Abstract
INTRODUCTION
Rectal douching/enema (RD) is a common practice among men who have sex with men (MSM) in preparation for sex. RD can break down the rectal mucosal barrier and potentially affect the rectal microbiome, however, it is unclear if this is associated with acquiring rectal infections (RI) with either rectal gonorrhoea (NG) and/or chlamydia (CT).
METHODS
From 2013–2015, 395 adult HIV-uninfected MSM were enrolled in a randomized controlled study for pre-exposure prophylaxis (PrEP) adherence with routine sexual risk survey and testing. Using data from this cohort, baseline differences by RI were assessed using Pearson’s Chi-square and Wilcoxon-Mann-Whitney test. Association between RD and RI was modelled using multivariable logistic regression adjusted for potential confounders (sexual behaviour, substance use, and age) selected a priori. Effect modification by number of male partners and sensitivity analysis to rule out reverse causality were also conducted.
RESULTS
Of 395 participants, 261 (66%) performed RD and 133 (33%) had at least one NG/CT RI over 48 weeks. Number of condomless anal receptive sex (med:4, p<0.001), male partners (med:6, p<0.001), and substance use (any of methamphetamine/hallucinogens/dissociative/poppers) (p<0.001) were associated with increased odds of RI. Controlling for potential confounders, odds of prevalent RI were 3.59 (p<0.001, 95% CI 1.90–6.78) and incident RI 3.87 (p=0.001, 95% CI 1.78–8.39) when douching weekly or more compared to not douching. MSM with more than six male partners had 5.41 (p=0.002, 95% CI 1.84–15.97) increased odds of RI when douching weekly or more compared to not douching.
CONCLUSION
Rectal hygiene with RD is a common practice (66%) among HIV-uninfected MSM on PrEP in this study, which increases the odds of acquiring rectal NG and/or CT independent of sexual risk behaviour, substance use and other factors. This suggests interventional approaches targeting rectal hygiene products and practices could reduce sexually transmitted infections.
INTRODUCTION
Close to a million people were infected with chlamydia, gonorrhoea, syphilis and/or trichomoniasis globally in 20161, all of which are curable. Chlamydia trachomatis and Neisseria gonorrhoeae are two common types of sexually transmitted infections (STIs) which facilitate transmission of human immunodeficiency virus (HIV)2,3. In the United States, chlamydia and gonorrhoea rates have been increasing and men who have sex with men (MSM) have been disproportionately affected by both STIs and the HIV epidemic4.
While the association between vaginal douching, HIV and STIs have been well studied5–8, evidence is conflicting for an association between rectal douching/enema (RD) and acquisition of HIV9–11, chlamydia12–15, and gonorrhoea15–17. Most RD studies have been in HIV-infected populations with limited data for risk associated with HIV-uninfected MSM and none on MSM on pre-exposure prophylaxis (PrEP). Introduction of PrEP as prevention for HIV has raised concerns regarding unintended negative consequences of increased STIs18, 19.
RD is the act of cleansing the rectum using a device to insert liquid into the rectum before anal receptive sex (ARS) and can be a potential risk for STIs. In a 2014 study by Noor and Rosser of 4,992 MSM, most commonly cited reasons for douching included cleanliness, request of sex partner, advice by friend and to prevent STIs11. Studies have also demonstrated an association of RD with breakdown of rectal mucosal barrier leading to increased risk of HIV and lymphogranuloma venereum (LGV)12, 20, 21. While condomless anal receptive sex (cARS) has been associated with high prevalence of HIV/STI among MSM22, it is unclear if douching is associated with acquiring rectal infections (RI) with gonorrhoea or non-LGV chlamydia. In a recent cross-sectional study surveying RD and sharing douching equipment among MSM, no significant association was found between RD and RI after adjusting for risk factors, although frequency of douching was not included in the analysis15. To further test the hypothesis that RD is independently associated with RI, we used prospective cohort data from the California Collaborative Treat Group randomized control trial (Daily Text Messages To Support Adherence to PrEP In At-Risk for HIV Individuals: The TAPIR Study) on PrEP adherence of high risk HIV-uninfected MSM to analyse if RD behaviour predicted prevalent and incident rectal gonorrhoea and/or rectal chlamydia, adjusting for differences in sexual risk behaviour, substance use and other factors.
METHODS
Study Setting
Data for this secondary analysis were obtained from sexual risk surveys and STI testing conducted for the TAPIR study enrolling from February 2013 to February 2015 at four Southern California medical centres (University of California, San Diego; University of Southern California; Harbor-University of California Los Angeles; and Long Beach Health Department). Subjects were followed for a minimum of 48 weeks ending in February 2016. Four hundred and thirty-five individuals screened for the study, 398 participants were eligible to enrol and 324 participants completed week 48 visit. Institutional review boards at each of the participating institutions approved the TAPIR study (NCT01761643).
Eligibility Criteria
English or Spanish speaking MSM or transgender woman without HIV infection aged 18 years or older at increased risk of HIV were eligible to participate in the main study. This analysis was limited to 395 MSM enrolled (Supplementary Figure 1). HIV-uninfected status was confirmed by a negative 4th generation antigen-antibody assay or an antibody assay in addition to HIV nucleic acid amplification test (Procleix). Persistent elevated risk of HIV infection was assessed by meeting one or more of the following criteria: 1) At least one HIV-infected sexual partner for ≥4 weeks; 2) No condom use during anal intercourse with ≥3 male sex partners who are HIV-infected or of unknown HIV status during the last 3 months; or 3) No condom use during anal sex with ≥1 male partner plus an STI diagnosis during the last 3 months. All participants were prescribed tenofovir disoproxil fumarate (TDF) combined with emtricitabine (FTC) for PrEP and received comprehensive preventive care per the Centers for Disease Control and Prevention guidance for PrEP23.
Study Measures
Douching frequency in the past 30 days was collected only at baseline with the following question, “In the past month, how frequently have you performed rectal douching or enema (cleaning out your rectum or colon)?” Response options were: “Never, once/twice, less than weekly, about weekly, more than once a week, do not know, and prefer not to answer.” Solution of RD was assessed by asking: “For rectal colonic cleansing in the past month what do you use?” Multiple response options were: “Nothing, soapy water, plain water, saline, mineral oil, do not know, prefer not to answer.” Participants were also asked if they used oral agents such as “polyethylene glycol-oral, oral colonic cleanser, other oral colonic cleanser” to promote bowel evacuation. For analytical purposes, responses on the frequency of douching were categorized as: 1) Never douching, 2) Douching weekly or more, and 3) Douching less than weekly. Responses on solution were dichotomized a priori to “Water only” and “Water and/or other solutions.”
Rectal chlamydia and gonorrhoea screening assessments were conducted using Hologic Aptima Combo2 and rectal swabs at baseline for all participants and weeks 4, 12, 24, 36 and 48 for most during follow-up. Participants with new STI diagnoses were notified and referred to their provider or local sexually transmitted disease clinic for treatment. The main outcome for this analysis was at least one RI by either chlamydia and/or gonorrhoea at any time point from baseline to week 48. Participants with multiple infections over 48-week period were counted once.
Study visits occurred at baseline and weeks 4, 12, 24, 36, and 48, and questionnaire responses were captured using a computer assisted self-report survey. Baseline data included sociodemographic characteristics (monthly income, education, age, race, ethnicity, employment and relationship status), HIV risk factor and antibiotic use. Risk behaviour on drug abuse (more than one drug at a time) and multiple substance use were collected every visit for past three months. Use of methamphetamines (e.g., crystal meth, ice, glass), hallucinogens (e.g., LSD, mushrooms, acid), dissociative (e.g., PCP, angel dust, ketamine) and/or poppers were combined into one substance use variable to collectively control for drugs associated with increased sexual risk.24 Participants answering “yes” to sex in the past three months were asked “How many male sexual partners have you had in past 3 months?” Participants answering “yes” to ARS, as defined as “their penis in you, sometimes referred to as being a ‘bottom’” were also asked, “How frequently did you use condoms with (these) partners in the past 3 months when having anal receptive (their penis in you) sex?” Response options were: “Never, Rarely, Sometimes, Often or Always.” MSM answering “yes” to sex with anyone in the last month were asked to list “Number of unprotected anal receptive (their penis in you) acts.” Only MSM engaging in ARS were included in the models.
Statistical Analysis
Baseline characteristics of 395 MSM with and without RI were summarized and compared using Pearson’s chi-squared or Fisher’s exact test for categorical variables and T-test and Wilcoxon Mann-Whitney test for continuous variables. Primary analysis on the effect of RD on RI by 48 weeks included 384 MSM and was conducted using a multivariable logistic regression adjusted for age, number of cARS, number of male sexual partners, use of antibiotics and substance use selected a priori based on causal structure of confounding25, 26. For analytical purposes, the median number of partners over 48 weeks was transformed to log base two to adjust for skewness and ease of interpretability. A sensitivity analysis was conducted to compare odds of incident cases (RI free at baseline and at least one positive test between weeks 4–48) with prevalent cases (at least one positive test between weeks 0–48) of RI to rule out reverse causality. A secondary analysis testing for effect modification by number of male sexual partners on RI was conducted by fitting interaction terms between RD and median number of male sexual partners (log 2 transformed) to the regression model. A p-value less than 0.05 was considered statistically significant. Goodness of fit and model selection was assessed using standardized Pearson residuals, likelihood ratio test, Hosmer Lemeshow and Akaike information criterion (AIC)/Bayesian information criterion (BIC) (Supplementary Tables 1-2, Supplementary Figures 2-3). Data management and statistical analysis was conducted using STATA 14.2, College Station, TX: StataCorp LP.
RESULTS
Sample Characteristics
Mean age of 395 participants was 35 years, ranging from 19 to 64 years. Mean age of MSM with RI was 33 years (SD 9), five years younger than MSM without a RI (p<0.001). Majority of participants were non-Hispanic (70%) and White (71%), with fewer African Americans (17%), and Asian or other racial identity (5%). Participants were generally well educated with majority having college or some college level education (71%). Most participants were either employed or working part-time (81%) and were single (65%) for relationship status.
Rectal Infection
One hundred and thirty-three of 395 participants (33%) had a RI over 48 weeks, majority with rectal chlamydia (81%), and less commonly with rectal gonorrhoea (41%). Twenty-two percent of participants with RI were infected with both rectal gonorrhoea and chlamydia. Among 133 participants with RI, 55 (41%) had a RI at baseline, 78 (59%) were RI free at baseline, but had a RI between weeks 4–48 (incident cases). Majority of incident cases were rectal chlamydia infections (81%), with fewer rectal gonorrhoea (37%), and 18% with rectal gonorrhoea and chlamydia.
Sexual, Drug Use and Douching Behaviour
Frequency of condom non-use for ARS was significantly associated with RI (p<0.017). Only 9% of participants without RI reported never using a condom for ARS compared to 15% of participants with a RI. Overall, participants with RI at baseline had higher: 1) Number of cARS (median 1, IQR 0–5 vs. median 0, IQR 0–2; p<0.001); 2) Number of male sexual partners (median 8, IQR 5–10 vs. median 5, IQR 3–10; p=0.001), and 3) Substance use (74% vs. 52%, p<0.001).
Total of 261 (66%) of 395 MSM reported RD at baseline, 81% MSM with RI were douching compared to 59% without a RI. While the majority of MSM used a water solution for douching/enema, 32% of MSM who douched used a combination of water and/or other solutions. Proportion of participants douching less than weekly in the past month were similar between MSM with and without RI (36% and 33%, respectively); however, 47% of MSM with RI douched more than weekly in the past month compared to 23% without any RI (p<0.001). Sociodemographic, sexual and behavioural risk factors are summarized by RI status in Table 1.
Table 1.
Baseline socio-demographic, sexual and behavioural characteristic of HIV-uninfected MSM on PrEP by rectal infection.
| Rectal Infection | |||||
|---|---|---|---|---|---|
| No (N=262) | Yes (N=133) | ||||
| n* | %** | n* | %** | p | |
| DEMOGRAPHICS | |||||
| Household Monthly Income Before Tax |
0.25 | ||||
| <$1000 | 27 | 10% | 8 | 6% | |
| $1,000-$1,999 | 27 | 10% | 22 | 17% | |
| $2,000-$2,999 | 38 | 15% | 22 | 17% | |
| $3,000-$3,999 | 31 | 12% | 13 | 10% | |
| $4,000-$7,999 | 72 | 28% | 30 | 23% | |
| >$8,000 | 26 | 10% | 17 | 13% | |
| Employment | 0.31 | ||||
| Employed | 161 | 62% | 87 | 66% | |
| Part-time | 46 | 18% | 26 | 20% | |
| Unemployed | 48 | 18% | 19 | 15% | |
| Retired/Disabled | 5 | 2% | 0 | 0% | |
| Relationship Status | 0.37 | ||||
| Committed | 91 | 35% | 37 | 28% | |
| Separated | 7 | 3% | 4 | 3% | |
| Single | 163 | 62% | 92 | 69% | |
| Education Level | 0.23 | ||||
| High school or less | 26 | 10% | 8 | 6% | |
| Some college | 89 | 34% | 58 | 44% | |
| College | 90 | 34% | 42 | 32% | |
| Some graduate/Adv | 57 | 22% | 25 | 19% | |
| Age (yrs), mean (SD) | 37 | ±9 | 33 | ±9 | <0.001 |
| Race | 0.036 | ||||
| White | 186 | 71% | 94 | 77% | |
| African American | 48 | 18% | 17 | 14% | |
| Asian/Other | 8 | 3% | 11 | 9% | |
| Ethnicity | 0.043 | ||||
| Hispanic | 68 | 26% | 48 | 36% | |
| Not Hispanic | 191 | 73% | 85 | 64% | |
| SEXUAL AND RISK BEHAVIOURS | |||||
| Drug Abuse | 50 | 19% | 36 | 27% | 0.063 |
| Alcohol Use | 214 | 82% | 110 | 84% | 0.68 |
| Marijuana Use | 116 | 45% | 72 | 55% | 0.053 |
| Tobacco Use | 83 | 32% | 54 | 41% | 0.069 |
| HIV Risk Factor | 0.906 | ||||
| MSM | 249 | 95% | 128 | 96% | |
| Heterosexual | 2 | 1% | 0 | 0% | |
| Both | 11 | 4% | 5 | 4% | |
| No. Male Partners, median (IQR)+ | 5 | 3–10 | 8 | 5–10 | 0.001 |
| Rectal Douching ++ | <0.001 | ||||
| Never | 104 | 40% | 24 | 18% | |
| Less than Weekly | 94 | 36% | 44 | 33% | |
| Weekly or More | 60 | 23% | 63 | 47% | |
| Cocaine Use | 38 | 15% | 22 | 17% | 0.57 |
| Heroin Use | 14 | 5% | 2 | 2% | 0.1 |
| Substance Use | 137 | 52% | 98 | 74% | <0.001 |
| Antibiotic Use+ | 71 | 27% | 39 | 29% | 0.69 |
| Condom Non-use for ARS+ | 0.017 | ||||
| Never | 24 | 9% | 20 | 15% | |
| Rarely/Sometimes | 63 | 24% | 60 | 45% | |
| Always/Often | 85 | 32% | 39 | 29% | |
| No. cARS, median (IQR)++ | 0 | 0–2 | 1 | 0–5 | <0.001 |
| Douching Solution++ | 0.623 | ||||
| Water and/or Other | 51 | 19% | 32 | 24% | |
| Water Only | 106 | 40% | 76 | 57% | |
Substance Use = Any of Methamphetamine/Hallucinogen/Dissociative/Popper
MSM = Men Who Have Sex with Men
ARS = Anal Receptive Sex
cARS = Condomless Anal Receptive Sex
Mean/median for continuous variables.
Standard deviation/interquartile range for continuous variables.
Past 3 months at baseline.
Past month at baseline.
Primary Analysis
Compared to not douching, douching less than weekly and weekly or more per month increased odds of RI to 1.97 (95% CI 1.11–3.49) and 4.57, (95% CI 2.58–8.09), respectively. Other factors associated with increased unadjusted odds of RI were: Substance use (OR 2.83, 95% CI 1.59–5.03), median number of male partners (OR 1.45, 95% CI 1.23–1.72), and number of cARS (OR 1.02, 95% CI 1.01–1.04). Controlling for age, number of cARS, number of male partners, use of antibiotic and substance use, MSM douching weekly or more had increased adjusted odds (aOR) of 3.59 (95% CI 1.90–6.78) for RI compared to not douching. Douching less than weekly was no longer significantly different than not douching with aOR of 1.75 (95% CI 0.96–3.20) (Table 2).
Table 2.
Adjusted/unadjusted odds of rectal infection among HIV-uninfected MSM on PrEP performing rectal douching and/or enema.
| Rectal Infection |
|||||||
|---|---|---|---|---|---|---|---|
| Total (N=384) |
Unadjusted OR | Adjusted OR* | |||||
| n (%) | OR | 95% CI | p | aOR | 95% CI | p | |
| Rectal Douching/Enema | |||||||
| Never | 125 (33%) | - | - | - | - | - | - |
| Less than weekly | 138 (36%) | 1.97 | 1.11–3.49 | 0.020 | 1.75 | 0.96–3.20 | 0.070 |
| Weekly or more | 121 (31%) | 4.57 | 2.58–8.09 | <0.001 | 3.59 | 1.90–6.78 | <0.001 |
| Substance Use | |||||||
| No | 92 (24%) | - | - | - | - | - | - |
| Yes | 292 (76%) | 2.83 | 1.59–5.03 | <0.001 | 1.97 | 1.06–3.67 | 0.033 |
| Number of Male Sexual
Partners**(med/IQR) |
6 (3–10) | 1.45 | 1.23–1.72 | <0.001 | 1.33 | 1.10–1.60 | 0.003 |
| Number cARS (med/IQR) | 4 (0–16) | 1.02 | 1.01–1.04 | <0.001 | 1.01 | 0.99–1.02 | 0.093 |
| Age (yrs) (mean/SD) | 35 (±9) | 0.96 | 0.93–0.98 | 0.001 | 0.95 | 0.92–0.98 | <0.001 |
| Antibiotic Use | |||||||
| No | 274 (71%) | - | - | - | - | - | - |
| Yes | 110 (29%) | 1.09 | 0.68–1.73 | 0.726 | 0.82 | 0.49–1.38 | 0.455 |
Substance Use = Methamphetamine/Hallucinogen/Dissociative/Popper
cARS = Condomless Anal Receptive Sex
Adjusted for age, number of condomless anal receptive sex, log2 median number of male sexual partners, use of antibiotics and substance use.
Log2 transformed median number of partners in past three months.
Sensitivity Analysis
Comparing RD to predict incident with prevalent RI, incident cases had slightly greater aOR 3.87 (95% CI 1.78–8.39, p=0.001) compared to prevalent aOR 3.59 (95% CI 1.90–6.78, p<0.001) for participants douching weekly or more (Figure 1). Odds of RI when douching less than weekly remained similar (aOR 1.78, 95% CI 0.83–3.84, p=0.142 vs. aOR 1.75, 95% CI 0.96–3.20, p=0.070) when comparing incident with prevalent RI, respectively. Considering prevalent rectal gonorrhoea and rectal chlamydia cases individually, aOR of rectal gonorrhoea was 2.11 (95% CI 0.84–5.26, p=0.110) and rectal chlamydia was 1.73 (95% CI 0.91–3.29, p=0.093), when douching less than weekly. Adjusted odds of rectal gonorrhoea and rectal chlamydia increased to 3.56 (95% CI 1.43–8.85, p=0.006) and 3.01 (95% CI 1.55–5.85, p=0.001), respectively, when douching weekly or more.
Figure 1.
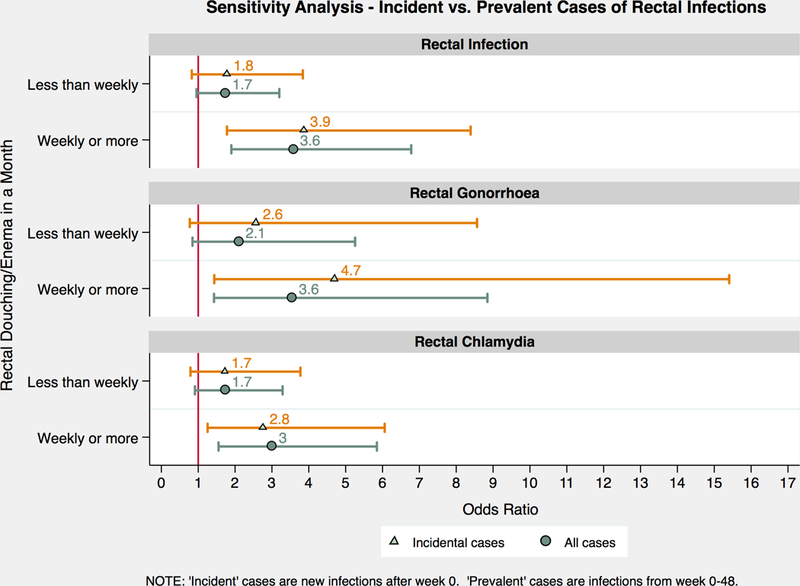
Sensitivity analysis comparing odds of incident versus prevalent cases of rectal infections, rectal gonorrhoea and rectal chlamydia. Incident cases are new infections after week 0, marked in triangles. Prevalent cases are infections from week 0 to 48, marked in circles.
Effect Modification
The effect of douching on RI was modified by the median number of male sexual partners in the past three months (p<0.05). Comparing MSM by median number of partners, ≤6 versus >6, aOR of RI was 0.99 (95% CI 0.45–2.18, p=0.988) and 3.99 (95% CI 1.40–11.41, p=0.010), respectively, when douching less than weekly compared to not douching. Significant increased odds are observed when douching weekly or more between MSM with ≤6 median number of partners (aOR 2.83, 95%1.23–6.50, p=0.014) compared to >6 median number of partners (aOR 5.41, 95% CI 1.84–15.97, p=0.002). This effect can be visualized by plotting the predicted probability of RI when douching at any frequency against median number of male sexual partners (Figure 2). The probability of RI ranges from 20–24% as the number of male sexual partner increase from 1 to 100 if not douching, while the probability of RI increases from 18% with one partner to 77% with 100 partners when douching at any frequency.
Figure 2.
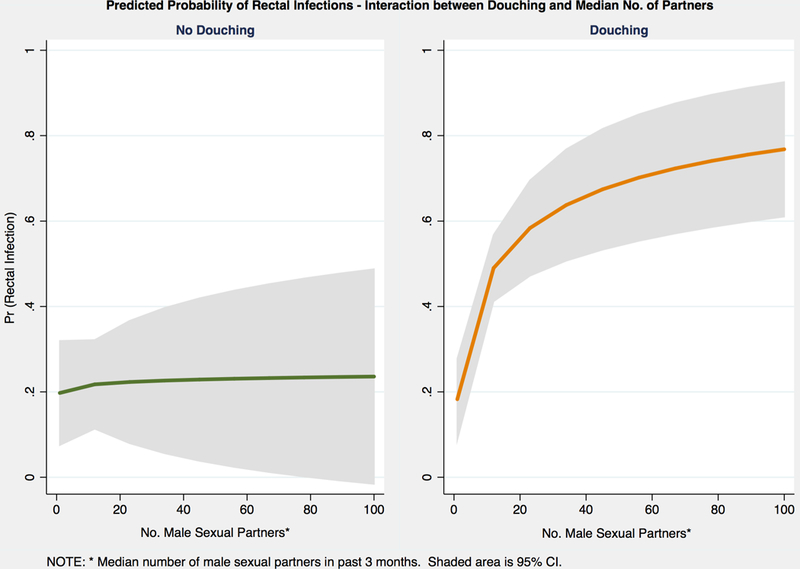
Predicted probability of rectal infections by interaction between douching and median number of male sexual partners in the past three months. The 95% CI is shaded in grey.
DISCUSSION
Our study found douching is common among MSM seeking PrEP in Southern California and the odds of RI increases to 3.59 when douching at least weekly and to 5.41 for MSM with more than six male sexual partners. Like other behavioural studies on douching in Europe15, 17 and the United States14, 27, we found douching to be popular among MSM engaging in ARS. We also had a high prevalence of RD (261 of 395, 66%), slightly higher than the prevalence of 14–53% found in previous studies10, 15, 16, 28, 29. Our cohort had a high prevalence of RI (133 of 395, 34%) with rectal chlamydia cases (27%) twice the prevalence of rectal gonorrhoea cases (14%).
Unlike other cross-sectional11, 14, 17 and case control12, 30 studies reporting an association between douching and RI, our cohort included HIV-uninfected MSM on PrEP followed prospectively and systematically tested for RI. Our results are higher than that of Heiligenberg et al where the adjusted odds of acquiring chlamydia or gonorrhoea were 2.4 (95% CI 1.3–4.4) among HIV-infected participants reporting enema use before sex16. While they did not control for number of cARS, it is lower than the aOR 3.59 (95% CI.190–6.78) in our HIV-uninfected MSM population.
Similar to other studies, individuals in our study used tap water or soap for douching14, 15. Previous research show type of solution to alter the rectal mucosa. In a repeated measure double blind study by Schmelzer et al, mucosal irritation by rectal biopsies after each enema by type of solution were compared20. Epithelium loss was found in rectal biopsies after soapsuds and tap water enemas, but not polyethylene glycol-electrolyte solution enemas in 24 healthy volunteers. We did not find any significant association between RI and douching with water compared to douching with water and/or other solutions but may have had limited power to detect this. Further research is needed to explore the effects of different douching solutions on the rectal mucosa among HIV-uninfected MSM and implications for prevention of STIs/HIV.
Strengths of our study include lab-confirmed routine testing for rectal gonorrhoea and chlamydia for all participants and control for high-risk behaviours, especially substance use associated with ARS, collected systematically over 48-weeks. The behavioural data collected by self-report using computer assisted self-interview made it less susceptible to interviewer and social bias, although recall bias may remain. The multivariate analysis was restricted to participants engaging in ARS as this group is at highest risk for RI. We conducted a sensitivity analysis to further examine incident RI and rule out concerns on reverse causality, such as douching because of exposure or known status of a STI. Results of the sensitivity analysis showed approximately the same increased odds when douching weekly or more with incident cases (aOR 3.87, p=0.001) as prevalent cases (aOR 3.59, p<0.001). Considering associations between douching weekly or more and prevalent rectal gonorrhoea or rectal chlamydia cases separately, aOR for rectal gonorrhoea was slightly higher (3.56, p=0.006) compared to aOR of rectal chlamydia (3.01, p=0.001).
This study had several limitations including information on the frequency of douching, which was only available for 30 days prior to baseline. As changes in any frequency of douching was not captured, we assumed douching behaviour tended to be consistent over time10. In addition, we did not collect information on the type of douching equipment, sharing behaviours, lubricant use, onset of douching and timing of douching (pre- or post-sexual activity) and previous STI, all of which remain as unmeasured confounders. Since gonorrhoea and chlamydia testing were conducted at assigned visit dates only, there was potential for undetected cases occurring between study visits. Finally, the results from this study were based on MSM at high risk for HIV seeking PrEP shortly after approval of TDF-FTC for this indication, and thus only representative of high-risk population.
Our study shows RD is a common practice among HIV-uninfected MSM on PrEP which increases the odds of acquiring rectal gonorrhoea and/or chlamydia. Furthermore, odds of RI may be increased with higher frequency of douching and increasing number of male sexual partners. It is frequently observed a previous STI is a risk factor for detecting prevalent STI. Within the conceptual framework of a microbiome and STI occurrence, this could be related to persistent susceptible microbiome or a change in the microbiome related to the STI or its treatment. Individuals may also be douching as a possible mitigating strategy for future STIs. Further research is needed to distinguish whether the increased odds are attributed to a change in the rectal microbiome or related to rectal trauma or local inflammation. This would be an important step to support development of rectal hygiene products to preserve the mucosal integrity and microbiome. In a population where behavioural prevention strategies like condom use are inconsistently accepted, STI prevention through modifications of current practices could be achieved by education on rectal hygiene frequency or possibly douche composition, if an alternative product was developed. These prevention strategies could lower biological transmission and acquisition risks in addition to PrEP and antiretrovirals to control for the HIV and STI epidemic.
Supplementary Material
Key Messages:
Rectal douching/enema is common among men who have sex with men (MSM), but unclear if it contributes to higher risk of sexual infection.
Among MSM in southern California, 66% practiced rectal douching/enema and 33% had at least one case of rectal gonorrhoea and/or chlamydia over 48 weeks.
Adjusted odds of rectal gonorrhoea and/or chlamydia increases by 3.59 when douching weekly or more and by 5.41 among MSM with more than six partners.
Rectal hygiene products could be used as interventional approaches to reduce sexually transmitted diseases.
Acknowledgements:
We gratefully acknowledge and thank all participants who contributed to the study and the CCTG 595 team at all clinical sites for implementation and data collection.
Funding: Data for this study was collected as part of the main CCTG 595 study supported by the California HIV Research Program (CHRP- MC08-SD-700 and EI-11-SD-005).
Footnotes
Competing Interest: None declared.
Data Sharing: Data can be available at request to corresponding author.
Ethical Approval: All participants were consented prior to participating.
REFERENCE
- 1.Organization WH. Sexually Transmitted Infections (STIs) Fact Sheet: 1.World Health Organization; 2016. [Available from: http://www.who.int/mediacentre/factsheets/fs110/en/ accessed September 2017.
- 2.Miller HG, Cain VS, Rogers SM, et al. Correlates of sexually transmitted bacterial infections among U.S. women in 1995. Family planning perspectives 1999;31(1):4–9, 23. [published Online First: 1999/02/25] [PubMed] [Google Scholar]
- 3.Fleming DT, Wasserheit JN. From epidemiological synergy to public health policy and practice: the contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sexually Transmitted Infections 1999;75(1):3–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2016: U.S. Department of Health and Human Services; 2016. [Available from: shttps://www.cdc.gov/std/stats accessed Sep 2017. [Google Scholar]
- 5.Scholes D, Stergachis A, Ichikawa LE, et al. Vaginal douching as a risk factor for cervical Chlamydia trachomatis infection. Obstetrics and gynecology 1998;91(6):993–7. [published Online First: 1998/06/04] [DOI] [PubMed] [Google Scholar]
- 6.Martino JL, Vermund SH. Vaginal douching: evidence for risks or benefits to women’s health. Epidemiologic reviews 2002;24(2):109–24. [published Online First: 2003/05/24] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Low N, Chersich MF, Schmidlin K, et al. Intravaginal practices, bacterial vaginosis, and HIV infection in women: individual participant data meta-analysis. PLoS medicine 2011;8(2):e1000416. doi: 10.1371/journal.pmed.1000416 [published Online First: 2011/03/02] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seidman D, Rusch M, Abramovitz D, et al. Intravaginal practices among HIV-negative female sex workers along the US–Mexico border and their implications for emerging HIV prevention interventions. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics 2016;133(2):212–16. doi: 10.1016/j.ijgo.2015.09.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moss AR, Osmond D, Bacchetti P, et al. Risk factors for AIDS and HIV seropositivity in homosexual men. American journal of epidemiology 1987;125(6):1035–47. [published Online First: 1987/06/01] [DOI] [PubMed] [Google Scholar]
- 10.Carballo-Dieguez A, Bauermeister JA, Ventuneac A, et al. The use of rectal douches among HIV-uninfected and infected men who have unprotected receptive anal intercourse: implications for rectal microbicides. AIDS Behav 2008;12(6):860–6. doi: 10.1007/s10461-007-9301-0 [published Online First: 2007/08/21] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Noor SW, Rosser BR. Enema use among men who have sex with men: a behavioral epidemiologic study with implications for HIV/STI prevention. Archives of sexual behavior 2014;43(4):755–69. doi: 10.1007/s10508-013-0203-0 [published Online First: 2013/12/19] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Vries HJ, van der Bij AK, Fennema JS, et al. Lymphogranuloma venereum proctitis in men who have sex with men is associated with anal enema use and high-risk behavior. Sex Transm Dis 2008;35(2):203–8. doi: 10.1097/OLQ.0b013e31815abb08 [published Online First: 2007/12/20] [DOI] [PubMed] [Google Scholar]
- 13.Tinmouth J, Gilmour MW, Kovacs C, et al. Is there a reservoir of sub-clinical lymphogranuloma venereum and non-LGV Chlamydia trachomatis infection in men who have sex with men? Int J STD AIDS 2008;19(12):805–9. doi: 10.1258/ijsa.2008.008260 [published Online First: 2008/12/04] [DOI] [PubMed] [Google Scholar]
- 14.Javanbakht M, Stahlman S, Pickett J, et al. Prevalence and types of rectal douches used for anal intercourse: results from an international survey. BMC Infect Dis 2014;14:95. doi: 10.1186/1471-2334-14-95 [published Online First: 2014/02/22] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Achterbergh R, van der Helm JJ, van den Boom W, et al. Is rectal douching and sharing douching equipment associated with anorectal chlamydia and gonorrhoea? A cross-sectional study among men who have sex with men. Sex Transm Infect 2017;93(6):431–37. doi: 10.1136/sextrans-2016-052777 [published Online First: 2017/0½2] [DOI] [PubMed] [Google Scholar]
- 16.Heiligenberg M, Rijnders B, Schim van der Loeff MF, et al. High prevalence of sexually transmitted infections in HIV-infected men during routine outpatient visits in the Netherlands. Sex Transm Dis 2012;39(1):8–15. doi: 10.1097/OLQ.0b013e3182354e81 [published Online First: 2011/12/21] [DOI] [PubMed] [Google Scholar]
- 17.Hambrick HR, Park SH, Goedel WC, et al. Rectal Douching Among Men Who Have Sex with Men in Paris: Implications for HIV/STI Risk Behaviors and Rectal Microbicide Development. AIDS Behav 2017. doi: 10.1007/s10461-017-1873-8 [published Online First: 2017/08/03] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kojima N, Davey DJ, Klausner JD. Pre-exposure prophylaxis for HIV infection and new sexually transmitted infections among men who have sex with men. Aids 2016;30(14):2251–2. doi: 10.1097/qad.0000000000001185 [published Online First: 2016/06/18] [DOI] [PubMed] [Google Scholar]
- 19.Kojima N, Davey DJ, Klausner J. Meta-Analysis Finds Association Between Pre-exposure Prophylaxis to Prevent Human Immunodeficiency Virus (HIV) Infection and Increased Risk of Sexually Transmitted Infection Acquisition Among Men Who Have Sex With Men. Open Forum Infectious Diseases, 2016:504–04. [Google Scholar]
- 20.Schmelzer M, Schiller LR, Meyer R, et al. Safety and effectiveness of large-volume enema solutions. Applied Nursing Research 2004;17(4):265–74. doi: 10.1016/j.apnr.2004.09.010 [DOI] [PubMed] [Google Scholar]
- 21.Fuchs EJ, Lee LA, Torbenson MS, et al. Hyperosmolar sexual lubricant causes epithelial damage in the distal colon: potential implication for HIV transmission. The Journal of infectious diseases 2007;195(5):703–10. doi: 10.1086/511279 [published Online First: 2007/01/31] [DOI] [PubMed] [Google Scholar]
- 22.Beyrer C, Baral SD, van Griensven F, et al. Global epidemiology of HIV infection in men who have sex with men. Lancet (London, England) 2012;380(9839):367–77. doi: 10.1016/s0140-6736(12)60821-6 [published Online First: 2012/07/24] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Service UPH. Preexposure Prophylaxis for the Prevention of HIV Infection in the United States - 2014 Clinical Practice Guideline: US Public Health Service, 2014. [Google Scholar]
- 24.Clatts MC, Goldsamt LA, Yi H. Club Drug Use Among Young Men Who Have Sex with Men in NYC: A Preliminary Epidemiological Profile. Substance use & misuse 2005;40(9–10):1317–30. doi: 10.1081/JA-200066898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Greenland S, Neutra R. Control of Confounding in the Assessment of Medical Technology. International Journal of Epidemiology 1980;9(4):361–67. doi: 10.1093/ije/9.4.361 [DOI] [PubMed] [Google Scholar]
- 26.Hernán MA, Hernández-Díaz S, Werler MM, et al. Causal Knowledge as a Prerequisite for Confounding Evaluation: An Application to Birth Defects Epidemiology. American journal of epidemiology 2002;155(2):176–84. doi: 10.1093/aje/155.2.176 [DOI] [PubMed] [Google Scholar]
- 27.Mitchell JW, Sophus AI, Lee JY, et al. Anal Douche Practices and Willingness to Use a Rectal Microbicide Enema for HIV Prevention and Associated Factors Among an Internet Sample of HIV-Negative and HIV-Discordant Male Couples in the US. AIDS Behav 2016;20(11):2578–87. doi: 10.1007/s10461-015-1250-4 [published Online First: 2016/10/21] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kinsler JJ, Galea JT, Lama JR, et al. Rectal douching among Peruvian men who have sex with men, and acceptability of a douche-formulated rectal microbicide to prevent HIV infection. Sex Transm Infect 2013;89(1):62. doi: 10.1136/sextrans-2012-050630 [published Online First: 2012/07/10] [DOI] [PubMed] [Google Scholar]
- 29.Carballo-Dieguez A, Bauermeister J, Ventuneac A, et al. Why rectal douches may be acceptable rectal-microbicide delivery vehicles for men who have sex with men. Sex Transm Dis 2010;37(4):228–33. doi: 10.1097/OLQ.0b013e3181bf9b2d [published Online First: 2009/12/05] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Macdonald N, Sullivan AK, French P, et al. Risk factors for rectal lymphogranuloma venereum in gay men: results of a multicentre case-control study in the U.K. Sex Transm Infect 2014;90(4):262–8. doi: 10.1136/sextrans-2013-051404 [published Online First: 2014/02/05] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


