Abstract
Opportunities are limited and the stakes are high in sexual reproduction. This inevitably leads to conflict. One pervasive conflict occurs within genomes between alternate alleles at heterozygous loci. Each gamete (e.g. sperm) and thus each offspring will inherit only one of the two alleles from a heterozygous parent. Most alleles play fair and have a 50% chance of being included in any given gamete. However, alleles gain an enormous advantage if they act selfishly to force their own transmission into more than half, sometimes even all, of the functional gametes. These selfish alleles are known as meiotic drivers and their cheating often exacts a high price on the fertility of eukaryotes ranging from plants to mammals. Here, we review how several types of meiotic drivers directly and indirectly contribute to infertility, and argue that a complete picture of the genetics of infertility will require focusing on both the standard alleles – those that play fair, as well as selfish alleles involved in genetic conflict.
In Brief:
Sarah Zanders and Rober Unckless review the ways in which selfish genetic elements contribute to infertility.
Introduction
The term ‘sexual conflict’ can evoke dramatic images of animals employing elaborate weaponry in mortal combat to secure access to mates. Less celebrated, but equally fascinating, are the conflicts raging within the gonads of the combatants and their potential mates. These internal, or intragenomic, conflicts can arise when the best interest of one part of the genome is at odds with the best interest of another part of the genome [1].
One such intragenomic conflict stems from the fact that only half of the alleles present in a diploid germ cell are represented in each gamete. Most alleles have a 50% chance to be transmitted into a given offspring. Given sufficient population sizes, these alleles can generally spread in a population if they are adaptive. There is, however, tremendous incentive for alleles to act selfishly and cheat during gametogenesis. Such cheating alleles that bias their own transmission into more than half of the functional gametes have been identified throughout eukaryotes. These selfish alleles are collectively known as meiotic drivers [2–4]. These alleles can spread in a population, even without promoting fitness. In fact, meiotic drivers can even spread if they cause decreased fertility.
In recent years, many novel meiotic drive systems have been discovered from plants to humans [3–10]. Studies applying advanced genetic and genomic approaches to this problem have only recently begun and it is likely that the number of identified drivers will grow greatly in the coming years [5, 6, 11], as will our understanding of the molecular mechanisms of these diverse drive systems. As more drivers are discovered, it is becoming clear that meiotic drivers are a widespread threat to eukaryotic genomes, rather than rare genetic anomalies. In this review, we provide a broad overview of meiotic drivers and explain how they can reduce fertility (Figure 1).
Figure 1:
Tree representing the genera containing drivers cited in this work. This is only a subset of the organisms in which drive has been observed.
I. Types of meiotic drivers
The term meiotic drive was first used to describe alleles that are able to bias meiotic chromosome segregation in their favor [2]. These drivers are sometimes called “true meiotic drivers” and there are two known types. The first type exploits asymmetric (i.e. female) meiosis in which one meiotic product is selected to become the gamete, while the other meiotic products become polar bodies. Standard alleles have a 50% chance of being transmitted into any given gamete. Drive loci, however, can manipulate asymmetric meiosis to increase their transmission into up to 100% of gametes. For example, in mice heterozygous for a “strong” and a “weak” centromere, the strong centromere exploits spindle asymmetry in the first meiotic division to promote its preferential transmission into eggs (Figure 2) [10, 12–14].
Figure 2:
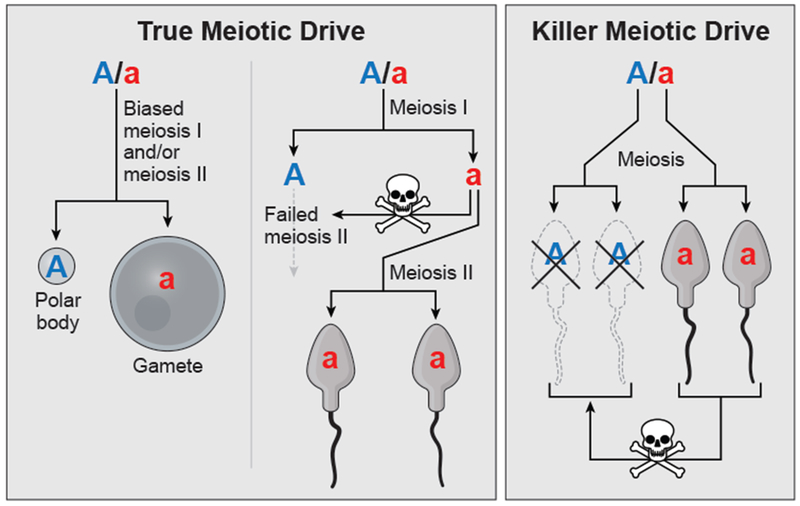
Models representing general mechanisms of meiotic drive. The box on the left depicts two types of ‘true’ meiotic drive. The example on left shows biased transmission favoring the ‘a’ allele during meiosis I and/or meiosis II. In the example on the right, the ‘a’ allele gains a transmission advantage by disrupting meiosis II of the chromosome that inherits ‘A.’ The box on the right depicts gamete killing meiotic drive where the developing gametes that inherit the ‘a’ allele destroy those that inherit ‘A.’
A second type of true meiotic drive is exemplified by the Paris sex-ratio meiotic drive system of Drosophila simulans. In this system, an X-linked drive system disrupts the segregation of the Y chromatids during meiosis II in males, resulting in the degradation of Y-bearing sperm and giving the X chromosome a transmission advantage (Figure 2) [15, 16]. While phenotypically similar to gamete killers described below, the mechanism of drive in the Paris system qualifies it as a true meiotic driver.
A final type of drive that may be considered true meiotic drive actually arises prior to the meiotic divisions during meiotic recombination. Meiotic recombination is initiated by programmed DNA double-strand breaks (DSBs). DSB sites are not random in most species, but instead occur more frequently in regions called “hotspots.” DNA sequence can be lost during DSB processing and meiotic breaks are preferentially repaired using the homologous chromosome, instead of the sister chromatid, as a template [17]. Together, gaps formed at DSBs and homolog directed repair lead to a “gene conversion bias” that favors the unbroken allele. In other words, alleles that incur DSBs less frequently (coldspots) could gain a transmission advantage over allelic sequences that act as hotspots. This type of drive is subtler than the other types of drive, but can have a significant impact over evolutionary timescales [18, 19]. Several synthetic gene drive approaches induce targeted DSBs and drive using a similar recombination mechanism [20].
While the meiotic drive field was still in its formative years, the definition of meiotic drive was expanded to include alleles that act after the meiotic divisions to promote their own biased transmission [21]. These drivers generally act to kill or disable gametes that do not inherit the driver from a heterozygote [4]. For example, in Oryza sativa (rice), a driving allele of the Sc locus acts in male gametogenesis by suppressing the expression of a gene essential for pollen development in pollen that inherit the non-driving allele [8]. These drivers are known by many names, but we refer to them here as “killer meiotic drivers” (Figure 2). Most known killer meiotic drivers act in symmetric meiosis (i.e. male) in which all the meiotic products are viable [3, 4, 22, 23]. There are, however, also killers that kill eggs that inherit the competing allele [24–26].
II. How do drivers contribute to infertility?
Gametogenesis is complex and there are a countless number of paths leading to infertility. Understanding how traits that limit the reproductive potential of an organism can arise and spread in a population has been (and still is) a major challenge of evolutionary biology [27]. Genetic conflict (and meiotic drive specifically) is emerging as a key factor to answering these questions as drivers and drive-associated proteins have been recurrently identified as causes of infertility within and between populations [7–9, 28–42]. Below, we break down how meiotic drivers can both directly and indirectly contribute to infertility (Figure 3).
Figure 3:
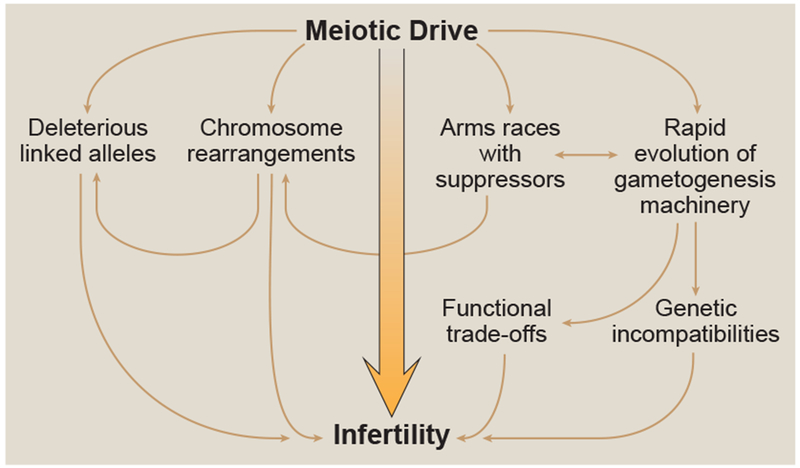
A schematic depicting how meiotic drivers can directly and indirectly contribute to infertility.
Meiotic drivers contribute to infertility directly
Infertility results from gamete destruction
The most dramatic examples of meiotic drivers directly contributing to infertility come from the killer meiotic drivers as these elements actively destroy gametes [4]. In organisms heterozygous for a single killer meiotic driver, up to half of the gametes will be destroyed. In some organisms, such as fungi, fertility is directly related to the number of meiotic products produced. For example, Neurospora crassa (red bread mold) gametogenesis generally produces 8 meiotic products (spores) which germinate to form new haploid individuals. N. crassa zygotes heterozygous for the Sk-2 killer meiotic drive locus, however, produce 4 spores on average as the others are destroyed by the driver [43, 44].
In other organisms where killer meiotic drivers act, fertility is not directly determined by how many gametes are produced [41]. In many species, males make sperm in dramatic excess relative to the number of offspring they will sire. This allows some eukaryotes, like the mosquito Aedes aegyptii, to maintain relatively high fertility despite a killer driver sabotaging sperm [45]. In other organisms with seemingly superfluous sperm, however, reducing sperm numbers can potentially impact fertility. In Drosophila melanogaster, for example, fertility of males heterozygous for the well-studied SD killer meiotic driver is reduced relative to wild-type controls, even in the absence of competition from other males [46, 47]. When males compete to fertilize the eggs of multiply mated females, the fertility costs of gamete killers are likely amplified [41].
Infertility results from off-target effects of the driver
The gametocidal properties of killer meiotic drivers are selected to specifically destroy gametes that do not inherit the drive locus. However, the processes ensuring gamete specificity are not perfect and even gametes that inherit the driver can be harmed by its toxicity [41, 48]. Phenotypes consistent with these off-target effects have been observed in several plant species. In Triticum aestivum (wheat), Silene latifolia (white campion) and Solanum lycopersicum (tomatoes), killer meiotic drivers are associated with decreased fertility of gametes carrying the driver, in addition to sabotaging the gametes not carrying the driver [25, 49, 50]. One particularly striking example is the X chromosome meiotic drive element in Drosophila affinis, a species where males lacking a Y chromosome (XO) are fertile. When the driving X is paired with a Y chromosome, the driver destroys Y-bearing sperm. However, when the driving X is found in an XO male, the driver kills the X-bearing sperm. This results in the production of sperm lacking a sex chromosome and, therefore, the production of only XO sons [51, 52].
The t haplotype drive locus found in the house mouse (Mus musculus) similarly exemplifies the possible off-target fertility costs of killer drive systems. The t haplotype driver encodes a set of alleles that disrupt sperm motility [53–56]. The locus also encodes an additional allele that rescues the motility of the sperm that inherit the t haplotype [57]. Importantly, the rescue is not complete. The rescued sperm carrying the t haplotype have persistent motility defects and heterozygous males carrying the t haplotypes are subfertile in a typical mating context [58–60].
Infertility results from the combination of multiple drivers
Several species encode distinct, unlinked killer meiotic drivers [29–31, 43, 61–63]. The fertility costs imposed by additional killers acting within an individual is context dependent. Infertility can be minimized in cases where one killer driver confers immunity against other drivers. This occurs in the fungus Podospora anserina, where the Spok1 driver provides spores that inherit it protection against the Spok2 driver [31]. In cases where distinct drivers do not confer cross-protective immunity, the fitness costs can be profound. In the fission yeast Schizosaccharomyces pombe, for example, multiple wtf drivers acting within one diploid can cause almost complete sterility [29, 30, 61]. Outcrossing between non-clonal individuals in S. pombe frequently generates infertile diploids [64]. The large number (sometimes >10) of heterozygous wtf drive loci found in these crosses is likely a major determinant of that infertility [65, 66].
Infertility associated with true meiotic drivers
Fewer true meiotic drivers have been discovered than killers, so less is known about their associated fitness costs. These drivers can, however, also directly contribute to infertility. The Paris sex-ratio meiotic driver of D. simulans described above, has similar effects on sperm production as killer meiotic drivers because it disrupts the formation of Y chromosome-bearing sperm [16]. In contrast, other true meiotic drivers that bias allele transmission into the one gamete produced by asymmetric meiosis could theoretically do so with no direct cost to fertility. Selfish centromeres, for example, could drive into all the gametes generated by a female without changing the absolute number of viable eggs produced [10, 67, 68]. It seems plausible, however, that altering chromosome structure or segregation to yield drive could disrupt meiosis (or mitosis) enough to affect fertility.
Determining how much infertility associated with driver loci is directly due to the action of the drivers versus how much is due to linked factors can be difficult. Because of this, it remains possible that some of the fitness costs discussed above are caused by linked alleles, discussed below, separate from those causing drive.
Meiotic drivers indirectly contribute to infertility
Population sizes are limited, environments are not always stable and genome maintenance mechanisms are not perfect. These factors can lead to the presence of maladapted alleles in a population. Meiotic drivers are not expected to change the rate at which alleles that decreas fertility arise, but drivers can promote the maintenance and even spread of such alleles in a population [69].
To illustrate this idea, consider a de novo mutation that decreases fertility. At a regular locus, natural selection can act to purge the allele from the population because individuals carrying it have a fitness disadvantage. However, if the mutation arose linked on the same haplotype as a meiotic driver, selection to remove the mutation would be less efficient because of the transmission bias conferred by the driver. This effect is exacerbated by the fact that many meiotic drivers are found in regions of low recombination (e.g. inversions), reducing the ability of selection to purge deleterious mutations linked to a driver. In theory, drivers can even promote the spread of driver-linked alleles that cause infertility in a population [69–71].
Deleterious mutations linked to meiotic drivers
Strongly deleterious alleles have been found linked to meiotic drivers. The large and intensely-studied mouse t haplotype region is rich with examples. Sixteen different complementation groups of lethal mutations have been observed in different t haplotype mouse lines [72]. In addition, viable males homozygous for different t haplotypes are sterile. This sterility was initially thought to result exclusively from the mutations causing drive, but additional unrelated recessive sterile mutations have been identified within the haplotype [73].
Chromosomal rearrangements associated with meiotic drivers
In addition to mutations that disrupt gene functions, meiotic drive loci also accumulate linked chromosome rearrangements [61, 74–77]. This can happen for the same reason described above for genic mutations: it can be difficult to purge mutations linked to drivers. In addition, chromosome inversions including or linked to drivers may make the drive phenotype stronger. There are two main reasons why drive alleles can benefit from chromosome rearrangements and they both stem from the fact that chromosome inversions suppress recombination. The first reason is that in many cases, distinct genetic components of driver systems must be in very strong linkage with each other. For example, the Sk-2 killer meiotic drive locus from Neurospora intermedia encodes both an (unidentified) poison and an antidote (the rsk protein) that rescues spores that inherit Sk-2 [44, 75]. Recombination between those factors could generate a suicide haplotype that encoded the poison, but not the antidote. Such recombination is prevented by an inversion [75]. A series of inversions also prevent separation of the genetic elements needed for drive of SD in D. melanogaster and the mouse t haplotype [74, 76].
The second reason drivers benefit from decreased recombination nearby is that it facilitates the evolution of genetic modifiers that enhance rather than suppress drive. Modifiers that enhance drive are generally favored if they arise on the same haplotype as the drive locus because the enhancer would also enjoy the transmission advantage of the driver [69]. For instance, the D. melanogaster SD driver is linked to multiple enhancers of drive [74]. At loci not on the same haplotype as the driver, suppressors of drive are generally favored. This is especially true at loci that are under-transmitted into progeny due to drivers, but is also true at unlinked loci [69].
Like genic mutations discussed above, chromosome rearrangements can also directly contribute to infertility. For example, several chromosome translocations and inversions linked to killer meiotic drive loci contribute to infertility within S. pombe populations [61, 65]. In addition, rearrangements can indirectly contribute to infertility by limiting the ability of recombination to purge deleterious mutations.
The compounding deleterious effects of drivers and chromosome rearrangements are perhaps best illustrated by the drive system found on an X chromosome variant in Drosophila recens. A series of inversions on the driving chromosome (XD) have led to chromosome-wide linkage to the driver. The costs of this linkage are steep. The XD chromosome already carries a recessive mutation that causes female sterility and will likely degenerate further in the absence of recombination [77].
Meiotic drive may lead to evolutionary tradeoffs and genetic incompatibilities between populations
The strength of selection for resistance to meiotic drive depends on several factors including the target locus (e.g. sex chromosome vs. autosome) and direct and indirect fertility costs associated with drive [78]. For example, if a driver on the X-chromosome results in female-biased population sex ratios, any mutation, regardless of chromosome, can be selected if it shifts the balance back toward 50% female [79, 80]. However, with an autosomal driver without direct fertility costs, only linked suppressors would be under strong selection for resistance. Also, the stronger the associated fertility costs, the stronger the selection for resistance to drive. This may lead to an arms race where resistance alleles in the rest of the genome are constantly arising to combat the evolving intricacies of the meiotic drive machinery.
Given that gametogenesis is a relatively fragile biological system, it stands to reason that some resistance mutations might come at a cost to fertility. In Drosophila affinis, for example, males carrying Y chromosomes that are resistant to an X-linked driver have lower fertility in the absence of drive compared to males carrying a Y that is susceptible to drive [51]. Decreased fertility associated with drive resistance has also been observed in S. pombe where some wtf genes encode only antidotes that can act as suppressors of other driving (poison and antidote) wtf genes. Some of these antidotes can decrease fertility when expressed in the absence of the driver they suppress [81].
Recent work in mice illustrates how evolutionary tradeoffs can arise due to the fact that variants that can suppress selfish alleles may be suboptimal for other facets of gametogenesis. A selfish centromere can exploit a delay between metaphase and anaphase of meiosis I to gain a transmission advantage into oocytes [14]. The authors suggest that an organism could suppress these selfish centromeres by weakening the spindle checkpoint to speed up the onset of meiosis I. The spindle checkpoint promotes fidelity of the meiosis I division, so weakening the checkpoint may thwart drivers, but it will likely come with an increased risk of meiosis I nondisjunction [82].
The evolution of genetic incompatibilities may be exacerbated when two populations (e.g. A and B) evolve in isolation from each other and then come into secondary contact. For example, drive and resistance to the driver could evolve in population A. When the two populations come back into contact, the driver from population A could be unleashed and reduce fertility. A related possibility is that mutations suboptimal for fertility are associated with a driver in population A. In response, compensatory mutations might become fixed in population A to restore fertility. However, upon secondary contact, both the mutations associated with the driver and the compensatory mutations might interact poorly with the alleles involved in gametogenesis from population B. This could lead to fertility defects [83–85]. This phenomenon is illustrated particularly clearly in D. subobscura where genetic incompatibilities between driving X chromosomes and autosomes are uncovered in crosses between individuals from populations on either side of the Strait of Gibraltar [86].
III. Implications for human fertility
A fundamental premise of basic science research is that knowledge gained in tractable model systems can often be applied directly or indirectly to humans. Meiotic drivers have been identified as causes of infertility in a wide-range of eukaryotes and there is no reason to suspect drive does not contribute to human infertility as well. There are, however, significant challenges associated with identifying meiotic drivers in humans and in discerning how they could affect fertility.
Known human meiotic drivers
There are several classes of known meiotic drivers in humans. The first are Robertsonian chromosome fusions [87]. These chromosomes result from fusions of two acrocentric chromosomes at the centromere-proximal end [88]. Women heterozygous for a Robertsonian fusion transmit the fused chromosome to more than half of their children [87]. The origins of the transmission advantage of Robertsonian fusions in humans are unknown, largely due to inherent challenges of studying humans. The transmission advantage is predicted to result from true meiotic drive biasing chromosome segregation in favor of fused chromosomes. The prevailing model is that the chromosome fusion generates a strong centromere, analogous to that described above in mouse, that allows the fused chromosome to exploit spindle asymmetry and gain preferential access to the egg [10, 12, 13, 87, 89].
Robertsonian fusions are the most common type of chromosome rearrangement in humans, present in 1/1000 live births [88]. Like other chromosome rearrangements, these fusions can contribute to infertility and congenital birth defects due to potential problems during gametogenesis and the generation of aneuploid gametes (i.e. missing or having too many chromosomes) [88, 90]. These fusions are more common in women with recurrent pregnancy losses [91]. The fertility costs of Robertsonian fusions may be stronger in males: the fusions are roughly 9-fold more common amongst infertile males [92, 93].
The second known example of meiotic drive in humans represents a unique type of true meiotic drive in which recombinant chromatids drive in the second meiotic division. Highlighting the herculean challenge of identifying selfish loci in humans, this meiotic drive was discovered by in-depth genotyping (~300,000 loci) of polar bodies and the oocytes produced by 23 individual meioses. This drive can be considered ‘unselfish’ because it generally favors recombinant over nonrecombinant chromatids, instead of favoring any particular selfish allele [5]. This breakthrough revealed an unexpected asymmetry of the second meiotic division in humans.
There is no known fertility cost of unselfish meiotic drive favoring recombinant chromatids [5]. The underlying mechanism could, however, be vulnerable to exploitation by undiscovered selfish alleles. For example, a selfish locus could mimic or hijack whatever feature marks recombinant chromatids and enables their preferential transmission [94]. This would generate a selfish locus that could contribute to infertility via the mechanisms described above for other drivers.
The last class of known meiotic drivers acting in humans are cold spots for meiotic DSBs. As described above, the coldspots have a small transmission advantage over allelic sites that act as DSB hotspots. This drive is subtle, but has been observed directly by genotyping human sperm [95] and in molecular evolution analyses [96, 97]. There is no reason to suspect that cold spot drive could directly cause infertility, but it could contribute indirectly [19, 40]. This likely occurs in mice where, like in humans, DSB hotspots are determined by PRDM9, a zinc-finger DNA-binding histone methyl transferase [19]. Drive against DSB hotspots leads to their inactivation via mutation into coldspots that no longer bind PRDM9 [98, 99]. When such a coldspot is heterozygous with an intact hotspot, the binding of PRDM9 is asymmetric between the homologous chromosomes at that site. Through unknown mechanisms, symmetric PRDM9 binding promotes proper meiotic recombination and meiotic progression [98]. Extensive asymmetric PRDM9 binding, as in hybrids, is associated with meiotic arrest and infertility [98–100].
Challenges detecting additional drivers in humans
Most species with well-characterized meiotic drivers are genetically tractable where minimal effort in the laboratory can yield hundreds of offspring allowing for both genetic confirmation of drive and considerable statistical power. Humans lack this genetic tractability. While the amount of genetic data is nearly limitless, offspring counts are exceedingly small and reliable pedigree data are rare (but see [101–103]). Drivers can also be detected via sequencing many gametes, but this is expensive and is, for now, uncommon [5].
Predicted human meiotic drivers
Because of these obstacles, some candidate meiotic drive loci in humans have been identified using indirect evidence, rather than actual observed allele transmission biases. One class of predicted human meiotic drivers are selfish centromeres not associated with chromosome rearrangements. As described above, selfish centromeres are predicted to be pervasive throughout eukaryotes, including primates [89, 104, 105]. In mice, differences in centromere strength can be attributed to differences in the centromeric satellite repeats [13]. Centromeric satellite repeats also vary in humans, setting up the possibility that some women could be heterozygous for a strong and a relatively weak centromere [106, 107]. The idea that human centromeres can vary in strength is supported by analyses of chromosomes with multiple possible centromere sites. On these chromosomes, one of the two chromosomes (the weaker one) is preferentially epigenetically inactivated [107]. It is currently unknown if or how human centromere heterozygosity impacts fertility.
The second class of loci currently predicted to be human drivers consist of the VCX and VCY gene families found on the X and Y chromosomes, respectively [108]. There are two copies of VCY and ~12 copies of VCX [108]. The VCX and VCY genes are all very similar (~96% identical). The major difference between them is that VCY genes carry a single copy of a 30 base pair sequence that is tandemly repeated in the VCX genes 2-30 times. VCX orthologs are only found in humans and simian primates [108]. In humans, expression of VCX and VCY was only observed in germ cells, but they may have additional expression as deletion of VCX is associated with intellectual disability [108, 109].
In addition to their germline expression, the hypothesis that these genes could be meiotic drivers is based on two observations. The first is that novel duplicates of VCX that occurred in the human lineages have evolved under positive selection [108, 110]. Positive selection is a hallmark of genes involved in genetic conflict, including several known meiotic drivers and their suppressors [28, 66, 111]. The second is that VCX and VCY share similarities to the Slx and Sly gene families of mouse [112]. Slx and Sly are also lineage restricted germline specific gene families. Slx is found in 25 copies (Slx and Slxl1) on the X chromosome and Sly is found in 126 copies on the Y chromosome [112, 113]. Both gene families promote drive of their respective chromosomes through unknown mechanisms [114–116].
Although the possible contributions of VCY and VCX to meiotic drive have not been tested, copy number variation of VCX has been tied to human infertility. In women, increased copy number of VCX is associated with premature ovarian failure [117]. In men, increased copy number is associated with non-obstructive azoospermia (no sperm in semen). Although the function of the gene is still unknown, overexpression of VCX in mouse and human cell culture led to decreased cell proliferation and apoptosis [118].
IV. Concluding thoughts
Evolutionary biologists have long recognized that the molecular genetic architecture of a trait or process is not the best engineering design possible. Instead, the machinery of life is the product of a long evolutionary history of gaining fitness incrementally in response to the available genetic resources and selective pressures at any given time [119]. Gametogenesis is no exception – selection has not simply designed gametogenesis to be as effective at producing viable gametes as possible. Instead, selection has had to work to find an uncomfortable and unstable state where enough good gametes are made to contribute to the next generation. It must do so while keeping the spread of selfish genetic elements in check. The fact that genes involved in reproduction are usually very fast evolving is consistent with this scenario [120, 121]. Both beneficial alleles and selfish parasitic alleles play major roles in gametogenesis and understanding the causes of infertility will require a greater understanding of both types of alleles.
Acknowledgements
We thank Mark Miller for the illustrations and are grateful to members of the Zanders labs for their comments on the manuscript. Research in the lab of SEZ is supported by The Stowers Institute for Medical Research, March of Dimes Foundation Grant No. 5-FY18-58, a Searle Scholars Award, and the National Institutes of Health (NIH) under the award numbers R00GM114436 and DP2GM132936. RLU is supported by NIH award numbers R00GM114714 and R01AI139154.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests
SEZ is an inventor on patent application based on wtf killers. Patent application serial 62/491,107. RLU declares that no competing interests exist.
References
- 1.Burt A and Trivers R, Genes in conflict : the biology of selfish genetic elements 2006, Cambridge, Mass.: Belknap Press of Harvard University Press; viii, 602 p., 8 p. of plates. [Google Scholar]
- 2.Sandler L and Novitski E, Meiotic Drive as an Evolutionary Force. The American Naturalist, 1957. 91(857): p. 105–110. [Google Scholar]
- 3.Lindholm AK, et al. , The Ecology and Evolutionary Dynamics of Meiotic Drive. Trends Ecol Evol, 2016. 31(4): p. 315–26. [DOI] [PubMed] [Google Scholar]
- 4.Bravo Nunez MA, Nuckolls NL, and Zanders SE, Genetic Villains: Killer Meiotic Drivers. Trends Genet, 2018. 34(6): p. 424–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ottolini CS, et al. , Genome-wide maps of recombination and chromosome segregation in human oocytes and embryos show selection for maternal recombination rates. Nat Genet, 2015. 47(7): p. 727–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Didion JP, et al. , A multi-megabase copy number gain causes maternal transmission ratio distortion on mouse chromosome 2. PLoS Genet, 2015. 11(2): p. e1004850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xie Y, et al. , Interspecific Hybrid Sterility in Rice Is Mediated by OgTPR1 at the S1 Locus Encoding a Peptidase-like Protein. Mol Plant, 2017. 10(8): p. 1137–1140. [DOI] [PubMed] [Google Scholar]
- 8.Shen R, et al. , Genomic structural variation-mediated allelic suppression causes hybrid male sterility in rice. Nat Commun, 2017. 8(1): p. 1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu X, et al. , A selfish genetic element confers non-Mendelian inheritance in rice. Science, 2018. 360(6393): p. 1130–1132. [DOI] [PubMed] [Google Scholar]
- 10.Chmatal L, et al. , Centromere strength provides the cell biological basis for meiotic drive and karyotype evolution in mice. Curr Biol, 2014. 24(19): p. 2295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wei KH, et al. , A Pooled Sequencing Approach Identifies a Candidate Meiotic Driver in Drosophila. Genetics, 2017. 206(1): p. 451–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akera T, et al. , Spindle asymmetry drives non-Mendelian chromosome segregation. Science, 2017. 358(6363): p. 668–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iwata-Otsubo A, et al. , Expanded Satellite Repeats Amplify a Discrete CENP-A Nucleosome Assembly Site on Chromosomes that Drive in Female Meiosis. Curr Biol, 2017. 27(15): p. 2365–2373 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akera TTA; Lampson M, Molecular and evolutionary strategies of meiotic cheating by selfish centromeres. bioRxiv, 2018. doi: 10.1101/405068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Helleu Q, et al. , Rapid evolution of a Y-chromosome heterochromatin protein underlies sex chromosome meiotic drive. Proc Natl Acad Sci U S A, 2016. 113(15): p. 4110–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cazemajor M, Joly D, and Montchamp-Moreau C, Sex-ratio meiotic drive in Drosophila simulans is related to equational nondisjunction of the Y chromosome. Genetics, 2000. 154(1): p. 229–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gray S and Cohen PE, Control of Meiotic Crossovers: From Double-Strand Break Formation to Designation. Annu Rev Genet, 2016. 50: p. 175–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boulton A, Myers RS, and Redfield RJ, The hotspot conversion paradox and the evolution of meiotic recombination. Proc Natl Acad Sci U S A, 1997. 94(15): p. 8058–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grey C, Baudat F, and de Massy B, PRDM9, a driver of the genetic map. PLoS Genet, 2018. 14(8): p. e1007479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Champer J, Buchman A, and Akbari OS, Cheating evolution: engineering gene drives to manipulate the fate of wild populations. Nat Rev Genet, 2016. 17(3): p. 146–59. [DOI] [PubMed] [Google Scholar]
- 21.Zimmering S, Sandler L, and Nicoletti B, Mechanisms of meiotic drive. Annu Rev Genet, 1970. 4: p. 409–36. [DOI] [PubMed] [Google Scholar]
- 22.Taylor DR and Ingvarsson PK, Common features of segregation distortion in plants and animals. Genetica, 2003. 117(1): p. 27–35. [DOI] [PubMed] [Google Scholar]
- 23.Jaenike J, Sex chromosome meiotic drive. Annual Review of Ecology and Systematics, 2001. 32: p. 25–49. [Google Scholar]
- 24.Endo TR, Gametocidal chromosomes and their induction of chromosome mutations in wheat. The Japanese Journal of Genetics, 1990. 65(3): p. 135–152. [Google Scholar]
- 25.Rick CM, Abortion of male and female gametes in the tomato determined by allelic interaction. Genetics, 1966. 53(1): p. 85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sano Y, The genic nature of gamete eliminator in rice. Genetics, 1990. 125(1): p. 183–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Presgraves DC, The molecular evolutionary basis of species formation. Nat Rev Genet, 2010. 11(3): p. 175–80. [DOI] [PubMed] [Google Scholar]
- 28.Phadnis N and Orr HA, A single gene causes both male sterility and segregation distortion in Drosophila hybrids. Science, 2009. 323(5912): p. 376–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nuckolls NL, et al. , wtf genes are prolific dual poison-antidote meiotic drivers. Elife, 2017. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu W, et al. , A large gene family in fission yeast encodes spore killers that subvert Mendel’s law. Elife, 2017. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grognet P, et al. , Genes that bias Mendelian segregation. PLoS Genet, 2014. 10(5): p. e1004387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Long Y, et al. , Hybrid male sterility in rice controlled by interaction between divergent alleles of two adjacent genes. Proc Natl Acad Sci U S A, 2008. 105(48): p. 18871–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dalstra HJ, et al. , Sexual transmission of the [Het-S] prion leads to meiotic drive in Podospora anserina. Proc Natl Acad Sci U S A, 2003. 100(11): p. 6616–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang J, et al. , A killer-protector system regulates both hybrid sterility and segregation distortion in rice. Science, 2012. 337(6100): p. 1336–40. [DOI] [PubMed] [Google Scholar]
- 35.Chen J, et al. , A triallelic system of S5 is a major regulator of the reproductive barrier and compatibility of indica-japonica hybrids in rice. Proc Natl Acad Sci U S A, 2008. 105(32): p. 11436–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tao Y, et al. , A sex-ratio meiotic drive system in Drosophila simulans. II: an X-linked distorter. PLoS Biol, 2007. 5(11): p. e293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tao Y, Hartl DL, and Laurie CC, Sex-ratio segregation distortion associated with reproductive isolation in Drosophila. Proc Natl Acad Sci U S A, 2001. 98(23): p. 13183–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tao Y, et al. , A sex-ratio meiotic drive system in Drosophila simulans. I: an autosomal suppressor. PLoS Biol, 2007. 5(11): p. e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin CJ, et al. , The hpRNA/RNAi Pathway Is Essential to Resolve Intragenomic Conflict in the Drosophila Male Germline. Dev Cell, 2018. 46(3): p. 316–326 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mihola O, et al. , A mouse speciation gene encodes a meiotic histone H3 methyltransferase. Science, 2009. 323(5912): p. 373–5. [DOI] [PubMed] [Google Scholar]
- 41.Price TA and Wedell N, Selfish genetic elements and sexual selection: their impact on male fertility. Genetica, 2008. 134(1): p. 99–111. [DOI] [PubMed] [Google Scholar]
- 42.McDermott SR and Noor MA, The role of meiotic drive in hybrid male sterility. Philos Trans R Soc Lond B Biol Sci, 2010. 365(1544): p. 1265–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Turner BC and Perkins DD, Spore killer, a chromosomal factor in neurospora that kills meiotic products not containing it. Genetics, 1979. 93(3): p. 587–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hammond TM, et al. , Molecular dissection of Neurospora Spore killer meiotic drive elements. Proc Natl Acad Sci U S A, 2012. 109(30): p. 12093–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wood RJ and Newton ME, Sex-ratio distortion caused by meiotic drive in mosquitoes. The American Naturalist, 1991. 137(3): p. 379–391. [Google Scholar]
- 46.Hartl DL, Dysfunctional sperm production in Drosophila melanogaster males homozygous for the segregation distorter elements. Proc Natl Acad Sci U S A, 1969. 63(3): p. 782–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nicoletti B, Trippa G, and DeMarco A, Reduced fertility in SD males and its bearing on segregation distortion in Drosophila melanogaster. Atti Accad. Naz. Lincei Mem. Cl. Sci. Fis. Mat. Nat 1967. 43: p. 383–392. [Google Scholar]
- 48.Wilkinson GS and Fry CL, Meiotic drive alters sperm competitive ability in stalk-eyed flies. Proc Biol Sci, 2001. 268(1485): p. 2559–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nasuda S, Friebe B, and Gill BS, Gametocidal genes induce chromosome breakage in the interphase prior to the first mitotic cell division of the male gametophyte in wheat. Genetics, 1998. 149(2): p. 1115–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Taylor DR, Saur MJ, and Adams E, Pollen Performance and Sex-Ratio Evolution in a Dioecious Plant. Evolution, 1999. 53(4): p. 1028–1036. [DOI] [PubMed] [Google Scholar]
- 51.Unckless RL, Larracuente AM, and Clark AG, Sex-ratio meiotic drive and Y-linked resistance in Drosophila affinis. Genetics, 2015. 199(3): p. 831–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Voelker RA, Preliminary characterization of “sex ratio” and rediscovery and reinterpretation of “male sex ratio” in Drosophila affinis. Genetics, 1972. 71(4): p. 597–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bauer H, et al. , The t complex-encoded GTPase-activating protein Tagapl acts as a transmission ratio distorter in mice. Nat Genet, 2005. 37(9): p. 969–73. [DOI] [PubMed] [Google Scholar]
- 54.Bauer H, et al. , The t-complex-encoded guanine nucleotide exchange factor Fgd2 reveals that two opposing signaling pathways promote transmission ratio distortion in the mouse. Genes Dev, 2007. 21(2): p. 143–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bauer H, et al. , The nucleoside diphosphate kinase gene Nme3 acts as quantitative trait locus promoting non-Mendelian inheritance. PLoS Genet, 2012. 8(3): p. e1002567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Olds-Clarke P, Models for male infertility: the t haplotypes. Rev Reprod, 1997. 2(3): p. 157–64. [DOI] [PubMed] [Google Scholar]
- 57.Herrmann BG, et al. , A protein kinase encoded by the t complex responder gene causes non-mendelian inheritance. Nature, 1999. 402(6758): p. 141–6. [DOI] [PubMed] [Google Scholar]
- 58.Manser A, et al. , Sperm competition suppresses gene drive among experimentally evolving populations of house mice. Mol Ecol, 2017. 26(20): p. 5784–5792. [DOI] [PubMed] [Google Scholar]
- 59.Sutter A and Lindholm AK, Detrimental effects of an autosomal selfish genetic element on sperm competitiveness in house mice. Proc Biol Sci, 2015. 282(1811). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sutter A and Lindholm AK, Meiotic drive changes sperm precedence patterns in house mice: potential for male alternative mating tactics? BMC Evol Biol, 2016. 16(1): p. 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zanders SE, et al. , Genome rearrangements and pervasive meiotic drive cause hybrid infertility in fission yeast. Elife, 2014. 3: p. e02630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Frank SA, Divergence of Meiotic Drive-Suppression Systems as an Explanation for Sex-Biased Hybrid Sterility and Inviability. Evolution, 1991. 45(2): p. 262–267. [DOI] [PubMed] [Google Scholar]
- 63.Hurst LD and Pomiankowski A, Causes of sex ratio bias may account for unisexual sterility in hybrids: a new explanation of Haldane’s rule and related phenomena. Genetics, 1991. 128(4): p. 841–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jeffares DC, et al. , Transient structural variations have strong effects on quantitative traits and reproductive isolation in fission yeast. Nat Commun, 2017. 8: p. 14061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lopez Hernandez JF and Zanders SE, Veni, vidi, vici: the success of wtf meiotic drivers in fission yeast. Yeast, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Eickbush MTY,JM; Zanders SE , Killer meiotic drive and dynamic evolution of the wtf gene family. biioRxiv, 2018. doi: 10.1101/461004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Henikoff S, Ahmad K, and Malik HS, The centromere paradox: stable inheritance with rapidly evolving DNA. Science, 2001. 293(5532): p. 1098–102. [DOI] [PubMed] [Google Scholar]
- 68.Fishman L and Saunders A, Centromere-associated female meiotic drive entails male fitness costs in monkeyflowers. Science, 2008. 322(5907): p. 1559–62. [DOI] [PubMed] [Google Scholar]
- 69.Crow JF, Why is Mendelian segregation so exact? Bioessays, 1991. 13(6): p. 305–12. [DOI] [PubMed] [Google Scholar]
- 70.Hartl DL, A mathematical model for recessive lethal segregation distorters with differential viabilities in the sexes. Genetics, 1970. 66(1): p. 147–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Curtsinger JW and Feldman MW, Experimental and Theoretical Analysis of the “Sex-Ratio” Polymorphism in DROSOPHILA PSEUDOOBSCURA. Genetics, 1980. 94(2): p. 445–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Klein J, Sipos P, and Figueroa F, Polymorphism of t-complex genes in European wild mice. Genetics Research, 1984. 44: p. 39–46. [Google Scholar]
- 73.Schimenti JC, Reynolds JL, and Planchart A, Mutations in Seracl or Synj2 cause proximal t haplotype-mediated male mouse sterility but not transmission ratio distortion. Proc Natl Acad Sci U S A, 2005. 102(9): p. 3342–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Larracuente AM and Presgraves DC, The selfish Segregation Distorter gene complex of Drosophila melanogaster. Genetics, 2012. 192(1): p. 33–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Harvey AM, et al. , A critical component of meiotic drive in Neurospora is located near a chromosome rearrangement. Genetics, 2014. 197(4): p. 1165–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Justice MJ and Bode VC, Genetic analysis of mouse t haplotypes using mutations induced by ethylnitrosourea mutagenesis: the order of T and qk is inverted in t mutants. Genetics, 1988. 120(2): p. 533–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dyer KA, Charlesworth B, and Jaenike J, Chromosome-wide linkage disequilibrium as a consequence of meiotic drive. Proc Natl Acad Sci U S A, 2007. 104(5): p. 1587–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hartl DL, Population dynamics of sperm and pollen killers. Theor Appl Genet, 1972. 42(2): p. 81–8. [DOI] [PubMed] [Google Scholar]
- 79.Fisher RA, The genetical theory of natural selection. 1930, Oxford,: The Clarendon press; xiv, 272 p. [Google Scholar]
- 80.Carvalho AB, et al. , An experimental demonstration of Fisher’s principle: evolution of sexual proportion by natural selection. Genetics, 1998. 148(2): p. 719–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bravo Núñez MA, Lange JJ, Zanders SE., A suppressor of a wtf poison-antidote meiotic driver acts via mimicry of the driver’s antidote. PLoS Genet. 2018. November 26;14(11):e1007836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gorbsky GJ, The spindle checkpoint and chromosome segregation in meiosis. FEBS J, 2015. 282(13): p. 2471–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Orr HA and Turelli M, The evolution of postzygotic isolation: accumulating Dobzhansky-Muller incompatibilities. Evolution, 2001. 55(6): p. 1085–94. [DOI] [PubMed] [Google Scholar]
- 84.Unckless RL and Orr HA, Dobzhansky-Muller incompatibilities and adaptation to a shared environment. Heredity (Edinb), 2009. 102(3): p. 214–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Welch JJ, Accumulating Dobzhansky-Muller incompatibilities: reconciling theory and data. Evolution, 2004. 58(6): p. 1145–56. [DOI] [PubMed] [Google Scholar]
- 86.Verspoor RL, et al. , Strong hybrid male incompatibilities impede the spread of a selfish chromosome between populations of a fly. Evol Lett, 2018. 2(3): p. 169–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pardo-Manuel de Villena F and Sapienza C, Transmission ratio distortion in offspring of heterozygous female carriers of Robertsonian translocations. Hum Genet, 2001. 108(1): p. 31–6. [DOI] [PubMed] [Google Scholar]
- 88.Morin SJ, et al. , Translocations, inversions and other chromosome rearrangements. Fertil Steril, 2017. 107(1): p. 19–26. [DOI] [PubMed] [Google Scholar]
- 89.Malik HS and Henikoff S, Major evolutionary transitions in centromere complexity. Cell, 2009. 138(6): p. 1067–82. [DOI] [PubMed] [Google Scholar]
- 90.Roux C, et al. , Segregation of chromosomes in sperm of Robertsonian translocation carriers. Cytogenet Genome Res, 2005. 111(3-4): p. 291–6. [DOI] [PubMed] [Google Scholar]
- 91.Fryns JP and Van Buggenhout G, Structural chromosome rearrangements in couples with recurrent fetal wastage. Eur J Obstet Gynecol Reprod Biol, 1998. 81(2): p. 171–6. [DOI] [PubMed] [Google Scholar]
- 92.Daniel A, Distortion of female meiotic segregation and reduced male fertility in human Robertsonian translocations: consistent with the centromere model of coevolving centromere DNA/centromeric histone (CENP-A). Am J Med Genet, 2002. 111(4): p. 450–2. [DOI] [PubMed] [Google Scholar]
- 93.De Braekeleer M and Dao TN, Cytogenetic studies in male infertility: a review. Hum Reprod, 1991. 6(2): p. 245–50. [PubMed] [Google Scholar]
- 94.Zanders SE and Malik HS, Chromosome segregation: human female meiosis breaks all the rules. Curr Biol, 2015. 25(15): p. R654–6. [DOI] [PubMed] [Google Scholar]
- 95.Jeffreys AJ and Neumann R, Reciprocal crossover asymmetry and meiotic drive in a human recombination hot spot. Nat Genet, 2002. 31(3): p. 267–71. [DOI] [PubMed] [Google Scholar]
- 96.Lesecque Y, et al. , The red queen model of recombination hotspots evolution in the light of archaic and modern human genomes. PLoS Genet, 2014. 10(11): p. e1004790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Myers S, et al. , Drive against hotspot motifs in primates implicates the PRDM9 gene in meiotic recombination. Science, 2010. 327(5967): p. 876–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Davies B, et al. , Re-engineering the zinc fingers of PRDM9 reverses hybrid sterility in mice. Nature, 2016. 530(7589): p. 171–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Smagulova F, et al. , The evolutionary turnover of recombination hot spots contributes to speciation in mice. Genes Dev, 2016. 30(3): p. 266–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gregorova S, et al. , Modulation of Prdm9-controlled meiotic chromosome asynapsis overrides hybrid sterility in mice. Elife, 2018. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liu Y, et al. , Identification of two maternal transmission ratio distortion loci in pedigrees of the Framingham heart study. Sci Rep, 2013. 3: p. 2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Huang LO, Infante-Rivard C, and Labbe A, Analysis of Case-Parent Trios Using a Loglinear Model with Adjustment for Transmission Ratio Distortion. Front Genet, 2016. 7: p. 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Meyer WK, et al. , Evaluating the evidence for transmission distortion in human pedigrees. Genetics, 2012. 191(1): p. 215–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pardo-Manuel de Villena F and Sapienza C, Female meiosis drives karyotypic evolution in mammals. Genetics, 2001. 159(3): p. 1179–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Schueler MG, et al. , Adaptive evolution of foundation kinetochore proteins in primates. Mol Biol Evol, 2010. 27(7): p. 1585–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Miga KH, et al. , Centromere reference models for human chromosomes X and Y satellite arrays. Genome Res, 2014. 24(4): p. 697–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sullivan LL, Chew K, and Sullivan BA, alpha satellite DNA variation and function of the human centromere. Nucleus, 2017. 8(4): p. 331–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lahn BT and Page DC, A human sex-chromosomal gene family expressed in male germ cells and encoding variably charged proteins. Hum Mol Genet, 2000. 9(2): p. 311–9. [DOI] [PubMed] [Google Scholar]
- 109.Van Esch H, et al. , Deletion of VCX-A due to NAHR plays a major role in the occurrence of mental retardation in patients with X-linked ichthyosis. Hum Mol Genet, 2005. 14(13): p. 1795–803. [DOI] [PubMed] [Google Scholar]
- 110.Han MV, et al. , Adaptive evolution of young gene duplicates in mammals. Genome Res, 2009. 19(5): p. 859–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.McLaughlin RN Jr. and Malik HS, Genetic conflicts: the usual suspects and beyond. J Exp Biol, 2017. 220(Pt 1): p. 6–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Soh YQ, et al. , Sequencing the mouse Ychromosome reveals convergent gene acquisition and amplification on both sex chromosomes. Cell, 2014. 159(4): p. 800–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mueller JL, et al. , The mouse Xchromosome is enriched for multicopy testis genes showing postmeiotic expression. Nat Genet, 2008. 40(6): p. 794–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cocquet J, et al. , A genetic basis for a postmeiotic X versus Y chromosome intragenomic conflict in the mouse. PLoS Genet, 2012. 8(9): p. e1002900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cocquet J, et al. , The multicopy gene Sly represses the sex chromosomes in the male mouse germline after meiosis. PLoS Biol, 2009. 7(11): p. e1000244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Conway SJ, et al. , Y353/B: a candidate multiple-copy spermiogenesis gene on the mouse Y chromosome. Mamm Genome, 1994. 5(4): p. 203–10. [DOI] [PubMed] [Google Scholar]
- 117.Quilter CR, et al. , Analysis ofXchromosome genomic DNA sequence copy number variation associated with premature ovarian failure (POF). Hum Reprod, 2010. 25(8): p. 2139–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ji J, et al. , Copy number gain of VCX, X-linked multi-copy gene, leads to cell proliferation and apoptosis during spermatogenesis. Oncotarget, 2016. 7(48): p. 78532–78540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dawkins R, The blind watchmaker. 1st American ed. 1986, New York: Norton; xiii, 332 p. [Google Scholar]
- 120.Swanson WJV, V. D, The rapid evolution of reproductive proteins. Nature Reviews Genetics, 2002. 3: p. 137–144. [DOI] [PubMed] [Google Scholar]
- 121.Anderson JA, Gilliland WD, and Langley CH, Molecular population genetics and evolution of Drosophila meiosis genes. Genetics, 2009. 181(1): p. 177–85. [DOI] [PMC free article] [PubMed] [Google Scholar]


