Abstract
Infection of rainbow trout with the parasitic ciliate Ichthyopthirius multifiliis induces differential responses in gills, skin and spleen. A controlled experimental infection was performed and expression of immune-relevant genes in skin, gills, and spleen were recorded by qPCR at day 1 and 8 after parasite exposure. Infection induced a marked reaction involving regulation of innate and adaptive immune genes in rainbow trout at day 8 post-infection. The expression level of a total of 22 out of 24 investigated genes was significantly higher in gills compared to skin reflecting the more sensitive and delicate structure of gills. Especially pro-inflammatory cytokines IL-6, IL-17 C1, regulatory cytokines IL-4/13A, IL-10, TGFβ, complement factor C5, chemokines CK10, CK12, acute phase proteins (precerebellin, hepcidin) and immunoglobulins (IgM, IgT) displayed differential expression levels. The spleen, a central immune organ with no trace of the parasite, showed elevated expression of IgM, IgT, complement factor C5 and chemokine CK10 (compared to skin and gills directly exposed to the parasite), indicating an interaction between the infected surface sites and central immune organs. This communication could be mediated by chemokines CK10 and CK12 and cytokine IL-4/13A and may at least partly explain the establishment of a systemic response in rainbow trout against the parasite.
Introduction
The parasitic ciliate Ichthyophthirius multifiliis is one of the most problematic parasites in aquaculture affecting a wide range of different freshwater fish species worldwide [1]. Rainbow trout (Oncorhynchus mykiss) is one of the main aquaculture species [2, 3] suffering from infections. A series of investigations have documented that fish hosts respond to infection with a protective immune response [4–11] but how the host regulates the different parasitic stimulations of the fish body surfaces and eventually establishes a systemic immunity is unknown. The present study addressed this issue by measuring immune reactions in rainbow trout surfaces and central immune organs at an early and late time point during I. multifiliis infection. Expression of a total of 24 immune genes (encoding cytokines, chemokines, complement factors, acute phase proteins, immune cell receptors, and immunoglobulins) were investigated in mucosal surfaces (gills, skin) and a central organ (spleen). The study thereby contributes to the understanding of how infections in the surface of a fish may elicit a protective systemic response.
Materials and methods
Ethics statement
The infection experiments were conducted at the Laboratory of Aquatic Pathobiology fish infection facilities at the University of Copenhagen (Frederiksberg C, Denmark). Animal care and investigations were performed according to license 2013-15-0201-00764a (The Experimental Animal Inspectorate under the Ministry of Food, Agriculture and Fisheries).
Fish and rearing conditions
Fish handling and infection procedures in this work were described previously [12]. Briefly, rainbow trout fry were produced and reared under pathogen-free conditions in a recirculated closed system (Bornholm Salmon hatchery, Nexø, Denmark). Fish were then transported to our experimental rainbow trout facility and kept in 200 L fish tanks (water temperature of 12–14°C) until initiation of the experiment. The experimental fish (body weight mean (SD): 7 (2) g) were acclimatized in 4 x 60 L tanks (20 fish in each) containing freshwater (municipal tap water, Frederiksberg) equipped with internal biofilters (Eheim, Germany), plastic plants (enrichment), and continuous aeration using air stones for two weeks before initiation of the infection experiment. Fish were fed commercial pelleted feed (Biomar, Denmark) every second day (1% of biomass). The aquaria were covered using a screen of dark plastic to avoid influence of stressors (light, movements) from the exterior. Rearing water temperature was set to 15–16°C and water quality (NH3, NO2, and pH) was monitored with standard test kits (Merck, Germany) throughout the experimental period.
Infection procedure and sampling
The experimental rainbow trout were randomly divided into two groups (infected/non-infected), each comprising two replicates. For the infection group, fish were exposed to infective theronts by adding a solution of parasites (2,400 theronts/fish). Theronts were produced from tomocysts developed from tomonts released from infected fish according to standard procedures [7, 13]. For the control group, the same treatments were conducted but the corresponding tanks were sham-infected by pouring a similar volume of pure water into the tanks.
A total of 5 fish from each tank were randomly sampled at day 1 and 8 post-infection (dpi). Fish were collected by a hand-net and subsequently euthanized in MS-222 (Cat. no. A5040, Sigma-Aldrich) (300 mg/L) followed by tissue collection. Tissues (skin, gills, and spleen) were aseptically sampled and immediately placed into 2 mL tubes containing RNAlater (Cat. no. R0901, Sigma Aldrich), pre-stored at 4°C for 24 h and then stored at -20°C until conducting gene expression analysis.
RNA extraction, cDNA synthesis and real-time PCR
Total RNA was extracted from tissue samples using GenEluteTM kit (Cat. no. RTN350-1KT, Sigma-Aldrich), according to the manufacturer’s instruction and subsequently DNase treated with DNase I (Cat. no. ENO521, Thermo Scientific). The quantity and purity of RNA were measured at 260/280 nm (NanoDrop 2000 Spectrophotometer, Thermo Scientific) and DNAse efficacy and RNA integrity were evaluated by electrophoresis on 1% agarose gels with ethidium bromide (EtBr) staining.
The first-strand cDNAs were synthesized using 1000 ng of total RNA, MultiScribe Reverse Transcription reagent (Thermo Fisher Scientific), and random hexamers (Roche) in a 20 μl setup. The reaction was placed at 37°C for 60 min in a Thermal Cycler (T100TM Thermal Cycler, BioRad). Subsequently, the synthesized cDNA was stored at -20°C until further use.
Presence of trophonts in skin and gills were confirmed visually under the dissection microscope (magnification x 7–40) but a quantitative estimation of the parasite burden was obtained by performing quantitative real-time PCR (qPCR) with specific primers and probes for the gene encoding the parasite’s I-antigen. Likewise the expression of immune-associated genes was evaluated by qPCR assays using the synthesized cDNA with specific primers and corresponding probes listed in S1 Table. The qPCR reactions were carried out in a 96 well plate containing 5 ng/μl of cDNA, 6.25 μl of Brilliant® II QPCR master mix (Agilent Technologies), forward and reverse primer (0.8 μM each), and TaqMan® probe (0.4 μM) in a total volume reaction of 12.5 μl. The reactions were performed on an AriaMx Real-Time PCR system (Agilent Technologies) under the following conditions: 95°C for 15 min followed by 45 cycles of 95°C for 10 s and 60°C for 45°C. The endogenous reference gene (EF1-α) was used to normalized the relative expression of the target genes.
Data analysis
The 2-ΔΔCt method was applied to determine the relative gene expression presented as the fold increase or decrease of the infected group relative to the time point control groups (mean expression level adjusted to 1). The absence of tank effect was tested and confirmed before pooling the gene expression data from duplicate tanks. To account for biological variation, only gene regulations with at least 2-fold change were considered significant. The statistical difference between groups was determined using a Student’s t-test applying a probability level of 5% (p<0.05). Additionally, correlations among the gene expression levels in investigated tissues at different time-points were analyzed by using Pearson correlation. The relative gene expression was visualized using a heat map, generated using gplots (v3.01) in R (v.3.44).
Results
The presence of parasites in infected tissues
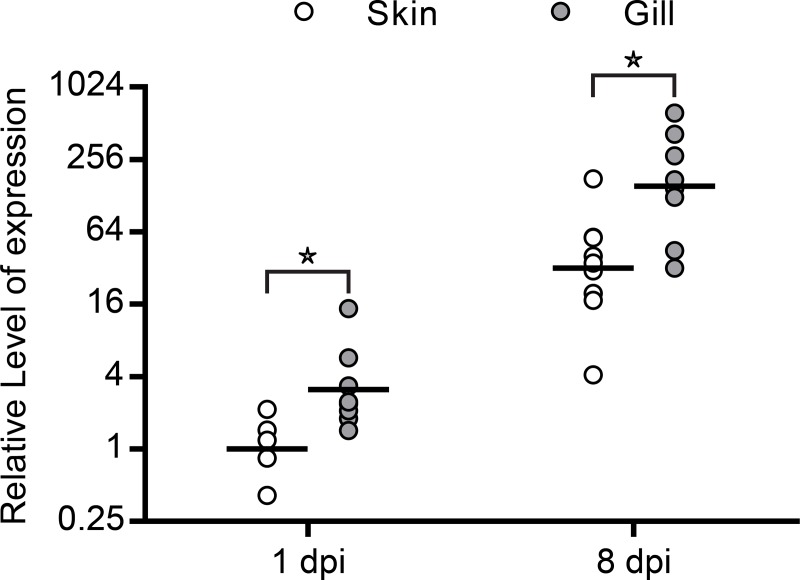
Presence of parasites in all examined tissues was confirmed by microscopy and quantifying the expression level of the gene encoding the specific Ichthyophthirius multifiliis I-antigen. The parasite gene transcript was measured in infected skin and gills at both day 1 and 8 after infection, but no expression was found in spleen. The transcript level in gills was higher when compared to skin at both time-points (3.1 and 4.8 fold, respectively) and the expression levels of I-antigen were increasing in both infected tissues indicating increased transcription during the experiment (Fig 1).
Fig 1. Relative levels of I. multifiliis is represented relative to the reference gene EF1α by calculating 2-ΔCq normalized to the group having the lowest expression level, skin at 1 dpi.
Student t-test (p<0.05) showed that gills had significantly higher level of I. multifiliis at both 1 dpi and 8 dpi (3.1 and 4.8 fold, respectively). No transcription of the gene was recorded in spleen.
Expression of immune-relevant genes
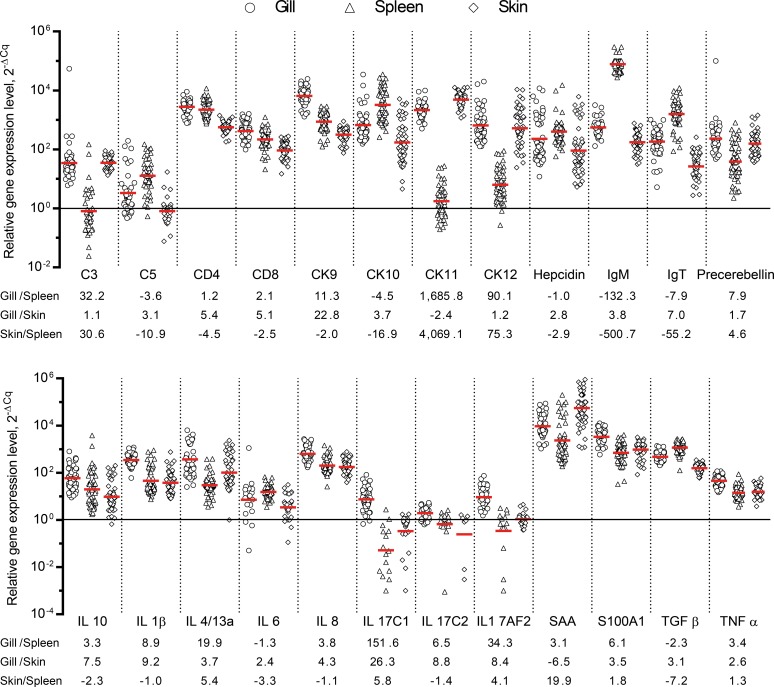
We measured the expression of a total of 24 immune-related genes encoding cytokines (IL-1β, IL-4/13A, IL-6, IL-8, IL-10, IL-17/C1, IL-17/C2, IL-17A/F2, TGFβ, and TNFα), chemokines (CK9, CK10, CK11, and CK12), complement factors (C3 and C5), acute phase proteins (hepcidin, precerebellin, SAA, and S100A1), immunoglobulins (IgM and IgT), and cellular receptors (CD4 and CD8). The overall percentage of samples having a Cq value was 94% in gills compared to 89% in both skin and spleen. The expression profiles of the genes encoding CD4, IL-1β, IL-8, IL-17C1, SAA, S100A1, TGFβ, and TNFα from gill tissue at 8 dpi were presented in a previous work [12]. The expression level differed from gene to gene in all tissues examined (Fig 2) and the majority of genes showed altered expression after infection. Genes encoding complement (C3 and C5) and cytokines IL-17 showed low expression particularly in skin and spleen. A total of 22 genes had higher expression in gills compared to skin (the genes encoding CK11 and SAA had a higher transcript level in skin). Seventeen genes in gills showed higher expression compared to the corresponding genes in spleen, but genes encoding C5, CK10, hepcidin, IL-6, TGFβ, IgM and IgT were expressed at higher level in spleen. A total of 10 genes encoding C3, CK11, CK12, precerebellin, IL-4/13A, IL-17/C1, IL-17A/F2, SAA, S100A1, and TNFα were expressed at a higher level in skin compared to spleen, whereas 14 genes showed higher expression in spleen.
Fig 2. Overall expression levels of genes.
The relative expression levels (both time points) were calculated as 2-ΔCq. The levels were normalized to the geometric mean of the least expressed gene (IL-17C2) (all tissues combined). All samples having valid Cq values are represented. Relative overall gene expression level (for each gene) between tissues is shown as the ratio (Gill/Spleen, Gill/Skin, Skin/Spleen).
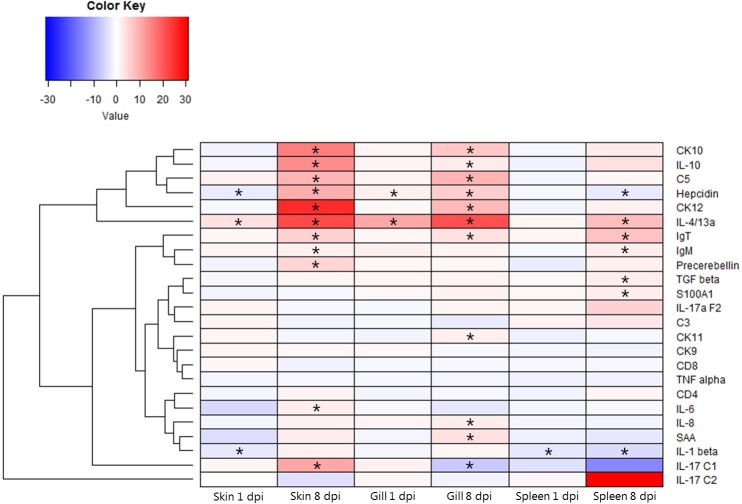
Expression levels in skin, gills and spleen at different time-points were correlated as shown by significant positive correlations of 8 genes (encoding CK10, CK12, hepcidin, SAA, IL-4/13A, IL-17A/F2, IgM, IgT) in gills and skin at 1 dpi. Genes encoding CK12, SAA, and IgM also showed significant positive correlation between the two mucosal surfaces at 8 dpi. The expression of genes encoding C3, C5, hepcidin, precerebellin, SAA, S100A1, IL-10, CD4, CD8, IgT in gills was positively correlated with expressions in spleen at 1 dpi, while only CD4 was positively correlated at 8 dpi in between these tissues. A significant positive correlation of the IgM gene expression in skin and spleen was found at 1 dpi, while the IL-10 gene expression was negatively correlated at day 8 after infection (S2 Table). Merely a small number of genes showed a significant regulation at day 1 after infection, some of which were up-regulated and others were down-regulated. In contrast, the majority of investigated genes at day 8 post-exposure showed a significant up-regulation associated with infection (Figs 3 and 4).
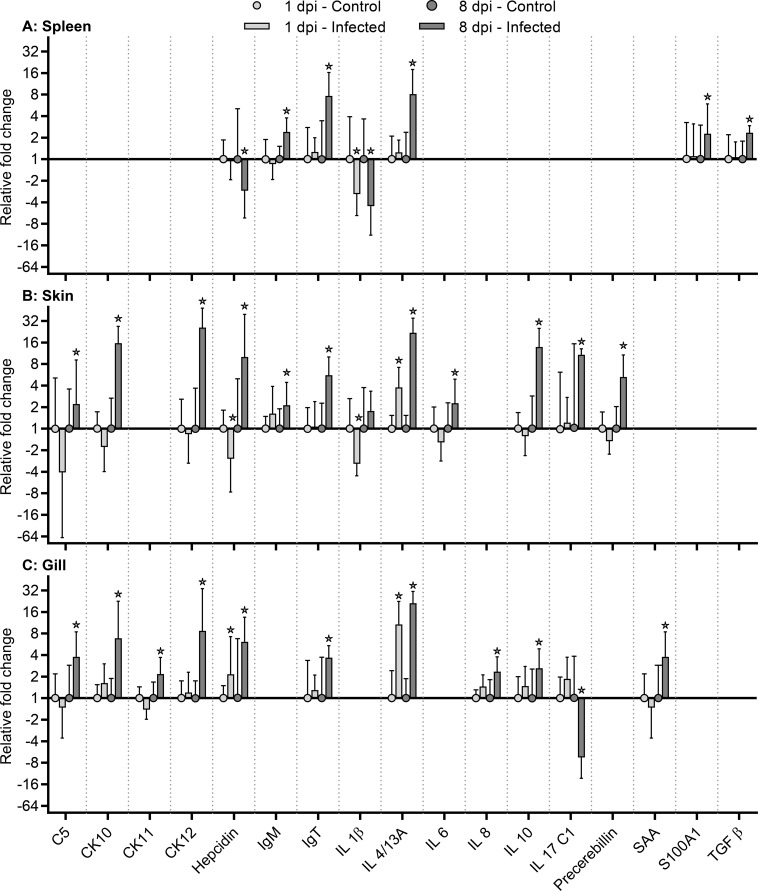
Fig 3. Gene expression analysis.
Relative fold change was calculated as 2-ΔΔCq. Due to their exponential nature, the geometric mean and geometric standard deviation were used. The EF1α was used as the reference gene (housekeeper). Only significant results are shown. A comprehensive summary of the gene expression study, including all the genes investigated, is present as supplementary material S3 Table. *: p<0.05 (Student’s t-test), fold change >2.
Fig 4. Heatmap showing the relative gene expression of immune associated genes in skin, gills, and spleen of rainbow trout, at different time points.
Infected fish compared to time point control fish. Red and blue indicate up-regulation and down-regulation, respectively. *: significant different from control group, p<0.05. The hierarchical clustering orders the rows based on similarity of expression levels (log2 fold change). The dendrogram indicates both similarity and the order that cluster are formed.
Cytokines
The expression of the gene encoding IL-1β was significantly decreased in the spleen at both time points. Its expression was also significantly decreased in the skin at day 1 pi (but not regulated at day 8 pi) whereas no significant regulation was observed in gills at any time point. The gene encoding IL-6 was significantly up-regulated in the skin at day 8 pi, but not at day 1 pi. Expression of the IL-6 gene did not differ between gills and spleen. The gene encoding IL-17/C1 showed significant up-regulation in the skin of infected fish at day 8 pi contrasting the down-regulation found in gills of infected fish. No significant regulation of the IL-17/C1 gene was seen in any of the tissues at day 1 after infection and in spleen during the experiment. A significant regulation of the gene encoding IL-4/13A was seen both in gills and skin, both at day 1 and 8 pi, but it was only significantly increased at day 8 pi in spleen. The gene encoding IL-10 was also significantly up-regulated in skin and gills at 8 dpi but not at 1 dpi and in spleen (both time points). Expression of the TGFβ gene was only significantly increased in the spleen at day 8 post-infection and not regulated in skin and gills at any time point. No significant regulation of genes encoding IL-17/C2, IL-17A/F2, and TNFα was observed for any of the tissues during the infection.
Complement factors
Two genes encoding complement factors (C3 and C5) were analyzed in this study, but only the C5 gene showed a significant regulation after infection as it was significantly up-regulated in skin and gills at day 8 pi but not at day 1. No significant changes of the C3 and C5 gene expression were observed in the spleen.
Chemokines
The genes encoding chemokines CK10 and CK12 and IL-8 were significantly expressed in the gill and skin of infected fish at day 8 pi but no significant regulation was seen in the spleen during infection. Expression of the CK11 gene was significantly up-regulated in gills of infected fish at day 8 pi, whereas no regulation of CK9 and CK11 genes in examined skin and spleen tissues was recorded.
Acute phase proteins
Regulation of the gene encoding hepcidin exhibited higher variability than the other acute phase proteins (precerebellin and serum amyloid A SAA). A significant increase in expression of the hepcidin gene was observed in the gills of infected fish at all sampling points and in skin at day 8. However, it was down-regulated in skin at day 1 pi and in spleen day 8 pi. A significant increase in expression of the precerebellin gene was only found in the skin of infected fish at day 8 pi whereas this gene showed a stable expression in gills and spleen during infection. A significant upregulation of the gene encoding SAA was found in gills of infected fish at day 8 pi, but not activated in skin and spleen at all. Similar to the cytokine TGFβ gene, the expression of the gene encoding S100A1 (involved in neurotransmitter signaling) was merely significantly elevated in the spleen at day 8 after infection, and not regulated in skin and gills at any sampling point.
Immunoglobulins and cellular receptors
The expression of immunoglobulins was clearly increased in the central immune organ compared to both skin and gills. A significant increase of the immunoglobulin T (IgT) gene transcription in examined tissues was observed especially at day 8 pi. The gene encoding IgM was up-regulated at day 8 pi in spleen and skin but in gills merely a slight IgM gene regulation was indicated. Significant regulations (more than 2-fold) for the genes encoding T cell receptors CD4 and CD8 were not observed.
Discussion
Rainbow trout responded, both at external surfaces and in the central immune organ spleen, to infection with the ciliated protozoan I. multifiliis by regulating both innate and adaptive immune genes. The reaction was correlated to the increasing severity of infection as the parasites increased their volume from theront size (30–40 μm) to maximum trophont size (> 500 μm) during the course of infection. In addition to the increased size of the parasite it is suggested that the elevated feeding and presentation of antigens from the growing trophont stimulate the infected tissue. Thus, I. multifiliis ciliates possess protein structures termed immobilization antigens (I-antigens) covering up to 60% of their surface [14]. Merely a few immune-related genes were regulated at day 1 pi compared to day 8 pi, at which time point these immune parameters dramatically changed. Regulation of immune genes in affected host organs have previously been reported [7, 15] but the present study demonstrated that gills (harbouring a higher infection) responded significantly stronger compared to skin having a lower parasite burden (as judged from the I-antigen expression). Antigen presenting cells in rainbow trout gills are sentinels for external stimuli [16] and gills may in general respond stronger to stimulation compared to skin. Exposure of trout to the bacterial pathogen Yersinia ruckeri elicited higher immune gene expression in gills [17] corresponding to findings in other fish models (non-infected) including miiuy croaker [18] and Japanese pufferfish [19].
In addition, the response in the spleen, which did not harbor any infection at all, was primarily concentrated about genes encoding immunoglobulins (IgM and IgT) and cytokines associated with humoral immunity (IL-4/13). This indicates that local protective responses raised in the mucosal surfaces (gills, skin) can transmit signals to central immune organs establishing a systemic response few days after exposure. Candidates involved in signaling between gills/skin and the spleen may be genes encoding chemokines CK10 and CK12 and the cytokine IL-4/13A. These genes were highly upregulated in gills and skin at day 8 concomitant with immunoglobulin gene upregulation in spleen. Despite the importance of a systemic protection based on mainly immunoglobulin IgM and IgT production [8, 20] a local reaction involving a series of innate factors is associated with both the primary and secondary response to the infection [4].
In this context it is noteworthy that genes expressed both in gills and skin encoded chemokines (CK10, CK12), APPs (hepcidin, SAA), cytokines (IL-4/13A, IL-17A/F2), and immunoglobulins (IgM, IgT) which indicate their role in the barrier function (early and late) against penetrating pathogens [21]. Also at the systemic level immune genes encoding complements (C3, C5), APPs (hepcidin, precerebellin, SAA, S100A1), cytokines IL-10, cell receptors (CD4, CD8), were expressed although at a lower level compared immunoglobulin genes. However, again the extrahepatic expression of complement varied considerably between tissues, showing that complement factor genes were mainly expressed in gills.
Some cytokine genes were mainly down-regulated including IL-1β, an important pro-inflammatory cytokine in fish [13]. Although this cytokine may play a role in the initiation of the early immune response and has multiple effects on gene expression during inflammation [22], IL-1β expression was likely suppressed in rainbow trout at later stages of the infection. Depression of pro-inflammatory cytokine production in rainbow trout macrophages infected with Renibacterium salmoninarum was previously demonstrated [23] and it cannot be excluded that a corresponding regulations is associated with I. multifiliis infection and at least partly connected to cortisol elevation [24–26]. Also TNFα, considered as an important component in the inflammatory response in fish [27] and activated in rainbow trout after i.p. injection of live theronts of I. multifiliis [15], was not induced by infection. Genes encoding cytokines IL-6 and IL-17 C1 were the only of pro-inflammatory cytokine genes slightly up-regulated in the skin at day 8 pi corresponding to the Ichthyobodo induced skin response after severe epidermal emaciation [28]. At the same time suppression of IL-17 C1 was found in gills at day 8 even though it showed a weak up-regulation at early of infection, which will frame the different responsiveness of gills and skin.
The complement system [29] is an essential part of the innate immune system [30] in alerting the host of the presence of potential pathogens [31] and playing a crucial role in the response or resistance against Ich [5, 32, 33]. In the present study mainly complement factor C5 played a role in the physical barriers skin and gills corresponding to previous studies of this parasite-host model [6, 7, 15] and against the bacterial pathogen Yersinia ruckeri [34]. C3 expression was less prominent in this work contrasting previous work showing high expression in liver, head kidney, skin, gill, and spleen [5–7, 15].
Chemokines are secreted immune factors attracting a diverse set of effector leukocytes to inflammatory sites [35]. The chemokines IL-8, CK10, CK11, and CK12 were significantly regulated in skin and gills at day 8 after infection and as previously shown the expression of chemokine genes in response to infection may differ between tissues [36–38]. In general regulation of chemokine genes CK10, CK11, and CK12 occurs during viral infection of rainbow trout [38, 39] and as shown by Munoz-Atienza et al. [40] CK11 may have direct antiparasitic effects and act as the first line of defense against infection.
The present study showed that some of the regulating or immune-modulatory cytokines such as IL-10 and TGFβ were activated. This would provide a control of pro-inflammatory actions during infection [41] limiting the potentially injurious effects elicited by excess inflammatory reactions [42]. Regulating cytokines such as IL-4/13A can suppress pro-inflammatory cytokine genes (such as IL-1 and TNF), and at the same time direct adaptive immune pathways involving B lymphocytes [43–46]. Interleukin IL-4 and IL-13 are closely related cytokines important for Th2 responses especially for defense against parasites [47].
The expression of acute phase response genes may have been induced by plasma-borne signals [48] some of which may stimulate leukocytes and hepatocytes to release acute phase proteins (APPs) into the bloodstream [29, 49]. Genes encoding serum Amyloid A (SAA), precerebellin, and Hepcidin were slightly elevated after infection in the present study. SAA has been suggested to directly influence the parasitic ciliate Ichthyophthirius multifiliis [6, 50] and the flagellate Ichthyobodo necator [28]. Expression of precerebellin which is part of the acute phase response in rainbow trout [51, 52] was elevated significantly in the skin, but involved to a lower extent in the gills and spleen. The link between inflammation and production of hepcidin has been demonstrated by previous studies [53–56]. During inflammation, the cytokine IL-6, a mediator of acute phase reaction [57], induces production of hepcidin [29] also in extrahepatic organs such as gills and skin as judged from the present work.
Activation of the gene encoding S100A1 in the spleen is less clear but it is indicated that it is part of the inflammatory response as suggested by zebrafish studies [58]. However, other functions may be exerted by the molecule S100A1 as it is a member of the S100 protein family expressed only in vertebrates [59], is calcium-binding, involved in plasma membrane transport [60] and essential in the acute response to hemodynamic stress [61]. In addition, in mammals the extracellular S100 proteins exert regulatory activities on immune cells, thereby participating in innate and adaptive immune responses, cell migration and chemotaxis, tissue development and repair, and leukocyte and tumor cell invasion [59].
Conclusion
The establishment of an immune response towards I. multifiliis occurs both at a local and a systemic level as seen by expression of immune genes in all examined tissue. Expression of immune genes in gills and skin followed the same pattern although gills showed a higher expression level suggesting that gills are more exposed or play a role as sentinels for external stimulation including exposure to pathogens. Despite absence of pathogens in the spleen at any time point the spleen showed elevated immune gene expression especially with regard to immunoglobulin production which suggests that signaling, associated with chemokines and cytokines, from infected mucosal surfaces may reach the spleen and establish a systemic protective response.
Supporting information
(PDF)
Correlation value and p-value are provided.
(XLSX)
(XLSX)
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This work was supported by the European Union as a Horizon 2020 project Parafishcontrol (www.parafishcontrol.eu) grant No. 634429 received by KB. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Dickerson HW, Findly RC. Immunity to Ichthyophthirius infections in fish: a synopsis. Developmental and comparative immunology. 2014;43(2):290–9. Epub 2013/07/03. 10.1016/j.dci.2013.06.004 . [DOI] [PubMed] [Google Scholar]
- 2.Wang Q, Yu Y, Zhang X, Xu Z. Immune responses of fish to Ichthyophthirius multifiliis (Ich): A model for understanding immunity against protozoan parasites. Developmental and comparative immunology. 2019;93:93–102. Epub 2019/01/11. 10.1016/j.dci.2019.01.002 . [DOI] [PubMed] [Google Scholar]
- 3.Jørgensen LVG. The fish parasite Ichthyophthirius multifiliis—Host immunology, vaccines and novel treatments. Fish Shellfish Immunol. 2017;67:586–95. Epub 2017/06/22. 10.1016/j.fsi.2017.06.044 . [DOI] [PubMed] [Google Scholar]
- 4.Jørgensen LVG, Korbut R, Jeberg S, Kania PW, Buchmann K. Association between adaptive immunity and neutrophil dynamics in zebrafish (Danio rerio) infected by a parasitic ciliate. PLoS One. 2018;13(9):e0203297 Epub 2018/09/12. 10.1371/journal.pone.0203297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heidarieh M, Diallo A, Moodi S, Taghinejad V, Akbari M, Monfaredan A. Gene expression analysis in rainbow trout (Oncorhynchus mykiss) skin: immunological responses to radiovaccine against Ichthyophthirius multifiliis. Revue Med Vet. 2015;166(7–8):233–42. [Google Scholar]
- 6.Olsen MM, Kania PW, Heinecke RD, Skjoedt K, Rasmussen KJ, Buchmann K. Cellular and humoral factors involved in the response of rainbow trout gills to Ichthyophthirius multifiliis infections: molecular and immunohistochemical studies. Fish Shellfish Immunol. 2011;30(3):859–69. Epub 2011/01/29. 10.1016/j.fsi.2011.01.010 . [DOI] [PubMed] [Google Scholar]
- 7.Sigh J, Lindenstrom T, Buchmann K. The parasitic ciliate Ichthyophthirius multifiliis induces expression of immune relevant genes in rainbow trout, Oncorhynchus mykiss (Walbaum). Journal of fish diseases. 2004;27(7):409–17. Epub 2004/07/02. 10.1111/j.1365-2761.2004.00558.x . [DOI] [PubMed] [Google Scholar]
- 8.Xu Z, Parra D, Gomez D, Salinas I, Zhang YA, von Gersdorff Jorgensen L, et al. Teleost skin, an ancient mucosal surface that elicits gut-like immune responses. Proc Natl Acad Sci U S A. 2013;110(32):13097–102. Epub 2013/07/26. 10.1073/pnas.1304319110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu D-H, Moreira GSA, Shoemaker CA, Zhang D, Beck BH. Expression of immune genes in systemic and mucosal immune tissues of channel catfish vaccinated with live theronts of Ichthyophthirius multifiliis. Fish & Shellfish Immunology. 2017;66:540–7. 10.1016/j.fsi.2017.05.051 [DOI] [PubMed] [Google Scholar]
- 10.Xu DH, Zhang QZ, Shoemaker CA, Zhang D, Moreira GS. Molecular immune response of channel catfish immunized with live theronts of Ichthyophthirius multifiliis. Fish Shellfish Immunol. 2016;54:86–92. Epub 2016/04/06. 10.1016/j.fsi.2016.03.166 . [DOI] [PubMed] [Google Scholar]
- 11.Dickerson HW, Clark TG. Immune response of fishes to ciliates. Annual Review of Fish Diseases. 1996;6:107–20. [Google Scholar]
- 12.Syahputra K, Kania PW, Al-Jubury A, Jafaar RM, Dirks RP, Buchmann K. Transcriptomic analysis of immunity in rainbow trout (Oncorhynchus mykiss) gills infected by Ichthyophthirius multifiliis. Fish Shellfish Immunol. 2019;86:486–96. Epub 2018/12/05. 10.1016/j.fsi.2018.11.075 . [DOI] [PubMed] [Google Scholar]
- 13.Sigh J, Lindenstrom T, Buchmann K. Expression of pro-inflammatory cytokines in rainbow trout (Oncorhynchus mykiss) during an infection with Ichthyophthirius multifiliis. Fish Shellfish Immunol. 2004;17(1):75–86. Epub 2004/05/18. 10.1016/j.fsi.2003.12.005 . [DOI] [PubMed] [Google Scholar]
- 14.Xu C, Clark TG, Leff AA, Dickerson HW. Analysis of the Soluble and Membrane-bound Immobilization Antigens of Ichthyophthirius multifiliis. The Journal of Eukaryotic Microbiology. 1995;42(5):558–64. 10.1111/j.1550-7408.1995.tb05905.x [DOI] [Google Scholar]
- 15.Jørgensen LVG, Nemli E, Heinecke RD, Raida MK, Buchmann K. Immune-relevant genes expressed in rainbow trout following immunisation with a live vaccine against Ichthyophthirius multifiliis. Dis Aquat Organ. 2008;80(3):189–97. Epub 2008/09/26. 10.3354/dao01935 . [DOI] [PubMed] [Google Scholar]
- 16.Kato G, Miyazawa H, Nakayama Y, Ikari Y, Kondo H, Yamaguchi T, et al. A Novel Antigen-Sampling Cell in the Teleost Gill Epithelium With the Potential for Direct Antigen Presentation in Mucosal Tissue. Front Immunol. 2018;9:2116 Epub 2018/10/09. 10.3389/fimmu.2018.02116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Skov J, Chettri JK, Jaafar RM, Kania PW, Dalsgaard I, Buchmann K. Effects of soluble immunostimulants on mucosal immune responses in rainbow trout immersion-vaccinated against Yersinia ruckeri. Aquaculture. 2018;492:237–46. 10.1016/j.aquaculture.2018.04.011 [DOI] [Google Scholar]
- 18.Yang Q, Sun Y, Su X, Li T, Xu T. Characterization of six IL-17 family genes in miiuy croaker and evolution analysis of vertebrate IL-17 family. Fish Shellfish Immunol. 2016;49:243–51. Epub 2016/01/02. 10.1016/j.fsi.2015.12.031 . [DOI] [PubMed] [Google Scholar]
- 19.Korenaga H, Kono T, Sakai M. Isolation of seven IL-17 family genes from the Japanese pufferfish Takifugu rubripes. Fish Shellfish Immunol. 2010;28(5–6):809–18. Epub 2010/02/11. 10.1016/j.fsi.2010.01.016 . [DOI] [PubMed] [Google Scholar]
- 20.Jørgensen LVG, Heinecke RD, Skjodt K, Rasmussen KJ, Buchmann K. Experimental evidence for direct in situ binding of IgM and IgT to early trophonts of Ichthyophthirius multifiliis (Fouquet) in the gills of rainbow trout, Oncorhynchus mykiss (Walbaum). Journal of fish diseases. 2011;34(10):749–55. Epub 2011/09/16. 10.1111/j.1365-2761.2011.01291.x . [DOI] [PubMed] [Google Scholar]
- 21.Matthews RA. Ichthyophthirius multifiliis Fouquet and Ichthyophthiriosis in Freshwater Teleosts. Advances in Parasitology Volume 59 Advances in Parasitology2005. p. 159–241. 10.1016/S0065-308X(05)59003-1 [DOI] [PubMed] [Google Scholar]
- 22.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–50. Epub 2009/03/24. 10.1146/annurev.immunol.021908.132612 . [DOI] [PubMed] [Google Scholar]
- 23.Grayson TH, Cooper LF, Wrathmell AB, Roper J, Evenden AJ, Gilpin ML. Host responses to Renibacterium salmoninarum and specific components of the pathogen reveal the mechanisms of immune suppression and activation. Immunology. 2002;106:273–83. 10.1046/j.1365-2567.2002.01420.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stoltze K, Buchmann K. Effect of Gyrodactylus derjavini infections on cortisol production in rainbow trout fry. Journal of Helminthology. 2001;75:291–4. 10.1079/JOH200157 [DOI] [PubMed] [Google Scholar]
- 25.Philip AM, Vijayan MM. Stress-Immune-Growth Interactions: Cortisol Modulates Suppressors of Cytokine Signaling and JAK/STAT Pathway in Rainbow Trout Liver. PLoS One. 2015;10(6):e0129299 Epub 2015/06/18. 10.1371/journal.pone.0129299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shepherd BS, Spear AR, Philip AM, Leaman DW, Stepien CA, Sepulveda-Villet OJ, et al. Effects of cortisol and lipopolysaccharide on expression of select growth-, stress- and immune-related genes in rainbow trout liver. Fish Shellfish Immunol. 2018;74:410–8. Epub 2018/01/13. 10.1016/j.fsi.2018.01.003 . [DOI] [PubMed] [Google Scholar]
- 27.Secombes CJ, Wang N, Hong S, Peddie S, Crampe M, Laing KJ, et al. Cytokines and innate immunity of fish. Developmental and comparative immunology. 2001;25:713–23. [DOI] [PubMed] [Google Scholar]
- 28.Chettri JK, Kuhn JA, Jaafar RM, Kania PW, Moller OS, Buchmann K. Epidermal response of rainbow trout to Ichthyobodo necator: immunohistochemical and gene expression studies indicate a Th1-/Th2-like switch. Journal of fish diseases. 2014;37(9):771–83. Epub 2013/08/21. 10.1111/jfd.12169 . [DOI] [PubMed] [Google Scholar]
- 29.Wood P. Understanding Immunology. 3rd ed: Pearson Education Limited, England; 2011. [Google Scholar]
- 30.Holland MCH, Lambris JD. The complement system in teleosts. Fish & Shellfish Immunology. 2002;12(5):399–420. 10.1006/fsim.2001.0408 [DOI] [PubMed] [Google Scholar]
- 31.Boshra H, Li J, Sunyer JO. Recent advances on the complement system of teleost fish. Fish Shellfish Immunol. 2006;20(2):239–62. Epub 2005/06/14. 10.1016/j.fsi.2005.04.004 . [DOI] [PubMed] [Google Scholar]
- 32.Buchmann K, Lindenstrøm T, Sigh J. Partial cross protection against Ichthyophthirius multifiliis in Gyrodactylus derjavini immunized rainbow trout. Journal of Helminthology. 1999;73(03):189–95. 10.1017/s0022149x9900030x [DOI] [PubMed] [Google Scholar]
- 33.Buchmann K, Sigh J, Nielsen CV, Dalgaard M. Host responses against the fish parasitizing ciliate Ichthyophthirius multifiliis. Veterinary Parasitology. 2001;100:105–16. [DOI] [PubMed] [Google Scholar]
- 34.Chettri JK, Raida MK, Kania PW, Buchmann K. Differential immune response of rainbow trout (Oncorhynchus mykiss) at early developmental stages (larvae and fry) against the bacterial pathogen Yersinia ruckeri. Developmental and comparative immunology. 2012;36(2):463–74. Epub 2011/09/29. 10.1016/j.dci.2011.08.014 . [DOI] [PubMed] [Google Scholar]
- 35.Esche C, Stellato C, Beck LA. Chemokines: key players in innate and adaptive immunity. J Invest Dermatol. 2005;125(4):615–28. Epub 2005/09/28. 10.1111/j.0022-202X.2005.23841.x . [DOI] [PubMed] [Google Scholar]
- 36.Bird S, Tafalla C. Teleost Chemokines and Their Receptors. Biology (Basel). 2015;4(4):756–84. Epub 2015/11/17. 10.3390/biology4040756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Castro R, Abos B, Pignatelli J, von Gersdorff Jorgensen L, Gonzalez Granja A, Buchmann K, et al. Early immune responses in rainbow trout liver upon viral hemorrhagic septicemia virus (VHSV) infection. PLoS One. 2014;9(10):e111084 Epub 2014/10/23. 10.1371/journal.pone.0111084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Montero J, Garcia J, Ordas MC, Casanova I, Gonzalez A, Villena A, et al. Specific regulation of the chemokine response to viral hemorrhagic septicemia virus at the entry site. J Virol. 2011;85(9):4046–56. Epub 2011/02/18. 10.1128/JVI.02519-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ballesteros NA, Rodriguez Saint-Jean S, Perez-Prieto SI, Aquilino C, Tafalla C. Modulation of genes related to the recruitment of immune cells in the digestive tract of trout experimentally infected with infectious pancreatic necrosis virus (IPNV) or orally vaccinated. Developmental and comparative immunology. 2014;44(1):195–205. Epub 2013/12/29. 10.1016/j.dci.2013.12.009 . [DOI] [PubMed] [Google Scholar]
- 40.Munoz-Atienza E, Aquilino C, Syahputra K, Al-Jubury A, Araujo C, Skov J, et al. CK11, a Teleost Chemokine with a Potent Antimicrobial Activity. J Immunol. 2019;202(3):857–70. Epub 2019/01/06. 10.4049/jimmunol.1800568 . [DOI] [PubMed] [Google Scholar]
- 41.Munoz C, Carlet J, Fitting C, Misset B, Bieriot J-P, Cavaillon J-m. Dysregulation of in vitro cytokine production by monocytes during sepsis. J Clin Invest. 1991;88:1747–54. 10.1172/JCI115493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Opal SM, DePalo VA. Anti-Inflammatory Cytokines. Chest. 2000;117(4):1162–72. 10.1378/chest.117.4.1162 [DOI] [PubMed] [Google Scholar]
- 43.Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–93. 10.1038/383787a0 [DOI] [PubMed] [Google Scholar]
- 44.D'Amico G, Frascaroli G, Bianchi G, Transidico P, Doni A, Vecchi A, et al. Uncoupling of inflammatory chemokine receptors by IL-10: generation of functional decoys. Nature immunology. 2000;1(5):387–91. 10.1038/80819 [DOI] [PubMed] [Google Scholar]
- 45.Dinarello CA. Proinflammatory Cytokines. Chest. 2000;118(2):503–8. 10.1378/chest.118.2.503 [DOI] [PubMed] [Google Scholar]
- 46.Zou J, Secombes CJ. The Function of Fish Cytokines. Biology (Basel). 2016;5(23):1–35. Epub 2016/05/28. 10.3390/biology5020023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takizawa F, Koppang EO, Ohtani M, Nakanishi T, Hashimoto K, Fischer U, et al. Constitutive high expression of interleukin-4/13A and GATA-3 in gill and skin of salmonid fishes suggests that these tissues form Th2-skewed immune environments. Mol Immunol. 2011;48(12–13):1360–8. Epub 2011/04/15. 10.1016/j.molimm.2011.02.014 . [DOI] [PubMed] [Google Scholar]
- 48.Bayne CJ, Gerwick L. The acute phase response and innate immunity of fish. Developmental and comparative immunology. 2001;25:725–43. [DOI] [PubMed] [Google Scholar]
- 49.Murata H, Shimada N, Yoshioka M. Current research on acute phase proteins in veterinary diagnosis: an overview. The Veterinary Journal. 2004;168(1):28–40. 10.1016/S1090-0233(03)00119-9 [DOI] [PubMed] [Google Scholar]
- 50.Gonzalez SF, Buchmann K, Nielsen ME. Ichthyophthirius multifiliis infection induces massive up-regulation of serum amyloid A in carp (Cyprinus carpio). Vet Immunol Immunopathol. 2007;115(1–2):172–8. Epub 2006/11/11. 10.1016/j.vetimm.2006.09.007 . [DOI] [PubMed] [Google Scholar]
- 51.Gerwick L, Reynolds WS, Bayne CJ. A precerebellin-like protein is part of the acute phase response in rainbow trout, Oncorhynchus mykiss. Developmental & Comparative Immunology. 2000;24(6–7):597–607. 10.1016/S0145-305X(00)00016-1. [DOI] [PubMed] [Google Scholar]
- 52.Gerwick L, Steinhauer R, Lapatra S, Sandell T, Ortuno J, Hajiseyedjavadi N, et al. The acute phase response of rainbow trout (Oncorhynchus mykiss) plasma proteins to viral, bacterial and fungal inflammatory agents. Fish & Shellfish Immunology. 2002;12(3):229–42. 10.1006/fsim.2001.0367 [DOI] [PubMed] [Google Scholar]
- 53.Ganz T., Hepcidin a key regulator of iron metabolism and mediator of anemia of inflammation. Blood. 2003;102(3):783–8. Epub 2003/03/29. 10.1182/blood-2003-03-0672 . [DOI] [PubMed] [Google Scholar]
- 54.Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan AW, D. M., Ganz T, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090–3. 10.1126/science.1104742 [DOI] [PubMed] [Google Scholar]
- 55.Raida MK, Buchmann K. Development of adaptive immunity in rainbow trout, Oncorhynchus mykiss (Walbaum) surviving an infection with Yersinia ruckeri. Fish Shellfish Immunol. 2008;25(5):533–41. Epub 2008/08/19. 10.1016/j.fsi.2008.07.008 . [DOI] [PubMed] [Google Scholar]
- 56.Singh B, Arora S, Gupta SK, Saxena A. Role of hepcidin in dysregulation of iron metabolism and anemia of chronic diseases, Iron Metabolism: InTech; 2012. 186 p. [Google Scholar]
- 57.Engelsma MY, Huising MO, van Muiswinkel WB, Flik G, Kwang J, Savelkoul HFJ, et al. Neuroendocrine-immune interactions in fish: a role for interleukin-1. Veterinary Immunology and Immunopathology. 2002;87:467–79. [DOI] [PubMed] [Google Scholar]
- 58.Haarder S, Kania PW, Lindebo Holm T, Ohtani M, Buchmann K. Comparison of two chemically-induced colitis-models in adult zebrafish, using optical projection tomography and novel transcriptional markers. Open Journal of Immunology. 2016;06(04):154–80. 10.4236/oji.2016.64016 [DOI] [Google Scholar]
- 59.Donato R, Cannon BR, Sorci G, Riuzzi F, Hsu K, Weber DJ, et al. Functions of S100 proteins. Curr Mol Med. 2013;13(1):24–57. [PMC free article] [PubMed] [Google Scholar]
- 60.Rescher U, Gerke V. S100A10/p11: family, friends and functions. Pflugers Arch. 2008;455(4):575–82. Epub 2007/07/20. 10.1007/s00424-007-0313-4 . [DOI] [PubMed] [Google Scholar]
- 61.Duarte-Costa S, Castro-Ferreira R, Neves JS, Leite-Moreira AF. S100A1: a Major Player in Cardiovascular Performance. Physiol Res. 2014;63:669–81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
Correlation value and p-value are provided.
(XLSX)
(XLSX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.