Abstract
Objective
We aimed to evaluate the comparative risk of hospitalized infection among patients with rheumatoid arthritis (RA) who initiated abatacept versus a TNF inhibitor (TNFi).
Methods
We identified RA patients aged ≥18 years with ≥2 RA diagnoses who initiated abatacept or a TNFi using claims data from Truven MarketScan database (2006–2015). The primary outcome was a composite endpoint of any hospitalized infection. Secondary outcomes were bacterial infection, herpes zoster, and infections affecting different organ systems. We performed 1:1 propensity score (PS) matching between the groups to control for baseline confounders. We estimated incidence rates (IR) and hazard ratio (HR) with 95% confidence intervals (CI) for hospitalized infection.
Results
We identified 11,248 PS-matched pairs of abatacept and TNFi initiators with median age of 56 years, and 83% female sex. The IR per 1,000 person-years for any hospitalized infection was 37 among abatacept initiators and 47 in TNFi initiators. The HR for the risk of any hospitalized infection associated with abatacept versus TNFi was 0.78 (95% CI 0.64–0.95) and remained lower when compared to infliximab (HR 0.63, 95% CI 0.47–0.85), while no significant difference was seen compared with adalimumab and etanercept. The risk of secondary outcomes was lower for abatacept for pulmonary infections, and similar to TNFi for the remaining outcomes.
Conclusions
In this large cohort of RA patients who used abatacept or TNFi as a first or second-line biologic agent, we found a lower risk of hospitalized infection after initiating abatacept versus TNFi which was driven mostly by infliximab.
Rheumatoid arthritis (RA) patients are at increased risk of infection compared to non-RA patients1–3. Part of this elevated risk is secondary to RA disease and due to the impaired ability of the immune system to recognize and fight off infections4,5. While new immunosuppressive therapies have led to dramatic improvements in controlling RA disease activity and damage, the risk of infection is further increased by immunosuppression, especially with the use of biologic disease-modifying anti-rheumatic drugs (DMARDs)6,7. Tumor necrosis factor inhibitors (TNFi) and abatacept are both biologic DMARDs, used either as monotherapy or in combination with a nonbiologic DMARD, with comparable efficacy in the treatment of RA8. However, the two therapies may differ in the risk of infection with their use.
Infections are frequent adverse events associated with TNFi therapy in RA and the risk of infection with TNFi use is higher than with non-biologic DMARD use6,9,10. While randomized clinical trial data for abatacept did not demonstrate increased risk of serious infections compared to placebo initially, subsequent studies have reported increased incidence of serious infections with its use, with reported IR for infection of 3.1 per 100-patient years 11–15 Although this rate is lower than the reported IR for TNFi of 6 per 100 patient-years6, there is a paucity of studies that have compared the two therapies directly. To date, no randomized clinical trial has compared the risk of infections between the different biologic therapies in RA. Previous observational studies have compared the risk of infections between abatacept and TNFi with mixed results16–19. While some of these studies have demonstrated a lower risk of infection with abatacept, in one study the risk was similar between abatacept and TNFi18.
Given similar efficacy between abatacept and TNFi as biologic therapies for treatment of RA, one of the main determinants in choosing between the medications is minimizing the risk of infection. Therefore, the aim of this study was to compare the risk of hospitalized infections among RA patients who initiate abatacept versus TNFi in the real-world setting using a large US nationwide claims database. We hypothesized that the rates and risk of hospitalized infection would be lower among RA patients initiating abatacept compared to TNFi.
METHODS
Data Source and Cohort Definitions
We used de-identified medical and pharmacy claims data from the Truven MarketScan database (1/1/2006–9/30/2015), which contains longitudinal, comprehensive healthcare data for mostly commercially insured individuals in the U.S. from all 50 states20.
We identified RA patients ages 18 and older with at least 2 RA International Classification of Diseases, 9th revision (ICD-9) codes (714.xx) separated by 7–365 days21. Among these RA patients, we selected new users of abatacept or TNFi (adalimumab, certolizumab, etanercept, golimumab, and infliximab) by the National Drug Codes or J codes, with no dispensing of the medication during at least 365 days of continuous enrollment preceding the date of first dispensing (i.e., index date) of abatacept or TNFi. Patients were required to have the second RA diagnosis code on or before the index date. A diagram of cohort and study design is presented in Supplemental Figure S1. For abatacept initiators, we allowed patients to have used non-biologic DMARDs or TNFi during the baseline period. For TNFi initiators, we allowed patients to have used non-biologic DMARDs or abatacept during the baseline period. We excluded patients who used rituximab, tocilizumab or tofacitinib prior to the index date from the two groups, as the use of other biologic DMARDs could affect the risk of infection during follow-up. We also excluded patients with malignancy, renal dialysis, HIV/AIDS, and history of solid or bone marrow transplantation at baseline, as these are other known comorbidities that would increase the risk of infection.
Study patients were followed from the day following index date, until the earliest event of death, end of enrollment, switching of therapy from abatacept to TNFi or from TNFi to abatacept or outcome occurrence. In our primary analysis, we censored patients using as-treated analysis which used a threshold of less than 30 days of treatment or dispensing gap. In a separate sensitivity analysis, we allowed for any gap in treatment and censored patients at the last drug available date.
Data Collection
During the 365-day period prior to the index date, we collected baseline covariates that may be related to infectious risk including demographics (age, sex, calendar year of index date, region of residence), comorbidities including hypertension, diabetes, obesity, smoking, alcohol use, depression, cardiovascular disease, chronic renal disease, chronic liver disease, pulmonary disease, viral hepatitis, inflammatory bowel disease, hospitalization for infection by ICD-9 codes, and calculated combined comorbidity index at baseline22. We measured healthcare utilization characteristics including number of outpatient physician visits to primary care providers and specialists, emergency department visits, acute care hospitalizations, history of influenza vaccination and pneumonia vaccination, and number of unique generic drug prescriptions at baseline.
We assessed use of non-biologic DMARDs including methotrexate, hydroxychloroquine, leflunomide, sulfasalazine, cyclosporine, tacrolimus, azathioprine, auranofin, and penicillamine. We also measured glucocorticoid use as any recent use in 30 days prior to the index date, any use during 365 days prior to the index date, and cumulative prednisone-equivalent dose for 365 days baseline period, calculated based on the total amount in milligrams of prednisone prescribed. We assessed any baseline use of non-steroidal anti-inflammatory drugs (NSAIDs), COX-2 enzyme inhibitors (COXIBs), and proton-pump inhibitors. We also assessed use of opioids, antibiotics, and zoster treatment during the 365 days of baseline, and recent use within 30 days prior to the index date.
Outcomes
The primary outcome was the composite end point of any hospitalized infection including bacterial, viral or opportunistic infection based on the principal diagnosis for hospitalization. We assessed secondary outcomes of bacterial infection, herpes zoster, and infections by affected organ system (bone/joint, cardiac, gastrointestinal, genitourinary, respiratory, skin/soft tissue, neurologic), based on the principal diagnosis for hospitalization. We used ICD-9 codes to identify hospitalized infection as previously described, with positive predictive value >80%16,23–25.
Statistical Methods
We compared the baseline characteristics of the abatacept and TNFi cohorts. To control for over 40 potential confounders simultaneously, we generated propensity score (PS) for the predicted probability of a patient initiating abatacept versus TNFi given patient characteristics at baseline. We then performed a 1:1 PS nearest neighbor matching using a caliper of 0.025 on the PS scale26. We compared the covariate balance after matching using standardized differences, and considered the absolute standard mean difference of <0.1 as balanced between the two matched groups27. After PS matching, we estimated the incidence rates (IR) of the primary and secondary outcomes per 1,000 person-years in the two treatment groups. We used Cox proportional hazards models to estimate the hazard ratios (HR) and 95% confidence intervals (CI) for primary and secondary outcomes. We ensured the proportional hazards assumption was not violated by including the interaction term of exposure medication and survival time as a time-dependent covariate in our Cox model.
We performed separate PS matching for abatacept versus the 3 most commonly prescribed TNFi (adalimumab, etanercept and infliximab) and calculated the IR per 1,000 person-years and HR for the primary outcome of any hospitalized infection. In another sensitivity analysis, we identified and PS-matched patients who were treatment naïve and had not received either TNFi or abatacept in the baseline period and calculated the IR and HR for primary and secondary outcomes.
All analyses were conducted using SAS 9.4 (SAS Institute, Cary, NC). The Institutional Review Board of the Brigham and Women’s Hospital approved this study.
RESULTS
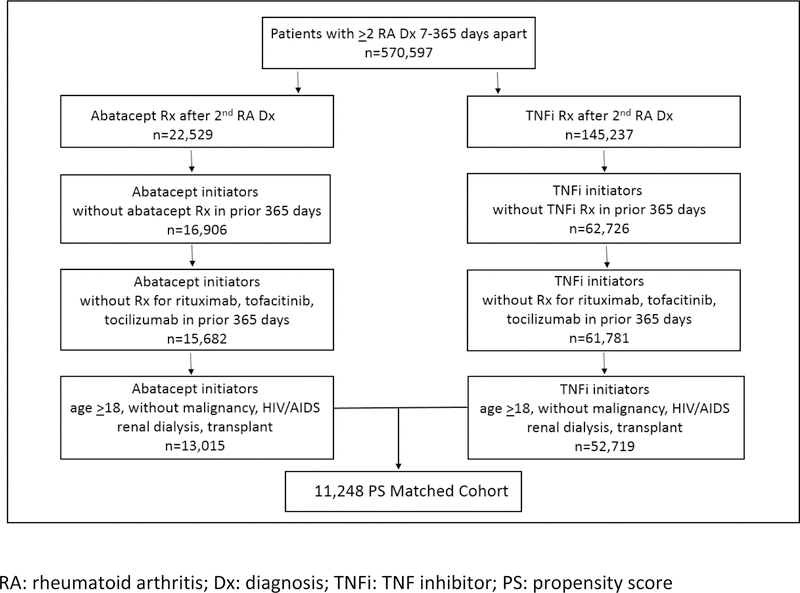
We identified 13,015 RA patients who were new initiators of abatacept and 52,719 RA patients who were new initiators of TNFi (Figure 1). After 1:1 PS matching, there were 11,248 pairs of patients initiating abatacept and TNFi. Baseline covariates were balanced after PS matching with absolute standardized mean difference <0.1. Prior to matching, the abatacept cohort was slightly older (54.8 ± 12.8 vs 52.1 ± 12.8) with a higher proportion of females (83% vs 76%) (Table 1). After PS matching, the abatacept cohort had a mean age of 55.3 ± 12.8 with 83% females, and TNFi cohort had a mean age of 55.5 ± 12.7 with 84% females.
Figure 1. Flow chart of study cohort selection.
RA: rheumatoid arthritis; Dx: diagnosis; TNFi: TNF inhibitor; PS: propensity score
Table 1.
Baseline characteristics of study cohorts, prior to and after 1:1 propensity score-matching
| Prior to matching | Propensity score-matched | |||
|---|---|---|---|---|
| Abatacept | TNFi | Abatacept | TNFi | |
| N=13,015 | N=52,719 | N=11,248 | N=11,248 | |
| Demographics | ||||
| Mean age (SD), years | 54.8 (12.8) | 52.1 (12.8) | 55.3 (12.8) | 55.5 (12.7) |
| Female sex | 83% | 76% | 83% | 84% |
| Region | ||||
| Northeast | 13% | 14% | 13% | 13% |
| South | 39% | 40% | 39% | 39% |
| North central | 21% | 22% | 21% | 21% |
| West | 15% | 16% | 15% | 15% |
| Unknown | 11% | 9% | 12% | 12% |
| Comorbidities | ||||
| Obesity | 9% | 9% | 9% | 9% |
| Smoking | 12% | 12% | 12% | 13% |
| Alcohol use | 1% | 1% | 1% | 1% |
| Depression | 12% | 11% | 12% | 12% |
| Diabetes | 17% | 15% | 18% | 18% |
| Hypertension | 42% | 37% | 43% | 43% |
| Hyperlipidemia | 32% | 30% | 32% | 32% |
| Cardiovascular disease | 56% | 49% | 57% | 57% |
| Heart failure | 4% | 2% | 4% | 5% |
| Pulmonary disease | 19% | 16% | 20% | 20% |
| Chronic kidney disease | 4% | 3% | 4% | 4% |
| Chronic liver disease | 5% | 5% | 5% | 5% |
| Viral hepatitis | 1% | 1% | 1% | 1% |
| Inflammatory bowel disease | 1% | 3% | 1% | 1% |
| Hospitalized infection | 3% | 2% | 3% | 3% |
| Mean combined comorbidity score (SD) | 0.54 (1.38) | 0.38 (1.18) | 0.57 (1.42) | 0.58 (1.43) |
| Healthcare utilization | ||||
| Mean No. PCP visits (SD) | 6.1 (7.8) | 5.3 (6.5) | 6.1 (7.9) | 6.1 (7.9) |
| Mean No. Rheumatology visits (SD) | 4.5 (4.7) | 3.4 (3.7) | 4.4 (4.7) | 4.2 (4.7) |
| ED visits | 31% | 28% | 31% | 31% |
| Any hospitalization | 17% | 13% | 18% | 18% |
| Mean No. unique prescriptions | 14.4 (8.2) | 13.2 (7.5) | 14.1 (8.3) | 14.1 (8.2) |
| Flu vaccination | 30% | 25% | 29% | 29% |
| Pneumonia vaccination | 7% | 8% | 7% | 7% |
The prevalence of several comorbidities was slightly higher among the abatacept cohort including diabetes, hypertension, cardiovascular disease, pulmonary disease but was well balanced between the two cohorts after PS matching. The combined comorbidity score was also higher among the abatacept cohort compared to the TNFi cohort (0.54 ± 1.38 vs 0.38 ± 1.18), and after PS matching was similar between the two cohorts (0.57 ± 1.42 vs 0.58 ± 1.43). Measures of healthcare utilization were also generally higher in the abatacept cohort but was balanced after PS matching. Baseline hospitalized infection prevalence was 3% for both PS matched cohorts.
Use of RA-related medication was well-balanced between the PS matched cohorts, although prior to matching there was a lower prior use of methotrexate, hydroxychloroquine, and sulfasalazine in the abatacept cohort (Table 2). Steroid use was prevalent among both cohorts at 30 days prior to the index date (44% for abatacept, 42% for TNFi), and at 365 days prior (70% vs 69%). Notably, the 58% of the abatacept cohort had prescription dispensing for TNFi in the baseline period, compared to 4% of TNFi cohort patients who had prescription dispensing for abatacept.
Table 2.
Baseline medications of study cohorts, prior to and after 1:1 propensity score-matching
| Prior to matching | Propensity score-matched | |||
|---|---|---|---|---|
| Abatacept | TNFi | Abatacept | TNFi | |
| N=13,015 | N=52,719 | N=11,248 | N=11,248 | |
| Prior use of biologic DMARDs | ||||
| Abatacept | 0% | 2% | 0% | 4% |
| TNFi | 64% | 0% | 58% | 0% |
| Adalimumab | 24% | 0% | 16% | 0% |
| Certolizumab | 4% | 0% | 2% | 0% |
| Etanercept | 24% | 0% | 16% | 0% |
| Golimumab | 4% | 0% | 3% | 0% |
| Infliximab | 21% | 0% | 20% | 0% |
| Prior use of non-biologic DMARDs | ||||
| Methotrexate | 56% | 69% | 55% | 55% |
| Hydroxychloroquine | 23% | 27% | 23% | 23% |
| Leflunomide | 18% | 14% | 18% | 18% |
| Sulfasalazine | 9% | 12% | 9% | 8% |
| Other* | 8% | 5% | 8% | 8% |
| Steroid use | ||||
| Steroid use | 70% | 69% | 68% | 67% |
| Recent steroid use (30 days prior) | 44% | 42% | 43% | 43% |
| Mean steroids cumulative dose 365 days in mg (SD) | 1235.9 (3484.3) | 1184.7 (9042.6) | 1192.9 (3572.8) | 1191.6 (6178.4) |
| Other Medications | ||||
| Antibiotics | 69% | 64% | 69% | 68% |
| Recent antibiotics (30 days prior) | 19% | 16% | 19% | 19% |
| Antiviral for zoster | 8% | 6% | 8% | 8% |
| Recent antiviral for zoster | 3% | 2% | 3% | 3% |
| NSAIDs | 44% | 54% | 43% | 42% |
| COXIBs | 11% | 11% | 11% | 11% |
| Opioids | 68% | 64% | 67% | 67% |
| Recent opioids (30 days prior) | 40% | 33% | 39% | 40% |
| Proton-pump inhibitors | 30% | 26% | 30% | 30% |
Other non-biologic DMARDs: cyclosporine, tacrolimus, azathioprine, auranofin, penicillamine
The overall IR of the primary outcome for the composite endpoint of any hospitalized infections in our PS matched cohorts was 36.7 per 1,000 person-years (95% CI 31.8–42.3) for abatacept compared to 47.4 per 1,000 person-years (95% CI 41.5–54.1) for TNFi using as-treated analysis allowing for <30 days gap in treatment (Table 3). In the primary as-treated analysis allowing for <30 days gap in treatment, the mean follow-up time on active treatment was 0.46±0.70 years for the abatacept group, and 0.41±0.66 years for the TNFi group. The risk of hospitalized infection in abatacept was lower compared to TNFi initiators with a HR of 0.78 (95% CI 0.64–0.95).
Table 3.
Risk of hospitalized infection in abatacept versus TNFi initiators: 1:1 PS-matched analysis
| Abatacept (N=11,248) | TNFi (N=11,248) | |||||||
|---|---|---|---|---|---|---|---|---|
| No. Events | Person-Years | IR, per 1000 PY (95% CI) | HR (95% CI) | No. Events | Person-years | IR, per 1000 PY (95% CI) | HR (95% CI) | |
| As-Treated (<30 days gap) | 188 | 5,126 | 36.7 (31.8–42.3) | 0.78 (0.64–0.95) | 219 | 4,621 | 47.4 (41.5–54.1) | 1.0 |
| As-Treated (Any gap) | 298 |
8,201 | 36.3 (32.4–40.7) | 0.86 (0.74–1.01) | 321 | 7,639 | 42.0 (37.7–46.9) | 1.0 |
In our PS matched sensitivity analyses between abatacept and the 3 most common TNFi, we found that the HR remained decreased for abatacept compared to infliximab (HR 0.63, 95% CI 0.47–0.85), but not statistically significantly higher or lower when compared to adalimumab (HR 0.78, 95% CI 0.57–1.06) and etanercept (HR 1.19, 95% CI 0.92–1.53) (Table 4). In a separate sensitivity analysis using as-treated analysis allowing for any gap in treatment the risk of infection was attenuated towards the null. When we broadened our outcome definition of infections using diagnosis codes at any position and not limited to the principal diagnosis code for hospitalization, the results were similar with HR of infection for abatacept compared to TNFi of 0.79 (95% CI 0.68–0.92). In secondary analyses assessing risk of separate types of infections, the HR was lower for respiratory infections among abatacept initiators compared to TNFi (Table 5). However, there was no significant difference in the risk of the other types of infections between the abatacept and TNFi cohorts.
Table 4.
Risk of hospitalized infection in abatacept versus adalimumab, etanercept and infliximab: 1:1 PS-matched analyses
| Abatacept | TNFi | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. Patients | No. Events | Person-Years | IR, per 1000 PY (95% CI) | HR (95% CI) | No. Patients | No. Events | Person-years | IR, per 1000 PY (95% CI) | HR (95% CI) | |
|
Abatacept vs. adalimumab |
3,737 |
77 |
2,668 |
28.9 (23.1–36.1) |
0.78 (0.57–1.06) |
3,737 |
85 |
2,306 |
36.9 (29.8–45.6) |
1.0 |
| Abatacept vs. etanercept | 4,550 | 128 | 3,161 | 40.5 (34.1–48.2) | 1.19 (0.92–1.53) | 4,550 | 112 | 3,284 | 34.1 (28.3–41.1) | 1.0 |
| Abatacept vs. infliximab | 2,118 | 70 | 1,677 | 41.8 (33.0–52.8) | 0.63 (0.47–0.85) | 2,118 | 104 | 1,573 | 66.1 (54.5–80.1) | 1.0 |
Table 5.
Risk of secondary outcomes for abatacept versus TNFi initiators: 1:1 PS-matched analysis for as-treated (<30 days gap)
| Abatacept (N=11,248) | TNFi (N=11,248) | |||||||
|---|---|---|---|---|---|---|---|---|
| No. Events | Person-Years | IR, per 1000 PY (95% CI) | HR (95% CI) | No. Events | Person-years | IR, per 1000 PY | HR (95% CI) | |
|
Bacterial infection |
100 |
5,160 |
19.4 (15.9–23.6) |
0.81 (0.62–1.06) |
112 |
4,621 |
24.0 (20.0–28.9) |
1.0 |
| Herpes zoster | 81 | 5,141 | 15.8 (12.7–19.6) | 1.00 (0.73–1.37) | 73 | 4,639 | 15.7 (12.5–19.8) | 1.0 |
|
By organ system | ||||||||
| Bone/Joint | 7 | 5,190 | 1.4 (0.6–2.8) | 0.91 (0.32–2.60) | 7 | 4,696 | 1.5 (0.7–3.1) | 1.0 |
| Cardiac | 0 | 5,191 | - | - | 1 | 4,697 | 0.2 (<0.1–1.5) | 1.0 |
| Gastrointestinal | 20 | 5,186 | 3.9 (2.5–6.0) | 0.75 (0.41–1.35) | 24 | 4,689 | 5.1 (3.4–7.6) | 1.0 |
| Genitourinary | 17 | 5,185 | 3.3 (2.0–5.3) | 1.04 (0.52–2.07) | 15 | 4,690 | 3.2 (1.9–5.3) | 1.0 |
| Respiratory | 65 | 5,160 | 12.6 (9.9–16.1) | 0.71 (0.52–0.99) | 83 | 4,666 | 17.8 (14.4–22.1) | 1.0 |
| Skin/soft tissue | 30 | 5,179 | 5.8 (4.1–8.3) | 0.68 (0.42–1.09) | 40 | 4,683 | 8.5 (6.3–11.6) | 1.0 |
| Neurologic | 1 | 5,191 | 0.2 (<0.1–1.4) | 0.91 (0.06–14.5) | 1 | 4,697 | 0.2 (<0.1–1.5) | 1.0 |
As our abatacept initiator could have been treated with TNFi previously, and TNFi initiators could similarly have been treated with abatacept, we conducted sensitivity analysis for our primary and secondary outcomes for only patients who were treatment naïve to both therapies during the baseline period (Supplemental Table S1). After PS-matching we identified 4,574 treatment-naïve abatacept initiators and equal number of TNFi initiators. The HR for any hospitalized infection for abatacept compared to TNFi was 0.87 (95% CI 0.68–1.11).
DISCUSSION
RA patients are at increased risk of infections and this risk is further increased with use of immunosuppressive therapy. Whether there is a difference in infectious risk conferred by different biologic DMARDs is unclear. In this US nationwide study of RA patients, we found that the IR and risk of hospitalized infections were lower among patients initiating abatacept compared to TNFi. This difference in risk appears to be mostly driven by lower infection risk when compared to infliximab. In our secondary outcome analysis, risk of respiratory infections (e.g., pneumonia, empyema, upper respiratory tract infections) was also lower among patients in the abatacept cohort compared to the TNFi cohort. However, there was no significant difference in the risk of hospitalized infections by the remaining types of infections or by affected organ systems.
The IR for infections found in our study are similar to what has been reported for abatacept and TNFi in the literature, with reported IR for abatacept of 31 per 1,000 person-years, and 60 per 1,000 person-years for TNFi in separate studies6,13. Further, the results are in line with most of the other studies that have compared the risk of infection between abatacept and TNFi. In a previous cohort study using Truven MarketScan data comparing the risk of infections in RA patients switching from first-line TNFi to rituximab, another TNFi or abatacept, the risk of infection was similar after switching to abatacept versus rituximab19. However, the infection risk was higher for those switching to another TNFi compared to rituximab. Similarly, a cohort study in Medicare data with RA patients who were previously treated with a biologic agent and newly switching to a different TNFi, rituximab, tocilizumab or abatacept found that the risk of one-year hospitalized infection was higher in the etanercept (HR 1.24, 95% CI 1.07–1.45), infliximab (HR 1.39, 95% CI 1.21–1.60) and rituximab (HR 1.36, 95% CI 1.21–1.53) groups compared to abatacept as reference17.
In another previous study using administrative claims data from 2005–2009, among RA patients who were initiating or switching biologic DMARD therapy, abatacept users had lower rates of hospitalized infection compared to infliximab (HR 0.68, 95% CI 0.48–0.96)16. The risk of infection was also lower for adalimumab and etanercept compared to infliximab in that study, while in our study we observed a lower infection risk for abatacept compared to infliximab (HR 0.63, 95% CI 0.47–0.85), a numerically lower risk for abatacept compared to adalimumab (HR 0.78, 95% CI 0.57–1.06), and similar risk for abatacept compared to etanercept (see Table 4).
In a predominantly male RA cohort of US veterans from 1998–2011, the risk of hospitalized bacterial infections was similar between abatacept compared to etanercept (HR 1.1, 95% CI 0.6–21), with IR for abatacept of 28 per 1,000 person-years (95% CI 17–47)18. These rates are in line with the hospitalized bacterial infection IR of 19.4 per 1,000 person-years (95% CI 15.9–23.6), and HR 0.81 (95% CI 0.62–1.06) in our study. Additionally, similar to our study results, previous reports comparing the risk of herpes zoster infections found no difference in the risk of zoster infections between abatacept and TNFi, with the IR of zoster infections slightly higher in previously reported literature for abatacept (IR 23.3 per 1,000 person-years, 95% CI 20.4–26.7 in one study28, and IR 18.7 per 1,000 person-years, 95% CI 15.8–22.0 in another29) compared to our study IR of 15.7 per 1,000 person-years (95% CI 12.7–19.6) in abatacept and 15.7 per 1,000 person-years (95% CI 12.5–19.8) in TNFi.
In our separate analysis comparing the risk of hospitalized infection in abatacept initiators versus the three most commonly prescribed TNFi, we found that the lower risk of hospitalized infection in abatacept initiators compared to TNFi was driven mostly by infliximab initiators (HR 0.63, 95% CI 0.47–0.85). The risk remained numerically, not statistically significantly, lower for abatacept in comparison to adalimumab initiators (HR 0.78, 95% 0.57–1.06), but the infection risk associated with abatacept was noted to be not significantly higher versus etanercept (HR 1.19, 95% CI 0.92–1.53). These results are in line with previously reported lower risk of serious infections in patients treated with etanercept compared to infliximab and adalimumab in a prospective cohort of RA patients in a Dutch registry30. There are several potential explanations for this observed difference among TNFi. The peak concentration of infliximab which is delivered as an intravenous infusion is higher than that of adalimumab and etanercept which are administered as subcutaneous injections31. Additionally, the clearance of etanercept is significantly higher with lower steady-state drug level compared to infliximab and adalimumab, and the binding avidity to TNF subunits is lower for etanercept (1:1 ratio), compared to infliximab (up to 3:1 ratio)31,32. Furthermore, etanercept is a TNF receptor fusion protein that binds only circulating TNF while infliximab is a monoclonal antibody against TNF and additionally has a cytotoxic effect by binding to cells that express TNF on their membranes33.
Our study has several strengths as a large cohort of US patients with RA in a nationwide database with a new user design with active comparator. In comparison to the previously mentioned studies, we used PS matching to minimize confounding by indication and control for imbalance among the abatacept and TNFi treatment groups to including patient characteristics, regional variation and adjusted for a large number of baseline confounders between the two cohorts such as prior antibiotic and antiviral use, and history of vaccinations. We directly compared the IR and risk of infection between abatacept and TNFi inhibitors and found lower IR of infections for abatacept compared to TNFi, similar to the reported IR in the literature, and lower risk of infection among abatacept initiators. The reason for this decreased risk of infection found in our study and previous studies is unknown but may potentially be due to the fact that abatacept indirectly blocks T-cell costimulation rather than the mechanism of directly inhibiting cytokines for TNFi11.
In a separate analysis using as-treated analysis allowing for any gap in treatment, there was no significant difference in risk of infection between the abatacept and TNFi cohorts. In our primary analysis, we used an as-treated analysis with <30 days of gap in treatment which requires high treatment adherence. Using this design, we observed a lower risk of hospitalized infection in abatacept initiators. This suggests that there may be selection bias for patients who are more adherent or continued on therapy versus those who discontinue their therapy for those on abatacept versus TNFi. Although it is not possible to ascertain the reasons for stopping the therapy after initiation using claims databases, one possibility is that there is a lower threshold to stop or switch from TNFi therapy if there was concern of risk of infection such as minor infections which may lower the rates of hospitalized infections for TNFi compared to abatacept.
It is notable that in our study which examined first-line or subsequent-line initiators of abatacept or TNFi, 58% of the abatacept cohort was previously on TNFi in the baseline period and would be classified as switchers, compared to only 4% of TNFi cohort who had previously used abatacept. In our sensitivity analysis, among patients who were treatment naïve to both abatacept and TNFi, the lower risk of infection among abatacept initiators compared to TNFi was attenuated toward the null. This suggests a potential role of RA disease severity of treatment history in addition to the types of biologics on the risk of infection in RA patients.
Our study has limitations. Although we used PS matching for confounding control, there is still a concern for partially measured covariates and lack of information on other covariates that may affect risk of infection. For instance, RA disease activity is an important factor that can affect the risk of infections34, but is a covariate we are unable to capture in a claims database35–38. Therefore, we included measures of steroid use, non-biologic DMARD use, number of rheumatology visits, and combined comorbidity index as covariates in our PS-matching. Steroid use was a covariate in our analysis, is another important factor in risk of infections and may be indirectly related to disease activity. However, there may be discrepancies between steroid dispensing measured in our study and actual use by patients39–41. We also attempted to assess sero-status as a covariate were limited by power as less than 3% of patients in each cohort had available laboratory results. Additionally, socioeconomic status and race and ethnicity are important demographic factors in the US that contribute to health disparities and outcomes that we were unable to collect and adjust for in our study42,43.
In this large nationwide cohort of RA patients in the U.S. initiating abatacept or a TNFi as a first- or second-line biologic therapy, we found a lower risk of any hospitalized infections associated with abatacept versus TNFi, particularly in comparison to infliximab, suggesting that RA patients with specific concerns about infections may benefit from use of abatacept compared to TNFi.
Supplementary Material
Significance and Innovation.
Comparative safety of infection risk associated with biologic immunosuppression with TNF inhibitors versus abatacept in RA patients is unclear
In this propensity score-matched study of 11,248 pairs of RA patients initiating abatacept or TNF inhibitor, the incidence rate and risk of hospitalized infections were lower among abatacept initiators (HR 0.78, 95% CI 0.64–0.95)
In subgroup analysis, the risk of infection for abatacept remained lower when compared to infliximab initiators, but not for etanercept and abatacept initiators
Use of abatacept is associated with a lower risk of hospitalized infections compared to TNF inhibitors among RA patients, in particular when compared to infliximab.
Acknowledgments
SOURCES OF FUNDING
This study was funded by an investigator-sponsored research grant from Bristol-Myers Squibb. The funding sources played no role in the study design, data analysis or interpretation of data or presentation of results. The funder was given the opportunity to make non-binding comments on a draft of the manuscript, but the authors retained the right of publication and to determine the final wording.
DISCLOSURES
SK Chen is supported by NIH Institutional Training Grant (T32 AR 7530–33).
KP Liao is supported by the Harold and DuVal Bowen Fund,
SC Kim received research support from Bristol-Myers Squibb, Roche, and Pfizer to the Brigham and Women’s Hospital.
J Liu has nothing to disclose.
REFERENCES
- 1.Doran MF, Crowson CS, Pond GR, O’Fallon WM, Gabriel SE. Frequency of infection in patients with rheumatoid arthritis compared with controls: a population-based study. Arthritis Rheum 2002;46(9):2287–2293. [DOI] [PubMed] [Google Scholar]
- 2.Mitchell DM, Spitz PW, Young DY, Bloch DA, McShane DJ, Fries JF. Survival, prognosis, and causes of death in rheumatoid arthritis. Arthritis Rheum 1986;29(6):706–714. [DOI] [PubMed] [Google Scholar]
- 3.Smitten AL, Choi HK, Hochberg MC, Suissa S, Simon TA, Testa MA, Chan KA. The risk of hospitalized infection in patients with rheumatoid arthritis. J Rheumatol 2008;35(3):387–393. [PubMed] [Google Scholar]
- 4.Mikuls TR. Co-morbidity in rheumatoid arthritis. Best Pract Res Clin Rheumatol 2003;17(5):729–752. [DOI] [PubMed] [Google Scholar]
- 5.Wagner UG, Koetz K, Weyand CM, Goronzy JJ. Perturbation of the T cell repertoire in rheumatoid arthritis. Proc Natl Acad Sci U S A 1998;95(24):14447–14452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Listing J, Strangfeld A, Kary S, Rau R, von Hinueber U, Stoyanova-Scholz M, Gromnica-Ihle E, Antoni C, Herzer P, Kekow J, Schneider M, Zink A. Infections in patients with rheumatoid arthritis treated with biologic agents. Arthritis Rheum 2005;52(11):3403–3412. doi: 10.1002/art.21386 [DOI] [PubMed] [Google Scholar]
- 7.Ruderman EM. Overview of safety of non-biologic and biologic DMARDs. Rheumatology 2012;51(suppl 6):vi37–vi43. [DOI] [PubMed] [Google Scholar]
- 8.Curtis JR, Singh JA. Use of Biologics in Rheumatoid Arthritis: Current and Emerging Paradigms of Care. Clin Ther 2011;33(6):679–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strangfeld A, Listing J, Herzer P, Liebhaber A, Rockwitz K, Richter C, Zink A. Risk of herpes zoster in patients with rheumatoid arthritis treated with anti-TNF-alpha agents. JAMA 2009;301(7):737–744. [DOI] [PubMed] [Google Scholar]
- 10.Kroesen S, Widmer AF, Tyndall A, Hasler P. Serious bacterial infections in patients with rheumatoid arthritis under anti-TNF-alpha therapy. Rheumatology (Oxford) 2003;42(5):617–621. [DOI] [PubMed] [Google Scholar]
- 11.Genovese MC, Becker J-C, Schiff M, Luggen M, Sherrer Y, Kremer J, Birbara C, Box J, Natarajan K, Nuamah I, Li T, Aranda R, Hagerty DT, Dougados M. Abatacept for rheumatoid arthritis refractory to tumor necrosis factor alpha inhibition. N Engl J Med 2005;353(11):1114–1123. [DOI] [PubMed] [Google Scholar]
- 12.Salliot C, Dougados M, Gossec L. Risk of serious infections during rituximab, abatacept and anakinra treatments for rheumatoid arthritis: meta-analyses of randomised placebo-controlled trials. Ann Rheum Dis 2009;68(1):25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simon TA, Askling J, Lacaille D, Franklin J, Wolfe F, Covucci A, Suissa S, Hochberg MC, Abatacept Epidemiology Study Group. Infections requiring hospitalization in the abatacept clinical development program: an epidemiological assessment. Arthritis Res Ther 2010;12(2):R67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weinblatt M, Combe B, Covucci A, Aranda R, Becker JC, Keystone E. Safety of the selective costimulation modulator abatacept in rheumatoid arthritis patients receiving background biologic and nonbiologic disease-modifying antirheumatic drugs: A one-year randomized, placebo-controlled study. Arthritis Rheum 2006;54(9):2807–2816. [DOI] [PubMed] [Google Scholar]
- 15.Kremer JM, Genant HK, Moreland LW, Russell AS, Emery P, Abud-Mendoza C, Szechinski J, Li T, Ge Z, Becker J-C, Westhovens R. Effects of abatacept in patients with methotrexate-resistant active rheumatoid arthritis: a randomized trial. Ann Intern Med 2006;144(12):865–876. [DOI] [PubMed] [Google Scholar]
- 16.Curtis JR, Xie F, Chen L, Baddley JW, Beukelman T, Saag KG, Spettell C, McMahan RM, Fernandes J, Winthrop K, Delzell E. The comparative risk of serious infections among rheumatoid arthritis patients starting or switching biological agents. Ann Rheum Dis 2011;70(8):1401–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yun H, Xie F, Delzell E, Levitan EB, Chen L, Lewis JD, Saag KG, Beukelman T, Winthrop KL, Baddley JW, Curtis JR. Comparative Risk of Hospitalized Infection Associated With Biologic Agents in Rheumatoid Arthritis Patients Enrolled in Medicare. Arthritis Rheumatol 2016;68(1):56–66. [DOI] [PubMed] [Google Scholar]
- 18.Curtis JR, Yang S, Patkar NM, Chen L, Singh JA, Cannon GW, Mikuls TR, Delzell E, Saag KG, Safford MM, DuVall S, Alexander K, Napalkov P, Winthrop KL, Burton MJ, Kamauu A, Baddley JW. Risk of Hospitalized Bacterial Infections Associated With Biologic Treatment Among US Veterans With Rheumatoid Arthritis. Arthritis Care Res (Hoboken) 2014;66(7):990–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnston SS, Turpcu A, Shi N, Fowler R, Chu B-C, Alexander K. Risk of infections in rheumatoid arthritis patients switching from anti-TNF agents to rituximab, abatacept, or another anti-TNF agent, a retrospective administrative claims analysis. Semin Arthritis Rheum 2013;43(1):39–47. [DOI] [PubMed] [Google Scholar]
- 20.Desai RJ, Rao JK, Hansen RA, Fang G, Maciejewski M, Farley J. Tumor Necrosis Factor- Inhibitor Treatment and the Risk of Incident Cardiovascular Events in Patients with Early Rheumatoid Arthritis: A Nested Case-control Study. J Rheumatol 2014;41(11):2129–2136. [DOI] [PubMed] [Google Scholar]
- 21.Kim S, Servi A, Polinski J. Validation of rheumatoid arthritis diagnoses in health care utilization data. Arthritis Res Ther 2011;13(1):R32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gagne JJ, Glynn RJ, Avorn J, Levin R, Schneeweiss S. A combined comorbidity score predicted mortality in elderly patients better than existing scores. J Clin Epidemiol 2011;64(7):749–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Curtis JR, Xie F, Yun H, Saag KG, Chen L, Delzell E. Risk of hospitalized infection among rheumatoid arthritis patients concurrently treated with a biologic agent and denosumab. Arthritis Rheumatol (Hoboken, NJ) 2015;67(6):1456–1464. [DOI] [PubMed] [Google Scholar]
- 24.Schneeweiss S, Robicsek A, Scranton R, Zuckerman D, Solomon DH. Veteran’s affairs hospital discharge databases coded serious bacterial infections accurately. J Clin Epidemiol 2007;60(4):397–409. [DOI] [PubMed] [Google Scholar]
- 25.Patkar NM, Curtis JR, Teng GG, Allison JJ, Saag M, Martin C, Saag KG. Administrative codes combined with medical records based criteria accurately identified bacterial infections among rheumatoid arthritis patients. J Clin Epidemiol 2009;62(3):321–327.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ripollone JE, Huybrechts KF, Rothman KJ, Ferguson RE, Franklin JM. Implications of the Propensity Score Matching Paradox in Pharmacoepidemiology. Am J Epidemiol 2018;187(9):1951–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Austin PC. Using the Standardized Difference to Compare the Prevalence of a Binary Variable Between Two Groups in Observational Research. Commun Stat - Simul Comput 2009;38(6):1228–1234. doi: 10.1080/03610910902859574 [DOI] [Google Scholar]
- 28.Curtis JR, Xie F, Yun H, Bernatsky S, Winthrop KL. Real-world comparative risks of herpes virus infections in tofacitinib and biologic-treated patients with rheumatoid arthritis. Ann Rheum Dis 2016;75(10):1843–1847. doi: 10.1136/annrheumdis-2016-209131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yun H, Xie F, Delzell E, Chen L, Levitan EB, Lewis JD, Saag KG, Beukelman T, Winthrop K, Baddley JW, Curtis JR. Risks of Herpes Zoster in Patients With Rheumatoid Arthritis According to Biologic Disease-Modifying Therapy. Arthritis Care Res (Hoboken) 2015;67(5):731–736. doi: 10.1002/acr.22470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Dartel SAA, Fransen J, Kievit W, Flendrie M, den Broeder AA, Visser H, Hartkamp A, van de Laar MAFJ, van Riel PLCM. Difference in the risk of serious infections in patients with rheumatoid arthritis treated with adalimumab, infliximab and etanercept: results from the Dutch Rheumatoid Arthritis Monitoring (DREAM) registry. Ann Rheum Dis 2013;72(6):895–900. [DOI] [PubMed] [Google Scholar]
- 31.Furst DE, Wallis R, Broder M, Beenhouwer DO. Tumor Necrosis Factor Antagonists: Different Kinetics and/or Mechanisms of Action May Explain Differences in the Risk for Developing Granulomatous Infection. Semin Arthritis Rheum 2006;36(3):159–167. [DOI] [PubMed] [Google Scholar]
- 32.Scallon B, Cai A, Solowski N, Rosenberg A, Song X-Y, Shealy D, Wagner C. Binding and functional comparisons of two types of tumor necrosis factor antagonists. J Pharmacol Exp Ther 2002;301(2):418–426. [DOI] [PubMed] [Google Scholar]
- 33.Scallon BJ, Moore MA, Trinh H, Knight DM, Ghrayeb J. Chimeric anti-TNF-alpha monoclonal antibody cA2 binds recombinant transmembrane TNF-alpha and activates immune effector functions. Cytokine 1995;7(3):251–259. [DOI] [PubMed] [Google Scholar]
- 34.Au K, Reed G, Curtis JR, Kremer JM, Greenberg JD, Strand V, Furst DE, CORRONA Investigators. High disease activity is associated with an increased risk of infection in patients with rheumatoid arthritis. Ann Rheum Dis 2011;70(5):785–791. [DOI] [PubMed] [Google Scholar]
- 35.Wolfe F, Michaud K, Simon T. Can severity be predicted by treatment variables in rheumatoid arthritis administrative data bases? J Rheumatol 2006;33(10):1952–1956. [PubMed] [Google Scholar]
- 36.Ting G, Schneeweiss S, Scranton R, Katz JN, Weinblatt ME, Young M, Avorn J, Solomon DH. Development of a health care utilisation data-based index for rheumatoid arthritis severity: a preliminary study. Arthritis Res Ther 2008;10(4):R95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Desai RJ, Solomon DH, Weinblatt ME, Shadick N, Kim SC. An external validation study reporting poor correlation between the claims-based index for rheumatoid arthritis severity and the disease activity score. Arthritis Res Ther 2015;17(1):83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vinet E, Kuriya B, Widdifield J, Bernatsky S. Rheumatoid Arthritis Disease Severity Indices in Administrative Databases: A Systematic Review. J Rheumatol 2011;38(11):2318–2325. [DOI] [PubMed] [Google Scholar]
- 39.Saag KG, Koehnke R, Caldwell JR, Brasington R, Burmeister LF, Zimmerman B, Kohler JA, Furst DE. Low dose long-term corticosteroid therapy in rheumatoid arthritis: an analysis of serious adverse events. Am J Med 1994;96(2):115–123. [DOI] [PubMed] [Google Scholar]
- 40.Doran MF, Crowson CS, Pond GR, O’Fallon WM, Gabriel SE. Predictors of infection in rheumatoid arthritis. Arthritis Rheum 2002;46(9):2294–2300. [DOI] [PubMed] [Google Scholar]
- 41.Schneeweiss S, Setoguchi S, Weinblatt ME, Katz JN, Avorn J, Sax PE, Levin R, Solomon DH. Anti–tumor necrosis factor α therapy and the risk of serious bacterial infections in elderly patients with rheumatoid arthritis. Arthritis Rheum 2007;56(6):1754–1764. [DOI] [PubMed] [Google Scholar]
- 42.Kawachi I, Daniels N, Robinson DE. Health disparities by race and class: why both matter. Health Aff (Millwood) 2005;24(2):343–352. [DOI] [PubMed] [Google Scholar]
- 43.Betancourt JR, Green AR, Carrillo JE, Ananeh-Firempong O. Defining cultural competence: a practical framework for addressing racial/ethnic disparities in health and health care. Public Health Rep 2003;118(4):293–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.