Abstract
The extracellular matrix is a complex network of hydrated macromolecular proteins and sugars that, in concert with bound soluble factors, comprise the acellular stromal microenvironment of tissues. Rather than merely providing structural information to cells, the extracellular matrix plays an instructive role in development and is critical for the maintenance of tissue homeostasis. In this chapter, we review the composition of the extracellular matrix and summarize data illustrating its importance in embryogenesis, tissue-specific development, and stem cell differentiation. We discuss how the biophysical and biochemical properties of the extracellular matrix ligate specific transmembrane receptors to activate intracellular signaling that alter cell shape and cytoskeletal dynamics to modulate cell growth and viability, and direct cell migration and cell fate. We present examples describing how the extracellular matrix functions as a highly complex physical and chemical entity that regulates tissue organization and cell behavior through a dynamic and reciprocal dialogue with the cellular constituents of the tissue. We suggest that the extracellular matrix not only transmits cellular and tissue-level force to shape development and tune cellular activities that are key for coordinated tissue behavior, but that it is itself remodeled such that it temporally evolves to maintain the integrated function of the tissue. Accordingly, we argue that perturbations in extracellular matrix composition and structure compromise key developmental events and tissue homeostasis, and promote disease.
1. INTRODUCTION
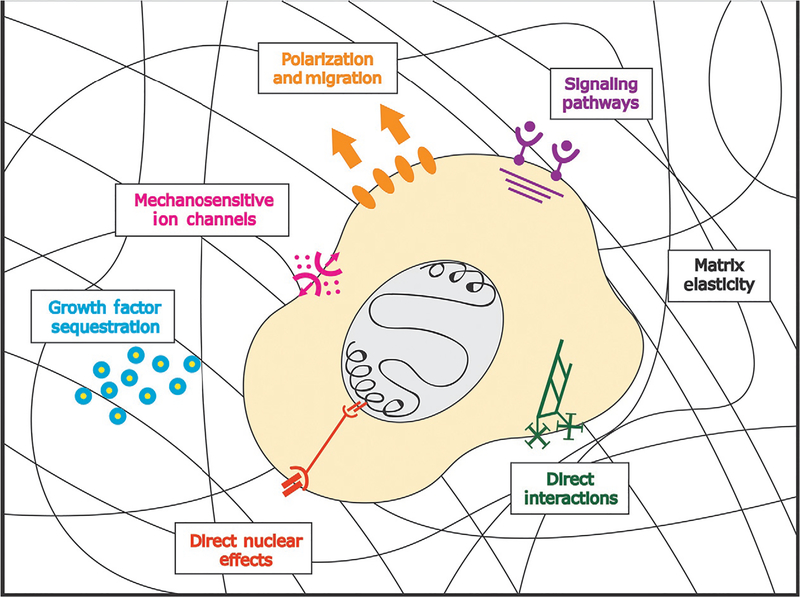
The extracellular matrix (ECM) is a complex network of proteins, polysaccharides, and water that comprise the acellular stromal microenvironment in all tissues and organs. Historically, the ECM was thought to provide structural information required to maintain the physical integrity of the tissue. However, it is now understood that the ECM is a biologically active component of all tissues that directs cell fate and influences tissue development and homeostasis (Fig. 1).
Fig. 1.
Illustration of the various physical and biochemical cues integrated by the extracellular matrix, which are simultaneously sensed by cells through parallel mechanisms and are critical for determining cell fate, inducing tissue-specific differentiation, and promoting developmental morphogenesis.
As organisms develop, they continuously generate and reorganize their ECM to provide the necessary structural framework to support the growth and development of emerging tissues. The ECM in turn provides critical biochemical and biophysical cues that guide cell fate, drive morphogenetic movements to sculpt the tissue, and induce tissue-specific differentiation.
The concept of “dynamic reciprocity” which maintains that the evolving ECM dictates cell and tissue fate which feedback to modulate ECM composition and organization represents a critical concept in developmental biology (Bissell, Hall, & Parry, 1982; Paszek & Weaver, 2004). “Tensional homeostasis” incorporates the viscoelasticity of the ECM and cell tension into the dynamic reciprocity paradigm thereby providing a unified working hypothesis with which to understand how the evolving biochemical and biophysical properties of the ECM direct development and maintain tissue homeostasis.
The ECM is also a key component of the adult stem cell niche and refers to the local microenvironment that sustains stem cell quiescence and facilitates the maintenance of stem cells through regulated self-replication and retention of multipotency. The ECM physically buffer stem cells that reside within the niche from differentiation cues sequesters critical growth factors and morphogens, and facilitates efficient nutrient exchange to sustain the long-term growth and survival and pluripotency of the stem cells.
2. ECM COMPOSITION
The main functional units of the ECM are cell-secreted macromolecular proteins. There are four general classes of ECM proteins: collagens, proteoglycans, glycoproteins, and elastins (Tsang, Cheung, Chan, & Cheah, 2010).
2.1. Collagens
Collagens provide the tissue with tensile strength and structural integrity (Gordon & Hahn, 2010; Lodish, Berk, Zipursky, et al., 2000). Collagens are composed of three alpha chains that assemble into heterotrimeric and homotrimeric molecules, various combinations which comprise the 28 recognized types of collagen. Fibrillar collagens are the most common type and are assembled in woven triple-helical structures that arise from long repeating stretches of Gly-X-Y residues in the alpha chains. In these repeated stretches, X is typically proline and Y is typically hydroxyproline. These triple helices then self-assemble into thin and thick fibrils. Nonfibrillar collagens arise from disruptions in the Gly-X-Y repeats of the alpha chains. Instead of forming fibrils, these nonfibrillar collagens form mesh-like networks in the ECM, such as collagen IV found in basement membranes.
The biochemical and mechanical properties of fibrillar collagens are largely dependent on posttranslational modifications that drive the crosslinking of the collagen fibrils, and thus dictate the tensile strength of the tissue. Proline and lysine residues on procollagens are hydroxylated intracellularly by specific enzymes (Yamauchi & Sricholpech, 2012). These hydroxylated residues can be further modified within the cell by enzyme-mediated glycosylation, resulting in the addition of galactose or glucose. Collagen glycosylation alters cell-collagen interactions, and thus broadly influences biological functions of collagen, such as the ability of collagens to direct angiogenesis or bone mineralization (Jurgensen et al., 2011; Palmieri et al., 2010; Tenni, Valli, Rossi, & Cetta, 1993). Once procollagens are secreted to the extracellular space and are self-assembled into fibrils, specific lysine and hydroxylysine residues are deaminated by an enzyme called lysyl oxidase (LOX) that produces reactive aldehyde groups that initiate covalent cross-links via condensation reactions. Lysyl oxidase and hydroxylase crosslinking are critical for the tensile strength and structural stability of tissues, and loss of their activity has deleterious consequences to the organism including bone fragility, cardiovascular disease, and in some instances lethality (Kagan & Li, 2003; Maki et al., 2002). Thus, extracellular posttranslational modifications to fibrillar collagen expand its structure function and modify its biological role in tissues.
2.2. Proteoglycans
Proteoglycans consist of a core protein domain covalently linked to glycosaminoglycans (GAGs) (Mouw, Ou, & Weaver, 2014). These GAGs form long, negatively charged, linear repeats of disaccharide units, and provide proteoglycans with their unique ability to bind water, which is critical for imparting compressive resistance to tissues. Aggrecan and versican are common examples of proteoglycans that are ubiquitously expressed in many tissues. Hyaluronan, or hyaluronic acid (HA), is a common GAG that is also abundantly expressed in all tissues but has unique properties because it does not covalently attach to peptides (Laurent & Fraser, 1992). Thus, while not a true proteoglycan, HA possesses similar biological functions exhibited by proteoglycans. HA is abundantly expressed in the brain, where it forms a relatively “loose” and unorganized network. However, HA can also bind and form cross-links with other proteoglycans and glycoproteins, such as versican and tenascin, to support more condensed perineural networks (PNNs) that stiffen the neuronal microenvironment (Kwok, Dick, Wang, & Fawcett, 2011). The formation of PNNs coincides with brain stiffening, reduced neuron growth and extension, and loss of plasticity in the developing brain, further illustrating how tissue-specific biochemical properties of the ECM guide cell fate and tissue development (Kwok et al., 2011; Yamaguchi, 2000).
2.3. Glycoproteins
Glycoproteins are similar to proteoglycans in that they consist of peptide units and covalently bound carbohydrate groups. However, whereas proteoglycans are characterized by long, linear chains of repeating disaccharides, glycoproteins contain bulky, branched carbohydrates with no, or few, repeating structures. Glycoproteins serve as connector molecules within the ECM because they contain functional groups that recognize and bind other ECM molecules, as well as to cell adhesion molecules, secreted growth factors, and morphogens. Fibronectin and laminin are two predominant and important glycoproteins found in the ECM stroma.
Fibronectin is secreted into the extracellular microenvironment and then forms polymerized fibrils with other fibronectin molecules or with collagen by a cell-dependent process termed fibrillogenesis (Kadler, Hill, & CantyLaird, 2008; Singh, Carraher, & Schwarzbauer, 2010). In fact, cross-linking of collagen by LOX depends upon fibronectin, emphasizing the importance of intimate interactions between ECM proteins in regulating its structure function (Kubow et al., 2009). The RGD sequence (Arg-Gly-Asp), which can bind to specific cellular transmembrane proteins called integrins, is a particularly important cell-binding motif within the fibronectin molecule. However, the strength of cell—integrin interactions with fibronectin is greatly enhanced through binding to the separate fibronectin synergy sequence (Schwarzbauer & DeSimone, 2011; Sechler, Corbett, & Schwarzbauer, 1997). Fibroblasts in particular are capable of unfolding fibronectin to reveal cryptic cell-binding sites, including the synergy sequence (Friedland, Lee, & Boettiger, 2009; Seong et al., 2013; Smith et al., 2007). Once revealed, the synergy sequence can then be ligated by cellular α5β1 integrin to potentiate intracellular tension and alter epithelial or endothelial morphogenesis or fibroblast function (Benito-Jardon et al., 2017; Friedland et al., 2009; Miroshnikova et al., 2017). In this way, fibronectin structure function can be discretely modulated by cell-generated force to alter cell and tissue function.
Laminins are composed of α, β, and γ heterotrimer chains that connect via central domains to form cross-shaped, Y-shaped, or single-arm structures (Mouw, Ou, et al., 2014; Timpl & Brown, 1994). Laminins are localized to the basal lamina of tissues and function primarily to connect various components of the ECM. Laminins are classified according to their subunit composition, such that laminin 111 refers to laminin trimers comprised of α1, β1, and γ1 chains (Aumailley et al., 2005). Laminin heterogeneity generates significant functional diversity. For instance, while laminin 111 polymerizes to form a meshwork that contributes to the tissue basement membrane, laminin 332, that is secreted during wound healing and hair follicle development, is subject to extensive proteolytic cleavage, which is critical for its ability to facilitate coordinated keratinocyte migration (Sugawara, Tsuruta, Ishii, Jones, & Kobayashi, 2008).
2.4. Elastin
Elastin is a unique structural protein that imparts, as its name suggests, elastic properties to tissues such as the skin and vasculature. Whereas fibrillar collagen provides tensile strength to resist deformation, elastin provides tissues with resilience so that when they are stretched they can return to their original conformation. Elastins are typically more hydrated and flexible in their relaxed, as compared to their stretched, state. The precise structure function relationship that attributes this unique property ofelastins is still a matter of debate, but is frequently described as the relaxed state being entropically favorable toward stretched configurations (Debelle & Tamburro, 1999).
Here, we reviewed the different types of ECM proteins as discrete units according to their structure and functional properties. Nevertheless, it is important to appreciate that the tissue-specific biochemical and physical properties of the ECM arise from its complex organization, posttranslational modifications, and its ability to be dynamically remodeled; and these are absolutely critical for the ECM’s ability to influence cell and tissue fate.
2.5. The Dynamic Nature of the ECM
The ECM is a fibrous network or scaffold within which tissues reside, prompting the perspective that the ECM is a static physical structure. In fact, the ECM is quite dynamic (Lu, Takai, Weaver, & Werb, 2011; Page-McCaw, Ewald, & Werb, 2007; Streuli, 1999). The protein components of the ECM are turned over regularly, and the cells within the tissue are actively engaged in remodeling their local ECM (Bonnans, Chou, & Werb, 2014). Virtually all cells produce and secrete ECM proteins into their microenvironment together with matrix metalloproteinases (MMPs), which degrade ECM proteins (Bonnans et al., 2014; Mecham, 2012; Page-McCaw et al., 2007). Cells also assemble and disassemble their local ECM by binding specific residues on matrix proteins and exerting physical forces generated by actomyosin contractility to modify the three-dimensional (3D) conformation and organization of these proteins (Ohashi, Kiehart, & Erickson, 2002; Pankov et al., 2000; Zhong et al., 1998). Cellular remodeling can promote interactions between different ECM molecules or reveal cryptic binding sites within the ECM molecule that promote cell adhesion and proliferation (Baneyx, Baugh, & Vogel, 2001, 2002; Zhong et al., 1998). The ability of the ECM to be constantly remodeled and reorganized is critical for development, tissue-specific differentiation, and stem cell fate specification.
Indeed, the ECM is a complex network of macromolecular structures whose physical and biochemical properties play a key role in cell and tissue biology. Moreover, the ECM is assembled and remodeled via cell-mediated processes, and its organization and reorganization are important in development and stem cell niche regulation. Rather than merely being a passive component of the stromal microenvironment within which cells and tissues exist, it is now appreciated that the ECM can directly regulate cell and tissue fate.
3. ECM REGULATES DEVELOPMENT AND MODULATES STEM CELL FATE
3.1. The ECM Regulates Development
Beginning in the 1980s and early 1990s, the ECM was recognized as a critical regulator of embryogenesis and tissue-specific development. Gene analysis and manipulation studies in model organisms revealed that several developmental defects could be mapped back to ECM proteins (Adams & Watt, 1993). In Caenorhabditis elegans, dpy-13 (dumpy) was identified as a member of the collagen gene family, and mutations to dpy-13 were shown to cause a short and chunky body shape (von Mende, Bird, Albert, & Riddle, 1988). Mutations to Sqt-1 and clb-2 were also identified as sequences for collagen proteins that caused significant developmental defects in body shape, with the clb-2 mutation causing embryonic lethality (Guo, Johnson, & Kramer, 1991; Kramer, Johnson, Edgar, Basch, & Roberts, 1988). Mutations to unc-6, a sequence for a laminin-related protein, caused defects in neural development that resulted from misguiding of axon extensions (Ishii, Wadsworth, Stern, Culotti, & Hedgecock, 1992). Similar studies in Drosophila identified mutations to the gene-coding sequences of laminin A and the Drosophila analog for fibrinogen, which both led to significant developmental defects (Baker, Mlodzik, & Rubin, 1990; Hortsch & Goodman, 1991). Furthermore, mutations to Drosophila integrins, the cellular receptors for ECM proteins, generated a host of defects ranging from wing, to eye, to muscle development (Brower & Jaffe, 1989; Leptin, Bogaert, Lehmann, & Wilcox, 1989; Volk, Fessler, & Fessler, 1990; Wilcox, DiAntonio, & Leptin, 1989; Zusman, Patel-King, Ffrench-Constant, & Hynes, 1990).
In addition to mapping genetic mutations to ECM proteins and receptors, function-blocking manipulation of cell—ECM interactions has served to illustrate the importance of cell-matrix adhesion to tissue development. Decoupling interactions between mesodermal progenitors and fibronectin using function-blocking antibodies in the blastocoel roof of amphibian embryos prevented development from proceeding beyond gastrulation (Boucaut et al., 1984, 1985). Later in development, injection of the integrin-binding motif of fibronectin, the RGD peptide, prevented the proper establishment of left/right asymmetry in Xenopus embryos (Yost, 1992). Similarly, injection of RGD-containing peptides in Gallus gallus domesticus (chick) embryos prevented neural crest cell migration (Boucaut et al., 1984).
3.2. ECM-Directed Cell Migration in Development
ECM-directed cell migration is a critical determinant of cell fate specification during development. This concept was demonstrated by studies exploring the impact of cell—ECM interactions in heart development. The heart originates as a bilayered tube formed by the ECM-dependent migration of myocardial precursors from the left and right lateral sides toward the midline of the organism. Zebrafish hearts exhibit serious developmental defects when mutations are induced in the fibronectin-encoding natter gene that impede myocardial precursor migration (Trinh & Stainier, 2004). Similarly, morpholino-mediated knockdown of zebrafish fibronectin in early embryogenesis compromised cardiac development that could be rescued by injection of exogenous fibronectin (Matsui et al., 2007). Coordinated and directed ECM deposition is also a critical aspect ofneural crest cell migration during embryogenesis. Neural crest cells migrate from the dorsal region of the developing neural tube and contribute to multiple cell types and tissues including neurons and osteoblasts (Knecht & Bronner-Fraser, 2002). Conditional knockout of β1 integrin in mouse neural crest precursors compromised fibronectin, laminin, tenascin, and collagen IV deposition, prevented neural crest cell migration, and led to lethal neuronal defects, emphasizing the link between ECM deposition and tissue development (Pietri et al., 2004). Indeed, ECM deposition is important for guiding and directing cell migration during development and by so doing plays a key role in shaping the embryo, as has been illustrated by the attractive and repellent effects of versican and aggrecan on neural crest cell migration (Perissinotto et al., 2000). Moreover, laminin α5 appears to restrict neural crest cell migration along confined pathways, as was illustrated by the unfettered broad migration of neural crest cells and compromised development of the organism when this laminin chain was knocked out (Coles, Gammill, Miner, & Bronner-Fraser, 2006). Not surprisingly, distinct ECM components direct cell migration in different tissues during development. For example, fibronectin facilitates migration of cranial neural crest cells, whereas vitronectin, collagen I, and laminin do not (Alfandari, Cousin, Gaultier, Hoffstrom, & DeSimone, 2003).
While these and other experiments emphasize the importance of the ECM in development, the main inference from these pioneering studies is that the ECM provides structural integrity to maintain cell adhesion-dependent tissue organization and that it facilitates directed cell migration. Over the past several decades this perspective has expanded to encompass a role for the ECM and its receptors as an instructive developmental regulator and important cue that directs stem cell differentiation.
3.3. ECM as a Critical Component of the Stem Cell Niche
The ECM not only regulates stem cell fate and tissue-specific differentiation but also modulates stem cell behavior in adult organisms. Stem cells are characterized by two unique properties: (i) they are capable of self-renewal, dividing to produce additional stem cells in a process termed “symmetric” division; (ii) they are also capable of giving rise to differentiated cells with specialized functions, termed “asymmetric” division (Fuchs & Chen, 2012). Despite the potential for both self-renewal and differentiation, adult stem cells often remain quiescent for long periods of time (Cheung & Rando, 2013; Li & Clevers, 2010). It is the specialized ECM microenvironment of these adult stem cells, the stem cell niche, which has been credited with regulating stem cell behavior, including the maintenance of their prolonged quiescence. Indeed, the ECM is a critical part ofthis niche, providing a microenvironment within which stem cells can maintain quiescence until they receive stimuli that promote their expansion and differentiation (Gattazzo, Urciuolo, & Bonaldo, 2014). Often, stem cell expansion and differentiation are preceded or accompanied by changes to the niche. Thus, the ECM both maintains stem cell populations in the adult tissue and regulates their differentiation.
4. CELL-ECM SIGNALING
The ECM influences cell fate by binding to cell surface receptors that recognize specific ECM moieties. Once bound to the ECM, these cellular receptors alter cell growth, survival, motility, and differentiation by initiating intracellular signaling and cytoskeletal reorganization. The cellular receptors that bind to the ECM include integrins as well as discoidin domain receptors (DDRs), syndecans, CD44, and receptor for hyaluronic acidmediated motility, as well as Robo receptors.
4.1. ECM-Integrin Binding
Integrins, which are the best-studied ECM receptors, are transmembrane heterodimer molecules that bind to specific ECM residues and interact with adhesion plaque proteins and cytoskeletal linker proteins via their cytoplasmic tail. There are 18 alpha subunits and 8 beta subunits, which combine to form 24 distinct integrin heterodimers (Hynes, 2002). The combination of the alpha and beta subunits that recognize and bind different ECM molecules imparts functional specificity to integrins. Not only do integrins facilitate the physical interaction of cells with the ECM, but they also induce intracellular signaling, alter cytoskeletal organization, and stimulate cellular tension to regulate cell growth and survival, promote invasion and migration, and direct cell differentiation and stem cell fate.
Integrin adhesion and signaling are highly context dependent. For instance, α5β1, αvβ5, α6β1, and α9β1 integrins are highly expressed in mouse embryonic stem cells (mESCs) (Lee et al., 2010), and their combined ligation to peptide-conjugated polyethylene glycol (PEG) hydrogels with fibronectin-derived peptide sequence RGDSP, CCN1-derived sequence TTSWSQ, and the tenascin-C-derived sequence AEIDGIE can maintain their pluripotency in culture. By contrast, preventing the initial, but not the late, ligation of the collagen-binding integrins α1β1 and α2β1, using function-blocking antibodies, inhibited osteoblast differentiation of both multipotent 2T3 cells and freshly harvested bone marrow cells, as was indicated by reduced alkaline phosphatase production and cellular mineralization (Jikko, Harris, Chen, Mendrick, & Damsky, 1999; Mizuno, Fujisawa, & Kuboki, 2000). Indeed, blocking integrin binding to collagen I reduced osteoblast differentiation even in the presence of a constitutively active bone morphogenetic protein 2 (BMP-2) receptor, presumably by blocking BMP-2 receptor activation. Similarly, knocking down the fibronectin receptor α5β1 integrin prevented osteoblast differentiation of human mesenchymal stem cells (MSCs), whereas its activation and ligation by collagen promoted osteoblast differentiation (Hamidouche et al., 2009). These findings underscore the critical context-dependent role of integrins in ECM-dependent regulation of stem cell fate.
Integrins are critical regulators of cell fate during tissue-specific development and differentiation. For instance, Fassler and colleagues used β1 integrin-deficient embryonic stem cells to demonstrate the importance of β1 integrin in early cardiogenesis (Fassler et al., 1996). Similarly, although conditional β1 integrin knockdown in mammary epithelial cells (MECs) had no effect on branching morphogenesis of the adolescent mammary gland, lack of β1 integrin prevented pregnancy-associated MEC alveolar differentiation (Klinowska et al., 1999; Naylor et al., 2005). Thus, in the absence of β1 integrin MECs failed to lactate, presumably because the cells could not respond to prolactin to activate transcription 5 (STAT5) signaling that is critically required for beta casein expression (Xu et al., 2009). Indeed, early studies identified β1 integrin signaling as necessary for MEC growth and survival (Boudreau, Sympson, Werb, & Bissell, 1995; Klinowska et al., 1999; Naylor et al., 2005).
4.2. Nonintegrin Receptors
Analogous to integrin receptors, the DDR subfamily of receptor tyrosine kinases that bind to extracellular collagens also modulate cell growth, survival, migration, and tissue morphogenesis by activating intracellular signaling and regulating gene transcription. For instance, DDR1 binding to collagen promotes the pluripotency of mESCs via Ras-mediated activation of phosphoinositide 3-kinase (PI3K)/Akt, and extracellular signal-regulated kinase (ERK) that regulate BMI-1 (Suh & Han, 2011). Similarly, DDR2 ligation by collagen and ERK activation is critical for runt-related transcription factor 2 (Runx2) induction of osteogenic differentiation that likely explains the dwarfism and aberrant bone formation observed in the DDR2-null mouse (Labrador et al., 2001; Zhang et al., 2011).
CD44 receptors, which bind to hyaluronan (HA), elicit profound effects on cell behavior by activating intracellular signaling and altering cytoskeletal organization. Thus, inhibiting CD44 binding to HA significantly reduced hematopoietic stem/progenitor cell (HSC/HPC) homing and engraftment into the bone marrow, presumably by impeding association with their endothelium and endosteum niche (Avigdor et al., 2004). Similarly, a role for CD44 in cell differentiation was underscored in CD44-null mice which exhibited severely compromised keratinocyte differentiation and epidermal barrier formation, presumably because HA ligation is critical for lamellar body formation and secretion and epidermal barrier development (Bourguignon et al., 2006). Interestingly, the CD44-null mice also had significantly reduced levels of extracellular HA, implicating cellular ligation in HA homeostasis.
Roundabout (Robo) proteins are another group of transmembrane receptors that bind to ECM glycoproteins termed Slits. Robo protein adhesion is critical for branching morphogenesis in the mammary gland (Macias et al., 2011), and Slit binding to Robo4 mediates HSC homing to the bone marrow niche and efficient bone marrow engraftment (Smith-Berdan et al.,2011). In Drosophila, Robo-Slit interactions play an integral role in mediating intestinal tissue homeostasis. Drosophila intestinal epithelial cells secrete Slit, which suppresses endocrine lineage differentiation by binding and activating Robo2 receptors on adjacent stem cell progenitors (Biteau & Jasper, 2014).
5. ECM-DEPENDENT MODULATION OF GROWTH FACTOR AND MORPHOGEN FUNCTION
The ECM controls the availability and presentation of growth factors and morphogens. This ECM-soluble factor-binding function is mediated primarily by the extracellular proteoglycans and heparin sulfates that contain binding sites for a multitude of growth factors and secreted morphogens and enzymes. The binding of soluble secreted factors to the ECM creates a signaling “reservoir” that can be rapidly accessed by the cells within the tissue. ECM binding of growth factors also modulates and sometimes potentiates the cellular signaling elicited by these molecules and can in some instances also bind inhibitors to temper signaling.
5.1. ECM-Transforming Growth Factor Beta Superfamily Interactions
Members of the transforming growth factor beta (TGF-β) superfamily, including BMPs and TGF-β, play a major role in regulating embryogenesis, tissue development, and stem cell fate. The BMPs and TGF-β bind to specific cellular receptors and activate intracellular signaling to alter cell and tissue behavior by regulating gene expression (ten Dijke & Hill, 2004). Through their receptor activation, BMPs and TGF-β regulate a multitude of developmental programs including embryonic gastrulation and tissue-specific differentiation such as cardiogenesis, chondrogenesis, and osteoblast differentiation (Abdel-Latif et al., 2008; Akhurst, Lehnert, Faissner, & Duffie, 1990; Chen, Deng, & Li, 2012; Dabovic et al., 2002; Dickson, Slager, Duffie, Mummery, & Akhurst, 1993; Erlebacher & Derynck, 1996; Goumans et al., 2008; van der Kraan, Blaney Davidson, Blom, & van den Berg, 2009). Given their critical role during embryogenesis, development, and tissue homeostasis, it is not surprising that the storage, release, and activation of TGF-β and BMPs are both tightly regulated by ECM binding (Robertson et al., 2015; Wipff Rifkin, Meister, & Hinz, 2007). To begin with, cells secrete TGF-β in its inactive form where it associates with a large macromolecular protein aggregate termed the latent TGF-β complex. Proteins within the latent complex contain the RGD peptide, which can then be recognized by cellular transmembrane receptors, including integrin αvβ6. Integrin-mediated binding of the latent protein complex can then release the active TGF-β ligand from the latent complex in an actomyosin tension-dependent manner (Hinz, 2015). BMPs also bind to the ECM and this binding influences tissue development by generating BMP gradients that critically direct cell migration and differentiation. For example, BMPs directly bind to specific collagen 11α and collagen IV domains (Wang, Harris, Bayston, & Ashe, 2008), and this bound BMP directs body axis formation, heart development, and sensory neuron development in the inner ear (Chang et al., 2008; Khetarpal, Robertson, Yoo, & Morton, 1994; Larraín et al., 2000; Li et al., 2005). Bone morphogenetic proteins also associate with fibrillins, which are ECM glycoproteins (Sengle et al., 2008), and this interaction is critical for limb digit formation patterning (Arteaga-Solis et al., 2001). Similarly, the simultaneous dynamic ECM remodeling and collagen fibrillogenesis that are absolutely critical for chondrogenesis and the cartilage-to-bone transition are tightly choreographed by ECM-bound BMP (Yoon et al., 2005; Zhu, Oganesian, Keene, &Sandell, 1999). The ECM-bound BMP not only stimulates cell migration and differentiation, but its ECM-bound inhibitors may also repress signaling and differentiation, as has been proposed by studies in a simplified embryogenesis model (Warmflash, Sorre, Etoc, Siggia, & Brivanlou, 2014).
5.2. ECM-Growth Factor Collaboration
ECM-bound growth factors can elicit profound effects on tissue development and differentiation. This concept was elegantly illustrated by studies conducted in Xenopus, in which platelet-derived growth factor subunit A (PDGFA) bound to ectodermal-generated fibronectin was shown to be absolutely critical for the directional migration of mesodermal cells during gastrulation (Keller, 2005). The importance of ECM binding of PDGFA was illustrated by showing that expression of either a dominant-negative PDGFA receptor or reducing the level of matrix-bound PDGFA severely compromised mesoderm migration toward the animal pole (Nagel, Tahinci, Symes, & Winklbauer, 2004). Similarly, IGF-I and IGF-II are often highly abundant in tissues where they associate with the ECM even when no insulin-like growth factor (IGF) mRNA can be easily detected (Han, D’Ercole, & Lund, 1987; Hill et al., 1989). This ECM-associated IGF has been shown to play an instructive role in directed neural development and was found to be critical for inducing oligodendrocyte differentiation from neural progenitors (Hsieh et al., 2004), and for the cooperative BMP-mediated induction of chondrogenesis from adipose-derived MSCs (An, Cheng, Yuan, & Li, 2010). The ECM also modulates IGF signaling through competitive binding of their carrier proteins the insulin growth factor-binding proteins (IGFBPs). The association of IGFBPs to the ECM not only retards their MMP-mediated degradation but additionally alters the cellular-binding affinity for IGF-1, as was illustrated by a sevenfold decrease in IGF-1 binding affinity induced by ECM-bound IGFBP-5 (Jones, Gockerman, Busby, Camacho-Hubner, & Clemmons, 1993; Martin, Fowlkes, Babic, & Khokha, 1999). Yet another example of how matrix-bound growth factors can influence tissue development is demonstrated by fibroblast growth factor (FGF), which associates strongly with ECM-localized or membrane-tethered proteoglycans that contain heparin sulfate side chains (Jia et al., 2009). In fact there are several different FGFs, each with different affinities for particular heparin sulfate side chains, so that variations in ECM composition can demonstrate unique specificity for sequestering particular FGFs (Harada et al., 2009). The proteoglycan association ofFGFs can also stabilize interactions between FGFs and FGF receptors to influence cellular FGF signaling and cell fate (Ornitz, 2000). More frequently, however, proteolytic release of FGFs from the heparin sulfate containing proteoglycans permits binding of the FGF ligand to cellular FGF receptors to stimulate cellular signaling and regulate cell growth, survival, and migration. Released FGF is key for tissue-specific differentiation, including lung and mammary gland branching morphogenesis (Cardoso, 2006; Patel et al., 2007; Pond et al., 2013; Tholozan et al., 2007).
6. ECM-DEPENDENT SPATIAL-MECHANICAL REGULATION OF CELL PHENOTYPE
6.1. Control of Cell Shape
ECM ligation-dependent changes in cell shape can significantly alter cell growth and survival and modulate cell fate. Initial evidence for a relationship between cell shape and ECM ligation and cell differentiation was provided using cultured explanted chick embryonic vertebrate chondrocytes. Over 50 years ago, Abbott and colleagues showed that when they plated isolated chondrocytes as clusters of “rounded” cells they retained their chondrocyte fate, as indicated by chondroitin sulfate and collagen production. However, if they plated the chondrocytes as single “spread” cells, sustained differentiation was inhibited (Abbott & Holtzer, 1966). Solursh and colleagues also reported that only when rounded but not spread MSCs were plated on a type I collagen matrix could the cells continue to express collagen II and stain positively for alcian blue (Solursh, Linsenmayer, &Jensen, 1982). Similarly, embryonic mesenchyme cells could only be efficiently differentiated into α-actin muscle myosin and desmin-expressing smooth muscle cells when they were plated as elongated cells on laminin basement membranes (Yang, Palmer, Relan, Diglio, & Schuger, 1998). Indeed, links between cell shape and mesenchymal cell smooth muscle cell differentiation were also indicated in another study in which the investigators used a microporous culture system that permitted precise control of ECM ligation, cell shape, and spreading to interrogate the impact of ECM context on cell fate specification (Yang, Relan, Przywara, & Schuger, 1999).
The advent ofmicrocontact printing, in which ECM ligands can be precisely patterned, permitted unprecedented assessment of the role of cell shape, ECM ligation, and cell differentiation, and facilitated delineation of molecular mechanism. In a seminal study by McBeath and colleagues, microcontact-printed fibronectin deconstructed the impact of cell shape on MSC fate, independent of ECM composition and density. McBeath and colleagues showed that plating MSCs on small fibronectin islands, in which the cells retained a “rounded” morphology, promoted their differentiation into adipocytes. By contrast, when the same cells were plated on large fibronectin islands that permitted their spreading, they activated more Ras homolog gene family member A (RhoA) and RhoA kinase (ROCK) and had higher cytoskeletal tension that fostered their osteogenic differentiation (McBeath, Pirone, Nelson, Bhadriraju, & Chen, 2004). More provocatively, when mesenchymal cells grown in mixed osteo/adipogenic media were plated on fibronectin patterns with concave edges and sharp vertices that enhanced cytoskeletal tension, they showed a bias toward osteogenesis. By contrast, mesenchymal cells grown on patterns with convex, rounded edges, which do not enhance cellular tension, preferentially underwent adipogenesis. These findings imply that cell shape may function as a cell fate “switch” by regulating cell tension (Kilian, Bugarija, Lahn, & Mrksich, 2010). Nevertheless, cell shape does not always unambiguously induce cell differentiation. For instance, keratinocytes plated on patterned ECM rectangles that restricted their spreading fostered their epidermal differentiation, whereas when they were plated on substrata that enhanced their spreading they maintained their multipotency (Connelly et al., 2010).
In recent years there has been a growing focus on understanding how the 3D microenvironment that cells experience in vivo results in different cell behaviors than those observed in studies of cell behavior in vitro using traditional two-dimensional (2D) cultures (Lund, Yener, Stegemann, & Plopper, 2009; Rubashkin, Ou, & Weaver, 2014; Sasai, 2013). In this regard, natural collagen and fibrin gels have been used for many years but are complex, lack reproducibility, and fail to control for cell shape and ligand concentration (Janmey, Winer, & Weisel, 2009; Lee, Kasper, & Mikos, 2014; Parenteau-Bareil, Gauvin, & Berthod, 2010). Decellularized tissues have also enjoyed some degree of success, but again are not well defined and cannot be easily controlled, despite showing exciting success in directing stem differentiation for cardiac tissue (Song & Ott, 2011). More recent developments including PEG, alginate, and HA hydrogels have been used extensively for encapsulation to improve cell survival and function both in vitro and in vivo, due to the ability to tightly control cross-linking, cell attachment, permeability, biodegradability, and numerous other physical properties through chemical modifications (Augst, Kong, & Mooney, 2006; Burdick & Prestwich, 2011; Phelps et al., 2012). However, these hydrogels, while highly defined, do not always faithfully recapitulate the architecture and pore size of native ECMs (Griffith, 2002; Griffith & Swartz, 2006; Rubashkin et al., 2014). Toward this objective, microprinting technologies that were originally developed for DNA and protein microarrays have been adapted to build 3D cellular environments with microscale control of the scaffold design (Barbulovic-Nad et al., 2006; MacBeath & Schreiber, 2000). These may prove instrumental in delineating the effects ofmicroenvironment composition and architecture in guiding cell fate and function (Flaim, Chien, & Bhatia, 2005; Flaim, Teng, Chien, & Bhatia, 2008; LaBarge et al., 2009; Montanez-Sauri, Beebe, & Sung, 2015).
6.2. ECM Stiffness and Cell Fate
All tissues within the body exhibit distinct mechanical properties that reflect their structure function and that are imparted predominantly by the composition and organization of their ECM. For example, the brain is quite soft and this compliant tissue primarily reflects the abundant hydrated HA found in the ECM. By contrast, bone is very rigid and this stiffness is mainly due to high amounts of densely packed, mineralized, cross-linked collagens.
The stiffness of the ECM in specific differentiated tissues is not only critical for the structure and function of the tissue but also regulates the growth, survival, and motility of the cellular constituents and may even direct their lineage-specific differentiation. For instance, human MSCs may be directed to differentiate down multiple independent lineages, apparently simply by varying substrate elasticity (Engler, Sen, Sweeney, & Discher, 2006). Thus, MSCs could be induced to display neurogenic markers when cultured on soft substrates (100–1000 Pa) analogous to the measured compliance of brain tissue, myogenic markers on substrates of intermediate stiffness (8000–17,000 Pa) reflecting the stiffness of skeletal muscle, and osteogenic markers on the most rigid substrates (25,000–40,000 Pa) representing the values measured in cortical bone tissue. Inhibition of nonmuscle myosin abolished elasticity-dependent differentiation of these MSCs, implying acto-myosin contractility is likely necessary to transmit information about the elasticity of the ECM into the cell to regulate its cell fate.
Neural stem cell differentiation was similarly found to be modulated by substrate stiffness (Saha et al., 2008) so that adult neural stem cells cultured in neurogenic media differentiated into neurons with the highest efficiency on substrates with an elasticity that is most similar to the brain (500 Pa). Even in mixed media that enables differentiation to multiple lineages, adult neural stem cells apparently only committed to a neuronal fate on softer substrates (100–500 Pa) and to a glial fate on stiffer substrates (1000–10,000 Pa). Importantly, only on the softest substrates (10 Pa) did the adult neural stem cells stop proliferating and develop prominent neural processes, recapitulating the distinctive behavior documented previously by neural vs glial cells on soft and stiff matrices (Flanagan, Ju, Marg, Osterfield, &Janmey, 2002).
Matrix elasticity can apparently directly regulate cell proliferation by modulating cyclin D1-dependent G1 cell cycle progression (Klein et al., 2009). The ability of ECM stiffness to modulate cell cycle progression could explain why MSC quiescence is induced by plating the cells on soft polyacrylamide gels with a compliance that is similar to that measured in the central bone marrow, whereas they proliferate and differentiate into adipocytes and osteoblasts when plated on stiffer substrates (Winer, Janmey, McCormick, & Funaki, 2009). Regardless, recognizing the impact of ECM compliance on cell cycle transit has permitted the development of culture conditions that maintain the pluripotency of both embryonic and induced pluripotent stem cells and may help to design new strategies to potentiate iPS cell generation (Dixon et al., 2014; Li et al., 2017).
Although it is entirely plausible that ECM compliance can directly specify stem cell fate, it is more likely these effects are mediated through modulation of cellular responses to growth factors and morphogens. This concept was recently illustrated by studies showing enhanced human embryonic stem cell (hESC) mesoderm progenitor differentiation mediated by culturing the cells on highly compliant substrates (Przybyla, Lakins, & Weaver, 2016). In this study, hESCs plated on soft (400 Pa) and stiff (60,000 Pa) substrates failed to undergo spontaneous differentiation. However, upon morphogen-induced differentiation, the hESCs plated on the softest substrates, recapitulating the elasticity of the early embryo, underwent highly efficient mesoderm progenitor differentiation as compared to those plated on the stiffer substrates. These findings suggest matrix elasticity may serve to “prime” cells for tissue-specific differentiation. Indeed, hESCs interacting with the softest matrix showed reduced cell—ECM integrin adhesions and developed strong cell-cell adhesions that promoted apical-basal polarity, sequestered β-catenin, P120 catenin, and Kaiso at the adherens junctions and reduced levels of secreted Wnt inhibitors. Upon exposure to BMPs and differentiation stimuli, the “primed” hESC colonies synchronously degraded their E-cadherin junctions, releasing large quantities of β-catenin, which was able to rapidly stimulate mesoderm gene expression upon translocation to the nucleus to induce mesoderm progenitor differentiation.
6.3. ECM Stiffness Modulates Morphogen Activity
Given that cells increase their actomyosin tension in response to the stiffness of the ECM, it is perhaps not surprising that the ability of cells to activate TGF-β can be potentiated by growing cells on or within a mechanically strained or stiffened ECM (Wipff et al., 2007). Moreover, once released, any activated TGF-β can then stimulate ECM synthesis and deposition and elevate the expression of enzymes such as LOX that further stiffen the ECM through a positive feedforward circuit that facilitates normal wound healing but can also drive pathological fibrosis (Cox & Erler, 2011; Frantz, Stewart, & Weaver, 2010). A more direct role for ECM stiffness in tension-mediated morphogen signaling was illustrated by its impact on Notch signaling. Notch activation is enhanced by mechanical force-mediated exposure of its cleavage site on the Notch receptor through ligand-receptor binding that is potentiated by cellular actomyosin contractility stimulated by integrin—ECM anchorage (D’Souza, Miyamoto, & Weinmaster, 2008).
6.4. ECM Stiffness Modulates Cell Behavior by Regulating Mechanosensitive Ion Channels
A stiff ECM can activate mechanosensitive ion channels to induce cell growth and survival, and stimulate cell migration and differentiation. For example, Piezo channels are ubiquitously expressed calcium ion channels that are exquisitely sensitive to mechanical activation (Coste et al.,2010). ECM ligation can activate Piezo transmembrane cation channels to regulate cell differentiation, provided the stiffness of the ECM is high enough that cellular actomyosin tension is substantially increased. This concept was illustrated by Pathak and colleagues who showed that ECM tension-mediated activation of Piezo1 could promote the calciumdependent neurogenesis of human neural stem cells and that its ablation or inhibition promoted astrogenesis (Pathak et al., 2014). Similarly, transient receptor potential (TRP) channels are calcium-permeable and voltageindependent ion channels that also influence stem cell growth, survival, migration, and differentiation and are thus important in tissue development and homeostasis (Muramatsu et al., 2007; Pla et al., 2005; Ramsey, Delling, & Clapham, 2006). TRP channels are highly sensitive to mechanical cues, and not surprisingly, their activity is also strongly induced by Rho GTPases (Mehta et al., 2003; Vriens et al., 2004).
6.5. ECM Stiffness Modulates Yes-Associated Protein and Transcriptional Coactivator With PDZ-Binding Motif
In recent years, signaling events that converge on the coactivators Yes- associated protein and transcriptional coactivator with PDZ-binding motif (YAP/TAZ) have become a major area of interest as evidence has emerged that their activity can be directly regulated by ECM stiffness (Hao et al., 2014). Further, YAP/TAZ are key components of the Hippo signaling pathway, which regulates organ size during development (Zhao, Tumaneng, & Guan, 2011). The relationship between the Hippo pathway and YAP/TAZ was first uncovered in Drosophila, where overexpression of the YAP homologue resulted in dramatic tissue overgrowth, phenocopying loss of Hippo signaling (Huang, Wu, Barrera, Matthews, & Pan, 2005). Conversely, mutation of the YAP homologue prevented normal growth and expansion of developing tissues. Later mechanistic studies revealed that activation of the Hippo signaling cascade ultimately results in phosphorylation of YAP and TAZ, preventing their nuclear localization and sequestering them to the cytoplasm where they undergo proteasomal degradation (Hong & Guan, 2012). In their unphosphorylated form, YAP/TAZ are shuttled to the nucleus, where they associate with other transcription factors and activate gene expression. Direct evidence for the role ofmechanotransduction in regulating YAP/TAZ signaling emerged when it was found that YAP nuclear translocation was induced by culturing cells on stiffsubstrates or by fostering cell spreading using large patterned ECM ligands (Dupont et al., 2011). By contrast, nuclear YAP translocation was prevented when cells were plated on soft substrates or when cell spreading was prevented (Dupont et al., 2011). Consistently, YAP/TAZ nuclear translocation was found to be necessary for the induction of MSC differentiation in a RhoA GTPase-dependent manner that required actomyosin-mediated cytoskeletal tension. Nevertheless, it is likely ECM-mediated regulation of Hippo signaling during development is mediated by both biochemical and biomechanical cues.
7. SUMMARY AND FUTURE DIRECTIONS
The ECM regulates embryogenesis, tissue-specific development, and stem cell fate by binding cellular receptors and activating ion channels to initiate intracellular signaling and gene transcription, by controlling the availability, activation, and presentation of soluble ligands, and by altering cellular tension. Nevertheless, tissue development and cell differentiation rely on tightly regulated epigenetics to induce sustained changes in gene transcription and cell behavior. Indeed, every cell within an organism is endowed with the same genetic code, so that it is the coordinated spatial and temporal regulation of gene expression that is responsible for directing tissue development, maintaining tissue homeostasis, and inducing tissue-specific differentiation.
Cell-ECM adhesion-dependent, tissue-specific differentiation may depend upon alterations in nuclear architecture and chromatin organization. For example, early studies that examined links between the ECM and MEC differentiation showed beta casein gene expression depended upon cell rounding that associated with alterations in higher order chromatin structure and epigenetic changes (Boudreau, Myers, & Bissell, 1995; Myers et al., 1998; Schmidhauser, Bissell, Myerst, & Caspersont, 1990). Consistently, lactogenic hormonal stimulation of the beta casein transcriptional enhancer could only be induced in MECs interacting with a laminin 111 ECM when the reporter cassette was stably expressed in the MECs, implying the chromatin architecture surrounding the promoter was necessary for proper regulation of beta casein expression (Boudreau, Myers, et al., 1995; Boudreau, Sympson, et al., 1995; Xu et al., 2009). ECM stiffness-dependent, tissue-specific gene expression also implies a causal link between cell shape, cytoskeletal organization, and chromatin organization (Engler et al., 2006; Flanagan et al., 2002; Gilbert et al., 2010; Xu et al., 2009). For instance, Chen and colleagues showed that cell shape regulates RhoA/Rac activity to modulate the osteogenic vs adipogenic vs chondrogenic, and myogenic fate of hMSCs (Gao, McBeath, & Chen, 2010; McBeath et al., 2004). Indeed, matrix metalloproteinase 14 (MMP14)-mediated ECM remodeling induces cell rounding to enhance histone acetylation marks of adipogenic cell fate, presumably by reducing epigenetic constraints imposed by type I collagen (Sato-Kusubata, Jiang, Ueno, & Chun, 2011). These studies raise the intriguing possibility that the ECM and its receptors regulate cell and tissue fate by modulating nuclear organization and chromatin. The question is how?
Ingber and colleagues have suggested that cells are “hard-wired,” such that force applied at cell-integrin adhesions is directly transduced to the nucleus through the cytoskeleton, resulting in rearrangements of nuclei and nuclear components (Maniotis, Chen, & Ingber, 1997). This idea was supported by the use of microsurgical techniques to pull on individual chromosomes and nucleoli, and the subsequent observation that pulling a single chromosome out of the nucleus led to sequential removal of the remaining chromosomes (Maniotis, Bojanowski, & Ingber, 1997). Further support for this model was provided by the identification of the linker of nucleoskeleton and cytoskeleton (LINC) complex, suggesting a mechanism through which physical interactions received through cellular integrin- ECM interactions might be directly transmitted to the nucleus to alter gene transcription (Crisp et al., 2006; Haque et al., 2006; Padmakumar et al., 2005; Wang, Tytell, & Ingber, 2009). Since nuclear chromatin is thought to be organized into dense heterochromatin and more open euchromatin, this paradigm suggests that alterations in the LINC complex stimulated by biomechanical cues could rapidly change nuclear shape and increase levels of transcriptionally accessible euchromatin to influence cell fate (Skinner & Johnson, 2017). The discovery of a set of nuclear receptor coactivators that were aptly named tension-induced proteins (TIPs) has illuminated another putative pathway through which biomechanical cues transmitted through cell-ECM receptors could regulate chromatin organization and alter nuclear processes to influence gene expression (Jakkaraju, Zhe, Pan, Choudhury, & Schuger, 2005). MSCs can be induced to differentiate toward a myogenic fate by exposing the cells to chronic stretch and toward an adipogenic fate by preventing their spreading (McBeath et al., 2004; Yang, Beqaj, Kemp, Ariel, & Schuger, 2000). Jakkaraju and colleagues found that myogenic differentiation of these MSCs depends upon histone acetylation and the recruitment of specific coactivators that bind to TIP proteins through a specific nuclear receptor box domain that is exposed in response to cell stretch, and that adipogenesis is the default pathway when these TIP proteins are not recruited (Jakkaraju et al., 2005). Thus, physical forces transmitted through the ECM could directly regulate the activity of nuclear transcriptional regulators to direct cell fate.
Importantly, chromatin organization is complex and dynamic, with distal sequences of DNA capable of interacting through topologically associated domains (TADs) and long sequences of DNA sequestered near the nuclear membrane in lamin-associated domains (LADs) (Gonzalez-Sandoval & Gasser, 2016; Nicodemi & Pombo, 2014). The precise organization of these TADs and LADs is cell-and tissue-type specific. Thus, while there may be physical connections between cell-ECM adhesions and the nucleus, chromatin remodeling also depends on dynamic interactions between DNA and its binding partners. For example, osteoblast-specific transcription factors such as Runx2 can physically associate with nuclear matrix components during osteoblast differentiation, whereas more ubiquitous transcription factors remain bound to nonmatrix nuclear compartments (Lindenmuth et al., 1997). These tissue-specific transcription factors apparently bind to the nuclear matrix through specific nuclear matrix-targeting sequences such that introducing mutations in these sequences compromise cell proliferation and differentiation of myeloid progenitor cells (Stein et al., 2007). Similarly, recent studies suggest cell density, cell migration, and fluid flow can all dramatically change nuclear shape to induce sustained changes in tissue-specific gene expression (Dahl, Ribeiro, & Lammerding, 2008; Denais et al., 2016; McBride & Knothe Tate, 2008).
Beyond DNA-protein interactions that regulate chromatin structure, it is becoming increasing clear that RNAs also play important roles in regulating chromatin organization and epigenetics. Studies of the human genome have revealed that while nearly all of the genome is capable of being transcribed, only about 2% of this genetic information consists of protein-encoding exons (Consortium, 2001; Djebali et al., 2012; ENCODE Consortium, 2007; Venter et al., 2001). The remainder of transcribed RNA is referred to as heteronuclear RNA and consists of numerous subclasses of RNA whose functions are still being uncovered (Guttman & Rinn, 2012; Salditt-Georgieff, Harpold, Wilson, & Darnell, 1981; Warner, Soeiro, Birnboim, Girard, & Darnell, 1966). Among these subclasses, long intergenic noncoding RNAs (lincRNAs) have emerged as a subclass of particular interest. These lincRNAs have been shown to act as important scaffolds for chromatinmodifying proteins that are responsible for establishing and maintaining the epigenetic state of the cell, and as might be expected, the expression of lincRNAs changes with differentiation and disease (Cesana et al., 2011; Kogo et al., 2011; Tsai et al., 2010; Wang et al., 2011). Although a clear demonstration oflincRNA regulation by cell-ECM interactions has yet to be reported, it seems likely that future studies will reveal such a mechanism given the number of ways the ECM is capable of regulating the comparatively few number of protein-encoding genes. Similarly, noncoding RNAs also regulate gene expression at the level of pre-RNA processing. For instance, small nucleolar RNA (snoRNA) base pair with pre-RNA to guide folding and splicing of transcripts, and some evidence even suggests snoRNAs can affect translation ofmRNA similar to microRNAs (miRNAs) (Ender et al., 2008; Gerbi & Borovjagin, 2004). We found ECM stiffness can regulate miRNAs, which modulate gene expression posttranscriptionally, downstream of activated integrins and through signaling cascades initiated by Robo-Slit interactions (Le et al., 2016; Mouw, Yui, et al., 2014). However, these were not broad regulatory effects, rather, we noted that a subgroup of miRNAs is highly sensitive to ECM stiffness, implying there may be a level of specificity to which sets of genes can be epigenetically regulated by miRNAs in response to stiffness. This raises the intriguing possibility of identifying novel tensionregulated pathways that modulate RNA biogenesis and modify chromatin and gene expression. Such interplay between the ECM and the epigenetic state of the cell could play a critical role in the spatial and temporal regulation of cell fate during embryogenesis, tissue-specific differentiation, wound healing, and homeostasis.
ACKNOWLEDGMENTS
The authors would like to acknowledge support from the California Institute for Regenerative Medicine (CIRM awards TR3–05542 and RB5–07409), the National Institutes of Health and National Cancer Institute (awards 1U01CA202241–01, R01CA192914, R01CA174929, and R01CA222508–01), and the Department of Defense Breast Cancer Research Program (USAMRAA DOD-BCRP award BC122990). J.M.M. is also grateful for support from NIH Training Grant: BioE T32 GM008155 and the UCSF Discovery Fellowship.
REFERENCES
- Abbott J, & Holtzer H (1966). The loss of phenotypic traits by differentiated cells. 3. The reversible behavior of chondrocytes in primary cultures. Journal of Cell Biology, 28(3), 473–487. 10.1083/jcb.283.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel-Latif A, Zuba-Surma EK, Case J, Tiwari S, Hunt G, Ranjan S, et al. (2008). TGF-β1 enhances cardiomyogenic differentiation of skeletal muscle-derived adult primitive cells. Basic Research in Cardiology, 103(6), 514–524. 10.1007/s00395-008-0729-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams JC, & Watt FM (1993). Regulation of development and differentiation by the extracellular matrix. Development (Cambridge, England), 117(4), 1183–1198. [DOI] [PubMed] [Google Scholar]
- Akhurst RJ, Lehnert SA, Faissner A, & Duffie E (1990). TGF beta in murine morphogenetic processes: The early embryo and cardiogenesis. Development (Cambridge, England), 108(4), 645–656. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/1696875. [DOI] [PubMed] [Google Scholar]
- Alfandari D, Cousin H, Gaultier A, Hoffstrom BG, & DeSimone DW (2003). Integrin α5β1 supports the migration of Xenopus cranial neural crest on fibronectin. Developmental Biology, 260(2), 449–464. 10.1016/S0012-1606(03)00277-X [DOI] [PubMed] [Google Scholar]
- An C, Cheng Y, Yuan Q, & Li J (2010). IGF-1 and BMP-2 induces differentiation of adipose-derived mesenchymal stem cells into chondrocytes-like cells. Annals of Biomedical Engineering, 38(4), 1647–1654. 10.1007/s10439-009-9892-x. [DOI] [PubMed] [Google Scholar]
- Arteaga-Solis E, Gayraud B, Lee SY, Shum L, Sakai L, & Ramirez F (2001). Regulation of limb patterning by extracellular microfibrils. Journal of Cell Biology, 154(2), 275–281. 10.1083/jcb.200105046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augst AD, Kong HJ, & Mooney DJ (2006). Alginate hydrogels as biomaterials. Macromolecular Bioscience, 6(8), 623–633. 10.1002/mabi.200600069. [DOI] [PubMed] [Google Scholar]
- Aumailley M, Bruckner-Tuderman L, Carter WG, Deutzmann R, Edgar D, Ekblom P, et al. (2005). A simplified laminin nomenclature. Matrix Biology, 24(5), 326–332. 10.1016/j.matbio.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Avigdor A, Goichberg P, Shivtiel S, Dar A, Peled A, Samira S, et al. (2004). CD44 and hyaluronic acid cooperate with SDF-1 in the trafficking of human CD34+ stem/ progenitor cells to bone marrow. Blood, 103(8), 2981–2989. 10.1182/blood-2003-10-3611. [DOI] [PubMed] [Google Scholar]
- Baker NE, Mlodzik M, & Rubin GM (1990). Spacing differentiation in the developing Drosophila eye: A fibrinogen-related lateral inhibitor encoded by scabrous. Science (New York, N.Y.), 250(4986), 1370–1377. 10.1126/science.2175046. [DOI] [PubMed] [Google Scholar]
- Baneyx G, Baugh L, & Vogel V (2001). Coexisting conformations of fibronectin in cell culture imaged using fluorescence resonance energy transfer. Proceedings of the National Academy of Sciences of the United States of America, 98(25), 14464–14468. 10.1073/pnas.251422998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baneyx G, Baugh L, & Vogel V (2002). Fibronectin extension and unfolding within cell matrix fibrils controlled by cytoskeletal tension. Proceedings ofthe National Academy of Sciences of the United States of America, 99(8), 5139–5143. 10.1073/pnas.072650799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbulovic-Nad I, Lucente M, Sun Y, Zhang M, Wheeler AR, & Bussmann M(2006). Bio-microarray fabrication techniques—A review. Critical Reviews in Biotechnology, 26(4), 237–259. 10.1080/07388550600978358. [DOI] [PubMed] [Google Scholar]
- Benito-Jardón M, Klapproth S, Gimeno-Lluch I, Petzold T, Bharadwaj M, Muller DJ, et al. (2017). The fibronectin synergy site re-enforces cell adhesion and mediates a crosstalk between integrin classes. eLife, 6, e22264 10.7554/eLife.22264.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissell MJ, Hall HG, & Parry G (1982). How does the extracellular matrix direct gene expression? Journal of Theoretical Biology, 99(1), 31–68. 10.1016/0022-5193(82)90388-5. [DOI] [PubMed] [Google Scholar]
- Biteau B, & Jasper H (2014). Slit/Robo signaling regulates cell fate decisions in the intestinal stem cell lineage of Drosophila. Cell Reports, 7(6), 1867–1875. 10.1016/j.celrep.2014.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnans C, Chou J, & Werb Z (2014). Remodelling the extracellular matrix in development and disease. Nature Reviews Molecular Cell Biology, 15(12), 786–801. 10.1038/nrm3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucaut JC, Darribere T, Li SD, Boulekbache H, Yamada KM, & Thiery JP (1985). Evidence for the role offibronectin in amphibian gastrulation. Journal of Embry- ology and Experimental Morphology, 89(Suppl), 211–227. [PubMed] [Google Scholar]
- Boucaut JC, Darribere T, Poole TJ, Aoyama H, Yamada KM, & Thiery JP (1984). Biologically active synthetic peptides as probes of embryonic development: A competitive peptide inhibitor of fibronectin function inhibits gastrulation in amphibian embryos and neural cell migration in avian embryos. Journal of Cell Biology, 99(5), 1822–1830. 10.1083/jcb.99.5.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau N, Myers C, & Bissell MJ (1995). From laminin to lamin: Regulation of tissue-specific gene expression by the ECM. Trends in Cell Biology, 5(1), 1–4. 10.1016/S0962-8924(00)88924-2. [DOI] [PubMed] [Google Scholar]
- Boudreau N, Sympson CJ, Werb Z, & Bissell MJ (1995). Suppression of ICE and apoptosis in mammary epithelial cells by extracellular matrix. Science, 267(5199), 891–893. 10.1126/science.7531366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourguignon LYW, Ramez M, Gilad E, Singleton PA, Man M-Q, Crumrine DA, et al. (2006). Hyaluronan-CD44 interaction stimulates keratinocyte differentiation, lamellar body formation/secretion, and permeability barrier homeostasis. The Journal of Investigative Dermatology, 126(6), 1356–1365. 10.1038/sj.jid.5700260. [DOI] [PubMed] [Google Scholar]
- Brower DL, & Jaffe SM (1989). Requirement for integrins during Drosophila wing development. Nature, 342(6247), 285–287. 10.1038/342285a0. [DOI] [PubMed] [Google Scholar]
- Burdick JA, & Prestwich GD (2011). Hyaluronic acid hydrogels for biomedical applications. Advanced Materials, 23(12), H41–56. 10.1002/adma.201003963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso WV (2006). Regulation of early lung morphogenesis: Questions, facts and controversies. Development, 133(9), 1611–1624. 10.1242/dev.02310. [DOI] [PubMed] [Google Scholar]
- Cesana M, Cacchiarelli D, Legnini I, Santini T, Sthandier O, Chinappi M, et al. (2011). A long noncoding RNA controls muscle differentiation by functioning as a competing endogenous RNA. Cell, 147(2), 358–369. 10.1016/j.cell.2011.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang W, Lin Z, Kulessa H, Hebert J, Hogan BLM, & Wu DK (2008). Bmp4 is essential for the formation of the vestibular apparatus that detects angular head movements. PLoS Genetics, 4(4), e1000050 10.1371/journal.pgen.1000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Deng C, & Li YP (2012). TGF-β and BMP signaling in osteoblast differentiation and bone formation. International Journal of Biological Sciences, 8(2), 272–288. 10.7150/ijbs.2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung TH, & Rando TA (2013). Molecular regulation of stem cell quiescence. Nature Reviews Molecular Cell Biology, 14(6), 329–40. 10.1038/nrm3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles EG, Gammill LS, Miner JH, & Bronner-Fraser M (2006). Abnormalities in neural crest cell migration in laminin alpha5 mutant mice. Developmental Biology, 289(1), 218–228. https://doi.org/S0012-1606(05)00759-1. [DOI] [PubMed] [Google Scholar]
- Connelly JT, Gautrot JE, Trappmann B, Tan DW-M, Donati G, Huck WTS, et al. (2010). Actin and serum response factor transduce physical cues from the microenvironment to regulate epidermal stem cell fate decisions. Nature Cell Biology, 12(7), 711–718. 10.1038/ncb2074. [DOI] [PubMed] [Google Scholar]
- Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ, et al. (2010). Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science, 330(6000), 55–60. 10.1126/science.1193270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox TR, & Erler JT (2011). Remodeling and homeostasis of the extracellular matrix: Implications for fibrotic diseases and cancer. Disease Models & Mechanisms, 4(2), 165–178. 10.1242/dmm.004077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisp M, Liu Q, Roux K, Rattner JB, Shanahan C, Burke B, et al. (2006). Coupling of the nucleus and cytoplasm: Role of the LINC complex. Journal of Cell Biology, 172(1), 41–53. 10.1083/jcb.200509124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabovic B, Chen Y, Colarossi C, Obata H, Zambuto L, Perle MA, et al. (2002). Bone abnormalities in latent TGF-β binding protein (Ltbp)-3-null mice indicate a role for Ltbp-3 in modulating TGF-β bioavailability. Journal of Cell Biology, 156(2), 227–232. 10.1083/jcb.200111080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl KN, Ribeiro AJS, & Lammerding J (2008). Nuclear shape, mechanics, and mechanotransduction. Circulation Research, 102(11), 1307–1318. 10.1161/CIRCRESAHA.108.173989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debelle L, & Tamburro AM (1999). Elastin: Molecular description and function. International Journal of Biochemistry and Cell Biology, 31(2), 261–272. 10.1016/S1357-2725(98)00098-3. [DOI] [PubMed] [Google Scholar]
- Denais CM, Gilbert RM, Isermann P, McGregor AL, te Lindert M, Weigelin B, et al. (2016). Nuclear envelope rupture and repair during cancer cell migration. Science, 352(6283), 353–358. 10.1126/science.aad7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson MC, Slager HG, Duffie E, Mummery CL, & Akhurst RJ (1993). RNA and protein localisations of TGF beta 2 in the early mouse embryo suggest an involvement in cardiac development. Development (Cambridge, England), 117(2), 625–39. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/7687212 [DOI] [PubMed] [Google Scholar]
- Dixon JE, Shah DA, Rogers C, Hall S, Weston N, Parmenter CDJ, et al. (2014). Combined hydrogels that switch human pluripotent stem cells from self-renewal to differentiation. Proceedings of the National Academy of Sciences of the United States of America, 111(15), 5580–5585. 10.1073/pnas.1319685111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djebali S, Davis CA, Merkel A, Dobin A, Lassmann T, Mortazavi A, et al. (2012). Landscape of transcription in human cells. Nature, 489(7414), 101–108. 10.1038/nature11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza B, Miyamoto A, & Weinmaster G (2008). The many facets of Notch ligands. Oncogene, 27(38), 5148–5167. 10.1038/onc.2008.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, et al. (2011). Role of YAP/TAZ in mechanotransduction. Nature, 474(7350), 179–183. 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- ENCODE Consortium. (2007). Identification and analysis of functional elements in 1% of the human genomeby the ENCODE pilotproject. Nature, 447(7146), 799–816. 10.1038/nature05874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ender C, Krek A, Friedlander MR, Beitzinger M, Weinmann L, Chen W, et al. (2008). A human snoRNA with microRNA-like functions. Molecular Cell, 32(4), 519–528. 10.1016/j.molcel.2008.10.017. [DOI] [PubMed] [Google Scholar]
- Engler AJ, Sen S, Sweeney HL, & Discher DE (2006). Matrix elasticity directs stem cell lineage specification. Cell, 126(4), 677–689. 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- Erlebacher A, & Derynck R (1996). Increased expression of TGF-β2 in osteoblasts results in an osteoporosis-like phenotype. Journal of Cell Biology, 132(1–2), 195–210. 10.1083/jcb.132.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassler R, Rohwedel J, Maltsev V, Bloch W, Lentini S, Guan K, et al. (1996). Differentiation and integrity of cardiac muscle cells are impaired in the absence of beta 1 integrin. Journal of Cell Science, 109(Pt. 1), 2989–2999. [DOI] [PubMed] [Google Scholar]
- Flaim CJ, Chien S, & Bhatia SN (2005).An extracellularmatrixmicroarrayforprobing cellular differentiation. Nature Methods, 2(2), 119–125. 10.1038/nmeth736. [DOI] [PubMed] [Google Scholar]
- Flaim CJ, Teng D, Chien S, & Bhatia SN (2008). Combinatorial signaling microenvironments for studying stem cell fate. Stem Cells and Development, 17(1), 29–40. 10.1089/scd.2007.0085. [DOI] [PubMed] [Google Scholar]
- Flanagan LA, Ju YE, Marg B, Osterfield M, & Janmey PA (2002). Neurite branching on deformable substrates. Neuro Report, 13(18), 2411–2415. 10.1097/00001756-200212200-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frantz C, Stewart KM, & Weaver VM (2010). The extracellular matrix at a glance. Journal of Cell Science, 123(24), 4195–4200. 10.1242/jcs.023820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedland JC, Lee MH, & Boettiger D (2009). Mechanically activated integrin switch controls 5 1 function. Science, 323(5914), 642–644. 10.1126/science.1168441. [DOI] [PubMed] [Google Scholar]
- Fuchs E, & Chen T (2012). A matter of life and death: Self-renewal in stem cells. EMBO Reports, 14(1), 39–48. 10.1038/embor.2012.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L, McBeath R, & Chen CS (2010). Stem cell shape regulates a chondrogenic versus myogenic fate through rac 1 and N-cadherin. Stem Cells, 28(3), 564–572. 10.1002/stem.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattazzo F, Urciuolo A, & Bonaldo P (2014). Extracellular matrix: A dynamic microenvironment for stem cell niche. Biochimica et Biophysica Acta—General Subjects,1840(8), 2506–2519. 10.1016/j.bbagen.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerbi S, & Borovjagin A (2004). Pre-ribosomal RNA processing in multicellular organisms. The Nucleolus, 170–198. [Google Scholar]
- Gilbert PM, Havenstrite KL, Magnusson KEG, Sacco A, Leonardi NA, Kraft P, et al. (2010). Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science, 329(5995), 1078–1081. 10.1126/science.1191035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Sandoval A, & Gasser SM (2016). On TADs and LADs: Spatial control over gene expression. Trends in Genetics, 32(8), 485–495. 10.1016/j.tig.2016.05.004. [DOI] [PubMed] [Google Scholar]
- Gordon MK, & Hahn RA (2010). Collagens. Cell and Tissue Research, 339(1), 247–257. 10.1007/s00441-009-0844-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goumans MJ, de Boer TP, Smits AM, van Laake LW, van Vliet P, Metz CHG, et al. (2008). TGF-β1 induces efficient differentiation of human cardiomyocyte progenitor cells into functional cardiomyocytes in vitro. Stem Cell Research, 1(2), 138–149. 10.1016/j.scr.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Griffith LG (2002). Tissue engineering—Current challenges and expanding opportunities.Science, 295(5557), 1009–1014. 10.1126/science.1069210. [DOI] [PubMed] [Google Scholar]
- Griffith LG, & Swartz MA (2006). Capturing complex 3D tissue physiology in vitro.Nature Reviews Molecular Cell Biology, 7(3), 211–224. 10.1038/nrm1858. [DOI] [PubMed] [Google Scholar]
- Guo X, Johnson JJ, & Kramer JM (1991). Embryonic lethality caused by mutations in basement membrane collagen of C. elegans. Nature, 349(6311), 707–709. 10.1038/349707a0. [DOI] [PubMed] [Google Scholar]
- Guttman M, & Rinn JL (2012). Modular regulatory principles of large non-coding RNAs. Nature, 482(7385), 339–346. 10.1038/nature10887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamidouche Z, Fromigué O, Ringe J, Häupl T, Vaudin P, Pagès J-C, et al. (2009). Priming integrin alpha5 promotes human mesenchymal stromal cell osteoblast differentiation and osteogenesis. Proceedings of the National Academy of Sciences of the United States of America, 106, 18587–18591. 10.1016/j.bone.2009.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han VK, D’Ercole AJ, & Lund PK (1987). Cellular localization ofsomatomedin (insulinlike growth factor) messenger RNA in the human fetus. Science, 236(4798), 193–197. [DOI] [PubMed] [Google Scholar]
- Hao J, Zhang Y, Wang Y, Ye R, Qiu J, Zhao Z, et al. (2014). Role of extracellular matrix and YAP/TAZ in cell fate determination. Cellular Signalling, 26(2), 186–191. 10.1016/j.cellsig.2013.11.006. [DOI] [PubMed] [Google Scholar]
- Haque F, Lloyd DJ, Smallwood DT, Dent CL, Shanahan CM, Fry AM, et al. (2006). SUN1 interacts with nuclear lamin A and cytoplasmic nesprins to provide a physical connection between the nuclear lamina and the cytoskeleton. Molecular and Cellular Biology, 26(10), 3738–3751. 10.1128/MCB.26.10.3738-3751.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada M, Murakami H, Okawa A, Okimoto N, Hiraoka S, Nakahara T, et al. (2009). FGF9 monomer-dimer equilibrium regulates extracellular matrix affinity and tissue diffusion. Nature Genetics, 41(3), 289–298. 10.1038/ng.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill DJ, Clemmons DR, Wilson S, Han VKM, Strain AJ, & Milner RDG (1989). Immunological distribution of one form of insulin-like growth factor (IGF)-binding protein and IGF peptides in human fetal tissues. Journal of Molecular Endocrinology, 2(1), 31–38. [DOI] [PubMed] [Google Scholar]
- Hinz B (2015). The extracellular matrix and transforming growth factor-β1: Tale of a strained relationship. Matrix Biology, 47, 54–65. 10.1016/j.matbio.2015.05.006. [DOI] [PubMed] [Google Scholar]
- Hong W, & Guan KL (2012). The YAP and TAZ transcription co-activators: Key downstream effectors of the mammalian hippo pathway. Seminars in Cell and Developmental Biology, 23(7), 785–793. https://doi.org/10.10167j.semcdb.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hortsch M, & Goodman CS (1991). Cell and substrate adhesion molecules in Drosophila. Annual Review of Cell Biology, 7(1), 505–557. [DOI] [PubMed] [Google Scholar]
- Hsieh J, Aimone JB, Kaspar BK, Kuwabara T, Nakashima K, & Gage FH (2004). IGF-I instructs multipotent adult neural progenitor cells to become oligodendrocytes. Journal of Cell Biology, 164(1), 111–122. 10.1083/jcb.200308101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Wu S, Barrera J, Matthews K, & Pan D (2005). The hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating yorkie, the Drosophila homolog of YAP. Cell, 122(3), 421–434. 10.1016/j.cell.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Hynes RO (2002). Integrins: Bidirectional, allosteric signaling machines. Cell, 110(6), 673–687. 10.1016/S0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- International Human Genome Sequencing Consortium. (2001). Initial sequencing and analysis of the human genome. Nature, 409, 860–921. 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- Ishii N, Wadsworth WG, Stern BD, Culotti JG, & Hedgecock EM (1992). UNC-6, a laminin-related protein, guides cell and pioneer axon migrations in C. elegans. Neuron, 9(5), 873–881. 10.1016/0896-6273(92)90240-E. [DOI] [PubMed] [Google Scholar]
- Jakkaraju S, Zhe X, Pan D, Choudhury R, & Schuger L (2005). TIPs are tension-responsive proteins involved in myogenic versus adipogenic differentiation. Developmental Cell, 9(1), 39–49. 10.1016/j.devcel.2005.04.015. [DOI] [PubMed] [Google Scholar]
- Janmey PA, Winer JP, & Weisel JW (2009). Fibrin gels and their clinical and bioengineering applications. Journal of the Royal Society Interface, 6(30), 1–10. 10.1098/rsif.2008.0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia J, Maccarana M, Zhang X, Bespalov M, Lindahl U, & Li JP (2009). Lack of L-iduronic acid in heparan sulfate affects interaction with growth factors and cell signaling. Journal of Biological Chemistry, 284(23), 15942–15950. 10.1074/jbc.M809577200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jikko A, Harris SE, Chen D, Mendrick DL, & Damsky CH (1999). Collagen integrin receptors regulate early osteoblast differentiation induced by BMP-2. Journal of Bone and Mineral Research, 14(7), 1075–1083. 10.1359/jbmr.1999.14.7.1075. [DOI] [PubMed] [Google Scholar]
- Jones JI, Gockerman A, Busby WH, Camacho-Hubner C, & Clemmons DR (1993). Extracellular matrix contains insulin-like growth factor binding protein-5: Potentiation of the effects of IGF-I. Journal of Cell Biology, 121(3), 679–687. 10.1083/jcb.121.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jürgensen HJ, Madsen DH, Ingvarsen S, Melander MC, Gardsvoll H, Patthy L, et al. (2011). A novel functional role of collagen glycosylation: Interaction with the endocytic collagen receptor uPARAP/ENDO180. Journal of Biological Chemistry, 286(37), 32736–32748. 10.1074/jbc.M111.266692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadler KE, Hill A, & Canty-Laird EG (2008). Collagen fibrillogenesis: Fibronectin, integrins, and minor collagens as organizers and nucleators. Current Opinion in Cell Biology, 20(5), 495–501. 10.1016/jxeb.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan HM, & Li W (2003). Lysyl oxidase: Properties, specificity, and biological roles inside and outside of the cell. Journal of Cellular Biochemistry, 88(4), 660–672. 10.1002/jcb.10413. [DOI] [PubMed] [Google Scholar]
- Keller R (2005). Cell migration during gastrulation. Current Opinion in Cell Biology, 17, 533–541. 10.1016/jxeb.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Khetarpal U, Robertson NG,Yoo TJ,& Morton CC (1994). Expression andlocalization ofCOL2A1 mRNA and type II collagen in human fetal cochlea. Hearing Research, 79(1–2), 59–73. 10.1016/0378-5955(94)90127-9. [DOI] [PubMed] [Google Scholar]
- Kilian KA, Bugarija B, Lahn BT, & Mrksich M (2010). Geometric cues for directing the differentiation of mesenchymal stem cells. Proceedings of the National Academy of Sciences of the United States of America, 107(11), 4872–4877. 10.1073/pnas.0903269107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein EA, Yin L, Kothapalli D, Castagnino P, Byfield FJ, Xu T, et al. (2009). Cell- cycle control by physiological matrix elasticity and in vivo tissue stiffening. Current Biology, 19(18), 1511–1518. 10.1016/jxub.2009.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinowska TC, Soriano JV, Edwards GM, Oliver JM, Valentijn AJ, Montesano R, et al. (1999). Laminin and beta 1 integrins are crucial for normal mammary gland development in the mouse. Developmental Biology, 215(1), 13–32. 10.1006/dbio.1999.9435. [DOI] [PubMed] [Google Scholar]
- Knecht AK, & Bronner-Fraser M (2002). Induction of the neural crest: a multigene process. Nature Reviews Genetics, 3(6), 453–461. 10.1038/nrg819. [DOI] [PubMed] [Google Scholar]
- Kogo R, Shimamura T, Mimori K, Kawahara K, Imoto S, Sudo T, et al. (2011). Long noncoding RNA HOTAIR regulates polycomb-dependent chromatin modification and is associated with poor prognosis in colorectal cancers. Cancer Research, 71(20), 6320–6326. 10.1158/0008-5472.CAN-11-1021. [DOI] [PubMed] [Google Scholar]
- Kramer JM,Johnson JJ, Edgar RS, Basch C, & Roberts S(1988). The sqt-1 gene of C. elegans encodes a collagen critical for organismal morphogenesis. Cell, 55(4), 555–565. 10.1016/0092-8674(88)90214-0. [DOI] [PubMed] [Google Scholar]
- Kubow KE, Klotzsch E, Smith ML, Gourdon D, Little WC, & Vogel V (2009). Crosslinking of cell-derived 3D scaffolds up-regulates the stretching and unfolding of new extracellular matrix assembled by reseeded cells. Integrative Biology, 1(11–12), 635 10.1039/b914996a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok JCF, Dick G, Wang D, & Fawcett JW (2011). Extracellular matrix and peri-neuronal nets in CNS repair. Developmental Neurobiology, 71(11), 1073–1089. 10.1002/dneu.20974. [DOI] [PubMed] [Google Scholar]
- La Barge MA, Nelson CM, Villadsen R, Fridriksdottir A, Ruth JR, Stampfer MR, et al. (2009). Human mammary progenitor cell fate decisions are products of interactions with combinatorial microenvironments. Integrative Biology, 1(1), 70–79. 10.1039/B816472J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrador JP, Azcoitia V, Tuckermann J, Lin C, Olaso E, Maries S, et al. (2001). The collagen receptor DDR2 regulates proliferation and its elimination leads to dwarfism. EMBO Reports, 2(5), 446–452. 10.1093/embo-reports/kve094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larraín J, Bachiller D, Lu B, Agius E, Piccolo S, & De Robertis EM (2000). BMP- binding modules in chordin: A model for signalling regulation in the extracellular space. Development (Cambridge, England), 127(4), 821–830. 10.1016/j.drugalcdep.2008.02.002.A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent TC, & Fraser JR (1992). Hyaluronan. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology, 6(7), 2397–2404. 10.1016/S0140-6736(01)35637-4. [DOI] [PubMed] [Google Scholar]
- Le LTN, Cazares O, Mouw JK, Chatterjee S, Macias H, Moran A, et al. (2016). Loss of miR-203 regulates cell shape and matrix adhesion through ROBO1/Rac/FAK in response to stiffness. Journal of Cell Biology, 212(6), 707–719. 10.1083/jcb.201507054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EJ, Kasper FK, & Mikos AG (2014). Biomaterials for tissue engineering. Annals of Biomedical Engineering, 42, 323–337. 10.1007/s10439-013-0859-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee ST, Yun JI, Jo YS, Mochizuki M, van der Vlies AJ, Kontos S, et al. (2010). Engineering integrin signaling for promoting embryonic stem cell self-renewal in a precisely defined niche. Biomaterials, 31(6), 1219–1226. 10.1016/j.biomaterials.2009.10.054. [DOI] [PubMed] [Google Scholar]
- Leptin M, Bogaert T, Lehmann R, & Wilcox M (1989). The function of PS integrins during Drosophila embryogenesis. Cell, 56(3), 401–408. 10.1016/0092-8674(89)90243-2. [DOI] [PubMed] [Google Scholar]
- Li L, & Clevers H (2010). Coexistence of quiescent and active adult stem cells in mammals. Science, 327(5965), 542–545. 10.1126/science.1180794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Corrales CE, Wang Z, Zhao Y, Wang Y, Liu H, et al. (2005). BMP4 signaling is involved in the generation of inner ear sensory epithelia. BMC Developmental Biology, 5, 16 10.1186/1471-213x-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Li L, Chen ZN, Gao G, Yao R, & Sun W (2017). Engineering-derived approaches for iPSC preparation, expansion, differentiation and applications. Bio fabrication, 9(3), 032001 10.1088/1758-5090/aa7e9a. [DOI] [PubMed] [Google Scholar]
- Lindenmuth DM, Van Wijnen AJ, Hiebert S, Stein JL, Lian JB, & Stein GS (1997). Subcellular partitioning of transcription factors during osteoblast differentiation: Developmental association of the AML/CBFα/PEBP2α-related transcription factor- NMP-2 with the nuclear matrix. Journal of Cellular Biochemistry, 66(1), 123–132. . [DOI] [PubMed] [Google Scholar]
- Lodish H, Berk A, Zipursky SL, et al. (2000). Molecular cell biology (4th ed.). (Section 22.3, Collagen: The fibrous proteins of the matrix). New York: W. H. Freeman. [Google Scholar]
- Lu P, Takai K, Weaver VM, & Werb Z (2011). Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harbor Perspectives in Biology, 3(12), 1–24. 10.1101/cshperspect.a005058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund AW, Yener B, Stegemann JP, & Plopper GE (2009). The naturaland engineered 3D microenvironment as a regulatory cue during stem cell fate determination. Tissue Engineering Part B, Reviews, 15(3), 371–380. 10.1089/ten.teb.2009.0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacBeath G, & Schreiber SL (2000). Printing proteins as microarrays for high- throughput function determination. Science, 289(September), 1760–1763. 10.1126/science.289.5485.1760. [DOI] [PubMed] [Google Scholar]
- Macias H, Moran A, Samara Y, Moreno M, Compton JE, Harburg G, et al. (2011). SLIT/ROBO1 signaling suppresses mammary branching morphogenesis by limiting basal cell number. Developmental Cell, 20(6), 827–840. 10.1016/j.devcel.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki JM, Rasanen J, Tikkanen H, Sormunen R, Makikallio K, Kivirikko KI, et al. (2002). Inactivation of the lysyl oxidase gene Lox leads to aortic aneurysms, cardiovascular dysfunction, and perinatal death in mice. Circulation, 106(19), 2503–2509. 10.1161/01.CIR.0000038109.84500.1E. [DOI] [PubMed] [Google Scholar]
- Maniotis AJ, Bojanowski K, & Ingber DE (1997). Mechanical continuity and reversible chromosome disassembly within intact genomes removed from living cells. Journal of Cellular Biochemistry, 65(1), 114–130. . [DOI] [PubMed] [Google Scholar]
- Maniotis AJ, Chen CS, & Ingber DE (1997). Demonstration of mechanical connections between integrins, cytoskeletal filaments, and nucleoplasm that stabilize nuclear structure. Proceedings of the National Academy of Sciences of the United States of America, 94(3), 849–854. 10.1073/pnas.94.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin DC, Fowlkes JL, Babic B, & Khokha R (1999). Insulin-like growth factor II signaling in neoplastic proliferation is blocked by transgenic expression of the metalloproteinase inhibitor TIMP-1. Journal of Cell Biology, 146(4), 881–892. 10.1083/jcb.146.4.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui T, Raya A, Callol-Massot C, Kawakami Y, Oishi I, Rodriguez-Esteban C, et al. (2007). miles-apart-Mediated regulation of cell-fibronectin interaction and myocardial migration in zebrafish. Nature Clinical Practice. Cardiovascular Medicine, 4(Suppl. 1), S77–82. 10.1038/ncpcardio0764. [DOI] [PubMed] [Google Scholar]
- McBeath R, Pirone DM, Nelson CM, Bhadriraju K, & Chen CS (2004). Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Developmental Cell, 6(4), 483–495. 10.1016/S1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- McBride SH, & Knothe Tate ML (2008). Modulation of stem cell shape and fate A: The role of density and seeding protocol on nucleus shape and gene expression. Tissue Engineering. Part A, 14(9), 1561–1572. 10.1089/ten.tea.2008.0112. [DOI] [PubMed] [Google Scholar]
- Mecham RP (2012). Overview of extracellular matrix. Current Protocols in Cell Biology, 57 10.1.1–10.1.16. 10.1002/0471143030.cb1001s57. [DOI] [PubMed] [Google Scholar]
- Mehta D, Ahmmed GU, Paria BC, Holinstat M, Voyno-Yasenetskaya T, Tiruppathi C, et al. (2003). RhoA interaction with inositol 1,4,5-trisphosphate receptor and transient receptor potential channel-1 regulates Ca2+ entry: Role in signaling increased endothelial permeability. Journal of Biological Chemistry, 278(35), 33492–33500. 10.1074/jbc.M302401200. [DOI] [PubMed] [Google Scholar]
- Miroshnikova YA, Rozenberg GI, Cassereau L, Pickup M, Mouw JK, Ou G, et al. (2017). α5Β1-Integrinpromotes tension-dependent mammary epithelial cell invasion by engaging the fibronectin synergy site. Molecular Biology of the Cell, 28(22), 2958–2977. mbc. E17–02-0126. 10.1091/mbc.E17-02-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno M, Fujisawa R, & Kuboki Y (2000). Type I collagen-induced osteoblastic differentiation of bone-marrow cells mediated by collagen-α2β1 integrin interaction. Jour- nal of Cellular Physiology, 184(2), 207–213. . [DOI] [PubMed] [Google Scholar]
- Montanez-Sauri SI, Beebe DJ, & Sung KE (2015). Micro scale screening systems for 3D cellular microenvironments: Platforms, advances, and challenges. Cellular and Molecular Life Sciences, 72(2), 237–249. 10.1007/s00018-014-1738-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouw JK, Ou G, & Weaver VM (2014). Extracellular matrix assembly: A multiscale deconstruction. Nature Reviews Molecular Cell Biology, 15(12), 771–785. 10.1038/nrm3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouw JK, Yui Y, Damiano L, Bainer RO, Lakins JN, Acerbi I, et al. (2014). Tissue mechanics modulate micro RNA-dependent PTEN expression to regulate malignant progression. Nature Medicine, 20(4), 360–367. 10.1038/nm.3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramatsu S, Wakabayashi M, Ohno T, Amano K, Ooishi R, Sugahara T, et al. (2007). Functional gene screening system identified TRPV4 as a regulator of chondrogenic differentiation. Journal of Biological Chemistry, 282(44), 32158–32167. 10.1074/jbc.M706158200. [DOI] [PubMed] [Google Scholar]
- Myers CA, Schmidhauser C, Mellentin-Michelotti J, Fragoso G, Roskelley CD, Casperson G, et al. (1998). Characterization ofBCE-1, a transcriptional enhancer regulated by prolactin and extracellular matrix and modulated by the state of his tone acetylation. Molecular and Cellular Biology, 18(4), 2184–2195. Retrieved from http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=121460&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel M, Tahinci E, Symes K, & Winklbauer R (2004). Guidance of mesoderm cell migration in the Xenopus gastrula requires PDGF signaling. Development (Cambridge, England), 131, 2727–2736. 10.1242/dev.01141. [DOI] [PubMed] [Google Scholar]
- Naylor MJ, Li N, Cheung J, Lowe ET, Lambert E, Marlow R, et al. (2005). Ablation of β1 integrin in mammary epithelium reveals a key role for integrin in glandular morphogenesis and differentiation. Journal of Cell Biology, 171(4), 717–728. 10.1083/jcb.200503144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicodemi M, & Pombo A (2014). Models of chromosome structure. Current Opinion in Cell Biology, 28(1), 90–95. 10.1016/jxeb.2014.04.004. [DOI] [PubMed] [Google Scholar]
- Ohashi T, Kiehart DP, & Erickson HP (2002). Dual labeling of the fibronectin matrix and actin cytoskeleton with green fluorescent protein variants. Journal of Cell Science, 115(Pt. 6), 1221–1229. [DOI] [PubMed] [Google Scholar]
- Ornitz DM (2000). FGFs, heparan sulfate and FGFRs: Complex interactions essential for development. Bio Essays, 22(2), 108–112. . [DOI] [PubMed] [Google Scholar]
- Padmakumar VC, Libotte T, Lu WL, Zaim H, Abraham S, Noegel AA, et al. (2005). The inner nuclear membrane protein Sun1 mediates the anchorage of Nesprin-2 to the nuclear envelope. Journal of Cell Science, 118(15), 3419–3430. 10.1242/jcs.02471. [DOI] [PubMed] [Google Scholar]
- Page-McCaw A, Ewald AJ, & Werb Z (2007). Matrix metalloproteinases and the regulation of tissue remodelling. Nature Reviews Molecular Cell Biology, 8(3), 221–233. 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmieri D, Valli M, Viglio S, Ferrari N, Ledda B, Volta C, et al. (2010). Osteoblasts extracellular matrix induces vessel like structures through glycosylated collagen I. Experimental Cell Research, 316(5), 789–799. 10.1016/j.yexcr.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Pankov R, Cukierman E, Katz BZ, Matsumoto K, Lin DC, Lin S, et al. (2000). Integrin dynamics and matrix assembly: Tensin-dependent translocation of α5β1 integrins promotes early fibronectin fibrillogenesis. Journal of Cell Biology, 148(5), 1075–1090. 10.1083/jcb.148.5.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parenteau-Bareil R, Gauvin R, & Berthod F (2010). Collagen-based biomaterials fort issue engineering applications. Materials, 3(3), 1863–1887. 10.3390/ma3031863. [DOI] [Google Scholar]
- Paszek MJ, & Weaver VM (2004). The tension mounts: Mechanics meets morphogenesis and malignancy. Journal of Mammary Gland Biology and Neoplasia, 9(4), 325–342. 10.1007/s10911-004-1404-x. [DOI] [PubMed] [Google Scholar]
- Patel VN, Knox SM, Likar KM, Lathrop CA, Hossain R, Eftekhari S, et al. (2007). Heparanase cleavage ofperlecan heparan sulfate modulates FGF10 activity during ex vivo submandibular gland branching morphogenesis. Development, 134(23), 4177–4186. 10.1242/dev.011171. [DOI] [PubMed] [Google Scholar]
- Pathak MM, Nourse JL, Tran T, Hwe J, Arulmoli J, Le DTT, et al. (2014). Stretch-activated ion channel Piezo1 directs lineage choice in human neural stem cells. Proceedings of the National Academy of Sciences of the United States of America, 111(45), 16148–16153. 10.1073/pnas.1409802111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perissinotto D, Iacopetti P, Bellina I, Doliana R, Colombatti A, Pettway Z, et al. (2000). Avian neural crest cell migration is diversely regulated by the two major hyaluronan-binding proteoglycans PG-M/versican and aggrecan. Development, 127(13), 2823–2842. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=10851128. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Enemchukwu NO, Fiore VF, Sy JC, Murthy N, Sulchek TA, et al. (2012). Maleimide cross-linked bioactive PEG hydrogel exhibits improved reaction kinetics and cross-linking for cell encapsulation and in situ delivery. Advanced Materials, 24(1), 64—70. 10.1002/adma.201103574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietri T, Eder O, Breau MA, Topilko P, Blanche M, Brakebusch C, et al. (2004). Conditional beta1-integrin gene deletion in neural crest cells causes severe developmental alterations of the peripheral nervous system. Development, 131(16), 3871—3883. 10.1242/dev.01264. [DOI] [PubMed] [Google Scholar]
- Pla AF,Maric D,Brazer S-C, Giacobini P, Liu X, Chang YH, et al. (2005). Canonical transient receptor potential 1 plays a role in basic fibroblast growth factor (bFGF)/FGF receptor-1-induced Ca2 + entry and embryonic rat neural stem cell proliferation. The Journal of Neuroscience,25(10),2687—2701. 10.1523/JNEUROSCI.0951-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pond AC, Bin X, Batts T, Roarty K, Hilsenbeck S, & Rosen JM (2013). Fibroblast growth factor receptor signaling is essential for normal mammary gland development and stem cell function. Stem Cells, 31(1), 178–189. 10.1002/stem.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przybyla L, Lakins JN, & Weaver VM (2016). Tissue mechanics orchestrate wntdependent human embryonic stem cell differentiation. Cell Stem Cell, 19(4), 462–475. 10.1016/j.stem.2016.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey IS, Delling M, & Clapham DE (2006). An introduction to TRP channels. doi: 10.1146/annurev.physiol.68.040204.100431. Annual Review of Physiology, 68(1), 619–647. 10.1146/annurev.physiol.68.040204.100431. [DOI] [PubMed] [Google Scholar]
- Robertson IB, Horiguchi M, Zilberberg L, Dabovic B, Hadjiolova K, & Rifkin DB (2015). Latent TGF-β-binding proteins. Matrix Biology, 47, 44–53. 10.1016/j.matbio.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubashkin MG, Ou G, & Weaver VM (2014). Deconstructing signaling in three dimensions. Biochemistry, 53(13), 2078–2090. 10.1021/bi401710d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha K, Keung AJ, Irwin EF, Li Y, Little L, Schaffer DV, et al. (2008). Substrate modulus directs neural stem cell behavior. Biophysical Journal, 95(9), 4426–4438. 10.1529/biophysj.108.132217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salditt-Georgieff M, Harpold MM, Wilson MC, & Darnell JE (1981). Large heterogeneous nuclear ribonucleic acid has three times as many 5’ caps as polyadenylic acid segments, and most caps do not enter polyribosomes. Molecular and Cellular Biology, 1(2), 179–187. 10.1128/MCB.1.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasai Y (2013). Next-generation regenerative medicine: Organogenesis from stem cells in 3D culture. Cell Stem Cell, 12(5), 520–530. 10.1016/j.stem.2013.04.009. [DOI] [PubMed] [Google Scholar]
- Sato-Kusubata K, Jiang Y, Ueno Y, & Chun T-H (2011). Adipogenic histone mark regulation by matrix metalloproteinase 14 in collagen-rich microenvironments. Molecular Endocrinology, 25(5), 745–753. 10.1210/me.2010-0429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidhauser C, Bissell MJ, Myerst CA, & Caspersont GF (1990). Extracellular matrix and hormones transcriptionally regulate bovine β-casein 5 sequences in stably transfected mouse mammary cells. Proceedings of the National Academy of Sciences of the United States of America, 87(December), 9118–9122. 10.1073/pnas.87.23.9118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzbauer JE, & DeSimone DW (2011). Fibronectins, their fibrillogenesis, and in vivo functions. Cold Spring Harbor Perspectives in Biology, 3, a005041 10.1101/cshperspect.a005041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sechler JL, Corbett SA, & Schwarzbauer JE (1997). Modulatory roles for integrin activation and the synergy site of fibronectin during matrix assembly. Molecular Biology of the Cell, 8(12), 2563–2573. 10.1091/mbc.8.12.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengle G, Charbonneau NL, Ono RN, Sasaki T, Alvarez J, Keene DR, et al. (2008). Targeting of bone morphogenetic protein growth factor complexes to fibrillin. The Journal of Biological Chemistry, 283(20), 13874–13888. 10.1074/jbc.M707820200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seong J, Tajik A, Sun J, Guan J-L, Humphries MJ, Craig SE, et al. (2013). Distinct biophysical mechanisms of focal adhesion kinase mechanoactivation by different extracellular matrix proteins. Proceedings of the National Academy of Sciences of the United States of America, 110(48), 19372–19377. 10.1073/pnas.1307405110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P, Carraher C, & Schwarzbauer JE (2010). Assembly of fibronectin extracellular matrix. Annual Review of Cell and Developmental Biology, 26, 397–419. 10.1146/annurev-cellbio-100109-104020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner BM, & Johnson EEP (2017). Nuclear morphologies: Their diversity and functional relevance. Chromosoma, 126(2), 195–212. 10.1007/s00412-016-0614-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ML, Gourdon D, Little WC, Kubow KE, Eguiluz RA, Luna-Morris S, et al. (2007). Force-induced unfolding of fibronectin in the extracellular matrix of living cells. PLoS Biology, 5(10), 2243–2254. 10.1371/journal.pbio.0050268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith-Berdan S, Nguyen A, Hassanein D, Zimmer M, Ugarte F, Ciriza J, et al. (2011). Robo4 cooperates with Cxcr4 to specify hematopoietic stem cell localization to bone marrow niches. Cell Stem Cell, 8(1), 72–83. 10.1016/j.stem.2010.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solursh M, Linsenmayer TF, & Jensen KL (1982). Chondrogenesis fromsinglelimb mesenchyme cells. Developmental Biology, 94(1), 259–264. 10.1016/0012-1606(82)90090-2. [DOI] [PubMed] [Google Scholar]
- Song JJ, & Ott HC (2011). Organ engineering based on decellularized matrix scaffolds.Trends in Molecular Medicine, 17, 424–432. 10.1016/j.molmed.2011.03.005. [DOI] [PubMed] [Google Scholar]
- Stein GS, Lian JB, van Wijnen AJ, Stein JL,Javed A, Montecino M, et al. (2007). Organization of transcriptional regulatory machinery in nuclear microenvironments: Implications for biological control and cancer. Advances in Enzyme Regulation, 47, 242–250. 10.1016/j.advenzreg.2006.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streuli C (1999).Extracellular matrix remodelling and cellular differentiation. Current Opinion in Cell Biology, 11, 634–640. 10.1016/S0955-0674(99)00026-5. [DOI] [PubMed] [Google Scholar]
- Sugawara K, Tsuruta D, Ishii M, Jones JCR, & Kobayashi H (2008). Laminin-332and-511 in skin. Experimental Dermatology, 17(6), 473–480. 10.1111/j.1600-0625.2008.00721.x. [DOI] [PubMed] [Google Scholar]
- Suh HN, & Han HJ (2011). Collagen I regulates the self-renewal of mouse embryonicstem cells through α2β1 integrin- and DDR1-dependent Bmi-1. Journal of Cellular Physiology, 226(12), 3422–3432. 10.1002/jcp.22697. [DOI] [PubMed] [Google Scholar]
- ten Dijke P, & Hill CS (2004). New insights into TGF-beta-Smad signalling. Trends in Biochemical Sciences, 29(5), 265–273. 10.1016/j.tibs.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Tenni R, Valli M, Rossi A, & Cetta G (1993). Possible role of overglycosylation in the type I collagen triple helical domain in the molecular pathogenesis of osteogenesis imperfecta. American Journal of Medical Genetics, 45, 252–256. 10.1002/ajmg.1320450219. [DOI] [PubMed] [Google Scholar]
- Tholozan FMD, Gribbon C, Li Z, Goldberg MW, Prescott AR, McKie N, et al. (2007). FGF-2 release from the lens capsule by MMP-2 maintains lens epithelial cell viability. Molecular Biology of the Cell, 18(11), 4222–4231. 10.1091/mbc.E06-05-0416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timpl R, & Brown JC (1994). The laminins. Matrix Biology, 14(4), 275–281. 10.1016/0945-053X(94)90192-9. [DOI] [PubMed] [Google Scholar]
- Trinh LA,& Stainier DYR (2004). Fibronectin regulates epithelial organization during myocardialmigrationinzebrafish. Developmental Cell, 6(3), 371–382. 10.1016/S1534-5807(04)00063-2. [DOI] [PubMed] [Google Scholar]
- Tsai MC, Manor O, Wan Y, Mosammaparast N, Wang JK, Lan F, et al. (2010). Long noncoding RNA as modular scaffold of histone modification complexes. Science, 329(5992), 689–693. 10.1126/science.1192002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang KY, Cheung MCH, Chan D, & Cheah KSE (2010). The developmental roles of the extracellular matrix: Beyond structure to regulation. Cell and Tissue Research,339(1), 93–110. 10.1007/s00441-009-0893-8. [DOI] [PubMed] [Google Scholar]
- van der Kraan PM, Blaney Davidson EN, Blom A, & van den Berg WB (2009). TGF-beta signaling in chondrocyte terminal differentiation and osteoarthritis. Modulation and integration of signaling pathways through receptor-Smads. Osteoarthritis and Cartilage, 17(12), 1539–1545. 10.1016/jjoca.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Venter JC, Adams MD, Myers EW, Li PW, Mural RJ, Sutton GG, et al. (2001). The sequence of the human genome. Science (New York, N.Y.), 291(5507), 1304–1351. 10.1126/science.1058040. [DOI] [PubMed] [Google Scholar]
- Volk T, Fessler LI, & Fessler JH (1990). A role for integrin in the formation of sarcomeric cytoarchitecture. Cell, 63(3), 525–536. 10.1016/0092-(90)90449-O. [DOI] [PubMed] [Google Scholar]
- von Mende N, Bird DM, Albert PS, & Riddle DL (1988). dpy-13: A nematode collagen gene that affects body shape. Cell, 55(4), 567–576. 10.1016/0092-8674(88)90215-2. [DOI] [PubMed] [Google Scholar]
- Vriens J, Watanabe H, Janssens A, Droogmans G, Voets T, & Nilius B (2004). Cell swelling, heat, and chemical agonists use distinct pathways for the activation of the cation channel TRPV4. Proceedings ofthe National Academy of Sciences of the United States of America, 101(1), 396–401. 10.1073/pnas.0303329101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Harris RE, Bayston LJ, & Ashe HL (2008). Type IV collagens regulate BMP signalling in Drosophila. Nature, 455(7209), 72–77. https://doi.org/nature07214. [DOI] [PubMed] [Google Scholar]
- Wang N, Tytell JD, & Ingber DE (2009). Mechanotransduction at a distance: Mechanically coupling the extracellular matrix with the nucleus. Nature Reviews Molecular Cell Biology, 10(1), 75–82. 10.1038/nrm2594. [DOI] [PubMed] [Google Scholar]
- Wang KC, Yang YW, Liu B, Sanyal A, Corces-Zimmerman R, Chen Y, et al. (2011). A long noncoding RNA maintains active chromatin to coordinate homeotic gene expression. Nature, 472(7341), 120–126. 10.1038/nature09819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warm flash A, Sorre B, Etoc F, Siggia ED, & Brivanlou AH (2014). A method to recapitulate early embryonic spatial patterning in human embryonic stem cells. Nature Methods, 11(8), 847–854. 10.1038/nmeth.3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner JR, Soeiro R, Birnboim HC, Girard M, & Darnell JE (1966). Rapidly labeled HeLa cell nuclear RNA: I. Identification by zone sedimentation of a heteroge neous fraction separate from ribosomal precursor RNA. Journal of Molecular Biology,19(2), 349–361. 10.1016/S0022-2836(66)80009-8. [DOI] [PubMed] [Google Scholar]
- Wilcox M, DiAntonio A, & Leptin M (1989). The function of PS integrins in Drosophila wing morphogenesis. Development, 107(4), 891–897. [DOI] [PubMed] [Google Scholar]
- Winer JP, Janmey PA, McCormick ME, & Funaki M (2009). Bone marrow-derived human mesenchymal stem cells become quiescent on soft substrates but remain responsive to chemical or mechanical stimuli. Tissue Engineering Part A, 15(1), 147–154. 10.1089/ten.tea.2007.0388. [DOI] [PubMed] [Google Scholar]
- Wipff PJ, Rifkin DB, Meister JJ, & Hinz B (2007). Myofibroblast contraction activates latent TGF-β1 from the extracellular matrix. Journal of Cell Biology, 179(6), 1311–1323. 10.1083/jcb.200704042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu R, Nelson CM, Muschler JL, Veiseh M, Vonderhaar BK, & Bissell MJ(2009). Sustained activation of STAT5 is essential for chromatin remodeling and maintenance of mammary-specific function. Journal of Cell Biology, 184(1), 57–66. 10.1083/jcb.200807021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi Y (2000). Lecticans: Organizers of the brain extracellular matrix. Cellular and Molecular Life Sciences, 57(2), 276–289. 10.1007/PL00000690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi M, & Sricholpech M (2012). Lysine post-translational modifications of collagen. Essays In Biochemistry, 52, 113–133. 10.1042/bse0520113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Beqaj S, Kemp P, Ariel I, & Schuger L (2000). Stretch-induced alternative splicing of serum response factor promotes bronchial myogenesis and is defective in lung hypoplasia. Journal of Clinical Investigation, 106(11), 1321–1330. 10.1172/JCI8893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Palmer KC, Relan N, Diglio C, & Schuger L (1998). Role oflaminin polymerization at the epithelial mesenchymal interface in bronchial myogenesis. Development (Cambridge, England), 125(14), 2621–2629. [DOI] [PubMed] [Google Scholar]
- Yang Y, Relan NK, Przywara DA, & Schuger L (1999). Embryonic mesenchymal cells share the potential for smooth muscle differentiation: Myogenesis is controlled by the cell’s shape. Development (Cambridge, England), 126(13), 3027–3033. [DOI] [PubMed] [Google Scholar]
- Yoon BS, Ovchinnikov DA, Yoshii I, Mishina Y, Behringer RR, & Lyons KM (2005). Bmpr1a and Bmpr1b have overlapping functions and are essential for chondrogenesis in vivo. Proceedings of the National Academy of Sciences of the United States of America, 102, 5062–5067. 10.1073/pnas.0500031102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yost HJ (1992). Regulation of vertebrate left-right asymmetries by extracellular matrix. Nature, 357(6374), 158–161. 10.1038/357158a0. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Su J, Yu J, Bu X, Ren T, Liu X, et al. (2011). An essential role of discoidin domain receptor 2 (DDR2) in osteoblast differentiation and chondrocyte maturation via modulation of Runx2 activation. Journal of Bone and Mineral Research, 26(3), 604–617. 10.1002/jbmr.225. [DOI] [PubMed] [Google Scholar]
- Zhao B, Tumaneng K, & Guan K-L (2011). The Hippo pathway in organ size control, tissue regeneration and stem cell self-renewal. Nature Cell Biology, 13(8), 877–883. 10.1038/ncb2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong C, Chrzanowska-Wodnicka M, Brown J, Shaub A, Belkin AM, & Burridge K (1998). Rho-mediated contractility exposes a cryptic site in fibronectin and induces fibronectin matrix assembly. Journal of Cell Biology, 141(2), 539–551. 10.1083/jcb.141.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Oganesian A, Keene DR, & Sandell LJ (1999). Type IIA procollagen containing the cysteine-rich amino propeptide is deposited in the extracellular matrix ofprechondrogenic tissue and binds to TGF-β1 and BMP-2. Journal of Cell Biology, 144(5), 1069–1080. 10.1083/jcb.144.5.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zusman S, Patel-King RS, Ffrench-Constant C, &Hynes RO (1990). Requirements for integrins during Drosophila development. Development (Cambridge, England), 108(3), 391–402. 10.1038/342285a0. [DOI] [PubMed] [Google Scholar]