Abstract
Host-associated microbial communities provide critical functions for their host. Transition metals are essential for both the mammalian host and the majority of commensal bacteria. As such, access to transition metals is an important component of host-microbe interactions in the gastrointestinal tract. In mammals, transition metal ions are often sequestered by metal biding proteins to limit microbial access under homeostatic conditions. In response to invading pathogens, the mammalian host further decreases availability of these micronutrients by regulating their trafficking or releasing high-affinity metal chelating proteins, a process termed nutritional immunity. Bacterial pathogens have evolved several mechanisms to subvert nutritional immunity. Here, we provide an overview on how metal ion availability shapes host-microbe interactions in the gut with a particular focus on intestinal inflammatory diseases.
Introduction
The intestinal microbiota serves critical functions to human body, ranging from aid of food digestion, providing critical nutrient, education and modulation of the immune system, and colonization resistance against invading pathogens. Perturbations such as antibiotic treatment and enteric pathogen overgrowth drive the gut microbiota into an imbalanced state termed dysbiosis. Microbiota dysbiosis is frequently associated with and may contribute to the exacerbation of disorders involving intestinal inflammation, such as infectious colitis and inflammatory bowel disease (IBD) (reviewed in (Pham and Lawley 2014; Tamboli et al. 2004; Winter and Baumler 2014)).
Nutrient availability in the large intestine is one key determinant of gut microbiota function and composition. With small metabolites being absorbed in the small intestine, the main carbon source for primary fermenters in the large intestine is dietary fiber, a mixture of complex polysaccharides. As such, changes in the macronutrient component of the diet have a profound impact on the microbiota composition (David et al. 2014). In addition to macronutrients, the diet contains micronutrients such as vitamins and the biologically relevant transition metals iron, manganese, molybdenum and zinc. These metal ions exhibit a versatile redox chemistry and therefore catalyze a wide range of reactions such as DNA replication, oxidative stress detoxification, respiration and energy generation. These properties render these metal ions essential for the host and gut microbes. Dietary micronutrient oversupply or limitation impact the composition and function of the gut microbiota on a broad scale. For example, infants receiving an iron fortified diet exhibit a decreased abundance of bifidobacterium and lactobacillus strains in their fecal microbiota and an increased risk for enteric infections (Jaeggi et al. 2015; Simonyte Sjodin et al. 2018).
To limit undesirable effects from their redox chemistry such as radical formation in the Fenton reaction, metal ions are bound by specific proteins during uptake, export, transfer, storage and recycling. During infection, inflammatory host responses further limit microbial access to metal ions. Metal trafficking and storage processes are modified and high-affinity, metal chelating proteins such as lactoferrin and calprotectin are released during inflammation. These ion withholding strategies are collectively termed nutritional immunity (recently reviewed in (Hood and Skaar 2012; Lopez and Skaar 2018)). Although most of the mechanisms involved in nutritional immunity were initially characterized in systemic sites where the maintenance of sterility is crucial, similar principles likely apply to the gastrointestinal track (Zackular et al. 2015). Yet, metal withholding in the intestinal tract is likely to impact both the pathogenic and the commensal bacteria. Of note, the presence of the metal-chelating proteins lactoferrin and calprotectin in the feces are commonly used as clinical markers of gut inflammation (Walsham and Sherwood 2016). Bacterial pathogens have evolved sophisticated mechanisms to subvert host nutritional immunity, but little is known about how commensal gut bacteria acquire transition metals. In this review, we will focus on how competition for iron, manganese, molybdenum, and zinc shapes host-microbe interactions in the inflamed intestine. In addition, we will use Salmonella to illustrate how nutritional immunity in the inflamed intestine can be overcome by enteric pathogens.
Mammalian iron metabolism during homeostasis and inflammatory diseases
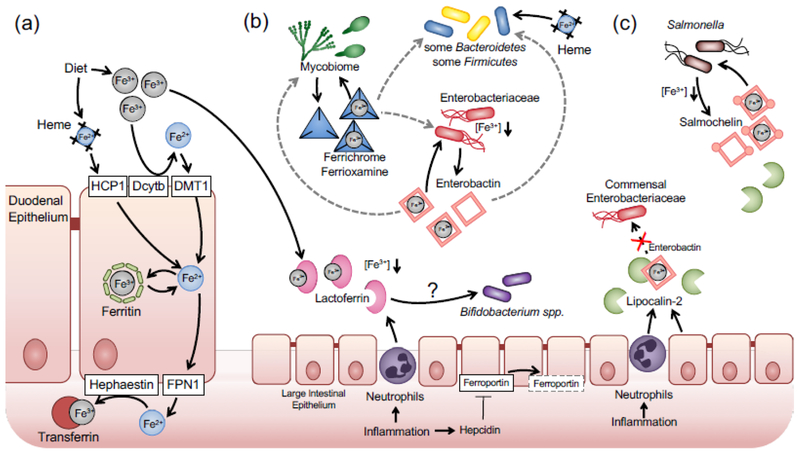
In mammals, iron is predominantly derived from the diet. Under physiological condition, iron exists in either reduced ferrous (Fe2+) or oxidized ferric state (Fe3+). Due to food processing techniques and the presence of oxygen in the stomach, dietary iron enters the small intestine predominantly in the form of ferric iron (Jacobs and Miles 1969). Uptake of dietary iron mainly occurs in the duodenum (Fig. 1A). Dietary iron can be grouped into two categories, heme iron and non-heme iron. Heme is taken up through the action of heme carrier protein 1 (HCP1) located on the brush-border membrane of duodenal enterocytes (Shayeghi et al. 2005) and subsequently degraded by heme oxygenase to yield ferrous iron intracellularly (Raffin et al. 1974). Non-heme ferric iron is reduced to the ferrous state by the cytochrome b reductase 1 (Dcytb) located in the brush-border membrane (McKie et al. 2001) and taken up through the divalent metal transporter 1 (DMT1) on the apical membrane of enterocytes (Gunshin et al. 1997). After uptake, ferrous iron can either be oxidized to ferric state by the ferroxidase activity of the iron storage protein ferritin and subsequently stored in ferritin (Lawson et al. 1991). Alternatively, iron is transported across the basolateral membrane by ferroportin into the plasma (Donovan et al. 2000; McKie et al. 2000). Plasma ferrous iron is then re-oxidized by the multicopper oxidase hephaestin (Griffiths et al. 2005), loaded onto the iron-binding glycoprotein transferrin and delivered to target tissue. In the body, most of the iron is bound by heme coordinated hemoglobin in the red blood cells. During events such as hemolysis, hemopexin and haptoglobin capture free heme and hemoglobin respectively to minimize free iron level in plasma (Alayash et al. 2013; Delanghe and Langlois 2001). Therefore, during iron absorption, export, transfer, storage and recycle, the concentration of free iron is maintained at low level (Fig. 1A).
Fig. 1. Iron uptake by the host and gut-associated microbial communities during inflammation.
(a) Under homeostatic conditions. Duodenal cytochrome B (Dcytb) converts Ferric iron to ferrous iron, which is transported into duodenal epithelial cells by the Divalent Metal Transporter 1 (DMT1). Iron is either stored intracellularly in complex with ferritin or released at the basolateral side through ferroportin (FPN1). Hephaestin oxidizes ferrous to ferric iron, which is bound by transferrin in the serum, (b) During intestinal inflammation, lactoferrin is released into the gut lumen. Hepcidin mediates degradation of ferroportin and decreased iron uptake by the host. Bacterial communities acquire iron through a number of different mechanisms, including heme and siderophoras. For example, Enlerobacteriaceae family members produce enterobactin during iron-limiting conditions. Iron-loaded enterobactin can be used by the original producer or possibly by other communities such as some Bacteroides strains or fungal members of the microbiota. a process termed iron piracy (gray arrows), (c) Enteric pathogens such as Salmonella produce several sidetophores such as enterobactin and salmochelin to overcome host iron sequestration. Neutrophil-derived lipocalin-2 sequesters enterobactin. Salmochelin, a glucosylated enterobactin derivative, is not recognized by lipocalin-2 and facilitates iron uptake by Salmonella during inflammatory conditions.
During episodes of inflammation, the host limits microbial access of iron. This process is in part controlled by the hormone hepcidin. Hepcidin controls iron concentration in extracellular fluid and blood plasma by regulating ferroportin-mediated iron export (McKie et al. 2000) (Fig. 1B). Hepcidin production is greatly enhanced in inflammatory diseases such as IBD and bacterial infections. By promoting the internalization and degradation of ferroportin, hepcidin reduces dietary iron absorption in the small intestine and the subsequent export into circulation, leading to hepcidin-induced-hypoferremia (HIF) (Nicolas et al. 2002). HIF is proposed as a defense mechanism against bacterial pathogens at systemic sites by limiting free iron in extracellular milieu and the circulation (Michels et al. 2015). For example, HIF is critical for controlling Vibrio vulnificus in a subcutaneous infection model (Arezes et al. 2015). However, how hepcidin influences enteric pathogen infection in the intestinal track remains unclear. Results from clinical studies on hepcidin levels and the severity of intestinal inflammation have not been consistent (reviewed in (Cherayil et al. 2011)). Infection of mice with Salmonella enterica serovar Typhimurium (S. Tm), a common cause of foodborne illness, induces hepcidin transcription. Interestingly, pharmaceutical inhibition of hepcidin transcription ameliorated Salmonella-induced and non-infectious colitis (Wang et al. 2009; Wang et al. 2012).
In addition to regulating iron trafficking, mammals also produce high-affinity iron binding proteins such as lactoferrin to sequester iron form microbes. Lactoferrin is a glycoprotein that exerts anti-microbial effects on a wide-range of bacterial species. Its bacteriostatic function is largely dependent upon its ability to chelate iron (Valenti and Antonini 2005). Lactoferrin is present in most mucosal surface throughout the body and its expression is induced by inflammation. In the lumen of the inflamed intestine, transmigrated neutrophils produce and release lactoferrin, which accumulates to levels exceeding 200 g/g under inflammatory conditions, while in control patients, lactoferrin levels are scarce (0.0–2.1 g/g)(Sipponen et al. 2008). The exact contribution of lactoferrin to generating an iron-limiting environment in the inflamed gut lumen remains to be shown experimentally. Curiously, human and bovine lactoferrin enhances growth of Bifidobacterium spp. in vitro (Petschow and Talbott 1991) and in piglets (Tang et al. 2009). Whether this “bifidogenic” effect is due to iron metabolism or degradation of apo-lactoferrin, a glycosylated peptide, is somewhat unclear (reviewed in (Oda et al. 2014)).
Bacterial iron acquisition
Gut bacteria encode a plethora of systems to acquire iron in the form of ferrous iron, ferric iron, heme, hemoglobin, transferrin, and lactoferrin (Fig. 1B). Specific examples of iron acquisition pathways in gut microbes are listed in Table 1. Iron acquisition by Enterobacteriaceae family members, such as E. coli and S. Tm, has been extensively studied in vitro and in vivo (reviewed in (Braun 2003)). Uptake of ferrous iron in E. coli is mediated by the FeoABC system (Kammler et al. 1993). Mutants defective in feoABC uptake are impaired for murine gut colonization (Pi et al. 2012; Stojiljkovic et al. 1993).
Table 1:
Examples of experimentally validated iron uptake systems in gut bacteria
| Phylum | Iron source or siderophore | Transporter/receptor (in exemplary organisms) | Reference |
|---|---|---|---|
| Bacteroidetes | Heme | HupA | (Chen and Wolin 1981) |
| FeoAB in conjunction with BtuS1 and BtuS2 (Bacteroides fragilis) | (Otto et al. 1996) (Rocha et al. 2018) | ||
| Ferrous iron | FeoAB (Bacteroides fragilis) | (Rocha et al. 1991) | |
| Ferric iron | Unknown | (Rocha et al. 1991) | |
| Ferrichrome | FchA1/FchA2 (B. fragilis) | (Rocha and Krykunivsky 2017) | |
| Enterobactin | Unknown | (Rocha and Krykunivsky 2017) | |
| Salmochelin | Unknown | (Rocha and Krykunivsky 2017) | |
| Firmicutes | Ferric citrate | YfmCDEF (Bacillus subtilis) | (Ollinger et al. 2006) |
| Ferrous iron | FeoAB (Clostridium perfringens) | (Awad et al. 2016) | |
| Heme | ChtD/ChtE (C. perfringens) | (Choo et al. 2016) | |
| Hemoglobin | Unknown (Prevotella intermedia) | (Leung et al. 1998) | |
| Bacillibactin/Enterobactin | FeuA (B. subtilis) | (Peuckert et al. 2011) | |
| Ferrichrome | FhuDBGC (B. subtilis) | (Schneider and Hantke 1993) | |
| Ferrioxamine B | FoxD FhuBGC (B. subtilis) | (Schneider and Hantke 1993) | |
| Schizokinen | YfhA/YfiYZ (B. subtilis) | (Ollinger et al. 2006) | |
| Transferrin and lactoferrin | Unknown (Enterococcus faecalis) | (Lisiecki 2017) | |
| Ferric-2-oxoacid complex | Unknown (E. faecalis) | (Lisiecki and Mikucki 2006) | |
| Hydroxamate | Unknown (Enterococcus spp.) | (Sobis-Glinkowska et al. 2001) | |
| Proteobacteria (Enterobacteriaceae) | Ferrous iron | FeoAB (Escherichia coli) | (Kammler et al. 1993) |
| Ferric citrate | FecABCDE (E. coli) | (Wagegg and Braun 1981) | |
| Heme | ChuA (EHEC) | (Torres and Payne 1997) | |
| Enterobactin | FepABCDG (S. Typhimurium) | (Rutz et al. 1991) | |
| Yersiniabactin | FyuA (Yersinia, EHEC) | (Schubert et al. 1998) | |
| Salmochelin | IroN (S. Typhimurium) | (Baumler et al. 1998) | |
| Dihydroxybenzoate | CirA (E. coli) | (Cardelli and Konisky 1974; Hantke 1990) | |
| Fiu (E. coli) | |||
| Ferrichrome | FhuA (S. Typhimurium) | (Tsolis et al. 1995) | |
| Bifidobacterium | Lactoferrin | Unknown | (Oda et al. 2014; Petschow and Talbott 1991) |
Siderophores, which are low molecular weight, high-affinity iron chelators, are produced when iron becomes limited. Examples of siderophores and their cognate uptake systems in common gut bacteria are listed in Table 1. Analysis of bacterial transcription profile in the murine intestinal tract revealed that E. coli in the colonic mucus layer express genes necessary siderophore biosynthesis and transport, suggesting that ferric iron is present in the lumen of the colon but its availability may be limited (Li et al. 2015).
Siderophores allow bacteria to scavenge iron from host chelators, such as transferrin and lactoferrin (reviewed in (Caza and Kronstad 2013)). E. coli and many other Gram-negative bacteria produce the catecholate siderophore enterobactin. Utilization of siderophores such as enterobactin (enterochelin) typically involves a TonB-dependent outer membrane receptor (e.g. FepA) which recognizes and translocates iron-laden siderophores (e.g. ferric enterobactin) into the periplasmic space using the energy transduced by the TonB-ExbB-ExbD system (Frost and Rosenberg 1975; Pugsley and Reeves 1976; Skare et al. 1993). In the periplasm, a periplasmic binding protein (e.g. FepB) relays the siderophore to an ABC-transporter imbedded in the inner membrane (e.g. FepCDG), which transports the siderophore into the cytoplasm.
Due to the lack of an outer membrane, Gram-positive bacteria possess a relatively simple siderophore transport system. These systems are typically composed of a siderophore-binding protein and a cognate permease embedded in the cell membrane (Wilson et al. 2016). Members of the human microbiota belonging to the phylum Firmicutes, such as Staphylococcus spp. and Bacillus spp. possess such siderophore transport system (Beasley et al. 2009; Miethke et al. 2006) (Table 1).
In both Gram-negative and Gram-positive bacteria, siderophore-bound iron needs to be released in the cytoplasm. Iron can be freed from siderophores by esterases that hydrolyze the siderophore backbone. For instance, Fes and IroD encoded by members of Enterobacteriaceae cleave iron-laden enterobactin and salmochelin respectively, into monomers or multimers of dihydroxybenzoate (DHBS). DHBS is a siderophore itself and can be re-secreted outside of the bacteria cells by specific efflux pumps like EntS and IroC (Caza et al. 2011). Alternatively, because ferrous iron has a significantly lower affinity for siderophores, some bacterial species employ ferric siderophore reductases to reduce ferric iron to ferrous state, to facilitate iron dissociation from siderophores (Schroder et al. 2003).
Pathogenic bacteria produce a wide range of siderophores to circumvent nutritional immunity and facilitate host colonization. Akin to E. coli, S. Tm produces enterobactin and the cognate uptake system (FepABCDG). The affinity of enterobactin for iron is high (Kd = 10−52), allowing S. Typhimurium to compete with host proteins such as lactoferrin (Kd = 10−20) for iron (Baker and Baker 2004). Mutants deficient for the outer membrane receptor FepA are attenuated for colonization of systemic sites in a mouse model of disseminated Salmonellosis (Nagy et al. 2013). During infection, neutrophils and epithelial cells produces the protein lipocalin-2, which sequesters iron-laden enterobactin (Flo et al. 2004; Goetz et al. 2002). To subvert host nutritional immunity, S. Typhimurium produces salmochelin, a “stealth” siderophore which incorporates two additional glucosyl moiety to the enterobactin backbone (Hantke et al. 2003). Salmochelin is not recognized by lipocalin (Fischbach et al. 2006) and thus allows S. Typhimurium to effectively escape nutritional immunity conferred by both lactoferrin and lipocalin (Fig. 1C). In mouse models, mutants lacking the receptor for Salmochelin uptake (IroN) are impaired for colonization of systemic sites and do not thrive in the inflamed gut lumen (Costa et al. 2016; Nagy et al. 2013; Raffatellu et al. 2009). Most of the commensal Enterobacteriaceae members produce enterobactin but not salmochelin. Therefore, inflammation dependent production of lipocalin-2 suppresses commensal Enterobacteriaceae, which contributes to colonization resistance against S. Typhimurium by competing for common nutrition resources (Kamada et al. 2013). By overcoming iron starvation mediated by lipocalin-2, S. Typhimurium sidesteps the competition with the commensal Enterobacteriaceae (Behnsen et al. 2014; Raffatellu et al. 2009) (Fig. 1C).
Interestingly, the E. coli Nissle 1917 strain produces and utilizes at least four distinct siderophores, enterobactin, salmochelin, aerobactin, and yersiniabactin (Patzer et al. 2003). Administration of Nissle 1917 as a probiotic intervention during murine S. Typhimurium infection reduces pathogen colonization and fecal shedding. The ability to displace S. Typhimurium was dependent on siderophore uptake systems in Nissle 1917, suggesting that competition for micronutrients is an important aspect of gut colonization during episodes of inflammation (Deriu et al. 2013).
Iron is also essential to bacteria belonging to other phyla such as Bacteroidetes and Firmicutes, the predominant phyla in the gut microbiome (Table 1). During homeostasis, unabsorbed dietary iron available in the colon should be sufficient to support the growth of commensal bacteria. Under this condition, commensals such as and Firmicutes can Bacteroidetes can acquire iron from heme, hemoglobin or by direct uptake of inorganic iron (Ho and Ellermeier 2015; Mazmanian et al. 2003; Rocha et al. 2018). However, these forms of iron are less microbially accessible during intestinal inflammation, as a E. coli mutant strain defective in siderophore utilization is severely attenuated in colonizing the inflamed gut. Interestingly, Bacteroidetes, do not appear to produce known siderophores (Otto et al. 1988; Rocha et al. 1991). Two recent in vitro studies demonstrated that some Bacteroides species are capable of utilizing siderophores that are produced by other bacteria or fungi (xenosiderophores), a phenomenon known as siderophore piracy (Moschen et al. 2016; Rocha and Krykunivsky 2017) (Fig. 1B). Conversely, Aspergillus strains can utilize bacteria-derived enterobactin through a specific siderophore transporter (Haas et al. 2003). Siderophore piracy also occurs during infection with enteric pathogens. For instance, S. Typhimurium encodes FhuA, a TonB-dependent outer membrane receptor to transport fungal siderophore ferrichrome (Killmann et al. 1998). However, the mechanisms underlying Bacteroides xenosiderophore utilization and whether siderophore piracy allows Bacteroides to overcome iron limitation in vivo remain to be explored.
Manganese
Manganese homeostasis in human health and disease
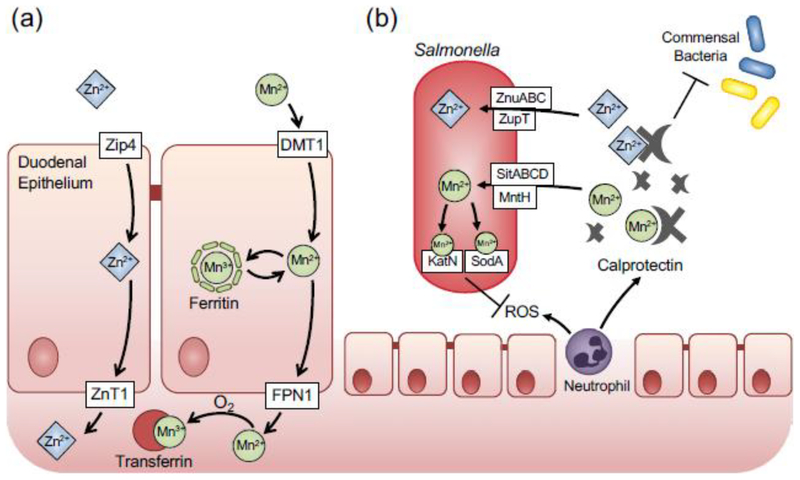
Manganese is essential for many cellular activities, as it functions as a cofactor for enzymes including arginase, glutamine synthetase, pyruvate carboxylase and manganese superoxide dismutase. Diet is the main source of manganese, and approximately 5% of the dietary manganese is absorbed in the gastrointestinal tract. DMT1, the divalent metal transporter responsible for uptake of the majority of dietary iron, is considered to be the major transporter for manganese influx (Illing et al. 2012) (Fig. 2A). Biochemical evidence suggests that manganese could be stored in ferritin (Ardini et al. 2018). Intracellular manganese is exported via ferreportin (Yin et al. 2010) and bound by transferrin for delivery to destination tissues (Davidsson et al. 1989). Manganese in the body is bound by gamma-globulin, albumin and transferrin (Aisen et al. 1969).
Fig. 2. Manganese and zinc homeostasis and subversion of nutritional immunity by Salmonella Typhimurium.
(a) Manganese and zinc uptake by the mammalian host in the duodemum. Zinc is imported into epithelial cells by Zrt- and Irt-like protein 4 (Zip4), and released on the basolateral side by Zinc Transporter 1 (ZnT1). Akin to iron, manganese is transported across the apical membrane of duodenal epithelial cells by the Divalent Metal Transporter 1 (DMT1). stored in ferritin. or exported on the basolateral side via ferroportin (FPN1). Free manganese is sequestered by transferring and transported to target organs, (b) During intestinal inflammation, zinc and manganese are sequestered by calprotectin. which is released by epithelial cells and neutrophils. In addition, zinc-binding protein metallothionein is expressed in the colonic mucosa in the inflamed intestine. Calprotectin-mediated metal withholding suppresses growth of commensal bacteria Enteric pathogen Salmonella uses several high-affinity transporters to subvert nutritional immunity and sidestep the competition with the commensal bacteria. Zinc-dependent superoxide dismutases and catalases help Salmonella defend against reactive oxygen species (ROS).
Many molecular mechanisms involved in manganese uptake and transport are shared with iron, which ensures manganese in the body is tightly sequestered from microbial use. During inflammation, the host can further sequester manganese in several ways. Phagocytic cells such as macrophages and neutrophils engulf bacteria and isolate them in phagosomal compartments, where manganese is depleted through the action of Natural Resistance-Associated Macrophage Protein 1 (NRAMP1) and related divalent metal transporters such as NRAMP2 (Jabado et al. 2000). Neutrophils can also limit bacterial access to manganese by producing lactoferrin and calprotectin, high affinity iron/manganese and manganese/zinc binding proteins respectively (Corbin et al. 2008). Calprotectin is member of the S100 protein family and a heterodimer of S100A8 and S100A9, each of which contains two EF-hand Ca2+ binding sites. The dimer interface forms two zinc binding sites and one manganese binding site (Damo et al. 2013). High level of calprotectin released by neutrophils during gut inflammation (125–250 g/ml) (Liu et al. 2012) contributes to manganese limitation in the inflamed intestine.
Bacterial manganese acquisition and virulence
During intestinal inflammation, reactive oxygen species (ROS) are released by inflammatory cells as an antimicrobial defense mechanism (Aviello and Knaus 2017). Bacterial defense against host oxidative stress is highly dependent on manganese. Manganese can efficiently catalyze superoxide disproportionation in the form of low molecular weight manganese complexes such as manganese phosphate and manganese carbonate (Barnese et al. 2008; Barnese et al. 2012), or serve as a cofactor in ROS detoxification enzymes such as manganese superoxide dismutase (Mn-SOD) and manganese-containing catalase. Manganese dependent, SOD-independent superoxide detoxification has been reported in Staphylococcus aureus and certain Lactobacillus strains (Archibald 1986; Kehl-Fie et al. 2011). Since some Lactobacillus strains lack SOD, they may depend on molecular weight manganese complexes to detoxify superoxide.
ROS-detoxifying enzymes that use manganese contribute to the virulence of enteric bacterial pathogens (Diaz-Ochoa et al. 2016; Wan et al. 2017). Enteric pathogens such S. Typhimurium possesses multiple manganese transporters (SitABCD, MntH and ZupT) to overcome calprotectin-mediated sequestration of manganese (Fig. 2B). Effective acquisition of manganese ensures proper functions of catalase and SOD, enabling S. Typhimurium to resist neutrophil killing and outcompete the commensals and thrive in the inflamed intestine (Diaz-Ochoa et al. 2016). Manganese acquisition is also important for the pathogenesis of Enterococcus faecalis, a member of the gut flora and a leading cause of nosocomial systemic infections (Ubeda et al. 2010). The ability to acquire manganese using high-affinity transport system is essential for E. faecalis virulence during systemic spread (Colomer-Winter et al. 2018).
In addition to defending against host-derived ROS, manganese could contribute to detoxification of superoxide produced during bacterial respiration. Depletion of butyrate-producing obligate anaerobes, a scenario frequently associated with antibiotic treatment or intestinal inflammation, drives metabolic switch from beta-oxidation to glycolysis in the gut epithelium (Donohoe et al. 2011; Donohoe et al. 2012). Switch in metabolic program in the gut epithelial cells leads to increased availability of oxygen in the gut lumen (Byndloss et al. 2017; Kelly et al. 2015). Under this circumstance, it is conceivable that Lactobacillus can use oxygen as the terminal electron acceptor and produce large quantity of hydrogen peroxide, with superoxide as the byproduct. Experimental evidence suggested that some Lactobacillus do produce hydrogen peroxide in the inflamed gut or in host NADPH oxidase deficiency (Archibald and Fridovich 1981; Pircalabioru et al. 2016; van Niel et al. 2002). It is conceivable that manganese -dependent enzymes such as Mn-SOD (Sanders et al. 1995) may be important for a respiratory metabolism of Lactobacillus in intestinal lumen.
Zinc
Zinc homeostasis in human health and disease
Homeostasis of zinc in humans is mostly maintained by absorption of dietary zinc in the gastrointestinal track, secretion and transport of endogenous zinc from the gastrointestinal system to target organs. Zinc deficiency represents significant clinical burden worldwide. It is known that dietary factors have a strong impact on zinc absorption by influencing the amount of zinc in the small intestine lumen (Gibson 1994a; Gibson 1994b). In this regard, zinc deficiency is most common in areas where animal-sourced food ingestion is low while plant consumption is high. It is important to note that the bioavailability of this metal in the gut rather than diet has direct impact on zinc deficiency. For instance, phytic acids, which are present in seeds and grains, bind zinc with high affinity. Phytic acid bound cannot be absorbed in the gut lumen (Hambidge et al. 2011). Zinc becomes available in the gut lumen postprandially and most of the zinc is absorbed in the small intestine (Cousins 1985; Krebs 1998; Lee et al. 1989; Matseshe et al. 1980) (Fig. 2A). After uptake, zinc is transported into the enterocytes in the duodenum and jejunum, translocated from apical to basolateral membrane then into the portal circulation (Reyes 1996). Zinc transport in mammalian cells is mediated by two protein families. The first class of proteins are mainly zinc importers (Krebs 2000). These proteins are known as Zip (Zrt- and Irt-like proteins (SLC39A)), which promote zinc transport from extracellular fluid or from intracellular vesicles into the cytoplasm (McMahon and Cousins 1998a; McMahon and Cousins 1998b). In the Zip family, Zip4, which is localized at the apical membrane of the enterocytes, is the major transporter responsible for uptake of dietary zinc (Wang and Zhou 2010). The second class of proteins involved in zinc transport is ZnT (solute-linked carrier 30 (SLC30A)), and their functions are to mediate zinc efflux from cells or influx into intracellular vesicles under the condition of zinc excess. The mammalian ZnT family consists of 10 members (ZnT1–10) (Cousins et al. 2006). The expression of the ZnTs is mostly regulated by changes in zinc concentration. These proteins are predominantly found in intracellular compartments, such as Golgi or endoplasmic reticulum, except for ZnT1 which is located on the plasma membrane. ZnT1 is expressed in the villi of the proximal small intestine, and studies in rats showed that expression of ZnT1 is increased in response to zinc supplementation trough diet (McMahon and Cousins 1998a; McMahon and Cousins 1998b), consistent with the idea that main role of ZnT1 is to regulate cellular zinc efflux (Palmiter and Findley 1995). These evidence also lead to the proposition that ZnT1 may be responsible for transporting dietary zinc from the basolateral side of the polarized enterocytes into circulation (Wang and Zhou 2010).
In addition to ZnTs and Zips, metallothionein also regulates zinc absorption (Cousins et al. 2006). Metallothioneins are intracellular metal binding proteins that are highly expressed in parenchymal cells of the intestine, liver, kidney and pancreas. Metallothionein expression is positively correlated to zinc availability, and serum zinc concentration was inversely related to metallothionein level. Davis et al. found that after dietary zinc supplementation, serum zinc concentration was higher in metallothionein-deficient mice compared to control animals. These data suggested that metallothionein serves as a zinc pool in case of elevated zinc intake (Davis et al. 1998).
Bacterial zinc acquisition and virulence
In the human body zinc is primarily distributed in the skeletal muscle and bone, but is also found in other organs such as spleen, liver and kidneys. Only a small percentage of the zinc is present in the blood, which is mostly bound to albumin (Wastney et al. 1986). In the tissue, nutritional immunity is imposed by mammalian zinc transporters or high-affinity zinc binding proteins. During acute phase of inflammation, IL-6 mediated upregulation of Zip8 and mobilization Zip14 to the sinusoidal membrane of hepatocyte contribute to the increased zinc uptake from plasma (Liu et al. 2013; Liuzzi et al. 2005). In addition, NRAMP family members function as general metal ion transporters and can sequester zinc from intracellular pathogens. IL-6 expression also induces expression of zinc-sequestering protein metallothionein (Gaetke et al. 1997). These mechanisms contribute to systemic hypozincemia as a component of acute phase response (Gabay and Kushner 1999). In the gut, metallothionein is expressed in the colonic submucosa in murine colitis models. Importantly, genetic ablation of metallothionein is associated with increased expression of inflammatory markers such as TNF-, suggesting an anti-inflammatory role of metallothionein (Al-Gindan et al. 2009; Tsuji et al. 2013). However, whether metallothionein-mediated alleviation of inflammation depends on zinc chelation remains to be determined.
Calprotectin is a key component to withholding zinc during bacterial infection in systemic sites and in the intestine (Corbin et al. 2008; Kehl-Fie and Skaar 2010; Liu et al. 2012). For instance, calprotectin inhibits Staphylococcus aureus growth in abscesses, and its chelation virtually renders the abscesses free of zinc (Corbin et al. 2008). Calprotectin makes up to 60% of the cytoplasmic proteins in neutrophils (Gebhardt et al. 2006) and its expression can be further induced by IL-22 and IL-17 during intestinal inflammation (Hood and Skaar 2012). Neutrophil transmigration is an important characteristic of immune responses during infectious and non-infectious colitis (Day et al. 1978; Zhou et al. 2018), and the resulting release of calprotectin can reach up to 250 g/ml in the fecal content, making it a sensitive biomarker for intestinal inflammation. Together, these data suggested that the inflamed intestine is a zinc limiting environment and calprotectin is an important contributing factor.
Zinc uptake in bacteria is regulated by Zur, zinc uptake regulator, which regulates intracellular zinc levels by controlling zinc uptake. Zur represses transcription of gene associated with zinc import when zinc is abundant, and derepresses its regulon in the absence of zinc (Outten et al. 2001). Bacterial pathogens such as S. Typhimurium, Brucella abortus, Listeria monocytogenes, E. coli and Yersinia pestis (Ammendola et al. 2007; Corbett et al. 2012; Desrosiers et al. 2010) express high-affinity zinc transporters homologous to the ZnuABC system in E. coli (Fig 2B). This system belongs to the ATP-binding cassette transporters family and is composed of three proteins: ZnuA, ZnuB, ZnuC, which are a soluble periplasmic metallochaperone, a membrane permease, and an ATPase receptively (Patzer and Hantke 1998). In the inflamed intestine, lipocalin-2 and calprotectin suppress the growth of commensal bacteria through metal limitation (Valeri and Raffatellu 2016).
Zinc availability and bacterial access to zinc in the gut influences enteric pathogen infection. The ZnuABC system allows S. Typhimurium to subvert host nutritional immunity to outcompete commensal bacteria and thrive in the inflamed gut (Behnsen et al. 2014; Liu et al. 2012). Clostridium difficile is a Gram-positive pathogen that infects the large intestine and causes a wide spectrum of diseases varying from mild diarrhea to pseudomembranous colitis, collectively called Clostridium difficile colitis (CDC) (Lessa et al. 2015). One of the most important risk factors for CDC is antibiotics use. In mice, antibiotic perturbation of the microbiota is required for C. difficile to establish infection and cause disease. In a recent report, Zackular et al. showed that excess dietary zinc introduced significant alterations to the composition of the microbiota, characterized by reduced alpha diversity and outgrowth of certain Enterococcus and Clostridium members. More importantly, excess zinc significantly lowered the threshold of antibiotic perturbation required for C. difficile infection. Detailed investigation into the dynamic of the microbiota structure associated showed that mice fed with normal zinc diet quickly recovered to baseline post low-dose antibiotic treatment while the mice fed with excess zinc failed to do so (Zackular et al. 2016; Zackular and Skaar 2018). This is likely to be the cause of the increase susceptibility to C. difficile infection, as a normal microbiota confers robust colonization resistance against C. difficile colonization (Buffie et al. 2015). These findings highlight the importance of zinc as a determinant of host-microbiome-pathogen integrations.
Molybdenum
Molybdenum homeostasis in health and disease
Molybdenum is a transition metal found in nearly all biological systems. Its capability to shuttle from between the +4/+5 and the +5/+6 redox states allows molybdenum-containing enzymes to catalyze reactions of a wide range of redox potentials under physiological conditions (Hille 2002; Mendel and Bittner 2006). Molybdenum can be taken up from diet and excreted in urine (Turnlund et al. 1995). Molybdenum is taken up in the form of the oxyanion. Two specific molybdate transporters identified in eukaryotes are the MOT1 and MOT2 from the alga Chlamydomonas reinhardtii. Homologues of MOT2 have been detected in mammals including humans (Tejada-Jimenez et al. 2011), however, molybdenum uptake and transport in mammalian cells is incompletely understood.
In prokaryotic and eukaryotic cells, molybdenum is only biologically active when it is properly coordinated by a organophosphate-dithiolate scaffold, named molybdopterin, to produce the molybdenum cofactor (Mo-co) (Hille 2002). Based on the type of pterin derivative coordinating the molybdenum atom, molybdoenzymes can be classified into three families: the xanthine oxidase (XO) family, the sulfite oxidase (SO) family, and the dimethyl sulfoxide (DMSO) reductase family (Hille et al. 2011). Members of these families utilize sulfurated MPT-cytosine dinucleotide (MCD), di-oxo Mo-co, and bis-MPT-guanine dinucleotide (bis-MGD) as their cofactors, respectively. Seven molybdenum enzymes have been identified in eukaryotes, which are sulfide oxidases, aldehyde oxidases, xanthine dehydrogenases, and mitochondria amidoxime reducing component (mARC) (Mendel and Kruse 2012). The importance of molybdenum to mammalian hosts is evidenced by the fact that Mo-co deficiency results in rare but severe inborn error of metabolism (reviewed in (Schwarz 2016)). However, how the bioavailability of molybdenum is regulated in mammalian hosts is not well understood, and no immune defense mechanism dedicated to sequestering molybdenum have been identified so far.
Molybdenum cofactor in host-microbe interactions in the gut
In bacteria, molybdate is acquired by a high-affinity ABC-type transporter (Kd ~50 nM), or two other promiscuous anion transporters (Self et al. 2001). Inside the cell, molybdenum is incorporated into the Mo-co in a highly conserved, multi-step process (reviewed in (Magalon and Mendel 2015)). Briefly, the biosynthesis of Mo-co in bacteria involves the synthesis of molybdopterin, incorporation of molybdenum to form Mo-co and the subsequent modifications to form Mo-co variants such as bis-MGD. The maturation of Mo-co is then followed by the insertion of Mo-co deep inside of the target enzymes in a molecular chaperon dependent manner (Genest et al. 2009).
In bacteria, molybdoenzymes catalyze a wide range of reactions related to the electron transport chain. Respiratory and fermentative formate dehydrogenases, nitrate reductases, and other anaerobic reductases are Mo-co-dependent. Among the gut bacterial phyla, Proteobacteria encode the highest amount of molybdoenzymes (Rothery and Weiner 2015). Proteobacteria are normally a minor (~ 0.1%) constituent of the gut microbial community, but undergo significant expansion during episodes of intestinal inflammation. In the inflamed intestine, formate oxidation and nitrate respiration independently contribute to the bloom of Enterobacteriaceae (Proteobacteria phylum) (Hughes et al. 2017; Winter et al. 2013). Importantly, these pathways are largely specific to Enterobacteriaceae and are only operational during intestinal inflammation. Tungsten, a transition element closely related to molybdenum, can replace molybdenum in Mo-co when tungsten is present in excess (Aurich 1959). Due to the difference in redox properties, the tungsten-substituted Mo-co inhibits the activities of proteobacterial molybdoenzymes including nitrate reductases and formate dehydrogenases (Gates et al. 2003). In mouse models of colitis, oral administration of sodium tungstate abrogates the fitness advantage conferred by molybdoenzyme-dependent respiration in Enterobacteriaceae (Zhu et al. 2018).
Conclusions and remarks
Over the past decades, numerous seminal studies have greatly advanced our understandings in the importance of transition metal ions in host-microbe interactions. In recent years, the microbiota became the new focus of transition metal ion research because its intimate association with human health and diseases. The complex nature of the microbial ecosystem in the gut hampers the development of microbiome-targeted intervention strategies to improve human health. Transition metal ions represent an important starting point for the development of such strategies, as they are crucial for the host, commensal bacteria, and enteric pathogens. Dietary supplement of metal ions has strong influence on the microbiota structure and function, but the outcomes of different studies have not been consistent and are poorly predictable a priori. This is largely due to the lack of mechanistic understanding on how metal ions impact bacterial physiology in such a complex ecosystem. For instance, it is unclear how commensals acquire metals in the intestinal tract during intestinal inflammation. Future experimental work can use the principles of host-microbe metal competition gained by studying model enteric pathogens to characterize mechanisms involved in commensals coping with metal starvation. These insights could be exploited to uncover novel treatment strategies to ameliorate diseases and promote homeostasis.
Acknowledgement
Work in S.E.W.’s lab was funded by the NIH (AI118807, AI128151), The Welch Foundation (I-1969-20180324), the Burroughs Wellcome Fund (1017880), and a Research Scholar Grant (RSG-17048-01-MPC) from the American Cancer Society. W.Z. was supported by a Research Fellows Award from the Crohn’s and Colitis Foundation of America (454921). The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the funding agencies. We want to thank the members of the Winter laboratory for helpful discussion. S.E.W. is listed as an inventor on patent US10092596B2, which describes a treatment to prevent the inflammation-associated expansion of Enterobacteriaceae. The other authors have no additional financial interests.
References:
- Aisen P, Aasa R, Redfield AG (1969) The chromium, manganese, and cobalt complexes of transferrin J Biol Chem 244:4628–4633 [PubMed] [Google Scholar]
- Al-Gindan Y, Shawarby M, Noto A, Taylor CG (2009) Intestinal inflammation in rats induces metallothionein in colonic submucosa J Clin Biochem Nutr 44:131–141 doi: 10.3164/jcbn.08-217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alayash AI, Andersen CB, Moestrup SK, Bulow L (2013) Haptoglobin: the hemoglobin detoxifier in plasma Trends Biotechnol 31:2–3 doi: 10.1016/j.tibtech.2012.10.003 [DOI] [PubMed] [Google Scholar]
- Ammendola S, Pasquali P, Pistoia C, Petrucci P, Petrarca P, Rotilio G, Battistoni A (2007) High-affinity Zn2+ uptake system ZnuABC is required for bacterial zinc homeostasis in intracellular environments and contributes to the virulence of Salmonella enterica Infect Immun 75:5867–5876 doi: 10.1128/IAI.00559-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archibald F (1986) Manganese: its acquisition by and function in the lactic acid bacteria Crit Rev Microbiol 13:63–109 doi: 10.3109/10408418609108735 [DOI] [PubMed] [Google Scholar]
- Archibald FS, Fridovich I (1981) Manganese and defenses against oxygen toxicity in Lactobacillus plantarum J Bacteriol 145:442–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardini M et al. (2018) Study of manganese binding to the ferroxidase centre of human H-type ferritin J Inorg Biochem 182:103–112 doi: 10.1016/j.jinorgbio.2018.02.003 [DOI] [PubMed] [Google Scholar]
- Arezes J et al. (2015) Hepcidin-induced hypoferremia is a critical host defense mechanism against the siderophilic bacterium Vibrio vulnificus Cell Host Microbe 17:47–57 doi: 10.1016/j.chom.2014.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aurich H (1959) [Inhibition of molybdenum-dependent nitrate reductase by wolfram in Neurospora crassa] Arch Mikrobiol 33:46–48 [PubMed] [Google Scholar]
- Aviello G, Knaus UG (2017) ROS in gastrointestinal inflammation: Rescue Or Sabotage? Br J Pharmacol 174:1704–1718 doi: 10.1111/bph.13428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awad MM, Cheung JK, Tan JE, McEwan AG, Lyras D, Rood JI (2016) Functional analysis of an feoB mutant in Clostridium perfringens strain 13 Anaerobe 41:10–17 doi: 10.1016/j.anaerobe.2016.05.005 [DOI] [PubMed] [Google Scholar]
- Baker HM, Baker EN (2004) Lactoferrin and iron: structural and dynamic aspects of binding and release Biometals 17:209–216 [DOI] [PubMed] [Google Scholar]
- Barnese K, Gralla EB, Cabelli DE, Valentine JS (2008) Manganous phosphate acts as a superoxide dismutase J Am Chem Soc 130:4604–4606 doi: 10.1021/ja710162n [DOI] [PubMed] [Google Scholar]
- Barnese K, Gralla EB, Valentine JS, Cabelli DE (2012) Biologically relevant mechanism for catalytic superoxide removal by simple manganese compounds Proc Natl Acad Sci U S A 109:6892–6897 doi: 10.1073/pnas.1203051109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumler AJ, Norris TL, Lasco T, Voight W, Reissbrodt R, Rabsch W, Heffron F (1998) IroN, a novel outer membrane siderophore receptor characteristic of Salmonella enterica J Bacteriol 180:1446–1453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beasley FC et al. (2009) Characterization of staphyloferrin A biosynthetic and transport mutants in Staphylococcus aureus Mol Microbiol 72:947–963 doi: 10.1111/j.1365-2958.2009.06698.x [DOI] [PubMed] [Google Scholar]
- Behnsen J, Jellbauer S, Wong CP, Edwards RA, George MD, Ouyang W, Raffatellu M (2014) The cytokine IL-22 promotes pathogen colonization by suppressing related commensal bacteria Immunity 40:262–273 doi: 10.1016/j.immuni.2014.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun V (2003) Iron uptake by Escherichia coli Front Biosci 8:s1409–1421 [DOI] [PubMed] [Google Scholar]
- Buffie CG et al. (2015) Precision microbiome reconstitution restores bile acid mediated resistance to Clostridium difficile Nature 517:205–208 doi: 10.1038/nature13828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byndloss MX et al. (2017) Microbiota-activated PPAR-gamma signaling inhibits dysbiotic Enterobacteriaceae expansion Science 357:570–575 doi: 10.1126/science.aam9949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardelli J, Konisky J (1974) Isolation and characterization of an Escherichia coli mutant tolerant to colicins Ia and Ib J Bacteriol 119:379–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caza M, Kronstad JW (2013) Shared and distinct mechanisms of iron acquisition by bacterial and fungal pathogens of humans Front Cell Infect Microbiol 3:80 doi: 10.3389/fcimb.2013.00080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caza M, Lepine F, Dozois CM (2011) Secretion, but not overall synthesis, of catecholate siderophores contributes to virulence of extraintestinal pathogenic Escherichia coli Mol Microbiol 80:266–282 doi: 10.1111/j.1365-2958.2011.07570.x [DOI] [PubMed] [Google Scholar]
- Chen M, Wolin MJ (1981) Influence of heme and vitamin B12 on growth and fermentations of Bacteroides species J Bacteriol 145:466–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherayil BJ, Ellenbogen S, Shanmugam NN (2011) Iron and intestinal immunity Curr Opin Gastroenterol 27:523–528 doi: 10.1097/MOG.0b013e32834a4cd1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choo JM et al. (2016) The NEAT Domain-Containing Proteins of Clostridium perfringens Bind Heme PLoS One 11:e0162981 doi: 10.1371/journal.pone.0162981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colomer-Winter C et al. (2018) Manganese acquisition is essential for virulence of Enterococcus faecalis PLoS Pathog 14:e1007102 doi: 10.1371/journal.ppat.1007102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett D et al. (2012) Two zinc uptake systems contribute to the full virulence of Listeria monocytogenes during growth in vitro and in vivo Infect Immun 80:14–21 doi: 10.1128/IAI.05904-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbin BD et al. (2008) Metal chelation and inhibition of bacterial growth in tissue abscesses Science 319:962–965 doi: 10.1126/science.1152449 [DOI] [PubMed] [Google Scholar]
- Costa LF et al. (2016) Iron acquisition pathways and colonization of the inflamed intestine by Salmonella enterica serovar Typhimurium Int J Med Microbiol 306:604–610 doi: 10.1016/j.ijmm.2016.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousins RJ (1985) Absorption, transport, and hepatic metabolism of copper and zinc: special reference to metallothionein and ceruloplasmin Physiol Rev 65:238–309 doi: 10.1152/physrev.1985.65.2.238 [DOI] [PubMed] [Google Scholar]
- Cousins RJ, Liuzzi JP, Lichten LA (2006) Mammalian zinc transport, trafficking, and signals J Biol Chem 281:24085–24089 doi: 10.1074/jbc.R600011200 [DOI] [PubMed] [Google Scholar]
- Damo SM et al. (2013) Molecular basis for manganese sequestration by calprotectin and roles in the innate immune response to invading bacterial pathogens Proc Natl Acad Sci U S A 110:3841–3846 doi: 10.1073/pnas.1220341110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- David LA et al. (2014) Diet rapidly and reproducibly alters the human gut microbiome Nature 505:559–563 doi: 10.1038/nature12820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidsson L, Lonnerdal B, Sandstrom B, Kunz C, Keen CL (1989) Identification of transferrin as the major plasma carrier protein for manganese introduced orally or intravenously or after in vitro addition in the rat J Nutr 119:1461–1464 doi: 10.1093/jn/119.10.1461 [DOI] [PubMed] [Google Scholar]
- Davis SR, McMahon RJ, Cousins RJ (1998) Metallothionein knockout and transgenic mice exhibit altered intestinal processing of zinc with uniform zinc-dependent zinc transporter-1 expression J Nutr 128:825–831 doi: 10.1093/jn/128.5.825 [DOI] [PubMed] [Google Scholar]
- Day DW, Mandal BK, Morson BC (1978) The rectal biopsy appearances in Salmonella colitis Histopathology 2:117–131 [DOI] [PubMed] [Google Scholar]
- Delanghe JR, Langlois MR (2001) Hemopexin: a review of biological aspects and the role in laboratory medicine Clin Chim Acta 312:13–23 [DOI] [PubMed] [Google Scholar]
- Deriu E et al. (2013) Probiotic bacteria reduce salmonella typhimurium intestinal colonization by competing for iron Cell Host Microbe 14:26–37 doi: 10.1016/j.chom.2013.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrosiers DC et al. (2010) Znu is the predominant zinc importer in Yersinia pestis during in vitro growth but is not essential for virulence Infect Immun 78:5163–5177 doi: 10.1128/IAI.00732-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Ochoa VE et al. (2016) Salmonella Mitigates Oxidative Stress and Thrives in the Inflamed Gut by Evading Calprotectin-Mediated Manganese Sequestration Cell Host Microbe 19:814–825 doi: 10.1016/j.chom.2016.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohoe DR, Garge N, Zhang X, Sun W, O’Connell TM, Bunger MK, Bultman SJ (2011) The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon Cell Metab 13:517–526 doi: 10.1016/j.cmet.2011.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohoe DR, Wali A, Brylawski BP, Bultman SJ (2012) Microbial regulation of glucose metabolism and cell-cycle progression in mammalian colonocytes PLoS One 7:e46589 doi: 10.1371/journal.pone.0046589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan A et al. (2000) Positional cloning of zebrafish ferroportin1 identifies a conserved vertebrate iron exporter Nature 403:776–781 doi: 10.1038/35001596 [DOI] [PubMed] [Google Scholar]
- Fischbach MA et al. (2006) The pathogen-associated iroA gene cluster mediates bacterial evasion of lipocalin 2 Proc Natl Acad Sci U S A 103:16502–16507 doi: 10.1073/pnas.0604636103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flo TH et al. (2004) Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron Nature 432:917–921 doi: 10.1038/nature03104 [DOI] [PubMed] [Google Scholar]
- Frost GE, Rosenberg H (1975) Relationship between the tonB locus and iron transport in Escherichia coli J Bacteriol 124:704–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabay C, Kushner I (1999) Acute-phase proteins and other systemic responses to inflammation N Engl J Med 340:448–454 doi: 10.1056/NEJM199902113400607 [DOI] [PubMed] [Google Scholar]
- Gaetke LM, McClain CJ, Talwalkar RT, Shedlofsky SI (1997) Effects of endotoxin on zinc metabolism in human volunteers Am J Physiol 272:E952–956 doi: 10.1152/ajpendo.1997.272.6.E952 [DOI] [PubMed] [Google Scholar]
- Gates AJ et al. (2003) Properties of the periplasmic nitrate reductases from Paracoccus pantotrophus and Escherichia coli after growth in tungsten-supplemented media FEMS Microbiol Lett 220:261–269 [DOI] [PubMed] [Google Scholar]
- Gebhardt C, Nemeth J, Angel P, Hess J (2006) S100A8 and S100A9 in inflammation and cancer Biochem Pharmacol 72:1622–1631 doi: 10.1016/j.bcp.2006.05.017 [DOI] [PubMed] [Google Scholar]
- Genest O, Mejean V, Iobbi-Nivol C (2009) Multiple roles of TorD-like chaperones in the biogenesis of molybdoenzymes FEMS Microbiol Lett 297:1–9 doi: 10.1111/j.1574-6968.2009.01660.x [DOI] [PubMed] [Google Scholar]
- Gibson RS (1994a) Content and bioavailability of trace elements in vegetarian diets Am J Clin Nutr 59:1223S–1232S doi: 10.1093/ajcn/59.5.1223S [DOI] [PubMed] [Google Scholar]
- Gibson RS (1994b) Zinc nutrition in developing countries Nutr Res Rev 7:151–173 doi: 10.1079/NRR19940010 [DOI] [PubMed] [Google Scholar]
- Goetz DH, Holmes MA, Borregaard N, Bluhm ME, Raymond KN, Strong RK (2002) The neutrophil lipocalin NGAL is a bacteriostatic agent that interferes with siderophore-mediated iron acquisition Mol Cell 10:1033–1043 [DOI] [PubMed] [Google Scholar]
- Griffiths TA, Mauk AG, MacGillivray RT (2005) Recombinant expression and functional characterization of human hephaestin: a multicopper oxidase with ferroxidase activity Biochemistry 44:14725–14731 doi: 10.1021/bi051559k [DOI] [PubMed] [Google Scholar]
- Gunshin H et al. (1997) Cloning and characterization of a mammalian proton-coupled metal-ion transporter Nature 388:482–488 doi: 10.1038/41343 [DOI] [PubMed] [Google Scholar]
- Haas H et al. (2003) Characterization of the Aspergillus nidulans transporters for the siderophores enterobactin and triacetylfusarinine C Biochem J 371:505–513 doi: 10.1042/BJ20021685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hambidge KM, Miller LV, Krebs NF (2011) Physiological requirements for zinc Int J Vitam Nutr Res 81:72–78 doi: 10.1024/0300-9831/a00052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantke K (1990) Dihydroxybenzoylserine--a siderophore for E. coli FEMS Microbiol Lett 55:5–8 [DOI] [PubMed] [Google Scholar]
- Hantke K, Nicholson G, Rabsch W, Winkelmann G (2003) Salmochelins, siderophores of Salmonella enterica and uropathogenic Escherichia coli strains, are recognized by the outer membrane receptor IroN Proc Natl Acad Sci U S A 100:3677–3682 doi: 10.1073/pnas.0737682100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille R (2002) Molybdenum and tungsten in biology Trends Biochem Sci 27:360–367 [DOI] [PubMed] [Google Scholar]
- Hille R, Nishino T, Bittner F (2011) Molybdenum enzymes in higher organisms Coord Chem Rev 255:1179–1205 doi: 10.1016/j.ccr.2010.11.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho TD, Ellermeier CD (2015) Ferric Uptake Regulator Fur Control of Putative Iron Acquisition Systems in Clostridium difficile J Bacteriol 197:2930–2940 doi: 10.1128/JB.00098-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood MI, Skaar EP (2012) Nutritional immunity: transition metals at the pathogen-host interface Nat Rev Microbiol 10:525–537 doi: 10.1038/nrmicro2836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes ER et al. (2017) Microbial Respiration and Formate Oxidation as Metabolic Signatures of Inflammation-Associated Dysbiosis Cell Host Microbe 21:208–219 doi: 10.1016/j.chom.2017.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illing AC, Shawki A, Cunningham CL, Mackenzie B (2012) Substrate profile and metal-ion selectivity of human divalent metal-ion transporter-1 J Biol Chem 287:30485–30496 doi: 10.1074/jbc.M112.364208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabado N, Jankowski A, Dougaparsad S, Picard V, Grinstein S, Gros P (2000) Natural resistance to intracellular infections: natural resistance-associated macrophage protein 1 (Nramp1) functions as a pH-dependent manganese transporter at the phagosomal membrane J Exp Med 192:1237–1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs A, Miles PM (1969) Intraluminal transport of iron from stomach to small-intestinal mucosa Br Med J 4:778–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeggi T et al. (2015) Iron fortification adversely affects the gut microbiome, increases pathogen abundance and induces intestinal inflammation in Kenyan infants Gut 64:731–742 doi: 10.1136/gutjnl-2014-307720 [DOI] [PubMed] [Google Scholar]
- Kamada N, Chen GY, Inohara N, Nunez G (2013) Control of pathogens and pathobionts by the gut microbiota Nat Immunol 14:685–690 doi: 10.1038/ni.2608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammler M, Schon C, Hantke K (1993) Characterization of the ferrous iron uptake system of Escherichia coli J Bacteriol 175:6212–6219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehl-Fie TE et al. (2011) Nutrient metal sequestration by calprotectin inhibits bacterial superoxide defense, enhancing neutrophil killing of Staphylococcus aureus Cell Host Microbe 10:158–164 doi: 10.1016/j.chom.2011.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehl-Fie TE, Skaar EP (2010) Nutritional immunity beyond iron: a role for manganese and zinc Curr Opin Chem Biol 14:218–224 doi: 10.1016/j.cbpa.2009.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly CJ et al. (2015) Crosstalk between Microbiota-Derived Short-Chain Fatty Acids and Intestinal Epithelial HIF Augments Tissue Barrier Function Cell Host Microbe 17:662–671 doi: 10.1016/j.chom.2015.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killmann H, Herrmann C, Wolff H, Braun V (1998) Identification of a new site for ferrichrome transport by comparison of the FhuA proteins of Escherichia coli, Salmonella paratyphi B, Salmonella typhimurium, and Pantoea agglomerans J Bacteriol 180:3845–3852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs NF (1998) Zinc supplementation during lactation Am J Clin Nutr 68:509S–512S doi: 10.1093/ajcn/68.2.509S [DOI] [PubMed] [Google Scholar]
- Krebs NF (2000) Overview of zinc absorption and excretion in the human gastrointestinal tract J Nutr 130:1374S–1377S doi: 10.1093/jn/130.5.1374S [DOI] [PubMed] [Google Scholar]
- Lawson DM et al. (1991) Solving the structure of human H ferritin by genetically engineering intermolecular crystal contacts Nature 349:541–544 doi: 10.1038/349541a0 [DOI] [PubMed] [Google Scholar]
- Lee HH, Prasad AS, Brewer GJ, Owyang C (1989) Zinc absorption in human small intestine Am J Physiol 256:G87–91 doi: 10.1152/ajpgi.1989.256.1.G87 [DOI] [PubMed] [Google Scholar]
- Lessa FC et al. (2015) Burden of Clostridium difficile infection in the United States N Engl J Med 372:825–834 doi: 10.1056/NEJMoa1408913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung KP, Subramaniam PS, Okamoto M, Fukushima H, Lai CH (1998) The binding and utilization of hemoglobin by Prevotella intermedia FEMS Microbiol Lett 162:227–233 doi: 10.1111/j.1574-6968.1998.tb13003.x [DOI] [PubMed] [Google Scholar]
- Li H et al. (2015) The outer mucus layer hosts a distinct intestinal microbial niche Nat Commun 6:8292 doi: 10.1038/ncomms9292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisiecki P (2017) Transferrin and Lactoferrin - Human Iron Sources for Enterococci Pol J Microbiol 66:419–425 doi: 10.5604/01.3001.0010.6495 [DOI] [PubMed] [Google Scholar]
- Lisiecki P, Mikucki J (2006) Iron supply of enterococci by 2-oxoacids and hydroxyacids Pol J Microbiol 55:195–202 [PubMed] [Google Scholar]
- Liu JZ et al. (2012) Zinc sequestration by the neutrophil protein calprotectin enhances Salmonella growth in the inflamed gut Cell Host Microbe 11:227–239 doi: 10.1016/j.chom.2012.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu MJ et al. (2013) ZIP8 regulates host defense through zinc-mediated inhibition of NF-kappaB Cell Rep 3:386–400 doi: 10.1016/j.celrep.2013.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liuzzi JP et al. (2005) Interleukin-6 regulates the zinc transporter Zip14 in liver and contributes to the hypozincemia of the acute-phase response Proc Natl Acad Sci U S A 102:6843–6848 doi: 10.1073/pnas.0502257102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez CA, Skaar EP (2018) The Impact of Dietary Transition Metals on Host-Bacterial Interactions Cell Host Microbe 23:737–748 doi: 10.1016/j.chom.2018.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magalon A, Mendel RR (2015) Biosynthesis and Insertion of the Molybdenum Cofactor EcoSal Plus 6 doi: 10.1128/ecosalplus.ESP-0006-2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matseshe JW, Phillips SF, Malagelada JR, McCall JT (1980) Recovery of dietary iron and zinc from the proximal intestine of healthy man: studies of different meals and supplements Am J Clin Nutr 33:1946–1953 doi: 10.1093/ajcn/33.9.1946 [DOI] [PubMed] [Google Scholar]
- Mazmanian SK et al. (2003) Passage of heme-iron across the envelope of Staphylococcus aureus Science 299:906–909 doi: 10.1126/science.1081147 [DOI] [PubMed] [Google Scholar]
- McKie AT et al. (2001) An iron-regulated ferric reductase associated with the absorption of dietary iron Science 291:1755–1759 doi: 10.1126/science.1057206 [DOI] [PubMed] [Google Scholar]
- McKie AT et al. (2000) A novel duodenal iron-regulated transporter, IREG1, implicated in the basolateral transfer of iron to the circulation Mol Cell 5:299–309 [DOI] [PubMed] [Google Scholar]
- McMahon RJ, Cousins RJ (1998a) Mammalian zinc transporters J Nutr 128:667–670 doi: 10.1093/jn/128.4.667 [DOI] [PubMed] [Google Scholar]
- McMahon RJ, Cousins RJ (1998b) Regulation of the zinc transporter ZnT-1 by dietary zinc Proc Natl Acad Sci U S A 95:4841–4846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendel RR, Bittner F (2006) Cell biology of molybdenum Biochim Biophys Acta 1763:621–635 doi: 10.1016/j.bbamcr.2006.03.013 [DOI] [PubMed] [Google Scholar]
- Mendel RR, Kruse T (2012) Cell biology of molybdenum in plants and humans Biochim Biophys Acta 1823:1568–1579 doi: 10.1016/j.bbamcr.2012.02.007 [DOI] [PubMed] [Google Scholar]
- Michels K, Nemeth E, Ganz T, Mehrad B (2015) Hepcidin and Host Defense against Infectious Diseases PLoS Pathog 11:e1004998 doi: 10.1371/journal.ppat.1004998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miethke M, Klotz O, Linne U, May JJ, Beckering CL, Marahiel MA (2006) Ferri-bacillibactin uptake and hydrolysis in Bacillus subtilis Mol Microbiol 61:1413–1427 doi: 10.1111/j.1365-2958.2006.05321.x [DOI] [PubMed] [Google Scholar]
- Moschen AR et al. (2016) Lipocalin 2 Protects from Inflammation and Tumorigenesis Associated with Gut Microbiota Alterations Cell Host Microbe 19:455–469 doi: 10.1016/j.chom.2016.03.007 [DOI] [PubMed] [Google Scholar]
- Nagy TA, Moreland SM, Andrews-Polymenis H, Detweiler CS (2013) The ferric enterobactin transporter Fep is required for persistent Salmonella enterica serovar typhimurium infection Infect Immun 81:4063–4070 doi: 10.1128/IAI.00412-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolas G et al. (2002) Severe iron deficiency anemia in transgenic mice expressing liver hepcidin Proc Natl Acad Sci U S A 99:4596–4601 doi: 10.1073/pnas.072632499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda H, Wakabayashi H, Yamauchi K, Abe F (2014) Lactoferrin and bifidobacteria Biometals 27:915–922 doi: 10.1007/s10534-014-9741-8 [DOI] [PubMed] [Google Scholar]
- Ollinger J, Song KB, Antelmann H, Hecker M, Helmann JD (2006) Role of the Fur regulon in iron transport in Bacillus subtilis J Bacteriol 188:3664–3673 doi: 10.1128/JB.188.10.3664-3673.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto BR, Kusters JG, Luirink J, de Graaf FK, Oudega B (1996) Molecular characterization of a heme-binding protein of Bacteroides fragilis BE1 Infect Immun 64:4345–4350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto BR, Verweij-van Vught AM, van Doorn J, Maclaren DM (1988) Outer membrane proteins of Bacteroides fragilis and Bacteroides vulgatus in relation to iron uptake and virulence Microb Pathog 4:279–287 [DOI] [PubMed] [Google Scholar]
- Outten CE, Tobin DA, Penner-Hahn JE, O’Halloran TV (2001) Characterization of the metal receptor sites in Escherichia coli Zur, an ultrasensitive zinc(II) metalloregulatory protein Biochemistry 40:10417–10423 [DOI] [PubMed] [Google Scholar]
- Palmiter RD, Findley SD (1995) Cloning and functional characterization of a mammalian zinc transporter that confers resistance to zinc EMBO J 14:639–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patzer SI, Baquero MR, Bravo D, Moreno F, Hantke K (2003) The colicin G, H and X determinants encode microcins M and H47, which might utilize the catecholate siderophore receptors FepA, Cir, Fiu and IroN Microbiology 149:2557–2570 doi: 10.1099/mic.0.26396-0 [DOI] [PubMed] [Google Scholar]
- Patzer SI, Hantke K (1998) The ZnuABC high-affinity zinc uptake system and its regulator Zur in Escherichia coli Mol Microbiol 28:1199–1210 [DOI] [PubMed] [Google Scholar]
- Petschow BW, Talbott RD (1991) Response of bifidobacterium species to growth promoters in human and cow milk Pediatr Res 29:208–213 doi: 10.1203/00006450-199102000-00021 [DOI] [PubMed] [Google Scholar]
- Peuckert F, Ramos-Vega AL, Miethke M, Schworer CJ, Albrecht AG, Oberthur M, Marahiel MA (2011) The siderophore binding protein FeuA shows limited promiscuity toward exogenous triscatecholates Chem Biol 18:907–919 doi: 10.1016/j.chembiol.2011.05.006 [DOI] [PubMed] [Google Scholar]
- Pham TA, Lawley TD (2014) Emerging insights on intestinal dysbiosis during bacterial infections Curr Opin Microbiol 17:67–74 doi: 10.1016/j.mib.2013.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pi H et al. (2012) Role of catecholate siderophores in gram-negative bacterial colonization of the mouse gut PLoS One 7:e50020 doi: 10.1371/journal.pone.0050020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pircalabioru G et al. (2016) Defensive Mutualism Rescues NADPH Oxidase Inactivation in Gut Infection Cell Host Microbe 19:651–663 doi: 10.1016/j.chom.2016.04.007 [DOI] [PubMed] [Google Scholar]
- Pugsley AP, Reeves P (1976) Iron uptake in colicin B-resistant mutants of Escherichia coli K-12 J Bacteriol 126:1052–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffatellu M et al. (2009) Lipocalin-2 resistance confers an advantage to Salmonella enterica serotype Typhimurium for growth and survival in the inflamed intestine Cell Host Microbe 5:476–486 doi: 10.1016/j.chom.2009.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffin SB, Woo CH, Roost KT, Price DC, Schmid R (1974) Intestinal absorption of hemoglobin iron-heme cleavage by mucosal heme oxygenase J Clin Invest 54:1344–1352 doi: 10.1172/JCI107881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes JG (1996) Zinc transport in mammalian cells Am J Physiol 270:C401–410 doi: 10.1152/ajpcell.1996.270.2.C401 [DOI] [PubMed] [Google Scholar]
- Rocha ER, Bergonia HA, Gerdes S, Jeffrey Smith C (2018) Bacteroides fragilis requires the ferrous-iron transporter FeoAB and the CobN-like proteins BtuS1 and BtuS2 for assimilation of iron released from heme Microbiologyopen:e00669 doi: 10.1002/mbo3.669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha ER, de Uzeda M, Brock JH (1991) Effect of ferric and ferrous iron chelators on growth of Bacteroides fragilis under anaerobic conditions FEMS Microbiol Lett 68:45–50 [DOI] [PubMed] [Google Scholar]
- Rocha ER, Krykunivsky AS (2017) Anaerobic utilization of Fe(III)-xenosiderophores among Bacteroides species and the distinct assimilation of Fe(III)-ferrichrome by Bacteroides fragilis within the genus Microbiologyopen 6 doi: 10.1002/mbo3.479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothery RA, Weiner JH (2015) Shifting the metallocentric molybdoenzyme paradigm: the importance of pyranopterin coordination J Biol Inorg Chem 20:349–372 doi: 10.1007/s00775-014-1194-6 [DOI] [PubMed] [Google Scholar]
- Rutz JM, Abdullah T, Singh SP, Kalve VI, Klebba PE (1991) Evolution of the ferric enterobactin receptor in gram-negative bacteria J Bacteriol 173:5964–5974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders JW, Leenhouts KJ, Haandrikman AJ, Venema G, Kok J (1995) Stress response in Lactococcus lactis: cloning, expression analysis, and mutation of the lactococcal superoxide dismutase gene J Bacteriol 177:5254–5260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider R, Hantke K (1993) Iron-hydroxamate uptake systems in Bacillus subtilis: identification of a lipoprotein as part of a binding protein-dependent transport system Mol Microbiol 8:111–121 [DOI] [PubMed] [Google Scholar]
- Schroder I, Johnson E, de Vries S (2003) Microbial ferric iron reductases FEMS Microbiol Rev 27:427–447 doi: 10.1016/S0168-6445(03)00043-3 [DOI] [PubMed] [Google Scholar]
- Schubert S, Rakin A, Karch H, Carniel E, Heesemann J (1998) Prevalence of the “high-pathogenicity island” of Yersinia species among Escherichia coli strains that are pathogenic to humans Infect Immun 66:480–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz G (2016) Molybdenum cofactor and human disease Curr Opin Chem Biol 31:179–187 doi: 10.1016/j.cbpa.2016.03.016 [DOI] [PubMed] [Google Scholar]
- Self WT, Grunden AM, Hasona A, Shanmugam KT (2001) Molybdate transport Res Microbiol 152:311–321 [DOI] [PubMed] [Google Scholar]
- Shayeghi M et al. (2005) Identification of an intestinal heme transporter Cell 122:789–801 doi: 10.1016/j.cell.2005.06.025 [DOI] [PubMed] [Google Scholar]
- Simonyte Sjodin K et al. (2018) Administration of ferrous sulfate drops has significant effects on the gut microbiota of iron-sufficient infants: a randomised controlled study Gut doi: 10.1136/gutjnl-2018-316988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipponen T, Savilahti E, Karkkainen P, Kolho KL, Nuutinen H, Turunen U, Farkkila M (2008) Fecal calprotectin, lactoferrin, and endoscopic disease activity in monitoring anti-TNF-alpha therapy for Crohn’s disease Inflamm Bowel Dis 14:1392–1398 doi: 10.1002/ibd.20490 [DOI] [PubMed] [Google Scholar]
- Skare JT, Ahmer BM, Seachord CL, Darveau RP, Postle K (1993) Energy transduction between membranes. TonB, a cytoplasmic membrane protein, can be chemically cross-linked in vivo to the outer membrane receptor FepA J Biol Chem 268:16302–16308 [PubMed] [Google Scholar]
- Sobis-Glinkowska M, Mikucki J, Lisiecki P (2001) [Hydroxamate siderophore effect on growth of enterococci] Med Dosw Mikrobiol 53:1–7 [PubMed] [Google Scholar]
- Stojiljkovic I, Cobeljic M, Hantke K (1993) Escherichia coli K-12 ferrous iron uptake mutants are impaired in their ability to colonize the mouse intestine FEMS Microbiol Lett 108:111–115 doi: 10.1111/j.1574-6968.1993.tb06082.x [DOI] [PubMed] [Google Scholar]
- Tamboli CP, Neut C, Desreumaux P, Colombel JF (2004) Dysbiosis in inflammatory bowel disease Gut 53:1–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Z et al. (2009) Effects of dietary supplementation with an expressed fusion peptide bovine lactoferricin-lactoferrampin on performance, immune function and intestinal mucosal morphology in piglets weaned at age 21 d Br J Nutr 101:998–1005 doi: 10.1017/S0007114508055633 [DOI] [PubMed] [Google Scholar]
- Tejada-Jimenez M, Galvan A, Fernandez E (2011) Algae and humans share a molybdate transporter Proc Natl Acad Sci U S A 108:6420–6425 doi: 10.1073/pnas.1100700108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres AG, Payne SM (1997) Haem iron-transport system in enterohaemorrhagic Escherichia coli O157:H7 Mol Microbiol 23:825–833 [DOI] [PubMed] [Google Scholar]
- Tsolis RM, Baumler AJ, Stojiljkovic I, Heffron F (1995) Fur regulon of Salmonella typhimurium: identification of new iron-regulated genes J Bacteriol 177:4628–4637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji T et al. (2013) Role of metallothionein in murine experimental colitis Int J Mol Med 31:1037–1046 doi: 10.3892/ijmm.2013.1294 [DOI] [PubMed] [Google Scholar]
- Turnlund JR, Keyes WR, Peiffer GL, Chiang G (1995) Molybdenum absorption, excretion, and retention studied with stable isotopes in young men during depletion and repletion Am J Clin Nutr 61:1102–1109 doi: 10.1093/ajcn/61.4.1102 [DOI] [PubMed] [Google Scholar]
- Ubeda C et al. (2010) Vancomycin-resistant Enterococcus domination of intestinal microbiota is enabled by antibiotic treatment in mice and precedes bloodstream invasion in humans J Clin Invest 120:4332–4341 doi: 10.1172/JCI43918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenti P, Antonini G (2005) Lactoferrin: an important host defence against microbial and viral attack Cell Mol Life Sci 62:2576–2587 doi: 10.1007/s00018-005-5372-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valeri M, Raffatellu M (2016) Cytokines IL-17 and IL-22 in the host response to infection Pathog Dis 74 doi: 10.1093/femspd/ftw111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Niel EW, Hofvendahl K, Hahn-Hagerdal B (2002) Formation and conversion of oxygen metabolites by Lactococcus lactis subsp. lactis ATCC 19435 under different growth conditions Appl Environ Microbiol 68:4350–4356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagegg W, Braun V (1981) Ferric citrate transport in Escherichia coli requires outer membrane receptor protein fecA J Bacteriol 145:156–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsham NE, Sherwood RA (2016) Fecal calprotectin in inflammatory bowel disease Clin Exp Gastroenterol 9:21–29 doi: 10.2147/CEG.S51902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan B et al. (2017) Type VI secretion system contributes to Enterohemorrhagic Escherichia coli virulence by secreting catalase against host reactive oxygen species (ROS) PLoS Pathog 13:e1006246 doi: 10.1371/journal.ppat.1006246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L et al. (2009) Selective modulation of TLR4-activated inflammatory responses by altered iron homeostasis in mice J Clin Invest 119:3322–3328 doi: 10.1172/JCI39939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L et al. (2012) The bone morphogenetic protein-hepcidin axis as a therapeutic target in inflammatory bowel disease Inflamm Bowel Dis 18:112–119 doi: 10.1002/ibd.21675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zhou B (2010) Dietary zinc absorption: A play of Zips and ZnTs in the gut IUBMB Life 62:176–182 doi: 10.1002/iub.291 [DOI] [PubMed] [Google Scholar]
- Wastney ME, Aamodt RL, Rumble WF, Henkin RI (1986) Kinetic analysis of zinc metabolism and its regulation in normal humans Am J Physiol 251:R398–408 doi: 10.1152/ajpregu.1986.251.2.R398 [DOI] [PubMed] [Google Scholar]
- Wilson BR, Bogdan AR, Miyazawa M, Hashimoto K, Tsuji Y (2016) Siderophores in Iron Metabolism: From Mechanism to Therapy Potential Trends Mol Med 22:1077–1090 doi: 10.1016/j.molmed.2016.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter SE, Baumler AJ (2014) Dysbiosis in the inflamed intestine: chance favors the prepared microbe Gut Microbes 5:71–73 doi: 10.4161/gmic.27129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter SE et al. (2013) Host-derived nitrate boosts growth of E. coli in the inflamed gut Science 339:708–711 doi: 10.1126/science.1232467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Z et al. (2010) Ferroportin is a manganese-responsive protein that decreases manganese cytotoxicity and accumulation J Neurochem 112:1190–1198 doi: 10.1111/j.1471-4159.2009.06534.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zackular JP, Chazin WJ, Skaar EP (2015) Nutritional Immunity: S100 Proteins at the Host-Pathogen Interface J Biol Chem 290:18991–18998 doi: 10.1074/jbc.R115.645085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zackular JP et al. (2016) Dietary zinc alters the microbiota and decreases resistance to Clostridium difficile infection Nat Med 22:1330–1334 doi: 10.1038/nm.4174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zackular JP, Skaar EP (2018) The role of zinc and nutritional immunity in Clostridium difficile infection Gut Microbes 9:469–476 doi: 10.1080/19490976.2018.1448354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou G et al. (2018) CD177(+) neutrophils as functionally activated neutrophils negatively regulate IBD Gut 67:1052–1063 doi: 10.1136/gutjnl-2016-313535 [DOI] [PubMed] [Google Scholar]
- Zhu W et al. (2018) Precision editing of the gut microbiota ameliorates colitis Nature 553:208–211 doi: 10.1038/nature25172 [DOI] [PMC free article] [PubMed] [Google Scholar]