Abstract
Background:
We previously reported that maternal alcohol use significantly increases the risk of sepsis in premature and term newborns. In the mouse, fetal ethanol exposure results in an immunosuppressed phenotype for the alveolar macrophage (AM) and decreases bacterial phagocytosis. In pregnant mice, ethanol decreased AM zinc homeostasis which contributed to immunosuppression and impaired AM phagocytosis. In this study, we explored whether ethanol-induced zinc insufficiency extended to the pup AM and contributed to immunosuppression and exacerbated viral lung infections.
Methods:
C57BL/6 female mice were fed a liquid diet with 25% ethanol-derived calories or pair-fed a control diet with 25% of calories as maltose-dextrin. Some pup AMs were treated in vitro with zinc acetate before measuring zinc pools or transporter expression and bacteria phagocytosis. Some dams were fed additional zinc supplements in the ethanol or control diets and then we assessed pup AM zinc pools, zinc transporters, and the immunosuppressant TGFβ1. On post-natal day 10, some pups were given intranasal saline or Respiratory Syncytial Virus (RSV) and then AM RSV phagocytosis and the RSV burden in the airway lining fluid were assessed.
Results:
Fetal ethanol exposure decreased pup AM zinc pools, zinc transporter expression, and bacterial clearance but in vitro zinc treatments reversed these alterations. In addition, the expected ethanol-induced increase in TGFβ1 and immunosuppression were associated with decreased RSV phagocytosis and exacerbated RSV infections. However, additional maternal zinc supplements blocked the ethanol-induced perturbations in the pup AM zinc homeostasis and TGFβ1 immunosuppression thereby improving RSV phagocytosis and attenuating the RSV burden in the lung.
Conclusion:
These studies suggest that, despite normal maternal dietary zinc intake, in utero alcohol exposure results in zinc insufficiency which contributes to compromised neonatal AM immune functions thereby increasing the risk of bacterial and viral infections.
Keywords: Fetal ethanol exposure, alveolar macrophage, zinc transporters, zinc insufficiency, phagocytic function
Graphical Abstract
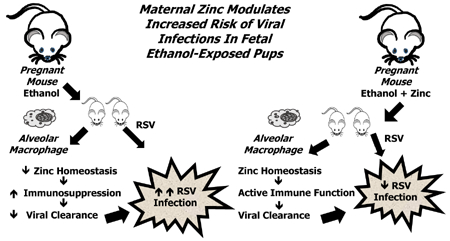
Introduction
Alcohol use prior to and during pregnancy continues to be a significant issue in our society. In a recent study, the estimated prevalence of fetal alcohol spectrum disorders among first-graders in four US communities ranged from 1.1% to 5.0% (May et al., 2018). However, the majority of infants with in utero alcohol exposure do not display the phenotypic characteristics associated with Fetal Alcohol Syndrome (Little, Snell, Rosenfeld, Gilstrap, & Gant, 1990) and, as a result, exposure often goes undetected by primary care providers. Consequently, a significant proportion of newborns are exposed to alcohol in utero but alcohol’s contribution to adverse outcomes remains unappreciated.
Chronic alcohol exposure in utero also results in a >34-fold higher risk of extreme premature delivery (<32 weeks gestational age) than non-exposed infants (Sokol et al., 2007); thereby, increasing the risk of the perinatal morbidity and mortality associated with prematurity (McCormick, 1985; Saigal & Doyle, 2008). In earlier studies, we reported that fetal alcohol exposure significantly increased the risk of sepsis in exposed premature and term newborns (Gauthier, 2015; Gauthier, Drews-Botsch, Falek, Coles, & Brown, 2005; Gauthier, Guidot, Kelleman, McCracken, & Brown, 2016). More recently, we reported that maternal report indicated that approximately one in three very low birthweight (<1500 g) premature newborns were exposed to alcohol in utero (Gauthier et al., 2016). Furthermore, alcohol exposure was associated with an alarming increased odds of developing late onset sepsis and bronchopulmonary dysplasia, two morbidities that continue to plague the very low birthweight infant.
In the airspace, the alveolar macrophage (AM) initiates and orchestrates the first immune responses against foreign particles (Fels & Cohn, 1986). However, the newborn is at higher risk for respiratory infections and injury because the AM is not as well equipped as adult AMs for essential immune responses such as chemotaxis, phagocytosis, pathogen killing, and restoration of homeostasis (Bellanti & Zeligs, 1995; Bohmwald, Espinoza, Pulgar, Jara, & Kalergis, 2017). We have shown in animal models that fetal alcohol exposure further exacerbates the already poor immune responses of the newborn lung and AM (Gauthier, Ping, Gabelaia, & Brown, 2010; Gauthier, Ping, et al., 2005; Ping, Harris, Brown, & Gauthier, 2007) resulting in exacerbation of experimental respiratory bacterial infections (Gauthier et al., 2009). The underlying mechanisms by which alcohol suppresses AM immune responses are multi-factorial but chronic oxidative stress is a central component in both the adult and fetal alcohol models (L. A. Brown, Ping, Harris, & Gauthier, 2007; Gauthier, Ping, et al., 2005; Harvey et al., 2011; Joshi et al., 2005; Liang, Harris, & Brown, 2014; Mehta et al., 2011; Mehta, Yeligar, Elon, Brown, & Guidot, 2013; Yeligar, Harris, Hart, & Brown, 2012, 2014). One mechanism observed in both adult and fetal models is that the alcohol-induced chronic oxidative stress upregulates AM expression and signaling of the immunosuppressant transforming growth factor β (TGFβ1) resulting in impaired bacterial clearance (S. D. Brown & Brown, 2012; Gauthier, Grunwell, Ping, Harris, & Brown, 2017; Yeligar et al., 2017). A central role for TGFβ1 in the AM immune suppression was further verified in these studies by demonstration that strategies to attenuate TGFβ1 signaling also restored AM pathogen clearance.
In adult models, chronic ethanol ingestion also decreases the AM zinc pool which was mimicked by treatment with TGFβ1 which similarly decreased the zinc pool (Mehta et al., 2011). The ethanol- or TGFβ1-induced decreases in the AM zinc pools were also linked to decreased expression of the cellular zinc transporters Zip1, Zip4 and ZNT1 (Curry-McCoy, Guidot, & Joshi, 2013). Equally important, zinc supplements blocked or reversed many of the negative effects of alcohol on the AM – decreased zinc transporters, decreased zinc pool, increased oxidative stress, increased TGFβ1, and impaired pathogen clearance (Curry-McCoy et al., 2013; Mehta et al., 2011).
During pregnancy, proper zinc nutrition is essential for fetal development, growth, and immune functions (Liu et al., 2018; Terrin et al., 2015; Young, Giesbrecht, Eskin, Aliani, & Suh, 2014). For example, zinc insufficiency during development results in decreased neutrophil chemotaxis and phagocytosis (Wellinghausen, 2001) as well as thymus atrophy (Dutz et al., 1976; Ferguson, 1978). In a mouse model, we demonstrated that ethanol ingestion prior to and during pregnancy decreased zinc homeostasis in the maternal AM resulting in impaired bacterial clearance, despite a well-balanced diet developed for the pregnant mouse (Konomi, Harris, Ping, Gauthier, & Brown, 2015). Whether the alcohol-induced zinc insufficiency observed in the pregnant mouse AM also results in zinc insufficiency in the newborn pup and impaired immune functions of its AM are the focus of this study.
In previous studies, we focused on the effects of fetal alcohol exposure on bacterial clearance and infection. However, lower respiratory tract infections with Respiratory Syncytial Virus (RSV) are more relevant for the newborn, particularly the premature newborn and often result in extended hospital stays, admission to intensive care, and the need for oxygen and mechanical ventilation, all of which are associated with increased hospital costs (Simoes, Anderson, Wu, & Ambrose, 2016). An immature immune system such as that observed in premature newborns results in exacerbation of RSV infections (Fonseca, Lukacs, & Ptaschinski, 2018; Paes, 2018). Further impaired development of the immune system through extrinsic and intrinsic factors such as fetal alcohol exposure may also predispose the lung to RSV infections as indicated in a fetal lamb model of in utero ethanol exposure (Ackermann, 2014; Lazic et al., 2007). Airway epithelial cells are the primary site for RSV infections but host innate immune responses such as phagocytosis and release of cytokines, chemokines, and other immune mediators are crucial in determining disease outcomes (Bohmwald et al., 2017). Since the AM is the most abundant phagocytic cell in the air space and orchestrates the respiratory immune response and inflammation, robust AM immune functions are critical for RSV clearance (Kolli et al., 2014; Ren et al., 2014). We have previously shown that TGFβ1 impairs the capacity of AM to clear RSV (Grunwell et al., 2018) but whether the increased TGFβ1 in the AM after ethanol exposure and the subsequent immunosuppression also impairs the capacity of the newborn AM to clear viruses has not been addressed.
In the current study, we examined whether the ethanol-induced zinc insufficiency observed in the AM of the pregnant mouse is extended to the ethanol-exposed pup AM and contributes to a decreased capacity to clear viruses resulting in exacerbation of an experimental RSV infection. The results of the current study demonstrated that in utero ethanol exposure decreased zinc transporters resulting in a decreased zinc pool in the pup AM. With in vitro zinc treatments, the AM zinc pools and zinc transporters were restored as well as bacterial clearance. Fetal ethanol exposure also impaired the capacity of the pup AM to phagocytose RSV resulting in exacerbation of RSV in the airway lining fluid. With supplementation of the maternal diet with additional zinc, the decreased zinc homeostasis, increased TGFβ1 and subsequent immunosuppression, impaired AM phagocytosis of RSV, and augmented RSV infection expected in the ethanol-exposed pup were normalized to control levels. Therefore, this study suggests that fetal ethanol exposure promotes dysregulation of zinc homeostasis within the alveolar space which subsequently promotes AM immunosuppression, impairs phagocytosis, and increases the risk of bacterial and viral infections.
Materials and Methods
Mouse model of in utero ethanol exposure prior to and during development.
Our model of fetal alcohol exposure is based on a continuous presentation of ethanol in a liquid diet (BioServ, Frenchtown, NJ), especially prepared for experimentation in pregnant mice. Female C57BL/6 mice shipped from the vendor (Charles River, Burlington, MA) were allowed to acclimate in the Emory Pediatrics facilities for a week. After acclimatization, the mice were introduced to the liquid diet for an additional week and then randomized to receive an isocaloric liquid diet ± 25% ethanol derived calories. For the ethanol group, the ethanol content of the diet was ramped from 0% to 12.5%, and then 25% of ethanol-derived calories over a 1 week timeframe. The control group was pair-fed to the ethanol group with 25% of the calories from maltose-dextrin. For some mice, the liquid diet was supplemented with zinc acetate (100 mg/L) as described previous for adult ethanol-fed male rats (Mehta et al., 2011; Mehta et al., 2013). The mice were placed on their assigned diet for three weeks prior to conception, during mating, throughout pregnancy, and nursing. Food consumption was recorded daily and the liquid food was changed daily. The assigned experimental liquid diet was the only access to food and water. Mice were weighed once a week prior to pregnancy. Pregnant mice were not disturbed except for food and cage changes; therefore, weights during days 8-20 of pregnancy (mouse equivalent to 2nd and 3rd trimester) were not recorded. At delivery, pups were checked for physical abnormalities and weighed. All animals were used with protocols reviewed and approved by the Emory University Institutional Animal Care Committee in accordance with NIH Guidelines (Guide for the Care and Use of Laboratory Animals).
Alveolar macrophage isolation.
After delivery and anesthesia with pentobarbital sodium intraperitoneally, the trachea of the pup was identified under a dissecting microscope and cannulated with a 27-G catheter. The lungs were serially lavaged with 40 μl sterile saline (5×) and to obtain samples of the alveolar lining fluid. After the initial lavage from each pup in a litter was pooled and centrifuged (402 g for 8 min), the supernatant was saved for further analysis. The subsequent lavages from each pup within a litter were also pooled and similarly centrifuged. The cell pellet obtained from the initial and the subsequent lavages was resuspended in RPMI 1640 1× media with 2% fetal bovine serum and antibiotics and pooled. Each litter represents an n of 1. Cell viability and count were determined with Trypan Blue stain (0.4%; Life Technologies, Grand Island, NY). DifQuik staining indicated the cells were predominantly AMs (>95%). Cells were then incubated 16 h in a 10% CO2 incubator at 37° C to mimic the airspace environment. Some pup AMs were treated with media containing 25 μM (final concentration) of sterile zinc acetate during the 16 h incubation, similar to the cells that received no additional zinc treatments. Each well received the same number of cells (1.25 × 105 cells/well).
Zinc levels in AMs.
Intracellular zinc levels in the isolated pup AMs were measured using a membrane permeable zinc specific dye, FluoZin-3AM (Invitrogen, Carlsbad, CA). FluoZin-3AM has a high affinity for zinc (Kd ~ 15 nM) and minimal interfering calcium sensitivity. FluoZin-3 is suitable for detection of zinc in the 1-100 nM range and is the most sensitive and zinc-specific of the different FluoZin fluorescent dyes. The cell permeable acetoxymethyl ester form of the fluorophore is useful for detecting low concentrations and small changes of intracellular zinc pools, has previously been shown to be an effective strategy to measure intracellular zinc pools in monocytes and AM (Haase et al., 2008; Joshi, Mehta, Jabber, Fan, & Guidot, 2009). After the AMs were incubated with FluoZin-3AM (45 min; 37°C), they were incubated for 30 min incubation with medium free of the fluorophore to allow for complete de-esterification of the intracellular acetoxymethyl ester form and subsequent fluorescence. Background fluorescence of unstained AMs was used to account for auto-fluorescence and subtracted from the RFU. Some AM were treated with the cell permeant chelating agent TPEN to confirm signal probe specificity. Cells were then washed and fixed with 3.7% paraformaldehyde and fluorescence was quantified using fluorescent microscopy (Olympus Corp, Melville, NY) and ImagePro Plus software for Windows. Data are presented as mean relative fluorescence units (RFU) per field ± S.E.M.
TGFβ1 and zinc transporter protein expression.
Some pup AM were cultured on slides, fixed with 3.7% paraformaldehyde, permeabilized with ice-cold methanol, and then evaluated for protein expression of TGFβ1 or the zinc transporters Zip1, ZnT1 and ZnT4 via immunostaining. After incubation with the primary antibody (1 h; 1:100 dilution; Santa Cruz Biotechnology, Inc., Santa Cruz, CA), the slides were washed three times with phosphate buffered saline (5 min) and then the secondary antibody was added (45 min; 1:200 dilution). Expression of TGFβ1, Zip1, ZnT1, or ZnT4 were quantified using fluorescent microscopy and ImagePro Plus for Windows. Background fluorescence of unstained AMs was used to account for autofluorescence and subtracted from the RFU for TGFβ1, Zip1, ZnT1, or ZnT4. Data are presented as mean RFU/field ± S.E.M.
Gene Expression Analysis.
For assessment in changes in mRNA expression, the AM cell pellet was resuspended in 1 mL RNALater (Sigma) and the samples were stored at −80° C until analysis. The RNA was isolated with the NucleoSpin RNA II kit with on-column genomic DNA digestion according to the manufacturer’s protocol (TaKaRa, Mountain View, CA) and mRNA quantified using a NanaDrop Fluorospectrometer (Thermo Scientific). cDNA was synthesized with a High Capacity cDNA Reverse Strand Synthesis kit (Applied Biosystems, Foster City, CA). The cDNA was then pre-amplified by PCR using the TaqMan PreAmp Master Mix according to the manufacturer’s protocol (Applied Biosystems/Thermo Fisher). Quantitative PCR (qPCR) was performed using TaqMan Gene Expression assays and Master Mix (Applied Biosystems/Thermo Fisher) on a StepOnePlus Real Time PCR System (Thermo Fisher). The mouse housekeeping gene Glucuronidase β (GUSB) was used for normalization (de Jonge et al., 2007). Each measurement was performed in duplicate and averaged. The data are reported as the mean ± SEM for pooled cells from at least three litters.
AM phagocytic index.
AMs isolated from pups were incubated overnight for 16 h, after which, pHrodo Staphylococcus aureus bioparticles conjugates (Life Technologies, Grand Island, NY) were added to the wells followed by an additional 4 h incubation. Cells were then washed with phosphate buffered saline and fixed with 3.7% paraformaldehyde. Fluorescence of phagocytosed TRITC-S.aureus was quantified by fluorescent microscopy (Olympus Corp, Melville, NY) and ImagePro Plus for Windows. Background fluorescence of unstained AMs was used to account for autofluorescence. The phagocytic index was calculated as previously described (Fitzpatrick, Holguin, Teague, & Brown, 2008b) with the percentage of cells positive for internalized fluorescence × mean relative fluorescent unit per field, as tallied from at least 10 experimental fields per set. Values are expressed as mean PI RFU/field ± S.E.M.
Experimental RSV lung Infections in the neonatal mouse.
On post-natal day 10, the neonatal mice (± ethanol and ± maternal zinc supplement) were given intranasal injections [Nanoliter Injector; 20 μl; each nasal nare; 2 × 105 plaque forming units (PFU)] of saline or RSV [line 19 RSV; generous gift from Dr. Martin Moore (Lukacs et al., 2006)]. The pups were then returned to their respective dams. After 48 h, the pups were sacrificed and the lungs lavaged. After the cells in the lavage were removed by centrifugation, the cell-free supernatant was then plated for PFU determination via the HEp-2 cells plaque assay. Some pup AM were cultured on slides, fixed with 3.7% paraformaldehyde, permeabilized with ice-cold methanol, and then evaluated for RSV phagocytosis via immunostaining. Cells were incubated with the primary antibody (1 h; a 1:100 dilution; Santa Cruz Biotechnology, Inc., Santa Cruz, CA). After washing with phosphate buffered saline, AM were incubated with the secondary antibody (anti-goat IgG; a 1:200 dilution; 45 min). RSV in the cell was quantified using fluorescent microscopy and ImagePro Plus for Windows. Background fluorescence of unstained AMs was used to account for autofluorescence and subtracted from the RFU obtained for the RSV phagocytosis. The phagocytic index was calculated as described above (Fitzpatrick, Holguin, Teague, & Brown, 2008a).
Statistical analysis.
SigmaPlot software (Systat Software; San Jose, CA) was used for statistical analysis and graph generation. ANOVA was used to detect overall differences between groups and post-hoc analysis was conducted (Tukey’s) for group comparisons. A p < 0.05 was (Konomi et al., 2015)deemed as statistically significant. Data are presented as mean ± S.E.M where each n represents one litter.
Results
Chronic ethanol ingestion by dams:
The liquid diet was well tolerated by the dams prior to conception, during mating, throughout pregnancy, and during the nursing period without significant loss of pregnancy or distress. As outlined in Table I, there were no statistical differences between the control and ethanol groups in food consumption before and during pregnancy, the number of successful pregnancies, or body weights of the dams before and after pregnancy. Similarly, there were no differences in those with zinc supplements. For the pups, there were no statistical differences between the four groups on the number of pups per litter, litter viability, or pup body weight (Table I). Fetal alcohol abnormalities were observed in some pups from one ethanol litter and no abnormalities were observed in the control group.
Table I.
Fetal data
| Control | Control + Zinc | Ethanol | Ethanol + Zinc | P | |
|---|---|---|---|---|---|
| DAMS | |||||
| Diet consumed (ml/day) | |||||
| Prior to mating | 10.1 ± 0.1 | 10.2 ± 0.1 | 10.3 ± 0.1 | 10.3 ± 0.1 | 0.218 |
| Prior to pregnancy | 12.0 ± 0.2 | 11.9 ± 0.3 | 11.4 ± 0.4 | 11.8 ± 0.2 | 0.212 |
| During pregnancy | 18.0 ± 1.1 | 18.6 ± 0.9 | 19.0 ± 0.5 | 19.1 ± 0.8 | 0.433 |
| Dam weight (g) | |||||
| Initial weight | 19.5 ± 0.2 | 19.3 ± 0.2 | 19.0 ± 0.3 | 19.2 ± 0.3 | 0.438 |
| Prior to pregnancy | 20.5 ± 0.4 | 20.6 ± 0.3 | 21.2 ± 0.5 | 20.8 ± 0.4 | 0.272 |
| After delivery | 25.9 ± 0.4 | 25.4 ± 0.3 | 25.1 ± 0.3 | 25.4 ± 0.4 | 0.301 |
| PUPS | |||||
| Successful pregnancies | 85.7% | 87.8% | 87.5% | 86.1% | |
| Total pups per litter | 8.7 ± 0.5 | 8.3 ± 0.4 | 7.4 ± 0.5 | 7.9 ± 0.6 | 0.463 |
| Live only | 7.3 ± 0.8 † | 7.5 ± 0.9 | 6.6 ± 0.8 †† | 7.0 ± 0.7 | 0.493 |
| Litter viability (%) | 84 ± 1% | 89 ± 1% | 90 ± 1% | 87 ± 0.1 | 0.591 |
| Average pup weight (g) | 1.3 ± 0.1 | 1.3 ± 0.1 | 1.3 ± 0.1 | 1.4 ± 0.1 | 0.521 |
| Litters with abnormal fetuses | 0 | 0 | 1 | 0 | |
| Cell count (cells/ml) | 1.75×105 ± 0.31×105 | 1.72×105 ± 0.33×105 | 1.68×105 ± 0.56×105 | 1.73×105 ±0.31×105 | 0.853 |
| Cell viability (%) | 73.6 ± 4.0 | 74.1 ± 3.9 | 73.9 ± 3.7 | 74.2 ± 3.8 | 0.961 |
Values represent mean ± S.E.M.
Dead pups in controls were fully developed, mom delivered past due date
Dead pups in ethanol group came from one litter and had the following abnormalities (frequency): cleft palate (1), eye abnormalities (2), very small in size (2)
Fetal ethanol exposure decreased zinc availability in the newborn pup AMs but these effects were reversed by in vitro zinc treatments.
Despite ad lib access to a liquid diet designed especially to meet the nutritional needs of pregnant mice, the AM intracellular zinc pools were decreased ~70% in the ethanol exposed group when compared to the AMs from unexposed pups (Figure 1A and 1B). However, in vitro zinc treatment of the ethanol-exposed AM restored the zinc pool to control levels. For the control group, in vitro zinc treatments did not statistically alter the AM zinc pools.
Figure 1. Fetal ethanol exposure decreased intracellular zinc levels in the AMs from newborn pups but was restored by zinc treatment.
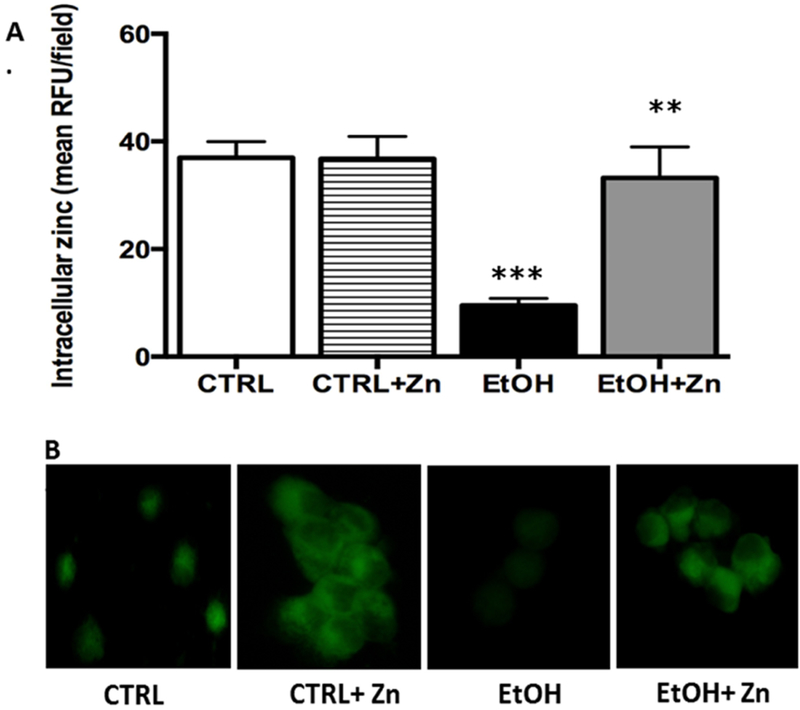
Dams were fed the control or ethanol diet during pregnancy and the AMs from the pups were isolated by lavage on the first day of life. The AM were cultured for 16 h with some AMs treated with media containing 25 μM zinc acetate. After incubation, FluoZin-3AM was added to the media (30 min.) before the cells were washed and fixed for confocal microscopy. RFUs were quantified by computerized analysis of the confocal fluorescent images and the bar heights represent the mean RFU/field ± S.E.M. from at least 6 different litters (A). Representative fluorescent images are shown for each condition (B). CTRL = control group; CTRL + Zn = control AMs treated in vitro with zinc acetate; EtOH = ethanol group; and EtOH + Zn = ethanol AMs treated in vitro with zinc acetate. *** denotes p = 0.05 when compared to the CTRL group and ** denotes p ≤ 0.05 when comparing the ETOH and ETOH + Zn groups.
Fetal ethanol exposure decreased AM protein expression of zinc transporters but these effects were ameliorated with in vitro zinc treatments.
Since the zinc pools were decreased in the ethanol-exposed neonatal AMs, we next examined whether this was due to altered expression of the zinc transporters. For Zip1, a transporter that imports zinc into the cell (Maywald, Wessels, & Rink, 2017; Wessels, Maywald, & Rink, 2017), in utero ethanol exposure significantly decreased its protein expression when compared to control AMs (Figure 2A and 2B). We next examined the ZnT transporter family, which decreases the cytoplasmic zinc pool by exporting zinc out of the cell or into intracellular compartments (Kambe, Yamaguchi-Iwai, Sasaki, & Nagao, 2004). Similar to Zip1, AMs isolated from fetal ethanol-exposed pups had ≥ 60% decrease in ZnT1 and ZnT4 expression when compared to controls (Figures 3A and 3B). These combined results suggested that the decreased zinc pools in the AM associated with fetal ethanol exposure correlated with decreased protein expression of the zinc transporters Zip1, ZnT1, and ZnT4. Furthermore, treatment of the newborn AMs with zinc restored protein expression of Zip1, ZnT1, and ZnT4 as well as restored zinc pools(Figures 2 – 4), further supporting the concept that the decreased AM zinc pools were related to the decreased protein expression of zinc transporters. Furthermore, these results suggested that the zinc concentration in the airway lining fluid modulated AM expression of zinc transporters which subsequently modulated the intracellular zinc homeostasis.
Figure 2. Fetal ethanol exposure decreased Zip1 protein expression in the AMs from newborn pups but expression was restored by zinc treatment.
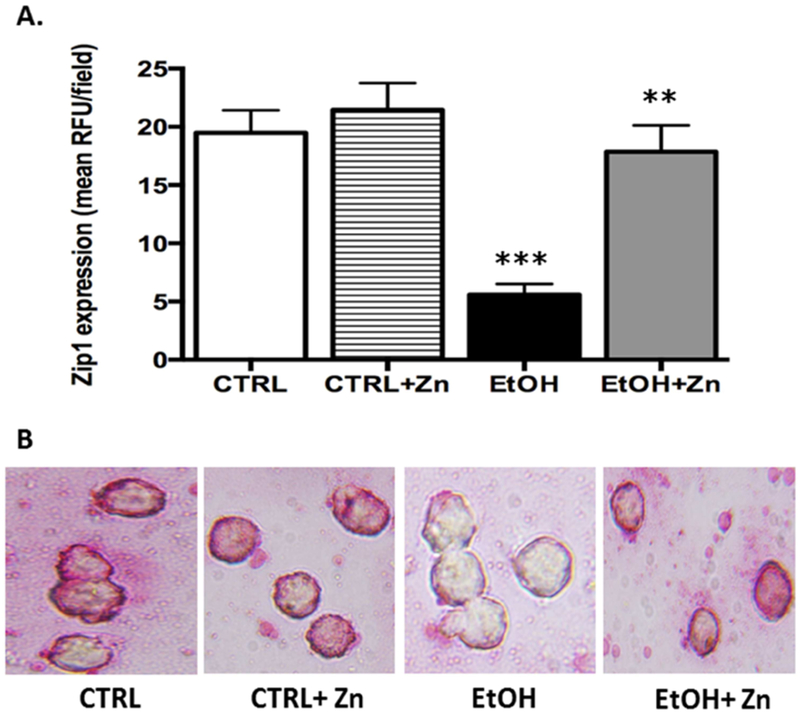
On the first day of life, the AMs from the pups (± fetal ethanol exposure) were isolated by lavage and cultured for 16 h. Zip1 protein expression in the AMs was determined via immunostaining and quantified using confocal fluorescent microscopy plus ImagePro Plus analysis. Bar heights represent mean RFU/field ± S.E.M. from at least 6 different litters per group (A). Representative fluorescent images are shown for each condition (B). CTRL = control group; CTRL + Zn = control AMs treated in vitro with zinc acetate; EtOH = ethanol group; and EtOH + Zn = ethanol AMs treated in vitro with zinc acetate. *** denotes p ≤ 0.05 when compared to the CTRL group and ** denotes p ≤ 0.5 EtOH + Zn vs. EtOH.
Figure 3. Fetal ethanol exposure decreased ZnT1 protein expression in the AMs from newborn pups but was restored by zinc treatment.
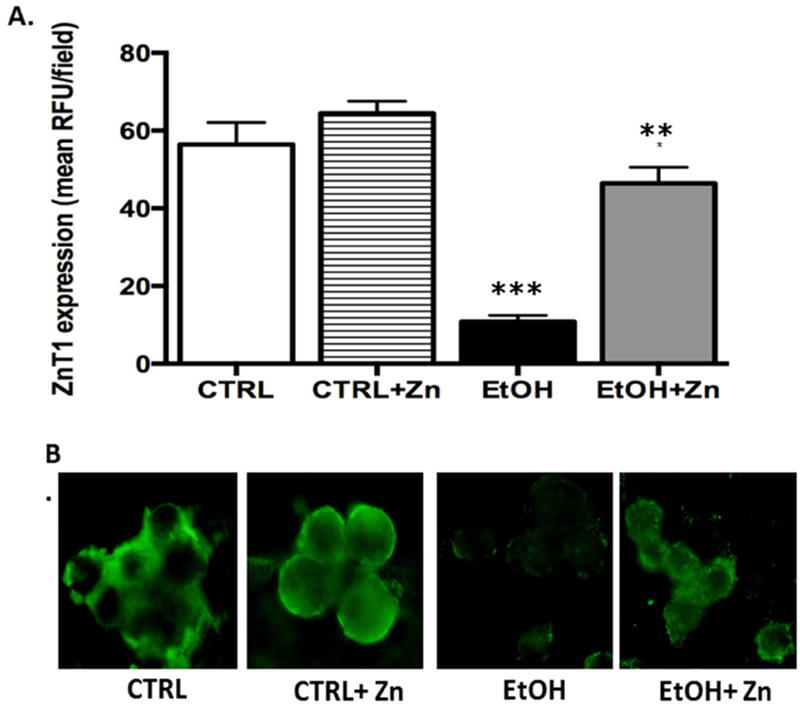
Protein expression of zinc transporter, ZnT1, in alveolar macrophages isolated from pups was determined via immunostaining and quantified using fluorescent microscope and ImagePro Plus analysis. Bar heights represent mean RFU/field ± S.E.M. from at least 5 different litters per group (A). Representative fluorescent images are shown for each condition (B). CTRL = control group; CTRL + Zn = control AMs treated in vitro with zinc acetate; EtOH = ethanol group; and EtOH + Zn = ethanol AMs treated in vitro with zinc acetate. *** denotes p ≤ 0.05 when compared to the CTRL group and ** denotes p ≤ 0.05 EtOH + Zn vs. EtOH.
Figure 4. Fetal ethanol exposure decreased ZnT4 protein expression in the AMs from newborn pups but expression was restored by zinc treatment.
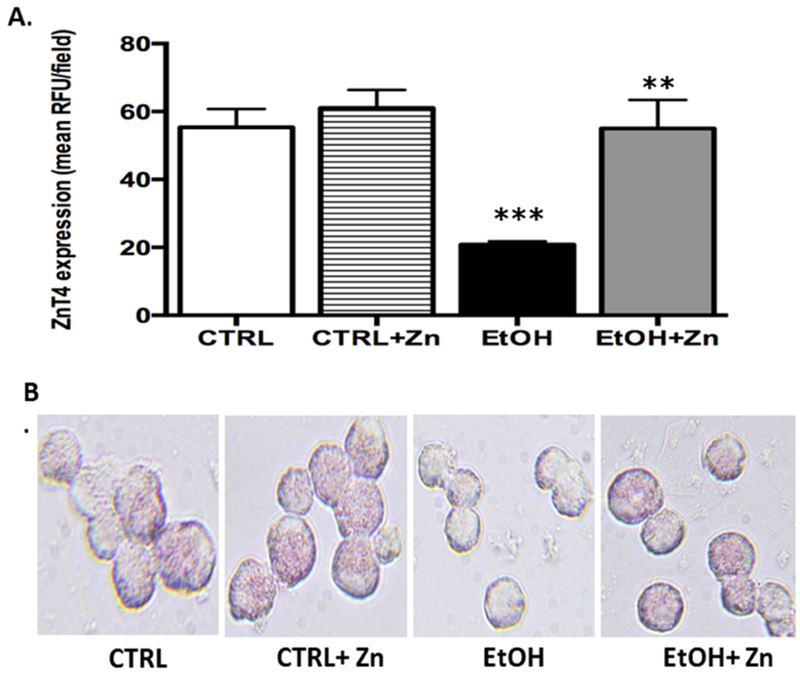
Protein expression of zinc transporter ZnT4 was determined and quantified similar to other transporters. Bar heights represent mean RFU/field ± S.E.M. from at least 5 different litters per group (A). Representative fluorescent images are shown for each condition (B). CTRL = control group; CTRL + Zn = control AMs treated in vitro with zinc acetate; EtOH = ethanol group; and EtOH + Zn = ethanol AMs treated in vitro with zinc acetate. *** denotes p ≤ 0.05 when compared to the CTRL group and ** denotes p ≤ 0.05 EtOH + Zn vs. EtOH.
Ethanol-induced decreases in zinc availability were associated with decreased AM phagocytosis but was improved with in vitro zinc treatments.
To determine if decreased zinc availability contributed to the expected decrease in bacterial clearance associated with ethanol-induced immunosuppression, pup AM were treated with zinc acetate. With the in vitro zinc treatments, the decreased bacterial phagocytosis expected for the ethanol-exposed AM was reversed (Figure 5A and B). The improvements in bacterial clearance by the ethanol-exposed AMs by the in vitro zinc treatments included the overall fluorescence per field indicating increased phagocytosis/cell and in the percent of cells that internalized bacteria when compared to the ethanol group (Table II). While the overall fluorescence for the ethanol + zinc group improved and were not statistically different from the control group, it remained significantly lower than the control + zinc group. For the control group, in vitro zinc treatments did not statistically affect phagocytosis.
Figure 5. The bacterial phagocytic index in the AMs from the newborn pups was decreased by fetal ethanol exposure but in vitro zinc treatments restored clearance.
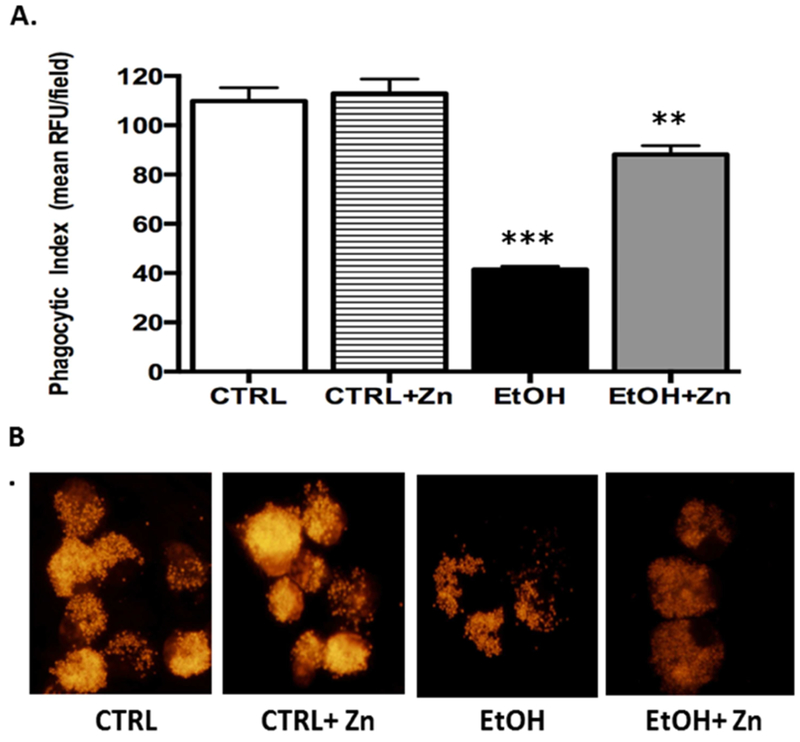
Inactivated TRITC-labeled Staphylococcus aureus bacteria was added to the cells and incubated for another 4 h. Internalization of TRITC-labeled S. aureus was determined using confocal fluorescent analysis. Phagocytic index (PI) was calculated as the percentage of cells with internalized fluorescence × the mean RFU per field. Bar heights represent mean PI relative to the control ± S.E.M. from at least 5 separate litters (A). Representative fluorescent images are shown for each condition (B). CTRL = control group; CTRL + Zn = AMs treated in vitro with zinc acetate; EtOH = ethanol group; and EtOH + Zn = ethanol AMs treated in vitro with zinc acetate. *** denotes p ≤ 0.05 when compared to the CTRL group and ** denotes p ≤ 0.05 EtOH + Zn vs. EtOH.
Figure 5 C.
Summary of bacterial clearance by alveolar macrophages
| CTRL | CTRL + Zn | EtOH | EtOH+Zn | p | |
|---|---|---|---|---|---|
| Mean RFU /field | 110.7 | 114 | 52.5 *** | 92.4 * | <0.001 |
| Percent of positive cells | 99.3 | 98.9 | 83.0 *** | 97.4 | <0.001 |
| Phagocytic index | 109.9 | 112.7 | 43.2 *** | 89.7 ** | <0.001 |
RFU = relative fluorescence units
Phagocytic index = mean RFU/field × % of positive cells
Values represent mean from at least 5 litters/group
ANOVA was performed for multiple comparisons followed by post-hoc tests (Tukey’s) for pairwise comparisons
p < 0.05 CTRL + Zn vs. EtOH + Zn
p < 0.01 CTRL, CTRL + Zn vs. EtOH + Zn
p < 0.001 CTRL, CTRL + Zn, EtOH + Zn vs. EtOH
Maternal zinc supplements blocked the expected decrease in homeostasis and subsequent AM immunosuppression associated with fetal ethanol exposure.
We first assessed the effects of maternal zinc supplements on mRNA expression of zinc transporters and the zinc pool in the pup AM. For the control group, addition of zinc supplements to the dam’s diet did not statistically alter Zip1 mRNA expression but resulted in a two-fold increase in ZnT1 mRNA expression (Figure 6). The signal for ZnT4 expression was too low to obtain statistical significance. The zinc pools in the AMs were not statistically altered when compared to the controls without additional zinc (Figure 7A and 7B) suggesting the increased expression of ZnT1 was necessary to export zinc and maintain zinc homeostasis. For the ethanol group, Zip1 and ZnT1 mRNA expression in the ethanol group were 13% and 48%, respectively, of that observed for the controls (Figure 6). However, addition of zinc supplements to the diet of ethanol-fed dams normalized Zip1 and ZnT1 expression and they were no longer statistically decreased when compared to the control or the control + zinc groups. Correspondingly, this normalization of the zinc transporters resulted in AM from ethanol-exposed pups with a normalization of the zinc pool (Figure 7A and 7B).
Figure 6. Fetal ethanol exposure decreased the relative gene expression of Zip1 and ZnT1 in the AMs from the newborn pups but these decreases were blocked by maternal zinc supplements.
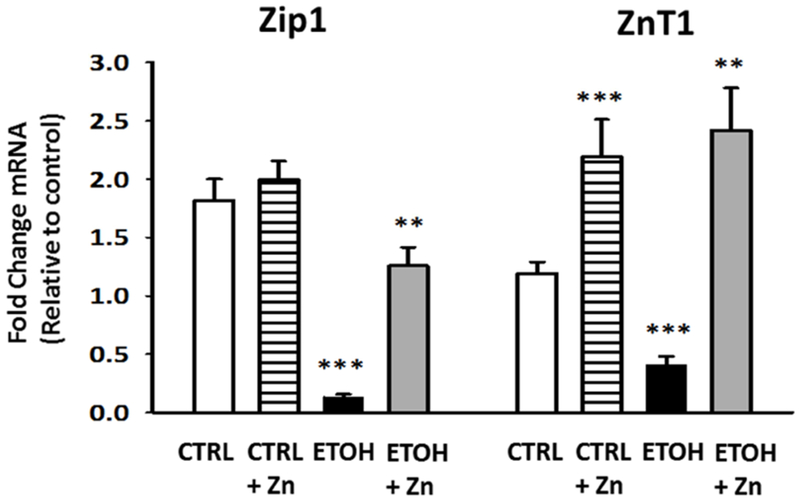
Dams were fed the control or ethanol diets ± zinc acetate supplements (100 mg/L). On day 10 of life, the AMs from the pups (± fetal ethanol exposure) were isolated by lavage and the relative gene expression of the zinc transporters Zip1 and ZnT1 was determined. The average relative gene expression of Zip1 and ZnT1 normalized to GUSB were determined by the comparative method (2−ΔΔCt), with the target gene expression in control AM set as 1 in each case. Each sample was analyzed in duplicate and each value represents the mean ± SEM of at least four separate litters. CTRL = pup AM from control dams; CTRL + Zn = pup AM from control dams with dietary zinc supplements; EtOH = pup AM from ethanol-fed dams; and EtOH + Zn = pup AM from ethanol-fed dams with dietary zinc supplements. *** denotes p ≤ 0.05 when compared to the CTRL group and ** denotes p ≤ 0.05 EtOH + Zn vs. EtOH.
Figure 7. Maternal zinc supplements maintained the neonatal AM zinc pools despite fetal ethanol exposure.
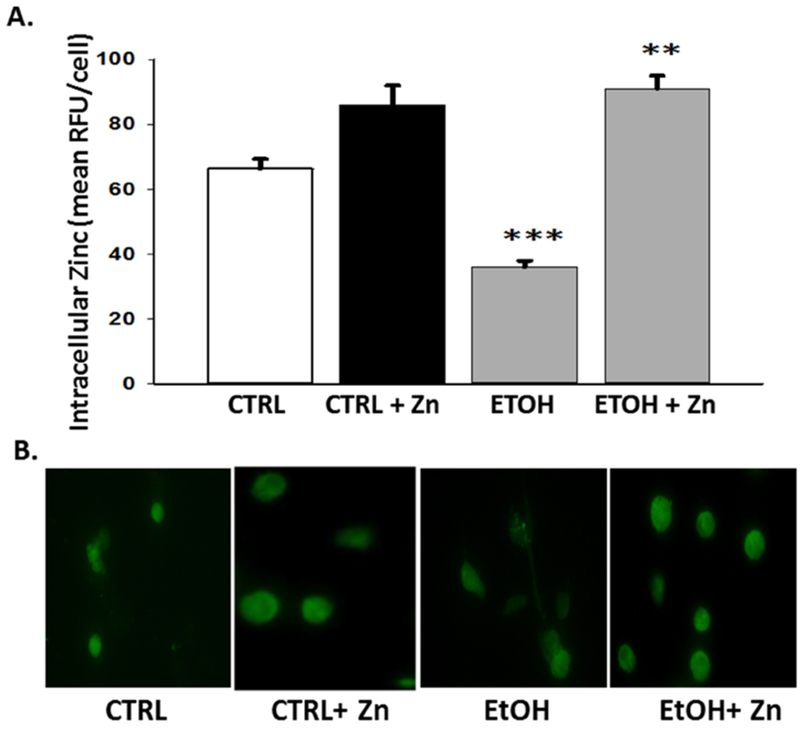
Dams were fed the control or ethanol diets ± zinc acetate supplements (100 mg/L) during pregnancy. On day 10 of life, the AMs from the pups (± fetal ethanol exposure) were isolated by lavage and FluoZin-3AM was added to the incubation media (45 min; 37°C). After a 30 min incubation with medium free of the fluorophore, the cells were fixed and fluorescence determined by confocal fluorescent microscopy. RFUs were quantified by computerized analysis and expressed relative to the values for the control group. Bar heights represent the mean RFU/field ± S.E.M. from at least 4 different litters (A). Representative fluorescent images are shown for each condition (B). CTRL = pup AM from control dams; CTRL + Zn = pup AM from control dams with dietary zinc supplements; EtOH = pup AM from ethanol-fed dams; and EtOH + Zn = pup AM from ethanol-fed dams with dietary zinc supplements. *** denotes p ≤ 0.05 when compared to the CTRL group and ** denotes p ≤ 0.05 EtOH + Zn vs. EtOH.
Since fetal ethanol exposure is expected to increase expression of the immunosuppressant TGFβ1 (Gauthier et al., 2017), we next determined if maternal zinc supplements normalized this immunosuppressed AM phenotype in pups with fetal ethanol exposure. For the ethanol-exposed pup AM, there was a 10.7 fold and 2-fold in TGFβ1 mRNA and protein expression, respectively (Figure 8A - C). However, maternal zinc supplements normalized both mRNA and protein expression of TGFβ1 in the AM from the ethanol-exposed pups suggesting that the immunosuppressed phenotype did not develop despite fetal ethanol exposure. For the control group, zinc supplements in the maternal diet did not significantly alter either TGFβ1 mRNA expression or protein expression in the AM.
Figure 8. Fetal ethanol exposure increased the relative gene and protein expression of the immunosuppressant TGFβ1 in the AMs from the newborn pups but was blocked by maternal zinc supplements.
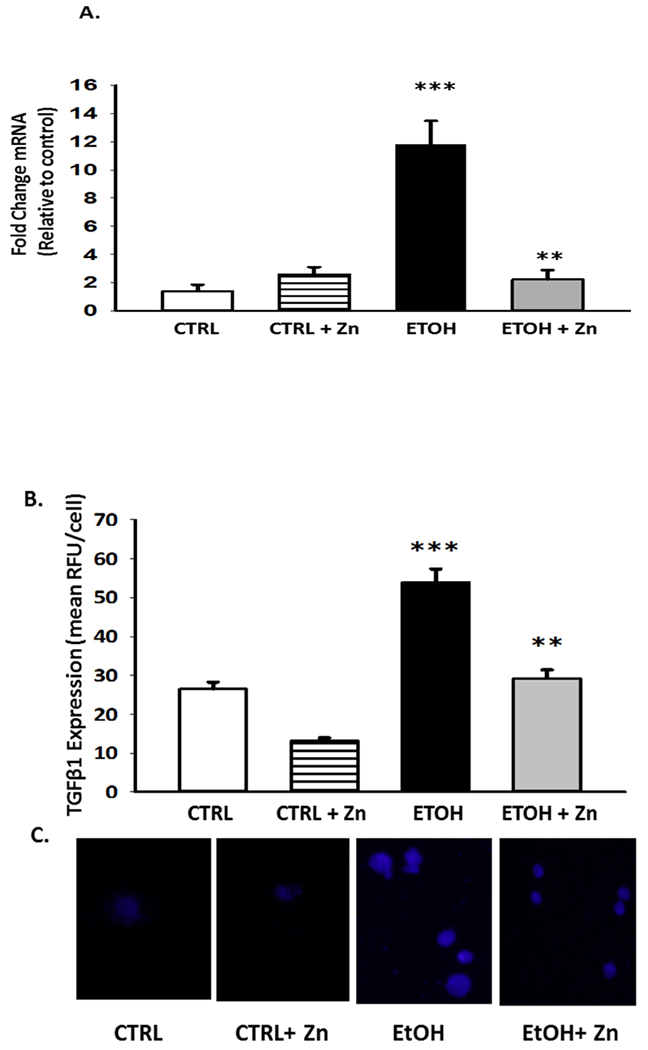
Dams were fed the control or ethanol diets ± zinc acetate supplements (100 mg/L) during pregnancy. On day 10 of life, the AMs from the pups (± fetal ethanol exposure) were isolated by lavage and the relative gene expression of TGFβ1 was determined (A). The average relative gene expression of TGFβ1 normalized to GUSB was determined by the comparative method (2−ΔΔCt), with the target gene expression in control AM set as 1 in each case. Each analysis was performed in duplicate and each value represents the mean ± SEM of at least four separate litters. Protein expression of TGFβ1 was determined and quantified similar to other transporters. Bar heights represent mean RFU/field ± S.E.M. from at least 5 different litters per group (B). Representative fluorescent images for protein expression are shown for each condition (C). CTRL = pup AM from control dams; CTRL + Zn = pup AM from control dams with dietary zinc supplements; EtOH = pup AM from ethanol-fed dams; and EtOH + Zn = pup AM from ethanol-fed dams with dietary zinc supplements. *** denotes p ≤ 0.05 when compared to the CTRL group and ** denotes p ≤ 0.01 EtOH + Zn vs. EtOH.
Fetal ethanol exposure decreased AM viral clearance and exacerbated the experimental lung RSV infection but maternal zinc supplements prevented these negative effects of ethanol in the pup.
We next determined if the decreased zinc pools, increased immunosuppressed phenotype, and decreased bacterial clearance associated with the AM after fetal ethanol exposure also impaired viral clearance and the risk of a viral infection. To address this question, RSV was introduced intranasally and then we assessed AM phagocytosis of the virus in vivo during the 24 incubation period. In this model, RSV phagocytosis in vivo by the AM was decreased by ~50% in pups with fetal ethanol exposure (Figure 9). However, the ethanol-induced suppression of RSV phagocytosis was blocked when zinc supplements were added to the maternal diet. This limited capacity of the fetal ethanol-exposed AM to phagocytose RSV was also associated with a 2-fold increase in the RSV burden in the bronchoalveolar lining fluid when compared to the control pups (Figure 10). A critical role for zinc availability in the immunosuppressed AM was demonstrated by the observation that these negative effects of fetal ethanol exposure on the RSV burden in the bronchoalveolar lining fluid were normalized to control values when additional zinc was added to the diet of the ethanol-fed pregnant dams. For the controls, dietary zinc supplements did not significantly alter AM virus phagocytosis or change the RSV burden in the lining fluid suggesting that the zinc concentration in the maternal diet was sufficient in the absence of ethanol.
Figure 9. The phagocytic index for RSV was decreased in the AMs from the ethanol-exposed pups but this decrease was blocked by maternal zinc supplements.
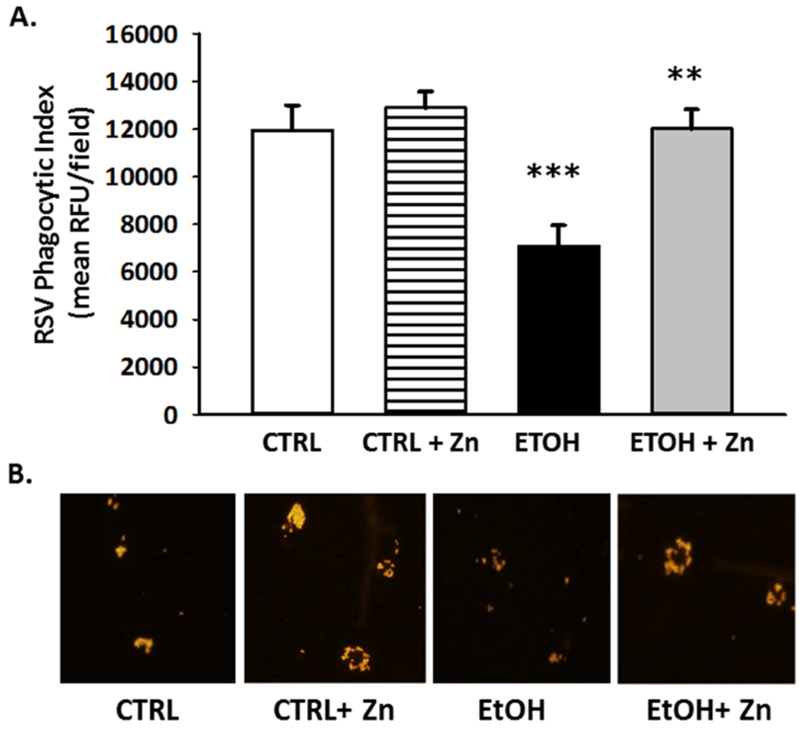
Dams were fed the control or ethanol diets ± zinc acetate supplements (100 mg/L) during pregnancy. On post-natal day 10, some pups (± ethanol and ± maternal zinc supplement) were given intranasal injections of saline or RSV (20 μl; each nasal nare; 2 × 105 PFU) and then returned to their respective dams. After 48 h, the pups were sacrificed and the lungs lavaged. After the cells in the lavage were removed by centrifugation and cultured on slides, they were fixed with 3.7% paraformaldehyde, permeabilized with ice-cold methanol, and then incubated with the primary antibody (1 h; a 1:100 dilution; Santa Cruz Biotechnology, Inc., Santa Cruz, CA). After washing the cells, AM were incubated with the secondary antibody (anti-goat IgG; a 1:200 dilution; 45 min). RSV in the cell was determined using confocal fluorescent analysis. Phagocytic index (PI) was calculated as the percentage of cells with internalized fluorescence × the mean RFU per field. Bar heights represent mean PI relative to the control ± S.E.M. from at least 6 separate litters (A). Representative fluorescent images are shown for each condition (B). CTRL = pup AM from control dams; CTRL + Zn = pup AM from control dams with dietary zinc supplements; EtOH = pup AM from ethanol-fed dams; and EtOH + Zn = pup AM from ethanol-fed dams with dietary zinc supplements. *** denotes p ≤ 0.05 when compared to the CTRL group and ** denotes p ≤ 0.01 EtOH + Zn vs. EtOH.
Figure 10. Fetal ethanol exposure exacerbated lung RSV growth but this increase was blocked by maternal zinc supplements.
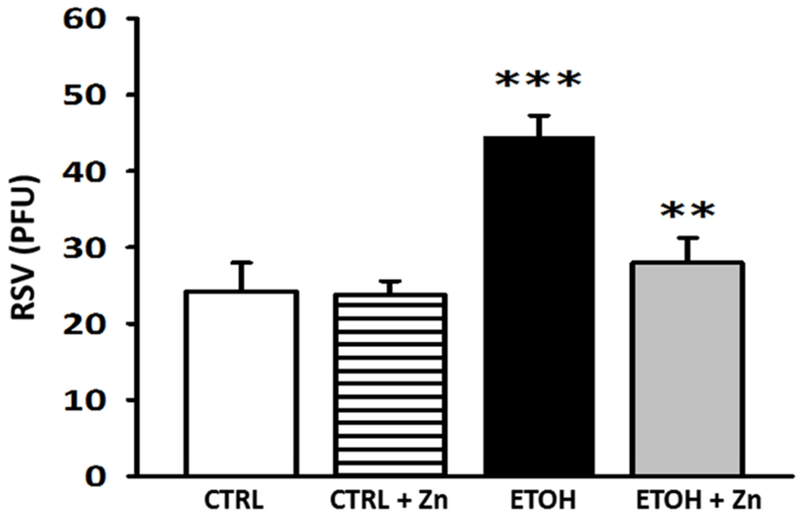
Dams were fed the control or ethanol diets ± zinc acetate supplements (100 mg/L) during pregnancy. On post-natal day 10, some pups (± ethanol and ± maternal zinc supplement) were given intranasal injections of saline or RSV (20 μl; each nasal nare; 2 × 105 PFU) and then returned to their respective dams. After 48 h, the pups were sacrificed and the lungs lavaged. After the cells in the lavage were removed by centrifugation, the cell-free supernatant was then plated for viral PFU determination (6 different litters). CTRL = pup AM from control dams; CTRL + Zn = pup AM from control dams with dietary zinc supplements; EtOH = pup AM from ethanol-fed dams; and EtOH + Zn = pup AM from ethanol-fed dams with dietary zinc supplements. *** denotes p ≤ 0.05 when compared to the CTRL group and ** denotes p ≤ 0.01 EtOH + Zn vs. EtOH.
Discussion
It is well established that in utero alcohol exposure has negative outcomes for the developing fetus (Akison, Kuo, Reid, Boyd, & Moritz, 2018; Gottesfeld, 1998). Although the lung has received little attention as a target of fetal alcohol exposure, growing evidence shows that the developing lung is also vulnerable to alcohol-induced toxicity and long term consequences (Gauthier et al., 2010; Gauthier, Ping, et al., 2005; Lazic et al., 2011; Ping et al., 2007; Sozo et al., 2009; Wang, Gomutputra, Wolgemuth, & Baxi, 2007). As noted above, fetal alcohol exposure is associated with an alarming increased odds of developing late onset sepsis and bronchopulmonary dysplasia in very low birthweight newborns (Gauthier et al., 2016), which has its own adverse consequences. Using animal models, our laboratory group has shown that in utero ethanol exposure results in fewer and less mature AM, decreased pools of the major antioxidant glutathione, an immunosuppressed phenotype, impaired phagocytosis of bacteria, and exacerbation of experimental respiratory bacterial infections (Gauthier et al., 2010; Gauthier, Ping, et al., 2005; Gauthier et al., 2009; Ping et al., 2007). In adult rats, chronic ethanol ingestion decreases the AM zinc pools and zinc transporter expression but dietary zinc supplements during ethanol ingestion prevented the expected decreases in zinc homeostasis and phagocytosis (Joshi et al., 2009). This decrease in lung zinc pools occurred despite an appropriate commercial rat diet and suggested that chronic ethanol ingestion increased the need for dietary zinc. The objective of the current study was to determine if the immunosuppressed AM phenotype associated with fetal ethanol exposure also includes impaired virus clearance, exacerbation of a viral infection, and if decreased zinc availability is a modulator of the ethanol-induced immunosuppressed phenotype.
Zinc is recognized as an essential trace metal required for human health and is a cofactor for approximately 300 enzyme-dependent processes involved in immunity, growth, cell differentiation, and metabolism (Cousins, 2006; King, 2011; Lee, 2018). Zinc homeostasis is tightly regulated through intestinal absorption, fecal excretion, renal reabsorption, and two families of transporters as well as storage/carrier proteins that tightly regulate its tissue, cellular, and organellar distribution (Krebs, 2000). Its storage across cells and extracellular fluids is not uniform and is present in two relatively distinct pools (Rahman et al., 2018). Approximately 90% of zinc is in a fixed pool bound to metalloproteins which turn over slowly. The other ~10% of total zinc is exchangeable through zinc transporters which serve as regulators of Zn uptake, efflux, and intracellular compartmentalization. They are categorized into two classes: 14 Zip (SLC39) family transporters that increase the cytosolic zinc pools by moving into the cytoplasm from the extracellular space or intracellular organelles and 10 ZnT (SLC30) family transporters that decrease cytosolic zinc by transporting zinc out of the cell or from the cytoplasm into cellular compartments (Bin, Seo, & Kim, 2018; Cousins, Liuzzi, & Lichten, 2006; Kambe et al., 2004). For the lung, the mRNA and protein for Zip1, Zip2, ZnT1, ZnT4 and ZnT7 are expressed (Hamon et al., 2014; Zalewski, 2006). In the current study, we focused on Zip1, ZnT1, and ZnT4 because these transporters were previously identified to modulate AM immune functions and to be sensitive to ethanol exposure (Curry-McCoy et al., 2013; Mehta et al., 2011).
In the current study, a maternal diet containing 25% of the calories as ethanol resulted in a significant decrease in the pup AM zinc pool when compared to AM from pair-fed control pups. This decrease in the zinc pool was associated with a similar decrease in the zinc transporters Zip1, ZnT1, and ZnT4. The central role of zinc availability in these observations was further demonstrated by the capacity of in vitro zinc treatments to restore both zinc transporter expression (Zip1, ZnT1 and ZnT4) and zinc pools to control values. This restoration of the zinc pool through in vitro zinc treatments also restored immune responses for the AM from ethanol-exposed pups. These positive effects of in vitro zinc treatments to restore the expected decrease in the zinc pool, zinc transporters, and bacteria phagocytosis for the AM after fetal ethanol-exposure were similar to that observed in AM from the ethanol-fed pregnant mouse (Konomi et al., 2015) and rat (Curry-McCoy et al., 2013; Mehta et al., 2011). While more stringent methods such as atomic absorption for assessing zinc concentrations would provide a more definitive understanding of zinc fluxes, these studies highlight the critical role for zinc availability in the extracellular lining fluid in maintaining AM zinc homeostasis, immune phenotype, and accompanying capacity to clear infectious particles.
As noted for the adult ethanol-fed rat, additional dietary zinc restored the AM zinc transporters, zinc pool, and phagocytosis (Curry-McCoy et al., 2013; Mehta et al., 2011). Therefore, we next examined the role of additional dietary zinc in the diet of the pregnant mouse as a strategy to negate the injurious effects of fetal ethanol exposure on the pup AM. Supplementation of the diet for the pregnant mouse with additional zinc acetate (100 mg/L) (Mehta et al., 2011; Mehta et al., 2013), normalized mRNA expression for Zip1 and ZnT4, the zinc pool, and the pup AM immune phenotype. While the protein expression was strong, the signal for ZnT4 mRNA expression was inconsistent and we were unable to assess the effects of ethanol and maternal zinc on its mRNA expression. The effects of ethanol and maternal zinc supplements on the other zinc transporters and carriers remain to be determined and we cannot rule out the possibility that also play contributing role to zinc homeostasis in the AM with fetal ethanol exposure.
In both adult and fetal models, we demonstrated that alcohol upregulates the immunosuppressant TGFβ1 resulting in impaired immune responses like phagocytosis (S. D. Brown & Brown, 2012; Gauthier et al., 2017; Yeligar et al., 2017). In the current study, additional zinc supplements in the maternal diet also blocked the upregulation of this important immunosuppressant which subsequently decreases AM bacterial and viral phagocytosis (Grunwell et al., 2018; Yeligar et al., 2016). The impact of ethanol exposure and zinc supplements on other aspects of AM immune responses and bacterial or viral clearance remain to be determined. The mechanisms by which ethanol-induced decreased zinc availability modulates TGFβ1 expression are unclear but may be related to the ethanol related oxidant stress. Although a redox-inert metal, zinc serves as an antioxidant through copper/zinc-superoxide dismutase, stabilization of membrane structure, protection of the protein sulfhydryl groups critical to redox signaling, and upregulation of the expression of metallothionein, which also exhibits antioxidant functions. In addition, zinc suppresses anti-inflammatory responses that would otherwise augment oxidative stress (Lee, 2018). In addition, zinc plays a role in the regulation of cellular glutathione and protection of cell membranes from oxidative damage (Go & Jones, 2013; Oteiza, 2012). With chronic ethanol ingestion, there is downregulation of the zinc-dependent nuclear factor (erythroid-derived 2)-like 2 (Nrf2), a master transcription factor that activates the antioxidant response element (ARE) and regulates antioxidant defenses including enzymes required for glutathione synthesis (Mehta et al., 2011). This is particularly intriguing, as we have published extensively on the role of oxidant stress and glutathione depletion in experimental models of chronic alcohol ingestion and in human subjects with alcohol abuse (Fan, Joshi, Koval, & Guidot, 2011; Gauthier, 2015; Gauthier & Brown, 2017; Gauthier, Drews-Botsch, et al., 2005; Guidot & Roman, 2002; Jensen, Fan, & Guidot, 2013; Joshi & Guidot, 2007; Mehta et al., 2013; Molina et al., 2002; Moss et al., 2000). Therefore, alcohol-induced zinc deficiency may play a central role in the oxidant stress, immunosuppression through upregulation of TGFβ1 in AM, and impaired phagocytosis of bacteria and viruses, thereby increasing the risk of a respiratory infection.
In previous studies (Gauthier et al., 2010; Gauthier, Ping, et al., 2005; Ping et al., 2007) as well as the current one, we have shown that fetal ethanol exposure impairs the capacity of AM to phagocytose bacteria. In the current study, we observed that fetal ethanol exposure also impaired the AM capacity for virus phagocytosis when the pups was given intra-nasal RSV. Equally important, maternal zinc supplements normalized RSV clearance by the ethanol-exposed pup AM, an event not unexpected given that it normalized the increased TGFβ1 expression, an event associated with immunosuppression and impaired RSV clearance (Grunwell et al., 2018). Impaired immune responses of the ethanol-exposed pup AM were also associated with exacerbation of experimental RSV infections as evidenced by a two-fold increase in the number of plaque forming units in the cell-free lavage fluid of the ethanol-exposed pup when compared to the control pup. Therefore, these results suggest that fetal ethanol exposure increases the risk of both bacterial and viral respiratory infections in the newborn. Equally important, a central role for zinc was demonstrated by the fact that zinc supplements in the maternal diet prevented the increased RSV infection associated with fetal ethanol exposure. However, zinc supplements in the maternal diet did not impact RSV growth in the control pups suggesting that the manufactured dietary zinc concentration was in the optimal range but fetal ethanol exposure increased the need for maternal dietary zinc. This was further supported by the observation of no significant change in the zinc pools or transporters for the control, control + zinc, or ethanol + zinc groups. Whether the observed decrease in the zinc pool of the pup was a direct effect of ethanol exposure, a decrease in maternal zinc availability or a combination remains to be determined.
Airway epithelial cells, whose immune functions are highly sensitive to alcohol and accompanying oxidant stress (Bailey et al., 2018; Price, Case, et al., 2018; Price, Gerald, et al., 2018), are the primary site for RSV infections. Indeed, fetal alcohol exposure has been shown to exacerbate a RSV infection in a lamb model (Ackermann, 2014; Lazic et al., 2007). However, robust AM immune functions are critical for RSV clearance and a major contributor to the outcome (Bohmwald et al., 2017; Kolli et al., 2014; Ren et al., 2014). With ethanol-induced decreases in RSV phagocytosis by the AM, an increase in the RSV burden in the airspace and the opportunity of the epithelial cells to be exposed and infected by the virus are not unexpected. Therefore, fetal ethanol exposure increases the risk of both viral and bacterial lung infections because it promotes an immunosuppressed phenotype in the AM and impairs the capacity of the AM to clear bacteria as well as viruses such as RSV. In addition, these studies suggested that zinc availability to the pup AM is a critical modulator of the AM immune phenotype, its capacity to clear infectious agents, and, subsequently, the risk of lung infections.
The underlying mechanisms by which ethanol ingestion during pregnancy resulted in zinc insufficiency in the newborn AM are unclear. Alcohol affects the status of many nutrients such as folate, cobalamin, iron, vitamin A, and zinc, all of which are needed for proper fetal development and overall growth (Keen et al., 2010). In pregnant women who consume alcohol, maternal plasma and fetal cord blood concentrations of zinc have been reported to be significantly decreased compared to those not consuming alcohol which is correlated with an increased risk of dysmorphogenesis (Young et al., 2014). In the U.S., it is estimated that only ~ 60% of pregnant women meet zinc requirements during pregnancy (Briefel et al., 2000) and mild to moderate zinc deficiency is not restricted to women of low socioeconomic status. There are no readily available zinc stores that can be released in response to dietary variations or intestinal uptake. Therefore, the ~10% of total zinc in the adult that is exchangeable becomes crucial during development (Hambidge, 1986; Prasad, 1985) and alcohol consumption during pregnancy would exacerbate the zinc insufficiency and oxidant stress for the mother and the developing fetus (Skalny, Skalnaya, Grabeklis, Skalnaya, & Tinkov, 2018).
Even marginal perturbations in maternal zinc status or immune system can have profound effects for development, particularly the fetal immune system which starts to develop in utero and continues to mature through infancy and childhood (Beach, Gershwin, & Hurley, 1982a; Shankar & Prasad, 1998), In addition, environmental factors such as malnutrition, infection, or alcohol can have enormous consequences for the fetal immune system, particularly if they occur during critical periods when precursor immune cells are forming (Marques, O’Connor, Roth, Susser, & Bjorke-Monsen, 2013). Moreover, gestational zinc deficiency in animal models and subsequent increased oxidative stress and reduced growth of spleen, thymus and lung, at a greater extent than other organs (Beach, Gershwin, & Hurley, 1982b; Keen et al., 2010). In animal models, gestational zinc deficiency also results in suppression of fetal immunoglobulin production, such as IgA and IgM, which persists into adulthood (Beach et al., 1982a; Chandra, 2002; Keen & Gershwin, 1990; Rink & Gabriel, 2000). Given that placental development requires zinc, decreased zinc availability and improper placental development could also contribute to impaired acquisition of maternal antibodies that are crucial for neonatal immunity in the first six months of life (Shankar & Prasad, 1998). In the adult rat model, chronic ethanol ingestion impaired intestinal cell uptake of zinc as well as altered intestinal barrier integrity, effects that were normalized by increasing the zinc content of the diet (Joshi et al., 2009). In addition, zinc supplements have been shown to prevent ethanol-induced hepatic endotoxin signaling and steatohepatitis through the protective effects of zinc on preserving intestinal barrier integrity (Zhong, Li, Sun, et al., 2015; Zhong, Li, Zhang, et al., 2015). Ethanol-induced alterations in intestinal zinc uptake and loss of barrier integrity in the pregnant mouse could potentially impact the developing fetus by resulting in zinc insufficiency as well as increasing endotoxin exposure (Bode & Bode, 2003). Therefore, there are multiple points during pregnancy at which alcohol-induced perturbations in maternal zinc homeostasis could account for our observed alterations in zinc homeostasis in the newborn lung, the fetal immune system in general and the AM immune response more specifically.
In summary, the current study demonstrated that in utero ethanol exposure resulted in a compromised AM immune response that was related to decreased zinc availability in the developing lung. The compromised zinc homeostasis is also supported by decreased protein expression of the zinc transporter, Zip1, in the ethanol-exposed pup AM and subsequent decreased intracellular zinc pools. There was also decreased AM expression of ZnT1 and ZnT4 transporters, which is not unexpected since decrease expression of these transporters would limit zinc export and help maintain intracellular zinc homeostasis. A causal role for decreased zinc availability in ethanol-induced suppression of neonatal AM functions was further demonstrated by the ability of in vitro zinc treatments to restore AM zinc transporter protein expression, zinc levels, and phagocytic function to control levels. This was further supported by the studies where additional zinc supplements in the maternal diet prevented ethanol-induced zinc depletion, expression of the immunosuppressant TGFβ1 in the neonatal AM, and normalized phagocytosis of RSV. Furthermore, these studies demonstrated that similar to respiratory bacterial infections, fetal ethanol exposure exacerbated viral infections from RSV in the pup which could be prevented through additional dietary zinc in the maternal diet. In this study, we focused on AMs and cannot rule out the possibility that other cell types also affected by ethanol exposure were positively impacted by the maternal zinc supplements and contributed to the improved RSV clearance. Additional studies are needed to determine if these effects of fetal ethanol exposure on zinc homeostasis or AM immune function improve with maturation or can be corrected postnatally.
Overall, this study provides provocative evidence that fetal ethanol exposure results in zinc depletion within the alveolar space which subsequently mediates dysregulation of the AM immune responses and risk of bacterial and viral infections. If these findings ultimately prove to be relevant in the clinical situation, then dietary zinc supplementation may provide a strategy to improve the AM immune responses and decrease the risk and severity of pulmonary infections in the highly vulnerable preterm infant with fetal alcohol exposure. Novel strategies such as zinc supplements may become particularly important in this era of antibiotic-resistant bacterial infections.
Fetal ethanol exposure dysregulated zinc homeostasis in alveolar macrophages.
Dysregulated zinc homeostasis resulted in immunosuppression of alveolar macrophages.
Ethanol exposure impaired the capacity of pup alveolar macrophages to clear viruses.
Fetal ethanol exposure exacerbated a lung Respiratory Syncytial Virus infection.
Maternal zinc supplements blocked fetal immunosuppression and lung viral infection.
Acknowledgements
This work was supported by the NIAAA through the Emory Alcohol and Lung Biology Center (1P50AA135757) and a R01 (5R01 012197).
Abbreviations
- AM
Alveolar macrophage
- ARE
Antioxidant response element
- CTRL
Control
- ETOH
Ethanol
- Nrf2
Nuclear factor (erythroid-derived 2)-like 2
- PFU
Plaque Forming Units
- PI
Phagocytic index
- RFU
Relative fluorescence units
- RSV
Respiratory syncytial virus
- TGFβ 1
Transforming growth factor β
- Zn
Zinc
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ackermann MR (2014). Lamb model of respiratory syncytial virus-associated lung disease: insights to pathogenesis and novel treatments. ILAR J, 55(1), 4–15. doi: 10.1093/ilar/ilu003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akison LK, Kuo J, Reid N, Boyd RN, & Moritz KM (2018). Effect of Choline Supplementation on Neurological, Cognitive, and Behavioral Outcomes in Offspring Arising from Alcohol Exposure During Development: A Quantitative Systematic Review of Clinical and Preclinical Studies. Alcohol Clin Exp Res, 42(9), 1591–1611. doi: 10.1111/acer.13817 [DOI] [PubMed] [Google Scholar]
- Bailey KL, Wyatt TA, Katafiasz DM, Taylor KW, Heires AJ, Sisson JH, … Burnham EL (2018). Alcohol and Cannabis Use Alter Pulmonary Innate Immunity. Alcohol. doi: 10.1016/j.alcohol.2018.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach RS, Gershwin ME, & Hurley LS (1982a). Gestational zinc deprivation in mice: persistence of immunodeficiency for three generations. Science, 218(4571), 469–471. [DOI] [PubMed] [Google Scholar]
- Beach RS, Gershwin ME, & Hurley LS (1982b). Reversibility of development retardation following murine fetal zinc deprivation. J Nutr, 112(6), 1169–1181. [DOI] [PubMed] [Google Scholar]
- Bellanti JA, & Zeligs BJ (1995). Developmental aspects of pulmonary defenses in children. Pediatr Pulmonol Suppl, 11, 79–80. [DOI] [PubMed] [Google Scholar]
- Bin BH, Seo J, & Kim ST (2018). Function, Structure, and Transport Aspects of ZIP and ZnT Zinc Transporters in Immune Cells. J Immunol Res, 2018, 9365747. doi: 10.1155/2018/9365747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode C, & Bode JC (2003). Effect of alcohol consumption on the gut. Best Pract Res Clin Gastroenterol, 17(4), 575–592. [DOI] [PubMed] [Google Scholar]
- Bohmwald K, Espinoza JA, Pulgar RA, Jara EL, & Kalergis AM (2017). Functional Impairment of Mononuclear Phagocyte System by the Human Respiratory Syncytial Virus. Frontiers in Immunology, 8, 1643. doi: 10.3389/fimmu.2017.01643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briefel RR, Bialostosky K, Kennedy-Stephenson J, McDowell MA, Ervin RB, & Wright JD (2000). Zinc intake of the U.S. population: findings from the third National Health and Nutrition Examination Survey, 1988-1994. J Nutr, 130(5S Suppl), 1367s–1373s. [DOI] [PubMed] [Google Scholar]
- Brown LA, Ping XD, Harris FL, & Gauthier TW (2007). Glutathione availability modulates alveolar macrophage function in the chronic ethanol-fed rat. Am J Physiol Lung Cell Mol Physiol, 292(4), L824–832. 10.1152/ajplung.00346.2006 [DOI] [PubMed] [Google Scholar]
- Brown SD, & Brown LA (2012). Ethanol (EtOH)-induced TGF-beta1 and reactive oxygen species production are necessary for EtOH-induced alveolar macrophage dysfunction and induction of alternative activation. Alcohol Clin Exp Res, 36(11), 1952–1962. doi: 10.1111/j.1530-0277.2012.01825.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra RK (2002). Nutrition and the immune system from birth to old age. Eur J Clin Nutr, 56 Suppl 3, S73–76. doi: 10.1038/sj.ejcn.1601492 [DOI] [PubMed] [Google Scholar]
- Cousins RJ (2006). Zinc In Bowman BA RR (Ed.), Present knowledge in nutrition (pp. 445–457). Washington, DC: ILSI Press. [Google Scholar]
- Cousins RJ, Liuzzi JP, & Lichten LA (2006). Mammalian zinc transport, trafficking, and signals. J Biol Chem, 281(34), 24085–24089. doi: 10.1074/jbc.R600011200 [DOI] [PubMed] [Google Scholar]
- Curry-McCoy TV, Guidot DM, & Joshi PC (2013). Chronic alcohol ingestion in rats decreases kruppel-like factor 4 expression and intracellular zinc in the lung. Alcohol Clin Exp Res, 37(3), 361–371. doi: 10.1111/j.1530-0277.2012.01946.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jonge HJ, Fehrmann RS, de Bont ES, Hofstra RM, Gerbens F, Kamps WA, … ter Elst A (2007). Evidence based selection of housekeeping genes. PLoS One, 2(9), e898. doi: 10.1371/journal.pone.0000898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutz W, Rossipal E, Ghavami H, Vessal K, Kohout E, & Post C (1976). Persistent cell mediated immune-deficiency following infantile stress during the first 6 months of life. Eur J Pediatr, 122(2), 117–130. [DOI] [PubMed] [Google Scholar]
- Fan X, Joshi PC, Koval M, & Guidot DM (2011). Chronic Alcohol Ingestion Exacerbates Lung Epithelial Barrier Dysfunction in HIV-1 Transgenic Rats. Alcohol Clin Exp Res. doi: 10.1111/j.1530-0277.2011.01531.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fels AO, & Cohn ZA (1986). The alveolar macrophage. J Appl Physiol, 60(2), 353–369. [DOI] [PubMed] [Google Scholar]
- Ferguson AC (1978). Prolonged impairment of cellular immunity in children with intrauterine growth retardation. J Pediatr, 93(1), 52–56. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick AM, Holguin F, Teague WG, & Brown LA (2008a). Alveolar macrophage phagocytosis is impaired in children with poorly controlled asthma. J Allergy Clin Immunol, 121(6), 1372–1378, 1378 e1371–1373. 10.1016/j.jaci.2008.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick AM, Holguin F, Teague WG, & Brown LA (2008b). Alveolar macrophage phagocytosis is impaired in children with poorly controlled asthma. J Allergy Clin Immunol, 121(6), 1372–1378, 1378 e1371–1373. doi: 10.1016/j.jaci.2008.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca W, Lukacs NW, & Ptaschinski C (2018). Factors Affecting the Immunity to Respiratory Syncytial Virus: From Epigenetics to Microbiome. Frontiers in Immunology, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier TW (2015). Prenatal Alcohol Exposure and the Developing Immune System. Alcohol Res, 37(2), 279–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier TW, & Brown LA (2017). In utero alcohol effects on foetal, neonatal and childhood lung disease. Paediatr Respir Rev, 21, 34–37. doi: 10.1016/j.prrv.2016.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier TW, Drews-Botsch C, Falek A, Coles C, & Brown LA (2005). Maternal alcohol abuse and neonatal infection. Alcohol Clin Exp Res, 29(6), 1035–1043. [DOI] [PubMed] [Google Scholar]
- Gauthier TW, Grunwell JR, Ping XD, Harris FL, & Brown LA (2017). Impaired defenses of neonatal mouse alveolar macrophage with cftr deletion are modulated by glutathione and TGFbeta1. Physiol Rep, 5(6). doi: 10.14814/phy2.13086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier TW, Guidot DM, Kelleman MS, McCracken CE, & Brown LA (2016). Maternal Alcohol Use During Pregnancy and Associated Morbidities in Very Low Birth Weight Newborns. Am J Med Sci, 352(4), 368–375. doi: 10.1016/j.amjms.2016.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier TW, Ping XD, Gabelaia L, & Brown LA (2010). Delayed neonatal lung macrophage differentiation in a mouse model of in utero ethanol exposure. Am J Physiol Lung Cell Mol Physiol, 299(1), L8–16. 10.1152/ajplung.90609.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier TW, Ping XD, Harris FL, Wong M, Elbahesh H, & Brown LA (2005). Fetal alcohol exposure impairs alveolar macrophage function via decreased glutathione availability. Pediatr Res, 57(1), 76–81. 10.1203/01.PDR.0000149108.44152.D3 [DOI] [PubMed] [Google Scholar]
- Gauthier TW, Young PA, Gabelaia L, Tang SM, Ping XD, Harris FL, & Brown LA (2009). In utero ethanol exposure impairs defenses against experimental group B streptococcus in the term Guinea pig lung. Alcohol Clin Exp Res, 33(2), 300–306. 10.1111/j.1530-0277.2008.00833.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go YM, & Jones DP (2013). Thiol/disulfide redox states in signaling and sensing. Crit Rev Biochem Mol Biol, 48(2), 173–181. doi: 10.3109/10409238.2013.764840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesfeld Z (1998). Sympathetic neural response to immune signals involves nitric oxide: effects of exposure to alcohol in utero. Alcohol, 16(2), 177–181. [DOI] [PubMed] [Google Scholar]
- Grunwell JR, Yeligar SM, Stephenson S, Ping XD, Gauthier TW, Fitzpatrick AM, & Brown LAS (2018). TGF-beta1 Suppresses the Type I IFN Response and Induces Mitochondrial Dysfunction in Alveolar Macrophages. J Immunol, 200(6), 2115–2128. doi: 10.4049/jimmunol.1701325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidot DM, & Roman J (2002). Chronic ethanol ingestion increases susceptibility to acute lung injury: role of oxidative stress and tissue remodeling. Chest, 122(6 Suppl), 309S–314S. [DOI] [PubMed] [Google Scholar]
- Haase H, Ober-Blobaum JL, Engelhardt G, Hebel S, Heit A, Heine H, & Rink L (2008). Zinc signals are essential for lipopolysaccharide-induced signal transduction in monocytes. J Immunol, 181(9), 6491–6502. [DOI] [PubMed] [Google Scholar]
- Hambidge KM, Casey CE & Krebs NF (1986). Zinc In: Trace Elements in Human and Animal Nutrition (5th ed Vol. 2). Orlando, FL: Academic Press. [Google Scholar]
- Hamon R, Homan CC, Tran HB, Mukaro VR, Lester SE, Roscioli E, … Hodge SJ (2014). Zinc and zinc transporters in macrophages and their roles in efferocytosis in COPD. PLoS One, 9(10), e110056. doi: 10.1371/journal.pone.0110056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey CJ, Thimmulappa RK, Sethi S, Kong X, Yarmus L, Brown RH, … Biswal S (2011). Targeting Nrf2 Signaling Improves Bacterial Clearance by Alveolar Macrophages in Patients with COPD and in a Mouse Model. Sci Transl Med, 3(78), 78ra32 10.1126/scitranslmed.3002042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen JS, Fan X, & Guidot DM (2013). Alcohol causes alveolar epithelial oxidative stress by inhibiting the nuclear factor (erythroid-derived 2)-like 2-antioxidant response element signaling pathway. Am J Respir Cell Mol Biol, 48(4), 511–517. doi: 10.1165/rcmb.2012-0334OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi PC, Applewhite L, Ritzenthaler JD, Roman J, Fernandez AL, Eaton DC, … Guidot DM (2005). Chronic ethanol ingestion in rats decreases granulocyte-macrophage colony-stimulating factor receptor expression and downstream signaling in the alveolar macrophage. J Immunol, 175(10), 6837–6845. [DOI] [PubMed] [Google Scholar]
- Joshi PC, & Guidot DM (2007). The alcoholic lung: epidemiology, pathophysiology, and potential therapies. Am J Physiol Lung Cell Mol Physiol, 292(4), L813–823. 10.1152/ajplung.00348.2006 [DOI] [PubMed] [Google Scholar]
- Joshi PC, Mehta A, Jabber WS, Fan X, & Guidot DM (2009). Zinc deficiency mediates alcohol-induced alveolar epithelial and macrophage dysfunction in rats. Am J Respir Cell Mol Biol, 41(2), 207–216. 10.1165/rcmb.2008-0209OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambe T, Yamaguchi-Iwai Y, Sasaki R, & Nagao M (2004). Overview of mammalian zinc transporters. Cell Mol Life Sci, 61(1), 49–68. doi: 10.1007/s00018-003-3148-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keen CL, & Gershwin ME (1990). Zinc deficiency and immune function. Annu Rev Nutr, 10, 415–431. doi: 10.1146/annurev.nu.10.070190.002215 [DOI] [PubMed] [Google Scholar]
- Keen CL, Uriu-Adams JY, Skalny A, Grabeklis A, Grabeklis S, Green K, … Chambers CD (2010). The plausibility of maternal nutritional status being a contributing factor to the risk for fetal alcohol spectrum disorders: the potential influence of zinc status as an example. Biofactors, 36(2), 125–135. doi: 10.1002/biof.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King JC (2011). Zinc: an essential but elusive nutrient. Am J Clin Nutr, 94(2), 679s–684s. doi: 10.3945/ajcn.110.005744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolli D, Gupta MR, Sbrana E, Velayutham TS, Chao H, Casola A, & Garofalo RP (2014). Alveolar macrophages contribute to the pathogenesis of human metapneumovirus infection while protecting against respiratory syncytial virus infection. Am J Respir Cell Mol Biol, 51(4), 502–515. doi: 10.1165/rcmb.2013-0414OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konomi JV, Harris FL, Ping XD, Gauthier TW, & Brown LAS (2015). Zinc Insufficiency Mediates Ethanol-Induced Alveolar Macrophage Dysfunction in the Pregnant Female Mouse. Alcohol and Alcoholism, 50(1), 30–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs NF (2000). Overview of zinc absorption and excretion in the human gastrointestinal tract. J Nutr, 130(5S Suppl), 1374s–1377s. [DOI] [PubMed] [Google Scholar]
- Lazic T, Sow FB, Van Geelen A, Meyerholz DK, Gallup JM, & Ackermann MR (2011). Exposure to ethanol during the last trimester of pregnancy alters the maturation and immunity of the fetal lung. Alcohol, 45(7), 673–680. doi: 10.1016/j.alcohol.2010.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazic T, Wyatt TA, Matic M, Meyerholz DK, Grubor B, Gallup JM, … Ackermann MR (2007). Maternal alcohol ingestion reduces surfactant protein A expression by preterm fetal lung epithelia. Alcohol, 41(5), 347–355. doi: 10.1016/j.alcohol.2007.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SR (2018). Critical Role of Zinc as Either an Antioxidant or a Prooxidant in Cellular Systems. Oxid Med Cell Longev, 2018, 9156285. doi: 10.1155/2018/9156285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y, Harris FL, & Brown LAS (2014). Alcohol induced mitochondrial oxidative stress and alveolar macrophage dysfunction. BioMed Res. Int, In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little BB, Snell LM, Rosenfeld CR, Gilstrap LC 3rd, & Gant NF (1990). Failure to recognize fetal alcohol syndrome in newborn infants. Am J Dis Child, 144(10), 1142–1146. [DOI] [PubMed] [Google Scholar]
- Liu E, Pimpin L, Shulkin M, Kranz S, Duggan CP, Mozaffarian D, & Fawzi WW (2018). Effect of Zinc Supplementation on Growth Outcomes in Children under 5 Years of Age. Nutrients, 10(3). doi: 10.3390/nu10030377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukacs NW, Moore ML, Rudd BD, Berlin AA, Collins RD, Olson SJ, … Peebles RS (2006). Differential immune responses and pulmonary pathophysiology are induced by two different strains of respiratory syncytial virus. American Journal of Pathology, 169(3), 977–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques AH, O’Connor TG, Roth C, Susser E, & Bjorke-Monsen AL (2013). The influence of maternal prenatal and early childhood nutrition and maternal prenatal stress on offspring immune system development and neurodevelopmental disorders. Front Neurosci, 7, 120. doi: 10.3389/fnins.2013.00120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Chambers CD, Kalberg WO, Zellner J, Feldman H, Buckley D, … Hoyme HE (2018). Prevalence of Fetal Alcohol Spectrum Disorders in 4 US Communities. Jama-Journal of the American Medical Association, 319(5), 474–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maywald M, Wessels I, & Rink L (2017). Zinc Signals and Immunity. Int J Mol Sci, 18(10). doi: 10.3390/ijms18102222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick MC (1985). The contribution of low birth weight to infant mortality and childhood morbidity. N Engl J Med, 312(2), 82–90. doi: 10.1056/nejm198501103120204 [DOI] [PubMed] [Google Scholar]
- Mehta AJ, Joshi PC, Fan X, Brown LA, Ritzenthaler JD, Roman J, & Guidot DM (2011). Zinc Supplementation Restores PU.1 and Nrf2 Nuclear Binding in Alveolar Macrophages and Improves Redox Balance and Bacterial Clearance in the Lungs of Alcohol-Fed Rats. Alcohol Clin Exp Res. doi: 10.1111/j.1530-0277.2011.01488.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta AJ, Yeligar SM, Elon L, Brown LA, & Guidot DM (2013). Alcoholism causes alveolar macrophage zinc deficiency and immune dysfunction. Am J Respir Crit Care Med, 188(6), 716–723. doi: 10.1164/rccm.201301-0061OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina PE, McClain C, Valla D, Guidot D, Diehl AM, Lang CH, & Neuman M (2002). Molecular pathology and clinical aspects of alcohol-induced tissue injury. Alcohol Clin Exp Res, 26(1), 120–128. [PubMed] [Google Scholar]
- Moss M, Guidot DM, Wong-Lambertina M, Ten Hoor T, Perez RL, & Brown LA (2000). The effects of chronic alcohol abuse on pulmonary glutathione homeostasis. Am J Respir Crit Care Med, 161(2 Pt 1), 414–419. [DOI] [PubMed] [Google Scholar]
- Oteiza PI (2012). Zinc and the modulation of redox homeostasis. Free Radio Biol Med, 53(9), 1748–1759. doi: 10.1016/j.freeradbiomed.2012.08.568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paes B (2018). Respiratory Syncytial Virus in Otherwise Healthy Prematurely Born Infants: A Forgotten Majority. American Journal of Perinatology, 35(6), 541–544. [DOI] [PubMed] [Google Scholar]
- Ping XD, Harris FL, Brown LA, & Gauthier TW (2007). In vivo dysfunction of the term alveolar macrophage after in utero ethanol exposure. Alcohol Clin Exp Res, 31(2), 308–316. 10.1111/j.1530-0277.2006.00306.x [DOI] [PubMed] [Google Scholar]
- Prasad AS (1985). Clinical and biochemical manifestation zinc deficiency in human subjects. J Pharmacol, 16(4), 344–352. [PubMed] [Google Scholar]
- Price ME, Case AJ, Pavlik JA, DeVasure JM, Wyatt TA, Zimmerman MC, & Sisson JH (2018). S-nitrosation of protein phosphatase 1 mediates alcohol-induced ciliary dysfunction. Sci Rep, 8(1), 9701. doi: 10.1038/s41598-018-27924-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price ME, Gerald CL, Pavlik JA, Schlichte SL, Zimmerman MC, Devasure JM, … Sisson JH (2018). Loss of cAMP-dependent stimulation of isolated cilia motility by alcohol exposure is oxidant dependent. Alcohol. doi: 10.1016/j.alcohol.2018.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman MM, Hossain KFB, Banik S, Sikder MT, Akter M, Bondad SEC, … Kurasaki M (2018). Selenium and zinc protections against metal-(loids)-induced toxicity and disease manifestations: A review. Ecotoxicol Environ Saf, 168, 146–163. doi: 10.1016/j.ecoenv.2018.10.054 [DOI] [PubMed] [Google Scholar]
- Ren J, Liu G, Go J, Kolli D, Zhang G, & Bao X (2014). Human metapneumovirus M2-2 protein inhibits innate immune response in monocyte-derived dendritic cells. PLoS One, 9(3), e91865. doi: 10.1371/journal.pone.0091865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rink L, & Gabriel P (2000). Zinc and the immune system. Proc Nutr Soc, 59(4), 541–552. [DOI] [PubMed] [Google Scholar]
- Saigal S, & Doyle LW (2008). An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet, 371(9608), 261–269. doi: 10.1016/s0140-6736(08)60136-1 [DOI] [PubMed] [Google Scholar]
- Shankar AH, & Prasad AS (1998). Zinc and immune function: the biological basis of altered resistance to infection. Am J Clin Nutr, 68(2 Suppl), 447S–463S. [DOI] [PubMed] [Google Scholar]
- Simoes EA, Anderson EJ, Wu X, & Ambrose CS (2016). Effects of Chronologic Age and Young Child Exposure on Respiratory Syncytial Virus Disease among US Preterm Infants Born at 32 to 35 Weeks Gestation. PLoS One, 11(11), e0166226. doi: 10.1371/journal.pone.0166226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalny AV, Skalnaya MG, Grabeklis AR, Skalnaya AA, & Tinkov AA (2018). Zinc deficiency as a mediator of toxic effects of alcohol abuse. Eur J Nutr, 57(7), 2313–2322. doi: 10.1007/s00394-017-1584-y [DOI] [PubMed] [Google Scholar]
- Sokol RJ, Janisse JJ, Louis JM, Bailey BN, Ager J, Jacobson SW, & Jacobson JL (2007). Extreme prematurity: an alcohol-related birth effect. Alcohol Clin Exp Res, 31(6), 1031–1037. [DOI] [PubMed] [Google Scholar]
- Sozo F, O’Day L, Maritz G, Kenna K, Stacy V, Brew N, … Harding R (2009). Repeated ethanol exposure during late gestation alters the maturation and innate immune status of the ovine fetal lung. Am J Physiol Lung Cell Mol Physiol, 296(3), L510–518. 10.1152/ajplung.90532.2008 [DOI] [PubMed] [Google Scholar]
- Terrin G, Berni Canani R, Di Chiara M, Pietravalle A, Aleandri V, Conte F, & De Curtis M (2015). Zinc in Early Life: A Key Element in the Fetus and Preterm Neonate. Nutrients, 7(12), 10427–10446. doi: 10.3390/nu7125542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Gomutputra P, Wolgemuth DJ, & Baxi L (2007). Effects of acute alcohol intoxication in the second trimester of pregnancy on development of the murine fetal lung. Am J Obstet Gynecol, 197(3), 269, e261–264. doi: 10.1016/j.ajog.2007.06.031 [DOI] [PubMed] [Google Scholar]
- Wellinghausen N (2001). Immunobiology of gestational zinc deficiency. Br J Nutr, 85 Suppl 2, S81–86. doi:S0007114501000952 [pii] [PubMed] [Google Scholar]
- Wessels I, Maywald M, & Rink L (2017). Zinc as a Gatekeeper of Immune Function. Nutrients, 9(12). doi: 10.3390/nu9121286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeligar SM, Chen MM, Kovacs EJ, Sisson JH, Burnham EL, & Brown LAS (2016). Alcohol and lung injury and immunity. Alcohol, 55, 51–59. doi: 10.1016/j.alcohol.2016.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeligar SM, Harris FL, Hart CM, & Brown LA (2012). Ethanol induces oxidative stress in alveolar macrophages via upregulation of NADPH oxidases. J Immunol, 188(8), 3648–3657. doi: 10.4049/jimmunol.1101278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeligar SM, Harris FL, Hart CM, & Brown LA (2014). Glutathione attenuates ethanol-induced alveolar macrophage oxidative stress and dysfunction by down regulating NADPH oxidases. Am J Physiol Lung Cell Mol Physiol. doi: 10.1152/ajplung.00159.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeligar SM, Ward JM, Harris FL, Brown LAS, Guidot DM, & Cribbs SK (2017). Dysregulation of Alveolar Macrophage PPARgamma, NADPH Oxidases, and TGFbeta1 in Otherwise Healthy HIV-Infected Individuals. AIDS Res Hum Retroviruses, 33(10), 1018–1026. doi: 10.1089/AID.2016.0030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JK, Giesbrecht HE, Eskin MN, Aliani M, & Suh M (2014). Nutrition implications for fetal alcohol spectrum disorder. Adv Nutr, 5(6), 675–692. doi: 10.3945/an.113.004846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalewski PD (2006). Zinc metabolism in the airway: basic mechanisms and drug targets. Curr Opin Pharmacol, 6(3), 237–243. [DOI] [PubMed] [Google Scholar]
- Zhong W, Li Q, Sun Q, Zhang W, Zhang J, Sun X, … Zhou Z (2015). Preventing Gut Leakiness and Endotoxemia Contributes to the Protective Effect of Zinc on Alcohol-Induced Steatohepatitis in Rats. J Nutr, 145(12), 2690–2698. doi: 10.3945/jn.115.216093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong W, Li Q, Zhang W, Sun Q, Sun X, & Zhou Z (2015). Modulation of Intestinal Barrier and Bacterial Endotoxin Production Contributes to the Beneficial Effect of Nicotinic Acid on Alcohol-Induced Endotoxemia and Hepatic Inflammation in Rats. Biomolecules, 5(4), 2643–2658. doi: 10.3390/biom5042643 [DOI] [PMC free article] [PubMed] [Google Scholar]


