Abstract
Objective:
We investigated risk of progression to rheumatoid arthritis (RA) in patients who were cyclic citrullinated antibody positive (CCP+) without RA at initial presentation.
Methods:
We performed a retrospective cohort study of CCP+ individuals seen at a US tertiary care system between 2009-2018 who were without RA or other systemic rheumatic disease by medical record review at time of CCP positivity. Progression to classifiable RA was determined through medical record review. We investigated risk of progression to RA overall and stratified by CCP level (low: >1 to 2 times upper limit of normal [x ULN]; medium: >2 to 3x ULN; high: >3x ULN). Multivariable Cox regression estimated the hazard ratio (HR) and 95% confidence interval (95%CI) for RA by CCP level.
Results:
We identified 340 CCP+ patients who were without RA or other rheumatic disease at baseline. During 1047 person-years of follow-up, 73 (21.5%) patients developed RA. Risk of progression to RA increased with CCP level, with 46.0% (95%CI 34.7-55.3) of high level CCP patients progressing to RA by 5 years. Compared to low CCP, medium (HR 3.00, 95%CI 1.32-6.81) and high (HR 4.83, 95%CI 2.51-9.31) CCP levels were strongly associated with progression to RA, adjusting for age, sex, body mass index, smoking, family history of RA, and rheumatoid factor level.
Conclusion:
Among CCP+ patients without RA, risk for progression to RA increased substantially with increasing CCP level. This study provides further support for close monitoring for development of RA among CCP+ patients and identifying strategies to mitigate this risk.
Keywords: cyclic citrullinated peptide antibody, rheumatoid arthritis, prevention
INTRODUCTION
Rheumatoid arthritis (RA) develops through preclinical phases prior to onset of classifiable RA (1). Previous studies have demonstrated presence of RA-specific antibodies like cyclic citrullinated peptide antibody (CCP) in the serum several years prior to RA onset (2-4). To date, much of what is known about CCP-positive (CCP+) individuals without classifiable RA comes from blood bank studies (5-7), studies of unaffected family members of patients with RA (8-15), and cohort studies of patients recruited from European arthralgia clinics (16-19).
A prospective cohort study (16) of undifferentiated arthritis (UA) patients showed that CCP+ was a significant risk factor for RA compared to CCP negativity. Similarly, another prospective cohort study of seropositive arthralgia patients (17) found that CCP+ predicted arthritis development compared to being CCP negative (CCP-) and that arthritis risk increased with high level CCP. While these European cohort studies have been instrumental in enhancing knowledge of how RA develops, the findings may not be generalizable to the US (where early arthritis or arthralgia clinics are uncommon) and typically were performed only among patients with UA or arthralgias who agreed to participate in research. Further, prior studies compared presence of CCP to absence of CCP so less is known about the effect of CCP level among a population who are all CCP+.
Therefore, we aimed to investigate risk for progression to RA in a clinical population in the US of CCP+ individuals without classifiable RA at time of initial CCP positivity. We first aimed to quantify the absolute risk of progression to RA among these patients. We then aimed to identify predictors at the time of initial CCP+ for subsequent progression to RA. We hypothesized that increasing CCP levels and presence of other arthritis-related traits would increase risk for progression to RA.
SUBJECTS AND METHODS
Study design and population
We performed a retrospective cohort study among outpatients or inpatients seen at Partners HealthCare, a tertiary health care system in Boston, Massachusetts. In August 2016, we queried the Partners Research Patient Data Registry, a research repository of all patients seen at Partners hospitals since 1990, to identify all individuals who tested positive for CCP (greater than upper limit of normal [ULN] of the laboratory assay) between 2009-2016. All aspects of this study were approved by the Partners HealthCare Institutional Review Board.
To be included in the study, CCP+ individuals ≥18 years of age had to be free of RA or other systemic rheumatic disease (SRD) at the index date, defined as the date of first positive CCP in the medical record. RA status at index date according to 2010 ACR/EULAR criteria was determined by medical record review (20). We excluded patients with other SRDs at the index date: systemic lupus erythematosus, scleroderma, spondyloarthritis (including ankylosing spondylitis, reactive arthritis, psoriatic arthritis), antiphospholipid syndrome, mixed connective tissue disease, Sjögren’s syndrome, systemic vasculitis, polymyalgia rheumatica, dermatomyositis, polymyositis, and juvenile idiopathic arthritis. We performed an initial brief medical record screen to filter out these conditions and then performed a more detailed review to confirm eligibility. Conditions at index date permitted in the study were: gout, pseudogout, osteoarthritis, inflammatory bowel disease, psoriasis, fibromyalgia, and palindromic rheumatism. We required sufficient detail in the medical record at index date related to possible RA and at least one follow-up visit to be included in the study. If a diagnosis of RA was made within 28 days of index date, the individual was considered as having prevalent RA at the index date and was excluded. All individuals included in the study were reviewed independently by two rheumatologists who agreed all analyzed patients were without RA or SRD at index date. Selection of the final analyzed study sample is depicted in the flow diagram (Figure 1).
Figure 1.
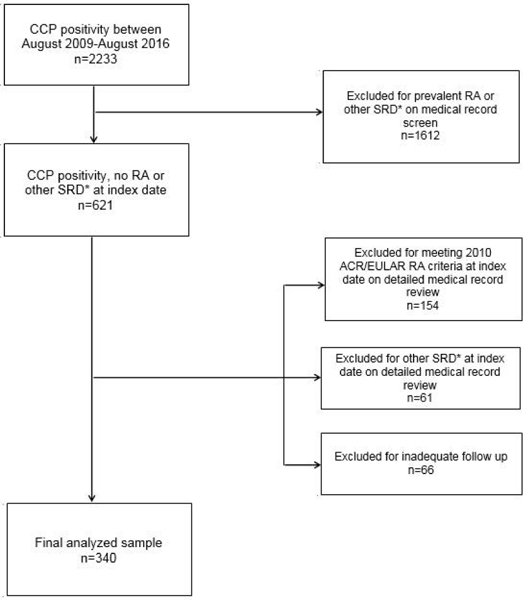
Flow diagram of study sample. CCP= cyclic citrullinated peptide antibody; RA= rheumatoid arthritis; SRD= systemic rheumatic disease; ACR/EULAR= American College of Rheumatology and European League Against Rheumatism. *Other SRD= systemic lupus erythematosus, scleroderma, dermatomyositis, polymyositis, seronegative spondyloarthropathies (including ankylosing spondylitis, reactive arthritis, psoriatic arthritis), antiphospholipid syndrome, mixed connective tissue disease, Sjögren’s syndrome, systemic vasculitis (including Takayasu arteritis, giant cell arteritis, polyarteritis nodosa, Behçet’s disease, Henoch-Schönlein purpura, ANCA-associated vasculitis), polymyalgia rheumatica, dermatomyositis, and juvenile idiopathic arthritis.
Primary exposure: CCP level
The primary exposure was CCP level at index date, measured as fold increased above the ULN of assay used. As the study population drew from different hospitals whose laboratories used different CCP assays over time, the ULN of assays varied by site and year. Therefore, we standardized all CCP results by dividing by the ULN of the assay used to obtain the fold-above ULN (x ULN). We stratified CCP level as low (>1 to 2x ULN), medium (>2 to 3x ULN), and high (>3x ULN) as clinically relevant cut points based on prior literature (21).
Outcome: RA diagnosis
The primary outcome was incident diagnosis of RA meeting 2010 ACR/EULAR criteria occurring after the index date, as determined by medical record review. All cases of incident RA and date of RA diagnosis were adjudicated by two rheumatologists.
Diagnosis and date of diagnosis of other SRDs were identified and reviewed independently by both rheumatologists. Date of death was recorded. We also recorded the date of last clinical follow-up, defined as the last clinical note from any physician (regardless of specialty) with a problem and medication list. Medical records were reviewed for all clinical follow-up as of February 2018.
Covariates
We identified possible confounders based on their associations with CCP and RA in prior literature (9,22-26). Data were collected as of the index date by medical record review. Body mass index (BMI) was calculated based on measured height and weight through clinical care within six months of the index date and further categorized as <25, 25-29.9, or ≥30 kg/m2. Race was dichotomized as white or non-white and education level as high school or less vs. some college or higher. Smoking status by medical record review was categorized as current smoker within one year of the index date vs. not current smoker (including never smoker, former smoker, or unknown smoking status). Family history (first and/or second-degree relatives) was categorized as present vs. absent family history of RA (which included unknown family history). We recorded rheumatoid factor (RF) and antinuclear antibody (ANA) levels that were measured clinically within 1 year of index date. Erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) were recorded if they were measured clinically within 3 months of index date. Comorbidities (hypertension, thyroid disease, interstitial lung disease, and osteoarthritis) were ascertained from the medical record.
Secondary exposures
We collected data on factors important in care delivery in these patients or early symptoms or signs of RA. We collected the reason for ordering initial CCP test as described by the ordering clinician (categorized as arthralgia, lung disease, abnormal laboratory result, axial pain, fatigue, fever, other clinical conditions). We noted the specialty of ordering physician (rheumatologist vs. non-rheumatologist), duration of joint symptoms (when available), and diagnosis of palindromic rheumatism (defined as diagnosis per treating physician of any specialty) prior to index date. Symptoms (pain, stiffness, or both) and signs (swelling, tenderness, or both) in RA-specific and non-RA-specific joints at index date were also collected through medical record review. RA-specific joints were defined as metacarpophalangeal joints, proximal interphalangeal joints, metatarsophalangeal joints, thumb interphalangeal joints, wrists, and elbows, as previously defined (27). Non-RA-specific joints were defined as shoulders, knees, and ankles.
We performed additional medical record review in the subset of patients whose reason for testing CCP was for lung disease. The nature of lung disease at index date (interstitial lung abnormalities, obstructive disease, nodules/lesions, or other) was determined by medical record review and agreed upon by two rheumatologists.
Statistical analysis
We reported baseline characteristics among the entire study sample and stratified by subsequent progression or non-progression to RA using descriptive statistics: mean and standard deviation for normal continuous variables, median and interquartile range (IQR) for non-normal continuous variables, and frequency and proportion for categorical variables. We tested for statistical differences between RA progressors and non-progressors using univariate tests (t-test for normally distributed continuous variables, Wilcoxon rank sum test for non-normally distributed continuous variables, chi-square test for categorical variables, and Fisher’s exact test for categorical variables with low cell size). We reported these baseline characteristics in the subset of patients whose reason for ordering CCP was for lung disease.
We reported the reason for ordering initial CCP in all patients and stratified by subsequent progression or non-progression to RA. We tested for statistical differences between RA progressors and non-progressors using Fisher’s exact test.
We created Kaplan-Meier curves to visualize RA-free survival after index date according to CCP level; CCP level and RF status; CCP level and family history of RA; and CCP level and presence of symptoms in RA-specific joints. We used log-rank tests to test for statistical differences between Kaplan-Meier curves.
We calculated the absolute risk of progressing to RA at fixed time intervals of 1 year, 3 years, and 5 years, due to their use in prior literature (28). Absolute risk of progression to RA was calculated in all patients, and stratified by CCP level (low, medium, high), RF (negative or not sent, low/medium positive, high positive), family history of RA, and presence of symptoms in RA-specific joints. We also calculated absolute risk of RA by combinations of dichotomized CCP level (low/medium vs. high) and RF, family history, or symptom status. We obtained estimates for RA risk and 95%CI bounds at each time point using the Kaplan-Meier curves.
We used Cox proportional hazards models to investigate the risk for progression to RA by CCP level. Person-time accrued from the start of index date. Censoring variables were as of the following dates, whichever came first: incident RA diagnosis (the primary outcome), diagnosis of other SRD, death, last documented follow-up note in the electronic medical record (end of follow-up). Therefore, we determined whether or not a patient had RA at all person-time that was analyzed. Initial models for CCP levels, potential confounders, and the secondary exposures were unadjusted to obtain HRs and 95%CIs. We used Cox regression to estimate the effect of CCP level on RA risk independent of potential confounders of age, sex, BMI, smoking, family history, and RF level, chosen based on prior literature (9,22-26). We did not include the following variables for the multivariable model because we considered them to be related to early symptoms and signs of RA, and not true confounders: specialty of ordering physician, testing for indication of arthralgia, palindromic rheumatism, and symptoms/signs/swelling involving RA-specific joints.
We tested for the proportional hazards assumption by including an interaction term between time after index date and CCP level for RA risk and verifying that there was no statistically significant interaction. The proportional hazards assumption was met in all analyses. Analyses were performed using SASv9.4. We set the threshold for statistical significance as a two-sided p value of <0.05.
RESULTS
Baseline characteristics
Among a total of 340 CCP+ patients who were without prevalent RA or SRD at baseline, we identified 73 (21.5%) incident RA cases during 1047 person-years of follow-up (incidence rate 69.7 per 1000 person-years), with median follow-up of 2.7 years/patient (IQR 1.1-4.6). Eleven patients (2.9%) developed SRD other than RA during follow-up (consisting of systemic lupus erythematosus, ANCA-associated vasculitis, polymyalgia rheumatica, and spondyloarthritis). Twenty patients (5.9%) died during follow-up.
Baseline characteristics of the study sample, overall and stratified by progressors to RA vs. non-progressors, are presented in Table 1. The study sample overall was 65.6% female and 74.7% white with mean age of 55.0 years (SD 15.3).
Table 1.
Characteristics at index date (initial positive CCP) in the entire study sample and stratified by those who later progressed or did not progress to rheumatoid arthritis (n=340).
| All patients (n=340) |
Progressed to RA (n=73) |
Non-progressors (n=267) |
p value | |
|---|---|---|---|---|
| Demographics | ||||
| Mean age, years (SD) | 55.0 (15.3) | 53.0 (14.2) | 55.6 (15.6) | 0.19 |
| Female, n (%) | 223 (65.6) | 56 (76.7) | 167 (62.5) | 0.02 |
| White, n (%) | 254 (74.7) | 49 (67.1) | 205 (76.8) | 0.09 |
| Some college education or higher | 148 (43.5) | 25 (34.2) | 123 (46.1) | 0.07 |
| Laboratory Data | ||||
| RF level by category, n (%) | ||||
| Not sent | 14 (4.1) | 0 (0.0) | 14 (5.2) | |
| Negative | 242 (71.2) | 39 (53.4) | 203 (76.0) | <0.0001 |
| >1 to 3x ULN (low/medium positive) | 38 (11.2) | 13 (17.8) | 25 (9.4) | |
| >3x ULN (high positive) | 46 (13.5) | 21 (28.8) | 25 (9.4) | |
| Mean CCP level, x ULN (SD) | 4.2 (4.7) | 7.5 (6.9) | 3.3 (3.5) | <0.0001 |
| CCP level by category, n (%) | ||||
| >1 to 2x ULN (low positive) | 167 (49.1) | 14 (19.2) | 153 (57.3) | |
| >2 to 3x ULN (medium positive) | 52 (15.3) | 10 (13.7) | 42 (15.7) | <0.0001 |
| >3x ULN (high positive) | 121 (35.6) | 49 (67.1) | 72 (27.0) | |
| ANA titer, n (%) | ||||
| Not sent | 39 (11.5) | 6 (8.2) | 33 (12.4) | |
| Negative | 104 (30.6) | 18 (24.7) | 86 (32.2) | 0.31 |
| 1:40- 1:160 | 147 (43.2) | 35 (47.9) | 112 (41.9) | |
| >1:160 | 50 (14.7) | 14 (19.2) | 36 (13.5) | |
| Mean ESR in mm/h (SD), n=269† | 22.5 (22.7) | 23.4 (20.6) | 22.2 (23.3) | 0.36 |
| Mean CRP in mg/L (SD), n=231† | 14.2 (38.3) | 16.5 (33.4) | 13.6 (39.6) | 0.03 |
| Lifestyle and Family History, n (%) | ||||
| Current smoker | 40 (11.8) | 14 (19.2) | 26 (9.7) | 0.03 |
| Body mass index category, n (%) | ||||
| <25 kg/m2 | 136 (40.0) | 30 (41.1) | 106 (39.7) | 0.97 |
| 25 to <30 kg/m2 | 101 (29.7) | 21 (28.8) | 80 (30.0) | |
| ≥30 kg/m2 | 103 (30.3) | 22 (30.1) | 81 (30.3) | |
| Positive family history of RA | 50 (14.7) | 18 (24.7) | 32 (12.0) | 0.01 |
| Comorbidities, n (%) | ||||
| Hypertension | 129 (37.9) | 21 (28.8) | 108 (40.4) | 0.07 |
| Osteoarthritis of any joint | 91 (26.8) | 16 (21.9) | 75 (28.1) | 0.29 |
| Hypothyroidism or hyperthyroidism | 40 (11.8) | 10 (13.7) | 30 (11.2) | 0.56 |
| Interstitial lung disease | 28 (8.3) | 0 (0.0) | 28 (10.5) | 0.004 |
| Clinical Presentation | ||||
| CCP ordered by rheumatologist, n (%) | 159 (46.8) | 44 (60.3) | 115 (43.1) | 0.01 |
| Palindromic rheumatism, n (%) | 32 (9.4) | 18 (24.7) | 14 (5.2) | <0.0001 |
| Mean symptom duration, weeks (SD), n=254† | 110.7 (216.6) | 115.9 (277.7) | 108.8 (191.4) | 0.69 |
| Symptoms* in RA-specific** joint, n (%) | 200 (58.8) | 54 (74.0) | 146 (54.7) | 0.003 |
| Signs*** in RA-specific* joint, n (%) | 81 (23.8) | 26 (35.6) | 55 (20.6) | 0.008 |
| Swelling in RA-specific* joint, n (%) | 46 (13.5) | 13 (17.8) | 33 (12.4) | 0.23 |
n patients with available data.
Symptoms= pain, stiffness, or both.
RA-specific joint is defined in this study as metacarpophalangeal joints, proximal interphalangeal joints, metatarsophalangeal joints, thumb interphalangeal joints, wrists, and elbows.
Signs= tenderness, swelling, or both.
CCP = cyclic citrullinated peptide antibody; CRP = C-reactive protein; ESR = erythrocyte sedimentation rate; RA = rheumatoid arthritis; RF = rheumatoid factor; SD = standard deviation; ULN = upper limit of normal.
Reason for ordering initial CCP test
The reasons for ordering initial CCP testing are presented in Table 2. Arthralgia (75.9%) was the most common reason, followed by lung disease (10.0%). For patients whose reason for checking CCP was an abnormal laboratory value, axial joint pain, fatigue, or fever, none progressed to RA.
Table 2.
Clinical reason for ordering initial CCP test (n=340).
| Reason | All patients, n(%) |
Progressed to RA, n(%) |
Non-progressors, n(%) |
p value |
|---|---|---|---|---|
| Arthralgia | 258 (75.9) | 70 (95.9) | 188 (70.4) | <0.0001 |
| Lung disease | 34 (10.0) | 1 (1.4) | 33 (12.4) | <0.0001 |
| Abnormal lab* | 16 (4.7) | 0 (0.0) | 16 (6.0) | <0.0001 |
| Axial pain (neck, back, or hip) | 4 (1.2) | 0 (0.0) | 4 (1.5) | 0.06 |
| Fatigue | 3 (0.9) | 0 (0.0) | 3 (1.1) | 0.13 |
| Fever | 2 (0.6) | 0 (0.0) | 2 (0.8) | 0.25 |
| Miscellaneous symptoms or other clinical conditions** | 20 (5.9) | 2 (2.7) | 18 (6.7) | 0.0002 |
Includes one or more of following: anti-nuclear antibody, erythrocyte sedimentation rate, C-reactive protein, rheumatoid factor, creatine phosphokinase, platelets, hepatitis C positivity, cryoglobulinemia
Includes rash, sicca symptoms, myalgia, paresthesia/neuropathy, scleritis, Raynaud’s, cirrhosis, pericarditis
CCP= cyclic citrullinated peptide antibody; RA= rheumatoid arthritis.
Characteristics of the subgroup of patients who had CCP checked for lung disease evaluation (n=34) are presented in Supplemental Table 1. Median duration of follow-up was 25.0 months (IQR 10.8-48.0), and seven patients (20.6%) died during follow-up. Only 38.2% were female, mean age was 66.9 years (SD 12.8), and most were white (76.5%). Current smokers comprised 8.8% of this subgroup, and 58.8% were former smokers. The proportion of patients with low level (47.1%), medium level (20.6%), or high level (32.4%) CCP was similar to the overall study sample. The underlying lung diseases were: interstitial lung abnormalities (70.6%), pleural effusion (8.8%), nodules/masses (5.9%), pulmonary hypertension (5.9%), chronic obstructive pulmonary disease (2.9%), bronchiectasis (2.9%), and hemoptysis (2.9%). Only 1 of these 34 (2.9%) patients progressed to RA during follow-up, and 1 patient developed another SRD (spondyloarthritis).
Absolute risk of progression to RA
The absolute risk of progression to RA at 1, 3, and 5 years is presented in Table 3 and corresponding Kaplan-Meier curves illustrating RA-free survival are in Figure 2. For all patients, 5-year risk was 25.3% (95%CI 19.6-30.6). Absolute risk of progression to RA increased with increasing CCP level: for example, among high level CCP, 1-year risk was 24.9% (95%CI 16.4-32.6), 3-year risk was 41.5% (95%CI 30.9-50.4), and 5-year risk was 46.0% (95%CI 34.7-55.3). Absolute risk of progression to RA also increased with increased RF level, having family history of RA, and presence of symptoms in RA-specific joints.
Table 3.
Absolute risk of progression to rheumatoid arthritis among patients with CCP positivity but no RA by specified follow-up.
| 1-year RA risk, % (95% CI) |
3-year RA risk, % (95% CI) |
5-year RA risk, % (95% CI) |
|
|---|---|---|---|
| All patients | 12.8 (9.1, 16.4) | 21.0 (16.0, 25.6) | 25.3 (19.6, 30.6) |
| CCP >1 to 2x ULN (low) | 5.6 (2.0, 9.2) | 7.3 (3.0, 11.5) | 10.2 (4.3, 15.7) |
| CCP >2 to 3x ULN (medium) | 8.2 (0.1, 15.6) | 18.0 (4.2, 29.8) | 27.4 (8.6, 42.3) |
| CCP >3x ULN (high) | 24.9 (16.4, 32.6) | 41.5 (30.9, 50.4) | 46.0 (34.7, 55.3) |
| RF negative or not sent | 9.1 (5.4, 12.6) | 14.9 (10.0, 19.6) | 18.3 (12.4, 23.7) |
| RF >1 to 3x ULN (low/medium) | 16.2 (3.4, 27.3) | 32.3 (14.2, 46.6) | 37.9 (16.9, 53.7) |
| RF >3x ULN (high) | 32.1 (15.8, 45.2) | 44.1 (25.5, 58.1) | 51.8 (31.2, 66.2) |
| No family history of RA | 11.0 (7.2, 14.6) | 17.9 (12.9, 22.6) | 21.3 (15.6, 26.6) |
| Family history of RA | 24.2 (10.5, 35.9) | 40.1 (21.6, 54.2) | 53.6 (25.8, 71.0) |
| No symptoms in RA-specific joint | 8.0 (3.1, 12.6) | 13.5 (6.7, 19.8) | 16.8 (8.7, 24.1) |
| Symptoms in RA-specific joint* | 16.1 (10.8, 21.2) | 25.9 (19.0, 32.1) | 30.6 (22.9, 37.6) |
| CCP low or medium level and RF negative | 6.5 (2.8, 9.9) | 9.4 (4.8, 13.8) | 12.8 (6.9, 18.3) |
| CCP low or medium level and RF positive | 5.0 (0.0, 14.1) | 12.3 (0.0, 27.2) | 22.1 (0.0, 42.1) |
| CCP high level and RF negative | 17.5 (7.0, 26.9) | 33.0 (18.4, 45.0) | 36.1 (20.6, 48.6) |
| CCP high level and RF positive | 31.4 (18.2, 42.5) | 47.7 (32.3, 59.7) | 53.3 (36.7, 65.6) |
| CCP low or medium level and negative family history of RA | 5.9 (2.5, 9.2) | 8.8 (4.4, 13.1) | 12.0 (6.3, 17.4) |
| CCP low or medium level and positive family history of RA | 10.5 (0.0, 23.4) | 18.0 (0.0, 34.9) | 38.5 (0.0, 66.6) |
| CCP high level and negative family history of RA | 21.6 (12.5, 29.8) | 36.6 (25.0, 46.5) | 40.3 (28.0, 50.5) |
| CCP high level and positive family history of RA | 34.4 (13.1, 50.4) | 55.5 (27.9, 72.5) | 61.8 (32.5, 78.4) |
| CCP low or medium level and no symptoms in RA-specific joint | 2.3 (0.0, 5.3) | 7.2 (0.7, 13.2) | 11.8 (2.8, 20.0) |
| CCP low or medium level and symptoms in RA-specific joint | 9.6 (4.0, 14.8) | 11.9 (5.5, 17.8) | 15.5 (7.5, 22.8) |
| CCP high level and no symptoms in RA-specific joint | 22.3 (7.3, 34.8) | 29.1 (11.9, 43.0) | 29.1 (11.9, 43.0) |
| CCP high level and symptoms in RA-specific joint | 25.6 (15.2, 34.7) | 45.9 (32.7, 56.6) | 52.0 (37.9, 62.9) |
CCP = cyclic citrullinated peptide antibody; RA = rheumatoid arthritis; RF = rheumatoid factor; ULN = upper limit of normal.
RA-specific joints were considered metacarpophalangeal joints, proximal interphalangeal joints, metatarsophalangeal joints, thumb interphalangeal joints, wrists, or elbows.
Figure 2.
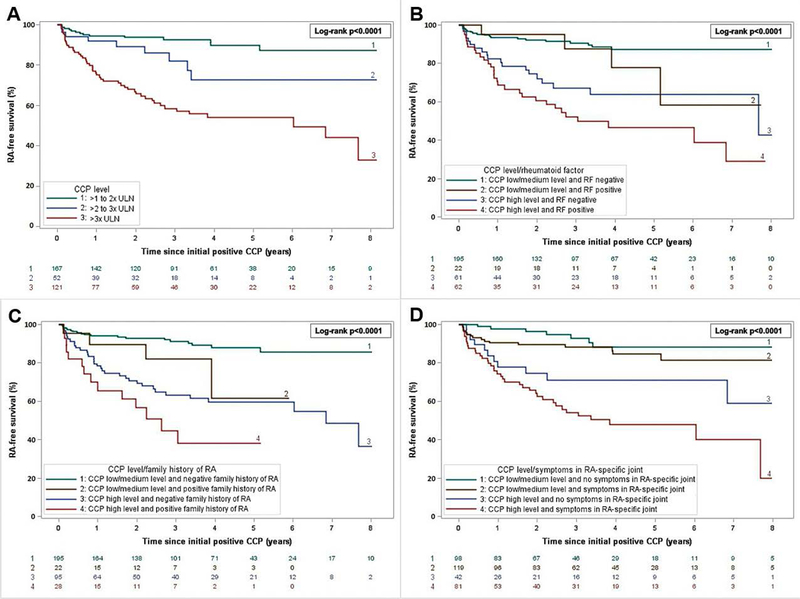
Kaplan-Meier curves for rheumatoid arthritis-free survival after index date of initial CCP positivity according to: A) CCP (cyclic citrullinated peptide) in 3 levels. B) CCP binary level and rheumatoid factor (RF) status. C) CCP binary level and presence/absence of family history of RA. D) CCP binary level and presence/absence of symptoms (pain, stiffness, or both) in RA-specific joints. RA-specific joints were defined as metacarpophalangeal joints, proximal interphalangeal joints, metatarsophalangeal joints, thumb interphalangeal joints, wrists, and elbows.
Risk of progression to RA according to CCP level in combination with RF status (positive or negative), family history of RA (positive or negative), or symptoms in RA-specific joints (present or absent) are presented in Table 3 as well as Figure 2. For example, with respect to CCP level and family history of RA, patients with high level CCP and positive family history were at highest risk (5-year risk 61.8%, 95%CI 32.5-78.4).
Cox regression models
Unadjusted and multivariable HRs for RA are presented in Table 4. Compared to low level CCP, medium level CCP (HR 2.75, 95%CI 1.22-6.19) and high level CCP (HR 6.18, 95%CI 3.40-11.2) were associated with increased RA risk in the unadjusted analysis. Increased CCP level remained associated with progression to RA when adjusted for age, sex, BMI, smoking, and family history (medium CCP: HR 2.94 [95%CI 1.30-6.69]; high CCP: HR 5.86 [95%CI 3.20-10.7], reference: low CCP). When also adjusted for RF level, CCP level remained predictive of RA (medium level CCP: HR 3.00 [95%CI 1.32-6.81]; high level CCP: HR 4.83 [95%CI 2.51-9.31]) compared to low CCP.
Table 4.
Unadjusted and multivariable hazard ratios for progression to rheumatoid arthritis among patients with CCP positivity (n=340).
| Cases/person -years |
Incidence rate per 1000 person-years |
Unadjusted HR (95% CI) |
Model 1*: Multivariable HR (95% CI) |
Model 2**: Multivariable HR (95% CI) |
|
|---|---|---|---|---|---|
| CCP level category | |||||
| >1 to 2x ULN (low) | 14/589 | 23.8 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| >2 to 3x ULN (medium) | 10/143 | 69.9 | 2.75 (1.22, 6.19) | 2.94 (1.30, 6.69) | 3.00 (1.32, 6.81) |
| >3x ULN (high) | 49/315 | 155.6 | 6.18 (3.40, 11.2) | 5.86 (3.20, 10.7) | 4.83 (2.51, 9.31) |
| Age (continuous, per year) | N/A | 0.99 (0.98, 1.01) | 1.00 (0.98, 1.01) | 1.00 (0.98, 1.01) | |
| Male | 17/359 | 47.4 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Female | 56/687 | 81.5 | 1.75 (1.01, 3.01) | 1.79 (1.01, 3.16) | 1.84 (1.04, 3.25) |
| Body mass index category | |||||
| <25 kg/m2 | 30/401 | 74.8 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| 25 to <30 kg/m2 | 21/333 | 63.1 | 0.89 (0.51, 1.55) | 1.15 (0.65, 2.06) | 1.00 (0.55, 1.83) |
| ≥30 kg/m2 | 22/312 | 70.5 | 0.96 (0.55, 1.66) | 0.91 (0.51, 1.62) | 0.86 (0.48, 1.53) |
| Not current smoker | 59/918 | 64.3 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Current smoker | 14/129 | 108.5 | 1.78 (0.99, 3.19) | 1.68 (0.92, 3.07) | 1.43 (0.76, 2.69) |
| No family history of RA | 55/946 | 58.1 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Family history of RA | 18/100 | 180.0 | 2.62 (1.53, 4.51) | 1.76 (1.00, 3.10) | 1.63 (0.92, 2.91) |
| RF level category Negative or not sent |
39/819 |
47.6 |
1.00 (Ref) |
- |
1.00 (Ref) |
| >1 to 3x ULN (low/medium) | 13/121 | 107.4 | 2.26 (1.20, 4.23) | 1.20 (0.60, 2.39) | |
| >3x ULN (high) | 21/106 | 198.1 | 3.80 (2.22, 6.49) | 1.84 (0.95, 3.56) | |
Model 1= adjusted for age, sex, body mass index, smoking, family history of RA.
Model 2= adjusted for variables in Model 1 and rheumatoid factor level.
BMI= body mass index; CCP= cyclic citrullinated peptide antibody; RA= rheumatoid arthritis; RF= rheumatoid factor; ULN = upper limit of normal.
Unadjusted HRs for RA with respect to ordering specialty, whether CCP was sent for arthralgias, presence of symptoms in RA-specific joints, signs in RA-specific joints, swelling in RA-specific joints, and palindromic rheumatism are presented in Supplemental Table 2. Testing CCP for arthralgias was associated with increased risk for RA (HR 7.84, 95%CI 2.47-24.9), as was presence of signs (swelling or tenderness) in RA-specific joints (HR 1.91, 95%CI 1.18-3.09).
DISCUSSION
In a US-hospital based cohort of 340 CCP+ patients who were without RA or other SRD at time of initial CCP positivity, 21.5% went on to develop RA by 2010 ACR/EULAR criteria. The strongest predictor of RA risk was CCP level, with high level CCP conferring a five-fold increased hazard for RA independent of age, sex, BMI, smoking, family history, and RF level. Our results suggest that about 46% of patients with high level CCP develop RA within five years.
Studies involving blood banks or asymptomatic first-degree relatives of patients with RA have demonstrated that CCP is significantly associated with increased risk of progression to RA. In a Swedish blood bank case-control study comparing 83 RA cases to age- and sex-matched controls, Rantapää et al (2) found that at any time prior to RA diagnosis, CCP was positive prior to diagnosis in 34% of RA subjects and was predictive of RA development (HR 16.1, 95%CI 3.3-76.7) compared to CCP negativity. Ramos-Remus et al (8) tested 819 healthy relatives of RA patients for CCP and RF and followed them longitudinally for 5 years with 2% developing RA during follow-up; CCP+ relatives had HR of 223.1 (95%CI 63.8, 779.9) for RA compared to CCP- relatives. As these studies investigated RA risk for seropositivity compared to seronegativity in a largely asymptomatic population, these findings may not be applicable to a clinical population who all have seropositivity and a clinical indication for CCP testing.
Cohort studies of patients recruited from arthralgia referral clinics in Europe have also advanced the understanding of the impact of CCP on RA risk. A prospective cohort study by van Gaalen and colleagues (16) of UA patients recruited to an early arthritis clinic in the Netherlands showed that 93% of CCP+ UA patients developed classifiable RA by 1987 ACR criteria within 3 years of follow-up (29). Being CCP+ was a significant risk factor for RA with an odds ratio of 37.8 (95% confidence interval [CI] 13.8-111.9) compared to being CCP-. However, this study was performed prior to development of 2010 ACR/EULAR classification criteria (20) which allowed for earlier RA detection, so it is possible that some of those with UA and CCP+ would have RA at baseline under the new criteria. We did not limit our study to patients with articular signs or symptoms, which may explain why the absolute RA risk was higher in that cohort compared to our study. In a prospective cohort study of 147 seropositive patients with arthralgias recruited in the Netherlands (17), CCP+ was associated with arthritis development (HR 6.0, 95%CI 1.8-19.8) compared to CCP-. Among CCP+ patients, RA risk increased with higher CCP level. Rakieh and colleagues (19) prospectively followed 100 CCP+ arthralgia patients in the United Kingdom for median 20 months and 43% developed RA per 2010 ACR/EULAR criteria. The majority (>80%) of that cohort had high level CCP and all had articular symptoms which may explain this higher RA incidence compared to our study. They analyzed a combined variable of “high level RF and/or CCP” (defined as >3x ULN for either assay) in multivariable analyses, and there was a HR of 4.52 (95%CI 1.07-19.15) for inflammatory arthritis compared to lower levels of these autoantibodies, similar to our findings. Overall, our study adds to the literature by investigating a CCP+ population tested through routine clinical care in the US in the current era of RA diagnosis. We were able to directly compare high to low level CCP+ and also had detailed granular data available related to reason for ordering the test and clinical characteristics at time of CCP positivity for predicting subsequent progression to RA.
Our study highlights the importance of CCP level on risk for progression to RA independent of other known risk factors. Because patients in our study had CCP ordered as part of routine clinical care, our findings may be helpful to clinicians confronted with interpretation of a positive CCP test. Knowledge of the absolute risk of progressing to RA among a CCP+ patient without RA based on their CCP level and other clinical factors could encourage lifestyle changes and affect subsequent screening. For example, at-risk patients could be encouraged (30,31) to quit smoking (24,25,27), increase fish intake (32-34), lose weight (24,35,36), and improve dental hygiene (37). Furthermore, there is increasing interest in primary prevention of RA in at-risk patient populations (38,39).
We found that only 1 of the 34 patients who had CCP tested for lung disease went on to develop RA. This group had high mortality (20.6% vs. 5.9% in overall study sample) so had shorter duration of follow-up (median 25.0 months vs. 32.4 months). Citrullination of protein can occur in the lung and is influenced by smoking which suggests that autoimmunity may begin in respiratory mucosa prior to articular involvement (40,41). Whether CCP+ in patients with ILD represents a “pre-RA” state, or CCP positivity is unrelated to future articular manifestations requires further study. Few patients in our study who had CCP tested for a clinical indication other than arthralgia went on to develop RA during follow-up. While CCP has high specificity for RA (42), we demonstrated that the clinical context in which CCP is tested clearly affects risk for progression to RA.
Our study has several limitations inherent to a retrospective cohort design. While we collected detailed data on clinical and demographic characteristics, there remains the possibility of unmeasured confounding. We had to rely on clinical notes which may not have included completely accurate details particularly for articular symptoms and signs. We were unable to perform analyses based on hand or foot radiographs since these were only performed in a minority of patients. Further, we had to rely on the treating clinician’s impression and choice not to diagnose and treat for RA at the index date. We mitigated this possibility by excluding patients who quickly progressed to RA since they were likely to have had prevalent RA at baseline. Diagnostic uncertainty is inherent to a complex disease such as RA so our study may aide clinicians faced with patients who have symptoms and CCP+ but without clear RA. As our medical record is exclusive to our health care system, we may not have captured whether RA was diagnosed outside of our system. However, we assessed all notes and problem lists for RA or RA-related medications and censored at the last RA-free note from a clinician regardless of specialty to maximize the likelihood that patients were free of RA or other SRDs during all analyzed person-time. While all patients in our study were CCP+, not all patients were tested using the same CCP assay. Therefore, we standardized CCP level based on the fold-above ULN. However, it is possible that the type of CCP assay used may affect diagnostic performance (43). Therefore, it is possible that some patients with low CCP+ were false positives or may have been negative on other CCP assays. In addition, our small sample size had limited power to detect associations of elevated body mass index and smoking for progression to RA. Finally, our study sample was drawn from a single tertiary care system in Boston, so it may not be generalizable to community care settings or other geographic regions. We did not collect detailed geographic information on included subjects, however it is likely that most resided in the greater Boston area. Further research is needed to extend these observations to other settings.
Furthermore, while we feel that studying patients where CCP was tested for clinical reasons is a strength of the study, we would emphasize that the predictive value of CCP level demonstrated in our study may not apply to other CCP-positive populations outside of a clinical setting.
A major strength of our study is the clinical setting in the US with detailed data on indication for testing CCP as well as demographics, lifestyle, symptoms, signs, comorbidities, laboratory results, and family history. Thus, we were able to quantify risk for progression to RA for a variety of clinical scenarios pertinent for treating clinicians. We were able to estimate absolute risk based on CCP level and combinations of RA risk factors in this “real world” population that may be applied to other patients who present clinically in the US. Unlike other studies, we did not restrict our study to patients referred to a specialty clinic with articular signs or symptoms. Furthermore, for all outcomes in our study, two rheumatologists agreed on RA outcome, date of diagnosis, and absence of prevalent RA at index date, to ensure that we truly captured incident RA in our cohort. Finally, we applied the 2010 ACR/EULAR criteria to determine prevalent and incident RA, whereas some prior studies utilized 1987 ACR criteria and most of the follow-up in our study occurred after 2010. Thus, our study quantifies RA risk for a clinical US CCP+ population in the current era of RA.
In conclusion, we found that CCP level was predictive of progression to RA in a clinical US cohort of CCP+ patients without classifiable RA. These findings provide evidence for close monitoring for development of RA in this population. Further research is needed to identify pharmacologic and non-pharmacologic strategies to prevent progression to RA among these patients at very elevated risk for RA.
Supplementary Material
SIGNIFICANCE & INNOVATIONS.
We performed a retrospective cohort study of cyclic citrullinated peptide antibody (CCP) positive patients without systemic rheumatic disease including rheumatoid arthritis (RA) to investigate progression to RA in this population.
Overall risk of progression to RA in the study sample was 21.5% over median of 2.7 years of follow-up.
Patients with CCP levels of 3-fold or higher than normal had 5-fold increased RA risk compared to patients with low level of CCP positivity (between 1 and 2-fold higher than normal). About 46.0% of patients with high CCP levels progressed to RA within 5 years.
These results quantify the risk of RA associated with elevated CCP level and other clinical characteristics and provide rationale for close monitoring of CCP+ patients for progression to RA.
ACKNOWLEDGMENTS
The authors thank the staff of the Immunology Laboratory at the Brigham and Women’s Hospital for helpful assistance.
Funding/Support: Dr. Sparks was supported by the National Institute of Arthritis and Skin and Musculoskeletal Diseases (grant numbers K23 AR069688, L30 AR066953, P30 AR070253, and P30 AR072577). The funders had no role in study design, data collection, analysis, decision to publish, or preparation of the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or Harvard University or its affiliated academic health care centers.
Footnotes
Study conception and design. Ford, Sparks.
Acquisition of data. Ford, Marshall, Zaccardelli, Prado, Wiyarand, Schur, Sparks.
Analysis and interpretation of data. Ford, Liu, Marshall, Zaccardelli, Prado, Wiyarand, Lu, Karlson, Schur, Deane, Sparks.
REFERENCES
- 1.Deane KD. Can rheumatoid arthritis be prevented? Best Pract Res Clin Rheumatol 2013;27:467–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rantapää-Dahlqvist S, Jong BAW de, Berglin E Hallmans G, Wadell G, Stenlund H, et al. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum 2003;48:2741–2749. [DOI] [PubMed] [Google Scholar]
- 3.Nielen MMJ, van Schaardenburg D, Reesink HW, van de Stadt RJ, van der Horst-Bruinsma IE, Koning MHMT de, et al. Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurements in blood donors. Arthritis Rheum 2004;50:380–6. [DOI] [PubMed] [Google Scholar]
- 4.Kokkonen H, Mullazehi M, Berglin E, Hallmans G, Wadell G, Ronnelid J, et al. Antibodies of IgG, IgA and IgM isotypes against cyclic citrullinated peptide precede the development of rheumatoid arthritis. Arthritis Res Ther 2011;13:R13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chibnik LB, Mandl LA, Costenbader KH, Schur PH, Karlson EW. Comparison of threshold cutpoints and continuous measures of anti-cyclic citrullinated peptide antibodies in predicting future rheumatoid arthritis. J Rheumatol 2009;36:706–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arkema EV, Goldstein BL, Robinson W, Sokolove J, Wagner CA, Malspeis S, et al. Anti-citrullinated peptide autoantibodies, human leukocyte antigen shared epitope and risk of future rheumatoid arthritis: a nested case-control study. Arthritis Res Ther 2013;15:R159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sokolove J, Bromberg R, Deane KD, Lahey LJ, Derber LA, Chandra PE, et al. Autoantibody epitope spreading in the pre-clinical phase predicts progression to rheumatoid arthritis. Matloubian M, ed. PLoS One 2012;7:e35296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramos-Remus C, Castillo-Ortiz JD, Aguilar-Lozano L, Padilla-Ibarra J, Sandoval-Castro C, Vargas-Serafin CO, et al. Autoantibodies in prediction of the development of rheumatoid arthritis among healthy relatives of patients with the disease. Arthritis Rheumatol 2015;67:2837–2844. [DOI] [PubMed] [Google Scholar]
- 9.Sparks JA, Chen C-Y, Hiraki LT, Malspeis S, Costenbader KH, Karlson EW. Contributions of familial rheumatoid arthritis or lupus and environmental factors to risk of rheumatoid arthritis in woman: a prospective cohort study. Arthritis Care Res (Hoboken) 2014;66:1438–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Young KA, Deane KD, Derber LA, Hughes-Austin JM, Wagner CA, Sokolove J, et al. Relatives without rheumatoid arthritis show reactivity to anti-citrullinated protein/peptide antibodies that are associated with arthritis-related traits: studies of the etiology of rheumatoid arthritis. Arthritis Rheum 2013;65:1995–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Demoruelle MK, Parish MC, Derber LA, Kolfenbach JR, Hughes-Austin JM, Weisman MH, et al. Performance of anti-cyclic citrullinated peptide assays differs in subjects at increased risk of rheumatoid arthritis and subjects with established disease. Arthritis Rheum 2013;65:2243–2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hughes-Austin JM, Deane KD, Derber LA, Kolfenbach JR, Zerbe GO, Sokolove J, et al. Multiple cytokines and chemokines are associated with rheumatoid arthritis-related autoimmunity in first-degree relatives without rheumatoid arthritis: Studies of the Aetiology of Rheumatoid Arthritis (SERA). Ann Rheum Dis 2013;72:901–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koppejan H, Trouw LA, Sokolove J, Lahey LJ, Huizinga TJW, Smolik IA, et al. Role of anti-carbamylated protein antibodies compared to anti-citrullinated protein antibodies in indigenous North Americans with rheumatoid arthritis, their first-degree relatives, and healthy controls. Arthritis Rheumatol (Hoboken, NJ) 2016;68:2090–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frisell T, Saevarsdottir S, Askling J. Family history of rheumatoid arthritis: an old concept with new developments. Nat Rev Rheumatol 2016;12:335–43. [DOI] [PubMed] [Google Scholar]
- 15.Jiang X, Frisell T, Askling J, Karlson EW, Klareskog L, Alfredsson L, et al. To what extent is the familial risk of rheumatoid arthritis explained by established rheumatoid arthritis risk factors? Arthritis Rheumatol (Hoboken, NJ) 2015;67:352–62. [DOI] [PubMed] [Google Scholar]
- 16.van Gaalen FA, Linn-Rasker SP, van Venrooij WJ, Jong BA de, Breedveld FC, Verweij CL, et al. Autoantibodies to cyclic citrullinated peptides predict progression to rheumatoid arthritis in patients with undifferentiated arthritis: A prospective cohort study. Arthritis Rheum 2004;50:709–715. [DOI] [PubMed] [Google Scholar]
- 17.Bos WH, Wolbink GJ, Boers M, Tijhuis GJ, Vries N de, van der Horst-Bruinsma IE, et al. Arthritis development in patients with arthralgia is strongly associated with anti-citrullinated protein antibody status: a prospective cohort study. Ann Rheum Dis 2010;69:490–4. [DOI] [PubMed] [Google Scholar]
- 18.Bizzaro N, Bartoloni E, Morozzi G, Manganelli S, Riccieri V, Sabatini P, et al. Anti-cyclic citrullinated peptide antibody titer predicts time to rheumatoid arthritis onset in patients with undifferentiated arthritis: results from a 2-year prospective study. Arthritis Res Ther 2013;15:R16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rakieh C, Nam JL, Hunt L, Hensor EMA, Das S, Bissell L-A, et al. Predicting the development of clinical arthritis in anti-CCP positive individuals with non-specific musculoskeletal symptoms: a prospective observational cohort study. Ann Rheum Dis 2015;74:1659–66. [DOI] [PubMed] [Google Scholar]
- 20.Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis 2010;69:1580–1588. [DOI] [PubMed] [Google Scholar]
- 21.Peoples C, Valiyil R, Davis RB, Shmerling RH. Clinical use of anti-cyclic citrullinated peptide antibody testing. J Clin Rheumatol 2013;19:351–352. [DOI] [PubMed] [Google Scholar]
- 22.Gabriel SE, Crowson CS, O’Fallon WM. The epidemiology of rheumatoid arthritis in Rochester, Minnesota, 1955-1985. Arthritis Rheum 1999;42:415–20. [DOI] [PubMed] [Google Scholar]
- 23.Crowson CS, Matteson EL, Myasoedova E, Michet CJ, Ernste FC, Warrington KJ, et al. The lifetime risk of adult-onset rheumatoid arthritis and other inflammatory autoimmune rheumatic diseases. Arthritis Rheum 2011;63:633–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hair MJH de, Landewé RBM, van de Sande MGH, van Schaardenburg D, van Baarsen LGM, Gerlag DM, et al. Smoking and overweight determine the likelihood of developing rheumatoid arthritis. Ann Rheum Dis 2013;72:1654–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karlson EW, Chang S-C, Cui J, Chibnik LB, Fraser PA, Vivo I De, et al. Gene-environment interaction between HLA-DRB1 shared epitope and heavy cigarette smoking in predicting incident rheumatoid arthritis. Ann Rheum Dis 2010;69:54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hemminki K, Li X, Sundquist J, Sundquist K. Familial associations of rheumatoid arthritis with autoimmune diseases and related conditions. Arthritis Rheum 2009;60:661–8. [DOI] [PubMed] [Google Scholar]
- 27.Sparks JA, Chang S-C, Deane KD, Gan RW, Kristen Demoruelle M, Feser ML, et al. Associations of smoking and age with inflammatory joint signs among unaffected first-degree relatives of rheumatoid arthritis patients: results from Studies of the Etiology of Rheumatoid Arthritis. Arthritis Rheumatol (Hoboken, NJ) 2016;68:1828–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van de Stadt LA, Witte BI, Bos WH, van Schaardenburg D. A prediction rule for the development of arthritis in seropositive arthralgia patients. Ann Rheum Dis 2013;72:1920–6. [DOI] [PubMed] [Google Scholar]
- 29.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315–24. [DOI] [PubMed] [Google Scholar]
- 30.Sparks JA, Iversen MD, Yu Z, Triedman NA, Prado MG, Miller Kroouze R, et al. Disclosure of personalized rheumatoid arthritis risk using genetics, biomarkers, and lifestyle factors to motivate health behavior improvements: a randomized controlled trial. Arthritis Care Res (Hoboken) 2018;70:823–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prado MG, Iversen MD, Yu Z, Miller Kroouze R, Triedman NA, Kalia SS, et al. Effectiveness of a web-based personalized rheumatoid arthritis risk tool with or without a health educator for knowledge of RA risk factors. Arthritis Care Res (Hoboken) 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shapiro JA, Koepsell TD, Voigt LF, Dugowson CE, Kestin M, Nelson JL. Diet and rheumatoid arthritis in women: a possible protective effect of fish consumption. Epidemiology 1996;7:256–63. [DOI] [PubMed] [Google Scholar]
- 33.Rosell M, Wesley A-M, Rydin K, Klareskog L, Alfredsson L, EIRA study group. Dietary fish and fish oil and the risk of rheumatoid arthritis. Epidemiology 2009;20:896–901. [DOI] [PubMed] [Google Scholar]
- 34.Gan RW, Demoruelle MK, Deane KD, Weisman MH, Buckner JH, Gregersen PK, et al. Omega-3 fatty acids are associated with a lower prevalence of autoantibodies in shared epitope-positive subjects at risk for rheumatoid arthritis. Ann Rheum Dis 2017;76:147–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu B, Hiraki LT, Sparks JA, Malspeis S, Chen C-Y, Awosogba JA, et al. Being overweight or obese and risk of developing rheumatoid arthritis among women: a prospective cohort study. Ann Rheum Dis 2014;73:1914–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qin B, Yang M, Fu H, Ma N, Wei T, Tang Q, et al. Body mass index and the risk of rheumatoid arthritis: a systematic review and dose-response meta-analysis. Arthritis Res Ther 2015;17:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Pablo P, Dietrich T, McAlindon TE. Association of periodontal disease and tooth loss with rheumatoid arthritis in the US population. J Rheumatol 2008;35:70–6. [PubMed] [Google Scholar]
- 38.Gonzalez-Lopez L, Gamez-Nava JI, Jhangri G, Russell AS, Suarez-Almazor ME. Decreased progression to rheumatoid arthritis or other connective tissue diseases in patients with palindromic rheumatism treated with antimalarials. J Rheumatol 2000;27:41–6. [PubMed] [Google Scholar]
- 39.Burgers LE, Allaart CF, Huizinga TWJ, Helm-van Mil AHM van der. Brief report: clinical trials aiming to prevent rheumatoid arthritis cannot detect prevention without adequate risk stratification: a trial of methotrexate versus placebo in undifferentiated arthritis as an example. Arthritis Rheumatol (Hoboken, NJ) 2017;69:926–931. [DOI] [PubMed] [Google Scholar]
- 40.Aubart F, Crestani B, Nicaise-Roland P, Tubach F, Bollet C, Dawidowicz K, et al. High levels of anti-cyclic citrullinated peptide autoantibodies are associated with co-occurrence of pulmonary diseases with rheumatoid arthritis. J Rheumatol 2011;38:979–82. [DOI] [PubMed] [Google Scholar]
- 41.Makrygiannakis D, Hermansson M, Ulfgren A-K, Nicholas AP, Zendman AJW, Eklund A, et al. Smoking increases peptidylarginine deiminase 2 enzyme expression in human lungs and increases citrullination in BAL cells. Ann Rheum Dis 2008;67:1488–92. [DOI] [PubMed] [Google Scholar]
- 42.Avouac J, Gossec L, Dougados M. Diagnostic and predictive value of anti-cyclic citrullinated protein antibodies in rheumatoid arthritis: a systematic literature review. Ann Rheum Dis 2005;65:845–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van Hoovels L, Jacobs J, Vander Cruyssen B, Van den Bremt S, Verschueren P, Bossuyt X. Performance characteristics of rheumatoid factor and anti-cyclic citrullinated peptide antibody assays may impact ACR/EULAR classification of rheumatoid arthritis. Ann Rheum Dis 2018;77:667–677. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


