EPIDEMIOLOGY OF PROLONGED POSTCONCUSSIVE SYMPTOMS
Since the early 20th century, when large numbers of World War I veterans returned from front lines with “shell shock” (1), there has been significant controversy regarding the nature of postconcussive symptoms that persist or emerge after a mild traumatic brain injury (mTBI). Symptoms such as headache, nausea, and blurry vision were originally thought to resolve in the first 7–10 days after injury for the vast majority (80%290%) of individuals (2, 3). More recent studies indicate that symptoms will persist in a significant minority of cases, with functional impairment in up to 33% at 3 months and 22% at 1 year, as recently described in a report from the Transforming Research and Clinical Knowledge in Traumatic Brain Injury (TRACK-TBI) research group (4). The pace and extent of recovery from mTBI are now understood to vary considerably depending on patient and injury characteristics, and recovery may take 6 months or longer (5). A predominant historical view holds that prolonged postconcussive symptoms represent a phenomenon with significant somatoform features that may have been present before the injury or may emerge well after the injury and that resemble the nonspecific symptoms experienced after any type of bodily trauma (1, 6). However, more contemporary research suggests that multiple etiologies contribute to prolonged postconcussive symptoms, including psychogenic factors (e.g., coping style and mood and anxiety disorders), cervicogenic factors (e.g., neck injury), and neurophysiological causes (e.g., cerebrovascular dysregulation, microscopic white matter damage) (7–9). As mTBI is a significant public health issue, with an estimated 42 million mTBIs sustained worldwide every year (10), even a conservative estimate of the prevalence of prolonged postconcussive symptoms (10%215% of mTBIs) suggests a substantial unrecognized burden of hundreds of thousands of new patients with such symptoms each year.
Current mTBI guidelines recommend psychiatric referral and treatment for prolonged postconcussive symptoms, without specificity regarding the nature of the psychiatric treatment (11, 12). With the elimination of the “postconcussional disorder” diagnostic entity in DSM-5, evidence-based recommendations for differential diagnosis and treatment are needed for psychiatrists who receive referrals of patients with prolonged postconcussive symptoms after mTBI.
DEFINITIONS
The Centers for Disease Control and Prevention definition of a TBI is “a disruption in the normal function of the brain that canbe caused by a bump, blow, or jolt to the head or a penetrating head injury” (2). Any one of the following is considered disruption of brain function: any period of loss of or decreased consciousness; any loss of memory for events before or after the injury (posttraumatic amnesia); any neurological deficits such as weakness, imbalance or dyscoordination, disruption of vision, change in speech or language, or sensory loss; or any alteration of consciousness at the time of injury, such as confusion, disorientation, slowed thinking, or difficulty with concentration (2).
Mild TBI (considered synonymous with the term “concussion” for this review) is defined by the American Congress of Rehabilitation Medicine as a traumatic brain injury that results in the following, if present: 1) loss of consciousness for up to 30 minutes, 2) alteration of consciousness for less than 24 hours, 3) posttraumatic amnesia for less than 24 hours, and 4) a Glasgow Coma Scale score of 13–15 at 30 minutes after injury (13). These variables are also used to distinguish moderate TBI (loss of consciousness for 30 minutes to 24 hours, alteration of consciousness or posttraumatic amnesia for 24 hours to 7 days, Glasgow Coma Scale score of 9–12) and severe TBI (loss of consciousness >24 hours, alteration of consciousness/posttraumatic amnesia for >7 days, Glasgow Coma Scale score <9), in which recovery is generally more prolonged and functional recovery less likely (14). Postconcussive symptoms typically fall into one of four categories: vestibular (e.g., imbalance, nausea, dizziness), sensory (e.g., blurry vision, migraines, tinnitus, photo/phonophobia), cognitive (e.g., difficulty focusing, forgetfulness), and emotional (e.g., fatigue, insomnia, irritability, depression) (2). Examples of commonly used validated rating scales for postconcussive symptoms include the Neurobehavioral Symptom Inventory, the Rivermead Post-Concussion Symptoms Questionnaire, and the Post-Concussion Symptom Scale (15–17).
There is no universal agreement on the time period required for postconcussive symptoms to become “prolonged.” The 2017 Berlin consensus statement on concussion in sports (18) specifies “persistent symptoms” as those lasting longer than 10–14 days in adults and 1 month in children. However, epidemiological studies are mixed regarding the validity of these duration criteria, demonstrating a range of outcomes, from complete recovery by 3 months (19) to prolonged recovery requiring up to 1 year (5). Postconcussional disorder was omitted from DSM-5, which instead instructs psychiatrists to diagnose either major or mild neurocognitive disorder due to traumatic brain injury, depending on the extent of cognitive and functional deficit. In the subsection “Development and Course,” DSM-5 states that in most cases of mTBI, symptoms have resolved completely by 3 months.
The only other standardized set of criteria for prolonged postconcussive symptoms is found in ICD-10, which does not specify a duration criterion and labels the condition “postconcussional syndrome.” Most guidelines now refer to postconcussive “symptoms” rather than a “syndrome” because of the nonspecificity of complaints (11, 12, 20). Whereas the DSM-5 criteria are oriented toward prolonged postconcussive symptoms as a neurological disorder (must have objective evidence of cognitive difficulties), the ICD-10 criteria approach prolonged postconcussive symptoms as a somatic symptom disorder (e.g., neuropsychological testing results may be normal, hypochondriasis may be present). While numerous studies demonstrate that prolonged postconcussive symptoms are highly associated with non-mTBI psychological factors (21, 22), there is also growing evidence from neuroimaging and neurophysiology studies that there is occult brain dysfunction underlying prolonged symptoms (23, 24). Therefore, an approach to prolonged postconcussive symptoms should recognize the neurophysiological, cervicogenic, and psychogenic contributors to the condition and assign probabilistic weights to these factors depending on how long symptoms have persisted after injury.
The ICD-10 criteria (20) for postconcussional syndrome (F07.2) are as follows:
The general criteria of F07 must be met (“Personality and behavior disorders due to known physiological condition”)
History of head trauma with loss of consciousness, preceding the onset of symptoms by a period of up to 4 weeks (objective evidence for brain damage may be lacking).
- At least three of the following must be present:
- Complaints of unpleasant sensations and pains, such as headache, dizziness (usually lacking the features of true vertigo), general malaise and excessive fatigue, or noise intolerance.
- Emotional changes, such as irritability, emotional lability, both easily provoked or exacerbated by emotional excitement or stress, or some degree of depression and/ or anxiety.
- Subjective complaints of difficulty in concentration and in performing mental tasks, and of memory complaints, without clear objective evidence (e.g., psychological tests) of marked impairment.
- Insomnia.
- Reduced tolerance to alcohol.
- Preoccupation with the above symptoms and fear of permanent brain damage, to the extent of hypochondriacal, overvalued ideas, and adoption of a sick role.
The DSM-5 criteria for major/mild neurocognitive disorder due to traumatic brain injury are as follows:
The criteria are met for major or mild neurocognitive disorder (i.e., significant/moderate cognitive decline based on clinical concern or testing; does/does not interfere with independence in daily living).
- There is evidence of a traumatic brain injury—that is, an impact to the head or other mechanisms of rapid movement or displacement of the brain within the skull, with one or more of the following:
- Loss of consciousness.
- Posttraumatic amnesia.
- Disorientation and confusion.
- Neurological signs (e.g., neuroimaging demonstrating injury; a newonset of seizures; a marked worsening of a preexisting seizure disorder; visual field cuts; anosmia; hemiparesis).
The neurocognitive disorder presents immediately after the occurrence of the traumatic brain injury or immediately after recovery of consciousness and persists past the acute postinjury period.
ASSESSMENT
To appropriately select treatments for a patient with prolonged postconcussive symptoms, in addition to taking a comprehensive medical and psychiatric history, evaluating psychiatrists should re-elicit the complete history of the mTBI to ensure that crucial diagnostic and prognostic details were not missed. They should perform a full physical and neurological examination, with special attention to smooth pursuits, saccades, accommodation, convergence, vestibulo-ocular reflex, balance, orthostatics, and neck examination (25, 26), to assess for signs of ongoing neurophysiological or cervicogenic contributors. The focus may then turn to the following key questions:
1. What were the characteristics, context, and mechanism of the mTBI?
TBI survivors may be unaware of the details of their injury owing to loss of consciousness or posttraumatic amnesia. Confirming the TBI severity (mild, moderate, severe) with outside records or collateral history improves prognostication regarding expected improvement. Whereas prolonged postconcussive symptoms are a phenomenon associated predominantly with mTBIs, moderate and severe TBIs are more likely to result in enduring neurological deficits, with up to 75% significantly disabled at 1 year, whereas with mTBIs, 75% are recovered at 1 year (4, 14).
The clinician should also ask about the context and mechanism of the injury, as these have varying associations with prolonged postconcussive symptoms. Sports injuries are unique in that they result from voluntary participation and may involve protective gear. These injuries are not typically accompanied by loss of consciousness, and they have high rates of full recovery (27). In contrast, mTBIs after assaults, after vehicular trauma, or during military service are highly associated with posttraumatic stress disorder (PTSD), which is associated with an increased risk of prolonged postconcussive symptoms (28, 29).
2. Is there evidence of traumatic brain damage on neuroimaging?
In the vast majority of mTBIs, as illustrated in the case presentation, results of structural neuroimaging on CT and T1- and T2-weighted MRI immediately after the injury will be normal. However, when there are findings such as hemorrhage, hematoma, contusion, or diffuse axonal injury (i.e., a “complicated” mTBI), the clinical course will be more consistent with a moderate TBI, with a higher likelihood of neurological deficit and disability (30, 31). In prolonged postconcussive symptoms, MRI without contrast may be considered if it has not already been obtained, given its greater sensitivity than CT for detecting evidence of contusions and shear foci stemming from the injury, while keeping in mind that intracranial abnormalities unrelated to the TBI in question may also be detected (30). Findings on MRI in mTBI are most often detected in frontotemporal areas, limbic structures, gray-white matter junctions, and long-coursing white matter tracts, such as the corpus callosum, superior longitudinal fasciculus, and corticospinal tract, and may haveassociated somatic, cognitive, and emotional effects (32). The MRI sequences most likely todetect traumatic abnormalities include fluid-attenuated inversion recovery sequences, gradient recalled echo T2*, and susceptibility-weighted imaging (33). Although numerous studies have found abnormalities in mTBI using functional MRI, diffusion tensor imaging, single-photon emission CT, and positron emission tomography, these are currently considered nonroutine and are not typically obtained during clinical evaluation (23, 34). EEG need not be obtained unless there is concern about ongoing seizures or encephalopathy, as slow waves on EEG after acute mTBI resolve within days (24).
3. How many previous mTBIs has the patient sustained, when did they occur, and how severe were they?
The more mTBIs one accumulates, the more severe prolonged postconcussive symptoms will be and the longer they will take to resolve (35, 36). This is especially true if a second mTBI occurs before a previous mTBI has fully resolved, leading to the potentially fatal phenomenon of “second-impact syndrome” (37).
4. What are the severity and trajectory of prolonged postconcussive symptoms, and what are the demographic, traumatic, and behavioral risk factors for them?
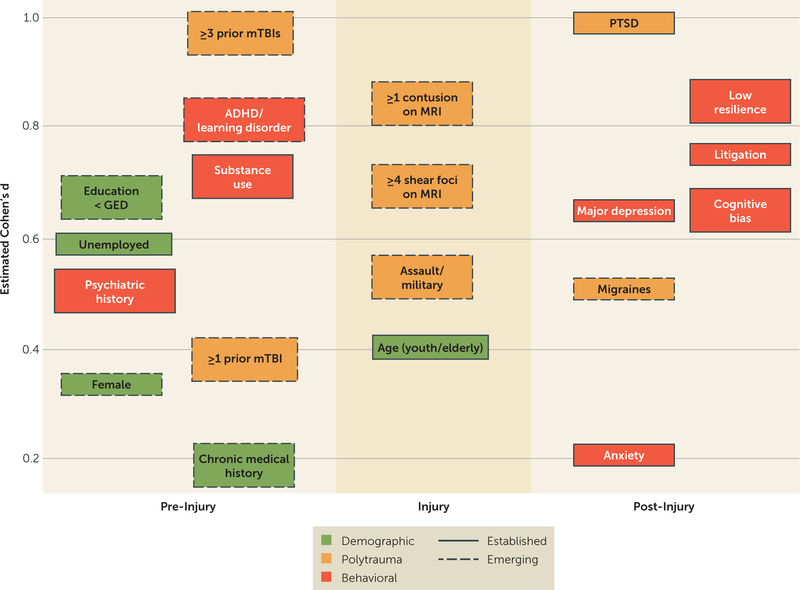
Clinicians should elicit the chronology of prolonged postconcussive symptoms in relation to time of injury, alleviating and exacerbating factors, and treatments pursued. A validated postconcussive symptom scale should be used to track symptoms, such as the Neurobehavioral Symptom Inventory, the Rivermead Post-Concussion Symptoms Questionnaire, or the Post-Concussion Symptom Scale. Graded aerobic exercise and its effect on symptom exacerbation is a valuable diagnostic maneuver that can inform treatment approaches (38). If not already done, formal neuropsychological testing should be performed to characterize cognitive deficits, effort, and symptom validity. Screening for major depressive disorder, anxiety, and PTSD with validated instruments such as the Patient Health Questionnaire, the Generalized Anxiety Disorder Questionnaire, and the PTSD Checklist is strongly recommended given the high rates of documented comorbidity with mTBI (4). Identifying risk factors for prolonged postconcussive symptoms (Figure 1) can lead to strategies for reducing symptoms. Preinjury factors that are well known to be associated with prolonged symptoms include age at time of injury (adolescents and elderly patients are at higher risk) (39, 40), female sex (41), lower education level (42), psychiatric history (43), chronic medical conditions (19), history of migraine (44), attentional, learning, or developmental disorders (45), and alcohol use disorder (46). Peri-injury factors include the mechanism of injury (military trauma versus sports injury) (28, 47). Postinjury factors include the presence of migraines (48), a diagnosis of major depressive disorder (49) or PTSD (50), a low level of resilience (51), cognitive distortions such as the “good old days” bias (52), negative expectations molded by contextual factors after diagnosis (“nocebo effects”) (53, 54), and litigation (55).
FIGURE 1. Risk Factors for Prolonged Postconcussive Symptomsa.
a Factors are arranged according to temporal relation to injury (x-axis) and estimated effect size (y-axis), calculated from articles cited in this review. When possible, preference was given to large, population-based studies. Boxes with solid lines indicate risk factors with substantial supporting literature, and boxes with dashed lines indicate risk factors supported by predominantly recent studies. Cohen’s d values were calculated from reported group means and standard deviations, odds ratios, correlation coefficients, and F statistics using established statistical methods. Effect sizes and study reference numbers are as follows: education < GED, 0.64 (30); unemployed, 0.59 (30); psychiatric history, 0.52 (19); female, 0.37 (41); ≥3 prior mTBIs, 1.0 (36); ADHD/learning disorder, 0.75 (45); substance use, 0.65 (46); ≥1 prior mTBI, 0.38 (30); chronic medical history, < 0.1 (19); ≥1 contusion on MRI, 0.83 (30); ≥4 shear foci on MRI, 0.64 (30); assault/military, 0.52 (28); age (youth/elderly), 0.40 (39); PTSD, 1.2 (29); major depression, 0.65 (49); migraines, 0.52 (48); anxiety, 0.14 (19); low resilience, 0.8 (51); litigation, 0.78 (55); and cognitive bias, 0.65 (52). Certain risk factors (e.g., major depression, migraine) are present in both the preinjury and postinjury states. For the purposes of this review, the temporal location for each risk factor was primarily determined based on the source articles from which effect sizes were determined. ADHD=attention deficit hyperactivity disorder; GED=General Equivalency Diploma; mTBI=mild traumatic brain injury; PTSD=posttraumatic stress disorder.
5. Are there other conditions masquerading as prolonged postconcussive symptoms?
Conditions unrelated to mTBI may mimic prolonged postconcussive symptoms, including medication side effects, nutritional deficiencies, neuro-degenerative disorders, infections, normal pressure hydrocephalus, and Alzheimer’s disease (56). Hypopituitarism may occur rarely after mTBI (57), and if it is suspected, laboratory screening tests may include thyroid-stimulating hormone, luteinizing hormone, follicle-stimulating hormone, insulin-like growth factor 1, early-morning cortisol, and prolactin levels. Although controversy still surrounds the clinical diagnosis of chronic traumatic encephalopathy (CTE) from repetitive mTBIs, psychiatrists should keep this diagnostic entity in mind when evaluating patients with prolonged postconcussive symptoms (58). Proposed guidelines state that if there is a decline in behavior, mood, or cognitive status following an asymptomatic period years after repetitive head injuries, with suggestive features such as apathy or motor symptoms in the absence of a more likely explanation, then CTE should be suspected (59). While the number of mTBIs necessary to increase risk of CTE is not known, there is no evidence to suggest that CTE is caused by a single mTBI (10). Definitive diagnosis of CTE at this time can only be made postmortem (58), and therapies should be directed toward any mood and cognitive manifestations.
DIFFERENTIAL DIAGNOSIS
It can be difficult to ascertain whether cognitive or emotional deficits are directly referable to the mTBI, as many associated problems (e.g., depression, pain, vestibular dysfunction) can independently result in similar symptoms. Differential diagnosis of cognitive and emotional prolonged postconcussive symptoms may fall under numerous diagnostic labels in DSM-5, depending on their features with regard to the inciting mTBI.
If there are cognitive deficits that are directly referable to structural brain damage or objective vestibular/visual dysfunction, the appropriate diagnoses are major or mild neurocognitive disorder due to mTBI, depending on the severity of deficits and functional impairment.
If prolonged postconcussive symptoms are exacerbated by a psychological factor, e.g., a coping style of denial leading to noncompliance with necessary medications for headache, then this is labeled “psychological factors affecting medical condition.”
If distress and anxiety related to the mTBI are out of proportion with the extent of objective impairment from the mTBI (e.g., significant anxiety about cognitive dysfunction despite normal neuropsychological testing results) then somatic symptom disorder may be diagnosed.
If symptoms meet full criteria for a depressive or anxiety disorder, this should be diagnosed as a primary psychiatric disorder. If the symptoms do not meet full criteria and are related to life events after the mTBI, e.g., loss of work, then adjustment disorder may be more appropriate.
If the prolonged symptoms involve neurological symptoms that have features suggestive of psychogenic etiology, e.g., sudden blindness with intact optokinetic nystagmus, and there is no evidence of factitiousness or malingering, then functional neurological symptom disorder may be applied.
If there is evidence of falsification or amplification of prolonged postconcussive symptoms, e.g., using nonprescribed medications to induce dizziness or blurry vision, and there is no evidence of material gain, factitious disorder imposed on self should be considered. Malingering should be considered if there is evidence of material gain.
TREATMENTS
There are no widely accepted, extensively studied, or Food and Drug Administration–approved treatments for prolonged postconcussive symptoms. However, small randomized controlled trials in several modalities, including medications, psychosocial interventions, metabolic supplements, physical therapies, and neurostimulation, provide clinicians a basis for formulating a comprehensive biopsychosocial treatment plan to target specific symptoms and risk factors.
Medications
To date, most medications prescribed, either on-label or off-label, are used to address physical symptoms or psychiatric syndromes occurring in patients with mTBI, such as major depressive disorder or PTSD (60). When initiating or titrating medications, the ruleof “start low, goslow” shouldbefollowed, as TBI patients will experience more medication side effects at lower doses than patients without TBI. Providers should avoid medications that blunt cognition and should use beneficial side effects to reduce physical postconcussive symptoms, e.g., sedating tricyclic antidepressants for insomnia. Because posttraumatic headaches/migraines and insomnia are among the most disabling physical postconcussive symptoms and canexacerbate cognitive and emotional symptoms, they should be treated early and aggressively (61–63). Posttraumatic headaches of nonmigrainous character (i.e., tension-type headache) may be treated with acetaminophen or nonsteroidal anti-inflammatory drugs such as ibuprofen (11), taking care to avoid more than twice weekly use to prevent rebound headaches (64). Migrainous headaches (i.e., throbbing in nature, associated with aura) may respond specifically to triptans as abortive agents, and to beta-blockers, tricyclic antidepressants, calcium channel blockers, and anticonvulsants as prophylactic agents. Insomnia may respond to trazodone, melatonin, mirtazapine, or tricyclic antidepressants, but oversedation or exacerbation of fatigue may result, and there is a paucity of evidence supporting their use after mTBI (65).
Small open-label and placebo-controlled trials have found sertraline, citalopram, milnacipran, and methylphenidate effective for post-TBI depression (66). In a meta-analysis of randomized controlled trials (67), methylphenidate was also found to be effective for attention deficits after mTBI. Other agents that may improve cognitive complaints in patients with prolonged postconcussive symptoms include selective serotonin reuptake inhibitors (SSRIs) (68), cholinesterase inhibitors (69), and lisdexamfetamine (70). Anxiety disorders and PTSD are significant contributors to prolonged symptoms (19, 28) and should be treated aggressively according to existing clinical guidelines (71). Comorbid fatigue, excessive daytime sleepiness, and apathy may respond favorably to modafinil, dopamine agonists, or traditional stimulants (72–74). There are few studies to guide the pharmacological treatment of irritability after mTBI, although SSRIs are generally tried first, before mood stabilizers (75).
Psychosocial Interventions
Numerous psychological mechanisms contribute to prolonged postconcussive symptoms, including coping styles featuring somatization, catastrophization, and an externalized locus of control; illness anxiety; contextual factors that contribute to negative expectations (“nocebo effects”) (53, 54); and cognitive biases such as “diagnosis threat” (feeling worse after receiving a diagnosis) and “good old days” bias (overestimating preinjury status) (19, 21, 43). There is a high rate of postconcussive symptoms in the non-mTBI general population (22), leading to potential misattribution of nonspecific symptoms to an mTBI when they may be more likely due to mundane causes. Patients’ expectations and concerns regarding prolonged postconcussive symptoms are a significant factor in prognosis, as distress about permanent brain damage, lost productivity, and disability can exacerbate a prolonged recovery. Education and information in the form of pamphlets, follow-up, or access to brain injury specialty care have been shown to reduce rates of prolonged symptoms (76).
Cognitive rehabilitation and training, which are typically delivered by occupational therapists or speech-language pathologists, are well studied and are formally recommended for problem solving, executive function, and mild memory impairments after TBI (77). Multimodal programs employing cognitive training designed to ameliorate postconcussive symptoms have shown benefits (78), even when delivered by computer (79), and patients should be offered holistic cognitive rehabilitation routinely if they demonstrate neuropsychological deficits.
Various types of psychotherapy for prolonged postconcussive symptoms have been studied, with cognitivebehavioral therapy (CBT) administered in person or by telephone shown in multiple trials to be effective for post-TBI depression(80), anxiety(81), andphysical symptoms (82). CBT should be routinely offered to patients with prolonged postconcussive symptoms. Problem-solving therapy has also been shown to decrease depression, anxiety, PTSD, and insomnia symptoms following mTBI in active-duty military personnel (83). A recent trial of collaborative care for prolonged postconcussive symptoms consisting of CBT, care management, and medication evaluation was shown to be effective for reducing prolonged symptoms in adolescents (84), suggesting that multidisciplinarybiopsychosocial treatment may beavalid treatment model for prolonged postconcussive symptoms.
Metabolic Treatments
In addition to medications, there may be utility in the use of metabolic supplements for prolonged postconcussive symptoms; they have a low risk of side effects, although data to support their effectiveness are lacking (85). N-acetylcysteine, pine bark (from Pinus radiata) extract, and the nootropic agent piracetam each showed benefits for postconcussive symptoms in single controlled studies (85, 86). Branched-chain amino acids have shown benefit in acute severe TBIbut have not been studied in mTBI or prolonged postconcussive symptoms (87). There has been significant controversy regarding the utility of hyperbaric oxygen therapy for prolonged postconcussive symptoms, with a recent review concluding that it was ineffective in four of five randomized controlled trials (88). While medical cannabis laws in various states permit its use in conditions comorbid with TBI, such as epilepsy, nausea, chronic pain, and PTSD, evidence of its benefits is still largely confined toanimal studies (89), and care must be takento avoid deleterious effects on cognition and mood. A large randomized controlled trial of dexanabinol for acute human brain injury was negative (90).
Physical Therapies
Physical therapy for somatic (especially vestibular and visual) postconcussive symptoms has increasing evidence for effectiveness and low risk of harm. Evaluation for somatic disturbances with the previously described physical, neurological, cervical, and vestibulo-ocular examinations and referral for specific physical therapy to address these symptoms and deficits is warranted (91, 92). Relief from somatic symptoms may result in an improvement in subjective cognition and effort. Diagnostic and therapeutic aerobic exercise is also warranted (93), as the exacerbation of symptoms during treadmill testing indicates need for further therapy, such as subthreshold graded exercise, before return to full activity (38). Several large studies support the introduction of aerobic exercise after a concussion and find that it leads to reductions in acute and chronic symptoms, particularly in children and adolescents (94). There is increasing concern that more than a few days of rest after the acute injury may not help alleviate postconcussive symptoms and may potentially prolong symptoms (95, 96).
Neurostimulation
Transcranial magnetic stimulation (TMS) and transcranial direct-current stimulation (tDCS) represent promising treatments for prolonged postconcussive symptoms (97). Two studies have demonstrated effectiveness of TMS for posttraumatic headaches and cognitive difficulties (98, 99), and tDCS has demonstrated mild positive effects on cognition in several small trials after mild to severe TBI (100, 101). Current barriers to the use of neurostimulation include their investigational status when used for prolonged postconcussive symptoms, lack of a Food and Drug Administration indication in the case of tDCS, and lack of third-party payer coverage.
CONCLUSIONS
Prolonged postconcussive symptoms after mTBI may have multiple etiologies, spanning the spectrum from the purely neurological to the purely psychogenic, and the clinician must be aware of preinjury, peri-injury, and postinjury contributors. Treatments of prolonged postconcussive symptoms should address specific symptoms with targeted interventions supported by reasonable levels of evidence and justifiable risk.
What is the usual prognosis of an acute postconcussive syndrome?
No change.
Continued deterioration.
Full resolution.
Improvement with combined therapies.
“Mr. A” had no significant medical or psychiatric history until his car collided with another vehicle. He recalled blacking out for possibly seconds to minutes before “waking up” slumped against the steering wheel. He had severe headache, nausea, dizziness, and difficulty focusing his eyes. First responders transported him to a hospital for evaluation. A CT scan of Mr. A’s head and neck showed no skull or cervical fracture or intracerebral bleeding. His vital signs and orthostatics were normal, and his physical examination demonstrated impaired horizontal tracking and convergence focusing. After being treated with fluids and antiemetics, Mr. A was discharged home with a diagnosis of concussion, with instructions to gradually increase his activities as tolerated.
Mr. A slept excessively for 2 days, taking acetaminophen and ibuprofen for throbbing whole-head pain, with mild relief. He returned to his job as a high school English teacher the following week but was fatigued, was unable to concentrate, and could not tolerate classroom noise. Although his vision was no longer blurry, while grading essays he found that the words seemed to move on the page. Unable to teach, he had totake a month off from work, applying for temporary medical leave. His primary care physician found that Mr. A had increased headache with convergence eye movements, but no saccadic, visual-acuity, or smooth-pursuit abnormalities and no physical abnormalities such as trigger points or neck tenderness. The physician prescribed nortriptyline for prophylaxis of posttraumatic headaches and sumatriptan as breakthrough therapy, and he cautioned the patient to monitor for serotonin toxicity. Mr. A asked whether an MRI scan would be necessary, and his physician said that the results would most likely be normal.
For 3 months, Mr. A’s concentration and energy continued to be impaired, making return to work impossible. Mr. A found the legal proceedings stemming from the collision to be extremely stressful. Neuropsychological testing indicated variable difficulties with processing speed, attention, and complex tasks, which were interpreted to be “due to inconsistent effort put forth,” thus calling into question the validity of the testing. Mr. A worried at night about losing his job and about financial ruin, which exacerbated hisdaytime symptoms. Prescribed graded aerobic exercise improved his headaches and fatigue tolerance but had little benefit on his cognition. After 6 months, Mr. A was informed that he would have to testify in court. A neurologist told him that prolonged postconcussive symptoms were almost always stress related and that he should see a psychiatrist.
The psychiatrist reviewed Mr. A’s history, medical records, imaging studies, and neuropsychological testing and repeated a physical examination to confirm resolution of the previous oculomotor findings. On further questioning regarding exacerbating factors, Mr. A recalled hitting his head on a cabinet the previous year, which caused dizziness but from which he recovered quickly. He scored in the clinically severe range on screening instruments for depression and anxiety. The psychiatrist requested an MRI scan, which, on susceptibility-weighted images, showed several areas of punctate hemorrhage consistent with axonal shear injury in the anterior dorsal corpus callosum. He prescribed methylphenidate, 5 mg, to be taken in the morning and early afternoon. Mr. A immediately noticed an improvement in his energy level and attention. The psychiatrist also referred Mr. A to cognitive-behavioral therapy for symptom management and coping with distress. By 1 year after the injury, Mr. A’s legal proceedings had concluded, he felt he had substantially improved, and he successfully returned to work full-time.
D. Improvement with combined therapies.
Want more? A CME course is available in the APA Learning Center at education.psychiatry.org
Acknowledgments
Dr. Quinn is supported by NIH grants 1P20-GM109089-01A1 and 1R01MH111826-01 and Department of Defense grant W81XWH-17-1-0432. Dr. Mayer is supported by NIH grants R01MH101512, R01NS098494, P20GM103472, R01AT007171, and P20GM109089 and Department of Defense grants W911NF-11-D-0001, W81XWH-13-2-0047, and W81XWH-17-1-0432. Dr. Master is supported by NIH grant 1 R01 NS097549-01A1, CDC grant 14IPA1405503, and Department of Defense grant W81XWH-BAA-14-1. Dr. Fann is supported by Veterans Health Administration grant 1I01RX001189, NIH grants 1R01NS091618 and R21HD089075, and Patient-Centered Outcomes Research Institute grants PCS-1604-35115 and R151133005.
Dr. Fann has served as a consultant for Quartet Health.
Footnotes
The other authors report no financial relationships with commercial interests.
REFERENCES
- 1.War Office Committee: Report of the War Office Committee of Enquiry Into “Shell-Shock” London, HM Stationery Office, 1922 [Google Scholar]
- 2.Centers for Disease Control and Prevention (CDC): Traumatic Brain Injury in the United States: Epidemiology and Rehabilitation (Report to Congress) Atlanta, Centers for Disease Control and Prevention, National Center for Injury Prevention and Control, Division of Unintentional Injury Prevention, 2015 [Google Scholar]
- 3.McCrory P, Meeuwisse WH, Aubry M, et al. : Consensus statement on concussion in sport: the 4th International Conference on Concussion in Sport held in Zurich, November 2012. PM R 2013; 5:255–279 [DOI] [PubMed] [Google Scholar]
- 4.McMahon P, Hricik A, Yue JK, et al. : Symptomatology and functional outcome in mild traumatic brain injury: results from the prospective TRACK-TBI study. J Neurotrauma 2014; 31:26–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carroll LJ, Cassidy JD, Cancelliere C, et al. : Systematic review of the prognosis after mild traumatic brain injury in adults: cognitive, psychiatric, and mortality outcomes: results of the International Collaboration on Mild Traumatic Brain Injury Prognosis. Arch Phys Med Rehabil 2014; 95(suppl):S152–S173 [DOI] [PubMed] [Google Scholar]
- 6.Larrabee GJ, Rohling ML: Neuropsychological differential diagnosis of mild traumatic brain injury. Behav Sci Law 2013; 31:686–701 [DOI] [PubMed] [Google Scholar]
- 7.Cassidy JD, Cancelliere C, Carroll LJ, et al. : Systematic review of self-reported prognosis in adults after mild traumatic brain injury: results of the International Collaboration on Mild Traumatic Brain Injury Prognosis. Arch Phys Med Rehabil 2014; 95(suppl):S132–S151 [DOI] [PubMed] [Google Scholar]
- 8.Leddy JJ, Baker JG, Merchant A, et al. : Brain or strain? Symptoms alone do not distinguish physiologic concussion from cervical/ vestibular injury. Clin J Sport Med 2015; 25:237–242 [DOI] [PubMed] [Google Scholar]
- 9.Tan CO, Meehan WP 3rd, Iverson GL, et al. : Cerebrovascular regulation, exercise, and mild traumatic brain injury. Neurology 2014; 83:1665–1672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gardner RC, Yaffe K: Epidemiologyof mild traumatic brainin jury and neurode generative disease. Mol Cell Neurosci 2015; 66(Pt B):75–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ontario Neurotrauma Foundation: Guidelines for Concussion/Mild Traumatic Brain Injury and Persistent Symptoms, 2nd ed. Toronto, Ontario Neurotrauma Foundation, 2013. http://onf.org/system/attachments/452/original/Complete_Guidelines_Oct._2017.pdf [Google Scholar]
- 12.Management of Concussion–Mild Traumatic Brain Injury Working Group: DoD Clinical Practice Guideline for the Management of Concussion–Mild Traumatic Brain Injury, version 2.0 Washington, DC, Department of Veterans Affairs and Department of Defense, February 2016. https://www.healthquality.va.gov/guidelines/Rehab/mtbi/mTBICPGFullCPG50821816.pdf [Google Scholar]
- 13.Menon DK, Schwab K, Wright DW, et al. : Position statement: definition of traumatic brain injury. Arch Phys Med Rehabil 2010; 91:1637–1640 [DOI] [PubMed] [Google Scholar]
- 14.Andriessen TMJC, Horn J, Franschman G, et al. : Epidemiology, severity classification, and outcome of moderate and severe traumatic brain injury: a prospective multicenter study. J Neurotrauma 2011; 28:2019–2031 [DOI] [PubMed] [Google Scholar]
- 15.Meterko M, Baker E, Stolzmann KL, et al. : Psychometric assessment of the Neurobehavioral Symptom Inventory–22: the structure of persistent postconcussive symptoms following deployment-related mild traumatic brain injury among veterans. J Head Trauma Rehabil 2012; 27:55–62 [DOI] [PubMed] [Google Scholar]
- 16.Potter S, Leigh E, Wade D, et al. : The Rivermead Post Concussion Symptoms Questionnaire: a confirmatory factor analysis. J Neurol 2006; 253:1603–1614 [DOI] [PubMed] [Google Scholar]
- 17.Kontos AP, Elbin RJ, Schatz P, et al. : A revised factor structure for the Post-Concussion Symptom Scale: baseline and postconcussion factors. Am J Sports Med 2012; 40:2375–2384 [DOI] [PubMed] [Google Scholar]
- 18.McCrory P, Meeuwisse W, Dvorak J, et al. : Consensus statement on concussion in sport: the 5th international conference on concussion in sport held in Berlin, October 2016. Br J Sports Med (Epub ahead of print, April 26, 2017) [DOI] [PubMed]
- 19.Ponsford J, Cameron P, Fitzgerald M, et al. : Predictors of postconcussive symptoms 3 months after mild traumatic brain injury. Neuropsychology 2012; 26:304–313 [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization: The ICD-10 Classification of Mental and Behavioural Disorders Diagnostic Criteria for Research Geneva, World Health Organization, 1993 [Google Scholar]
- 21.Broshek DK, De Marco AP, Freeman JR: A review of post-concussion syndrome and psychological factors associated with concussion. Brain Inj 2015; 29:228–237 [DOI] [PubMed] [Google Scholar]
- 22.Iverson GL, Lange RT: Examination of “postconcussion-like” symptoms in a healthy sample. Appl Neuropsychol 2003; 10:137–144 [DOI] [PubMed] [Google Scholar]
- 23.Mayer AR, Bellgowan PSF, Hanlon FM: Functional magnetic resonance imaging of mild traumatic brain injury. Neurosci Biobehav Rev 2015; 49:8–18 [DOI] [PubMed] [Google Scholar]
- 24.Rapp PE, Keyser DO, Albano A, et al. : Traumatic brain injury detection using electrophysiological methods. Front Hum Neurosci 2015; 9:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mucha A, Collins MW, Elbin RJ, et al. : A Brief Vestibular/Ocular Motor Screening (VOMS) assessment to evaluate concussions: preliminary findings. Am J Sports Med 2014; 42:2479–2486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matuszak JM, McVige J, McPherson J, et al. : A practical concussion physical examination toolbox. Sports Health 2016; 8:260–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heyer GL, Schaffer CE, Rose SC, et al. : Specific factors influence postconcussion symptom duration among youth referred to a sports concussion clinic. J Pediatr 2016; 174:33–38.e2 [DOI] [PubMed] [Google Scholar]
- 28.Vanderploeg RD, Belanger HG, Curtiss G: Mild traumatic brain injury and posttraumatic stress disorder and their associations with health symptoms. Arch Phys Med Rehabil 2009; 90:1084–1093 [DOI] [PubMed] [Google Scholar]
- 29.Haarbauer-Krupa J, Taylor CA, Yue JK, et al. : Screening for posttraumatic stress disorder in a civilian emergency department population with traumatic brain injury. J Neurotrauma 2017; 34: 50–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yuh EL, Mukherjee P, Lingsma HF, et al. : Magnetic resonance imaging improves 3-month outcome prediction in mild traumatic brain injury. Ann Neurol 2013; 73:224–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levin HS: Prediction of recovery from traumatic brain injury. J Neurotrauma 1995; 12:913–922 [DOI] [PubMed] [Google Scholar]
- 32.Bigler ED, Maxwell WL: Neuropathology of mild traumatic brain injury: relationship to neuroimaging findings. Brain Imaging Behav 2012; 6:108–136 [DOI] [PubMed] [Google Scholar]
- 33.Currie S, Saleem N, Straiton JA, et al. : Imaging assessment of traumatic brain injury. Postgrad Med J 2016; 92:41–50 [DOI] [PubMed] [Google Scholar]
- 34.Raji CA, Tarzwell R, Pavel D, et al. : Clinical utility of SPECT neuroimaging in the diagnosisand treatment of traumatic brain injury: a systematic review. PLoS One 2014; 9:e91088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dams-O’Connor K, Spielman L, Singh A, et al. : The impact of previous traumatic brain injury on health and functioning: a TRACK-TBI study. J Neurotrauma 2013; 30:2014–2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dretsch MN, Silverberg ND, Iverson GL: Multiple past concussions are associated with ongoing post-concussive symptoms but not cognitive impairment in active-duty Army soldiers. J Neurotrauma 2015; 32:1301–1306 [DOI] [PubMed] [Google Scholar]
- 37.Kamins J, Giza CC: Concussion–mild traumatic brain injury. Neurosurg Clin N Am 2016; 27:441–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ellis MJ, Leddy JJ, Willer B: Physiological, vestibulo-ocular, and cervicogenic post-concussion disorders: an evidence-based classification system with directions for treatment. Brain Inj 2015; 29:238–248 [DOI] [PubMed] [Google Scholar]
- 39.Zemek R, Barrowman N, Freedman SB, et al. : Clinical risk score for persistent postconcussion symptoms among children with acute concussion in the ED. JAMA 2016; 315:1014–1025 [DOI] [PubMed] [Google Scholar]
- 40.Jacobs B, Beems T, Stulemeijer M, et al. : Outcome prediction in mild traumatic brain injury: age and clinical variables are stronger predictors than CT abnormalities. J Neurotrauma 2010; 27:655–668 [DOI] [PubMed] [Google Scholar]
- 41.Sandel NK, Schatz P, Goldberg KB, et al. : Sex-based differences in cognitive deficits and symptom reporting among acutely concussed adolescent lacrosse and soccer players. Am J Sports Med 2017; 45: 937–944 [DOI] [PubMed] [Google Scholar]
- 42.Karr JE, Areshenkoff CN, Garcia-Barrera MA: The neuropsychological outcomes of concussion: a systematic review of meta-analyses on the cognitive sequelae of mild traumatic brain injury. Neuropsychology 2014; 28:321–336 [DOI] [PubMed] [Google Scholar]
- 43.Ponsford J, Cameron P, Fitzgerald M, et al. : Long-term outcomes after uncomplicated mild traumatic brain injury: a comparison with trauma controls. J Neurotrauma 2011; 28:937–946 [DOI] [PubMed] [Google Scholar]
- 44.Tator CH, Davis HS, Tartaglia MC, et al. : Postconcussion syndrome: demography and predictors in 221 patients. Can J Neurol Sci 2015; 42(S1):S25 [Google Scholar]
- 45.Miller JH, Gill C, Kuhn EN, et al. : Predictors of delayed recovery following pediatric sports-related concussion: a case-control study. J Neurosurg Pediatr 2015; 17:491–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lange RT, Iverson GL, Franzen MD: Short-term neuropsychological outcome following uncomplicated mild TBI: effects of day-of-injury intoxication and pre-injuryalcohol abuse. Neuropsychology 2007; 21: 590–598 [DOI] [PubMed] [Google Scholar]
- 47.McCrea M, Guskiewicz KM, Marshall SW, et al. : Acute effects and recovery time following concussion in collegiate football players: the NCAA Concussion Study. JAMA 2003; 290:2556–2563 [DOI] [PubMed] [Google Scholar]
- 48.Kontos AP, Elbin RJ, Lau B, et al. : Posttraumatic migraine as a predictor of recovery and cognitive impairment after sport-related concussion. Am J Sports Med 2013; 41:1497–1504 [DOI] [PubMed] [Google Scholar]
- 49.Lange RT, Iverson GL, Rose A: Depression strongly influences postconcussion symptom reporting following mild traumatic brain injury. J Head Trauma Rehabil 2011; 26:127–137 [DOI] [PubMed] [Google Scholar]
- 50.Vasterling JJ, Brailey K, Proctor SP, et al. : Neuropsychological outcomes of mild traumatic brain injury, post-traumatic stress disorder, and depression in Iraq-deployed US Army soldiers. Br J Psychiatry 2012; 201:186–192 [DOI] [PubMed] [Google Scholar]
- 51.Losoi H, Silverberg ND, Wäljas M, et al. : Resilience is associated with outcome from mild traumatic brain injury. J Neurotrauma 2015; 32:942–949 [DOI] [PubMed] [Google Scholar]
- 52.Iverson GL, Lange RT, Brooks BL, et al. : “Good old days” bias following mild traumatic brain injury. Clin Neuropsychol 2010; 24: 17–37 [DOI] [PubMed] [Google Scholar]
- 53.Silver JM: Effort, exaggeration, and malingering after concussion. J Neurol Neurosurg Psychiatry 2012; 83:836–841 [DOI] [PubMed] [Google Scholar]
- 54.Vanderploeg RD, Belanger HG, Kaufmann PM: Nocebo effects and mild traumatic brain injury: legal implications. Psychol Inj Law 2014; 7:245–254 [Google Scholar]
- 55.Belanger HG, Curtiss G, Demery JA, et al. : Factors moderating neuropsychological outcomes following mild traumatic brain injury: a meta-analysis. J Int Neuropsychol Soc 2005; 11:215–227 [DOI] [PubMed] [Google Scholar]
- 56.Knopman DS, DeKosky ST, Cummings JL, et al. : Practice parameter: diagnosis of dementia (an evidence-based review): Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2001; 56:1143–1153 [DOI] [PubMed] [Google Scholar]
- 57.Benvenga S, Campenní A, Ruggeri RM, et al. : Hypopituitarism secondary to head trauma. J Clin Endocrinol Metab 2000; 85: 1353–1361 [DOI] [PubMed] [Google Scholar]
- 58.Hanlon FM, McGrew CA, Mayer AR: Does a unique neuropsychiatric profile currently exist for chronic traumatic encephalopathy? Curr Sports Med Rep 2017; 16:30–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Montenigro PH, Bernick C, Cantu RC: Clinical features of repetitive traumatic brain injuryand chronic traumatic encephalopathy. Brain Pathol 2015; 25:304–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Silver JM, McAllister TW, Arciniegas DB: Depression and cognitive complaints following mild traumatic brain injury. Am J Psychiatry 2009; 166:653–661 [DOI] [PubMed] [Google Scholar]
- 61.Fann JR, Hart T, Schomer KG: Treatment for depression after traumatic brain injury: a systematic review. J Neurotrauma 2009; 26:2383–2402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Martindale SL, Morissette SB, Rowland JA, et al. : Sleep quality affects cognitive functioning in returning combat veterans beyond combat exposure, PTSD, and mild TBI history. Neuropsychology 2017; 31:93–104 [DOI] [PubMed] [Google Scholar]
- 63.Wickwire EM, Williams SG, Roth T, et al. : Sleep, sleep disorders, and mild traumatic brain injury: what we know and what we need to know: findings from a National Working Group. Neurotherapeutics 2016; 13:403–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Diener H-C, Holle D, Solbach K, et al. : Medication-overuse headache: risk factors, pathophysiology, and management. Nat Rev Neurol 2016; 12:575–583 [DOI] [PubMed] [Google Scholar]
- 65.Kemp S, Biswas R, Neumann V, et al. : The value of melatonin for sleep disorders occurring post-head injury: a pilot RCT. Brain Inj 2004; 18:911–919 [DOI] [PubMed] [Google Scholar]
- 66.Salter KL, McClure JA, Foley NC, et al. : Pharmacotherapy for depression posttraumatic brain injury. J Head Trauma Rehabil 2016; 31:E21–E32 [DOI] [PubMed] [Google Scholar]
- 67.Huang C-H, Huang C-C, Sun C-K, et al. : Methylphenidate on cognitive improvement in patients with traumatic brain injury: a meta-analysis. Curr Neuropharmacol 2016; 14:272–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fann JR, Uomoto JM, Katon WJ: Cognitive improvement with treatment of depression following mild traumatic brain injury. Psychosomatics 2001; 42:48–54 [DOI] [PubMed] [Google Scholar]
- 69.McAllister TW, Zafonte R, Jain S, et al. : Randomized placebo-controlled trial of methylphenidate or galantamine for persistent emotional and cognitive symptoms associated with PTSD and/or traumatic brain injury. Neuropsychopharmacology 2016; 41:1191–1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tramontana MG, Cowan RL, Zald D, et al. : Traumatic brain injuryrelated attention deficits: treatment outcomes with lisdexamfetamine dimesylate (Vyvanse). Brain Inj 2014; 28:1461–1472 [DOI] [PubMed] [Google Scholar]
- 71.American Psychiatric Association: Practice Guideline forthe Treatment of Patients With Acute Stress Disorder and Posttraumatic Stress Disorder Washington, DC, American Psychiatric Association, March 2009 [Google Scholar]
- 72.Kraus MF, Maki PM: Effect of amantadine hydrochloride on symptoms of frontal lobe dysfunction in brain injury: case studies and review. J Neuropsychiatry Clin Neurosci 1997; 9:222–230 [DOI] [PubMed] [Google Scholar]
- 73.Kaiser PR, Valko PO, Werth E, et al. : Modafinil amelioratesexcessive daytime sleepiness after traumatic brain injury. Neurology 2010; 75: 1780–1785 [DOI] [PubMed] [Google Scholar]
- 74.Johansson B, Wentzel A-P, Andréll P, et al. : Methylphenidate reduces mental fatigue and improves processing speed in persons suffered a traumatic brain injury. Brain Inj 2015; 29:758–765 [DOI] [PubMed] [Google Scholar]
- 75.Arciniegas DB, Anderson CA, Topkoff J, et al. : Mild traumatic brain injury: a neuropsychiatric approach to diagnosis, evaluation, and treatment. Neuropsychiatr Dis Treat 2005; 1:311–327 [PMC free article] [PubMed] [Google Scholar]
- 76.Ponsford J, Willmott C, Rothwell A, et al. : Impact of early intervention on outcome following mild head injury in adults. J Neurol Neurosurg Psychiatry 2002; 73:330–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cicerone KD, Langenbahn DM, Braden C, et al. : Evidence-based cognitive rehabilitation: updated review of the literature from 2003 through 2008. Arch Phys Med Rehabil 2011; 92:519–530 [DOI] [PubMed] [Google Scholar]
- 78.Twamley EW, Thomas KR, Gregory AM, et al. : CogSMART compensatory cognitive training for traumatic brain injury: effects over 1 year. J Head Trauma Rehabil 2015; 30:391–401 [DOI] [PubMed] [Google Scholar]
- 79.Cooper DB, Bowles AO, Kennedy JE, et al. : Cognitiverehabilitationfor military service members with mild traumatic brain injury. J Head Trauma Rehabil 2017; 32:E1–E15 [DOI] [PubMed] [Google Scholar]
- 80.Gertler P, Tate RL, Cameron ID: Non-pharmacological interventions for depression in adults and children with traumatic brain injury. Cochrane Database Syst Rev 2015; (12):CD009871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Soo C, Tate R: Psychological treatment for anxiety in people with traumatic brain injury. Cochrane Database Syst Rev 2007; (3): CD005239. [DOI] [PubMed] [Google Scholar]
- 82.Potter SDS, Brown RG, Fleminger S: Randomised, waiting list controlled trial of cognitive-behavioural therapy for persistent postconcussional symptoms after predominantly mild-moderate traumatic brain injury. J Neurol Neurosurg Psychiatry 2016; 87:1075–1083 [DOI] [PubMed] [Google Scholar]
- 83.Bell KR, Fann JR, Brockway JA, et al. : Telephone problem solving for service members with mild traumatic brain injury: a randomized, clinical trial. J Neurotrauma 2017; 34:313–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McCarty CA, Zatzick D, Stein E, et al. : Collaborative care for adolescents with persistent postconcussive symptoms : a randomized trial. Pediatrics 2016; 138:e20160459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shen Q, Hiebert JB, Hartwell J, et al. : Systematic reviewof traumatic brain injury and the impact of antioxidant therapy on clinical outcomes. Worldviews Evid Based Nurs 2016; 13:380–389 [DOI] [PubMed] [Google Scholar]
- 86.Hakkarainen H, Hakamies L: Piracetam in the treatment of postconcussional syndrome: adouble-blind study. Eur Neurol 1978; 17:50–55 [DOI] [PubMed] [Google Scholar]
- 87.Sharma B, Lawrence DW, Hutchison MG: Branched chain amino acids (BCAAs) and traumatic brain injury. J Head Trauma Rehabil (Epub ahead of print, Jan 5, 2017) [DOI] [PubMed]
- 88.Hawkins JR, Gonzalez KE, Heumann K: The effectiveness of hyperbaric oxygen therapy as a treatment for postconcussion symptoms. J Sport Rehabil 2016; 32:1–14 [DOI] [PubMed] [Google Scholar]
- 89.Hill KP: Medical marijuana for treatment of chronic pain and other medical and psychiatric problems. JAMA 2015; 313:2474–2483 [DOI] [PubMed] [Google Scholar]
- 90.Maas AIR, Murray G, Henney H 3rd, et al. : Efficacy and safety of dexanabinol in severe traumatic brain injury: results of a phase III randomised, placebo-controlled, clinical trial. Lancet Neurol 2006; 5:38–45 [DOI] [PubMed] [Google Scholar]
- 91.Scheiman MM, Talasan H, Mitchell GL, et al. : Objective assessment of vergence after treatment of concussion-related CI: a pilot study. Optom Vis Sci 2017; 94:74–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schneider KJ, Meeuwisse WH, Nettel-Aguirre A, et al. : Cervicovestibular rehabilitation in sport-related concussion: a randomised controlled trial. Br J Sports Med 2014; 48:1294–1298 [DOI] [PubMed] [Google Scholar]
- 93.Leddy JJ, Cox JL, Baker JG, et al. : Exercise treatment for postconcussion syndrome: a pilot study of changes in functional magnetic resonance imaging activation, physiology, and symptoms. J Head Trauma Rehabil 2013; 28:241–249 [DOI] [PubMed] [Google Scholar]
- 94.Grool AM, Aglipay M, Momoli F, et al. : Association between early participation in physical activity following acute concussion and persistent postconcussive symptoms in children and adolescents. JAMA 2016; 316:2504–2514 [DOI] [PubMed] [Google Scholar]
- 95.Buckley TA, Munkasy BA, Clouse BP: Acute cognitive and physical rest may not improve concussion recovery time. J Head Trauma Rehabil 2016; 31:233–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Thomas DG, Apps JN, Hoffmann RG, et al. : Benefits of strict rest after acute concussion: a randomized controlled trial. Pediatrics 2015; 135:213–223 [DOI] [PubMed] [Google Scholar]
- 97.Li S, Zaninotto AL, Neville IS, et al. : Clinical utility of brain stimulation modalities following traumatic brain injury: current evidence. Neuropsychiatr Dis Treat 2015; 11:1573–1586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Koski L, Kolivakis T, Yu C, et al. : Noninvasive brain stimulation for persistent postconcussion symptoms in mild traumatic brain injury. J Neurotrauma 2015; 32:38–44 [DOI] [PubMed] [Google Scholar]
- 99.Leung A, Shukla S, Fallah A, et al. : Repetitive transcranial magnetic stimulation in managing mild traumatic brain injury-related headaches. Neuromodulation 2016; 19:133–141 [DOI] [PubMed] [Google Scholar]
- 100.Ulam F, Shelton C, Richards L, et al. : Cumulative effects of transcranial direct current stimulation on EEG oscillations and attention/working memory during subacute neurorehabilitation of traumatic brain injury. Clin Neurophysiol 2015; 126:486–496 [DOI] [PubMed] [Google Scholar]
- 101.Sacco K, Galetto V, Dimitri D, et al. : Concomitant use of transcranial direct current stimulation and computer-assisted training for the rehabilitation of attention in traumatic brain injured patients: behavioral and neuroimaging results. Front Behav Neurosci 2016; 10:57. [DOI] [PMC free article] [PubMed] [Google Scholar]