Abstract
Blunted reward sensitivity and life stress are each depressogenic. Additionally, individuals with clinical and psychosocial vulnerabilities are prone to experience or evoke dependent life stressors (e.g., interpersonal conflict) that, in turn, increase depression risk. However, no previous study has investigated the role of neural vulnerability factors in generating life stress. Therefore, the current study investigated whether a neural measure of reward sensitivity prospectively predicts the generation of life stress, which in turn mediates effects of these neural processes on subsequent depression. Participants were 467 never-depressed adolescent girls. Using event-related potentials, neural sensitivity to the difference between monetary reward and loss (the Reward Positivity [RewP]) was assessed at baseline. Negative life events were assessed twice via interview over the ensuing 18 months, yielding an index of total life stress over the follow-up period. A self-report dimensional measure of depression symptoms was administered at baseline and follow-up. After accounting for baseline age, depression, and race, a blunted RewP predicted greater dependent, but not independent, life stress over the follow-up. Mediation analyses revealed a significant indirect effect of the RewP on follow-up depression through dependent, but not independent, life stress. Our results suggest that neural processing reward and loss plays a crucial role in depressogenic stress generation.
Keywords: Stress generation, depression, life stress, reward processing, Reward Positivity (RewP)
General Scientific Summary:
The present study demonstrates that a blunted neural response to reward predicts the occurrence of behaviorally-dependent stressful life events over the subsequent 18-months, and that this “stress generation” effect partially explains the association between neural reward dysfunction and later depression. These findings provide insight into one mechanism by which a blunted response to reward may contribute to the development of later depression.
Neural response to reward and future life stress: Stress generation pathway to depression Numerous studies indicate that life stress is implicated in the etiology of depressive disorders (Brown, 1978; Kendler, Karkowski, & Prescott, 1999). Dependent life events, in which the individual’s actions may have contributed to the occurrence of the event (e.g., a relationship ending), are particularly influential in the onset of depression and tend to have a greater impact on depressive disorders than independent events (e.g., relocating to a new area due to a change in parent’s job; Kendler et al., 1999). Stressful life events increase during adolescence (Ge, Lorenz, Conger, Elder, & Simons, 1994; Rudolph & Hammen, 1999), and this increase in life stress contributes to the increased rates of depression during this time (Ge et al., 1994). This is especially important for adolescent females due to their greater exposure (Ge, Conger, & Elder, 2001; Hankin, Mermelstein, & Roesch, 2007; Rudolph & Hammen, 1999) and greater depressive vulnerability to dependent life events (Rudolph & Hammen, 1999) compared to male adolescents.
Stress Generation
Hammen’s stress generation model posits that the relationship between life stress and depression is bidirectional; while increases in life stress confer risk for the development and exacerbation of depression, risk factors for, and symptoms of, depression increase the likelihood that an individual will experience stressful life events (Hammen, 1991, 2006). Specifically, the stress generation model hypothesizes that depressed individuals behave in ways that contribute to the occurrence of additional stressors (i.e., they generate dependent life events; Hammen, 1991), which in turn maintain depression or increase the risk of relapse. Investigation of the stress generation hypothesis has demonstrated that depressed adolescents and adults experience more dependent stressful life events than their healthy peers (Hammen, 1991; Liu & Alloy, 2010). Moreover, the stress generation model has been extended beyond active symptoms to include a number of risk factors for the development of psychopathology, such as personality traits (Kercher, Rapee, & Schniering, 2009), cognitive styles (Safford, Alloy, Abramson, & Crossfield, 2007), coping styles (Holahan, Moos, Holahan, Brennan, & Schutte, 2005) and problem solving (Davila, Hammen, Burge, Paley, & Daley, 1995); for a review of stress generation, see (Liu & Alloy, 2010). Only two studies have examined biological measures that predict stress generation. Pupil dilation in response to angry faces in the offspring of depressed, but not never-depressed, mothers (Feurer, Burkhouse, Siegle, & Gibb, 2017) and serotonin transporter gene polymorphisms (5-HTTLPR; Starr, Hammen, Brennan, & Najman, 2013) prospectively predicted the generation of dependent life events. Fewer studies have addressed the second part of the stress generation model, but those that have found support for the indirect effect of risk factors on subsequent depression via stress generation (Davila et al., 1995; Hammen, Davila, Brown, Ellicott, & Gitlin, 1992; Hammen, Shih, & Brennan, 2004; Hankin, Kassel, & Abela, 2005; Holahan et al., 2005).
Associations Between Depression and Neural Response to Reward Versus Loss
Abnormalities in neural processing of reward are also associated with concurrent and future depression (Goldstein & Klein, 2014; Keren et al., 2018). For example, fMRI studies have demonstrated that blunted neural activation in response to rewards is associated with depression in adults (Pizzagalli et al., 2009) and prospectively predicts depressive onsets and symptoms during adolescence (Stringaris et al., 2015).
Event-related potential (ERP) investigations have also provided evidence that abnormalities in the processing of gain and loss are related to depression symptoms. The Reward Positivity (RewP; also named the Feedback Negativity [FN] and Feedback-Related Negativity [FRN]) is an ERP component reflecting neural sensitivity to the difference between reward and loss and is scored as the neural response to gain minus loss. It is a positive deflection in the ERP signal occurring approximately 250–350 ms following feedback indicating monetary gain or loss, and is larger in response to gains due to the absence of a response to loss feedback (Proudfit, 2015). Research has indicated that a blunted RewP is cross-sectionally associated with, and prospectively predicts, depressive symptoms and episodes during childhood and adolescence (Belden et al., 2016; Bress, Foti, Kotov, Klein, & Hajcak, 2013; Bress, Meyer, & Proudfit, 2015; Bress, Smith, Foti, Klein, & Hajcak, 2012; Keren et al., 2018; Nelson, Perlman, Klein, Kotov, & Hajcak, 2016). Thus, the literature on the RewP indicates that individuals with, or at risk for, depression exhibit diminished sensitivity to the difference between gain and loss.
Life Stress and Reward Dysfunction
Life stress, including early childhood maltreatment, disrupts neural processing of reward (Admon et al., 2012; Auerbach, Admon, & Pizzagalli, 2014; Casement et al., 2014; McCrory, Gerin, & Viding, 2017; Novick et al., 2018), and these neurodevelopmental changes in reward processing influence later behavior (Masten & Cicchetti, 2010). However, there is a dearth of research investigating the converse relationship; that is, whether neural processing of reward and loss influences the occurrence of subsequent life stressors. Auerbach and colleagues (2014) recently hypothesized that abnormalities in the processing of reward and loss contribute to the generation of life stress due to their association with approach and avoidance behaviors. Adolescents exhibiting avoidance-related deficits may withdraw from social situations, eroding relationships with peers and family, whereas teens demonstrating approach-related abnormalities may fail to recognize opportunities to engage in rewarding or enjoyable activities (Auerbach et al., 2014). Hence, abnormalities in the processing of rewards and loss have the potential to contribute to the generation of life stress.
The Current Study
While previous studies have provided evidence implicating a blunted RewP as a neural marker of vulnerability for adolescent depression (Nelson et al., 2016), it is plausible that this is, in part, due to the role of reward processing in stress generation (Auerbach et al., 2014). However, we are not aware of any studies prospectively investigating individual differences in neural response to reward in predicting subsequent life events. The goal of the current study is to examine whether neural sensitivity to reward and loss, as indexed by the RewP, prospectively predicts the occurrence of dependent and independent stressful life events over the subsequent 18 months in a large sample of adolescent girls. Consistent with the stress generation model, we hypothesized that abnormalities in processing of reward and loss would predict subsequent dependent, but not independent, life stress, although we did not have specific hypotheses as to whether stress generation effects would be driven more by neural response to gain, loss, or the difference between the two. Moreover, as the stress generation model posits that generation of life stress leads to subsequent depression, we also tested whether stress generation mediated the effects of neural sensitivity to the difference between reward and loss on subsequent depression symptoms. Our study extends prior stress generation investigations by examining a neural risk factor for depression on subsequent dependent life stress. In order to control for any pre-existing stress generation effects on depression, we adjusted for baseline levels of depression symptoms in all analyses. Because participant race was significantly associated with life stress in our sample, we controlled for race in all analyses. Finally, due to age-related changes in rates of life stress (Ge et al., 1994) and neural reward system development during adolescence (Galvan, 2010), age was included as a covariate in all models.
Method
Participants
The sample was drawn from a cohort of 550 adolescent females and their parents who were recruited from the community to participate in a study of predictors of first-incident depression1. Eligibility requirements included being female, between 13.5 and 15.5 years of age, fluency in English, and a co-participating biological parent. Exclusion criteria included a lifetime history of major depressive disorder or dysthymia, because the study aimed to predict first-onset depression, or developmental disabilities. Parents and adolescents provided informed consent and assent, respectively, and the Stony Brook University Institutional Review Board approved the study. All families were compensated for their participation.
The analysis sample for this report included 467 adolescent girls with an average age of 14.39 years (SD = 0.63). The majority of participants were of non-Hispanic Caucasian background (88.0%) and 66.4% of participants had at least one parent who had obtained at a bachelors degree or greater. Participants were excluded from the analyses if they were missing self-report data on depressive symptoms at the baseline (T1; N = 3) or 18-month (T3; N = 4) assessment, life stress interview data at the 9-month (T2) or T3 assessment (N = 48), did not complete the doors task (described below; N = 25) or had outlier RewP values that were more than three standard deviations from the mean (N = 3).
Measures
Adolescent Depression Symptoms.
Adolescent symptoms of depression were assessed using the expanded version of the Inventory of Depression and Anxiety Symptoms (IDAS-II; Watson et al., 2012). The IDAS-II is a self-report inventory that consists of 99 items comprising 18 factor-analytically derived scales (e.g., ill temper, well-being, mania, panic, social anxiety) cutting across internalizing disorders in a manner consistent with the Hierarchical Taxonomy of Psychopathology (Hi-TOP; Kotov et al., 2017). The current study operationalized depression symptoms using the IDAS-II Dysphoria subscale, which includes 10 items that are rated on a Likert-type scale ranging from 1 (not at all) to 5 (extremely) based on the previous two weeks. Dysphoria is the core symptom dimension of depression (Watson, 2009), and the IDAS-II Dysphoria subscale captures the depression symptoms of depressed mood, anhedonia, cognitive disturbance, psychomotor changes, self-esteem, guilt, and worry. The IDAS-II Dysphoria scale demonstrated excellent reliability at both assessments (Cronbach’s α’s = .89).
Stressful Life Events.
Negative stressful life events were assessed using the Stressful Life Events Schedule for adolescents (SLES; Williamson et al., 2003), a structured clinical interview that probes for 77 events in a number of domains (e.g. school, health, relationships). The SLES includes follow-up probes for each event in order to elicit additional information about the experience. Final ratings of contextual objective threat and behavioral dependence are each ranked on a 4-point scale ranging from ‘little or no effect’ to ‘great effect’, and ‘completely independent’ to ‘completely dependent’, respectively based on an extensive rating manual developed by the authors of the instrument. All ratings were determined at a consensus meeting by three trained interviewers who were kept blind to the participant’s subjective responses to the events. Objective threat ratings were summed separately based on consensus ratings of event dependence to create dependent and independent life stress scores. The SLES demonstrates substantial agreement with other widely used and validated measures such as the Life Events and Difficulties Schedule (κ = 0.77) and the Life Events Checklist (ICC = 0.83; Williamson et al., 2003). Because all ratings were made via team consensus, we could not calculate reliability for the current study without re-rating each of the life stress interviews with a completely independent consensus team. However, evidence suggests that consensus ratings of interviews has significantly higher validity when compared to individual interviewer ratings (Pulakos, Schmitt, Whitney, & Smith, 1996).
Procedure
At T1, adolescents completed the IDAS-II and the doors task (described below). Participants also completed the IDAS-II at the T3 assessment. The SLES was administered at T2 in order to assess negative episodic life stress between T1 and T2, and again at T3 in order to assess negative episodic life stress between T2 and T3. SLES scores from T1-T2 and T2-T3 were summed based on event dependence to create cumulative T1-T3 dependent and T1-T3 independent life stress scores.
Doors Task.
The doors task was administered on a computer via Presentation, version 17.2 (Neurobehavioral Systems, Albany, Calif.), and consists of three 20-trial blocks. Each trial began with two identical doors presented on the screen. Participants were told that they could either win $0.50 or lose $0.25 on each trial and were asked to select the right or left door by clicking the right or left mouse, respectively. Rewards trials are twice as large in magnitude compared to loss trials because losses are subjectively about twice as valuable as gains (Tversky & Kahneman, 1992), while also ensuring that participants accrue money over the course of the task. The doors were presented on the screen until the participant made a selection. Following the participant’s selection, a fixation cross was presented for 1000 ms, and feedback was subsequently presented for 2000 ms. A green arrow pointing upward represented a gain, whereas a red arrow pointing downward indicated a loss. The feedback, which was randomly determined, was followed by a fixation cross presented for 1500 ms, subsequently followed by the message “Click for next round”. In order to ensure that participants remained engaged, the “Click for next round” message remained on the screen until the participant responded to begin the next trial. All participants received 30 gain and 30 loss trials.
EEG Recording and Processing.
The same EEG recording and processing parameters utilized in previous studies were implemented in the current study (Bress et al., 2013; Nelson et al., 2016). Continuous EEG was recoded with a 34-electrode elastic cap with sites placed according to the 10/20 system. Electrooculography (EOG) was recorded using four additional facial electrodes: one placed approximately 1 cm outside both the left and right eyes and two placed approximately 1 cm above and below the right eye. Sintered Ag/AgCl electrodes were used. The ActiveTwo system was used to record EEG and EOG, and the signal was digitized with a sampling rate of 1024 Hz using a low-pass fifth-order sinc filter with a half-power cutoff of 204.8 Hz. A common mode sense active electrode producing a monopolar (nondifferential) channel was used as a recording reference for the EEG electrodes. The EOG electrodes produced two bipolar channels measuring horizontal and vertical eye movement.
BrainVision Analyzer, version 2.1 (Brain Products, Gilching, Germany), was used to analyze EEG data. An average of the left and right mastoids, band-pass filtered (0.1 to 30 Hz), and corrected for eye movement artifacts, were used as an offline reference (Gratton, Coles, & Donchin, 1983). Feedback-locked epochs, each with a duration of 1000 ms, were extracted beginning 200 ms before feedback presentation, with the 200 ms interval prior to feedback utilized as the baseline. Epochs containing a maximum voltage difference of less than 0.5 mV within 100 ms intervals, a voltage greater than 50 mV between sample points, or a voltage difference of 300 mV within a segment were automatically rejected. Additional artifacts were identified and removed based on visual inspection.
Feedback-locked ERPs were scored as the mean amplitude from 250–350 ms following feedback at the FCz electrode site because it is where the difference between gains and losses was maximal. Scores were averaged separately for gain and loss trials. The RewP was then quantified as the difference between gain and loss trials (gains minus losses). Split-half reliability was calculated for each of these components. Spearman-Brown [SB] coefficients indicated that internal consistency was excellent for neural response to gain (SB = .91) and loss (SB =.89), but poorer for the RewP (SB =.52). These results are consistent with previous studies investigating internal consistency of ERP and fMRI measures (Luking et al., 2017), and lower internal consistency for difference scores such as the RewP are expected due to accumulation of measurement error (Chiou & Spreng, 1996).
Data Analytic Strategy
Attrition analyses were conducted to examine whether there were differences between participants included and excluded from the current analyses. Next, preliminary analyses included bivariate correlations between predictor and outcome variables. Then, using linear regression, T1 age, race (coded as minority background vs. non-Hispanic Caucasian background, with minority background being coded as smaller values), T1 depression (indexed by the IDAS-II Dysphoria scale), and the RewP were entered simultaneously as variables predicting T1-T3 life stress. This resulted in two separate regression models, one predicting dependent and one predicting independent life stress. In order to determine whether a stress generation effect of the RewP is driven by response to gain, loss, or a combination of both, we ran follow-up analyses replacing the RewP with the individual neural response to gain and loss components in prediction of dependent and independent life stress. Regression models in which the RewP and neural response to gain or loss demonstrated significant stress generation effects were then examined in a mediation model with two mediators (T1-T3 dependent and T1-T3 independent life stress) predicting T3 depression symptoms, adjusting for T1 age, race, and T1 depression symptoms. We hypothesized that if the RewP or neural response to gain or loss demonstrated a significant stress generation effect (X), it would also indirectly influence subsequent depression symptoms (Y) via dependent (M1), but not independent life stress (M2). All variables were z-scored before being entered into the mediation model so that their relative influence could be compared.
All analyses were conducted using IBM SPSS 24 for Macintosh (IBM, Armonk, N.Y.). The SPSS macro PROCESS (model 4) was utilized to conduct mediation analyses.
Results
Attrition Analyses
Attrition analyses demonstrated that participants excluded from the current analyses did not differ from those included in terms of race, ethnicity, T1 age, or T1 depression symptoms.
Descriptive Statistics and Bivariate Associations
The ERP waveforms showing neural response to gain, loss, the RewP difference score, and the three-dimensional scalp distribution of the RewP are displayed in Figure 1. Bivariate correlations between predictor and outcome variables, as well as the mean and standard deviation for each measure, are shown in Table 1. As expected, depression symptoms (indexed by the IDAS-II Dysphoria scale) were moderately and significantly correlated across assessments. Similarly, depression symptoms were positively correlated with life stress. This relationship was stronger for dependent than independent life stress, and for T3 compared to T1 depression symptoms. Individuals from minority backgrounds experienced more dependent life stress. Consistent with previous manuscripts utilizing data from this study (e.g., Nelson et al., 2016), the RewP was significantly negatively correlated with T3 depression.
Figure 1.
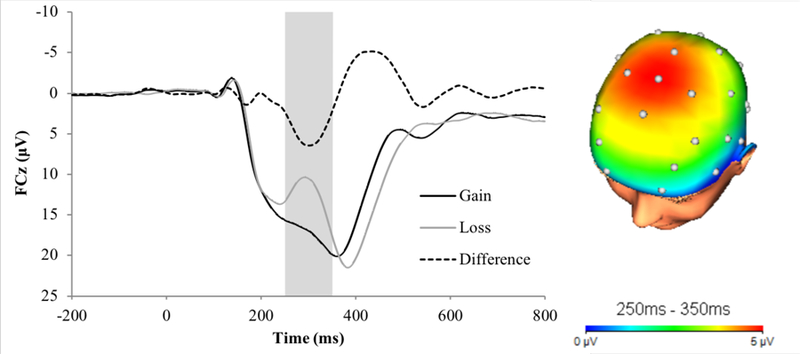
RewP Waveform and Scalp Distribution. Baseline assessment event-related potential (ERP) waveforms (left) and three-dimensional rendered scalp distributions of the Reward Positivity difference score (RewP; i.e., gains minus losses) at electrode FCz (right). The shaded region shows the segment where the RewP was scored.
Table 1.
Means, Standard Deviations, and Correlations Among Factors
| Factor | M/N | SD/% | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. T1 Age | 14.39 | 0.63 | -- | |||||||
| 2. Race | 56/467 | 12% | −.05 | -- | ||||||
| 3. T1 Depression | 16.42 | 7.20 | −.00 | −.09 | -- | |||||
| 4. T3 Depression | 15.78 | 6.93 | .06 | −.03 | 45*** | -- | ||||
| 5. T1 RewP | 4.98 | 5.65 | .12* | −.07 | −.01 | −.09* | -- | |||
| 6. T1 Gain | 17.26 | 9.35 | .12** | .02 | −.03 | .02 | .46*** | -- | ||
| 7. T1 Loss | 12.28 | 8.44 | .06 | .06 | −.03 | .09 | −.16*** | .80*** | -- | |
| 8. T1-T3 Dependent Life Stress | 2.06 | 1.84 | .11* | −.10* | .27*** | .42*** | −.08 | .03 | .08 | -- |
| 9. T1-T3 Independent Life Stress | 3.12 | 2.37 | .04 | −.03 | .18*** | .37*** | .02 | .02 | .01 | .33*** |
p < .05.
p < .01.
p < .001.
T1 = Time 1 (baseline visit). T3 = Time 3 (18-month follow-up visit). Race was coded as Non- Caucasian vs. Non-Hispanic Caucasian with higher values reflecting individuals from Non-Hispanic Caucasian backgrounds. The number and percentages reported for race reflect the number of participants from minority backgrounds out of the entire sample.
Linear Regression Analyses
RewP Predicting Life Stress
Results for linear regression analyses examining the association between T1 RewP and T1-T3 dependent and independent life stress are presented in Table 2 (top). T1 age was positively associated with T1-T3 dependent, but not T1-T3 independent, life stress. T1 depression was positively associated with both T1-T3 dependent and T1-T3 independent life stress. Race was not significantly associated with dependent nor independent life stress when considering the effects of other variables. After adjusting for T1 age, race, and T1 depression, T1 RewP was still significantly associated with T1-T3 dependent, but not T1-T3 independent, life stress. These results suggest that a blunted RewP predicts the generation of dependent, but not independent, life stress over the subsequent 18-months, which is consistent with the stress generation model.
Table 2.
RewP and Neural Response to Gain and Loss as a Predictors of Stress Generation
| T1-T3 Dependent Life Stress |
T1-T3 Independent Life Stress |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R2 | b | SE B | β | t | p | R2 | b | SE B | β | t | p | |
| RewP Models | .10 | .04 | ||||||||||
| T1 Age | 0.33 | 0.13 | 0.11 | 2.52 | .01 | 0.15 | 0.17 | 0.04 | 0.89 | .38 | ||
| Race | −0.42 | 0.25 | −0.07 | −1.66 | .10 | −0.06 | 0.34 | −0.01 | −0.18 | .86 | ||
| T1 Depression | 0.66 | 0.11 | 0.26 | 5.83 | <.001 | 0.60 | 0.15 | 0.18 | 3.96 | <.001 | ||
| T1 RewP | −0.03 | 0.02 | −0.09 | −2.03 | .04 | 0.01 | 0.02 | 0.01 | 0.30 | .77 | ||
| Gain & Loss Models | .10 | .04 | ||||||||||
| T1 Age | 0.31 | 0.13 | 0.11 | 2.38 | .02 | 0.15 | 0.17 | 0.04 | 0.85 | .39 | ||
| Race | −0.44 | 0.25 | −0.08 | −1.76 | .08 | −0.07 | 0.34 | −0.01 | −0.20 | .85 | ||
| T1 Depression | 0.67 | 0.11 | 0.26 | 5.89 | <.001 | 0.60 | 0.15 | 0.18 | 3.96 | <.001 | ||
| T1 Gain | −0.03 | 0.02 | −0.13 | −1.71 | .09 | 0.01 | 0.02 | 0.03 | 0.36 | .72 | ||
| T1 Loss | 0.04 | 0.02 | 0.19 | 2.60 | .01 | 0.00 | 0.02 | −0.01 | −0.10 | .92 | ||
T1 = Time 1 (baseline visit). T3 = Time 3 (18-month follow-up visit). RewP = Reward Positivity. Race was coded as non-Caucasian vs. Caucasian with higher values reflecting individuals identifying as Caucasian.
To investigate the specificity of our findings, we constructed two path models, each with four predictors (T1 Age, Race, T1 Dysphoria, and T1 RewP) and two outcomes (T1-T3 Dependent Life Stress and T1-T3 Independent Life Stress). In the first model we allowed all paths to be estimated freely, whereas the effects of the predictor variables on the two outcome variables were constrained to equality in the second model. We then compared model fit using a likelihood-ratio test to investigate whether the fit of the constrained model was significantly different from the unconstrained model. Although we observed a non-significant trend for a difference, we could not reject the null hypothesis that the constrained and unconstrained models were equivalent, χ2(1) = 3.00, p=0.08. Therefore, while the stress generation effect of the RewP was only observed for dependent life stress, the findings were not significantly stronger than those for independent life stress, although this low powered test for specificity approached statistical significance.
Neural Response to Gain and Loss Predicting Life Stress
To examine whether the stress generation effect was driven by a blunted response to gain, and enhanced response to loss, or a combination of both, we examined the associations between T1 neural response to gain and loss separately with T1-T3 dependent and independent life stress (see Table 2, bottom). T1 age was positively associated with T1-T3 dependent, but not T1-T3 independent, life stress. Again, T1 depression was positively associated with both T1-T3 dependent and T1-T3 independent life stress. Race was not significantly associated with dependent nor independent life stress after considering the effects of other variables in the models. After controlling for T1 age, race, and T1 depression, T1 neural response to gain was not significantly associated with T1-T3 dependent nor T1-T3 independent life stress. However, T1 neural response to loss was positively associated with T1-T3 dependent, but not T1-T3 independent, life stress. These findings suggest that even after accounting for covariates and the variance contributed by T1 neural response to gain, an enhanced T1 neural response to loss predicts greater levels of dependent life stress over the subsequent 18-months.2
Indirect effects of Neural Processing on Depression
Indirect Effects of the RewP on Depression
Our regression analyses supported our hypothesis that a blunted T1 RewP predicted significantly higher levels of T1-T3 dependent life stress. Additionally, our results demonstrated significant bivariate associations between T1 RewP and T3 depression and T1-T3 dependent life stress and T3 depression. Therefore, we decided to examine whether the effects of T1 RewP on T3 depression symptoms operated through T1-T3 dependent, but not independent, life stress. We conservatively included T1 age, race, and T1 depression symptoms as covariates of the mediator and outcome variables, with all variables z-scored prior to being entered into the model. Results (Figure 2) indicate that approximately 35% of the variance in T3 depression was accounted for by the predictors (R2 = .35). T1 RewP significantly predicted T1-T3 dependent life stress, and that T1-T3 dependent life stress significantly predicted T3 depression. Conversely, the RewP did not significantly predict T1-T3 independent life stress, although T1-T3 independent life stress did significantly predict T3 depression. In addition, there was a significant total effect of the RewP on T3 depression, including a significant direct effect of the T1 RewP on T3 depression. The indirect effect of T1 RewP on T3 depression symptoms was tested using a bootstrap estimation approach with 5,000 samples (Preacher & Hayes, 2004). Importantly, the indirect effect of T1 RewP on T3 depression through T1-T3 dependent life stress was statistically significant, whereas the indirect effect of T1 RewP on T3 depression through T1-T3 independent life stress was not. These findings suggest that a blunted RewP influences behavior in a manner that generates dependent, but not independent, life stress, and that dependent life stress in turn influences subsequent depression symptoms.
Figure 2.
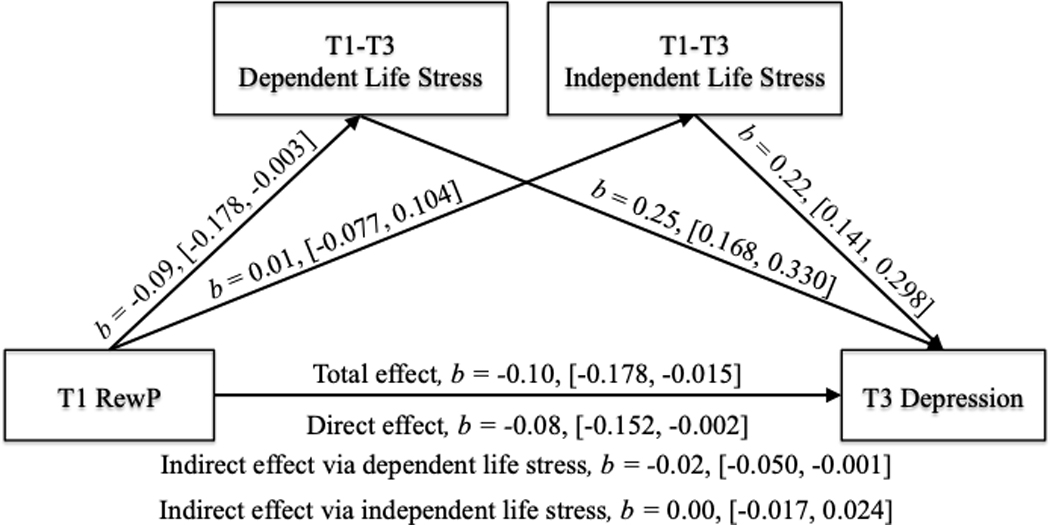
Indirect effects of T1 RewP on T3 Depression Operate Through T1-T3 Dependent Life Stress. The effects of T1 RewP on T3 depression are significant operating indirectly through dependent, but not independent, life stress, providing support for the stress generation hypothesis. All values were z-scored before being entered into the model. T1 = Time 1 (baseline visit). T3 = Time 3 (18-month follow-up visit). Values in brackets reflect 95% confidence intervals.
Indirect Effects of the Neural Response to Loss on Depression
Regression analyses demonstrated that neural response to loss predicted significantly higher levels of T1-T3 dependent life stress. Additionally, T1-T3 dependent life stress and T3 depression were significantly correlated. Therefore, we decided to examine whether T1 neural response to loss influenced T3 depression symptoms via T1-T3 dependent, but not independent, life stress. We conservatively included T1 age, race, and T1 depression symptoms as covariates of the mediator and outcome variables, with all variables z-scored prior to being entered into the model. Consistent with the pattern of findings observed in the RewP model, results indicate that approximately 35% of the variance in T3 depression was accounted for by the predictors (R2 = .35). Response to loss significantly predicted T1-T3 dependent life stress (b = 0.09, SE = 0.04, p = .04), and T1-T3 dependent life stress significantly predicted T3 depression (b = 0.25, SE = 0.04, p < .001). Conversely, the response to loss did not significantly predict T1-T3 independent life stress (b = 0.01, SE = 0.05, p = .75), although T1-T3 independent life stress did significantly predict T3 depression (b = 0.22, SE = 0.04, p < .001). In addition, there was a significant total effect of neural response to loss on T3 depression (b = 0.10, SE = 0.04, p = .02), although the direct effect of T1 loss on T3 depression only approached significance (b = 0.07, SE = 0.04, p = .06). The indirect effect of T1 neural response to loss on T3 depression symptoms was tested using a bootstrap estimation approach with 5,000 samples (Preacher & Hayes, 2004). The indirect effect of T1 response to loss on T3 depression through T1-T3 dependent life stress was statistically significant (b = 0.02, SE = 0.01, 95% CI = .003, .049), whereas the indirect effect of T1 RewP on T3 depression through T1-T3 independent life stress (b = 0.00, SE = 0.01, 95% CI = −.017, .023) was not. These findings suggest that neural response to loss influences behavior in a manner that generates dependent life stress, and this life stress in turn influences subsequent depression symptoms.
Discussion
The current study examined whether the difference between neural response to reward and loss, as indexed by the RewP, prospectively predicted the generation of dependent and independent stressful life events up to 18 months later, and whether there was an indirect effect of reward processing on subsequent depression symptoms that operated via stress generation. Results demonstrated that the RewP is a prospective marker of stress generation such that individuals with a blunted RewP generate greater levels of dependent, but not independent, life stress over the subsequent 18 months. This effect was driven more by response to loss than response to gain. Subsequent analyses of the total and indirect effects of the RewP on depression symptoms indicated that there was a significant total effect of the RewP on depression symptoms at the 18-month follow-up assessment, and that this effect operated indirectly via the RewP’s stress generation effect. This pattern of findings was also true when examining the effect of neural response to loss; a significant indirect effect of neural response to loss on subsequent depression via dependent, but not independent, life stress was observed. Results in both models remained significant even when adjusting for initial level of depression symptoms, suggesting that these associations are at least partially independent of the well-established stress generation effects of preexisting depression symptoms (Hammen, 1991; Liu & Alloy, 2010).
Several aspects of the bivariate correlations warrant comment. First, while the RewP and dependent life stress were not significantly correlated at the bivariate level, regression models including T1 age and race as covariates revealed a significant stress generation effect of the RewP. This is likely due to suppression effects, in which inclusion of covariates control for criterion-irrelevant variance in a predictor (Watson, Clark, Chmielewski, & Kotov, 2013). In the current study, T1 age is significantly positively correlated with the RewP and dependent stress, but the relationship between the RewP and dependent life is in the opposite direction. Similarly, participants from minority backgrounds report significantly more dependent events and a non-significantly larger RewP, but the association between the RewP and dependent stress is in the opposite direction. This pattern suggests that age and race suppressed an association between the RewP and T1-T3 dependent life stress, which appeared only when these covariates were adjusted. However, it will be important to replicate these findings.
Second, although the RewP predicted T3 depression, the cross-sectional association between RewP and T1 depression was non-significant. It may be that abnormalities in reward processing play a greater role after the transition from childhood/early adolescence into mid-adolescence and beyond, once the reward system is more fully developed (Casey, Jones, & Hare, 2008; Diedenhofen & Musch, 2015; Galván, 2013; Guyer, Silk, & Nelson, 2016; van Duijvenvoorde, Peters, Braams, & Crone, 2016). Finally, the absence of bivariate associations between T1 neural response to loss and both T1 and T3 depression suggests that the difference between the neural response to gain and loss may have a stronger association with depression symptoms than the gain and loss components alone, although the association between neural response to loss and T3 Depression approached significance (r=.08, p=.07).
The RewP, as well as neural response to loss, preceded subsequent behaviorally-dependent stressful life events, which is partially consistent with Auerbach et al.’s (2014) hypothesis that disrupted processing of reward and loss stimuli contributes to the generation of life stress. It is not yet clear if the neural system responsible for generating the RewP contributes directly or indirectly to the behaviors that increase risk for stressful life events (Walsh & Anderson, 2012). The reinforcement learning account of the RewP (Holroyd & Coles, 2002) provides a potential explanation for how a blunted RewP influences behavior and contributes to the increased generation of dependent life stress. Feedback indicating that outcomes are better or worse than expected evokes phasic increases and decreases, respectively, in midbrain dopamine release, which aids in learning and updating responses in later situations (Holroyd & Coles, 2002). Diminished sensitivity to the difference between loss and gains, perhaps due to the decreased salience of loss, may lead to less efficient learning following feedback, resulting in continued engagement in maladaptive approach- or avoidance-related behaviors. These maladaptive behaviors may, in turn, contribute to the generation of dependent stressful life events (Auerbach et al., 2014). The accumulation of dependent stressors over time resulting from inefficient learning of contingencies may increase the risk for developing depression symptoms, which are among the most powerful predictors of subsequent major depressive episodes (Klein et al., 2013).
A rapidly growing literature indicates that stress influences the neural circuitry associated with processing of rewarding and emotional stimuli (Novick et al., 2018; Swartz, Williamson, & Hariri, 2015). The present findings extend that literature by raising the possibility of bidirectional effects between life stress and neural response to reward and loss. Stress may alter functioning of neural systems involved in processing information about reward and loss, which subsequently leads to changes in behavior that generate additional life stress, resulting in a cycle and increasing vulnerability for depression. Future studies should test for bidirectional effects between life stress, neural reward dysfunction, and depression. This would require at least three waves of life stress, neural measures of reward and loss processing, and depression assessments.
A blunted neural response to reward and a blunted RewP have been shown to be associated with greater levels of depressive symptoms (Keren et al., 2018). However, when examining the individual effects of response to gain and loss in the current study, neural response to loss, but not to gain, was associated with later dependent life stress after adjusting for age, race, and existing symptoms of depression. This finding contributes to a growing literature suggesting the importance of processing negative and loss-related feedback. For example, using time-frequency analyses of ERP data, depressed adolescents differed from healthy adolescents in their neural response to losses, but not gains, on a monetary gambling task (Webb et al., 2017). Furthermore, fMRI findings have demonstrated an association between neural response to loss and risk for depression in children and adolescents (Gotlib et al., 2010; Luking, Pagliaccio, Luby, & Barch, 2016), and some studies report response to loss being more strongly related to depression risk than blunted response to gain (Luking et al., 2016). Together with our findings that dependent life events mediate the association between neural response to loss and subsequent depressive symptoms, this literature raises the possibility that the mechanisms linking response to gain and loss with depression may differ. For example, response to loss may reflect reinforcement of maladaptive approach or withdrawal behaviors despite those behaviors contributing to the generation of life stress. Conversely, a blunted response to gain may reflect a lack of reinforcement of positive experiences leading to a reduction in seeking out and taking advantage of opportunities for pleasurable experiences. This could result in an absence of positive life events rather than the generation of negative events, and would not be detected in standard stressful life events assessments.
The current study had several noteworthy strengths. Although cognitive, interpersonal, personality, genetic, and pupillary markers of stress generation have been identified, the present investigation is the first study to identify a neural marker of stress generation. This provides insight into one potential mechanism by which sensitivity to the difference between neural processing of reward and loss works together with life stress to confer risk for depression. Second, our longitudinal design allowed us to test whether the increased life stress generated by individuals with a blunted RewP contributed indirectly to later symptoms of depression. This is significant because the stress generation model posits that stress generation contributes to the maintenance and relapse of depression. However, most previous stress generation investigations have been limited to examining the effects of depression and associated risk factors on life stress and have assumed, but not tested, indirect effects of stress generation on later depression. Third, examination of the separate effects of gain and loss provides clues into what is driving the stress generation effect beyond what can be gathered from looking at the RewP difference score. Finally, we used repeated semi-structured life events interviews and consensus ratings using the contextual threat method, which are considered the methodological gold standard for life-stress assessment.
However, the current study also has several limitations. First, as the ERP data were collected prior to the administration of the life stress interview, it is not possible to examine whether these processes influence each other in a reciprocal, transactional manner. Second, neural response to gain and loss feedback were highly correlated (r=.80, Table 1), and the association between neural response to gain and dependent life stress approached significance. Therefore, we cannot conclude that an enhanced response to loss is necessarily of greater importance to the generation of dependent life stress than a blunted response to gain, and further work on this question is needed. Also, we did not examine diagnoses based on structured clinical interviews, choosing instead to use of dimensional scores because of their greater sensitivity and power, and the elimination of arbitrary thresholds for caseness in a relatively young non-clinical sample. Finally, the current sample is composed entirely of adolescent females from the community, and potential participants were excluded if they had a history of Major Depressive or Dysthymic Disorder at baseline. Therefore, we do not know whether the current findings apply to males, children or adults, or clinical samples.
Conclusion
These results extend the stress generation literature by providing evidence that blunted reward sensitivity in a monetary gambling task prospectively predicts the generation of dependent, but not independent, life stress. Moreover, the RewP and neural response to loss is indirectly associated with later depression symptoms via their effects on dependent life stress. If replicated, these findings provide insight into one mechanism by which neural sensitivity to the difference between gain and loss may be related to later symptoms of depression and may provide early targets for the prevention and treatment of depressive disorders.
Acknowledgements
This study was supported by the National Institute of Mental Health grant R01 MH093479 awarded to R.K. We gratefully acknowledge the support of all study participants. We are also indebted to the dedicated efforts of the ADEPT study coordinators and team for their work on the project.
This study was supported by National Institute of Mental Health grant R01 MH093479 awarded to R.K. All procedures in the current study (Personality Development and Vulnerability to First Episode Depression) were approved by the Stony Brook University Institutional Review Board. Findings from the current manuscript, including the stress generation effect of the RewP and the indirect effect of the RewP on subsequent depression via dependent life stress, were presented as a poster at the 32nd annual meeting of the Society for Research in Psychopathology.
Footnotes
Two published papers have examined reward processing and depression in this sample. The first study (Nelson et al., 2016) reported on the association between the Reward Positivity (RewP) and the first onset of depression and dysphoria symptoms in a subset of this sample. However, that paper excluded cases who were diagnosed with depression NOS at the baseline visit and did not examine life stress. The second study (Nelson et al., 2018) also reported on the association between the RewP and the first onset of depression and dysphoria symptoms in a subset of this sample. Again, this excluded cases who were diagnosed with depression NOS at the baseline visit, focused on time-frequency, rather than time-domain measures of response to gains and losses, and did not include any measures of life stress. Thus, both studies addressed very different questions than the current manuscript.
The stress generation effects of time-frequency measures of neural response to gain and loss were also examined. Results from these analyses indicate that neither delta power in response to gains (β = 0.02, p = .74), nor theta power in response to losses (β = −0.01, p = .77), were significantly associated with T1-T3 dependent life stress.
References
- Admon R, Lubin G, Rosenblatt JD, Stern O, Kahn I, Assaf M, & Hendler T (2012). Imbalanced neural responsivity to risk and reward indicates stress vulnerability in humans. Cerebral Cortex, 23(1), 28–35. [DOI] [PubMed] [Google Scholar]
- Auerbach RP, Admon R, & Pizzagalli DA (2014). Adolescent Depression: Stress and Reward Dysfunction. Harvard review of psychiatry, 22(3), 139–148. doi: 10.1097/HRP.0000000000000034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belden AC, Irvin K, Hajcak G, Kappenman ES, Kelly D, Karlow S, … Barch DM (2016). Neural Correlates of Reward Processing in Depressed and Healthy Preschool- Age Children. Journal of the American Academy of Child & Adolescent Psychiatry, 55(12), 1081–1089. doi: 10.1016/j.jaac.2016.09.503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bress JN, Foti D, Kotov R, Klein DN, & Hajcak G (2013). Blunted neural response to rewards prospectively predicts depression in adolescent girls. Psychophysiology, 50(1), 74–81. [DOI] [PubMed] [Google Scholar]
- Bress JN, & Hajcak G (2013). Self-report and behavioral measures of reward sensitivity predict the feedback negativity. Psychophysiology, 50(7), 610–616. doi: 10.1111/psyp.12053 [DOI] [PubMed] [Google Scholar]
- Bress JN, Meyer A, & Proudfit GH (2015). The stability of the feedback negativity and its relationship with depression during childhood and adolescence. Dev Psychopathol, 27(4 Pt 1), 1285–1294. doi: 10.1017/s0954579414001400 [DOI] [PubMed] [Google Scholar]
- Bress JN, Smith E, Foti D, Klein DN, & Hajcak G (2012). Neural response to reward and depressive symptoms in late childhood to early adolescence. Biol Psychol, 89(1), 156–162. doi: 10.1016/j.biopsycho.2011.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GW, Harris TO (1978). Social Origins of Depression: A Study of Psychiatric Disorder in Women London: Free Press. [Google Scholar]
- Casement MD, Guyer AE, Hipwell AE, McAloon RL, Hoffmann AM, Keenan KE, & Forbes EE (2014). Girls’ challenging social experiences in early adolescence predict neural response to rewards and depressive symptoms. Dev Cogn Neurosci, 8, 18–27. doi: 10.1016/j.dcn.2013.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Jones RM, & Hare TA (2008). The adolescent brain. Ann N Y Acad Sci, 1124, 111–126. doi: 10.1196/annals.1440.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiou J, & Spreng RA (1996). The reliability of difference scores: A re-examination. Journal of Consumer Satisfaction Dissatisfaction and Complaining Behavior, 9, 158–167. [Google Scholar]
- Davila J, Hammen C, Burge D, Paley B, & Daley SE (1995). Poor interpersonal problem solving as a mechanism of stress generation in depression among adolescent women. J Abnorm Psychol, 104(4), 592–600. [DOI] [PubMed] [Google Scholar]
- Diedenhofen B, & Musch J (2015). cocor: A comprehensive solution for the statistical comparison of correlations. PLoS ONE, 10(4), e0121945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feurer C, Burkhouse KL, Siegle G, & Gibb BE (2017). Increased pupil dilation to angry faces predicts interpersonal stress generation in offspring of depressed mothers. J Child Psychol Psychiatry, 58(8), 950–957. doi: 10.1111/jcpp.12739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A (2010). Adolescent Development of the Reward System. Frontiers In Human Neuroscience, 4, 6. doi: 10.3389/neuro.09.006.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galván A (2013). The Teenage Brain: Sensitivity to Rewards. Current Directions in Psychological Science, 22(2), 88–93. doi: 10.1177/0963721413480859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge X, Conger RD, & Elder GH Jr (2001). Pubertal transition, stressful life events, and the emergence of gender differences in adolescent depressive symptoms. Developmental Psychology, 37(3), 404. [DOI] [PubMed] [Google Scholar]
- Ge X, Lorenz FO, Conger RD, Elder GH, & Simons RL (1994). Trajectories of stressful life events and depressive symptoms during adolescence. Developmental Psychology, 30(4), 467. [Google Scholar]
- Goldstein BL, & Klein DN (2014). A review of selected candidate endophenotypes for depression. Clinical Psychology Review, 34(5), 417–427. doi: 10.1016/j.cpr.2014.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib IH, Hamilton JP, Cooney RE, Singh MK, Henry ML, & Joormann J (2010). Neural processing of reward and loss in girls at risk for major depression. Arch Gen Psychiatry, 67(4), 380–387. doi: 10.1001/archgenpsychiatry.2010.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton G, Coles MG, & Donchin E (1983). A new method for off-line removal of ocular artifact. Electroencephalogr Clin Neurophysiol, 55(4), 468–484. [DOI] [PubMed] [Google Scholar]
- Guyer AE, Silk JS, & Nelson EE (2016). The neurobiology of the emotional adolescent: From the inside out. Neurosci Biobehav Rev, 70, 74–85. doi: 10.1016/j.neubiorev.2016.07.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammen C (1991). Generation of stress in the course of unipolar depression. J Abnorm Psychol, 100(4), 555–561. [DOI] [PubMed] [Google Scholar]
- Hammen C (2006). Stress generation in depression: reflections on origins, research, and future directions. J Clin Psychol, 62(9), 1065–1082. doi: 10.1002/jclp.20293 [DOI] [PubMed] [Google Scholar]
- Hammen C, Davila J, Brown G, Ellicott A, & Gitlin M (1992). Psychiatric history and stress: Predictors of severity of unipolar depression [Press release] [DOI] [PubMed] [Google Scholar]
- Hammen C, Shih JH, & Brennan PA (2004). Intergenerational Transmission of Depression: Test of an Interpersonal Stress Model in a Community Sample [Press release] [DOI] [PubMed] [Google Scholar]
- Hankin BL, Kassel JD, & Abela JRZ (2005). Adult Attachment Dimensions and Specificity of Emotional Distress Symptoms: Prospective Investigations of Cognitive Risk and Interpersonal Stress Generation as Mediating Mechanisms. Personality and Social Psychology Bulletin, 31(1), 136–151. doi: 10.1177/0146167204271324 [DOI] [PubMed] [Google Scholar]
- Hankin BL, Mermelstein R, & Roesch L (2007). Sex Differences in Adolescent Depression: Stress Exposure and Reactivity Models. Child Development, 78(1), 279–295. [DOI] [PubMed] [Google Scholar]
- Holahan CJ, Moos RH, Holahan CK, Brennan PL, & Schutte KK (2005). Stress generation, avoidance coping, and depressive symptoms: a 10-year model. Journal of Consulting and Clinical Psychology, 73(4), 658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holroyd CB, & Coles MGH (2002). The neural basis of human error processing: reinforcement learning, dopamine, and the error-related negativity. Psychol Rev, 109(4), 679–709. doi: 10.1037/0033-295x.109.4.679 [DOI] [PubMed] [Google Scholar]
- Kendler KS, Karkowski LM, & Prescott CA (1999). Causal relationship between stressful life events and the onset of major depression. Am J Psychiatry, 156(6), 837–841. doi: 10.1176/ajp.156.6.837 [DOI] [PubMed] [Google Scholar]
- Kercher AJ, Rapee RM, & Schniering CA (2009). Neuroticism, life events and negative thoughts in the development of depression in adolescent girls. J Abnorm Child Psychol, 37(7), 903–915. doi: 10.1007/s10802-009-9325-1 [DOI] [PubMed] [Google Scholar]
- Keren H, O’Callaghan G, Vidal-Ribas P, Buzzell GA, Brotman MA, Leibenluft E, … Wolke S (2018). Reward Processing in Depression: A Conceptual and Meta-Analytic Review Across fMRI and EEG Studies. American Journal of Psychiatry, appi. ajp 2018.17101124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotov R, Krueger RF, Watson D, Achenbach TM, Althoff RR, Bagby RM, … Zimmerman M (2017). The Hierarchical Taxonomy of Psychopathology (HiTOP): A dimensional alternative to traditional nosologies. J Abnorm Psychol, 126(4), 454–477. doi: 10.1037/abn0000258 [DOI] [PubMed] [Google Scholar]
- Klein DN, Glenn CR, Kosty DB, Seeley JR, Rohde P, & Lewinsohn PM (2013). Predictors of first lifetime onset of major depressive disorder in young adulthood. Journal of Abnormal Psychology, 122(1), 1–6. doi: 10.1037/a0029567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RT, & Alloy LB (2010). Stress generation in depression: A systematic review of the empirical literature and recommendations for future study. Clin Psychol Rev, 30(5), 582–593. doi: 10.1016/j.cpr.2010.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luking KR, Nelson BD, Infantolino ZP, Sauder CL, & Hajcak G (2017). Internal consistency of functional magnetic resonance imaging and electroencephalography measures of reward in late childhood and early adolescence. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 2(3), 289–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luking KR, Pagliaccio D, Luby JL, & Barch DM (2016). Depression Risk Predicts Blunted Neural Responses to Gains and Enhanced Responses to Losses in Healthy Children. J Am Acad Child Adolesc Psychiatry, 55(4), 328–337. doi: 10.1016/j.jaac.2016.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten AS, & Cicchetti D (2010). Developmental cascades. Development and Psychopathology, 22(3), 491–495. doi: 10.1017/S0954579410000222 [DOI] [PubMed] [Google Scholar]
- McCrory E,J, Gerin M,I, & Viding E (2017). Annual Research Review: Childhood maltreatment, latent vulnerability and the shift to preventative psychiatry – the contribution of functional brain imaging. Journal of Child Psychology and Psychiatry, 58(4), 338–357. doi:doi:10.1111/jcpp.12713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson BD, Perlman G, Klein DN, Kotov R, & Hajcak G (2016). Blunted Neural Response to Rewards as a Prospective Predictor of the Development of Depression in Adolescent Girls. Am J Psychiatry, 173(12), 1223–1230. doi: 10.1176/appi.ajp.2016.15121524 [DOI] [PubMed] [Google Scholar]
- Novick AM, Levandowski ML, Laumann LE, Philip NS, Price LH, & Tyrka AR (2018). The effects of early life stress on reward processing. Journal of Psychiatric Research, 101, 80–103. doi: 10.1016/j.jpsychires.2018.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Holmes AJ, Dillon DG, Goetz EL, Birk JL, Bogdan R, … Fava M (2009). Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. American Journal of Psychiatry, 166(6), 702–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher KJ, & Hayes AF (2004). SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behavior research methods, instruments, & computers, 36(4), 717–731. [DOI] [PubMed] [Google Scholar]
- Proudfit GH (2015). The reward positivity: from basic research on reward to a biomarker for depression. Psychophysiology, 52(4), 449–459. doi: 10.1111/psyp.12370 [DOI] [PubMed] [Google Scholar]
- Pulakos ED, Schmitt N, Whitney D, & Smith M (1996). Individual differences in interviewer ratings: The impact of standardization, consensus discussion, and sampling error on the validity of a structured interview. Personnel Psychology, 49(1), 85–102. [Google Scholar]
- Rudolph KD, & Hammen C (1999). Age and Gender as Determinants of Stress Exposure, Generation, and Reactions in Youngsters: A Transactional Perspective. Child Development, 70(3), 660–677. [DOI] [PubMed] [Google Scholar]
- Safford SM, Alloy LB, Abramson LY, & Crossfield AG (2007). Negative cognitive style as a predictor of negative life events in depression-prone individuals: a test of the stress generation hypothesis. J Affect Disord, 99(1–3), 147–154. doi: 10.1016/j.jad.2006.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr LR, Hammen C, Brennan PA, & Najman JM (2013). Relational security moderates the effect of serotonin transporter gene polymorphism (5-HTTLPR) on stress generation and depression among adolescents. Journal of Abnormal Child Psychology, 41(3), 379–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringaris A, Vidal-Ribas Belil P, Artiges E, Lemaitre H, Gollier-Briant F, Wolke S, … Paillere-Martinot ML (2015). The Brain’s Response to Reward Anticipation and Depression in Adolescence: Dimensionality, Specificity, and Longitudinal Predictions in a Community-Based Sample. Am J Psychiatry, 172(12), 1215–1223. doi: 10.1176/appi.ajp.2015.14101298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz JR, Williamson DE, & Hariri AR (2015). Developmental Change in Amygdala Reactivity During Adolescence: Effects of Family History of Depression and Stressful Life Events. American Journal of Psychiatry, 172(3), 276–283. doi: 10.1176/appi.ajp.2014.14020195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tversky A, & Kahneman D (1992). Advances in prospect theory: Cumulative representation of uncertainty. Journal of Risk and uncertainty, 5(4), 297–323. [Google Scholar]
- Walsh MM, & Anderson JR (2012). Learning from experience: event-related potential correlates of reward processing, neural adaptation, and behavioral choice. Neuroscience & Biobehavioral Reviews, 36(8), 1870–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D (2009). Differentiating the Mood and Anxiety Disorders: A Quadripartite Model. Annu Rev Clin Psychol, 5(1), 221–247. doi: 10.1146/annurev.clinpsy.032408.153510 [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Chmielewski M, & Kotov R (2013). The value of suppressor effects in explicating the construct validity of symptom measures. Psychol Assess, 25(3), 929–941. doi: 10.1037/a0032781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, O’Hara MW, Naragon-Gainey K, Koffel E, Chmielewski M, Kotov R, … Ruggero CJ (2012). Development and validation of new anxiety and bipolar symptom scales for an expanded version of the IDAS (the IDAS-II). Assessment, 19(4), 399–420. doi: 10.1177/1073191112449857 [DOI] [PubMed] [Google Scholar]
- Webb CA, Auerbach RP, Bondy E, Stanton CH, Foti D, & Pizzagalli DA (2017). Abnormal neural responses to feedback in depressed adolescents. J Abnorm Psychol, 126(1), 19–31. doi: 10.1037/abn0000228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson DE, Birmaher B, Ryan ND, Shiffrin TP, Lusky JA, Protopapa J, … Brent DA (2003). The Stressful Life Events Schedule for children and adolescents: development and validation. Psychiatry Research, 119(3), 225–241. doi: 10.1016/s0165-1781(03)00134-3 [DOI] [PubMed] [Google Scholar]
- van Duijvenvoorde AC, Peters S, Braams BR, & Crone EA (2016). What motivates adolescents? Neural responses to rewards and their influence on adolescents’ risk taking, learning, and cognitive control. Neurosci Biobehav Rev, 70, 135–147. doi: 10.1016/j.neubiorev.2016.06.037 [DOI] [PubMed] [Google Scholar]


