Abstract
Attention involves three distinct networks for alerting, orienting, and executive control. Interventions targeting the specific attentional networks remain lacking. Transcranial direct current stimulation (tDCS) has been shown to modulate cortical excitability, which potentially serves as an interventional tool to treat individuals with attention impairment. The purpose of this study was to examine the effects of applying tDCS over the right posterior parietal cortex (PPC) on the performance of the three attentional networks. 26 healthy young adults performed the Attention Network Test (ANT) before and after anodal or sham tDCS stimulation over the right PPC. The alerting, orienting, and executive effects were assessed before and after the stimulation. The results demonstrated that the orienting effect was significantly improved after real tDCS relative to sham, whereas the alerting and executive control effects remained unaffected. Consistent with previous clinical and functional imaging studies, this suggests that the right PPC is actively engaged with the spatial orienting of attention.
Keywords: Transcranial direct current stimulation (tDCS), Right posterior parietal cortex (PPC), Orienting attention, Attention Network Test (ANT)
Introduction
Attention refers to a series of cognitive operations in selecting, filtering, and using information for further processing. Posner and Peterson (1990; 2012) describe attention as a system and categorize attention into three networks - alerting, orienting and executive – each with its own anatomy, circuitry and function. The alerting network involves triggering and sustaining an arousal status and engages the reticular activating system located in the brainstem and thalamus as well as the parietal and frontal cortical sites related to the norepinephrine system (Moruzzi & Magoun, 1995; Aston-Jones & Cohen, 2005). The (spatial) orienting network modulates the ability to prioritize sensory inputs by selecting a location relevant to a behavioral goal. This network relies on cortical areas such as the superior and inferior parietal areas and the frontal eye fields, as well as subcortical areas, such as the pulvinar thalamus and the superior colliculus (Posner, 1980; Corbetta & Shulman, 2002). The executive network is associated with conflict resolution and mainly relies on the anterior cingulate gyrus and prefrontal cortex (Dosenbach et al., 2008; Cieslik et al., 2013). Although these three systems are independent, they work closely together to accomplish goal-directed tasks (Raz & Buhle, 2006; Petersen & Posner, 2012).
Neuroimaging, neurophysiology, and neuropsychology studies have consistently supported the critical role of the posterior parietal cortex (PPC) for directing attention (Rosner & Mittleman, 1996; Eimer, 1998; Corbetta et al., 2000; Corbetta & Shulman, 2002; Behrmann et al., 2004; Thakral & Slotnick, 2009). The PPC is located at the portion of parietal cortex posterior to the primary somatosensory cortex. The intraparietal sulcus (IPS) separates the PPC into the superior parietal lobe (SPL) and the inferior parietal lobe (IPL). The SPL and IPL represent two biasing mechanisms: SPL, along with the superior frontal cortex, contributes to top-down or goal-directed actions, whereas IPL, along with the inferior frontal cortex, contributes to bottom-up or stimulus-driven actions (Corbetta & Shulman, 2002). It has been shown that the neural activity in the PPC increases prior to visual stimulus appearance, indicating that the signals are biased in favor of the attended location (Kastner et al., 1999). Moreover, Kravitz and colleagues (2011) have suggested that the PPC contributes to the selection of relevant visuospatial signals for goal-directed movements and that there are hemispheric specializations to these contributions. Specifically, the right PPC carries and processes visuospatial information from both the left and right visual fields (Heilman & Van Den Abell, 1980) and directs spatial attention signals toward either side of space (Szczepanski et al., 2010), whereas the left PPC contributes to only the right visual field and does not carry spatial attention signals.
Transcranial direct current stimulation (tDCS) is a non-invasive and well-tolerated brain stimulation technique to modulate cortical excitability and to probe cortical function (Nitsche & Paulus, 2000). Through animal studies, it has been shown that applying positive or negative direct currents to the cortical surface leads to neuronal activation or inhibition, respectively (Creutzfeldt et al., 1962; Bindman et al., 1964). Furthermore, these polarizing effects can last for 15–30 minutes after 10–15 minutes of continuous stimulation (Bishop & O’leary, 1950; Creutzfeldt et al., 1962; Bindman et al., 1964; Purpura & McMurtry, 1965). Human studies also found neurophysiological intra- and after- effects of tDCS (Nitsche & Paulus, 2011; Stagg & Nitsche, 2011; Lauro et al., 2014). Nitsche and colleagues (2000; 2004; 2005) used transcranial magnetic stimulation (TMS) to verify cortico-spinal and intra-cortical excitability changes during and after a short period of tDCS stimulation applied over the motor cortex. Their results suggested that anodal stimulation of the motor cortex enhanced cortical excitability and cathodal stimulation inhibited cortical excitability based on significant differences in the TMS input-output curve (I-O curve), short interval intra-cortical inhibition (SICI) and intra-cortical facilitation (ICF) (Nitsche et al., 2005). In particular, during stimulation, tDCS was thought to modulate the resting membrane potential as anodal tDCS enhanced motor-evoked potential (MEP) amplitudes relative to sham tDCS values whereas cathodal stimulation diminished MEP amplitudes relative to sham tDCS. After tDCS, its effects were suggested to be dependent upon the shifts in intra-cortical inhibition and facilitation as anodal tDCS reduced SICI and enhanced ICF, whereas cathodal tDCS enhanced SICI and reduced ICF. These modulations could be further explained by changes in sodium and calcium ion channel permeability (Nitsche et al., 2004; 2005), neurotransmitter release (Cambieri et al., 2012) and/or neurotrophic factors (Antal, Chaieb, et al., 2010; Antal, Terney, et al., 2010; Fritsch et al., 2010).
How tDCS modulates the PPC, especially in its effects on the attention components, remains unclear. Behavioral studies suggest anodal tDCS to the right parietal lobe could enhance multisensory spatial orienting (Bolognini et al., 2010), visual memory (Jones & Berryhill, 2012), visuospatial localization (Wright & Krekelberg, 2014) and learning for concealed object detection (Clark et al., 2012). tDCS has been shown to enhance the alerting effect when delivered over the right ventrolateral prefrontal cortex (Coffman et al., 2012), but there is surprisingly no work examining the impact of tDCS delivered over PPC sites on attentional network function. Neurophysiological studies provided the baseline effects on enhanced neurotransmission, large-scale network connectivity and cerebral excitability after anodal tDCS over the PPC via proton magnetic resonance spectroscopy (1H-MRS), resting-state functional magnetic resonance imaging (rs-fMRI) and a combination of TMS and electroencephalography (EEG). Studies have found that tDCS over right parietal cortex increased glutamatergic concentration under the electrode (Clark et al., 2011) and functional connectivity in the parietal-frontal network (Hunter et al., 2015) but these findings were not linked with alterations in the attention network components. Finally, Lauro and coworkers (2014) found that anodal tDCS over the right PPC increased global cortical excitability for up to 15 minutes after the end of the stimulation. Whether these neurophysiological after-effects modulate attention, and particularly which component of attention, is currently unclear.
The purpose of this study therefore was to apply anodal tDCS over the right PPC and examine the effects on several different components of attention. In particular, we used the Attention Network Test (ANT) to probe the alerting, orienting, and executive attentional networks. We hypothesized that the orienting network would be enhanced after anodal tDCS stimulation to the right PPC due to its central role in the processing of visuospatial attention signals. We also hypothesized the alerting network would be influenced after anodal tDCS to the right PPC because it is an important node in this network. By contrast, we hypothesized that the executive network would be much less affected by this stimulation.
Methods
Subjects
26 healthy young adults participated in this study (13 males/13 females; age: 24.4±4.0 years; education: 16.8±1.9 years). All subjects had no known history of neurological or psychiatric disorders, had normal or corrected-to-normal vision and were right-handed as determined via the Edinburgh Handedness Inventory (Oldfield, 1971). Subjects were asked to avoid any intake of alcohol or caffeine for the 24 hours prior to testing. Written informed consent form was obtained prior to their participation in the study. The ethical approval of the study was granted by the Institutional Review Board at the University of Oregon.
Protocol
Subjects visited the laboratory twice at least one week apart for anodal or sham transcranial direct current stimulation (tDCS). The stimulation condition order was randomized for each subject. For each visit, the Attention Network Test (ANT) was performed before and after receiving stimulation (Figure 1). During the anodal condition, a constant current of 1.5mA was applied for 20 minutes. For the sham condition, no constant current was delivered except in the first and last 30 seconds ramping down and up, respectively. A Soterix Medial 1×1 tDCS device (Sotetix Medical Inc., New York, NY) was used to deliver low-intensity direct current. The anode electrode was placed over P4 according to the international 10–20 EEG system for the right posterior parietal cortex (PPC). The reference site was placed above the left supraorbital ridge. The electrodes were inserted into a 5 cm × 7 cm EASYpadTM (Soterix Medical Inc., New York, NY) soaked with approximately 14 ml saline per sponge. Prior to participation, each subject was screened by a safety questionnaire to avoid any potential risks. Following each session, questionnaire was administrated to monitor possible side effects.
Figure 1. Experimental protocol.
Subjects performed the attention network test (ANT) before and after anodal or sham tDCS. Two visits were made for anodal and sham tDCS conditions.
Attention Network Test (ANT)
The Attention Network Test (ANT) (Fan et al., 2002) measures the efficiency of three attentional networks - alerting, orienting, and executive attention - in a ~20-min testing session which was presented to the subject through a Java program developed by the Sackler Institute for Developmental Psychobiology. During testing, subjects sat in front of a 13-inch laptop monitor (Toshiba, Portege R835) with a 50 cm viewing distance in a quiet and isolated cubicle. The instruction was to focus on the fixation cross and to press the keyboard arrow (right or left) corresponding to the direction of a target arrow appearing above or below the center fixation cross, as quickly and accurately as possible. Figure 2 displays the general sequence of one trial. First, the screen would display a central fixation cross for 400 to 1600 ms followed by a pre-cue event for 100 ms. There were four possible pre-cue conditions: no, central, double, or spatial cues. No cues indicated that no asterisk but only the central fixation cross appeared. Central cues indicated that an asterisk appeared on top of the central fixation cross. Double cues indicated that two asterisks appeared both 5° above and 5° below the central fixation cross. Spatial cues indicated that an asterisk appeared at either 5° above or 5° below the central fixation cross. These spatial cues provided valid information regarding the location of the target arrow that would subsequently be displayed after the second fixation view. Three possible flanker conditions - neutral, congruent, or incongruent – would then be displayed. In the neutral condition the target arrow appeared in isolation either above or below the central fixation cross. In the congruent condition the target arrow (the arrow in the center) and the flanker arrows (four other same-sized arrows, two to the left and two to the right of the target arrow) pointed in the same direction, whereas in the incongruent condition the target arrow pointed in the opposite direction to the flanker arrows. Upon the appearance of the arrows, subjects were instructed to press the correct keyboard arrow to match the direction of the target arrow (pointing either to right or left) as quickly and accurately as possible. For this purpose, the subjects pressed the right arrow key with the right index finger and the left arror key with the left index finger. Once the subject responded, the target arrow disappeared and the central fixation would appear until the next trial. If no response was detected, the target arrow disappeared after 1700 ms. The total duration of each trail was approximately 4 seconds.
Figure 2. Experimental procedure of Attention Network Test (ANT).
Subjects focus on the fixation cross and respond with the keyboard right or left based on the target arrow appearing above or below the fixation cross.
Each subject completed a series of 24 practice trials with visual accuracy feedback prior to the experimental trials without visual accuracy feedback. Three blocks of experimental trials were then conducted. Each block was composed of 96 trials (4 pre-cue conditions × 2 target locations (above or below) × 2 target directions (right or left) × 3 flanker conditions × 2 repetitions) for a total of 288 experimental trials. Subjects were allowed to rest between blocks until they felt ready to continue to the next block. All the subjects completed the ANT within 30 minutes.
The alerting, orienting, and executive effects were calculated based on the reaction time (RT) of the accurate experimental trials. In order to avoid the influence of the outliers and wrong responses, median reaction times for ANT conditions of no, double, central, spatial, incongruent, and congruent cues were first calculated per experimental block. Grand mean of the reaction times from the three blocks were then used to calculate the alerting, orienting, and executive effects for each person. The alerting effect was measured by the RT difference between no cue and double cues, as alerting effect refers to the ability to achieve and maintain vigilance status. The orienting effect was measured by the RT difference between central and spatial cues, as the orienting effect indicates the ability to select spatial information. The executive effect was measured by the RT difference between incongruent and congruent conditions, as executive control refers to the ability to process conflicting stimuli (Fan et al., 2002). A greater value in the alerting or orienting effects indicates more efficiency in alerting and orienting attention networks due to faster cue-related performance. A lower executive effect value indicates a better executive attention network due to a faster response to conflicting situations.
Dependent Variables and Statistical Analysis
The alerting, orienting, and executive effects derived from the ANT and their absolute changes before and after the stimulation were the dependent variables. Two-way repeated-measures ANOVAs were used to analyze the effects of tDCS on alerting, orienting, and executive attention. Model effects included tDCS conditions (real, sham), time (pre, post-tDCS), and their interaction. Tukey-Kramer post-hoc tests were further applied on significant models to identify differences between means within each dependent variable. A linear model was lastly used to detect the differences in the absolute tDCS effects between real and sham conditions. Effect sizes were also calculated to quantify the differences between two tDCS conditions. Significance level was set to p<0.05 for all analyses. All statistical analyses were conducted using JMP Pro version 13.0 (SAS Institute, Cary, NC).
Results
All 26 participants completed both real and sham visits. Stimulation was well-tolerated by all participants and no major side effects or adverse events were reported. The data were normally distributed and had acceptable homogeneity of variance.
Mean and standard error of reaction times (RTs) for each cue type and attention effect before and after real and sham tDCS were summarized in Table 1. The model effect was found only for the orienting effect [F(3,103)=3.43; p=.02] but not for the alerting [F(3,103)=2.32; p=0.08] or executive effect [F(3,103)=0.49; p=.69]. The orienting effect showed a significant interaction [F(1,103)=4.54; p=.04], a trend for significant time effect [F(1,103)=3.22; p=.07], and no significant condition effect [F(1,103)=2.52; p=.12]. The post-hoc pairwise comparison suggested that the orienting effect was significantly enhanced from 37.66 ms to 53.36 ms after real tDCS (p=.003) but not after sham tDCS (p=.82). For the alerting effect, although the interaction [F(1,103)=0.04; p=0.85] and time [F(1,103)=1.90; p=.17] effects were not significant, the condition effect was significant [F(1,103)=5.02; p=.02]. For the executive effect, no significant difference was found for time [F(1,103)=0.01; p=.90], condition [F(1,103)=1.43; p=.23], or the interaction [F(1,103)=0.03; p=.86]. None of the attention effects were changed after the sham stimulation.
Table 1.
Mean (Standard Error, SE) of reaction time (RT, ms) for each stimulation group according to six cue types and three attentional effects before and after real tDCS and sham stimulation.
| Real tDCS | Sham Stimulation | |||||
|---|---|---|---|---|---|---|
| Pre | Post | p-value | Pre | Post | p-value | |
| No cue | 569.39 | 75.74 | .63 | 589.74 | 580.00 | .49 |
| (8.6) | (9.9) | (10.9) | (9.0) | |||
| Double cue | 512.56 | 511.61 | .94 | 521.60 | 506.31 | .20 |
| (6.9) | (9.6) | (9.5) | (7.1) | |||
| Alerting effect | 56.83 | 64.13 | .28 | 68.14 | 73.69 | .40 |
| (4.5) | (4.9) | (4.8) | (4.4) | |||
| Central cue | 527.53 | 537.98 | .45 | 550.42 | 530.77 | .27 |
| (9.2) | (10.3) | (15.2) | (9.1) | |||
| Spatial cue | 489.87 | 484.62 | .67 | 497.88 | 479.58 | .20 |
| (8.8) | (8.7) | (12.4) | (7.0) | |||
| Orienting effect | 37.66 | 53.36 | .003* | 52.53 | 51.19 | .82 |
| (2.7) | (4.4) | (4.8) | (3.8) | |||
| Congruent cue | 511.89 | 512.05 | .99 | 523.06 | 509.42 | .21 |
| (8.3) | (8.3) | (9.1) | (6.6) | |||
| Incongruent cue | 622.24 | 622.18 | .99 | 639.87 | 627.12 | .45 |
| (10.4) | (10.5) | (12.4) | (11.5) | |||
| Executive effect | 110.35 | 110.12 | .97 | 116.03 | 117.69 | .85 |
| (4.7) | (5.0) | (5.6) | (6.6) | |||
indicates significant difference (p<.05).
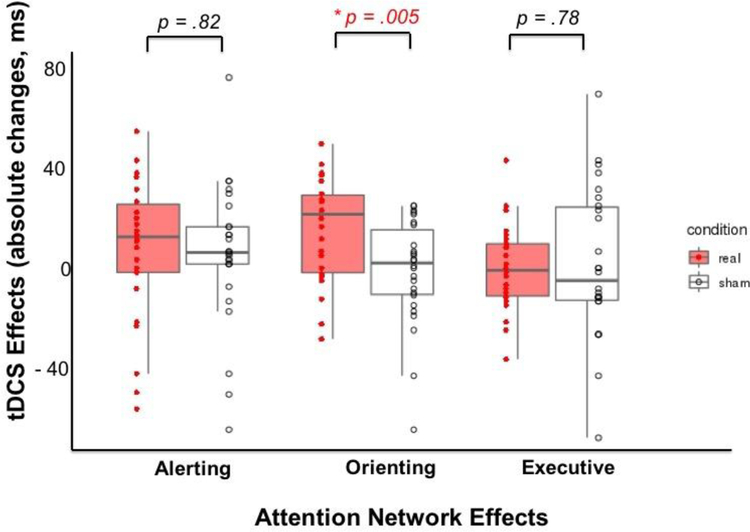
The tDCS effects for group mean and individual participants are presented in Table 2 and Figure 3. The absolute tDCS changes demonstrated that only the orienting effect was significantly improved after the real tDCS, as compared to sham tDCS [F(1,51)=8.45, p=.005, Cohen’s d=0.81]. No significant differences were observed between the real and sham stimulation for the absolute changes in the alerting [F(1,51)=0.05, p=.82, Cohen’s d =0.06] or executive effects [F(1,51)=0.07, p=.78, Cohen’s d=0.08].
Table 2.
Mean (Standard Error, SE), and their condition differences, for tDCS effects on alerting, orienting, and executive effect for real and sham conditions.
| Real | Sham | Mean difference (95% confidence interval) | p value | Cohen’s d | |
|---|---|---|---|---|---|
| Alerting | 7.30 (5.5) | 5.54(5.6) | 1.76 (−13.99, 17.5) | .82 | 0.06 |
| Orienting | 15.7(4.0) | −1.35(4.2) | 17.04 (5.3, 28.2) | .005* | 0.81 |
| Executive | −0.23(3.4) | 1.67(6.0) | 1.89 (−11.8, 15.6) | .78 | 0.08 |
indicates significant difference (p<.05).
Figure 3. tDCS effects on the alerting, orienting, and executive effects in the real (red) and sham (white) conditions.
The red and white boxplots with whiskers indicate the data values fall between the 25th and 75th percentile of the tDCS effects. The horizontal line segment inside the box represents the median. The red and white dots indicate the tDCS effects for each participant. The orienting effect was significantly enhanced after real tDCS as compared to sham tDCS.
* indicates the significant difference between real and sham stimulation.
Discussion
The purpose of this study was to examine the effects of anodal tDCS over the right PPC on the attention network. The results demonstrated a significant enhancement of the visuospatial orienting of attention after the anodal tDCS, but no changes in the function of the alerting or executive networks. The findings support our hypothesis on the critical role of PPC in orienting attention.
The ANT orienting effect was enhanced by 41.7% after a 20-minute period of anodal stimulation. Previous studies found evidence for the involvement of the parietal cortex in orienting attention through other neurophysiological tools such as fMRI (Corbetta et al., 2002), TMS (Chica et al., 2011) and intermittent theta burst stimulation (iTBS) (He et al., 2013). Roy et al. (2015) reported a significant improvement in reorienting attention after the application of anodal tDCS. Previous findings, together with our data, suggest that non-invasive brain stimulation appears to be a promising tool to enhance the visuospatial orienting of attention through modulating the cortical activity of the right PPC (Benwell et al., 2015; Moos et al., 2012; Sparing et al, 2009)
We did not find any significant tDCS effects on the alerting and executive effects. Although the posterior parietal cortex is also known as a key node of the attention network (Posner & Rothbart, 2007), our stimulating montage only influenced the orienting effect. Yin et al. (2012) investigated the posterior parietal cortex and its role with three distinct attention components with both anatomical neuroimaging and diffusion tensor imaging (DTI) data. The team reported that the alerting effect was mainly linked to the right thalamus and supplementary motor area, while the orienting effect was significantly linked with the right parietal cortex, especially the inferior parietal regions.
Interpretations of the present findings should be made with caution as the baseline values of the orienting effect prior to stimulation were noticeably different between the real and sham conditions (37.66 ms and 52.53 ms, respectively). Despite having a lower baseline value in the pre-anodal condition, a greater orienting effect was still observed in the post-anodal than in the post-sham stimulations (53.36 vs. 51.19 ms). Further analysis also confirmed that this unequal baseline did not affect the significant improvement of the oritneing effect after the real tDCS. We also noted that the increased orienting effect was attributed to the slower reaction time toward central cues after anodal tDCS, which was slower by 10.45 ms after real tDCS but was faster by about 19.65 ms after sham tDCS. However, the reaction times after the stimulation were not significantly different between the real and sham condition. We therefore suspect that these noticeable yet insignificant differences may due to the measurement or recording errors.
We hypothesized that all reaction times would be improved after anodal tDCS but that did not occur when subjects responded to central cues after anodal tDCS. Several limitations were considered. First, inter-subject variability was high. Within the orienting effect, the range of the tDCS effect was 78.5 ms (from −28.5 to 50 ms) for anodal tDCS Although most of subjects (18 out of 26) improved their orienting effect after anodal tDCS, there were some who had no improvement (1 out of 26, 0 ms) or poorer performance ( 7 out of 26, from −2.5 to −28.5). This high variability suggested there might be both responders and non-responders to anodal tDCS. In particular, different subjects could have slight variations in the brain anatomy at the P4 site, functional connectivity at that site, or tolerence for stimulation. Depending on only ANT behaviors without verifying them through other neuroimaging techniques was a limitation of this study. In this study, the same researcher placed the anodal electrode over the right PPC on every subject, based on the 10–20 EEG international system, and marked the site on the swim cap to localize the same location for the second visit. However, due to the lack of a neural navigation system and a subject-specific head model, we could not quantitatively confirm the stimulation sites between the two visits or among subjects.
This study did not include tDCS with cathode stimulation nor measure any potential effect caused by the reference electrode even though previous studies suggested cathodal tDCS over PPC could disrupt visuospatial processing (Schweid et al., 2008) or induce a neglect-like effect (Giglia et al., 2011). Although most of the studies did not consider the reference electrode influencing the cognitive behavior, this cathodal reference electrode could theoretically impact the direction of the direct current and induce inconsistent cognitive behaviors. Lastly, the ceiling effect of cognitive performance could play a role in young healthy adults. The accuracy rate of the ANT test was above 96% for all the subjects for either pre- or post-test, for both the anodal and sham conditions. There may be very little room for improvement with such a relatively easy task. Due to the enhancement nature of the anodal tDCS, the effects could thus be limited.
Acknowledgement
This study was supported in part by the Eugene and Clarissa Evonuk Memorial Graduate Fellowship.
Footnotes
Conflict of interest statement:
We, the authors of this manuscript, affirm that we have no financial affiliation or involvement with any commercial organization that has a direct financial interest in any matter included in this manuscript.
Ethical board review statement:
This study was reviewed and approved by the Institutional Review Board at University of Oregon.
Publisher's Disclaimer: This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process, which may lead to differences between this version and the Version of Record.
Reference
- Antal A, Chaieb L, Moliadze V, Monte-Silva K, Poreisz C, Thirugnanasambandam N, Nitsche MA, Shoukier M, Ludwig H, & Paulus W (2010) Brain-derived neurotrophic factor (BDNF) gene polymorphisms shape cortical plasticity in humans. Brain Stimul, 3, 230–237. [DOI] [PubMed] [Google Scholar]
- Antal A, Terney D, Kuhnl S, & Paulus W (2010) Anodal transcranial direct current stimulation of the motor cortex ameliorates chronic pain and reduces short intracortical inhibition. J. Pain Symptom Manage, 39, 890–903. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G & Cohen JD (2005) An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu. Rev. Neurosci, 28, 403–450. [DOI] [PubMed] [Google Scholar]
- Behrmann M, Geng JJ, & Shomstein S (2004) Parietal cortex and attention. Curr. Opin. Neurobio, 14, 212–217. [DOI] [PubMed] [Google Scholar]
- Benwell CS, Learmonth G, Miniussi C, Harvey M, Thut G (2015) Non-linear effects oftranscranial direct current stimulation as a function of individual baseline performance: evidence from biparietal tDCS influence on lateralized attention bias. Cortex, 69: 152–165. [DOI] [PubMed] [Google Scholar]
- Bindman LJ, Lippold OC, & Redfearn JW (1964) The action of brief polarizing currents on the cerebral cortex of the rat (1) during current flow and (2) in the production of long-lasting after-effects. J. Physiol, 172, 369–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop GH & O’leary JL (1950) The effects of polarizing currents on cell potentials and their significance in the interpretation of central nervous system activity. Electroencephalogr. Clin. Neurophysiol, 2, 401–416. [DOI] [PubMed] [Google Scholar]
- Bolognini N, Olgiati E, Rossetti A, & Maravita A (2010) Enhancing multisensory spatial orienting by brain polarization of the parietal cortex. Eur. J. Neurosci, 31, 1800–1806. [DOI] [PubMed] [Google Scholar]
- Cambieri C, Scelzo E, Pietro Li Voti, Priori A, Accornero N, & Inghilleri M (2012) Transcranial direct current stimulation modulates motor responses evoked by repetitive transcranial magnetic stimulation. Neurosci. Lett, 522, 167–171. [DOI] [PubMed] [Google Scholar]
- Chica AB, Bartolomeo P, & Valero-Cabre A (2011) Dorsal and Ventral Parietal Contributions to Spatial Orienting in the Human Brain. J. Neurosci, 31, 8143–8149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cieslik EC, Zilles K, Caspers S, Roski C, Kellermann TS, Jakobs O, Langner R, Laird AR, Fox PT, & Eickhoff SB (2013) Is there “one” DLPFC in cognitive action control? Evidence for heterogeneity from co-activation-based parcellation. Cerebral Cortex, 23, 2677–2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark VP, Coffman BA, Mayer AR, Weisend MP, Lane TDR, Calhoun VD, Raybourn EM, Garcia CM, & Wassermann EM (2012) TDCS guided using fMRI significantly accelerates learning to identify concealed objects. NeuroImage, 59, 117–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark VP, Coffman BA, Trumbo MC, & Gasparovic C (2011) Transcranial direct current stimulation (tDCS) produces localized and specific alterations in neurochemistry: A 1H magnetic resonance spectroscopy study. Neurosci. Lett, 500, 67–71. [DOI] [PubMed] [Google Scholar]
- Coffman BA, Trumbo MC, Clark VP (2012) Enhancement of object detection with transcranial direct current stimulation is associated with increased attention. BMC Neurosci, 13, 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M & Shulman GL (2002) Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci, 3, 201–215. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Kincade JM, & Shulman GL (2002) Neural systems for visual orienting and their relationships to spatial working memory. J. Cogn. Neurosci, 14, 508–523. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Kincade JM, Ollinger JM, McAvoy MP, & Shulman GL (2000) Voluntary orienting is dissociated from target detection in human posterior parietal cortex. Nat. Neurosci, 3, 292–297. [DOI] [PubMed] [Google Scholar]
- Creutzfeldt OD, Fromm GH, & Kapp H (1962) Influence of transcortical dc currents on cortical neuronal activity. Exp. Neurol, 5, 436–452. [DOI] [PubMed] [Google Scholar]
- Dosenbach NUF, Fair DA, Cohen AL, Schlaggar BL, & Petersen SE (2008) A dual-networks architecture of top-down control. Trends. Cogn. Sci, 12, 99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eimer M (1998) Mechanisms of visuospatial attention: Evidence from event-related brain potentials. Vis. Cogn, 5, 257–286. [Google Scholar]
- Fan J, McCandliss BD, Sommer T, Raz A, & Posner MI (2002) Testing the efficiency and independence of attentional networks. J. Cogn. Neurosci, 14, 340–347. [DOI] [PubMed] [Google Scholar]
- Fritsch B, Reis J, Martinowich K, Schambra HM, Ji Y, Cohen LG, & Lu B (2010) Direct Current Stimulation Promotes BDNF-Dependent Synaptic Plasticity: Potential Implications for Motor Learning. Neuron, 66, 198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giglia G, Mattaliano P, Puma A, Rizzo S, Fierro B, & Brighina F (2011) Neglect-like effects induced by tDCS modulation of posterior parietal cortices in healthy subjects. Brain Stimul, 4, 294–299. [DOI] [PubMed] [Google Scholar]
- He X, Lan Y, Xu G, Mao Y, Chen Z, Huang D, & Pei Z (2013) Frontoparietal regions may become hypoactive after intermittent theta burst stimulation over the contralateral homologous cortex in humans. J. Neurophysiol, 110, 2849–2856. [DOI] [PubMed] [Google Scholar]
- Heilman KM, Van Den Abell T (1980) Right hemisphere dominance for attention: the mechanism underlying hemispheric asymmetries of inattention (neglect). Neurology, 30, 327–330. [DOI] [PubMed] [Google Scholar]
- Hunter MA, Coffman BA, Gasparovic C, Calhoun VD, Trumbo MC, & Clark VP (2015) Baseline effects of transcranial direct current stimulation on glutamatergic neurotransmission and large-scale network connectivity. Brain Research, 1594, 92–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KT & Berryhill ME (2012) Parietal contributions to visual working memory depend on task difficulty. Front Psychiatry, 3, 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastner S, Pinsk MA, De Weerd P, Desimone R, & Ungerleider LG (1999) Increased activity in human visual cortex during directed attention in the absence of visual stimulation. Neuron, 22, 751–761. [DOI] [PubMed] [Google Scholar]
- Kravitz DJ, Saleem KS, Baker CI, & Mishkin M (2011) A new neural framework for visuospatial processing. Nat. Rev. Neurosci, 12, 217–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauro LJR, Rosanova M, Mattavelli G, Convento S, Pisoni A, Opitz A, Bolognini N, & Vallar G (2014) TDCS increases cortical excitability: Direct evidence from TMS-EEG. Cortex, 58, 99–111. [DOI] [PubMed] [Google Scholar]
- Moos K, Vossel S, Weidner R, Sparing R, Fink GR Modulation of top-down control of visual attention by cathodal tDCS over right IPS. J Neurosci, 32(46):16360–16368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moruzzi G & Magoun HW (1995) Brain Stem Reticular Formation and Activation of the EEG. 1949. J. Neuropsychiatry Clin. Neurosci, 7, 251–267. [DOI] [PubMed] [Google Scholar]
- Nitsche MA & Paulus W (2000) Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J. Physiol, 527, 633–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA & Paulus W (2011) Transcranial direct current stimulation--update 2011. Restor. Neurol. Neurosci, 29, 463–492. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Fricke K, Henschke U, Schlitterlau A, Liebetanz D, Lang N, Henning S, Tergau F, & Paulus W (2004) Pharmacological Modulation of Cortical Excitability Shifts Induced by Transcranial Direct Current Stimulation in Humans. J. Physiol, 553, 293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Seeber A, Frommann K, Klein CC, Rochford C, Nitsche MS, Fricke K, Liebetanz D, Lang N, Antal A, Paulus W, & Tergau F (2005) Modulating parameters of excitability during and after transcranial direct current stimulation of the human motor cortex. J. Physiol, 568, 291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC (1971) The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia, 9, 97–113. [DOI] [PubMed] [Google Scholar]
- Petersen SE & Posner MI (2012) The attention system of the human brain: 20 years after. Annu. Rev. Neurosci, 35, 73–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI (1980) Orienting of attention. Q. J. Exp. Psychol, 32, 3–25. [DOI] [PubMed] [Google Scholar]
- Posner MI & Petersen SE (1990) The attention system of the human brain. Annu. Rev. Neurosci, 13, 25–42. [DOI] [PubMed] [Google Scholar]
- Posner MI & Rothbart MK (2007) Research on attention networks as a model for the integration of psychological science. Annu. Rev. Psychol,58, 1–23. [DOI] [PubMed] [Google Scholar]
- Purpura DP & McMurtry JG (1965) Intracellular activities and evoked potential changes during polarization of motor cortex. J. Neurophysiol, 28, 166–185. [DOI] [PubMed] [Google Scholar]
- Raz A & Buhle J (2006) Typologies of attentional networks. Nat. Rev. Neurosci, 7, 367–379. [DOI] [PubMed] [Google Scholar]
- Rosner AL & Mittleman G (1996) Visuospatial attention in the rat and posterior parietal cortex lesions. Behav. Brain Res, 79, 69–77. [DOI] [PubMed] [Google Scholar]
- Roy LB, Sparing R, Fink GR, & Hesse MD (2015) Modulation of attention functions by anodal tDCS on right PPC. Neuropsychologia, 74, 96–107. [DOI] [PubMed] [Google Scholar]
- Schweid L, Rushmore RJ, & Valero-Cabre A (2008) Cathodal transcranial direct current stimulation on posterior parietal cortex disrupts visuo-spatial processing in the contralateral visual field. Exp. Brain Res, 186, 409–417. [DOI] [PubMed] [Google Scholar]
- Sparing R, & Thimm M (2009) Bidirectional alterations of interhemispheric parietal balance by non-invasive cortical stimulation. Brain, 132, 3011–3020. [DOI] [PubMed] [Google Scholar]
- Stagg CJ & Nitsche MA (2011) Physiological Basis of Transcranial Direct Current Stimulation. The Neuroscientist, 17, 37–53. [DOI] [PubMed] [Google Scholar]
- Szczepanski SM, Konen CS, & Kastner S (2010) Mechanisms of Spatial Attention Control in Frontal and Parietal Cortex. J. Neurosci, 30, 148–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakral PP & Slotnick SD (2009) The role of parietal cortex during sustained visual spatial attention. Brain Research, 1302, 157–166. [DOI] [PubMed] [Google Scholar]
- Wright JM & Krekelberg B (2014) Transcranial direct current stimulation over posterior parietal cortex modulates visuospatial localization. J. Vis, 14, 5–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin X, Zhao L, Xu J, Evans AC, Fan L, Ge H, Tang Y, Khundrakpa B, Wang J, Liu S (2012) Anatomical substrates of the alerting, orienting and executive control components of attention: focus on the posterior parietal lobe. PLoS One,7(11): e50590. [DOI] [PMC free article] [PubMed] [Google Scholar]