarkers. Blood Purif. 2018;45:270–277 10.1159/000485591 [DOI] [PubMed] [Google Scholar]
- 38.Kashani K, Al-Khafaji A, Ardiles T, Artigas A, Bagshaw SM, Bell M et al. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care. 2013;17(1): R25 10.1186/cc12503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoste EAJ, McCullough PA, Kashani K, Chawla LS, Joannidis M, Shaw AD et al. Derivation and validation of cutoff for clinical use of cycle arrest biomarkers. Nephrol Dial Transplant.2014; 29: 2054–2061. 10.1093/ndt/gfu292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bihorac A, Chawla LS, Shaw AD, Al-Khafaji A, Davison DL, Demuth GE et al. Validation of cell-cycle arrest biomarkers for acute kidney injury using clinical adjudication. Am J Respir Crit Care Med. 2014; 189:932–9. 10.1164/rccm.201401-0077OC [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
(DOCX)
(CSV)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.