Introduction
Chronic ocular pain is an often overlooked and poorly treated problem, as many patients with this condition will visit an optometrist or ophthalmologist, who may have little training or experience in the management of pain. Frequently, those who present with complaints of persistent “burning” or “itching” eye discomfort are preliminarily given a diagnosis of dry eye (DE) disease, and undergo a series of tests to assess the quality and quantity of tears and the integrity of the ocular surface.[41] However, several studies have reported weak relationships between these DE signs and reports of DE-related symptoms [16,28,37], and many patients with DE-related symptoms often do not obtain relief from treatments aimed at enriching the ocular surface environment.[15,36]
Recent research from several groups has led to the recognition that many patients who have been diagnosed with DE based on symptom report may be more accurately described as suffering from chronic ocular pain. [3,32] This evidence includes associations between endorsements of pain-specific descriptors and allodynia [21] and the co-occurrence of other pain conditions associated with central sensitization, sometimes referred to as “chronic overlapping pain conditions.”[23,24,33] Thus, the mechanisms underlying their “DE” symptoms may be more closely related to other chronic pain conditions, rather than to the tear dysfunction implied by the term DE. However, convergent evidence to support this assertion is hampered by the paucity of validated questionnaires that can be used to elucidate characteristics specific to eye pain. Validation of such an instrument would be valuable for research seeking to understand the potential mechanisms associated with different types of eye pain that may be shared with other forms of chronic pain [40], as well as and facilitating clinical trials in this evolving field.
Due to the dearth of eye pain specific questionnaires, assessments validated for ocular surface disease (e.g., Dry Eye Questionnaire-5 and Ocular Surface Disease Index) have often been used to also identify individuals with eye pain/discomfort. Recently, however, the Ocular Pain Assessment Survey (OPAS) was validated specifically for ocular pain [31]. A limitation of this questionnaire is that it gives a generalized evaluation of eye pain and does not specifically examine many of the descriptors that appear in validated neuropathic pain questionnaires (e.g., Neuropathic Pain Symptom Inventory [8], Pain Quality Assessment Scale [19]). Because self-reported spontaneous “burning” or “itching” eye pain symptoms are often accompanied by endorsement of allodynic and hyperalgesic responses (i.e., heightened painful sensations to wind, cold, or light), and because the ocular anatomical structures lend themselves to increased risk for peripheral nerve damage, it is plausible that nerve injury contributes to a substantial proportion of ocular pain reports that are not otherwise accounted for.
The present study was undertaken with the goal of evaluating the utility of a modified version of the Neuropathic Pain Symptom Inventory (NPSI) to assess the severity of neuropathic-like eye pain. Although the descriptors of neuropathic-like eye pain should theoretically be similar to those of neuropathic pain referred to other body areas, the stimulus modalities evoking allodynic or hyperalgesic responses differ for the cornea compared to other body areas. Thus, a modification of these questions was incorporated based on clinical experience from patient reports.
Methods
Study Population
Patients with normal eyelid and corneal anatomy were prospectively recruited from the Miami Veterans Affairs (VA) Healthcare System eye clinic between October 2013 and October 2017. Patients with scheduled appointments for regular check-ups, for new symptoms related to the eye, or for follow-up appointments, regardless of DE diagnosis, were included as potential participants.
Patients were excluded from participation if they had risk factors accounting for their dry eye symptoms, including: contact lens use, use of ocular medications other than artificial tears, history of refractive surgery, HIV, sarcoidosis, graft-versus host disease or a collagen vascular disease, presence of an active external ocular process, cataract surgery within the last 6 months, history of any glaucoma, or retinal surgery. Participants who did not speak and understand English well were also excluded. Informed consent was obtained from all subjects. Miami VA Institution Review Board approval was obtained to allow the prospective evaluation of subjects. The study was conducted in accordance to the principles of the Declaration of Helsinki and complied with the requirements of the United States Health Insurance Portability and Accountability Act.
In the original NPSI validation paper [8], the authors included only individuals with at least moderate levels of pain severity (visual analogue scale scores of ≥ 30mm out of 100mm), and for whom pain was due to peripheral or central nervous system injury as indicated by clinical history, laboratory data, and/or imaging. For ocular pain, there is currently no gold standard method to diagnose neuropathic ocular pain nor to verify ocular nervous system lesion. As such, in this paper, we took an inclusive approach, in order to provide a wide range of NPSI scores, and analyzed data from all individuals with reported eye pain, defined as a Numerical Rating Scale (NRS) score of ≥ 1 for average eye pain intensity during the past week.
Procedures
Individuals who were qualified, and agreed to take part in the study, completed the informed consent process. For the majority of participants, the study included one visit during which they completed questionnaires, assessments of evoked corneal mechanical pain sensitivity, a standard tear film evaluation, and an assessment of analgesic responsiveness to corneal topical anesthetic. For most participants, a trained research staff member read instructions for each questionnaire and then the participant completed the questionnaire on paper independently. The research assistant was present during the administration to answer any questions the participant might have. For those individuals who had trouble seeing/reading the questionnaires, the research staff member read the questions and response selections to them, and recorded their responses. All participants completed the questionnaires in the same order each time they were administered: demographics and medical history questionnaire; mental health questionnaires; dry eye symptom questionnaire; and pain-specific questionnaires including the NPSI-eye. Measures of tear function were then collected by a trained technician. For the analgesic responsiveness assessment, subjects were asked to rate the intensity of their eye pain “right now” using a 0–10 NRS, then one drop of a topical anesthetic (proparacaine hydrochloride ophthalmic solution; Alcaine®) was administered to each eye, and after a period of 15 seconds, the participant was again asked to rate his/her pain “right now.”
A subgroup of participants (n = 20) completed an additional administration of the NPSI-Eye questionnaire at the end of the study visit (approximately 3 to 4 hours later) in order to provide information for the assessment of short-term test-retest reliability. A separate subset of 26 individuals, who received treatment for their ocular pain subsequent to their first study visit, also underwent a second study visit approximately 4 months later, during which they completed the same questionnaires as during study visit 1. Data from this subgroup of participants was used to evaluate the sensitivity of the NPSI-Eye to changes in neuropathic-like eye pain.
Measures
For each individual, demographic information (age, sex, race, ethnicity), past and current ocular, medical, and pain conditions, and medication information was collected. A standard tear film assessment was conducted on all participants, including measurement of (1) tear osmolarity (TearLAB, San Diego, CA) (once in each eye); (2) tear evaporation measured via tear breakup time (TBUT) (5 μl fluorescein placed, 3 measurements taken in each eye and averaged);(3) corneal epithelial cell disruption measured via corneal staining (National Eye Institute (NEI) scale [27], 5 areas of cornea assessed; score 0–3 for each area and total score 0–15);and (4) tear production measured via Schirmer’s strips with anesthesia. High tear osmolarity and corneal staining, and low TBUT and Schirmer’s scores are indicative of more severe DE.
NPSI-Eye:
As mentioned above, the NPSI [8] was modified to be more consistent with clinical experience of patient-reported neuropathic-like ocular pain: 1) The initial instruction script was altered to emphasize that we were interested in responses to the questions related only to their ocular pain condition; and 2) questions 8, 9, and 10 were modified to reflect the stimuli typically reported to produce allodynic or hyperalgesic responses when presented on, or to, the eye (wind, light, contact with something hot or cold), in place of the cutaneous stimuli examined in the original NPSI (i.e., brushing, pressure, contact with something cold). Thus, as in the original NPSI [8], the NPSI-Eye was composed of 12 questions, with 10 of the questions scored, and a total score range of 0 to 100.
Comparison pain questionnaires:
Two general assessments of pain, the NRS and the short-form McGill Pain Questionnaire (sf-MPQ) [26], were used to measure the intensity and quality of ocular pain. A NRS (0 = “no pain”; 10 = “most intense pain imaginable”) was used to assess the “average intensity of eye pain during the past week.” The NRS is a universally-used assessment of pain severity, and is recommended as the primary pain outcome measure for clinical trials.[12] The sf-MPQ includes ratings of the severity (none, mild, moderate, severe) of 11 sensory descriptors (throbbing, shooting, stabbing, sharp, cramping, gnawing, hot-burning, aching, heavy, tender, splitting) and 4 affective descriptors (tiring-exhausting, sickening, fearful, and punishing-cruel). The sf-MPQ has shown good psychometric properties across clinical pain conditions.[22,42]
Dry eye symptom questionnaire:
Participants filled out the Dry Eye Questionnaire 5 (DEQ-5) [9] which consists of 5 questions regarding the intensity and frequency of symptoms of “discomfort,” “dryness,” and “watery eyes.” Scores can range from 0 to 22, with higher scores indicating more severe symptoms of DE. It has been validated in DE patients with measurable tear film abnormalities that are due to differing underlying causes.[9] Though it has been shown that high scores on the DEQ-5 frequently accompany DE signs (i.e., abnormal measures of tear film function), it has also been shown that high DEQ-5 scores can be present even when signs of dry eye are minimal.[16,28] Thus, scores on the DEQ-5 can reflect symptoms related to dry eye pathology but may also reflect symptoms of other conditions, including neuropathic ocular pain, independent of dry eye pathology.
Mental health questionnaires:
Symptoms of post-traumatic stress disorder (PTSD) were assessed via the PTSD Checklist – Military Version (PCL-M) (score 17–85) [5,17] and symptoms of depression were assessed using the Patient Health Questionnaire 9 (PHQ9) (score 0–27).[34]
Ocular quantitative sensory testing (QST)/Belmonte aesthesiometry testing:
Testing of sensitivity to air-puff/pressure presented at the cornea was performed to evaluate the validity of self-report ratings of eye pain exacerbated by wind (Q8 of the NPSI-Eye). For all testing, the tip of the modified Belmonte aesthesiometer [2] (0.5 mm in diameter) was placed perpendicular to, and 4 mm from, the surface of the cornea. Stimulation consisted of brief pulses of air at room temperature (approximately 23 to 26ºC) applied to the central corneal surface. Two measures of pain sensitivity were recorded: 1) rating of ocular pain intensity (NRS, 0–10) after the presentation of a suprathreshold noxious stimulus (120 ml/min) to the left eye; and 2) ocular pain threshold in the right eye. Pain threshold was determined by averaging two ascending series during which the flow rate of the aesthesiometer was increased in 10 mL/min increments until the subject reported the stimulus as painful, or the maximum allowable flow rate (400mL/min) was reached. All ocular QST measures were obtained during the morning hours by the same operator.
Responsiveness to corneal topical anesthetic:
Intensity ratings of “pain right now,” using a 0–10 NRS, were collected before, and 15 seconds after administration of anesthetic eye drops. Based on these ratings, three groups of participants were defined: 1) “no pain before” = participants who did not have current pain (i.e., those who rated eye pain a “0” before anesthetic was applied); 2) “complete response” = participants whose current pain was completely relieved by anesthetic drops (purportedly reflecting only peripheral mechanisms of ocular pain [10,11]); and 3) “no, or partial, response” = participants whose pain was not relieved, or only partially relieved, by anesthetic drops (purportedly reflecting a mix of both peripheral and central mechanisms of ocular pain). [10,11]
Dry eye discordance score:
Based on previous work [30, 39], we calculated a DE discordance score to reflect the imbalance between signs of DE and symptom report. A detailed description of the calculation for this score can be found in Ong et al., 2018 [30]. Briefly, a composite DE signs severity score was calculated from measures of tear function and rank ordered across participants. Similarly, DE symptom report scores were rank ordered across all participants. The “discordance score” was the difference between the individual’s symptom score rank and their composite signs severity score rank (each transformed to a 0 to 1 scale). Thus, the DE discordance score ranged from −1 (minimal symptoms, maximal signs) to 1 (maximal symptoms, minimal signs). A positive discordance score indicates more severe DE symptoms than signs, which is suggestive of a central pain condition [20, 30].
Statistical Analysis
Statistical analyses were performed using the SPSS 22.0 (SPSS Inc, Chicago, IL) statistical package. Descriptive statistics were used to summarize patient demographic and clinical information. Where appropriate, means and standard deviations (SD) are expressed as mean ± SD, and p < 0.05 is considered statistically significant. Normality of distributions was assessed using the Kolmogorov-Smirnov (K-S) test statistic. As some parameters were not normally distributed, we applied both parametric and non-parametric strategies where appropriate.
Test-retest reliability:
The short-term test–retest reliability of each item and of the total score of the NPSI-Eye were calculated using Intraclass Correlation Coefficients (ICC) and Bland-Altman calculations of the 95% limits of agreement [6] based on possible range of scores (0 to 10 for individual NPSI-Eye questions, and 0 to 100 for total NPSI-Eye score). Data from a subgroup of individuals who underwent two administrations of the NPSI-Eye, with 2–4 hours in between administration, were used for this evaluation. Individuals who underwent this part of the study consisted of all individuals who were enrolled during the last two months (September-October, 2017) of the larger study.
Construct validity (convergent and divergent validities):
Relationships were examined between the total score of the NPSI-Eye and scores from the comparison pain severity questionnaires (the NRS rating of eye pain during the past week and the sf-MPQ total score), a comparison eye symptom questionnaire (DEQ-5), and psychological questionnaires (PHQ-9 and PCL-M) using Spearman’s rank-order (non-parametric) correlation coefficients. It was expected that the pain severity indices would be highly related, the dry eye symptom index would be less related, and the psychological health indices would have the lowest correlation with the NPSI-Eye.
Concurrent validity:
Concurrent validity for question 8 of the NPSI-Eye (self-report of severity of wind-evoked hyperalgesia) was evaluated by assessing its relationship with evoked pain sensitivity at the eye (ratings of evoked pain using a pre-set air-puff stimulus delivered to the cornea and evoked pain thresholds to air-puff stimulation measured via the method of limits).
Criterion-related validity:
Criterion validity of the NPSI-Eye was assessed using DE discordance scores and response to anesthetic drops. The association between DE discordance scores and total NPSI-Eye scores was evaluated with Spearman’s rho. Comparisons of the total score on the NPSI-Eye between groups of participants that were differentiated based on their response to eye drop anesthetic, purportedly reflecting the neuropathic mechanisms of their ocular pain condition [10,11], were also performed, using an ANOVA, followed by Bonferroni-corrected post-hoc pairwise tests.
Analysis of sensitivity to change:
Twenty-six individuals who initiated clinical treatment for ocular pain after the first study visit were identified and asked to return for a second study session approximately 4 months after the first visit. Treatment was administered based on clinical judgment and standard of care, individualized for each patient, ranging from eye drops to gabapentin, as this study was not a clinical trial. We made use of a convenience sample of those participants who were, when initially enrolled in the study, prescribed new treatment, and who returned for follow-up clinic appointments approximately 4 months after initiating the treatment.
To evaluate the sensitivity of the NPSI-Eye to change due to treatment, we assessed the relationship (using Spearman’s rho) between change in NPSI-Eye total score and change in the NRS rating of pain intensity.
Factor analysis:
We performed exploratory factor analyses using principal component analysis (PCA) as a method to investigate whether the 10 items of the NPSI-Eye could be combined into independent factors indicating different aspects of neuropathic pain, as has been evaluated with the original NPSI.[8] Only components with eigenvalues ≥ 1 were retained. We then validated the PCA by constructing 1000 random bootstrap samples with 497 observations each. These were used to obtain bootstrap estimates and 95% confidence intervals.
Results:
Study population:
Of the 511 people otherwise eligible for the study, 114 (22.3%) were excluded from analyses based on a self-report rating of average eye pain during the past week equal to 0. Comparison of demographic differences between those included in the analysis (those with average eye pain ≥ 1) and those excluded based on an average eye pain rating of 0 revealed that age, race, and ethnicity did not differ between these groups (p > 0.05), but that the percentage of males was greater in the group reporting a “0” for average eye pain during the past week (97.1% vs 89.5%, p = 0.015).
The study sample included 397 individuals with ocular pain (NRS rating of average eye pain intensity during the past week ≥ 1). Table 1 lists descriptive statistics for the sample, including demographic, clinical, pain, and tear film characteristics.
Table 1.
Demographic and clinical data of the study population N=397
| Demographics | |
|---|---|
| Age in years; mean ± SD (range) | 61.2 ± 10.1 (27–89) |
| Gender, % male (n) | 89.2% (354) |
| Race, % white (n) | 43.3% (172) |
| Ethnicity, % Hispanic (n) | 25.2% (100) |
| Current-Smoking, % (n) | 36.8% (146) |
| Past-smoking, % (n) | 50.1% (199) |
| Co-Morbidities, % (n) | |
| Diabetes | 31.9% (127) |
| Hypertension | 68.0% (270) |
| Depression | 65.5% (260) |
| Sleep Apnea | 24.2% (96) |
| Medications, % (n) | |
| Beta blocker | 19.1% (76) |
| Cholesterol lowering medication | 45.6% (181) |
| Antidepressant | 52.9% (210) |
| Anxiolytic | 52.6% (209) |
| Anti-histamine | 22.9% (91) |
| Alpha 2 Delta Ligands (gabapentin and pregabalin) | 30.2% (120) |
| Analgesics | 67.2% (266) |
| Serotonin and norepinephrine reuptake inhibitors (duloxetine and venlafaxine) | 1% (4) |
| Artificial tears | 68.5% (272) |
| Average ocular pain intensity during the past week | |
| NRS, mean ± SD (range) | 4.33 ± 2.3 (1–10) |
| NRS 1 to 3 (mild pain), % (n) | 40.6% (161) |
| NRS 4 to 6 (moderate pain), % (n) | 39.0% (155) |
| NRS ≥ 7 (severe pain), % (n) | 20.4% (81) |
| Dry eye symptoms | |
| DEQ5, mean ± SD (range) | 13.0 ± 3.94 (0–22) |
| DEQ5 6 to 11, % (n) | 27.5% (109) |
| DEQ5 ≥ 12, % (n) | 68.8% (273) |
| Dry eye signs | |
| Osmolarity, mean ± SD (range) | 300.8 ± 13.4 (275–380) |
| TBUT, mean ± SD (range) | 9.5 ± 5.0 (0–33) |
| TBUT ≤5, % (n) | 16.4% (65) |
| Corneal staining, mean ± SD (range) | 2.0 ± 2.6 (0–14) |
| Corneal staining ≥ 2, % (n) | 42.3% (168) |
| Schirmer, mean ± SD (range) | 13.1 ± 7.6 (0–45) |
| Schirmer ≤ 5, %(n) | 15.7% (62) |
SD= Standard deviation; NRS = numerical rating scale; DEQ5 = Dry Eye Questionnaire – 5; TBUT = tear break up time
Face validity:
Of the participants included in this analysis based on having an NRS score ≥1, 97% also had a NPSI-Eye total score ≥ 1. Six of the ten symptom items on the NPSI-Eye were endorsed by at least 60% of the sample. Table 2 lists the frequencies of positive response (rating ≥1) for each item.
Table 2.
Frequency of responses with ratings ≥ 1 for each item of the NPSI-Eye
| NPSI-Eye item | Percent of participants (n = 397) |
|---|---|
| Burning | 76.2 |
| Squeezing | 50.9 |
| Pressure | 72.7 |
| Electric shocks | 36.3 |
| Stabbing | 45.6 |
| Provoked by wind | 72.9 |
| Provoked by light | 80.7 |
| Provoked by contact with cold | 60.9 |
| Pins and needles | 40.1 |
| Tingling | 60.4 |
| Total score | 97.0 |
Test-retest reliability:
Short-term test-retest reliability was evaluated in a subset of 20 individuals who filled out the NPSI-Eye twice during a single visit with an interval of 2 to 4 hours in between (Table 3). ICCs were ≥ 0.90 for the NPSI-Eye total score and for responses on 8 of the 10 individual questions (all p < 0.001). ICCs for ratings for the two questions regarding abnormal sensations (“pins and needles” and “tingling”) were 0.79 and 0.77, respectively (p <0.001). Similarly, Bland-Altman calculations for the 95% limits of agreement were less than 3 in absolute value for all items, with the exception of “pins and needles” and “tingling” (< 5). The NPSI-Eye total score had 95% limits of agreement from −5 to 8, 13% of the possible range of scores.
Table 3.
Test-retest analysis of each item and NPSI-Eye total score measured twice within 4 hours
| Bland Altman |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| NPSI-Eye Item | ICC | p-value | Lower LoA | Upper LoA | 95% range | Possible range | LoA % of range | Mean diff | SD diff |
| Burning | 0.99 | < 0.001 | −1.1 | 0.8 | 2.0 | 11 | 18% | −0.15 | 0.49 |
| Squeezing | 0.96 | < 0.001 | −1.6 | 1.2 | 2.8 | 11 | 25% | −0.20 | 0.70 |
| Pressure | 0.99 | < 0.001 | −1.3 | 1.0 | 2.3 | 11 | 21% | −0.15 | 0.59 |
| Electric shocks | 0.89 | < 0.001 | −1.2 | 0.8 | 2.1 | 11 | 19% | −0.20 | 0.52 |
| Stabbing | 0.99 | < 0.001 | −0.7 | 0.8 | 1.6 | 11 | 14% | 0.05 | 0.39 |
| Provoked by wind | 0.98 | < 0.001 | −1.3 | 1.6 | 3.0 | 11 | 27% | 0.15 | 0.75 |
| Provoked by light | 0.97 | < 0.001 | −1.4 | 2.2 | 3.5 | 11 | 32% | 0.40 | 0.88 |
| Provoked by cold | 0.92 | < 0.001 | −2.4 | 3.1 | 5.5 | 11 | 50% | 0.35 | 1.39 |
| Pins and needles | 0.78 | < 0.001 | −3.2 | 4.7 | 7.9 | 11 | 72% | 0.75 | 1.97 |
| Tingling | 0.77 | < 0.001 | −3.2 | 4.0 | 7.2 | 11 | 65% | 0.40 | 1.79 |
| Total score | 0.98 | < 0.001 | −5.2 | 8.0 | 13.2 | 101 | 13% | 1.40 | 3.30 |
ICC = Intraclass correlation coefficient; LoA = limits of agreement
Construct validity:
There were moderate-to-high correlations between the total score on the NPSI-Eye and the comparison pain intensity questionnaires (NRS, sf-MPQ total score: Spearman’s rho= 0.69 and 0.65, respectively, p < 0.001). The correlation between NPSI-Eye and DEQ-5 scores was 0.56 (p < 0.001), and correlations with mental health questionnaires were 0.32 (PHQ-9) and 0.37 (PCL-M), respectively (Table 4).
Table 4.
Pearson and Spearman correlation coefficients
| Type of Validity | Correlation factors with NPSI-Eye | Spearman’s rho | P Value |
|---|---|---|---|
| Construct Validity | Rating (0–10) of average pain intensity during the past week (NRS) with NPSI-Eye total score | 0.69 | <0.001 |
| sf-MPQ total score with NPSI-Eye total score | 0.65 | <0.001 | |
| DEQ-5 with NPSI-Eye total score | 0.56 | <0.001 | |
| PHQ-9 with NPSI-Eye total score | 0.32 | <0.001 | |
| PCL-M with NPSI-Eye total score | 0.37 | <0.001 | |
| Concurrent Validity | Ocular air-puff pain threshold with NPSI-Eye wind hyperalgesia rating (Q8) | −0.15 | 0.007 |
| Ocular pain intensity rating of supra-threshold air-puff stimulus with NPSI-Eye wind hyperalgesia rating (Q8) | 0.11 | 0.035 | |
NPSI-Eye=Neuropathic Pain Symptom Inventory modified for the eye; NRS = numerical rating scale; sf-MPQ=short form McGill Pain Questionnaire; DEQ-5 = Dry Eye Questionnaire - 5; PHQ-9 = Patient Health Questionnaire - 9; PCL-M = Post-traumatic stress Checklist – Military version
Concurrent validity.
We evaluated correlations between the NPSI-Eye self-reported “wind”-exacerbated pain ratings (question 8) and air-puff/mechanical pain sensitivity measured at the corneal surface. We found significant, but modest, correlations in the expected directions (Table 4): Individuals with higher self-reported wind-exacerbated pain had higher pain intensity ratings to a fixed stimulus (rho = 0.11, p = 0.035) and had lower pain thresholds (rho= −0.15, p = 0.007).
Criterion-related validity:
A moderate-high correlation (rho = 0.48, p < 0.001) was found between DE discordance scores and NPSI-Eye total scores (Figure 1). Measures of DE signs/tear function (osmolarity, TBUT, corneal staining, and Shirmer’s scores) were not correlated with NPSI-Eye scores (all rho’s < 0.1, p > 0.05).
Figure 1. Relationship between NPSI-Eye and DE symptoms/signs discordance score.
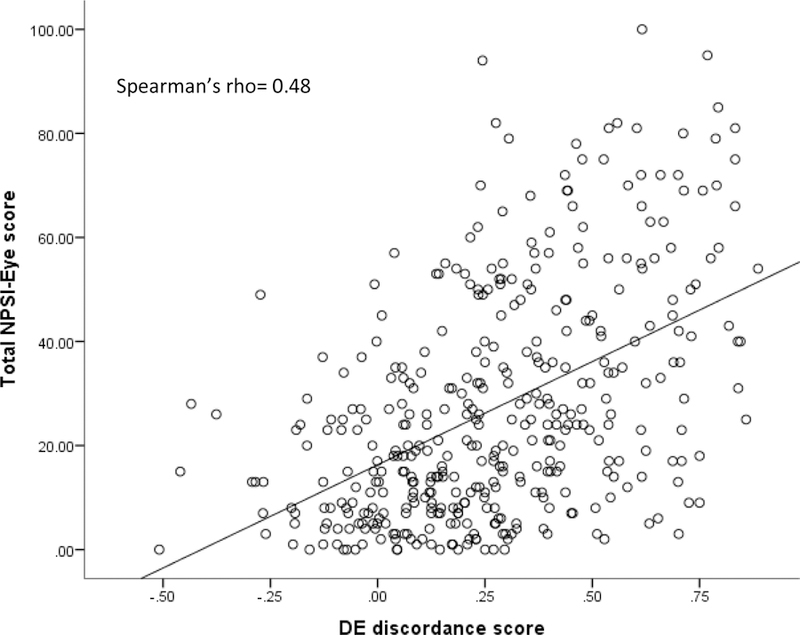
Participants were rank ordered based on the difference in severity of their reported DE symptoms and a composite measure of signs of tear dysfunction. A higher number on the DE discordance score reflects a greater difference in the severity of symptoms compared to signs. A higher score on the NPSI-Eye reflects greater neuropathic-like pain symptoms. The Spearman correlation coefficient was significant at p < 0.05.
Mean total NPSI-Eye scores for the “no pain before” group (n = 251) was 23.9 ± 20.4, for the “complete response” group (n = 29) was 27.4 ± 20.9, and for the “no, or partial, response” group (n = 66) was 40.1 ± 24.0. The ANOVA was significant across the groups (p < 0.001), and post-hoc, Bonferroni-corrected comparisons revealed a significant difference between the “no, or partial, response” group and both the “complete response” group (p = 0.024) and the “no pain before” group (p < 0.001), though no significant difference was found between the “complete response” and “no pain before” groups (p = 1.00) (Figure 2). However, differences between the DEQ-5 scores, reflecting intensity of symptoms associated with dry eye, were not significant between the “complete response” and “no, or partial, response” groups (14.0 ± 2.8 vs 14.5 ± 3.8, p = 1.00).
Figure 2. NPSI-Eye total score across subgroups based on response to eye drop anesthetic.
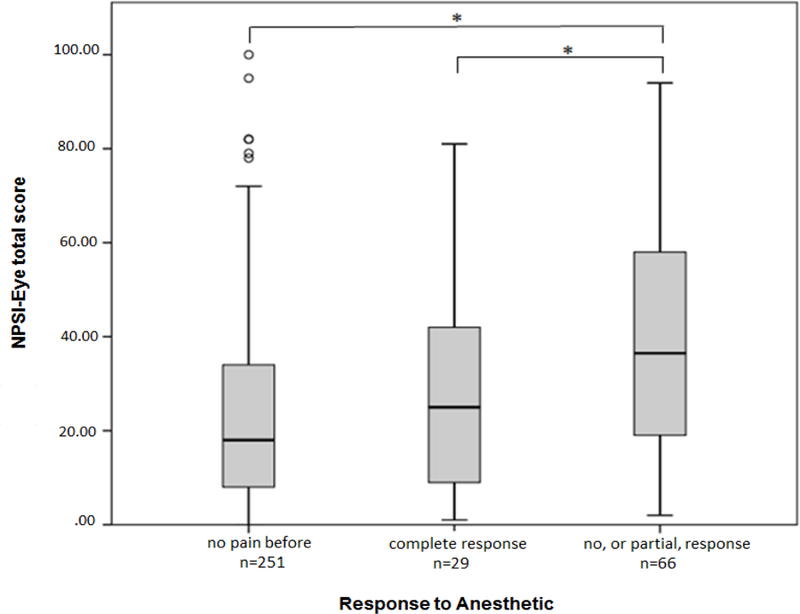
NSPI-Eye total scores were compared across three groups of participants based on their response to anesthetic eye drops: 1) those with intermittent eye pain symptoms (“no pain before”); 2) those with eye pain presumably due to peripheral mechanisms alone (“complete response”); and 3) those with eye pain presumably including at least partial central pain mechanisms (“no, or partial, response”). The box plots show the median (bold horizontal line inside the box), the limits of the 25th and 75th percentile (lower and upper limits of the boxes, respectively), and the upper and lower error bars indicate the limits of 95% of scores. Open circles indicate outliers. Significant comparisons between groups are noted with * (p< 0.05).
Sensitivity to change due to treatment:
In 26 individuals who received clinical treatment after their initial visit and returned after 4 months, a moderate positive correlation was noted between the change in total NPSI-Eye score and change in ratings of the average eye pain during the past week (rho = 0.35, p < 0.01). (Figure 3)
Figure 3. Relationship between change in total NPSI-Eye and NRS comparing 2 points in time.
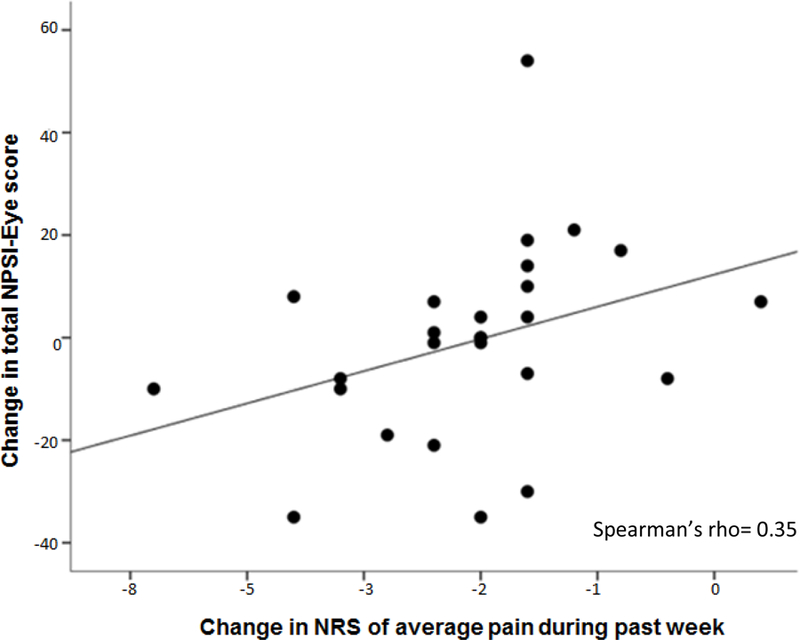
A subgroup of study participants (n = 26) completed repeated measurement (approximately 4 months after the initial visit) of the NPSI-Eye and ratings (NRS) of average eye pain intensity. Filled circles represent changes in scores across this time period on these measures, with a regression line fitted to the data. The Spearman correlation coefficient was significant at p < 0.05.
Factor analysis:
Factor analysis revealed that the NPSI-Eye had two main determinative factors. The first component accounted for 56% of the total variance in NPSI-Eye variables. All loadings were positive and similar, likely corresponding to a measure of average overall eye pain and discomfort. The second component accounted for 11% of the total variance. In component 2, “burning” and evoked pain loaded positively, while “electric shock like pain”, “stabbing pain”, and “pins and needles” loaded negatively. There was no strong loading of either “pressure” or “squeezing” pain. Loading variables were similar in magnitude and sign between our bootstrap estimates and the initial analysis (Supplemental Table 1a&b).
Discussion:
With the recent emergence of evidence that “dry eye” in many patients may more accurately be labeled a chronic pain condition involving both peripheral and central neuropathic mechanisms, the need for a specific measure of neuropathic pain intensity for ocular pain is essential to move the field forward. With this in mind, we undertook the present study, modeled after the approach of Bouhassira et al. [8] in the initial NPSI validation paper, to assess the psychometric properties of the NPSI-Eye. The results of this study show that NPSI-Eye has good-to-very good short-term reliability and convergent, divergent, and criterion-related validity, has moderate sensitivity to change, and exhibits fair concurrent validity for self-reported evoked allodynia and hyperalgesia due to wind/air.
All items on the NPSI-Eye were endorsed by at least 36% of participants, and six of ten were endorsed by more than 60% of the sample, suggesting the items are relevant and meaningful to patients with eye pain. These results not only support the applicability of the NPSI-Eye for evaluating eye pain intensity, but also support the potential for future studies to examine the utility of neuropathic pain diagnostic questionnaires (e.g., Douleur Neuropathique 4 (DN4) [7], pain DETECT questionnaire (PD-Q) [14], Leeds Assessment of Neuropathic Signs and Symptoms (LANSS) [4] as a neuropathic ocular pain screening tool that can be used clinically.
The relatively recently published OPAS [31] is a questionnaire developed to quantify multidimensional aspects of ocular pain, and related quality of life, without a specific focus on neuropathic pain qualities. It does, however, share some items similar to the NPSI-Eye. As in our population, response frequency to any occurrence of “light sensitivity,” “burning sensation,” or any increase in pain due to “wind, dry air, heat, air conditioning” was high, with a frequency of 88%, 86%, 94%, respectively on the OPAS. Because the OPAS was not published prior to the start of the present study, we were not able to administer it for direct comparison with the NPSI-Eye.
Regarding the reliability of the items on the NPSI-Eye, ICC’s for total score and for 8 of 10 questions were ≥ 0.90, and for the remaining two items was ≥ 0.77. These results are very similar in magnitude to those reported for the original NPSI, [7] in which ICCs for the total score and all 10 items were ≥ 0.87 when repeat administration was separated by 3 hours, and generally better than those reported for the German version of the NPSI (ICCs ≥ 0.66) over a 24 hour test-retest period.[35]
The modified version of the NPSI for the eye demonstrated good convergent validity when assessed via correlations with other measures of general pain intensity (NRS of average eye pain during the past week and the sf-MPQ total score). These correlations (rho ≥ 0.65) are similar in magnitude, or greater, compared to correlations between general pain intensity and the total score on the original French version [8] (r = 0.60), the Portuguese version [1] (r = 0.40 and 0.53), and the Japanese version of the NPSI [25] (r = 0.41). We were also able to demonstrate moderate relationships between the NPSI-Eye and the DEQ-5 (rho = 0.56). The DEQ-5 targets the assessment of dry eye symptoms, and, as eye “discomfort” is one of the symptoms captured in this questionnaire, it was expected that scores on this measure would be related to the NPSI-Eye, but less so than the questionnaires created for the specific assessment of pain intensity (i.e., NRS and sf-MPQ). With regard to divergent validity, correlations between the NPSI-Eye and the PHQ-9 (depression symptoms) and the PCL-M (PTSD symptoms) were lower than those found for the NRS and sf-MPQ, as has also been demonstrated for the original NPSI and depression and anxiety [8] (Spearman’s rho = 0.32 and 0.27), the Japanese version of the NPSI and physical and mental component scores of the SF-36 (Pearson’s r = −0.06 and −0.14) [25], and the German version of the NPSI and depression (Pearson’s r = 0.32) [35].
The evaluation of criterion-related validity for the NPSI-Eye was an important component of the psychometric assessment of this questionnaire, as there is currently no established gold-standard for diagnosing neuropathic ocular pain. Using response to a topical anesthetic as a test to compare participants with differing degrees of underlying peripheral and central nociceptive processing components to their eye pain, we were able to show that the NPSI-Eye was capable of reflecting neuropathic pain-specific symptom intensity. This was supported by the combination of two findings: 1) a significant difference in NPSI-Eye total scores between the subgroup whose eye pain was at least partially due to central factors (“no, or partial, response” to topical anesthetic) and the subgroups representing pain due to peripheral factors (“complete response” to topical anesthetic) and intermittent pain (“no pain before” anesthetic testing); and 2) a lack of a significant difference in DEQ-5 scores between the subgroups with current pain (“no, or partial, response” vs. “complete response”). In addition, an elevated discordance score (indicative of DE-associated symptoms that outweigh DE signs) has been associated with various identified risk factors for NOP (presence of a chronic pain condition, depression, use of antidepressants) [30, 39] so it is encouraging that a similar positive correlation was seen between discordance score and the NPSI-Eye.
Although self-report of allodynic/hyperalgeic response to wind (Q8 of NPSI-Eye) was significantly related to air-puff pain threshold (p < 0.05) and suprathreshold ratings (p < 0.005) obtained via ocular QST methods, the effect size was modest (Spearman’s rho of −0.15 and 0.11, respectively). This small effect size may be due to a number of factors, including: inclusion of a heterogeneous sample of ocular pain conditions contributing error variance to this relationship; imposing a ceiling value for pain threshold which artificially limits the range of values for this measure; and low construct validity for this question of the NPSI-Eye. In addition, a large proportion of individuals in the study were taking analgesic medications (67.2%), which may have affected their sensitivity to the air-puff stimuli. Based on these results, it is clear that there is a need for additional assessment of the wind-evoked pain question of the NPSI-Eye.
Overall, the results of this investigation provide support for the reliability and validity of a modified version of the NPSI for the eye. These findings are also supported by our previous work, in which we reported that individuals with DE symptoms had responses on the NPSI-Eye that were comparable to data from the literature in individuals with established neuropathic pain in locations outside the eye, using the original form of the NPSI [8]. Furthermore, when individuals were divided into those who endorsed a “burning” quality to their eye pain (question #1 of the NPSI-Eye), and those who did not, we showed that those with burning pain had a more chronic DE symptom course [29] that was less responsive to therapy with artificial tears [15] (suggesting an underlying central component to their ocular pain).
The factor analysis performed on our sample of participants, however, did not replicate the findings of Bouhassira et al.’s five factor solution when the original NPSI was administered to individuals with a wide variety of underlying diagnoses (e.g. nerve trauma, post-herpetic neuralgia, non-diabetic and diabetic polyneuropathy, post-stroke pain, spinal cord trauma, nerve entrapment, multiple sclerosis, myelitis).[8] Responses to questions from the NPSI-Eye in a predominantly male veteran sample with eye pain produced only two factors, with the first potentially representing generalized pain intensity, and the second composed of spontaneous burning pain and evoked pain to wind, light, and temperature (hot/cold). Other qualities examined via the NPSI-Eye (“squeezing,” “electric shocks,” “stabbing,” and “pins and needles”) were less relevant as seen in both face validity assessment and factor analysis.
It is important to consider our study findings bearing in mind its limitations, which included a specific study population (majority male, middle to older age, seen at a VA hospital) with the majority of individuals being seen at one point in time. As dry eye is more predominant in women (at least 65% of the dry eye population) [13, 18], and veterans are more likely to exhibit chronic pain conditions overall compared to the general population (44% vs. 26%) [38], the generalizability of our psychometric findings regarding the use of the NPSI-Eye is limited. Additionally, individuals with mild to severe eye pain intensity (NRS ≥ 1) were included, whereas in the original NPSI validation paper, only individuals with moderate or greater pain (visual analogue scale ≥ 30mm) were enrolled, which may limit the direct comparison of results between the two studies. Furthermore, we included individuals with self-report of any type of unexplained ocular pain, as there is a lack of gold standard for the diagnosis of neuropathic pain in the eye. Thus, the study sample in the present study likely included at least some individuals who did not have ocular pain due to nervous system lesion, whereas the Bouhassira et al. study included only those with pain that “could be clearly attributed to a peripheral or central nervous system injury” (p.249) [8].
Despite these limitations, we conclude that the NPSI-Eye can be used to quantify specific dimensions of ocular pain and may be more specific for the presence of neuropathic ocular pain. The present analyses suggest that the elimination of items with low frequency of endorsement and indiscriminant factor loading could improve the clinical and research utility of this questionnaire for measuring neuropathic ocular pain severity. Future studies will be needed to replicate our findings in other ocular pain patient populations, and to validate diagnostic tools for this condition, potentially taking advantage of questionnaires currently in use in non-ocular neuropathic pain conditions (e.g., DN4, painDETECT, LANSS).
Supplementary Material
Acknowledgements
The authors thank the eye clinic staff and patients at the Miami Bruce W. Carter Veteran’s Affairs Medical Center for their involvement in this study. This study was supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Clinical Sciences Research EPID-006–15S (A. Galor, PI), R01EY026174 (A. Galor, PI), NIH Center Core Grant P30EY014801, Research to Prevent Blindness Unrestricted Grant; NIH NINDS R21 NS105880 (R. Levitt, PI/PD); NIDCR 1R01DE022903 (R. Levitt, PI/PD, E. Martin, PI). The authors have no conflicts of interest to disclose.
References
- 1.de Andrade DC, Ferreira KA, Nishimura CM, Yeng LT, Batista AF, de Sá K, Araujo J, Stump PR, Kaziyama HH, Galhardoni R, Fonoff ET, Ballester G, Zakka T, Bouhassira D, Teixeira MJ. Psychometric validation of the Portuguese version of the Neuropathic Pain Symptoms Inventory. Health Qual Life Outcomes 2011;9:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belmonte C, Acosta MC, Schmelz M, Gallar J. Measurement of corneal sensitivity to mechanical and chemical stimulation with a CO2 esthesiometer. Invest Ophthalmol Vis Sci 1999;40(2):513–9. [PubMed] [Google Scholar]
- 3.Belmonte C, Nichols JJ, Cox SM, Brock JA, Begley CG, Bereiter DA, Dartt DA, Galor A, Hamrah P, Ivanusic JJ, Jacobs DS, McNamara NA, Rosenblatt MI, Stapleton F, Wolffsohn JS. TFOS DEWS II pain and sensation report. Ocul Surf 2017;15(3):404–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bennett M The LANSS Pain Scale: the Leeds assessment of neuropathic symptoms and signs. Pain 2001;92(1–2):147–57. [DOI] [PubMed] [Google Scholar]
- 5.Bjornestad AG, Schweinle A, Elhai JD. Measuring secondary traumatic stress symptoms in military spouses with the posttraumatic stress disorder checklist military version. J Nerv Ment Dis 2014;202(12):864–9. [DOI] [PubMed] [Google Scholar]
- 6.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods oc linical measurement. Lancet 1098;327 (8476):307–310. [PubMed] [Google Scholar]
- 7.Bouhassira D, Attal N, Alchaar H, Boureau F, Brochet B, Bruxelle J, Cunin G, Fermanian J, Ginies P, Grun-Overdyking A, Jafari-Schluep H, Lantéri-Minet M, Laurent B, Mick G, Serrie A, Valade D, Vicaut E. Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4). Pain 2005;114(1–2):29–36. [DOI] [PubMed] [Google Scholar]
- 8.Bouhassira D, Attal N, Fermanian J, Alchaar H, Gautron M, Masquelier E, Rostaing S, Lanteri-Minet M, Collin E, Grisart J, Boureau F. Development and validation of the Neuropathic Pain Symptom Inventory. Pain 2004;108(3):248–57. [DOI] [PubMed] [Google Scholar]
- 9.Chalmers RL, Begley CG, Caffery B. Validation of the 5-Item Dry Eye Questionnaire (DEQ-5): Discrimination across self-assessed severity and aqueous tear deficient dry eye diagnoses. Cont Lens Anterior Eye 2010;33(2):55–60. [DOI] [PubMed] [Google Scholar]
- 10.Crane AM, Feuer W, Felix ER, Levitt RC, McClellan AL, Sarantopoulos KD, Galor A. Evidence of central sensitisation in those with dry eye symptoms and neuropathic-like ocular pain complaints: incomplete response to topical anaesthesia and generalised heightened sensitivity to evoked pain. Br J Ophthalmol 2017;101(9):1238–43. [DOI] [PubMed] [Google Scholar]
- 11.Dieckmann G, Goyal S, Hamrah P. Neuropathic Corneal Pain: Approaches for Management. Ophthalmology 2017;124(11s):S34–s47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dworkin RH, Turk DC, Farrar JT, Haythornthwaite JA, Jensen MP, Katz NP, Kerns RD, Stucki G, Allen RR, Bellamy N, Carr DB, Chandler J, Cowan P, Dionne R, Galer BS, Hertz S, Jadad AR, Kramer LD, Manning DC, Martin S, McCormick CG, McDermott MP, McGrath P, Quessy S, Rappaport BA, Robbins W, Robinson JP, Rothman M, Royal MA, Simon L, Stauffer JW, Stein W, Tollett J, Wernicke J, Witter J; IMMPACT. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain 2005;113(1–2):9–19. [DOI] [PubMed] [Google Scholar]
- 13.Farrand KF, Fridman M, Stillman IÖ, Schaumberg DA. Prevalence of Diagnosed Dry Eye Disease in the United States Among Adults Aged 18 Years and Older. Am J Ophthalmol 2017. October;182:90–98. [DOI] [PubMed] [Google Scholar]
- 14.Freynhagen R, Baron R, Gockel U, Tolle TR. painDETECT: a new screening questionnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin 2006;22(10):1911–20. [DOI] [PubMed] [Google Scholar]
- 15.Galor A, Batawi H, Felix ER, Margolis TP, Sarantopoulos KD, Martin ER, Levitt RC. Incomplete response to artificial tears is associated with features of neuropathic ocular pain. Br J Ophthalmol 2016;100(6):745–9. [DOI] [PubMed] [Google Scholar]
- 16.Galor A, Felix ER, Feuer W, Shalabi N, Martin ER, Margolis TP, Sarantopoulos CD, Levitt RC. Dry eye symptoms align more closely to non-ocular conditions than to tear film parameters. Br J Ophthalmol 2015;99(8):1126–9. [DOI] [PubMed] [Google Scholar]
- 17.Gaylord KM, Cooper DB, Mercado JM, Kennedy JE, Yoder LH, Holcomb JB. Incidence of posttraumatic stress disorder and mild traumatic brain injury in burned service members: preliminary report. J Trauma 2008;64(2 Suppl):S200–5; discussion S5–6. [DOI] [PubMed] [Google Scholar]
- 18.Gayton JL. Etiology, prevalence, and treatment of dry eye disease. Clin Ophthalmol 2009;3:405–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jensen MP, Gammaitoni AR, Olaleye DO, Oleka N, Nalamachu SR, Galer BS. The pain quality assessment scale: assessment of pain quality in carpal tunnel syndrome. J Pain 2006;7(11):823–32. [DOI] [PubMed] [Google Scholar]
- 20.Kalangara JP, Galor A, Levitt RC, Felix ER, Alegret R, Sarantopoulos CD. Burning Eye Syndrome: Do Neuropathic Pain Mechanisms Underlie Chronic Dry Eye? Pain Med 2016. April;17(4):746–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalangara JP, Galor A, Levitt RC, Covington DB, Mc Manus KT, Sarantopoulos CD, Felix ER. Characteristics of Ocular Pain Complaints in Patients With Idiopathic Dry Eye Symptoms. Eye Contact Lens 2017;43(3):192–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kitisomprayoonkul W, Klaphajone J, Kovindha A. Thai Short-form McGill Pain Questionnaire. J Med Assoc Thai 2006;89(6):846–53. [PubMed] [Google Scholar]
- 23.Lee CJ, Levitt RC, Felix ER, Sarantopoulos CD, Galor A. Evidence that dry eye is a comorbid pain condition in a U.S. veteran population. Pain Rep 2017;2(6):e629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levitt AE, Galor A, Chowdhury AR, Felix ER, Sarantopoulos CD, Zhuang GY, Patin D, Maixer W, Smith SB, Martin ER, Levitt RC. Evidence that Dry Eye Represents a Chronic Overlapping Pain Condition. Mol Pain 2017;13:1744806917729306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsubayashi Y, Takeshita K, Sumitani M, Oshima Y, Tonosu J, Kato S, Ohya J, Oichi T, Okamoto N, Tanaka S. Psychometric Validation of the Japanese Version of the Neuropathic Pain Symptom Inventory. PLoS One 2015;10(11):e0143350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Melzack R The short-form McGill Pain Questionnaire. Pain 1987;30(2):191–7. [DOI] [PubMed] [Google Scholar]
- 27.Methodologies to diagnose and monitor dry eye disease: Report of the Diagnostic Methodology Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf 2007;5:108–152. 7. [DOI] [PubMed] [Google Scholar]
- 28.Nichols KK, Nichols JJ, Mitchell GL. The lack of association between signs and symptoms in patients with dry eye disease. Cornea 2004;23(8):762–70. [DOI] [PubMed] [Google Scholar]
- 29.Ong ES, Alghamdi YA, Levitt RC, McClellan AL, Lewis G, Sarantopoulos CD, Felix ER, Galor A. Longitudinal Examination of Frequency of and Risk Factors for Severe Dry Eye Symptoms in US Veterans. JAMA Ophthalmol 2017;135(2):116–123. [DOI] [PubMed] [Google Scholar]
- 30.Ong ES, Felix ER, Levitt RC, Feuer WJ, Sarantopoulos CD, Galor A. Epidemiology of discordance between symptoms and signs of dry eye. Br J Ophthalmol 2018;102(5):674–679. [DOI] [PubMed] [Google Scholar]
- 31.Qazi Y, Hurwitz S, Khan S, Jurkunas UV, Dana R, Hamrah P. Validity and Reliability of a Novel Ocular Pain Assessment Survey (OPAS) in Quantifying and Monitoring Corneal and Ocular Surface Pain. Ophthalmology 2016;123(7):1458–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosenthal P, Baran I, Jacobs DS. Corneal pain without stain: is it real? Ocul Surf 2009;7(1):28–40. [DOI] [PubMed] [Google Scholar]
- 33.Shtein RM, Harper DE, Pallazola V, Harte SE, Hussain M, Sugar A, Williams DA, Clauw DJ. Discordant Dry Eye Disease (An American Ophthalmological Society Thesis). Trans Am Ophthalmol Soc 2016. August;114:T4. [PMC free article] [PubMed] [Google Scholar]
- 34.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16(9):606–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sommer C, Richter H, Rogausch JP, Frettlöh J, Lungenhausen M, Maier C. A modified score to identify and discriminate neuropathic pain: a study on the German version of the Neuropathic Pain Symptom Inventory (NPSI). BMC Neurol 2011;11:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stonecipher K, Perry HD, Gross RH, Kerney DL. The impact of topical cyclosporine A emulsion 0.05% on the outcomes of patients with keratoconjunctivitis sicca. Curr Med Res Opin 2005;21(7):1057–63. [DOI] [PubMed] [Google Scholar]
- 37.Sullivan BD, Crews LA, Messmer EM, Foulks GN, Nichols KK, Baenninger P, Geerling G, Figueiredo F, Lemp MA. Correlations between commonly used objective signs and symptoms for the diagnosis of dry eye disease: clinical implications. Acta Ophthalmol 2014;92(2):161–6. [DOI] [PubMed] [Google Scholar]
- 38.Toblin RL, Mack KA, Perveen G, Paulozzi LJ. A population-based survey of chronic pain and its treatment with prescription drugs. Pain 2011;152(6):1249–55. [DOI] [PubMed] [Google Scholar]
- 39.Vehof J, Sillevis Smitt-Kamminga N, Nibourg SA, Hammond CJ. Predictors of Discordance between Symptoms and Signs in Dry Eye Disease. Ophthalmology 2017;124(3):280–286. [DOI] [PubMed] [Google Scholar]
- 40.Vehof J, Zavos HM, Lachance G, Hammond CJ, Williams FM. Shared genetic factors underlie chronic pain syndromes. Pain 2014. August;155(8):1562–8. [DOI] [PubMed] [Google Scholar]
- 41.Willcox MDP, Argüeso P, Georgiev GA, Holopainen JM, Laurie GW, Millar TJ, Papas EB, Rolland JP, Schmidt TA, Stahl U, Suarez T, Subbaraman LN, Uçakhan OÖ, Jones L. TFOS DEWS II Tear Film Report. Ocul Surf 2017;15(3):366–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wright KD, Asmundson GJ, McCreary DR. Factorial validity of the short-form McGill pain questionnaire (SF-MPQ). Eur J Pain 2001;5(3):279–84. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


