Abstract
Rationale
Embryonic heart is characterized by of rapidly dividing cardiomyocytes required to build a working myocardium. Cardiomyocytes retain some proliferative capacity in the neonates but lose it in adulthood. Consequently, a number of signaling hubs including microRNAs are altered during cardiac development that adversely impacts regenerative potential of cardiac tissue. Embryonic stem cell cycle (ESCC) miRs are a class of microRNAs exclusively expressed during developmental stages however their effect on cardiomyocyte proliferation and heart function in adult myocardium has not been previously studied.
Objective
To determine whether transient reintroduction of ESCC miR-294 promotes cardiomyocyte cell cycle reentry enhancing cardiac repair after myocardial injury.
Methods and Results
miR-294 is expressed in the heart during development, prenatal stages, lost in the neonate and adult heart confirmed by qRT-PCR and in situ hybridization. Neonatal ventricular myocytes (NRVMs) treated with miR-294 showed elevated expression of Ki67, p-histone H3 and Aurora B confirmed by immunocytochemistry compared to control cells. miR-294 enhanced oxidative phosphorylation and glycolysis in NRVMs measured by seahorse assay. Mechanistically, miR-294 represses Wee1 leading to increased activity of the cyclin B1/CDK1 complex confirmed by qRT-PCR and immunoblot analysis. Next, a doxycycline (dox)-inducible AAV-9-miR-294 vector was delivered to mice for activating miR-294 in myocytes for 14 days continuously after myocardial infarction. miR-294 treated mice significantly improved left ventricular (LV) functions together with decreased infarct size and apoptosis 8 wks after MI. Myocyte cell cycle reentry increased in miR-294 hearts analyzed by Ki67, p-H3 and AurB expression parallel to increased small myocyte number in the heart. Isolated adult myocytes from miR-294 hearts showed increased EdU+ cells and upregulation of cell cycle markers and miR-294 targets 8 wks after MI.
Conclusions
Ectopic transient expression of miR-294 recapitulates developmental signaling and phenotype in cardiomyocytes promoting cell cycle reentry that leads to augmented cardiac function in mice after myocardial infarction.
Keywords: Myocardial regeneration, microRNAs, myocardial infarction, proliferation, cell cycle reentry, Basic Science Research, Myocardial Infarction, Myocardial Regeneration
INTRODUCTION
Heart failure is the leading cause of morbidity and mortality worldwide manifested by inability of the cardiac tissue to activate a regenerative response in face of widespread cardiomyocyte death. Consequently, remaining myocytes undergo adverse structural and functional remodeling leading to compromised pump function. In contrast, embryonic heart consists of cardiomyocytes capable of cell division and proliferation that extends briefly into postnatal cardiac development1–3. Evidence shows that new cardiomyocytes replace dead myocardial tissue after injury to the neonatal heart4 that is associated with restoration of cardiac function. Nevertheless, during transition into adulthood, cardiomyocytes adopt a differentiated morphology optimized for cardiac pump function yet lose ability to undergo replication. Identification of signaling hubs mediating myocyte cell cycle regulation during cardiac development may offer clues for novel strategies for reactivation of cardiomyocyte regeneration in pathologic heart.
MicroRNAs have recently emerged as important regulators of cardiac development and function. Expression of various microRNA families directly corresponds to physiological and pathological changes in cardiac milieu5, 6, while loss of certain microRNAs is associated with development of cardiomyopathies and heart failure7. Of interest, manipulation of microRNA targets directly via gain or loss of function studies have demonstrated cardiomyocyte proliferation and cell division8, 9. Additionally, delivery strategies based on microRNA mimics have been effective in driving cardiomyocyte proliferation and augmentation of cardiac function after myocardial injury10, 11. Previously, we have shown that delivery of embryonic factors via embryonic stem cell derived exosomes to the heart improves cardiac structure and function after myocardial infarction12. Importantly, ESC exosomes treated hearts showed an endogenous proliferative response and significant augmentation of cardiomyocyte cell cycle activation. Analysis of exosomes revealed enrichment for embryonic cell cycle specific microRNAs including miR-294. Studies conducted recently demonstrate a critical role for miR-294 in maintenance of ESC cell cycle13, pluripotency14, self-renewal15 and metabolism16. Nevertheless, the role of miR-294 in the context of the adult heart has never been studied and given the well-documented properties of the miR regulating cell cycle, may potentially be a target for reactivation of the adult cardiomyocyte cell cycle.
In this article, we report that transient introduction of miR-294 in the heart leads to significant augmentation of cardiac structure and function concurrent with cardiomyocyte cell cycle reentry. Our data suggests, miR-294 as a novel therapeutic target for cardiomyocyte cell cycle reentry and cardiac repair following myocardial injury and may potentially be utilized for other cell types that are refractory to optimal proliferation.
METHODS
The authors declare that all supporting data are available within the article and its Online Data Supplement. Raw data are available from the corresponding author on reasonable request.
Cell culture
Neonatal rat ventricular myocytes (NRVMs) were isolated from 1–2 day old rat pups as previously described17 and cultured in F-10 medium (Gibco) supplemented with 10% Fetal Bovine Serum (FBS). Adult feline ventricular myocytes (AFM) were isolated as described previously18, 19 and cultured in M199 medium supplemented with penicillin, streptomycin and gentamicin.
MicroRNA treatment and quantification
NRVMs were transfected with miRNA mimic for miR-294–3p (Life Technologies) or negative control mimic (Life Technologies). NRVMs were grown in F10 media without antibiotics and transfected with either miRNA mimics or controls (50nM, Invitrogen, CA, USA) using Lipofectamine RNAiMAX (Invitrogen, CA, USA) for 24 hrs as per manufacturer’s instructions12. Total RNA was isolated using miRNeasy Mini Kit (Qiagen) according to manufacturer’s protocol. Real time reactions were performed in triplicate on ABI stepOne plus Real-time PCR system (Applied Biosystem, CA, USA). Detailed methods are provided in the online supplement.
Western blot
Immunoblot analysis was performed as described previously12, 20 with additional detail in the online supplement.
Immunohistochemistry
Immunocytochemistry, TUNEL assays, EdU labeling assay and immunohistochemistry were performed as described previously12, 20, 21 with additional detail in online supplement including a list of antibodies in Online Table II.
RT2 Profiler PCR arrays
NRVMs were tested for expression of cell cycle associated genes by using RT2 profiler PCR arrays (Qiagen). Briefly, Single-stranded cDNA was synthesized from all samples using the RT2 First Strand Kit (Qiagen) as described in the Qiagen protocol for RT2 profiler array sample preparation. The reverse transcribed product was used to run real time PCR reactions using RT2 SYBR Green ROX qPCR mastermix on a ABI stepOneplus system (Applied Biosystems).
In situ hybridization
Sections from a 14.5-day-old mouse embryo and AAV9 administered hearts were labeled with a double DIG LNA miR-294–3p probe followed by detection by miRCURY LNA microRNA ISH optimization Kit (Exiqon) to detect endogenous levels for miR-294–3p according to the manufacturer’s instructions.
CDK1 Kinase activity
Activity of CDK1 kinase was measured in NRVMs treated with miR-294 mimic or control using MESACUP Cdc2/Cdk1 Kinase assay Kit (MBL, Japan) according to the manufacturer’s protocol. Briefly, 2 × 106 NRVMs/group were treated with either miR-294–3p or negative control followed by addition of biotinylated MV peptides. Samples were then transferred to 96-well plate and treated with anti-phosphoMV to capture phosphorylated peptides followed by incubation with streptavidin for color development. Plates were read at OD492 in a spectrophotometer.
Luciferase target validation
NRVMs were transfected with mouse Wee1 3’-UTR and control luciferase reporter plasmids (GeneCopoeia) followed by treatment with miR-294–3p mimic. After 48hrs, Firefly and Renilla luciferase reporter activity was measured using Luc-Pair Duo-Luciferase Assay Kit according to the manufacturer’s instructions.
Live cell imaging
Live cell imaging was performed on NRVMs cultured on gelatin coated slides respectively for 72hrs after treatment with miR-294 on a leica TCS Sp8 confocal microscope. Additional details in the online supplement.
Oxygen consumptions rates and ECAR measurement
A seahorse Bioscience XF96 extracellular flux analyzer was utilized to measure oxygen consumption rates (OCR) and extracellular acidification rates (ECAR) in NRVMs treated with miR-294 mimic or control using the mito stress and glycolysis stress kit (Agilent Technologies) respectively.
Induction of Acute MI, AAV administration and Osmotic pump implantation
All animal procedures involving animal surgeries, miRNA administration and AAV delivery were carried out in a blinded manner. Animals (C57BL/6 8–12 weeks old male mice) were divided into two groups (n=25/group) receiving either AAV9-Ctrl or AAV9-miR-294 retro-orbital injections 2 weeks before the surgery. At the day of surgery, Animals underwent myocardial infarction procedure by permanent ligation of the left anterior descending artery (LAD) as described previously12, 20. All animals were implanted subcutaneously with mini-osmotic pumps filled with EdU for 7 days. Additional detail regarding AAV generation, delivery and dose optimization is included in the online data supplement. Male mice were used for the study due to their high susceptibility to cardiac dysfunction in response to LAD ligation that allows detection of changes associated with cardiomyocyte proliferation without confounding factors such as gross anatomical and physiological characteristics of the female heart22, 23.
Single cell isolation and EdU labeling
Single cell adult cardiomyocytes were isolated from hearts injected with AAV9-Ctrl and AAV9-miR-294 8 weeks after myocardial infarction. Isolated myocytes were stained with Click-iT EdU Alexa Fluor 488 Imaging Kit (Life Technologies) and DAPI as per the manufacturer’s instructions. Images were acquired on a Nikon TiE eclipse inverted microscope.
Echocardiography and hemodynamic measurements
Mice underwent serial echocardiography at week 1, 2, 3, 4, 6 and 8 as described previously12, 20, 21. In brief, Transthoracic two-dimensional M-mode echocardiography was performed using the Vevo770 (VisualSonics, Toronto, ON, Canada) equipped with a 30-MHz transducer to record B and M-mode measurements in mice under anesthesia. Left ventricular pressure was measured with a 1.4-Fr Millar pressure catheter (SPR-671, Millar Instruments, Houston, TX) connected to an ADInstruments PowerLab 16/30 (ADInstruments, Colorado Springs, CO) with LabChart Pro 6.0 software as described previously20.
Statistics
Statistical analysis is performed using unpaired Student’s t test for data comparing 2 groups and 1-way or 2-way ANOVA with Bonferroni post-hoc test for comparing more than 2 groups for data exhibiting normal distribution. For data that do not exhibit normal distribution Mann-Whitney test was used. All data sets were assessed for normality using Shapiro-Wilk test for normality. P < 0.05 is considered statistically significant. Error bars represent ±SD. Statistical analysis is performed using Graph Pad prism v 7.0 software.
RESULTS
Temporal expression of miR-294 during cardiac development
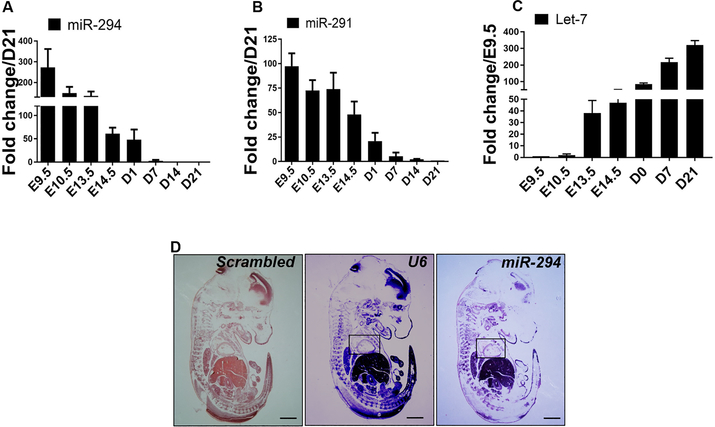
miR-294 is known to be highly expressed during embryonic stages24, 25 but has never been studied in the context of cardiac biology. We isolated RNA from developing mouse heart (E9.5 – 14.5), neonatal heart (D1 and D7) and adult heart (D21) for characterization of miRNA expression. Increased expression of miR-290 family members’ miR-291, albeit at a lower level and miR-294 was observed in the heart at E9.5 that declined over time with complete abrogation at postnatal day 7 (Figure 1A-B) while no expression was observed for miR-295 (data not shown). miR-294 is known to target let-7 via intermediate signaling components15 and we hypothesized that upregulation of miR-294 in embryonic heart would repress let-7 expression. Indeed, let-7 was poorly expressed at E9.5 and 10.5 followed by a gradual increase with maximum expression observed at postnatal day 21 (Figure 1C). We confirmed miR-294 expression in the embryonic heart by staining E14.5 embryo for miR-294 using in situ hybridization (ISH) (Figure 1D and Online Figure I A-B). Additionally, myocardial injury had no effect on promoting miR-294 levels in the heart early after infarction (Online Figure I C). Collectively, miR-294 and let-7 expression show opposing patterns that coincides with embryonic cardiac development and rapidly alter after maturation of cardiac tissue during transition from postnatal to adult cardiac stages.
Figure 1: miR-294 expression in the heart during embryonic and adult cardiac development.
A) Increased expression of miR-294 in the embryonic heart from E9.5 – E14.5 that progressively declines with birth (D1) and postnatal development (D7-D21) (n=3). B) Other miR-290 family members such as miR-291 are also expressed during embryonic heart development albeit at a lower level and decreasing after birth (n=3). C) Let-7 expression is low during E9.5 and 10.5 but starts to increase after that with maximum expression in the adult heart (D21) (n=3). D) miR-294 expression validated in a E14.5 whole embryo including embryonic heart (inset) by in situ hybridization. Scale bar = 100μm.
miR-294 promotes cell cycle activity in neonatal and adult cardiomyocytes
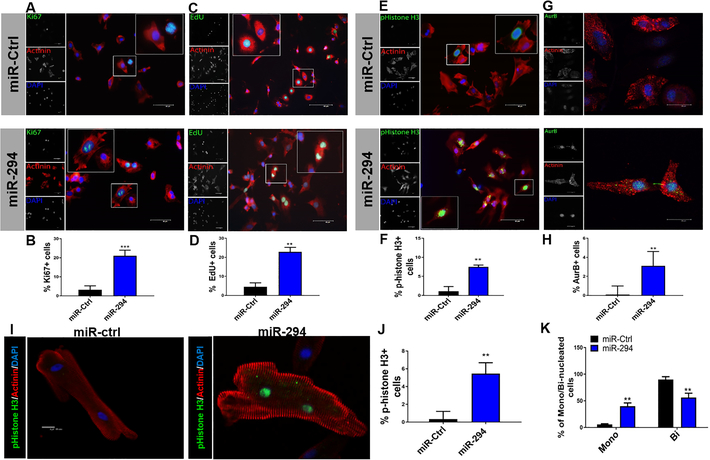
miR-290 family members are known as embryonic stem cell cycle (ESCC) microRNAs and regulate cell cycle and proliferation. Embryonic heart expresses miR-290 family members, miR-291 and miR-294 (Figure 1) and given the role of miR-290 family in cell cycle regulation, it was hypothesized that miR-290 family may affect cardiomyocyte cell cycle. To determine which microRNA is more potent, Neonatal rat ventricular myocytes (NRVMs) were transfected with miR-291, miR-294 and miR-295 alone or in combination followed by analysis of cell cycle markers. Increased expression of Cyclin B1, Cdk2 and Cyclin E1 was observed with miR-294 treatment compared to other conditions (Online Figure I D) thereby selecting miR-294 for further experiments. To test whether miR-294 promotes cardiomyocyte cell cycle reentry; NRVMs were treated with miR-294–3p mimic to assess expression of cell cycle markers. Increased Ki67+ NRVMs were observed in miR-294 treated (21.0%) group compared to negative control mimic (3.2%) (Figure 2A-B). Similarly, NRVMs were pulse chased with 5-ethynyl-2’-deoxyuridine (EdU), treated with miR-294 along with negative control and assessed 24hrs later. miR-294 treated NRVMs showed increased EdU+ cells (22.9%) compared to negative control (4.6%) (Figure 2C-D). Additionally, p-histone H3 staining was significantly enhanced in miR-294 (7.5%) compared to negative control mimic (1.1%) (Figure 2E-F). Interestingly, we observed aurora B+ NRVMs after miR-294 treatment (3.1%) compared to control (0.1%) (Figure 2G-H), indicating miR-294 treatment not only promotes G1/S transition but pushes cells towards cytokinesis. Further confirmation of NRVMs cell cycle was done by live cell imaging for 72 hours post miR-294 treatment (Online Video I). Concurrently, miR-294 promoted survival of NRVMs challenged with H2O2 stress as evidenced by reduced TUNEL+ cells together with increased AKT phosphorylation that may be related to the overall proliferative phenotype (Online Figure II A-C). To validate the purity of our NRVM cultures, cells were stained with cardiac troponin T (cTnT) together with Ki67 that showed concurrent results as describe above (Online Figure II D). Together, results indicate that miR-294 increases cell cycle reentry in NRVMs however the main question is whether a similar response is observed in adult cardiomyocytes. For this purpose, feline adult cardiomyocytes were used as described previously18, 19, allowing an optimal experimental system for studying the effect of miR-294 on adult myocyte cell cycle. Moreover, miR-294 is highly conserved among mammals with conserved sites across various species for cell cycle target genes (Online Figure II E). Adult myocytes treated with miR-294–3p led to a significant increase in p-histone H3+ adult cardiomyocytes (Figure 2I, J) compared to control miR treated cells. In parallel, enhancement of mononucleated fraction was observed in adult cardiomyocytes with corresponding reduction in binucleated cells after treatment with miR-294 (Figure 2K). Collectively, results show that miR-294 possesses ability to drive cell cycle reentry in neonatal and adult cardiomyocytes.
Figure 2: miR-294 promotes cell cycle progression in cardiomyocytes.
A) Increased expression of Ki67+/actinin+ cells was observed in NRVMs treated with miR-294–3p mimic compared to control mimic after 24hrs with quantification in (B) (n=5). Ki67 (green), α-actinin (red) and nuclei (blue). Scale bar = 40μm. C-D) NRVMs were labeled with EdU (G1/S transition) followed by miR-294 mimic or control treatment. EdU detection 24 hrs later showed increased % of EdU+/actinin+ cells in miR-294–3p group compared to control mimic. EdU (green), α-actinin (red) and nuclei (blue) (n=5). Scale bar = 40μm. E-F) Analysis of M-phase marker p-histone H3 showed increased %expression in miR-294 treated group compared to control 24hrs treatment. p-histone 3 (green), α-actinin (red) and nuclei (blue) (n=5). Scale bar = 40μm. Insets show higher magnification. G) Expression of aurora B in miR-294 treated NRVMs shows a late telophase NRVM stained for aurora B in the cytoplasmic body, (H) Significant increase in miR-294 group compared to control after 24hrs (n=5). Aurora B (green), α-actinin (red) and nuclei (blue). Scale bar = 20μm. I-J) Adult feline cardiomyocytes treated with miR-294 mimic show increased expression of p-histone H3 24hrs treatment. p-histone H3 (green), α-actinin (red) and nuclei (blue) (n=4). Scale bar = 40μm. K) miR-294 treatment of adult cardiomyocytes leads to significant % increase in mononucleated cells and a corresponding % decrease in binucleated myocytes (n=3). miR-Ctrl vs. miR-294 *p < 0.05, **p < 0.01, ***p < 0.001, data was assessed using unpaired student’s t test.
Increased cell cycle activity drives energy generation in miR-294 treated myocytes
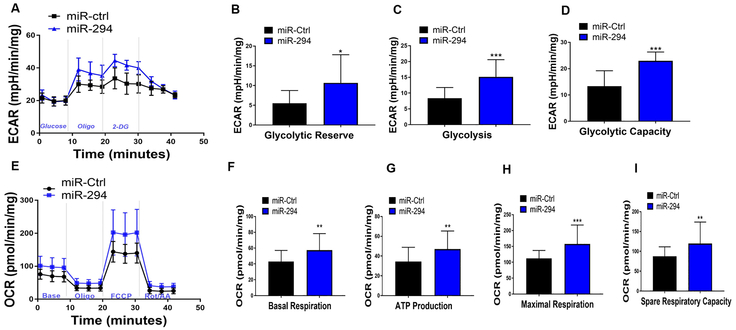
Recent studies indicate that cell transition through cell cycle carries a high metabolic demand26. Cell proliferation generally favors high glycolytic metabolism with many cell cycle regulators promoting expression of glycolytic intermediates27. However, neonatal cardiac tissue is highly proliferative at postnatal day 1 and increases both oxidative phosphorylation and glycolysis to meet the high energy demand required to sustain proliferative rates1. Since miR-294 is active predominately in the embryonic stages14, 15 and regulates cell cycle and proliferation, it was hypothesized that enhanced cell cycle reentry in cardiomyocytes may increase cellular bioenergetics. For this purpose, NRVMs were treated with miR-294 and control mimic followed by seahorse analysis for oxidative phosphorylation and glycolysis. miR-294 treatment led to significant upregulation of extracellular acidification rate (ECAR), glycolytic capacity, glycolysis and glycolytic reserve compared to control mimic 24hrs after treatment (Figure 3A-D). Interestingly, oxidative phosphorylation (OCR) including ATP production, maximal respiration and spare capacity was also enhanced in NRVMs 24hrs after treatment with miR-294 compared to control cells (Figure 3E-I). Taken together, results show that miR-294 increases both glycolysis and oxidative phosphorylation in NRVMs as opposed to favoring glycolysis only, possibly to cope with increased cell cycle demand reminiscent of developmental cardiac tissue.
Figure 3: miR-294 increases NRVM bioenergetics.
A) Analysis of glycolysis, showed increased extra-cellular acidification rate (ECAR) including significant upregulation of glycolytic capacity (B), glycolysis (C) and glycolytic reserve (D) in NRVMs treated with miR-294 24hrs after treatment. E) miR-294 treatment significantly increased oxygen consumption rate (OCR) including basal respiration (F), ATP production (G), maximal respiration (H) and spare respiratory capacity (I) as measured by mito stress test using XF seahorse analyzer. Data for OCR and ECAR was normalized to protein content. miR-ctrl; n=30 and miR-294; n=32/ three independent experiments. miR-Ctrl vs. miR-294 *p < 0.05, **p < 0.01, ***p < 0.001, data was assessed using non-parametric Mann-Whitney test.
miR-294 targets Wee1/CDK1-CyclinB1 axis to drive proliferative changes in myocytes
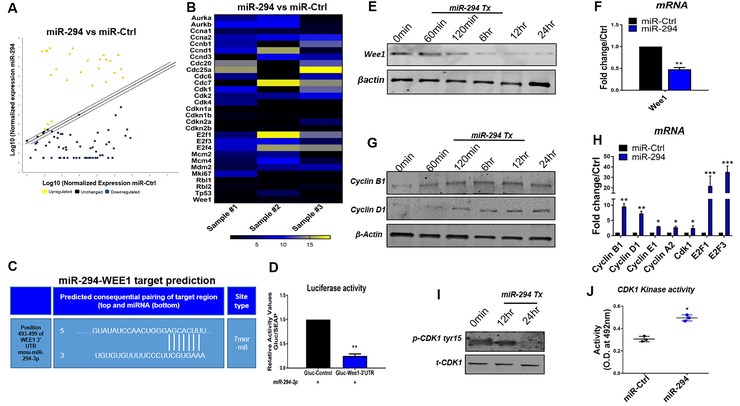
miR-294 is part of embryonic cell cycle specific (ESCC) miRs, regulating cell cycle and proliferation in ESCs13, 28 including downstream signaling via Lin28a25, 29 and let-715. Therefore, the next set of studies set out to test the mechanistic basis for increased cell cycle reentry in miR-294 treated NRVMs. For this purpose, cell cycle array analysis was carried out for NRVMs treated with miR-294 and control mimic. In total, expression of 84 cell cycle regulators changed significantly with upregulated, downregulated or unchanged genes after miR-294 treatment, shown in Figure 4A, online figure III A and Online Table III. Increased expression of several markers for G1/S transition and M phase was observed in miR-294 treated NRVMs compared to control including blunting of negative regulators of cell cycle such as Wee1 (Figure 4B). Since miRs act through transcriptional repression and targeting of mRNA 3’-UTR, a miRNA target prediction search was conducted using targetscan 7.2 that identified Wee1 as a potential target with only one putative 7mer site within Wee1- 3’-UTR (Figure 4C) amongst the cell cycle genes altered after miR-294 treatment in NRVMs. To confirm, whether miR-294 targets Wee1- 3’-UTR, a reporter assay was performed using 3’-UTR of Wee1 that drives luciferase expression. NRVMs were transfected with mouse Wee1 3’-UTR luciferase reporter plasmid together with control plasmid and treated with miR-294 mimic. Treatment with miR-294 significantly reduced luciferase activity validating miR-294 targeting of Wee1 (Figure 4D). Immunoblot and mRNA analysis further showed reduced expression of Wee1 (Figure 4E-F) together with reduced let-7 (Online Figure III B) and increased Lin28a and c-myc expression (Online figure III C-D), known downstream targets for miR-294. Additionally, NRVMs were treated with siRNAs for Wee1 (Online Figure III E) stained for cell cycle markers. Increased expression of Ki67 and p-histone H3 was observed in siWee1 NRVMs compared to scramble cells, albeit at a much lower level than miR-294 treatment suggesting other mechanisms for miR-294 cell cycle effect on NRVMs (Online Figure III F-G).
Figure 4: Analysis of molecular signaling in miR-294 treated NRVMs.
A) Cell cycle array analysis of NRVMs with miR-294–3p mimic or control treatment from 3 independent experiments (n=3). B) Heat map representation of miR-294 fold change over miR-Ctrl. C) Target prediction analysis for miR-294 putative sites on 3-UTR of target genes, identified a 7mer-8m target site in 3-UTR of Wee1 (position 493–499). D) Dual luciferase reporter activity assay for validation of miR-294 targeting of 3UTR-Wee1 in the presence of miR-294 mimic indicates reduced luciferase activity in NRVMs transfected with plasmid carrying 3-UTR-Wee1 (n=3). E) Immunoblot analysis show reduced Wee1 expression in NRVMs after treatment with miR-294 mimic together with mRNA expression in (F) (n=3). G) Elevated proteins levels for cyclin B1 and D1 in miR-294 treated NRVMs (n=3). H) miR-294 treatment of NRVMs shows increased mRNA expression of Cyclin B1,D1, E1, A2, CDK1, E2F1 and E2F3 compared to control cells (n=3). I) Decreased phosphorylation of CDK1tyr15 to total CDK1 ratio (n=3). (J) Elevated CDK1 kinase activity in NRVMs treated with miR-294 compared to control (n=3). miR-Ctrl vs. miR-294 *p < 0.05, **p < 0.01, ***p < 0.001, data was assessed using unpaired student’s t test.
Wee1 is known to inactivate CDK1-CyclinB1 complex preventing entry into G2/M phase by phosphorylation of CDK1 at tyr1530. Since miR-294 represses Wee1, it was hypothesized that miR-294 mediated cell cycle reentry in NRVMs may be connected to release of CDK1-CyclinB1 complex and its downstream signaling. Indeed, increased cyclin B1 expression was observed in NRVMs treated with miR-294 together with cyclin D1, cyclin E1, A2, CDK1, E2F1 and E2F3 as confirmed by immunoblot and mRNA analysis respectively (Figure 4G-H). Moreover, decreased phosphorylation of CDK1 at tyr15 was observed after miR-294 treatment (Figure 4I) concomitant with increased CDK1 kinase activity as measured by kinase activity assay (Figure 4J). Similar cell cycle signaling was observed in adult feline myocytes that show increased miR-294 levels after transfection (Online Figure II F) together with increased mRNA expression of cyclins (D1, E1, B1) and CDK1 in response to miR-294 treatment (Figure II G). In summary, miR-294 treatment of NRVMs results in repression of Wee1 allowing CDK1-CyclinB1 complex activity that promotes expression of cell cycle markers together with activation of the canonical miR-294 downstream signaling pathways.
Transient activation of miR-294 in the heart after myocardial injury
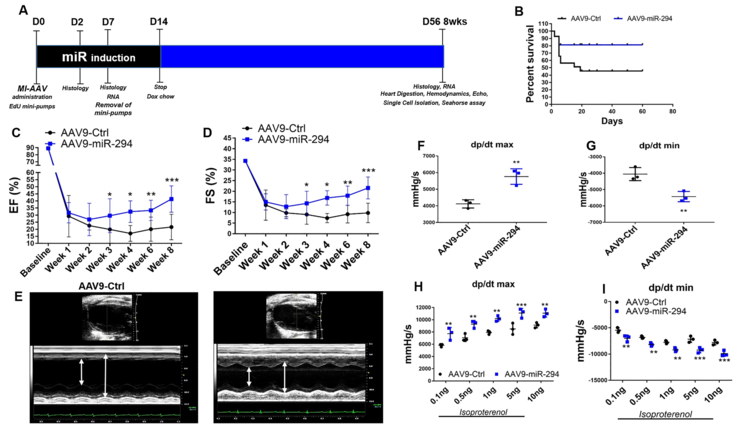
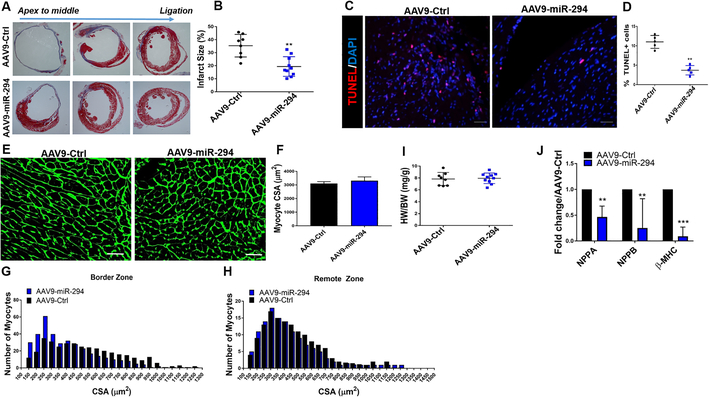
To assess whether miR-294 is a viable therapeutic option for cardiac regeneration after myocardial injury, a two-pronged transient delivery strategy was devised. In the first approach, a single intramyocardial injection of LNA-miR-294–3p mimic (Exiqon) was administered to the heart after myocardial infarction injury (Online Figure IV A). Analysis of cardiac function indicated significant improvement early at 2 weeks that was lost over time (Online Figure IV B-C). There was increase in expression of the transfected miR-294 at day 2 (Online Figure IV D) concurrent with increased number of BrdU+ cells (Online Figure V A-B), BrdU+/SMA+ smooth muscle cells (Online Figure V C-D) and reduced TUNEL+ cells (Online Figure V E-F) at day 2 after MI but no significant reduction in infarct size (Online Figure IV E-F) at 8 weeks. The overall underperformance of miR-294 in the single injection strategy prompted us to design a more robust delivery approach. Thus, the second approach was based on delivery of AAV9-TRE-miR-294-αMHC-rtTA vector to the heart followed by doxycycline mediated activation of miR-294 under the control of αMHC promoter for cardiomyocyte only expression for 2 weeks after infarction while control animals received AAV9 control vector (Figure 5A and Online Figure VI). Analysis of mice 8 weeks after injury demonstrated increased survival (Figure 5B) and significant augmentation of ejection fraction (EF), fractional shortening (FS) (Figure 5C-E, Online Figure VII A), left ventricular anterior wall thickness (LVAW) (Online Figure VII B) and reduction in left ventricular internal diameter at end-systole (LVIDs) (Online Figure VII C) in AAV9-miR-294 animals compared to controls as measured by echocardiography. Cardiac function assessment shows significant improvement starting at 3 weeks post-MI that could be due to a combination of increased cardiomyocyte survival and enhanced proliferative signaling during the first week after MI that ultimately allows cardiomyocyte cell cycle activity observed at 8 weeks. Hemodynamic measurements showed increased cardiac contractility under baseline and stimulation (Figure 5F-I, Online Figure VII E-F) and a comparable heart rate during measurements for mice in both groups (Online Figure VII D). In parallel, AAV9-miR-294 administered heart showed decreased infarct size (Figure 6 A-B) and increased new vessel formation (Online Figure IX D) at 8 weeks and apoptosis at day 2 (Figure 6 C-D) after injury compared to control. Analysis of myocyte size (Figure 6 E-F) and HW/BW ratio (Figure 6 I) showed no difference between the groups but increased number of small myocytes in border zone (Figure 6 G-H). Expression of hypertrophic markers such as NPPA, NPPB and β-MHC was downregulated in AAV9-miR-294 hearts 8 weeks after injury (Figure 6 J). Taken together, transient activation of miR-294 in the heart seems to be better than single injection resulting in significant enhancement of cardiac structure and function after myocardial injury.
Figure 5: AAV9-miR-294 administration augments cardiac function after myocardial infarction in mice.
A) Schematic illustration of experimental design. Animals were divided into two groups and administered AAV9-GFP (n=20) and AAV9-miR-294 (n=20) two weeks prior to myocardial infarction surgery. At the day of surgery (D0) mice were subjected to LAD ligation followed by administration of doxycycline chow to induce miR-294 expression that was removed at day14 and EdU mini-pump implantation removed at D7. Hearts were harvested at D2 and D7 for histology and RNA analysis. Heart were analyzed for echos/hemodynamic measurements, histology and RNA analysis at terminal time point was 8 weeks or D56 after MI. B) Kaplan-Meir survival curve analysis shows increased %survival in AAV9-miR-294 group compared to AAV9-GFP. C) Increased ejection fraction and fractional shortening (D) in AAV9-miR-294 (n=10) administered hearts compared to AAV9-GFP (n=8) animals at 8 weeks after MI. E) Increased wall contractility in AAV9-miR-294 animals compared to controls. Hemodynamic measurements show increased dp/dtmax and reduced dp/dtmin under baseline (F-G) and stimulated (H-I) conditions 8 weeks after MI (n=3). AAV9-Ctrl vs. AAV9-miR-294 *p < 0.05, **p < 0.01, ***p < 0.001, data in panels C,D, were assessed using one-way analysis of variance H and I was assessed using two-way ANOVA with bonferroni post-hoc test while panels F and G were assessed using unpaired student’s t test.
Figure 6: Infarct size, apoptosis and myocyte size analysis.
A) Reduced infarct size in AAV9-miR-294 (n=10) administered animals 8 weeks after MI compared to control animals (n=8) as measured by masson’s trichrome staining. Quantification of infarct size in (B). Decreased TUNEL+ cells in the heart treated with AAV9-miR-294 2days after MI compared to AAV9-Ctrl. TUNEL (red), nuclei (blue), scale bar = 40μm. Corresponding quantification in D. E-F) Analysis of myocyte cross-sectional area indicates no significant difference between AAV9-miR-294 (n=10) and AAV9-Ctrl (n=8) animals as determined by wheat germ agglutinin (WGA) staining (green). Scale bar = 40μm. G-H) Size distribution analysis indicated higher number of small sized myocytes in Border zone and remote area of AAV9-miR-294 (n=10) hearts compared to control (n=8) 8 weeks after myocardial infarction. I) HW/BW ratio was not significantly increased in AAV9-miR-294 (n=10) hearts compared to control (n=8) at 8 weeks. J) mRNA expression for markers of hypertrophy was significantly downregulated in AAV9-miR-294 treated (n=3) hearts compared to control (n=3). AAV9-Ctrl vs. AAV9-miR-294 *p < 0.05, **p < 0.01, ***p < 0.001, data was assessed using unpaired student’s t test.
Increased myocyte cell cycle activity in the heart after miR-294 delivery
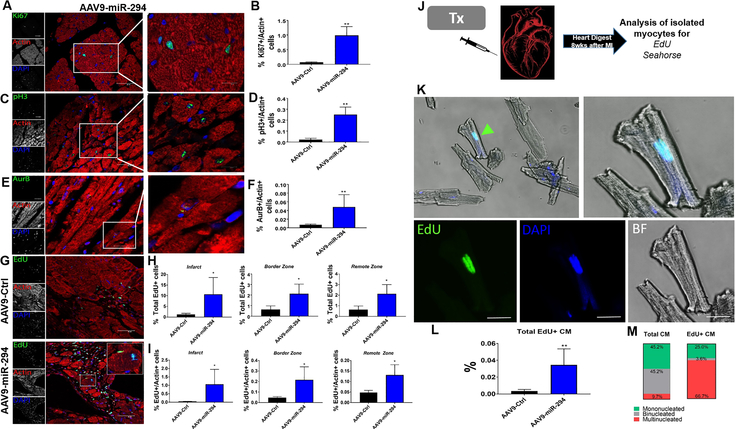
Administration of AAV9-miR-294 to the heart increased cardiac function, so the next question was whether there was increase in myocyte cell cycle activity. Analysis of heart samples at day7 after dox induction and MI, showed significant upregulation of miR-294 as observed by in situ hybridization (Online Figure VIII A-B) and miRNA qRT-PCR analysis (Online Figure VIII C) compared to control hearts. Similarly, miR-294 targets, Lin28a was upregulated while let-7 was blunted (Online Figure VIII D). miR-294 downstream target Wee1 was also observed to be downregulated in miR-294 hearts compared to control as confirmed by qRT-PCR (Online Figure VIII D) and immunostaining (Online Figure VIII E). Increased expression of cyclins (D1, D2, E1, A2, B1) and CDK1 (Online Figure VIII F) together with upregulation in enzymes for glycolysis and OXPHOS (Online Figure IX A-B) was observed in AAV9-miR-294 hearts compared to controls at D7 after MI and Dox induction. To analyze myocyte cell cycle, sections from 8 weeks-old MI animals were stained for cell cycle markers. Increased % expression of Ki67 (Figure 7A-B) p-histone+ (Figure 7C-D, Online Video II) and AurB+ myocytes (Figure 7E-F) were observed in the AAV9-miR-294 hearts compared to controls. Additionally, EdU mini pumps were implanted in all mice at the time of MI and dox induction for 7 days. EdU detection revealed increased EdU+ cells (Figure 7G-H) parallel with significantly high number of EdU+ myocytes (Figure 7I) in infarct, border zone and remote zone of AAV9-miR-294 hearts compared to controls. Moreover, hearts from both AAV9-miR-294 and AAV9-Ctrl administered groups were digested for myocyte isolation and stained for EdU expression (Figure 7J). Increased number of EdU+ adult mono/bi/tri-nucleated myocytes were derived from AAV9-miR-294 hearts compared to controls (Figure 7K-M). Metabolic analysis of isolated adult myocytes from miR-294 hearts at 8 weeks revealed decreased ECAR rates compared to AAV control animals suggesting cardiac energetics reminiscent of fully differentiated adult cardiomyocytes (Online Figure IX C). Collectively, robust expression of miR-294 during first 2 weeks after MI (regenerative window) leads to increased cardiomyocyte cell cycle reentry possibly via induction of miR-294 downstream signaling targets.
Figure 7: AAV9-miR-294 enhances cardiomyocyte cell cycle in vivo after myocardial injury.
A) Histological analysis of heart sections reveals increased Ki67+/actin+ cells 8 weeks after MI in AAV9-miR-294 group compared to control. Ki67 (green), Actin (red) and nuclei (blue), scale bar = 40μm. Insets represent higher magnification at 20μm. Quantification in (B) (n=5). C) Increased p-histone 3/Actin+ small myocytes in AAV9-miR-294 hearts compared to controls. Inset shows small pH3 myocytes in the border zone area (scale bar = 20μm). p-histone H3 (green), actin (red), nuclei (blue). Quantification in (D) (n=5). E) Analysis of cytokinesis marker auroraB shows increased aurB+/actin+ cells in AAV9-miR-294 hearts compared to controls (n=5) (Scale bar = 40μm). Inset shows a AurB+ myocyte in the border zone area (scale bar = 20μm). AuroraB (green), actin (red) and nuclei (blue). Quantification in (F). G-H) EdU detection of the hearts at 8 weeks showed high number of EdU+ cells in infarct, border zone and remote zone in AAV9-miR-294 group compared to control with significantly higher number of EdU+ myocytes respectively (I) (n=5). EdU (green), actin (red), nuclei (blue), scale bar = 40μm. J) Schematic representation of single myocyte isolation from both AAV9-GFP and AAV9-miR-294 hearts followed by EdU detection and seahorse assays on the isolated myocytes 8 weeks after MI (n=3). K) AAV9-miR-294 hearts showed significantly higher number of EdU labeled adult myocytes compared to control hearts. EdU (green), nuclei (blue) bright field (BF). Inset shows an EdU+ isolated adult myocyte from an AAV9-miR-294 heart. Quantification in (L) (n=3). M) Distribution of EdU+ myocytes in the AAV9-miR-294 hearts showed higher % of mononucleated and tri-nucleated EdU+ myocytes. AAV9-Ctrl vs. AAV9-miR-294 *p < 0.05, **p < 0.01, ***p < 0.001, data was assessed using unpaired student’s t test
DISCUSSION
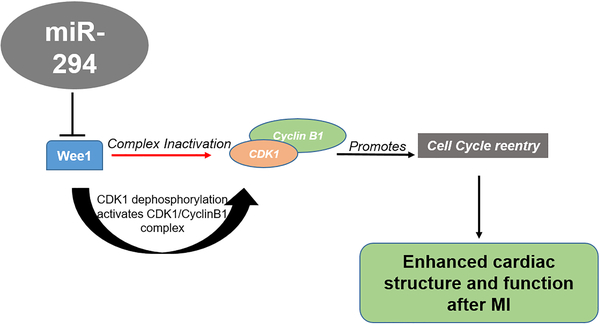
Our findings here demonstrate that, transient activation of the embryonic cell cycle miR-294 in the heart for 14 days post injury leads to induction of pro-reparative changes in the heart that lead to significant enhancement of cardiac repair after myocardial injury. Moreover, salutary effects of miR-294 are multifaceted and include increased cardiomyocyte cell cycle reentry mediated through targeting of cell cycle regulators and in particular activation of the Wee1/CyclinB-CDK1 complex as well as enhanced cell survival, induction of angiogenesis and restriction of infarct size, that collectively enhances cardiac structure and function after myocardial injury (Figure 8).
Figure 8: Schematic representation of the working hypothesis.
Wee1 inhibits proliferation (red arrow) by inactivating CDK1/CyclinB1 complex. microRNA-294 delivery to the infarcted heart represses Wee1 activates CDK1/Cyclin B1 complex (Black arrows) through dephosphorylation of CDK1 that promotes cell cycle reentry together with a proliferative phenotype that includes high energy generation and survival ultimately augmenting cardiomyocyte replenishment in the heart after myocardial infarction.
The mammalian heart is known to grow by cardiomyocyte proliferation during embryogenesis with the last wave of DNA replication at day 14 without cytokinesis resulting in binucleation31. The adult cardiomyocytes retain turnover ability31 with the adult myocyte pool replaced 15–20 times between ages 20 and 100 years but grow only by hypertrophy32, 33. In contrast, neonatal heart is a proliferative organ, able to regenerate itself after myocardial injury4, 32, 34. Recent evidence shows a distinct molecular signature of neonatal cardiomyocytes compared to their adult counterparts regulating myocyte proliferation32, 35. Reintroduction of embryonic or developmental factors active during cardiac development to induce regeneration in the adult heart has become an attractive strategy lately. Cell cycle regulators such as cyclins36, cyclin-dependent kinases32, proto-oncoproteins35 and microRNAs6, 11, 37 are highly expressed in the embryonic heart and their re-expression in adult heart enhances cardiomyocyte cell cycle reentry, cardiomyocyte dedifferentiation and redifferentiation into new myocytes. Studies have shown that inhibition of negative microRNA regulators8 of proliferation such as miR-15 family or conversely enhancing proliferative microRNAs9, 11 such miR-590, 199a and 302–367 in the heart leads to increased cardiomyocyte proliferation and improved cardiac structure and function. Previously, we have shown that delivery of embryonic factors directly to the heart proves to be an effective therapy for reactivation of endogenous proliferative and reparative processes following myocardial injury12. The pro-reparative effects were tied to delivery of cell cycle regulating microRNAs, in particular miR-294. Whether, targeted delivery of miR-294 to cardiomyocytes enhances cell cycle reentry and repair following injury was not tested. Here, we show a novel role for pluripotent microRNA-294 to induce pro-reparative changes in the heartthat enhance cardiac structure and function following injury.
miR-294 belongs to embryonic stem cell cell cycle (ESCC) miRNAs that are implicated in regulating key ESC properties such as pluripotency14, self-renewal15 and cell cycle13. This miRNA family consists of 14 miRNAs with a common seed sequence AAAGUGC and constitute about 70% of the entire microRNAs in ESCs24. Studies show miR-294 to promote G1/S transition via transcriptional repression of cell cycle inhibitors14 and maintaining ESCs in S/G2 phases25. Nevertheless, embryonic cell cycle miRNA-294 as a candidate to drive pro-reparative changes in the heart after myocardial injury has never been tested in the heart. Our results show that miR-294 promotes cell cycle reentry in neonatal and adult cardiomyocytes based on detection of cell cycle markers such as Ki67, pHH3, EdU and Aurora b in accordance with studies showing the use of cell cycle markers to determine cell cycle activity9, 11. However, our approach does not directly test new myocyte formation or cytokinesis that may indicate additional benefits of miR-294 for enhancement of cardiac function that go beyond cell cycle reentry. Nevertheless, the particularly unique and novel aspect of the study is the use of miR-294 to drive pro-reparative changes in the heart following injury. Our results show miR-294 promotes cardiomyocytes cell cycle activity together with increased cell survival, enhanced angiogenesis and restriction of infarct size in the heart after injury. Interestingly, our results also show reduced apoptosis in hearts of animals administered miR-294 that may suggest miR-294 ability to target cellular survival in concordance with published studies showing pro-survival and anti-apoptotic effect of miR-29438, 39. Mechanistically, the increased cell cycle reentry in cardiomyocyte in response to miR-294 was due to targeting of multiple cell cycle regulators (Online Table III) and in particular Wee1-Cyclin B1/CDK1 complex. Previously, Bicknell and colleagues have shown that forced expression of cyclin B1-CDK1 complex re-initiates cell division in adult rat cardiomyocytes40. More recently, overexpression of a combination of cell cycle regulators CDK4/CyclinD-CDK1/CyclinB1 was shown to induce adult cardiomyocytes cell cycle reentry and cytokinesis41. Authors showed that CyclinB1-CDK1 alone or in combination with aurora B overexpression results in small increase in EdU/pHH3+ myocytes that may contradict our findings. Nevertheless, we believe that the salutary effects of miR-294 are mediated via Wee1 repression and the consequent activation of CyclinB1-CDK1 complex. Additionally, miR-294 changes expression of a number of cell cycle regulators as shown in the cardiomyocyte global profiling data in Online Table III suggesting that miR-294 effects are not limited to Wee1-Cyclin B1/CDK1.
Another unique and novel aspect of the study is the use of a transient miRNA delivery strategy designed to express miR-294 for 14 days in cardiomyocytes after injury. Single injections or short-term introduction of microRNAs have been shown to drive functional changes in the heart after injury10, 11 and a recent study employed improved transfection protocol to enhance miRNA persistence in the heart for 12 days following single injection10. Our strategy reported here goes further and prolongs persistence of miR-294 for 14 days with the idea that enhancing miRNA persistence during the regenerative window in the heart promotes cardiac repair. Most of the studies with miRNA in vivo delivery rely on either constitutive activation/deletion of the miRNA of choice that leads to a continuous expression of the transgene increasing off-target effects while consecutive injections of the miRNA of interest though beneficial maybe limited in driving myocyte cell cycle. Additionally, we found that single injection of the miRNA leads to a transient increase in cardiac function but this increment fades over time suggesting requirement for a more robust strategy for miRNA delivery and therapeutic efficacy. However, we recognize that pluripotent microRNA activation in the heart may lead to adverse or uncontrolled proliferation and for this reason, our delivery strategy employed doxycycline inducible cardiac specific vector to allow transient on/off strategy specifically in the cardiomyocytes. Our strategy incorporating a regulated delivery approach for miR-294 restricted to cardiomyocytes is novel as to date none of the studies have employed a regulated approach for miRNA delivery to induce cardiomyocyte cell cycle activation. This is particularly important as regulated cell specific miRNA expression system allows better control and advantage over unregulated approaches employed in the past and may reduce miRNA off-target effects. Interestingly, our data showed increased EdU+ multinucleated cells in the miR-294 hearts compared to controls suggesting either a multinucleation event or cells undergoing cell cycle reentry/karyokinesis. Whether the cell actually completed the cell division remains untested. Analysis of the hearts after AAV9 delivery suggested unusually high transduction rates yet the frequency of cardiomyocytes undergoing a cell cycle event was low. Various studies have shown heterogenic cardiomyoycte age and structure/physiological properties42 together with proliferation potential in the mammalian heart43, urodeles44 and zebrafish45 including spatially heterogeneous pattern of proliferation46 suggesting transcriptional heterogeneity47 in the cardiomyocytes, factors governing which remain unclear. Therefore, for any transgene delivery based approach, expression of a tag (GFP in our study) would provide evidence for incorporation of the transgene in the cells. Whether expression of GFP would mean that every cardiomyocyte undergoes a cell cycle event remains unpredictable due to underlying heterogeneity in cardiomyocyte proliferative behavior as discussed above.
In conclusion, we report here a novel role for pluripotent derived microRNA-294 in cardiomyocyte cell cycle and cardiac repair. Transient activation of miR-294 leads to myocyte cell cycle reactivation that together with pro-reparative changes in the heart, thereby enhancing cardiac structure and function following myocardial injury.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What Is Known?
Embryonic heart is a proliferative organ but cellular replacement is largely restricted as the heart transitions into adulthood.
MicroRNAs play an important role during cardiac development and homeostasis thereby representing a viable strategy for cardiac repair following injury
What New Information Does This Article Contribute?
Embryonic cell cycle miR-294 is expressed in the heart during embryonic development and is lost in the adult heart
miR-294 increases cell cycle reentry in neonatal and adult cardiomyocytes
Transient expression of miR-294 in the heart augments cardiac function after myocardial infarction
Salutary effects of miR-294 are linked to increased cell cycle reentry, enhanced survival, induction of angiogenesis, restriction of infarct size and induction of developmental signaling
The neonatal cardiac tissue is reminiscent of proliferative cardiomyocytes yet the adult heart transitions to a post-mitotic state, with limited cellular turnover. Re-introduction of developmental signaling including regulators of cell cycle has been an attractive strategy for promoting cardiac repair in the heart. Here, we report that embryonic cell cycle miR-294 has the ability to modulate cardiac repair after myocardial infarction.. Expression of miR-294 in neonatal and adult cardiomyocyte promoted cell cycle reentry together with induction of cell cycle related molecular signaling. Transient introduction of the miR-294 in the cardiomyocyte specifically for 14 days post myocardial infarction led to significant enhancement of cardiac structure and function. The salutary effects of miR-294 were tied to enhancement of cell cycle reentry, survival, angiogenesis, and restriction of infarct size and induction of developmental signaling in the heart. Cardiac repair potential of embryonic cell cycle miR-294 represents a novel strategy for induction of pro-reparative changes in the heart.
ACKNOWLEDGEMENTS
We thank all members for Khan and Kishore laboratories for their valuable discussions.
SOURCES OF FUNDING
The work was supported by National Institute of Health grant HL135177, American Heart Association Scientific Development Grant 15SDG22680018 to M. Khan, HL137850 and 15SDG25550038 to S. Mohsin, and HL091983, HL126186 and HL134608 to R. Kishore.
Nonstandard Abbreviations and Acronyms
- ESCC
Embryonic stem cell cycle
- ISH
In situ hybridization
- EdU
5-ethynyl-2’-deoxyuridine
- AurB
Aurora B kinase
- TUNEL
Terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick end labeling
- OXPHOS
Oxidative phosphorylaiton
- OCR
Oxygen consumption rate
- ECAR
Extracellular acidification rate
- NRVM
Neonatal rat ventricular myocytes
- EF
Ejection fraction
- FS
Fractional shortening
- LVID
Left ventricular internal diameter
- LVAW
Left ventricular anterior wall
- LNA
Locked nucleic acid
- NPPA
Atrial natriuretic peptide
- NPPB
Brain natriuretic peptide
- β-MHC
Beta myosin heavy chain
Footnotes
DISCLOSURES
None.
This manuscript was sent to Mark A. Sussman, Consulting Editor, for review by expert referees, editorial decision, and final disposition.
REFERENCES
- 1.de Carvalho A, Bassaneze V, Forni MF, Keusseyan AA, Kowaltowski AJ and Krieger JE. Early Postnatal Cardiomyocyte Proliferation Requires High Oxidative Energy Metabolism. Sci Rep. 2017;7:15434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mollova M, Bersell K, Walsh S, Savla J, Das LT, Park SY, Silberstein LE, Dos Remedios CG, Graham D, Colan S and Kuhn B. Cardiomyocyte proliferation contributes to heart growth in young humans. Proc Natl Acad Sci U S A. 2013;110:1446–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yutzey KE. Cardiomyocyte Proliferation: Teaching an Old Dogma New Tricks. Circ Res. 2017;120:627–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Porrello ER, Mahmoud AI, Simpson E, Hill JA, Richardson JA, Olson EN and Sadek HA. Transient regenerative potential of the neonatal mouse heart. Science. 2011;331:1078–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen JF, Murchison EP, Tang R, Callis TE, Tatsuguchi M, Deng Z, Rojas M, Hammond SM, Schneider MD, Selzman CH, Meissner G, Patterson C, Hannon GJ and Wang DZ. Targeted deletion of Dicer in the heart leads to dilated cardiomyopathy and heart failure. Proc Natl Acad Sci U S A. 2008;105:2111–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thum T, Galuppo P, Wolf C, Fiedler J, Kneitz S, van Laake LW, Doevendans PA, Mummery CL, Borlak J, Haverich A, Gross C, Engelhardt S, Ertl G and Bauersachs J. MicroRNAs in the human heart: a clue to fetal gene reprogramming in heart failure. Circulation. 2007;116:258–67. [DOI] [PubMed] [Google Scholar]
- 7.van Rooij E, Sutherland LB, Liu N, Williams AH, McAnally J, Gerard RD, Richardson JA and Olson EN. A signature pattern of stress-responsive microRNAs that can evoke cardiac hypertrophy and heart failure. Proc Natl Acad Sci U S A. 2006;103:18255–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Porrello ER, Mahmoud AI, Simpson E, Johnson BA, Grinsfelder D, Canseco D, Mammen PP, Rothermel BA, Olson EN and Sadek HA. Regulation of neonatal and adult mammalian heart regeneration by the miR-15 family. Proc Natl Acad Sci U S A. 2013;110:187–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eulalio A, Mano M, Dal Ferro M, Zentilin L, Sinagra G, Zacchigna S and Giacca M. Functional screening identifies miRNAs inducing cardiac regeneration. Nature. 2012;492:376–81. [DOI] [PubMed] [Google Scholar]
- 10.Lesizza P, Prosdocimo G, Martinelli V, Sinagra G, Zacchigna S and Giacca M. Single-Dose Intracardiac Injection of Pro-Regenerative MicroRNAs Improves Cardiac Function After Myocardial Infarction. Circ Res. 2017;120:1298–1304. [DOI] [PubMed] [Google Scholar]
- 11.Tian Y, Liu Y, Wang T, Zhou N, Kong J, Chen L, Snitow M, Morley M, Li D, Petrenko N, Zhou S, Lu M, Gao E, Koch WJ, Stewart KM and Morrisey EE. A microRNA-Hippo pathway that promotes cardiomyocyte proliferation and cardiac regeneration in mice. Sci Transl Med. 2015;7:279ra38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khan M, Nickoloff E, Abramova T, Johnson J, Verma SK, Krishnamurthy P, Mackie AR, Vaughan E, Garikipati VN, Benedict C, Ramirez V, Lambers E, Ito A, Gao E, Misener S, Luongo T, Elrod J, Qin G, Houser SR, Koch WJ and Kishore R. Embryonic stem cell-derived exosomes promote endogenous repair mechanisms and enhance cardiac function following myocardial infarction. Circ Res. 2015;117:52–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Y, Baskerville S, Shenoy A, Babiarz JE, Baehner L and Blelloch R. Embryonic stem cell-specific microRNAs regulate the G1-S transition and promote rapid proliferation. Nat Genet. 2008;40:1478–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lichner Z, Pall E, Kerekes A, Pallinger E, Maraghechi P, Bosze Z and Gocza E. The miR-290–295 cluster promotes pluripotency maintenance by regulating cell cycle phase distribution in mouse embryonic stem cells. Differentiation. 2011;81:11–24. [DOI] [PubMed] [Google Scholar]
- 15.Melton C, Judson RL and Blelloch R. Opposing microRNA families regulate self-renewal in mouse embryonic stem cells. Nature. 2010;463:621–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cao Y, Guo WT, Tian S, He X, Wang XW, Liu X, Gu KL, Ma X, Huang D, Hu L, Cai Y, Zhang H, Wang Y and Gao P. miR-290/371-Mbd2-Myc circuit regulates glycolytic metabolism to promote pluripotency. EMBO J. 2015;34:609–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cannavo A, Rengo G, Liccardo D, Pun A, Gao E, George AJ, Gambino G, Rapacciuolo A, Leosco D, Ibanez B, Ferrara N, Paolocci N and Koch WJ. beta1-Blockade Prevents Post-Ischemic Myocardial Decompensation Via beta3AR-Dependent Protective Sphingosine-1 Phosphate Signaling. J Am Coll Cardiol. 2017;70:182–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bailey BA and Houser SR. Calcium transients in feline left ventricular myocytes with hypertrophy induced by slow progressive pressure overload. J Mol Cell Cardiol. 1992;24:365–73. [DOI] [PubMed] [Google Scholar]
- 19.Nuss HB and Houser SR. T-type Ca2+ current is expressed in hypertrophied adult feline left ventricular myocytes. Circ Res. 1993;73:777–82. [DOI] [PubMed] [Google Scholar]
- 20.Khan M, Mohsin S, Avitabile D, Siddiqi S, Nguyen J, Wallach K, Quijada P, McGregor M, Gude N, Alvarez R, Tilley DG, Koch WJ and Sussman MA. beta-Adrenergic regulation of cardiac progenitor cell death versus survival and proliferation. Circ Res. 2013;112:476–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mohsin S, Khan M, Toko H, Bailey B, Cottage CT, Wallach K, Nag D, Lee A, Siddiqi S, Lan F, Fischer KM, Gude N, Quijada P, Avitabile D, Truffa S, Collins B, Dembitsky W, Wu JC and Sussman MA. Human cardiac progenitor cells engineered with Pim-I kinase enhance myocardial repair. J Am Coll Cardiol. 2012;60:1278–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blenck CL, Harvey PA, Reckelhoff JF and Leinwand LA. The Importance of Biological Sex and Estrogen in Rodent Models of Cardiovascular Health and Disease. Circ Res. 2016;118:1294–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cavasin MA, Tao Z, Menon S and Yang XP. Gender differences in cardiac function during early remodeling after acute myocardial infarction in mice. Life Sci. 2004;75:2181–92. [DOI] [PubMed] [Google Scholar]
- 24.Landgraf P, Rusu M, Sheridan R, Sewer A, Iovino N, Aravin A, Pfeffer S, Rice A, Kamphorst AO, Landthaler M, Lin C, Socci ND, Hermida L, Fulci V, Chiaretti S, Foa R, Schliwka J, Fuchs U, Novosel A, Muller RU, Schermer B, Bissels U, Inman J, Phan Q, Chien M, Weir DB, Choksi R, De Vita G, Frezzetti D, Trompeter HI, Hornung V, Teng G, Hartmann G, Palkovits M, Di Lauro R, Wernet P, Macino G, Rogler CE, Nagle JW, Ju J, Papavasiliou FN, Benzing T, Lichter P, Tam W, Brownstein MJ, Bosio A, Borkhardt A, Russo JJ, Sander C, Zavolan M and Tuschl T. A mammalian microRNA expression atlas based on small RNA library sequencing. Cell. 2007;129:1401–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yuan K, Ai WB, Wan LY, Tan X and Wu JF. The miR-290–295 cluster as multi-faceted players in mouse embryonic stem cells. Cell Biosci. 2017;7:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kalucka J, Missiaen R, Georgiadou M, Schoors S, Lange C, De Bock K, Dewerchin M and Carmeliet P. Metabolic control of the cell cycle. Cell Cycle. 2015;14:3379–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Attwooll C, Lazzerini Denchi E and Helin K. The E2F family: specific functions and overlapping interests. EMBO J. 2004;23:4709–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zovoilis A, Smorag L, Pantazi A and Engel W. Members of the miR-290 cluster modulate in vitro differentiation of mouse embryonic stem cells. Differentiation. 2009;78:69–78. [DOI] [PubMed] [Google Scholar]
- 29.Hanina SA, Mifsud W, Down TA, Hayashi K, O’Carroll D, Lao K, Miska EA and Surani MA. Genome-wide identification of targets and function of individual MicroRNAs in mouse embryonic stem cells. PLoS Genet. 2010;6:e1001163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harvey SL, Charlet A, Haas W, Gygi SP and Kellogg DR. Cdk1-dependent regulation of the mitotic inhibitor Wee1. Cell. 2005;122:407–20. [DOI] [PubMed] [Google Scholar]
- 31.Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabe-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H, Jovinge S and Frisen J. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ahuja P, Sdek P and MacLellan WR. Cardiac myocyte cell cycle control in development, disease, and regeneration. Physiol Rev. 2007;87:521–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maillet M, van Berlo JH and Molkentin JD. Molecular basis of physiological heart growth: fundamental concepts and new players. Nat Rev Mol Cell Biol. 2013;14:38–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xin M, Olson EN and Bassel-Duby R. Mending broken hearts: cardiac development as a basis for adult heart regeneration and repair. Nat Rev Mol Cell Biol. 2013;14:529–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pasumarthi KB and Field LJ. Cardiomyocyte cell cycle regulation. Circ Res. 2002;90:1044–54. [DOI] [PubMed] [Google Scholar]
- 36.Chaudhry HW, Dashoush NH, Tang H, Zhang L, Wang X, Wu EX and Wolgemuth DJ. Cyclin A2 mediates cardiomyocyte mitosis in the postmitotic myocardium. J Biol Chem. 2004;279:35858–66. [DOI] [PubMed] [Google Scholar]
- 37.Chen J, Huang ZP, Seok HY, Ding J, Kataoka M, Zhang Z, Hu X, Wang G, Lin Z, Wang S, Pu WT, Liao R and Wang DZ. mir-17–92 cluster is required for and sufficient to induce cardiomyocyte proliferation in postnatal and adult hearts. Circ Res. 2013;112:1557–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guo WT, Wang XW, Yan YL, Li YP, Yin X, Zhang Q, Melton C, Shenoy A, Reyes NA, Oakes SA, Blelloch R and Wang Y. Suppression of epithelial-mesenchymal transition and apoptotic pathways by miR-294/302 family synergistically blocks let-7-induced silencing of self-renewal in embryonic stem cells. Cell Death Differ. 2015;22:1158–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zheng GX, Ravi A, Calabrese JM, Medeiros LA, Kirak O, Dennis LM, Jaenisch R, Burge CB and Sharp PA. A latent pro-survival function for the mir-290–295 cluster in mouse embryonic stem cells. PLoS Genet. 2011;7:e1002054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bicknell KA, Coxon CH and Brooks G. Forced expression of the cyclin B1-CDC2 complex induces proliferation in adult rat cardiomyocytes. Biochem J. 2004;382:411–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mohamed TMA, Ang YS, Radzinsky E, Zhou P, Huang Y, Elfenbein A, Foley A, Magnitsky S and Srivastava D. Regulation of Cell Cycle to Stimulate Adult Cardiomyocyte Proliferation and Cardiac Regeneration. Cell. 2018;173:104–116 e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rota M, Hosoda T, De Angelis A, Arcarese ML, Esposito G, Rizzi R, Tillmanns J, Tugal D, Musso E, Rimoldi O, Bearzi C, Urbanek K, Anversa P, Leri A and Kajstura J. The young mouse heart is composed of myocytes heterogeneous in age and function. Circ Res. 2007;101:387–99. [DOI] [PubMed] [Google Scholar]
- 43.Patterson M, Barske L, Van Handel B, Rau CD, Gan P, Sharma A, Parikh S, Denholtz M, Huang Y, Yamaguchi Y, Shen H, Allayee H, Crump JG, Force TI, Lien CL, Makita T, Lusis AJ, Kumar SR and Sucov HM. Frequency of mononuclear diploid cardiomyocytes underlies natural variation in heart regeneration. Nat Genet. 2017;49:1346–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bettencourt-Dias M, Mittnacht S and Brockes JP. Heterogeneous proliferative potential in regenerative adult newt cardiomyocytes. J Cell Sci. 2003;116:4001–9. [DOI] [PubMed] [Google Scholar]
- 45.Lien CL, Schebesta M, Makino S, Weber GJ and Keating MT. Gene expression analysis of zebrafish heart regeneration. PLoS Biol. 2006;4:e260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Soufan AT, van den Berg G, Ruijter JM, de Boer PA, van den Hoff MJ and Moorman AF. Regionalized sequence of myocardial cell growth and proliferation characterizes early chamber formation. Circ Res. 2006;99:545–52. [DOI] [PubMed] [Google Scholar]
- 47.Quaife-Ryan GA, Sim CB, Ziemann M, Kaspi A, Rafehi H, Ramialison M, El-Osta A, Hudson JE and Porrello ER. Multicellular Transcriptional Analysis of Mammalian Heart Regeneration. Circulation. 2017;136:1123–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.