Abstract
Barth syndrome (BTHS) is a rare, multi-systemic genetic disorder caused by mutations in the TAZ gene. TAZ encodes a mitochondrial enzyme that remodels the acyl chain composition of newly synthesized cardiolipin, a phospholipid unique to mitochondrial membranes. The clinical abnormalities observed in BTHS patients are caused by perturbations in various mitochondrial functions that rely on remodeled cardiolipin. However, the contribution of different cardiolipin-dependent mitochondrial functions to the pathology of BTHS is not fully understood. In this review, we will discuss recent findings from different genetic models of BTHS, including the yeast model of cardiolipin deficiency that has uncovered the specific in vivo roles of cardiolipin in mitochondrial respiratory chain biogenesis, bioenergetics, intermediary metabolism, mitochondrial dynamics, and quality control. We will also describe findings from higher eukaryotic models of BTHS that highlight a link between cardiolipin-dependent mitochondrial function and its impact on tissue and organ function.
INTRODUCTION
About four decades ago, physician Peter Barth reported a Dutch family with a history of inherited infantile cardiomyopathy involving abnormal mitochondria (1). This rare X-linked genetic disorder later came to be known as Barth syndrome (BTHS) (OMIM 302060). BTHS occurs at a frequency of 1 in 300,000 to 400,000 live births, but evidence is accumulating that this number is highly under-estimated (2). BTHS is primarily characterized by cardiomyopathy, skeletal muscle myopathy, neutropenia, and growth delay in the affected individuals (2). Initial observations indicating that BTHS is a mitochondrial disorder came from the electron microscopic examinations of patient tissues, which showed the presence of abnormal mitochondria with aberrant cristae morphology and reduced respiratory chain function (1). The disease-causing mutation was subsequently mapped to the TAZ gene on the X chromosome (3). Later, biochemical studies led to the identification of TAZ as an evolutionarily conserved phospholipid-lysophospholipid transacylase that remodels the acyl chains of cardiolipin (CL) (4), a signature phospholipid of the mitochondria. CL has a unique dimeric structure consisting of two phosphatidyl-moieties linked by a glycerol backbone (Fig.1). The acyl chains of the newly synthesized CL undergo remodeling, a process by which saturated acyl chains are replaced with unsaturated forms to generate a fully mature form of CL (4). In the heart, CL is predominantly present as tetralinoleoyl-CL, however a remodeling defect due to TAZ mutations leads to deficiency of tetralinoleoyl-CL in BTHS patients (5).
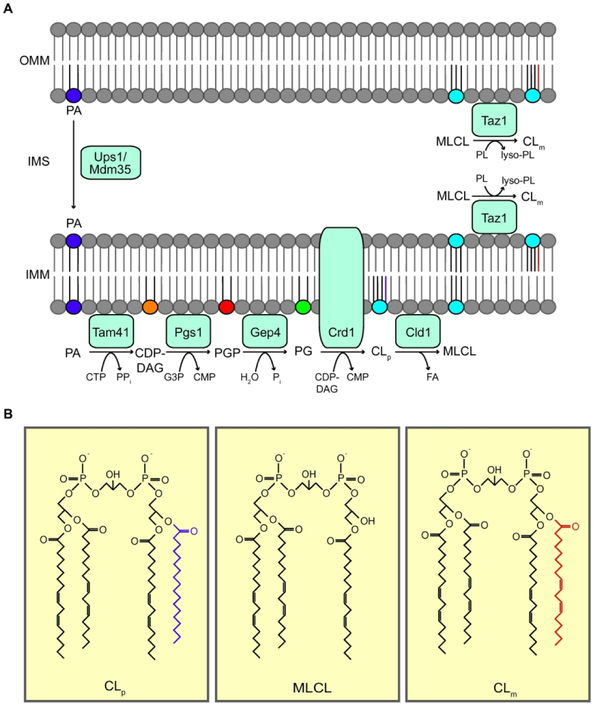
Figure 1: Cardiolipin biosynthetic pathway.
(A) CL biosynthesis occurs exclusively in the mitochondria. CL precursor, PA, is transported from the OMM to the IMM via Ups1/Mdm35 to initiate the CL biosynthetic pathway. PA is converted to PG through three subsequent enzyme-catalyzed reactions by Tam41, Pgs1, and Gep4. Crd1 biosynthesizes nascent CL (CLp) from PG. CLp must then be remodeled to form mature CL (CLm) through sequential deacylation and reacylation reactions catalyzed by Cld1 and Taz1, respectively. Taz1 is localized to the OMM and the IMM facing the IMS. (B) Structures of CLp, MLCL, and CLm. ATP, adenosine triphosphate; ADP, adenosine diphosphate; CTP, cytidine triphosphate; PPi, pyrophosphate; CDP-DAG, cytidine diphosphate-diacylglycerol; CMP, cytidine monophosphate; PA, phosphatidic acid; CLp, premature cardiolipin; CLm, mature cardiolipin; MLCL, monolysocardiolipin; PG, phosphatidylglycerol; PGP, phosphatidylglycerol phosphate; G3P, glycerol-3-phosphate; PL, phospholipid; FA, fatty acid; OMM, outer mitochondrial membrane; IMM, inner mitochondrial membrane; IMS, intermembrane space.
Although a number of causative mutations in TAZ have been identified, the large variability in clinical presentations is not fully understood, pointing to a gap in our knowledge about various contributing factors underlying the pathophysiology of BTHS (2). The primary biochemical defect in BTHS is perturbation in the CL biosynthetic process; therefore, determining all CL-dependent mitochondrial functions can provide clues to the underlying causes of the variability in pathophysiology of this disorder. In this review, we highlight diverse CL-dependent mitochondrial functions that are key to understanding the clinical heterogeneity of BTHS. These include respiratory chain biogenesis, bioenergetics, intermediary metabolism, mitochondrial quality control, and mitochondrial dynamics. We will discuss findings from a number of BTHS models, including yeast, fly, zebrafish, and mice, as well as organoid models, which provide the biochemical and physiological underpinnings necessary to understand the clinical features associated with BTHS.
BIOSYNTHESIS OF CARDIOLIPIN
Cardiolipin biosynthesis is highly conserved and confined to the mitochondrial membranes (reviewed recently by Gaspard and McMaster, Ref. 6) (Fig. 1 and Table 1). Briefly, CL biosynthesis requires phosphatidic acid (PA), which is transported from the outer mitochondrial membrane (OMM) to the inner mitochondrial membrane (IMM) via the Ups1/Mdm35 lipid transport protein complex in yeast and PRELID1/TRIAP1 in humans (Fig. 1). PA in the IMM is then converted to CDP-diacylglycerol (CDP-DAG) by the cytidylyltransferase Tam41/TAMM41 in yeast and humans, respectively (7). In the committed step, the yeast mitochondrial enzyme Pgs1 and its mammalian counterpart PGS1 convert CDP-DAG into phosphatidylglycerolphosphate (PGP) through transfer of the phosphatidyl group from CDP-DAG onto the glycerol-3-phosphate. PGP is subsequently dephosphorylated to phosphatidylglycerol (PG) via a reaction catalyzed by the yeast enzyme Gep4 and its human homolog, PTPMT1. Nascent CL (CLp) is then biosynthesized via the condensation of PG and a phosphatidyl group from another CDP-DAG molecule by Crd1 in yeast and CLS1 in humans.
Table 1:
CL biosynthesis and remodeling enzymes in yeast and humans.
| Yeast | Human | Function |
|---|---|---|
| Tam41 | TAMM41 | phosphatidate cytidylyltransferase |
| Pgs1 | PGS1 | phosphatidylglycerol synthase |
| Gep4 | PTPMT1 | phosphatidylglycerophosphatase |
| Crd1 | CLS1 | cardiolipin synthase |
| Cld1 | ? | cardiolipin-specific deacylase |
| Taz1 | TAZ | transacylase |
Remodeling of cardiolipin
The acyl chains of newly synthesized CL (CLp) are remodeled to yield mature CL (CLm), by a process involving sequential deacylation and reacylation reactions in which the saturated acyl chains are replaced with unsaturated fatty acyl chains (5,8). In yeast, CLp deacylation and reacylation reactions are catalyzed by the mitochondrial enzymes Cld1 and Taz1, respectively (Fig. 1). Cld1 initiates the first step in CL remodeling by deacylating nascent CL to form monolysocardiolipin (MLCL) (8). In Drosophila, the phospholipase iPLA2 performs the function of Cld1 (9). The human homolog of Cld1 has not yet been identified (Table 1). MLCL generated from the deacylation of CLp is then reacylated by Taz1 to form mature CLm (4). In vitro experiments demonstrated that Drosophila TAZ can transfer acyl groups from various phospholipids including phosphatidylcholine (PC), phosphatidylethanolamine (PE), and CL to many different lysophospholipids (MLCL, LPA, LPE, LPC), but the transfer rates of linoleoyl acyl chain from PC to MLCL were several folds higher than the other pairs (4). TAZ-deficient mitochondria from all investigated BTHS disease models are characterized by an increased level of MLCL along with a decrease in total and remodeled CL levels (9-11). Deletion of CLD1 in yeast taz1Δ cells prevents accumulation of MLCL, a finding that provides in vivo evidence for the deacylation-reacylation cycle of CL remodeling (12,13).
MOLECULAR BASIS OF BARTH SYNDROME
The primary defect in BTHS is the accumulation of MLCL and a decrease in remodeled as well as total CL. Thus, the ensuing mitochondrial dysfunction could be attributed to any combination of one or more of the above perturbations in CL species. An increased MLCL/CL ratio or a perturbation in content and composition of CL are proposed to be the molecular basis of BTHS. The strongest evidence for the detrimental role of an abnormal MLCL/CL ratio comes from studies on the yeast remodeling mutants cld1Δ and taz1Δ, where mitochondrial dysfunction associated with the yeast model of BTHS, taz1Δ, could be corrected by restoring the MLCL/CL ratio through deletion of CLD1 (12,13). In addition, yeast cld1Δ cells, which have an MLCL/CL ratio similar to wild type cells but contain an aberrant CL composition, do not have any mitochondrial phenotypes associated with BTHS (8,12,13). Further evidence in support of the role of the elevated MLCL/CL ratio in BTHS pathology comes from Drosophila studies, where deletion of the Drosophila equivalent of CLD1 gene in a fly model of BTHS rescues the male sterility phenotype while partially restoring the MLCL/CL ratio (9). A human genetic study on BTHS patients with ameliorated clinical phenotypes showed that the severity of clinical presentations correlates with the MLCL/CL ratio (14). In fact, the MLCL/CL ratio has proven to be a robust diagnostic marker for BTHS (14).
GENETICS OF BARTH SYNDROME
Over 100 different pathogenic mutations in TAZ have been identified to date (www.barthsyndrome.org). These pathogenic mutations include a wide range of missense, nonsense, and splicing mutations, full or partial deletions, and frame-shifts throughout the entire open reading frame (15,16). The development of a genetically tractable yeast model of BTHS, taz1Δ, has greatly facilitated the study of these loss-of-function mutations because human TAZ can complement yeast taz1Δ cells (17). Claypool and colleagues classified a number of BTHS patient mutations by expressing them in taz1Δ cells (16). The majority of BTHS-associated mutations give rise to non-functional TAZ proteins. However, it has been difficult to establish a correlation between the type of mutation and the clinical presentations. In fact, Ronvelia et al. showed that a single mutation (genotype) gives rise to different clinical symptoms (phenotypes) (18). Similarly, 9 out of 14 BTHS pedigrees showed no correlation between the type of mutation and clinical presentations (15). These observations indicate that there are additional modifying factors that act as key determinants of BTHS pathogenesis. It will be crucial to identify these additional factors or processes that lead up to the disease state in order to develop therapeutic strategies.
MITOCHONDRIAL DYSFUNCTIONS IN BARTH SYNDROME
The primary cause of BTHS is a disruption in the mitochondrial membrane phospholipid composition, which impairs the functions of many proteins embedded therein. Thus, BTHS-associated disease phenotypes originate from a disruption in membrane-related functions including mitochondrial bioenergetics and dynamics. To gain a better understanding of BTHS pathophysiology and to answer questions regarding the source of variability in the clinical symptoms presented in different patients, it is imperative to catalog the wide range of mitochondrial dysfunctions caused by CL deficiency.
Cardiolipin and mitochondrial ultrastructure:
The most direct evidence for the role of mitochondria in BTHS pathology comes from the electron microscopic studies on BTHS patient tissues and various BTHS models. Electron micrographs of heart, liver, and skeletal muscle mitochondria from BTHS patients have shown enlarged mitochondria with stacked and disarrayed cristae (19). Ultrastructural deformities have also been observed in the flight muscle mitochondria of a Drosophila model of BTHS, which is characterized by hyperdense cristae (20). Similarly, a murine model of BTHS exhibited enlarged mitochondria harboring circularly stacked cristae in cardiac and skeletal muscles (11).
A decrease in CL, as observed in BTHS mitochondria, is expected to perturb mitochondrial cristae structure owing to its non-bilayer promoting propensities (21). Although in theory the non-bilayer promoting properties of CL could explain the observed ultrastructure defects in BTHS mitochondria, yeast mitochondria completely devoid of CL have only minor defects in cristae length (13). However, it is difficult to directly correlate mitochondrial ultrastructure abnormalities to CL levels because CL deficiency in yeast cells triggers accumulation of PE, another non-bilayer forming phospholipid that may mitigate the ultrastructure phenotype (21). Notably, synthetic lethal interaction between CL and mitochondrial PE biosynthetic pathways has been reported, which indicates overlapping roles of these two phospholipids in maintaining mitochondrial integrity (22).
Another possible explanation for the abnormal cristae morphology of BTHS mitochondria is the interaction of CL with the Mitochondrial Contact Site and Cristae Organizing System (MICOS) complex, which has been implicated in the maintenance of cristae shape and organization (23,24). A molecular basis for this observation is provided by an in vitro study by Rampelt et al., which showed that Mic10, a MICOS subunit, requires CL for its oligomerization, a process essential for mature MICOS complex formation (23). Thus, deficiency of CL could alter mitochondrial morphology by impairing MICOS function. Together, these observations point to the crucial role of CL in maintaining mitochondrial ultrastructure, which in turn is required for efficient respiratory chain functions (24).
Cardiolipin and mitochondrial dynamics:
Mitochondria exist as a network, which is maintained by two opposing processes: fusion and fission. Fusion involves the merging of two mitochondria, whereas fission involves division of mitochondria. Both of these processes are mediated by dynamin-related GTPases, including OPA1 for fusion (25), and DRP1 for fission (26). OPA1 is critical for fusion of the IMM, which is enriched in CL. A recent in vitro study showed that membrane fusion of OPA1-containing liposomes is dependent on the presence and abundance of CL (25). Interestingly, it was found that the presence of OPA1 in one liposome and a critical amount of CL in the other was sufficient to induce membrane fusion, indicating the novel finding that CL alone is capable of priming membranes for fusion (25). Importantly, the authors went on to show that the longer length and higher degree of unsaturation of CL acyl chains facilitated the fusion process, thus implicating a crucial role for CL remodeling by TAZ in the mitochondrial fusion process. Mgm1, a yeast homolog of OPA1, has also been shown to require CL for its dimerization and activation of GTPase activity for membrane fusion (27). Thus, CL has an evolutionarily conserved role in mitochondrial fusion.
Mitochondrial fission is mediated by Drp1, which requires CL for its recruitment onto the mitochondrial membranes and activation of its GTPase activity through lipid-protein interactions (28). Drp1 is recruited to a CL dense region of the OMM where CL molecules organize around the protein. This organization induces phase transition of the membrane from a bilayer arrangement to an inverted hexagonal non-bilayer configuration, resulting in localized OMM constrictions that lead to fission (28). A balance between fusion and fission is required to maintain mitochondrial homeostasis. Thus, an important role of CL in both of these processes suggests defects in mitochondrial dynamics are potential contributors to BTHS pathology.
Cardiolipin and mitochondrial respiratory chain biogenesis:
The mitochondrial respiratory chain (MRC) consists of four multimeric protein complexes (complex I-IV) that are integral to the IMM. The MRC couples respiration to the generation of a proton gradient, which is harnessed by ATP synthase to produce mitochondrial ATP. The MRC complexes assemble into supramolecular structures, known as supercomplexes, which increase their stability as well as efficiency. In yeast, MRC complexes III and IV associate in different stoichiometries, forming large (III2/IV2) and small (III2/IV) supercomplexes (29). In mammals, different combinations of individual MRC complexes can produce different supercomplexes, of which the cryo-electron microscopic structures of I/III2/IV and I/III2 have been determined (30). Blue native gel electrophoresis on mitochondria isolated from CL-deficient yeast cells demonstrated a critical requirement of CL for the stability of supercomplexes (31). Consistent with this role, supercomplex levels are decreased in the yeast model of BTHS and patient lymphoblasts (32,33).
Recent cryo-electron microscopy based structural studies have provided clues to the potential role of CL in supercomplex formation. CL, along with two abundant mitochondrial phospholipids PE and PC, have been found in purified yeast and mammalian supercomplexes (30,34). It has been proposed that these phospholipid molecules might occupy the space between the individual complexes to stabilize the supercomplex (30). Phospholipid quantification of the purified yeast III2/IV2 supercomplex revealed the presence of ~50 molecules of CL (34). Direct evidence for the role of CL in maintaining the stability of yeast supercomplexes came from a study by Wenz et al., which showed that mutation of a specific CL binding site in a yeast complex III subunit results in a loss of III2/IV2 supercomplex formation (35). The elimination of CL binding to complex III results in a weakened interaction with complex IV and the subsequent destabilization of the supercomplex. The authors suggest that this phenomenon arises primarily due to the charge sensitivity of supercomplex formation, because simultaneously mutating the adjacent positively charged CL-binding residues restores supercomplex assembly by neutralizing the loss of two negative charges contributed by CL (35). The in vitro reconstitution of yeast complexes III and IV into proteoliposomes exhibited an absolute requirement of CL for the formation of stable III2/IV and III2/IV2 supercomplexes (36). The presence of PE and PC alone in the proteoliposome was not sufficient for supercomplex formation, while the addition of CL alone restored the same (36). Recent in vivo studies in yeast have suggested that supercomplex formation is a function of the overall abundance of complex III and IV subunits (37). Thus, it is possible that the decreased abundance of supercomplexes in CL deficient cells is due to a reduced expression or enhanced turnover of individual MRC subunits. Notably, decreased translation of MRC complex IV subunit in CL deficient yeast cells has been reported (38) and forced overexpression of the same subunit restored supercomplex formation (37,39). Moreover, pharmacological stimulation of mitochondrial biogenesis also led to enhanced supercomplex formation in BTHS lymphoblasts (40).
A number of studies performed on mammalian systems have demonstrated a CL requirement for maintaining the steady state levels of individual MRC complexes. For example, complex I and IV levels were found to be lowered in BTHS patient lymphoblasts (33) and the abundance and activity of complex II is reduced in BTHS murine cardiomyocytes (41). Finally, a reduction in complex III activity was also reported in the mouse model of BTHS (41,42). Thus, CL deficiency results in a general disintegration of MRC structure and function.
Cardiolipin and mitochondrial bioenergetics:
CL has been shown to be essential for the efficient coupling of respiration to ATP synthesis, classically defined as the P/O ratio (amount of ATP formed per molecule of oxygen consumed). A complete loss of CL in yeast crd1Δ mitochondria led to reduced energetic coupling during fast respiration driven by NADH as the substrate (43). The same group later went on to show that a similar respiratory coupling defect was also present in CL-deficient taz1Δ cells (17). While oxidative phosphorylation was not severely impacted under optimal conditions, CL was required for efficient energy transformation under osmotic and temperature stress (43). In addition to the coupling defect, a reduction in the activity of complex V (ATP synthase) was observed in a BTHS mouse model (42). In yeast, the assembly and activity of the mitochondrial ATP/ADP carrier, AAC2, which mediates transport of ATP from the matrix to the intermembrane space in exchange for ADP, was shown to be highly dependent on CL (44). A subsequent study showed that yeast AAC2 also interacts with supercomplexes and that this interaction is impaired in yeast mitochondria lacking CL (45). Thus, impaired function of AAC2 might act synergistically with the disruption of MRC activity to compromise mitochondrial energy generation.
Mitochondria undergo swelling and shrinking during respiration. Yeast mitochondria lacking CL exhibits increased swelling as compared to wild type mitochondria when placed in a hypotonic solution, suggesting a possible role of CL in maintaining mitochondrial osmotic stability (17,43). Interestingly, CL deficient mitochondria also lose their ability to shrink back to their original size when returned to an isotonic medium, suggesting that CL deficient mitochondrial membranes lose their elasticity. Deterioration of CL-dependent biophysical properties of the IMM also likely contributes to membrane “leakiness,” leading to decreased membrane potential and overall efficiency of mitochondrial energy generation. Thus, CL deficiency impairs mitochondrial bioenergetics, which perturbs the function of high energy demanding tissues specifically affected in BTHS, such as the heart and skeletal muscles. Recent advances in tools and technologies to simultaneously assess different mitochondrial bioenergetic functions, such as the extracellular flux analyzer (Agilent Technologies) and high-resolution respirometry (Oroboros Instruments), may facilitate higher resolution mapping of CL-dependent mitochondrial bioenergetic functions.
Cardiolipin and oxidative stress:
Reactive oxygen species (ROS) are potentially toxic by-products of oxidative metabolism in aerobic organisms generated by aberrant transfer of electrons to oxygen to form superoxide radicals and other reactive intermediates. Mitochondria are one of the major sources of ROS owing to the oxidative metabolism that takes place in this organelle, and MRC complexes I and III have been shown to be the principal sites of ROS generation. In wild type mitochondria, the assembly of MRC supercomplexes is proposed to confine the movement of electrons within the MRC and minimize their escape into the surrounding membrane, thereby reducing the generation of ROS. CL deficiency-mediated destabilization of supercomplexes in the yeast model of BTHS, taz1Δ, leads to increased protein carbonylation, suggesting an increase in the steady state levels of ROS (46). Increased protein carbonylation could be the result of an increase in ROS production or a decrease in the antioxidant defense systems. Taz1 deficient yeast cells do not exhibit enhanced sensitivity to various oxidative insults, suggesting that the likely cause for oxidative damage is increased ROS production and not decreased ROS quenching mechanisms (46). A similar increase in mitochondrial ROS levels was also observed in mammalian cellular BTHS models (47,48). Thus, increased ROS is one of the biochemical hallmarks of BTHS.
Cardiolipin and intermediary metabolism:
Until recently, studies uncovering various CL-dependent mitochondrial dysfunctions have mostly been targeted towards mitochondrial structure and bioenergetics, often overlooking mitochondrial and cellular metabolites. Recent lipidomic and metabolomic studies on different BTHS models have identified perturbations in the levels of both polar and lipid metabolites. Lipidomics on the TAZ-KD mouse model of BTHS showed alterations in the acyl chain compositions of other abundant mitochondrial phospholipids, especially PC and PE (42). The overall distribution of linoleic (18:2) and arachidonic (20:4) fatty acyl chains were altered such that the incorporation of linoleic and arachidonic acids was more favored in PC and arachidonic acids into PE (42). The authors also reported perturbation in the generation of oxidized derivatives of unsaturated fatty acids, which have been implicated to act as signaling molecules in the maintenance of calcium homeostasis, mitokine signaling, inflammation, and vascular regulation. In the same BTHS mouse model, eicosanoid metabolism from arachidonic acid was disrupted with an increase in the levels of hydroxyeicosatetraenoic acids (HETE), including 5- and 11-HETE, and a decrease in 15-HETE. Moreover, prostaglandin synthesis was upregulated with an increase in the levels of PGE2, PGF1α, PGF2α, and TXB2 in the BTHS mice (42). In addition, this study also found a shift in mitochondrial metabolism with regards to substrate utilization. L-palmitoyl carnitine or succinate-stimulated oxidation was reduced whereas glutamate-stimulated oxidation was increased, pointing towards an impairment in fatty acid oxidation in BTHS mice mitochondria (42). Genome-wide transcriptional profiling of cardiac mitochondria from the BTHS mice identified genes in metabolic pathways that are likely perturbed. For example, genes involved in amino acid synthesis, protein translation, and GTP hydrolysis were upregulated, whereas genes involved in the catabolism of branched chain amino acids (leucine, isoleucine, and valine) were downregulated (42). Consistent with the perturbation in the expression of genes involved in amino acid metabolism in TAZ-KD mice, Sandlers et al. found alterations in the amino acid profile in plasma from BTHS patients (49). Specifically, they showed an increase in proline and tyrosine and a decrease in arginine levels (49). Although the mechanism for increased levels of proline in BTHS in not known, a defect in mitochondrial proline uptake could explain the observed decrease in arginine levels because proline contributes to arginine biosynthesis. In a recent study, glucose uptake and triacylglycerol synthesis were found to be increased in BTHS patient lymphoblasts compared to age-matched controls (50). Higher glucose utilization is suggested to be a compensatory mechanism for energy generation in BTHS mitochondria where mitochondrial ATP synthesis is attenuated (50). Since metabolites directly determine the cellular and organismal physiology, a more exhaustive metabolomics study may uncover novel pathways that contribute to BTHS pathophysiology and provide targets for pharmacological interventions.
Cardiolipin and mitochondrial protein import:
The majority of mitochondrial proteins are encoded by the nuclear genome, biosynthesized as precursors in the cytosol, and are subsequently imported into the mitochondria. Highly sophisticated mitochondrial protein import machineries are present in both the mitochondrial membranes. Translocase of the Outer Membrane (TOM) acts as the main entry point for all mitochondrial proteins. The OMM contains two classes of import proteins - α-helical and β-barrel proteins. Once through the TOM complex, β-barrel proteins of the OMM need to pass through the Sorting and Assembly Machinery (SAM) for their correct integration. In a yeast model of BTHS, both TOM and SAM have been shown to require CL for their stability and function (51). Consistent with this result, the authors found a reduction in the assembly kinetics of β-barrel containing proteins in BTHS patient lymphoblasts, indicating that requirement of CL for mitochondrial protein import is evolutionarily conserved (51). It has been shown that in crd1Δ mitochondria, the association of the Tom20 receptor with the core of the TOM complex is weakened, which results in reduced stability of the TOM complex (51). The authors also report a decreased stability and function of the SAM complex in crd1Δ mitochondria, resulting in impaired β-barrel protein integration into the OMM. The CL-dependent role of the TOM and SAM complexes is further highlighted by the fact that the majority of double deletion mutants of TOM/SAM proteins with Crd1/Taz1 proteins exhibit synthetic sickness, with tom5Δcrd1Δ and tom5Δtaz1Δ being synthetic lethal (51). Interestingly, the import of α-helical proteins was found to be largely unaffected by the loss or deficiency of CL. It is also important to note that mitochondrial protein import is dependent on the mitochondrial membrane potential; thus, CL deficient cells with low membrane potential have an indirect impairment of their protein import machinery (44).
Cardiolipin in mitochondrial metal homeostasis:
Fe-S clusters are ubiquitously present in cells as cofactors for several biochemical reactions, including those of electron transport and the TCA cycle in the mitochondria. Several key mitochondrial enzymes, including MRC complexes I, II, III, and aconitase, require Fe-S cofactors to function. The role of CL in Fe-S cluster biogenesis came from a genome-wide expression profiling of CL-deficient crd1Δ yeast cells (52). The genes responsible for cellular iron uptake were highly upregulated in CL deficient mutants, leading to increased mitochondrial iron content. Perturbation in mitochondrial iron homeostasis was accompanied by Fe-S cluster biogenesis defects that were inferred from synthetic genetic interactions between CRD1 and ISU1, a mitochondrial Fe-S scaffolding protein, as well as a decrease in the activity of many mitochondrial and cytosolic Fe-S-containing proteins, including aconitase, succinate dehydrogenase (MRC complex II), sulfite reductase and isopropylmalate isomerase (52). While this study identifies a novel role of CL in Fe-S cluster biogenesis, at present it is not clear how CL deficiency stimulates mitochondrial iron accumulation and upregulation of iron uptake genes. Nevertheless, owing to the critical role of Fe-S cofactors in a variety of cellular processes, the relationship between CL and iron metabolism needs to be further investigated in different BTHS models.
Cardiolipin and mitochondrial quality control:
Quality control mechanisms act at the organellar and molecular levels to monitor and maintain mitochondrial integrity. Damaged mitochondria are removed from cells by mitophagy, a specialized form of autophagy, which ensures that mitochondrial quality in the cells is not compromised. In order to be incorporated into autophagosomes, mitochondria need to be separated from the reticular network through mitochondrial fission. As mentioned in a previous section, mitochondrial fission is dependent on CL, thus highlighting an important role of CL in the initiation of mitophagy. Exciting evidence for the involvement of CL in mitophagy came from Chu and colleagues who showed that externalization of CL to the OMM of neurons serves as a signal for mitophagy (53). It was shown that externalized CL on the OMM is recognized by microtubule associated protein-1 light chain 3 (LC3), which promotes engulfment of mitochondria by autophagosomes. A recent study showed that CL deficiency perturbs mitophagy in yeast cells via decreased activation of the protein kinase C pathway, suggesting an evolutionarily conserved role of CL in mitophagy (54). Mitophagy defects in BTHS mitochondria were recently uncovered by Hsu et al., who showed that mitophagosome biogenesis was defective in CL-depleted TAZ-KD mouse embryonic fibroblasts (55). An accumulation of swollen and dysfunctional mitochondria in BTHS cells is suggestive of defective mitochondrial quality control; however, the contribution of mitophagy to BTHS pathology has not yet been investigated (1). Mitophagy has been proposed to be crucial for cardiac functions under stress conditions (56); therefore, it is possible that the cardiac defects in BTHS are caused at least in part by defective mitophagy.
Mitochondrial AAA proteases play a key role in mitochondrial proteostasis and quality control by regulating the biogenesis of mitochondrial proteins and ensuring selective turnover of misfolded proteins (57). Recently, the role of an i-AAA protease, Yme1, in BTHS pathology was identified through a genetic screen in yeast (58). There were pronounced mitochondrial ultrastructural and mitophagy defects in cells lacking both Yme1 and Taz1, which led to reduced respiratory growth and increased sensitivity to oxidative stress (58). Thus, this study highlighted a possible role of CL in maintaining mitochondrial proteostasis.
BARTH SYNDROME MODELS
Pioneering studies in a simple eukaryotic yeast model of BTHS have uncovered many key mitochondrial defects, as described in previous sections. However, yeast cells do not exhibit the complexity of mammalian cells and cannot be used to study tissue and organ specific defects observed in BTHS patients. Over the last decade, multiple invertebrate and vertebrate models of BTHS, including the fly, fish, and mouse, have been developed. All these available model systems display the characteristic biochemical defects underlying BTHS (Table 2), including reduced levels of mitochondrial CL, increased MLCL, and altered acyl chain composition of CL. The availability of these model organisms, which faithfully recapitulate many of the biochemical defects observed in BTHS patients (Table 2), have proved critical in developing our understanding of BTHS pathology and they could also serve as useful tools in developing therapeutics for BTHS.
Table 2.
BTHS-associated mitochondrial dysfunctions in different BTHS disease models. Data taken from Refs. 32, 46, 54, 43, 20, 11, 62, 63, and 42.
| Model organism | Mitochondrial defects |
|---|---|
| Yeast (Saccharomyces cerevisiae) | ○ Reduced stability of MRC supercomplexes ○ Increased oxidative stress ○ Reduced mitophagy ○ Partial uncoupling ○ Reduced osmotic stability |
| Fly (Drosophila melanogaster) | ○ Abnormal mitochondrial morphology in flight muscles ○ Swelling and disruption of cristae membrane ○ Reduced ATP synthase dimer rows in cristae membrane |
| Mouse (Mus musculus) | ○ Abnormal mitochondrial morphology ○ Reduced cristae density ○ Disruption of the parallel alignment between mitochondria and sarcomeres ○ Reduced MRC supercomplex levels in cardiac mitochondria ○ Reduced MRC complex II levels and activity in cardiac mitochondria |
Cellular and organoid models of BTHS:
Many mammalian cellular models are available for BTHS including patient-derived lymphoblasts (33), fibroblasts (59), and induced-Pluripotent Stem Cells (iPSCs) (48,60). Recently, a CRISPR-mediated stable TAZ-KO mouse C2C12 myoblast cell line has also been generated (47). Abnormal mitochondrial morphology is observed in patient-derived fibroblasts (59) and BTHS iPSC cardiomyocytes (60). A hallmark of mitochondrial CL deficiency is the loss of MRC supercomplexes. Consistent with this role of CL, studies with patient-derived BTHS lymphoblasts and BTHS iPSCs from dermal fibroblasts showed reduced stability of MRC supercomplexes containing complex I, III, and IV (33,48). Another common feature observed in multiple cellular models of BTHS is increased oxidative stress (48). TAZ-KO in mouse C2C12 myoblasts displayed mitochondrial defects consistent with other models of BTHS and was associated with impairment of myocyte differentiation to myotubes, which may explain the skeletal myopathy observed in BTHS patients (47).
Advances in the study of cardiac disorders are restricted by the lack of available organoid models. The invasive nature of the methods used to obtain cardiac tissue is a major obstacle for research in this area. However, Wang et al. developed a heart-on-chip model of BTHS (60). In this organoid model system, the BTHS iPSC tissues developed a significantly lower twitch and peak systolic stress, suggesting reduced contractile functions compared to the control tissue, indicating that engineered myocardial tissue recapitulates the BTHS myopathic phenotype. Analysis of mitochondrial function in this model is required to assess the role of mitochondrial bioenergetics on various physiological phenotypes reported.
Drosophila model of BTHS:
Drosophila is a very useful model system for BTHS because it has specialized flight muscles with abundant mitochondria and, like humans, it expresses several TAZ transcripts (20). The Drosophila model of BTHS was constructed by the imprecise excision of a P element inserted upstream of the coding region of the TAZ gene, which caused similar changes in CL species profile as seen in Barth syndrome patients (20). BTHS flies showed reduced locomotor activity, and their flight muscles displayed frequent mitochondrial abnormalities, mostly in the cristae membranes (20). In a subsequent study, the same group found that TAZ deficiency in Drosophila disrupts the final stage of spermatogenesis- spermatid individualization, causing male sterility (9). Interestingly, it was found that male sterility in TAZ-deficient flies can be genetically suppressed by the inactivation of a calcium-independent phospholipase, iPLA2-VIA, which prevents MLCL accumulation, restoring the MLCL/CL ratio (9).
Zebrafish model of BTHS:
Zebrafish embryos offer a powerful model for studying pediatric disorders and are particularly suitable for studying infantile hypertrophic cardiomyopathy because: 1) they are transparent and develop outside of the mother, enabling observation of organ development; 2) they do not require a functional cardiovascular system for the first 4-5 days post-fertilization, as their small size allows sufficient passive oxygen diffusion; and 3) compared to mouse models, zebrafish are inexpensive and knockdowns and knockouts can be easily generated and titrated. The zebrafish BTHS model was generated by antisense morpholino-based knockdown of Taz protein (61). taz-KD zebrafish embryos exhibit severe bradycardia, pericardial effusions, and generalized edema that resembles cardiac failure in BTHS patients. In this study, mitochondrial bioenergetic functions were not measured; therefore, it is difficult to assign cardiac defects to mitochondrial dysfunction. However, mitochondrial dysfunction in zebrafish does give rise to a similar cardiac phenotype as described in BTHS zebrafish (61); therefore, it is likely that the cardiac defects are caused by a disruption in CL-dependent mitochondrial functions. In the future, a stable CRISPR based taz-KO model of zebrafish could prove valuable in understanding the developmental defects observed in BTHS.
Mouse model of BTHS:
A doxycycline-induced shRNA-mediated TAZ-KD mouse recapitulates many of the disease phenotypes of BTHS, including cardiomyopathy and skeletal muscle myopathy (11,41,62,63). These studies were performed on the same mouse model, but they differed in the dosage, timing, and mode of doxycycline administration. Two independent studies by Acehan et al. (11) and Soustek et al. (62) reported initial characterization of inducible shRNA TAZ-KD mice. Mice were administered doxycycline in their diets pre- and post-natally. Remarkably, these mice did not exhibit an overt phenotype until much later in life. Soustek et al. reported skeletal muscle defects in 2-month old TAZ-KD mice, but cardiac defects were apparent only after 7-10 months of age. Similarly, Acehan et al. also observed mitochondrial defects in skeletal muscle at 2 months of age, and cardiac mitochondrial defects became evident at 8 months. Both of these studies reported defects in cardiac function, including left ventricular dilation, left ventricular mass reduction, and ejection fraction in TAZ-KD mice. In a study by Phoon et al., pregnant dams were treated with doxycycline early in gestation leading to the knockdown of TAZ in the embryonic stage. These mice exhibited increased pre- and post-natal death, suggesting that the mode of delivery of doxycycline has a major impact on the severity of phenotype, because unlike previous studies, where doxycycline was administered in chow, in this study doxycycline was administered in water (63). Electron microscopy of cardiac tissue from these mice demonstrated ultrastructural abnormalities in mitochondria at both embryonic and newborn stages. Early diastolic dysfunction was evident from echocardiography studies. Histological examination of embryonic day 13.5 and newborn TAZ-KD mice showed myocardial thinning, hypertrabeculation and noncompaction, and defective ventricular septation (63). A more recent report by Dudek et al. identified reduced MRC supercomplexes at a pre-onset stage of the disease in the TAZ-KD mice. Moreover, their analyses demonstrated a cardiac-specific loss of succinate dehydrogenase (MRC complex II) in mouse and patient cell-derived cardiomyocytes (41). Notably, these MRC defects were not observed in mitochondria isolated from kidneys and livers of age-matched TAZ-KD mice, suggesting that MRC complex II dysfunction is an important contributor to BTHS-associated cardiomyopathy (41).
Recently, TAZ-KO mice have also been generated, which display reproductive defects in males; the testes from the TAZ-KO mice were smaller than their control counterparts, and this was associated with a disruption of spermatogenesis by meiosis (64). Spermatocytes in the mutant animals failed to progress past the pachytene stage of meiosis, had higher levels of DNA double strand damage, and mutant males were infertile, a phenotype similar to the Drosophila model of BTHS (20). Together, these data reveal an evolutionarily conserved role of TAZ in male fertility. The results from various BTHS models indicate that CL-dependent mitochondrial functions are critical for heart and muscle development and function. The usefulness of the mouse models of BTHS lies in the fact that in TAZ-depleted mice, the cardiac defects observed reproduce many of the relevant cardiac parameters noted in BTHS patients, providing an excellent opportunity to test potential therapeutic targets in a mammalian model system.
FUTURE PERSPECTIVES
Despite the tremendous increase in our understanding of CL metabolism and its role in mitochondrial physiology, currently no effective treatment exists for BTHS. There is an urgent unmet need to develop therapeutics for the treatment of BTHS. The primary defect in BTHS is altered CL content and acyl species composition; therefore, mitochondrial membrane phospholipid composition is an obvious target for correcting the pathophysiology associated with BTHS. Unfortunately, there is no obvious solution to replicating TAZ function in BTHS patients short of gene therapy. Because perturbation in CL results in wide-ranging impacts on mitochondrial function, the successes of therapeutic interventions likely depends on the ability to correct many, if not all of these mitochondrial dysfunctions.
Past attempts to rectify a particular mitochondrial defect among all the CL-associated mitochondrial defects have not improved organismal phenotypes. For example, alleviating oxidative stress, one of the hallmarks of BTHS, has been targeted as a potential therapeutic strategy, but has not been effective when tested on BTHS organismal models (65). Valianpour et al. first suggested that increasing the amount of linoleic acid in the diet might be beneficial for patients with BTHS (66). This idea was tested recently on the BTHS iPSC cardiomyocyte disease model, where it was found that linoleic acid and other mitochondria targeted anti-oxidants could rescue the defect in the organization and biophysical characteristics of sarcomeres (60). While promising, the results from this in vitro study did not replicate in a mouse model of BTHS, where cardiomyopathy and skeletal muscle myopathy phenotypes of catalase-overexpressing TAZ-KD mice did not improve, even though oxidative stress was reduced in them (65). These findings indicate that resolving oxidative stress is not sufficient to suppress cardioskeletal myopathy associated with BTHS. The role and regulation of ROS in BTHS needs to be carefully considered in light of a recent study showing that under hypoxic conditions ROS levels are reduced in TAZ-KO MEFs and BTHS patient-derived iPSC cardiomyocytes, which leads to decreased transcription of HIF-1α and its target genes (67). The transcriptional downregulation of HIF-1α was also observed in the TAZ-KD BTHS mouse model, which undergo maladaptive hypertrophy in response to pressure overload challenge. This study points to an unexpected role of HIF-1α in BTHS-associated cardiac defects and represents a potential therapeutic target.
A promising strategy emerged from the finding that supramolecular assembly of MRC complexes can be stimulated by bezafibrate treatment in a mouse model of BTHS (68). Indeed, Xu et al. were able to demonstrate an increase in supercomplex assembly in patient B-lymphoblasts and, interestingly, the increase in supercomplex levels was accompanied by a reduced turnover of CL and a decrease in MLCL (40). In an in vivo study, bezafibrate was found effective in substantially ameliorating the development of left ventricular systolic dysfunction in TAZ-KD mice that were exposed to beta-adrenergic stress (68). Without beta-adrenergic stress, TAZ-KD mice develop dilated cardiomyopathy much later in life, around the age of 7 months. These mice underwent prolonged treatment with a supra-pharmacological dose of bezafibrate (0.5% w/w in rodent diet) over a 4-month period that effectively prevented left ventricular dilation. Surprisingly, these positive outcomes of bezafibrate treatment were associated with increased oxidative stress in cardiomyocytes. Moreover, improvement of systolic function in bezafibrate-treated mice was accompanied with simultaneous reduction of CL content and increase of MLCL levels in cardiac muscle (68). Therefore, at this moment, it is difficult to realize the therapeutic potential of bezafibrate in ameliorating mitochondrial pathophysiology in BTHS patients.
Recent studies from the yeast model system have shown that it is possible to genetically or nutritionally enhance supercomplex formation in CL deficient cells without altering the levels of CL. Forced overexpression of MRC complex III and IV subunits, including Rip1, Cox4, and Cox5A, or Odc1, a conserved IMM oxodicarboxylic acid carrier, stabilized MRC supercomplexes in CL deficient yeast cells (37,69). In addition, studies from our group found that a small molecule metabolite, ethanolamine, can ameliorate mitochondrial defects in a yeast model of BTHS by increasing the expression of MRC subunits and promoting MRC supercomplex assembly (39). Interestingly, a partial inhibition of cytosolic protein synthesis also fully restored MRC supercomplex levels and the activity of the oxidative phosphorylation system in a yeast model of BTHS (70). These results identify protein translation machinery as a potential therapeutic target for the treatment of BTHS.
A number of groups focused on preventing the accumulation of MLCL as a strategy to combat mitochondrial dysfunctions in BTHS. For example, inactivation of iPLA2-VIA, the gene encoding a calcium-independent phospholipase, rescues the male sterility defect in TAZ-deficient flies by preventing CL depletion and MLCL accumulation (9). Furthermore, the same study showed that treatment of BTHS patient lymphoblasts in tissue culture with a phospholipase inhibitor, bromoenol lactone, partially restored CL homeostasis. Although such genetic interventions or treatment with a non-specific phospholipase inhibitor like bromoenol lactone are not feasible on patients, these studies identify CL-specific phospholipase as a potential target for therapeutic intervention. In an orthogonal approach, Mejia and colleagues utilized TAZ-independent remodeling ability of monolysocardiolipin acyl transferase 1 (MLCL AT-1) to suppress mitochondrial defects in BTHS patient lymphoblasts (71). Transfection of BTHS lymphoblasts with an MLCL AT-1 overexpression construct increased CL levels, improved mitochondrial basal respiration, and decreased the proportion of cells producing superoxides. However, CL molecular species composition and MRC supercomplex assembly were not restored in MLCL AT-1 overexpressing cells. This interesting finding provides a potential avenue for the treatment of BTHS.
The fine resolution mapping of metabolic perturbations emanating from TAZ mutation is still lacking, which may discover new biochemical pathways that underlie some of the clinical phenotypes. For example, the biochemical basis of neutropenia, which is typically observed in BTHS patients, is still poorly understood (2). The increased understanding of various mitochondrial defects in CL-deficient cells combined with the availability of multiple BTHS model systems have placed us in an excellent position to develop therapeutic approaches to treat this rare but often debilitating genetic disorder.
ACKNOWLEDGMENTS
We thank John Neff, Alison Vicary, and Constanze Rowe for their valuable comments in the preparation of this manuscript. This work was supported by the Welch Foundation Grant (A-1810) and the National Institutes of Health award (R01GM111672) to V.M.G. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations:
- ATP
Adenosine triphosphate
- MRC
Mitochondrial respiratory chain
- CDP-DAG
Cytidinediphospho-diacylglycerol
- CL
Cardiolipin
- MLCL
monolysocardiolipin
- PE
Phosphatidylethanolamine
- PA
Phosphatidic acid
- PC
Phosphatidylcholine
- PG
Phosphatidylglycerol
- BTHS
Barth syndrome
REFERENCES
- 1.Barth PG, Scholte HR, Berden JA, Van der Klei-Van Moorsel JM, Luyt-Houwen IE, et al. (1983) An X-linked mitochondrial disease affecting cardiac muscle, skeletal muscle and neutrophil leucocytes. J. Neurol. Sci. 62, 327–355. [DOI] [PubMed] [Google Scholar]
- 2.Clarke SL, Bowron A, Gonzalez IL, Groves SJ, Newbury-Ecob R, et al. (2013) Barth syndrome. Orphanet. J. Rare Dis. 8, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bione S, D’Adamo P, Maestrini E, Gedeon AK, Bolhuis PA, et al. (1996) A novel X-linked gene, G4.5. is responsible for Barth syndrome. Nat. Genet. 12, 385–389. [DOI] [PubMed] [Google Scholar]
- 4.Xu Y, Malhotra A, Ren M, and Schlame M (2006) The enzymatic function of tafazzin. J. Biol. Chem. 281, 39217–39224. [DOI] [PubMed] [Google Scholar]
- 5.Schlame M, Towbin JA, Heerdt PM, Jehle R, DiMauro S, et al. (2002) Deficiency of tetralinoleoyl-cardiolipin in Barth syndrome. Ann. Neurol. 51, 634–637. [DOI] [PubMed] [Google Scholar]
- 6.Gaspard GJ, McMaster CR (2015) Cardiolipin metabolism and its causal role in the etiology of the inherited cardiomyopathy Barth syndrome. Chem. Phys. Lipids. 193, 1–10. [DOI] [PubMed] [Google Scholar]
- 7.Blumson NJ, Gomez-Espinosa E, Ashlin TG, and Cockcroft S (2018) Mitochondrial CDP-diacylglycerol synthase activity is due to the peripheral protein, TAMM41 and not due to the integral membrane protein, CDP-diacylglycerol synthase 1. Biochim. Biophys. Acta, Mol. Cell. Biol. Lipids. 1863, 284–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beranek A, Rechberger G, Knauer H, Wolinski H, Kohlwein SD, et al. (2009) Identification of a Cardiolipin-specific Phospholipase Encoded by the Gene CLD1 (YGR110W) in Yeast. J. Biol. Chem. 284, 11572–11578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malhotra A, Edelman-Novemsky I, Xu Y, Plesken H, Ma J, et al. (2009) Role of calcium-independent phospholipase A2 in the pathogenesis of Barth syndrome. Proc Natl Acad Sci U S A. 106, 2337–2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gu Z, Valianpour F, Chen S, Vaz FM, Hakkaart GA, et al. (2004) Aberrant cardiolipin metabolism in the yeast taz1 mutant: a model for Barth syndrome. Mol. Microbiol. 51, 149–158. [DOI] [PubMed] [Google Scholar]
- 11.Acehan D, Vaz F, Houtkooper RH, James J, Moore V, et al. (2011) Cardiac and skeletal muscle defects in a mouse model of human Barth syndrome. J. Biol. Chem. 286, 899–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ye C, Lou W, Li Y, Chatzispyrou IA, Hüttemann M, et al. (2014) Deletion of the cardiolipin-specific phospholipase Cld1 rescues growth and life span defects in the tafazzin mutant: implications for Barth syndrome. J. Biol. Chem. 289, 3114–3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baile MG, Sathappa M, Lu YW, Pryce E, Whited K, et al. (2014) Unremodeled and remodeled cardiolipin are functionally indistinguishable in yeast. J. Biol. Chem. 289, 1768–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bowron A, Honeychurch J, Williams M, Tsai-Goodman B, Clayton N, et al. (2015) Barth syndrome without tetralinoleoyl cardiolipin deficiency: a possible ameliorated phenotype. J. Inherit. Metab. Dis. 38, 279–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnston J, Kelley RI, Feigenbaum A, Cox GF, Iyer GS, et al. (1997) Mutation characterization and genotype-phenotype correlation in Barth syndrome. Am. J. Hum. Genet. 61, 1053–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whited K, Baile MG, Currier P, and Claypool SM (2013) Seven functional classes of Barth syndrome mutation. Hum. Mo.l Genet. 22, 483–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma L, Vaz FM, Gu Z, Wanders RJ, and Greenberg ML (2004) The human TAZ gene complements mitochondrial dysfunction in the yeast taz1Delta mutant. Implications for Barth syndrome. J. Biol. Chem. 279, 44394–44399. [DOI] [PubMed] [Google Scholar]
- 18.Ronvelia D, Greenwood J, Platt J, Hakim S, and Zaragoza MV (2012) Intrafamilial variability for novel TAZ gene mutation: Barth syndrome with dilated cardiomyopathy and heart failure in an infant and left ventricular noncompaction in his great-uncle. Mol. Genet. Metab. 107, 428–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bissler JJ, Tsoras M, Göring HH, Hug P, Chuck G, et al. (2005) Infantile dilated X-linked cardiomyopathy, G4.5 mutations, altered lipids, and ultrastructural malformations of mitochondria in heart, liver, and skeletal muscle. Lab Invest. 82, 335–344 [DOI] [PubMed] [Google Scholar]
- 20.Xu Y, Condell M, Plesken H, Edelman-Novemsky I, Ma J, et al. (2006) A Drosophila model of Barth syndrome. Proc. Natl. Acad. Sci. U S A 103, 11584–11588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Basu Ball W, Neff JK, and Gohil VM (2017) The role of nonbilayer phospholipids in mitochondrial structure and function. FEBS Lett. 592, 1273–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gohil VM, Thompson MN, and Greenberg ML (2005) Synthetic lethal interaction of the mitochondrial phosphatidylethanolamine and cardiolipin biosynthetic pathways in Saccharomyces cerevisiae. J. Biol. Chem. 280, 35410–35416. [DOI] [PubMed] [Google Scholar]
- 23.Rampelt H, Wollweber F, Gerke C, de Boer R, van der Klei IJ, et al. (2018) Assembly of the Mitochondrial Cristae Organizer Mic10 Is Regulated by Mic26-Mic27 Antagonism and Cardiolipin. J. Mol. Biol. 430, 1883–1890. [DOI] [PubMed] [Google Scholar]
- 24.Friedman JR, Mourier A, Yamada J, McCaffery JM, and Nunnari J (2015) MICOS coordinates with respiratory complexes and lipids to establish mitochondrial inner membrane architecture. Elife. 28, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ban T, Ishihara T, Kohno H, Saita S, Ichimura A, et al. (2017) Molecular basis of selective mitochondrial fusion by heterotypic action between OPA1 and cardiolipin. Nat. Cell Biol. 19, 856–863. [DOI] [PubMed] [Google Scholar]
- 26.Francy CA, Clinton RW, Fröhlich C, Murphy C, and Mears JA (2017) Cryo-EM Studies of Drp1 Reveal Cardiolipin Interactions that Activate the Helical Oligomer. Sci. Rep. 7, 10744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DeVay RM, Dominguez-Ramirez L, Lackner LL, Hoppins S, Stahlberg H, et al. (2009) Coassembly of Mgm1 isoforms requires cardiolipin and mediates mitochondrial inner membrane fusion. J. Cell Biol. 186, 793–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stepanyants N, Macdonald PJ, Francy CA, Mears JA, Qi X, et al. (2015) Cardiolipin’s propensity for phase transition and its reorganization by dynamin-related protein 1 form a basis for mitochondrial membrane fission. Mol. Biol. Cell. 26, 3104–3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schägger H and Pfeiffer K (2000) Supercomplexes in the respiratory chains of yeast and mammalian mitochondria. EMBO J. 19, 1777–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu M, Gu J, Guo R, Huang Y, and Yang M (2016) Structure of Mammalian Respiratory Supercomplex I1III2IV1. Cell. 167, 1598–1609. [DOI] [PubMed] [Google Scholar]
- 31.Pfeiffer K, Gohil V, Stuart RA, Hunte C, Brandt U, et al. (2003) Cardiolipin stabilizes respiratory chain supercomplexes. J. Biol. Chem. 278, 52873–52880. [DOI] [PubMed] [Google Scholar]
- 32.Brandner K, Mick DU, Frazier AE, Taylor RD, Meisinger C, et al. (2005) Taz1, an outer mitochondrial membrane protein, affects stability and assembly of inner membrane protein complexes: implications for Barth Syndrome. Mol. Biol. Cell. 16, 5202–5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McKenzie M, Lazarou M, Thorburn DR, and Ryan MT (2006) Mitochondrial respiratory chain supercomplexes are destabilized in Barth Syndrome patients. J. Mol. Biol. 361, 462–469. [DOI] [PubMed] [Google Scholar]
- 34.Mileykovskaya E, Penczek PA, Fang J, Mallampalli VK, Sparagna GC, et al. (2012) Arrangement of the respiratory chain complexes in Saccharomyces cerevisiae supercomplex III2IV2 revealed by single particle cryo-electron microscopy. J. Biol. Chem. 287, 23095–23103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wenz T, Hielscher R, Hellwig P, Schägger H, Richers S, et al. (2009) Role of phospholipids in respiratory cytochrome bc(1) complex catalysis and supercomplex formation. Biochim. Biophys. Acta. 1787, 609–616. [DOI] [PubMed] [Google Scholar]
- 36.Bazán S, Mileykovskaya E, Mallampalli VK, Heacock P, Sparagna GC, et al. (2013) Cardiolipin-dependent reconstitution of respiratory supercomplexes from purified Saccharomyces cerevisiae complexes III and IV. J. Biol. Chem. 288, 401–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cui TZ, Conte A, Fox JL, Zara V, and Winge DR (2014) Modulation of the respiratory supercomplexes in yeast: enhanced formation of cytochrome oxidase increases the stability and abundance of respiratory supercomplexes. J. Biol. Chem, 289, 6133–6141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Su X, and Dowhan W (2006) Translational regulation of nuclear gene COX4 expression by mitochondrial content of phosphatidylglycerol and cardiolipin in Saccharomyces cerevisiae. Mol. Cell Biol. 26, 743–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Basu Ball W, Baker CD, Neff JK, Apfel GL, Lagerborg KA, et al. (2018) Ethanolamine ameliorates mitochondrial dysfunction in cardiolipin-deficient yeast cells. J. Biol. Chem. 293, 10870–10883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu Y, Phoon CK, Berno B, D’Souza K, Hoedt E, et al. (2016) Loss of protein association causes cardiolipin degradation in Barth syndrome. Nat. Chem. Biol. 12, 641–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dudek J, Cheng IF, Chowdhury A, Wozny K, Balleininger M, et al. (2016). Cardiac-specific succinate dehydrogenase deficiency in Barth syndrome. EMBO Mol. Med, 8, 139–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kiebish MA, Yang K, Liu X, Mancuso DJ, Guan S, et al. (2013) Dysfunctional cardiac mitochondrial bioenergetic, lipidomic, and signaling in a murine model of Barth syndrome. J. Lipid Res. 54, 1312–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koshkin V, and Greenberg ML (2002) Cardiolipin prevents rate-dependent uncoupling and provides osmotic stability in yeast mitochondria. Biochem. J. 364, 317–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jiang F, Ryan MT, Schlame M, Zhao M, Gu Z, et al. (2000) Absence of cardiolipin in the crd1 null mutant results in decreased mitochondrial membrane potential and reduced mitochondrial function. J. Biol. Chem. 275, 22387–22394. [DOI] [PubMed] [Google Scholar]
- 45.Claypool SM, Oktay Y, Boontheung P, Loo JA, and Koehler CM (2008) Cardiolipin defines the interactome of the major ADP/ATP carrier protein of the mitochondrial inner membrane. J. Cell Biol. 182, 937–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen S, He Q, and Greenberg ML (2008) Loss of tafazzin in yeast leads to increased oxidative stress during respiratory growth. Mol. Microbiol. 68, 1061–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lou W, Reynolds CA, Li Y, Liu J, Hüttemann M, et al. (2018). Loss of tafazzin results in decreased myoblast differentiation in C2C12 cells: A myoblast model of Barth syndrome and cardiolipin deficiency. Biochim. Biophys. Acta, 1863, 857–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dudek J, Cheng IF, Balleininger M, Vaz FM, Streckfuss-Bomeke K, et al. (2013). Cardiolipin deficiency affects respiratory chain function and organization in an induced pluripotent stem cell model of Barth syndrome. Stem Cell Res. 11, 806–819. [DOI] [PubMed] [Google Scholar]
- 49.Sandlers Y, Mercier K, Pathmasiri W, Carlson J, McRitchie S, et al. (2016) Metabolomics Reveals New Mechanisms for Pathogenesis in Barth Syndrome and Introduces Novel Roles for Cardiolipin in Cellular Function. PLoS One. 11:e0151802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mejia EM, Zinko JC, Hauff KD, Xu FY, Ravandi A, et al. (2017) Glucose Uptake and Triacylglycerol Synthesis Are Increased in Barth Syndrome Lymphoblasts. Lipids. 52, 161–165. [DOI] [PubMed] [Google Scholar]
- 51.Gebert N, Joshi AS, Kutik S, Becker T, McKenzie M, et al. (2009) Mitochondrial cardiolipin involved in outer-membrane protein biogenesis: implications for Barth syndrome. 23. Curr. Biol. 19, 2133–2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Patil VA, Fox JL, Gohil VM, Winge DR, and Greenberg ML (2013) Loss of cardiolipin leads to perturbation of mitochondrial and cellular iron homeostasis. J. Biol. Chem. 288, 1696–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chu CT, Ji J, Dagda RK, Jiang JF, Tyurina YY, et al. (2013) Cardiolipin externalization to the outer mitochondrial membrane acts as an elimination signal for mitophagy in neuronal cells. Nat. Cell Biol. 15, 1197–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shen Z, Li Y, Gasparski AN, Abeliovich H, and Greenberg ML (2017) Cardiolipin Regulates Mitophagy through the Protein Kinase C Pathway. J. Biol. Chem. 292, 2916–2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hsu P, Liu X, Zhang J, Wang HG, Ye JM, et al. (2015) Cardiolipin remodeling by TAZ/tafazzin is selectively required for the initiation of mitophagy. Autophagy. 11, 643–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kubli DA, and Gustafsson ÅB (2012) Mitochondria and mitophagy: the yin and yang of cell death control. Circ. Res. 111, 1208–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gerdes F, Tatsuta T, and Langer T (2012) Mitochondrial AAA proteases--towards a molecular understanding of membrane-bound proteolytic machines. Biochim. Biophys. Acta. 1823, 49–55. [DOI] [PubMed] [Google Scholar]
- 58.Gaspard GJ, and McMaster CR (2015) The mitochondrial quality control protein Yme1 is necessary to prevent defective mitophagy in a yeast model of Barth syndrome. J. Biol. Chem. 290, 9284–9298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Barth PG, Van Den Bogert C, Bolhuis PA, Scholte HR, Van Gennip AH, et al. (1996) X-linked cardioskeletal myopathy and neutropenia (Barth syndrome): respiratory-chain abnormalities in cultured fibroblasts. J. Inherit. Metab. Dis. 19, 157–160. [DOI] [PubMed] [Google Scholar]
- 60.Wang G, Mccain ML, Yang L, He A, Pasqualini FS, et al. (2014). Modeling the mitochondrial cardiomyopathy of Barth syndrome with induced pluripotent stem cell and heart-on-chip technologies. Nat. Med. 20, 616–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Khuchua Z, Yue Z, Batts L, and Strauss AW (2006) A zebrafish model of human Barth syndrome reveals the essential role of tafazzin in cardiac development and function. Circ. Res. 99, 201–208. [DOI] [PubMed] [Google Scholar]
- 62.Soustek MS, Falk DJ, Mah CS, Toth MJ, Schlame M, et al. (2011). Characterization of a transgenic short hairpin RNA-induced murine model of Tafazzin deficiency. Hum. Gene Ther. 22, 865–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Phoon CK, Acehan D, Schlame M, Stokes DL, Edelman-Novemsky I, et al. (2012). Tafazzin knockdown in mice leads to a developmental cardiomyopathy with early diastolic dysfunction preceding myocardial noncompaction. J. Am. Heart Assoc. pii: jah3–e000455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cadalbert LC, Ghaffar FN, Stevenson D, Bryson S, Vaz FM, et al. (2015) Mouse Tafazzin Is Required for Male Germ Cell Meiosis and Spermatogenesis. PLoS One. 10:e0131066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Johnson JM, Ferrara PJ, Verkerke ARP, Coleman CB, Wentzler EJ, et al. (2018) Targeted overexpression of catalase to mitochondria does not prevent cardioskeletal myopathy in Barth syndrome. J. Mol. Cell Cardiol. 121, 94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Valianpour F, Wanders RJ, Overmars H, Vaz FM, Barth PG, et al. (2003) Linoleic acid supplementation of Barth syndrome fibroblasts restores cardiolipin levels: implications for treatment. J. Lipid Res. 44, 560–566. [DOI] [PubMed] [Google Scholar]
- 67.Chowdhury A, Aich A, Jain G, Wozny K, Lüchtenborg C, et al. (2018) Defective mitochondrial cardiolipin remodeling dampens HIF-1α expression in hypoxia. Cell Rep. 25, 561–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huang Y, Powers C, Moore V, Schafer C, Ren M, et al. (2017) The PPAR pan-agonist bezafibrate ameliorates cardiomyopathy in a mouse model of Barth syndrome. Orphanet J. Rare Dis. 12, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.de Taffin de Tilques M, Tribouillard-Tanvier D, Tétaud E, Testet E, di Rago JP, et al. (2017). Overexpression of mitochondrial oxodicarboxylate carrier (ODC1) preserves oxidative phosphorylation in a yeast model of Barth syndrome. Dis. Model Mech. 10, 439–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.de Taffin de Tilques M, Lasserre JP, Godard F, Sardin E, Bouhier M, et al. (2018). Decreasing cytosolic translation is beneficial to yeast and human Tafazzin-deficient cells. Microb. Cell. 5, 220–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mejia EM, Zegallai H, Bouchard ED, Banerji V, Ravandi A, et al. (2018) Expression of human monolysocardiolipin acyltransferase-1 improves mitochondrial function in Barth syndrome lymphoblasts. J. Biol. Chem. 293, 7564–7577. [DOI] [PMC free article] [PubMed] [Google Scholar]