Abstract
In non-APL AML, identification of a signaling signature would predict potentially actionable targets to enhance differentiation effects of all-trans-retinoic acid (RA) and make combination differentiation therapy realizable. Components of such a signaling machine/signalsome found to drive RA-induced differentiation discerned in a FAB M2 cell line/model (HL-60) were further characterized and then compared against AML patient expression profiles. FICZ, known to enhance RA-induced differentiation, was used to experimentally augment signaling for analysis. FRET revealed novel signalsome protein associations: CD38 with pS376SLP76 and caveolin-1 with CD38 and AhR. The signaling molecules driving differentiation in HL-60 cluster in non-APL AML de novo samples, too. Pearson correlation coefficients for this molecular ensemble are nearer 1 in the FAB M2 subtype than in non-APL AML. SLP76 correlation to RXRα and p47phox were conserved in FAB M2 model and patient subtype but not in general non-APL AML. The signalsome ergo identifies potential actionable targets in AML.
Keywords: retinoic acid, differentiation, neutrophil, FICZ, HL-60
Introduction
Differentiation therapy with all-trans retinoic acid (RA), the standard of care for acute promyelocytic leukemia (APL), remains an effective treatment for this once highly lethal disease [1]. RA promotes the conversion of promyelocytic cells into mature neutrophils, characterized by cell cycle arrest, CD38 and CD11b cell surface marker expression, respiratory burst, and metabolic modification (as indicated by ALDH1 activity and glucose uptake) [2–4]. Significant research and clinical efforts (including 12 currently open clinical trials) have sought to apply RA therapy to non-APL acute myeloid leukemia (AML) indications, but RA-based therapies still yield highly variable results in this context [3]. There is thus a need to find actionable targets to make non-APL AML susceptible to differentiation therapy.
In a recent publication [3], we explored the heterogeneity of AML and provided evidence that the HL-60 model bears fidelity to a subtype of non-APL AML with leukemic blasts. HL-60 is derived from a patient with FAB M2 leukemia, a subtype of non-APL leukemia. This myeloblastic leukemia model cell line does not harbor any RARα mutations or fusion proteins, yet is highly responsive to RA, which induces differentiation of blasts into neutrophils. RA-induced differentiation requires sustained activation of MAPK signaling [5–9], and involves kinases, adaptors, and GEF signaling regulatory molecules, including the Lyn and Fgr Src-family protein tyrosine kinases (SFKs), PI3K, c-Cbl, SLP76, Vav1, as well as the aryl hydrocarbon receptor (AhR) transcription factor, here performing a putative novel cytosolic signaling function [4, 10–14]. These factors are embedded in a signalsome which is activated by RA to drive differentiation.
For several of these putative signalsome components, their ability to drive differentiation has been directly demonstrated. For example, during RA-induced differentiation, ectopic expression of c-Raf [15, 16], c-Cbl [11], and AhR [4] has been shown to enhance MAPK signal activation and promote RA-induced differentiation and G0-arrest. SFKs have also been found to be functionally significant [10]. Expression of Lyn and Fgr, regulators of MAPK signaling, is upregulated, and Lyn is phosphorylated after RA treatment of HL-60 [17]. Lyn can regulate c-Cbl via phosphorylation [18]. c-Cbl associates with AhR, which is upregulated by RA and promotes RA-induced differentiation, especially when an AhR ligand is present [4, 19]. Moreover, members of the signalsome were implicated in RA responsiveness in de novo blasts [3]. AhR is a receptor within the signalsome. FICZ is an endogenous AhR ligand which we found enhances RA-induced differentiation of HL-60 cells to neutrophils [3, 19, 20]. The mechanism by which FICZ propels differentiation is not well understood.
In this study, we performed an analysis of known signalsome components, integrating data from both patient mRNA datasets from the TARGET database and the RA-responsive HL-60 model, to aid in our understanding of the actionable targets for RA-induced differentiation in AML. We also add new mechanistic insight toward RA and FICZ as a co-treatment for differentiation induction therapy that could expand the therapeutic value of RA. We report that for the signalsome (1) FICZ augments RA-induced connectivity relationships rather than elicits new ones; (2) AhR associates with caveolin, mobilizing it to the inner plasma membrane; and (3) previously identified signalsome components within AML patient mRNA datasets cluster into modules, where some of these modules also occur as clusters for HL-60.One such module, representing the MAPK signaling axis/network, is particularly prominent. More specifically, the signaling network that drives RA-induced differentiation in the FAB M2 HL-60 model, exists in non-APL AML populations and clusters with greater correlation in FAB M2 myeloid leukemia subtype. Notable as a linkage from the signalsome to transcription or differentiation markers, SLP76 correlation to RXRα and p47phox are conserved in the FAB M2 model and patient cells but not in non-APL AML. We suggest that the signalsome defined in HL-60 is of import for identifying potential actionable targets in the FAB M2, non-APL AML patient population.
Our laboratory has described an RA-driven signalsome that propels RA-induced differentiation and maturation in the patient-derived HL-60 myeloblastic cell line [3, 8, 19, 20]. We recently clarified that HL-60 serves as a faithful model system for an RA-responsive subtype of non-APL AML, which is still not well characterized because of its novelty [3]. In this study, an overarching question we address is whether there are signaling molecules that distinguish RA-responsiveness from non-responsiveness in non-APL AML. First, we report several novel protein interactions in the signalsome using the HL-60 model. Next, we apply hierarchical clustering analysis to the family of signaling factors known to be part of the RA-induced signalsome in HL-60, comparing available data for RA treatment vs. combination RA and FICZ treatment. Here, FICZ—a potential therapeutic agent—is an experimental probe for discerning actionable targets in the signalsome that contribute to its function in driving differentiation. Finally, we analyze the TARGET mRNA sample database across non-APL AML patients for clustering features within the ensemble of RA-induced signalsome components. This analysis provides a global picture of mRNA expression across a spectrum of AML patients, and allows an early stage integration of clinical samples and the RA-responsive HL-60 model system to get insights on signalsome architecture.
Materials and Methods
Cell culture and treatments
The HL-60 human myeloblastic leukemia cell line, derived from the original patient isolate, was a generous gift of Dr. Robert Gallagher and maintained in this laboratory and was certified and tested for mycotoxin by Bio-Synthesis (Lewisville, TX, USA) in August 2017. The cells were grown in RPMI 1640 (Invitrogen, Carlsbad, CA) supplemented with heat inactivated 5% fetal bovine serum (Hyclone, Logan,UT) and 1× antibiotic/antimycotic (Sigma, St. Louis, MO) in a 5% CO2 humidified atmosphere at 37◦C. The cells were cultured in constant exponential growth as previously described [21]. The experimental cultures were initiated at a density of 0.1 × 106 cells/ml. Viability was monitored by 0.2% trypan blue (Invitrogen, Calsbad, CA) exclusion and routinely exceeded 95%.
For treatments, all-trans-retinoic acid (RA) (Sigma, St. Louis, MO) was added from a 5 mM stock solution in 100% ethanol to a final concentration of 1 μM in culture. 6-Formylindolo(3,2-b)carbazole (Abcam, Cambridge, MA ab141631), was added from a 100 μM DMSO stock to a final concentration of 100 nM in culture for 48h. This dose was previously established to enhance RA-induced differentiation with no evidence of toxicity [19, 20].
Direct conjugation of Alexa Fluors to primary antibodies
Conjugation of Alexa Fluor succinimidyl esters to primary antibodies was performed using the manufacturer’s protocol (Invitrogen) as previously described [22]. Briefly, 30 μL 0.75 M sodium bicarbonate, pH 8.3, was added to 200 μL primary antibody. 1 mg of Alexa Fluor was dissolved in 100 μL DMSO, and 20 μL Alexa Fluor 488 or 594 was added to one vial of primary antibody while vortexing. The tubes were then shaken at room temperature with an orbital shaker for 1 h at 200 RPM. 20 μL 1.5 M hydroxylamine, pH 8.5, was then added to each tube and then shaken for an additional 1 h. Finally, the conjugated antibodies were dialyzed in 2 L of PBS in the dark at room temperature overnight.
Fluorescence resonance energy transfer
Fluorescence resonance energy transfer (FRET) experiments were performed on fixed cells [19] with directly conjugated antibodies [22]. Briefly, 1 × 106 cells from each sample were centrifuged at 700 RPM for 5 min, washed twice with PBS, fixed for 10 min with 2% paraformaldehyde, and permeabilized with ice cold methanol as previously described [23]. After washing, cells were resuspended in 200 μl of PBS containing 5 μL of a 1:1 mixture of Alexa Fluor 488- and 594-conjugated primary antibodies. The antibodies used were as follows: rabbit AhR (Santa Cruz, sc-5578, H-211), mouse AhR (Abcam, RPT9, ab2769), rabbit c-Cbl (Santa Cruz, sc-170, C-15), mouse Cbl-b (Santa Cruz, sc-8006, G-1), mouse CD38 (BD Pharmingen, 5554580), rabbit RARα (Abcam, ab76074), mouse RXRα (Abcam, ab118329), rabbit monoclonal caveolin-1 (Abcam, ab192869), rabbit pS376SLP76 (Cell Signaling, 92711), rabbit SLP76 (Abcam, ab196599), mouse monoclonal IgG1 (Abcam, ab91353), and rabbit polyclonal IgG (Cell Signaling, 2729). Samples were incubated for 1 h at 37 °C in the dark. Samples were analyzed using a Becton Dickinson FACS Aria III SORP (San Jose, CA). To measure the FRET signal, a 488 nm laser line was used to excite Alexa Fluor 488, which in turn excited Alexa Fluor 594. Cells stained with one primary antibody conjugated to Alexa Fluor 488 or 594 were used for compensation controls for spillover into all fluorescence collection channels. At least 10,000 cells in each population were measured. The efficiency of FRET is dependent on the inverse sixth power of the intermolecular separation: E=1/[1+(r/R0)6], where r is the distance between the donor and acceptor and R0 is the Forster distance, the distance at which the E is 50% for this pair of acceptor-donor. The R0 for Alexa Fluor 488 and Alexa Fluor 594 is 60 Å. The statistical analysis was performed using GraphPad Prism (GraphPad software, San Diego, CA). Means of treatment groups were compared using one-way ANOVA with Tukey’s multiple comparisons test. The data represents the means of three repeats ± S.E.M. A p-value of < 0.05 was considered significant.
TARGET RNA-Seq data processing
TARGET RNA-Seq data set was downloaded from National Cancer Institute (NCI)’s data portal (https://ocg.cancer.gov/programs/target/data-matrix, May/18/2017). 264 patient samples from the BCCA cohort were used in this study. The processed fragments per kilobase of transcript per million mapped reads (FPKM) obtained directly from TARGET was converted to transcripts per million (TPM) by exp(log(FPKM) - log(sum(FPKM)) + log(106)), and then log2 transformed for further analysis.
Correlation and Clustering analysis
All correlation and clustering statistics were performed using R (version 3.3.3; http://www.r-project.org/). Pearson correlation was used and the correlation was calculated by the ‘rcorr’ function in ‘Hmisc’ package. The data analyzed for expression, activation and protein-protein association of signalsome members and differentiation markers from HL-60 samples included the protein-protein interactions reported here and all the data previously obtained for HL-60 model induced to differentiate with RA versus RA and FICZ (primary data reported in [19, 20]), normalized to untreated control. These data, segregated for each treatment, were analyzed using the ‘heatmap.2’ function available in the ‘gplots’ package for R [24]. TARGET cohort gene expression profiles and the absolute correlation values were further used for hierarchical clustering using a method we already reported [3]. The heatmap was generated using the ‘aheatmap’ function in R package ‘NMF’ (https://github.com/renozao/NMF) [24].
Results
AhR is part of the extra-nuclear signalsome responsible for RA-induced differentiation.
AhR, SLP76, CD38 and c-Cbl are established components of the RA-induced signalsome, and FICZ augments the activation of this signalsome to drive differentiation [3, 10, 11, 19, 20, 23]. Interestingly, we previously noted that RA treatment results in a very striking increase in phosphorylation of SLP76 [12], a protein that interacts with CD38, AhR and c-Cbl [19, 23, 25]. Given that AhR and CD38 promote RA-induced signaling and differentiation, and SLP76 appears to be an adaptor that facilitates this signaling, we were motivated to determine whether pS376SLP76 associates with AhR or CD38, and if this protein interaction is inducible with RA or RA and FICZ. SLP76 is reported to be located at the plasma membrane in various cell types including T cells and neutrophils [19, 23, 25, 26]. We chose to pursue the pS376 site because it is known to regulate helper T cell function [27], but a role in neutrophils is not yet established.
We assessed the pS376SLP76/AhR and pS376SLP76/CD38 interactions using fluorescence resonance energy transfer (FRET) measured by flow cytometry. FRET is a very sensitive method (see Materials and Methods) for detecting the distance-dependent interaction between two targets, by capturing the resonance energy transfer of donor-acceptor excited states of two target antibody-conjugated fluorophores. The large number of individual cells analyzed by flow cytometry provides credibility of observed changes in the measured populations.
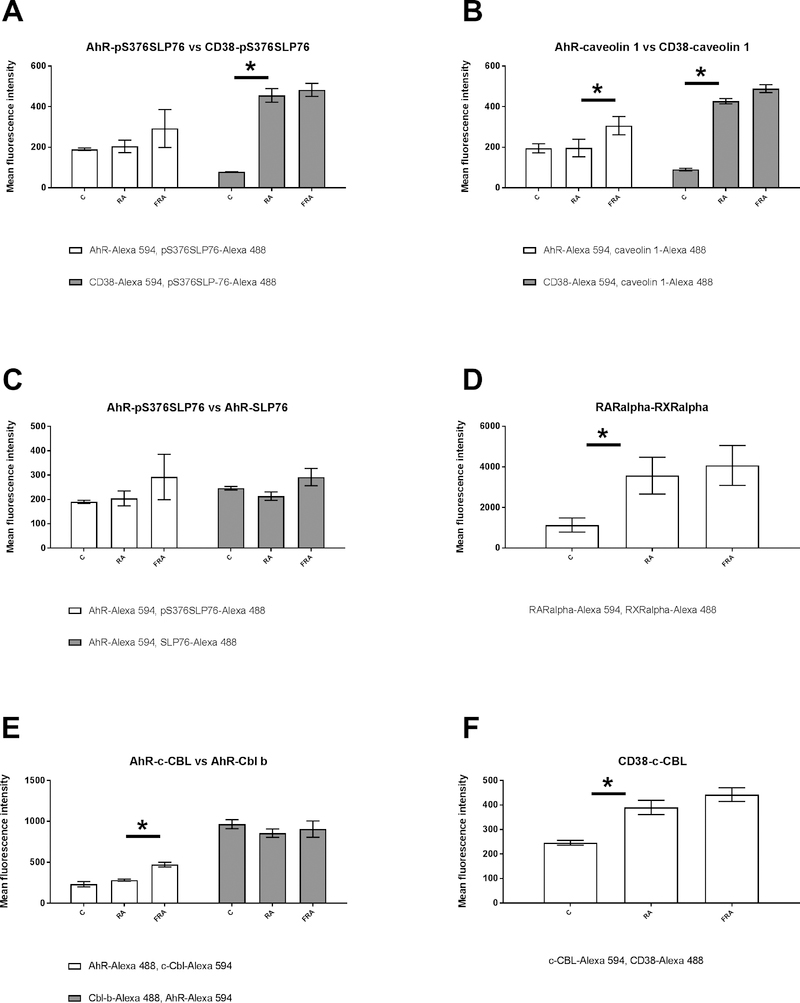
AhR associates with pS376SLP76 in untreated HL-60 samples (Fig 1A). The basal AhR-pS376SLP76 interaction is not enhanced by RA, but is modestly, albeit not significantly, enhanced by FICZ+RA. The interaction between CD38 and pS376SLP76, on the other hand, is significantly augmented by RA, p=0.0078 (Fig. 1A). We also compared the association of AhR with total SLP76 in HL-60 cells that were untreated, treated with RA alone or a combination of RA and FICZ (Fig. 1C). The interaction of these two proteins is not significantly affected by either treatment (Fig. 1C).
Figure 1: RA and RA+FICZ modulation of protein-protein associations in the RA induced signalsome.
HL-60 cells were initiated in culture at 0.1 × 106 cells/ml and treated with 1 μM RA, 100 nM FICZ, as indicated. Cells were harvested, fixed for 10 min with 2% paraformaldehyde, and permeabilized with ice cold methanol. Cells were labeled with primary antibodies (or isotype controls) directly conjugated to Alexa Fluor 488 or 594, as indicated. The immunocomplexes were analyzed using flow cytometry (BD FACS Aria III SORP, BD Biosciences). Mean fluorescence intensity for the FRET signal is presented. (A) comparison of AhR-pS376SLP76 and CD38-pS376SLP76 FRET for control, RA and RA+FICZ treated cells. (B) comparison of caveolin-1 – AhR and caveolin-1- CD38 interactions. (C) comparison of AhR interactions with either SLP76 or pS376SLP76. (D) comparison of RARα-RXRα interactions. (E) comparison of AhR interactions with either c-Cbl or Cbl b.(F) comparison of CD38 interactions with c-Cbl.
AhR has traditionally been considered a ligand-activated nuclear transcription factor, but the paradigm under consideration indicates it relates to membrane signaling molecules. We tested the anticipation that AhR was associated with the cell membrane using the membrane-specific molecule caveolin-1. Although primarily known for caveolin-mediated endocytosis, caveolin-1 is of significance here because it directs the interaction of membrane microdomains, including lipid rafts that provide putative anchors for signaling proteins [28]. We also tested for interaction of caveolin-1 with the membrane receptor CD38, which augments RA-induced differentiation, although its absence does not hinder differentiation [22, 25, 29]. In untreated HL-60 samples, AhR associates with caveolin-1 (Fig. 1B). Interestingly, in response to treatment with RA and FICZ the interaction of AhR or CD38 with caveolin-1 exhibits the same trend as their interaction with pS376SLP76. The basal level of AhR/caveolin-1 interaction is not enhanced by RA, but enhanced by FICZ+RA p=0.005. In contrast, the CD38/caveolin-1 association is significantly augmented by RA (Fig. 1B) p=0.003.
Two nuclear transcription factors responding to RA-induced signaling are the retinoic acid receptor (RAR) isoforms (α/β/γ) and their heterodimeric binding partners, the retinoid X receptor (RXR) isoforms. Hierarchical clustering analysis reveals that the expression of RXRα is highly correlated with SLP76 expression (Fig. 2). This indicates a link from the signalsome to downstream transcriptional regulation. It is known that RARα associates with RXRα after RA treatment, hence we investigated if this important interaction is modulated by RA and FICZ. We show here that there is a significant increase in RAR-RXR FRET signaling, reflecting a more favorable spatial orientation, between RARα and RXRα after RA treatment p=0.03, but this is not further augmented by combination treatment with RA and FICZ (Fig. 1D). On the chromatin, the RARα and RXRα, in the absence of the ligand provide allosteric inhibition and in the presence of the ligand (RA) undergo conformational changes that relieve allosteric inhibition and recruitment of co-activators [30]. Interestingly, there is also evidence for extra nuclear RARα localization, and the various pools of RARα and RXRα are not fully understood [31, 32].
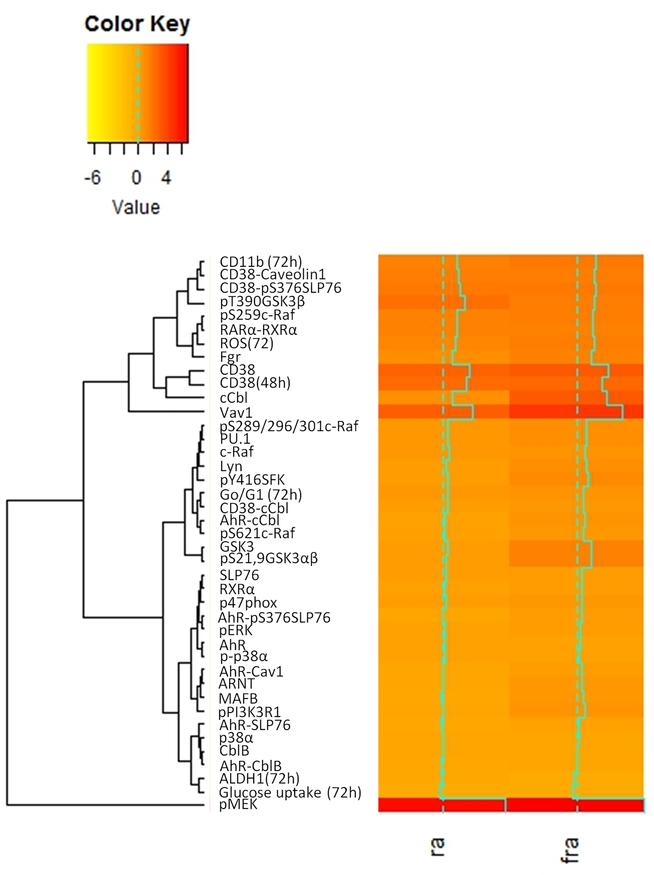
Figure 2. Hierarchical clustering of proteins in HL-60 model of non-APL AML.
Clustering based on phenotypic data, signaling protein expression, protein activation and protein association in HL-60 model was performed using the ‘heatmap.2’ function available in the ‘gplots’ package for R.
c-Cbl is an adaptor protein with E3 ligase activity and appears to be a crucial node within the RA-induced signalsome, due to the number of confirmed interactions with other signalsome factors, including SLP76, AhR and CD38. We used FRET to investigate whether three interactions already reported [19] (c-Cbl/AhR, Cbl-b/AhR and c-Cbl/CD38) were of the same amplitude with the new interactions assessed here. When we assessed the FRET signal between c-Cbl and AhR, we confirmed that the mean fluorescence intensity and thus the c-Cbl/AhR interaction was not increased by RA but was enhanced by RA and FICZ after 48 hours p,0.05 (Fig. 1E). We observed that the association of Cbl-b with AhR is constitutive, with a FRET signal about 4 times weaker than for the RARα/RXRα interaction, and no observed modulation by RA or RA and FICZ (Fig. 1E). Of note, all other interactions assessed here are about 10 times weaker than the RARα/RXRα interaction (Fig. 1). Increased CD38/c-Cbl interaction is detected (p < 0.05) after RA treatment (Fig. 1F), as anticipated [19] and consistent with RA-induced CD38 expression.
These FRET-derived interaction results elaborate on our working model of the existence of a signalsome that propels RA-induced differentiation. These data suggest that AhR and SLP76 are in the immediate proximity of plasma membrane-bound proteins CD38 and caveolin-1. More specifically, there are highly RA-inducible interactions between CD38 and pS376SLP76, CD38 and caveolin-1, and CD38 and c-Cbl. Formation and activation of this membrane-associated signaling complex involves relocalization of its constituents (Fig. 1G). RA-upregulated, CD38-associated interaction partners could serve as additional therapeutic targets to augment RA-induced differentiation therapy. Thus, we were motivated to further investigate the expression and hierarchical clustering of these signalsome factors within available datasets from RA-responsive versus non-responsive AML cells.
Clustering based on phenotypic biomarkers, protein expression, protein activation and protein association in the HL-60 model.
RA-induced differentiation is driven by a signalsome that, in addition to the CD38 receptor, includes downstream kinase modules, reflecting MAPK and PI3K pathways, and their regulators, including SFKs and adaptors [8, 9, 19, 20]. FICZ has been shown to enhance signaling and subsequent differentiation; hence it can be used as an experimental tool to probe for action elements of the signalsome. To search for functional modules within this signaling machine, hierarchical clustering analysis was performed to identify correlated factors within the current and historically accumulated data on phenotypic markers, protein expression, protein activation and protein associations. Comparing HL-60 cells treated with RA versus RA and FICZ, for example, might reveal modules of action elements seminal to signalsome activation. We started by analyzing the above FRET data and previously obtained expression, phosphorylation and functional biomarker data [19, 20] at 48 h post treatment time points in order to refine our model [19] of action modules (Fig. 2).
Phosphorylated MEK (pMEK) was the signalsome component that exhibited the greatest increase in expression after RA treatment, and combined RA and FICZ treatment augmented this increase (Fig. 2). Vav1 exhibited the next greatest increase in response to RA, which was also augmented by addition of FICZ. In contrast, the stem-like and malignant transformation markers ALDH1 expression and glucose uptake clustered distally to all other markers—these markers decreased in the RA-treated HL-60 model and decreased further in RA and FICZ-treated cells compared to untreated cells. We note that SLP76 clusters with phosphorylated (activated) ERK (pERK), which is immediately downstream of (activated) pMEK, suggesting their collaboration.
We previously reported that MAPK pathway signaling regulates transcriptional activation by RAR/RXR [16, 33, 34]. We have also reported that there are differential effects with respect to signaling attributes and cellular outcome in terms of differentiation and cell cycle progression [12, 35, 36]. We observe now that clustering analysis identifies differential coupling of specific MAPK signaling members with RARα/RXRα and differentiation markers (Fig. 2). Namely, c-Raf and its phosphorylated forms exhibit distinct correlations. pS259Raf phosphorylation, Fgr expression, RARα/RXRα interaction and reactive oxygen species (ROS) production cluster closely together and are thus strongly correlated, with pS259Raf and RARα/RXRα having one of the tightest couplings observed among clusters of all analyzed entities. In contrast, total c-Raf and pS289/296/301Raf are most highly correlated with PU.1, Lyn and pY416SFK. This cluster is closely linked to its nearest neighboring cluster, which couples G0/G1 cell cycle arrest closely with CD38/c-Cbl interaction (Fig. 2). Meanwhile, pS621Raf is better correlated with AhR/c-Cbl interaction, in a cluster most immediately linked to the group containing G0/G1 arrest and CD38/c-Cbl association. These data suggest that c-Raf and its different phosphorylated forms may differentially interact with various signalsome components to drive different features of cell differentiation.
While the clusterings thus far discussed represent primarily very tight couplings, we note that the clusterings between signaling molecules vary. This may reflect the functional connection between entities. In this regard, SLP76 is notable (Table 2). In arguably the tightest cluster, SLP76 is tightly coupled with other signaling molecules, including pERK and RXRα. pS376SLP76, through its interaction with CD38, exists in a cluster that includes CD11b and CD38/caveolin-1 interaction. While these putative interactions are novel, reflecting a significance for SLP76 in driving differentiation via the CD38-associated signalsome, this is consistent with SLP76 being a facilitator of membrane-associated signaling complexes colocalized with receptors.
Table 2.
The proteins that correlate with SLP76 in non-APL AML and FAB M2 TARGET AML data set analyzed.
| Non-APL-AML | FAB M2 |
|---|---|
| Vav1 | Vav1 |
| Raf1 | Raf1 |
| GSK3β | GSK3β |
| ARNT | ARNT |
| p38α | p38α |
| GSK3α | GSK3α |
| cCbl | cCbl |
| ERK2 | ERK2 |
| Fgr | |
| CD11b | |
| RXRα | |
| NCF1 |
We also observed that pS376SLP76 association with AhR was linked to pERK in a 2-element group with high Pearson correlation. SLP76 ergo appears to be a multifaceted functionary, in this specific instance linking AhR and ERK activation. This is consistent with previous report that the AhR ligand FICZ enhances ERK activation and drives differentiation [20]. Notably, the most prominent upregulated signalsome components induced by FICZ are c-Cbl and Vav1, where AhR, c-Cbl and SLP76 are a known interacting triad of proteins [19, 37]. Hence the clustering data suggest mechanistic detail to previous effects and point to a significant role in particular for SLP76 in mediating signaling seminal to RA-induced differentiation. Interestingly, in the instance of GSK3, which has been shown to promote RA-induced differentiation, pT390-GSK3β increases with both treatments compared to untreated samples but decreases with RA and FICZ compared to RA alone.
Gene expression data from non-APL AML patients reveals modular architecture of RA-induced signalsome
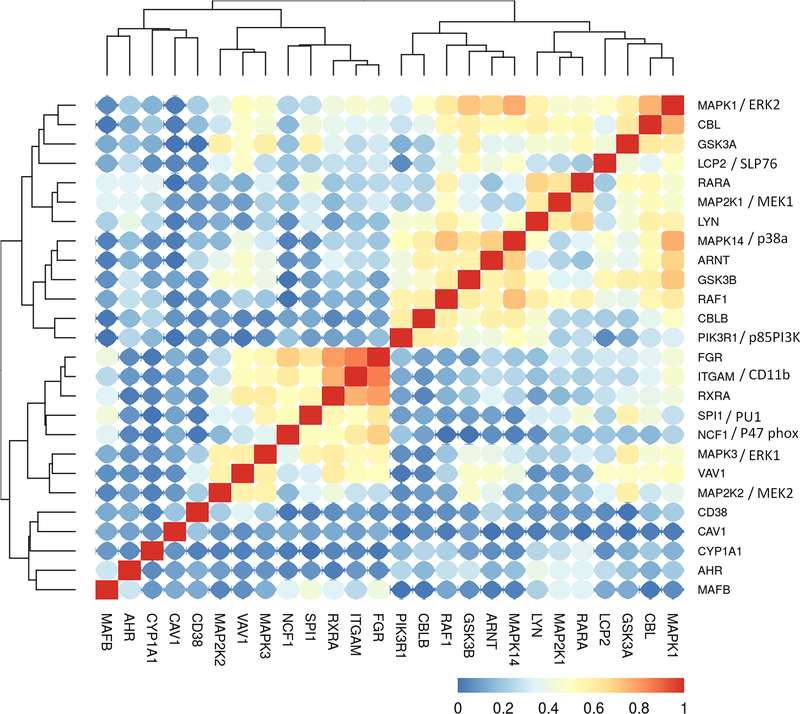
Using the HL-60 model as a guide to identify the prominent molecules and interactions seminal to RA response, we next interrogated the TARGET data base to ascertain if the expression observed in clinical patient data is consistent with our model motivated by the cell line. We first analyzed the expression correlation of the signalsome components in a non-APL AML TARGET BCCA cohort, which consists of 264 patient mRNAseq samples collected at the first presentation/diagnosis (Fig. 3). The composition of the AML dataset in the TARGET database according to FAB classification is presented in Table 1.
Figure 3:
Clustering analysis of the TARGET non-APL AML (BCCA cohort) RNA-Seq data set, using the gene expression data for the genes we analyzed in HL-60 model. The Pearson correlation between each gene pairs were calculated and then a hieratical clustering was performed on the absolute correlation. Red color represents higher correlation (either positive or negative) and blue color represents less correlation. 264 patient samples were included in the analysis.
Table 1.
Sample distribution (FAB classification) of the TARGET AML data set analyzed
| FAB classification | n patients |
|---|---|
| M0 | 7 |
| M1 | 36 |
| M2 | 71 |
| M4 | 66 |
| M5 | 54 |
| M6 | 4 |
| M7 | 9 |
| NOS | 17 |
There are several prominent distinct cluster groups of signaling molecules evident within the clustered patient datasets. Looking at the Raf/MEK/ERK axis, we observe that ERK1 and ERK2 expression correlates with all RA-induced signalsome molecules, except for CD38, caveolin-1, AhR and MAFB. Additionally, ERK1 and ERK2 do not correlate with cytochrome P4501A1, a downstream target of AhR transcriptional activity. ERK1 is in a tight cluster with MEK2 and Vav1, whereas ERK2 is in a tight cluster with c-Cbl and GSK3α, coupled to SLP76. Interestingly, MEK1 and MEK2 are sequestered in two distinct clusters. MEK1 is highly correlated with Lyn and RARα, whereas MEK2 strongly clusters with ERK1 and Vav1. The MEK1 cluster is coupled to a cluster of ERK2, c-Cbl, GSK3α and SLP76. MEK1 expression correlates with c-Raf, but MEK2 does not. c-Raf clusters closely with GSK3β, p38, and ARNT with the connected tight cluster of p85PI3K and Cbl-b. c-Raf expression also correlates positively with c-Cbl, Lyn, MEK1, ERK2 and RARα. Lyn, RARα and MEK1 form a tight cluster. It also associates with c-Cbl, SLP76, GSK3α and ERK2. Fgr is another SFK in the signalsome, and CD11b, Fgr and RXRα are highly correlated. Fgr also correlates with Vav1, ERK1, p47phox and PU.1, thus having a set of associations distinct from Lyn. Hence there is apparent linkage between signalsome entities in the patient samples, and the clustering suggests some degree of modular structure within the signalsome.
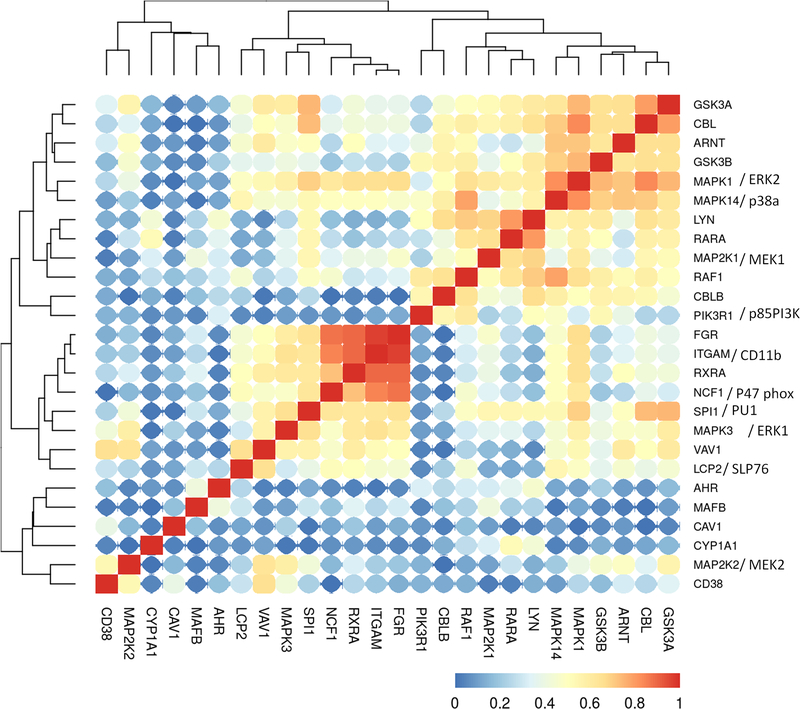
Having characterized the hierarchical clustering of RA-induced signalsome proteins from a non-APL AML patient cohort, we next analyzed clustering of the same molecules in FAB M2 AML, the subtype to which HL-60 is ascribed (Fig. 4). Knowing that most non-APL AML patients are not RA responsive, we asked if a distinguishing feature for the FAB M2 subtype of AML could be discerned when subject to the same hierarchical clustering analysis. Accordingly, we analyzed the same ensemble of signalsome molecules in an FAB M2 subset consisting of data from 71 AML patients. We make the general observation that the clusterings in this subcategory are generally similar to that of non-APL AML; however, the correlations values are closer to 1.0 compared to the clustering across all non-APL AML patients. Also, some differences emerge.
Figure 4:
Clustering analysis of the TARGET M2 FAB classification AML (BCCA cohort) RNA-Seq data set, using the gene expression data for the genes we analyzed in HL-60 model. The Pearson correlation between each gene pairs were calculated and then a hieratical clustering was performed on the absolute correlation. 71 patient samples were analyzed.
ERK1 and ERK2 in the FAB M2 population exhibited largely the same correlations or lack of couplings as observed above for the non-APL AML (Fig. 4). However, although there was a lack of correlation with AhR, MAFB, caveolin-1, and cytochrome P450 1A1 observed, there was coupling detected with CD38, unlike the case for all non-APL AML. MEK2 still correlates with ERK1 and Vav1, but now correlates tightly with CD38 as well. We note that in the FAB M2 AML population, CD38 exhibits coupling to more partners, akin to the case for HL-60 cells, compared to the non-APL AML population analyzed above. c-Raf shows essentially the same correlations that were observed in the broad non-APL AML cohort. SLP76 clusters in FAB M2 with the same set of molecules as in non-APL AML as well as with Fgr, CD11b, RXRα, and p47phox. Lyn clusters with RARα and MEK1 in FAB M2 as it does in broad non-APL AML. However, in the FAB M2 subtype dataset, Lyn also associates with many other signalsome molecules, including c-Cbl, p85 PI3K, p38α, c-Raf, MEK1, ERK2, AhR, as well as with Cbl-b, GSK3α/β and the transcription factor PU.1. Notably, GSK3 [38] and PU.1 [8] are downstream drivers of myeloid differentiation. Fgr clusters with the same ensemble of molecules as it did for non-APL AML, showing no appreciable differences for APL.
Discussion
Currently, a great impediment to non-APL AML diagnosis and treatment is the lack of reliable markers and actionable targets to exploit for therapeutic intervention and predict outcome. Medical management of the disease has been challenging. Remission rates have been poor, and the disease is not susceptible to RA differentiation therapy. The APL subtype of AML, in contrast, has a marker that is also an actionable target, namely the PML-RARα fusion protein, the sine qua non of APL. RA induces remission in almost all PML-RARα positive APL patients [39, 40]. However, the remission is not durable, and the relapsed cases are resistant to retinoid treatment [41]. The current standard of care is RA in combination with arsenic trioxide. In a fraction of cases, there is the further pathological sequela of treatment, retinoic acid syndrome (RAS), that can result in fatal cardiopulmonary failure [42, 43]. Thus, even for APL there is still a need for RA-based therapies with higher efficacy and minimized toxicity.
In the hope of reducing relapses in APL patients and extending the use of RA therapy to non-APL AML patients, combination therapy including RA is in use for APL and being explored for non-APL AML [41]. However, rational optimization of therapeutic regimens is challenging and empirically derived in the absence of specific molecular targets. A study analyzing the dataset derived from the North American Intergroup Study INT0129, calculated for the first time that for APL, the estimated duration of RA needed to eliminate the leukemic stem population is a year [44]. RA monotherapy has been effective in both induction and maintenance of remission in some cases of APL [45, 46]. In a pulsed RA clinical study, RA was administered 45 mg/m2/day for 21 days, then for 1 week every 2 weeks [45]. An earlier study established that a single oral RA dose of 15 mg/m2 led to a plasma concentration of 1 μM, a concentration sufficient to induce APL blast differentiation [47]. We chose to use 1 μM RA to induce differentiation in HL-60 cells, a FAB M2 model system of RA-responsive non-APL AML. We recently reported that this model bears fidelity to a subset of primary non-APL AML patients [3]. As proof of principle in this model we have found that combination therapy can enhance RA monotherapy [3].
The present study sought to gain insight into the workings of the signalsome responsible for RA induced differentiation. To that end, we analyzed the putatively significant couplings of signalsome components identified in the RA-responsive, FAB M2 HL-60 model cell line, followed by clustering analysis of the same entities in largely non-responsive non-APL AML patient population. We then sought conserved or prominently divergent features in the FAB M2 AML patient populations. Such an analysis would hopefully point to elements of the signalsome vulnerable to therapeutic intervention. This, in turn, may motivate rational design of combination therapies using RA with other agents to enhance RA action. The data revealed certain novel findings toward this end.
AhR was one such molecule of interest. It is a receptor within the signalsome. FICZ is an endogenous AhR ligand which we found enhances RA-induced differentiation of HL-60 cells to neutrophils [3, 19, 20]. The mechanism by which FICZ propels differentiation is not well understood. While it might cause changes in signalsome composition that result in global alterations of molecular linkages, it might also use the existing signaling machine and enhance connectivity of its components, akin to the function of an adaptor, for example. Traditionally considered a ligand activated nuclear transcription factor, we provide evidence that it also functions at the plasma membrane to promote RA-induced differentiation. We previously reported that the AhR ligand FICZ combined with RA augments RA-induced differentiation [19, 20] and that AhR is instrumental in RA-induced differentiation [4] through activity beyond its transcriptional activity [19]. We hypothesized that AhR is part of the RA-induced signalsome and localized close to the plasma membrane, possibly as a scaffold. Our results confirm that AhR is closely associated with caveolin-1 and shares protein interaction partners (c-Cbl, SLP76 and pS376SLP76) with the CD38 surface protein. This contributes to a mechanistic rationalization for its action and motivates it as a target to use in RA-combination therapy.
c-Cbl is a functionally prominent component of the signalsome, and also provides prognostic stratification in AML patients [3, 48–52]. c-Cbl undergoes CD38-dependent phosphorylation during RA-induced HL-60 differentiation [53]. Moreover, overexpression of c-Cbl augments CD38 basal levels and propels RA-induced differentiation and MAPK activation, whereas c-Cbl knockdown blunts differentiation [11]. A c-Cbl tyrosine kinase binding domain mutant (G306E) is unable to complex with CD38 and drive MAPK signaling and cell differentiation, suggesting that c-Cbl is important in effecting signaling seminal to RA-induced differentiation [23]. Recently we analyzed the Therapeutically Applicable Research to Generate Effective Treatments (TARGET) database (National Cancer Institute) and showed that c-Cbl expression levels stratify the survival rates in mutant-NPM1 AML patients [3].
CD38 is a type II transmembrane protein; RA induces its expression and ectopic overexpression augments RA-induced differentiation [29]. However, enigmatically CD38 knockdown does not impair RA-induced differentiation [22]. The fact that CD38 and caveolin-1 anchor related elements of the RA-induced signalsome (AhR, c-Cbl and SLP76) to the plasma membrane may explain how CD38 potentiates a differentiation signal without being necessary. Significantly, while CD38 is coupled to a number of signalsome partners in HL-60 cells and FAB M2 patients, CD38 was largely devoid of such correlations in non-APL AML patients. Analyzing the TARGET cohorts (Fig. 3 and 4) revealed that in non-APL AML subtypes, CD38 does not cluster with MAPK pathway members; however, for the FAB M2 subtype it does. Clinically, the presence of CD34highCD38low AML blasts indicates a poor prognosis [54, 55].
Caveolin-1 is a membrane lipid raft protein known to be a scaffold protein involved in caveolin-mediated endocytosis. However, caveolin-1 is also known to promote c-Raf signaling [56]. Prolonged MAPK signaling involving nuclear translocation of c-Raf propels RA-induced myeloid cell differentiation [16, 33, 57]. The putative interaction of AhR and CD38 with signaling molecule adaptors like c-Cbl, SLP76 and pS376SLP76 along with caveolin-1 may provide the cohesion of signaling molecules seminal to durable activation. Here, CD38 and caveolin-1 may provide a plasma membrane scaffold to anchor the RA-induced signalsome (Fig. 1).
Conclusions
Taken together our results show that the HL-60 model of non-APL AML is a valuable model instrumental in understanding the complexity of non-APL AML. AhR, positioned close to the plasma membrane, is part of the RA-induced signalsome responsible for propelling differentiation. Generally, FICZ in combination with RA augments RA-induced changes rather than elicits new changes. By itself FICZ causes no enhancement in signaling or differentiation. In this regard, it was previously reported that FICZ by itself did not affect signaling seminal to differentiation or cell differentiation/proliferation state [20]. The signalsome components of patient mRNA (TARGET database) cluster into modules, some being similar to protein clusters previously observed in HL-60 samples, with a significant module being MAPK signaling molecules correlating with CD38. The results of our analysis of the signalsome components both in the TARGET database and in the RA-responsive model contribute insights on the potentially actionable targets for RA-induced differentiation in AML that could expand the therapeutic use of RA.
Acknowledgments
This work was supported by grant R01 CA152870 from the National Institutes of Health (AY) and by the Center on the Physics of Cancer Metabolism through Award Number 1U54CA210184-01 from the National Cancer Institute.
Source(s) of support. This work was supported by grant R01 CA152870 from the National Institutes of Health (AY) and by the Center on the Physics of Cancer Metabolism through Award Number 1U54CA210184-01 from the National Cancer Institute.
Abbreviations:
- AML
acute myelocytic leukemia
- APL
acute promyelocytic leukemia
- RA
retinoic acid
- FICZ
6-Formylindolo(3,2-b)carbazole
- AhR
aryl hydrocarbon receptor
- MAPK
mitogen-activated protein kinase
- SFK
Src-family-kinases
- ALDH1
aldehyde dehydrogenase 1
- FRET
fluorescence resonance energy transfer
Footnotes
Conflict of Interest declaration. None of the authors have any financial conflict of interests.
Potential conflict of interest
None of the authors have any financial conflict of interests.
Disclaimers. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Ades L, Guerci A, Raffoux E, Sanz M, Chevallier P, Lapusan S, Recher C, Thomas X, Rayon C, Castaigne S, Tournilhac O, de Botton S, Ifrah N, Cahn JY, Solary E, Gardin C, Fegeux N, Bordessoule D, Ferrant A, Meyer-Monard S, Vey N, Dombret H, Degos L, Chevret S, Fenaux P & European APLG (2010) Very long-term outcome of acute promyelocytic leukemia after treatment with all-trans retinoic acid and chemotherapy: the European APL Group experience, Blood. 115, 1690–6. [DOI] [PubMed] [Google Scholar]
- 2.Yen A, Powers V & Fishbaugh J (1986) Retinoic acid induced HL-60 myeloid differentiation: dependence of early and late events on isomeric structure, Leukemia research. 10, 619–29. [DOI] [PubMed] [Google Scholar]
- 3.Bunaciu RP, MacDonald RJFG, Johnson LM, Varner JD, Wang X, Nataraj S, Guzman ML & Yen A (2018) Potential for subsets of wt-NPM1 primary AML blasts to respond to retinoic acid treatment, Oncotarget. 9, 4134–4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bunaciu RP & Yen A (2011) Activation of the aryl hydrocarbon receptor AhR Promotes retinoic acid-induced differentiation of myeloblastic leukemia cells by restricting expression of the stem cell transcription factor Oct4, Cancer research. 71, 2371–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yen A, Roberson MS, Varvayanis S & Lee AT (1998) Retinoic acid induced mitogen-activated protein (MAP)/extracellular signal-regulated kinase (ERK) kinase-dependent MAP kinase activation needed to elicit HL-60 cell differentiation and growth arrest, Cancer research. 58, 3163–72. [PubMed] [Google Scholar]
- 6.Hong HY, Varvayanis S & Yen A (2001) Retinoic acid causes MEK-dependent RAF phosphorylation through RARalpha plus RXR activation in HL-60 cells, Differentiation; research in biological diversity. 68, 55–66. [DOI] [PubMed] [Google Scholar]
- 7.Yen A & Varvayanis S (2000) Retinoic acid increases amount of phosphorylated RAF; ectopic expression of cFMS reveals that retinoic acid-induced differentiation is more strongly dependent on ERK2 signaling than induced GO arrest is, In vitro cellular & developmental biology Animal. 36, 249–55. [DOI] [PubMed] [Google Scholar]
- 8.Tasseff R, Jensen HA, Congleton J, Dai D, Rogers KV, Sagar A, Bunaciu RP, Yen A & Varner JD (2017) An Effective Model of the Retinoic Acid Induced HL-60 Differentiation Program, Scientific reports. 7, 14327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jensen HA, Bunaciu RP, Varner JD & Yen A (2015) GW5074 and PP2 kinase inhibitors implicate nontraditional c-Raf and Lyn function as drivers of retinoic acid-induced maturation, Cellular signalling. 27, 1666–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Congleton J, MacDonald R & Yen A (2012) Src inhibitors, PP2 and dasatinib, increase retinoic acid-induced association of Lyn and c-Raf (S259) and enhance MAPK-dependent differentiation of myeloid leukemia cells, Leukemia : official journal of the Leukemia Society of America, Leukemia Research Fund, UK. 26, 1180–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shen M & Yen A (2008) c-Cbl interacts with CD38 and promotes retinoic acid-induced differentiation and G0 arrest of human myeloblastic leukemia cells, Cancer research. 68, 8761–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yen A, Varvayanis S, Smith JL & Lamkin TJ (2006) Retinoic acid induces expression of SLP-76: expression with c-FMS enhances ERK activation and retinoic acid-induced differentiation/G0 arrest of HL-60 cells, European journal of cell biology. 85, 117–32. [DOI] [PubMed] [Google Scholar]
- 13.Shen M, Bunaciu RP, Congleton J, Jensen HA, Sayam LG, Varner JD & Yen A (2011) Interferon regulatory factor-1 binds c-Cbl, enhances mitogen activated protein kinase signaling and promotes retinoic acid-induced differentiation of HL-60 human myelo-monoblastic leukemia cells, Leukemia & lymphoma. 52, 2372–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marchisio M, Bertagnolo V, Colamussi ML, Capitani S & Neri LM (1998) Phosphatidylinositol 3-kinase in HL-60 nuclei is bound to the nuclear matrix and increases during granulocytic differentiation, Biochemical and biophysical research communications. 253, 346–51. [DOI] [PubMed] [Google Scholar]
- 15.Yen A, Williams M, Platko JD, Der C & Hisaka M (1994) Expression of activated RAF accelerates cell differentiation and RB protein down-regulation but not hypophosphorylation, European journal of cell biology. 65, 103–13. [PubMed] [Google Scholar]
- 16.Wang J & Yen A (2008) A MAPK-positive feedback mechanism for BLR1 signaling propels retinoic acid-triggered differentiation and cell cycle arrest, The Journal of biological chemistry. 283, 4375–86. [DOI] [PubMed] [Google Scholar]
- 17.Katagiri K, Yokoyama KK, Yamamoto T, Omura S, Irie S & Katagiri T (1996) Lyn and Fgr protein-tyrosine kinases prevent apoptosis during retinoic acid-induced granulocytic differentiation of HL-60 cells, The Journal of biological chemistry. 271, 11557–62. [DOI] [PubMed] [Google Scholar]
- 18.Wu J, Meng F, Lu H, Kong L, Bornmann W, Peng Z, Talpaz M & Donato NJ (2008) Lyn regulates BCR-ABL and Gab2 tyrosine phosphorylation and c-Cbl protein stability in imatinib-resistant chronic myelogenous leukemia cells, Blood. 111, 3821–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bunaciu RP, Jensen HA, MacDonald RJ, LaTocha DH, Varner JD & Yen A (2015) 6-Formylindolo(3,2-b)Carbazole (FICZ) Modulates the Signalsome Responsible for RA-Induced Differentiation of HL-60 Myeloblastic Leukemia Cells, PloS one. 10, e0135668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bunaciu RP & Yen A (2013) 6-Formylindolo (3,2-b)carbazole (FICZ) enhances retinoic acid (RA)-induced differentiation of HL-60 myeloblastic leukemia cells, Molecular cancer. 12, 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brooks SC, Kazmer S, Levin AA & Yen A (1996) Myeloid differentiation and retinoblastoma phosphorylation changes in HL-60 cells induced by retinoic acid receptor- and retinoid X receptor-selective retinoic acid analogs, Blood. 87, 227–37. [PubMed] [Google Scholar]
- 22.MacDonald RJ, Shrimp JH, Jiang H, Zhang L, Lin H & Yen A (2017) Probing the requirement for CD38 in retinoic acid-induced HL-60 cell differentiation with a small molecule dimerizer and genetic knockout, Scientific reports. 7, 17406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shen M & Yen A (2009) c-Cbl tyrosine kinase-binding domain mutant G306E abolishes the interaction of c-Cbl with CD38 and fails to promote retinoic acid-induced cell differentiation and G0 arrest, The Journal of biological chemistry. 284, 25664–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.R Core Team (2016) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: [Google Scholar]
- 25.Congleton J, Shen M, MacDonald R, Malavasi F & Yen A (2014) Phosphorylation of c-Cbl and p85 PI3K driven by all-trans retinoic acid and CD38 depends on Lyn kinase activity, Cellular signalling. 26, 1589–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Noraz N, Schwarz K, Steinberg M, Dardalhon V, Rebouissou C, Hipskind R, Friedrich W, Yssel H, Bacon K & Taylor N (2000) Alternative antigen receptor (TCR) signaling in T cells derived from ZAP-70-deficient patients expressing high levels of Syk, The Journal of biological chemistry. 275, 15832–8. [DOI] [PubMed] [Google Scholar]
- 27.Navas VH, Cuche C, Alcover A & Di Bartolo V (2017) Serine Phosphorylation of SLP76 Is Dispensable for T Cell Development but Modulates Helper T Cell Function, PloS one. 12, e0170396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lockwich TP, Liu X, Singh BB, Jadlowiec J, Weiland S & Ambudkar IS (2000) Assembly of Trp1 in a signaling complex associated with caveolin-scaffolding lipid raft domains, The Journal of biological chemistry. 275, 11934–42. [DOI] [PubMed] [Google Scholar]
- 29.Lamkin TJ, Chin V, Varvayanis S, Smith JL, Sramkoski RM, Jacobberger JW & Yen A (2006) Retinoic acid-induced CD38 expression in HL-60 myeloblastic leukemia cells regulates cell differentiation or viability depending on expression levels, Journal of cellular biochemistry. 97, 1328–38. [DOI] [PubMed] [Google Scholar]
- 30.Westin S, Kurokawa R, Nolte RT, Wisely GB, McInerney EM, Rose DW, Milburn MV, Rosenfeld MG & Glass CK (1998) Interactions controlling the assembly of nuclear-receptor heterodimers and co-activators, Nature. 395, 199–202. [DOI] [PubMed] [Google Scholar]
- 31.Piskunov A & Rochette-Egly C (2012) A retinoic acid receptor RARalpha pool present in membrane lipid rafts forms complexes with G protein alphaQ to activate p38MAPK, Oncogene. 31, 3333–45. [DOI] [PubMed] [Google Scholar]
- 32.Piskunov A, Al Tanoury Z & Rochette-Egly C (2014) Nuclear and extra-nuclear effects of retinoid Acid receptors: how they are interconnected, Sub-cellular biochemistry. 70, 103–27. [DOI] [PubMed] [Google Scholar]
- 33.Wang J & Yen A (2004) A novel retinoic acid-responsive element regulates retinoic acid-induced BLR1 expression, Molecular and cellular biology. 24, 2423–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Geil WM & Yen A (2014) Nuclear Raf-1 kinase regulates CXCR5 promoter by associating with NFATc3 to drive retinoic acid-induced leukemic cell differentiation, The FEBS journal. 281, 1170–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yen A, Forbes ME, Varvayanis S, Tykocinski ML, Groger RK & Platko JD (1993) C-FMS dependent HL-60 cell differentiation and regulation of RB gene expression, Journal of cellular physiology. 157, 379–91. [DOI] [PubMed] [Google Scholar]
- 36.Yen A, Sturgill R & Varvayanis S (1997) Increasing c-FMS (CSF-1 receptor) expression decreases retinoic acid concentration needed to cause cell differentiation and retinoblastoma protein hypophosphorylation, Cancer research. 57, 2020–8. [PubMed] [Google Scholar]
- 37.Ibabao CN, Bunaciu RP, Schaefer DM & Yen A (2015) The AhR agonist VAF347 augments retinoic acid-induced differentiation in leukemia cells, FEBS open bio. 5, 308–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wallace AS, Supnick HT, Bunaciu RP & Yen A (2016) RRD-251 enhances all-trans retinoic acid (RA)-induced differentiation of HL-60 myeloblastic leukemia cells, Oncotarget. 7, 46401–46418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nilsson B (1984) Probable in vivo induction of differentiation by retinoic acid of promyelocytes in acute promyelocytic leukaemia, British journal of haematology. 57, 365–71. [DOI] [PubMed] [Google Scholar]
- 40.Douer D, Zickl LN, Schiffer CA, Appelbaum FR, Feusner JH, Shepherd L, Willman CL, Bloomfield CD, Paietta E, Gallagher RE, Park JH, Rowe JM, Wiernik PH & Tallman MS (2013) All-trans retinoic acid and late relapses in acute promyelocytic leukemia: very long-term follow-up of the North American Intergroup Study I0129, Leukemia research. 37, 795–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cornic M, Delva L, Castaigne S, Lefebvre P, Balitrand N, Degos L & Chomienne C (1994) In vitro all-trans retinoic acid (ATRA) sensitivity and cellular retinoic acid binding protein (CRABP) levels in relapse leukemic cells after remission induction by ATRA in acute promyelocytic leukemia, Leukemia : official journal of the Leukemia Society of America, Leukemia Research Fund, UK. 8, 914–7. [PubMed] [Google Scholar]
- 42.Ahmed Z, Shaikh MA, Raval A, Mehta JB, Byrd RP Jr. & Roy TM (2007) All-trans retinoic acid syndrome: another cause of drug-induced respiratory failure, Southern medical journal. 100, 899–902. [DOI] [PubMed] [Google Scholar]
- 43.Breccia M, Latagliata R, Carmosino I, Cannella L, Diverio D, Guarini A, De Propris MS, Petti MC, Avvisati G, Cimino G, Mandelli F & Lo-Coco F (2008) Clinical and biological features of acute promyelocytic leukemia patients developing retinoic acid syndrome during induction treatment with all-trans retinoic acid and idarubicin, Haematologica. 93, 1918–20. [DOI] [PubMed] [Google Scholar]
- 44.Werner B, Gallagher RE, Paietta EM, Litzow MR, Tallman MS, Wiernik PH, Slack JL, Willman CL, Sun Z, Traulsen A & Dingli D (2014) Dynamics of leukemia stem-like cell extinction in acute promyelocytic leukemia, Cancer research. 74, 5386–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Visani G, Buonamici S, Malagola M, Isidori A, Piccaluga PP, Martinelli G, Ottaviani E, Grafone T, Baccarani M & Tura S (2001) Pulsed ATRA as single therapy restores long-term remission in PML-RARalpha-positive acute promyelocytic leukemia patients: real time quantification of minimal residual disease. A pilot study, Leukemia : official journal of the Leukemia Society of America, Leukemia Research Fund, UK. 15, 1696–700. [DOI] [PubMed] [Google Scholar]
- 46.Finsinger P, Breccia M, Minotti C, Carmosino I, Girmenia C, Chisini M, Volpicelli P, Vozella F, Romano A, Montagna C, Colafigli G, Cimino G, Avvisati G, Petti MC, Lo-Coco F, Foa R & Latagliata R (2015) Acute promyelocytic leukemia in patients aged >70 years: the cure beyond the age, Annals of hematology. 94, 195–200. [DOI] [PubMed] [Google Scholar]
- 47.Chen GQ, Shen ZX, Wu F, Han JY, Miao JM, Zhong HJ, Li XS, Zhao JQ, Zhu J, Fang ZW, Chen SJ, Chen Z & Wang ZY (1996) Pharmacokinetics and efficacy of low-dose all-trans retinoic acid in the treatment of acute promyelocytic leukemia, Leukemia : official journal of the Leukemia Society of America, Leukemia Research Fund, UK. 10, 825–8. [PubMed] [Google Scholar]
- 48.Ibanez M, Such E, Cervera J, Luna I, Gomez-Segui I, Lopez-Pavia M, Dolz S, Barragan E, Fuster O, Llop M, Rodriguez-Veiga R, Avaria A, Oltra S, Senent ML, Moscardo F, Montesinos P, Martinez-Cuadron D, Martin G & Sanz MA (2012) Rapid screening of ASXL1, IDH1, IDH2, and c-CBL mutations in de novo acute myeloid leukemia by high-resolution melting, The Journal of molecular diagnostics : JMD. 14, 594–601. [DOI] [PubMed] [Google Scholar]
- 49.Ghassemifar R, Thien CB, Finlayson J, Joske D, Cull GM, Augustson B & Langdon WY (2011) Incidence of c-Cbl mutations in human acute myeloid leukaemias in an Australian patient cohort, Pathology. 43, 261–5. [DOI] [PubMed] [Google Scholar]
- 50.Caligiuri MA, Briesewitz R, Yu J, Wang L, Wei M, Arnoczky KJ, Marburger TB, Wen J, Perrotti D, Bloomfield CD & Whitman SP (2007) Novel c-CBL and CBL-b ubiquitin ligase mutations in human acute myeloid leukemia, Blood. 110, 1022–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sargin B, Choudhary C, Crosetto N, Schmidt MHH, Grundler R, Rensinghoff M, Thiessen C, Tickenbrock L, Schwable J, Brandts C, August B, Koschmieder S, Bandi SR, Duyster J, Berdel WE, Muller-Tidow C, Dikic I & Serve H (2007) Flt3-dependent transformation by inactivating c-Cbl mutations in AML, Blood. 110, 1004–12. [DOI] [PubMed] [Google Scholar]
- 52.Brizzi MF, Rosso A, Dentelli P, Ferrero D, Lanfrancone L & Pegoraro L (1998) c-Cbl tyrosine phosphorylation and subcellular localization in human primary leukemic cells, Experimental hematology. 26, 1229–39. [PubMed] [Google Scholar]
- 53.Kontani K, Kukimoto I, Nishina H, Hoshino S, Hazeki O, Kanaho Y & Katada T (1996) Tyrosine phosphorylation of the c-cbl proto-oncogene product mediated by cell surface antigen CD38 in HL-60 cells, The Journal of biological chemistry. 271, 1534–7. [DOI] [PubMed] [Google Scholar]
- 54.Haase D, Feuring-Buske M, Schafer C, Schoch C, Troff C, Gahn B, Hiddemann W & Wormann B (1997) Cytogenetic analysis of CD34+ subpopulations in AML and MDS characterized by the expression of CD38 and CD117, Leukemia : official journal of the Leukemia Society of America, Leukemia Research Fund, UK. 11, 674–9. [DOI] [PubMed] [Google Scholar]
- 55.Jentzsch M, Bill M, Nicolet D, Leiblein S, Schubert K, Pless M, Bergmann U, Wildenberger K, Schuhmann L, Cross M, Ponisch W, Franke GN, Vucinic V, Lange T, Behre G, Mrozek K, Bloomfield CD, Niederwieser D & Schwind S (2017) Prognostic impact of the CD34+/CD38- cell burden in patients with acute myeloid leukemia receiving allogeneic stem cell transplantation, American journal of hematology. 92, 388–396. [DOI] [PubMed] [Google Scholar]
- 56.Xu L, Wang L, Wen Z, Wu L, Jiang Y, Yang L, Xiao L, Xie Y, Ma M, Zhu W, Ye R & Liu X (2016) Caveolin-1 is a checkpoint regulator in hypoxia-induced astrocyte apoptosis via Ras/Raf/ERK pathway, American journal of physiology Cell physiology. 310, C903–10. [DOI] [PubMed] [Google Scholar]
- 57.Smith J, Bunaciu RP, Reiterer G, Coder D, George T, Asaly M & Yen A (2009) Retinoic acid induces nuclear accumulation of Raf1 during differentiation of HL-60 cells, Experimental cell research. 315, 2241–8. [DOI] [PMC free article] [PubMed] [Google Scholar]